- 1School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
- 2Research Center of Mental and Neurological Disorders, School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
- 3Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, China
- 4School of Science, Beijing Technology and Business University, Beijing, China
The antidepressant mechanism of acupuncture has not been fully elucidated recently. Thus, the objective of the present study is to investigate the antidepressant mechanism of acupuncture of modulating the neuroinflammation induced by high mobility group box-1 (HMGB1) in rats subjected to chronic restraint stress (CRS). Forty-four male Sprague Dawley rats were randomly divided into control, model, escitalopram, and acupuncture group. Except for rats in the control group, all rats were exposed to CRS for 21 days continuously. Rats in the escitalopram group were subjected to a suspension of escitalopram and saline. One hour before CRS procedures, acupuncture was performed at Baihui (GV20) and Yintang (GV29) for rats in the acupuncture group, 20 min per day for 21 days. All rats in each group were conducted to detect the body weight, sucrose preference test at 0, 7, 14, 21 days to evaluate the depression-like behaviors. The expression of microglial activation and HMGB1 in the hippocampus was detected by immunofluorescence. The expression of hippocampal interleukin-10 (IL-10) was detected by western blot. And the content of serum tumor necrosis factor-α (TNF-α) was detected by the enzyme-linked immunosorbent assay method. CRS-exposed rats showed obviously decreased body weight and sucrose preference when compared with the control group, which was reversed by acupuncture. The results have also shown that acupuncture ameliorated the CRS-induced activation of microglia and HMGB1 in the hippocampus CA1 region. Furthermore, acupuncture reduced the stress-induced upregulation of TNF-α in serum. Collectively, the current study highlights the role of acupuncture in alleviating depressive behavior associated with stress-induced neuroinflammation mediated by HMGB1 in the CRS model of depression.
Introduction
Depression is one of the most common mental disorder diseases, which is also a leading cause of global burden (1, 2). Depression often presents with durable and significant sadness, lack of interest and pleasure, or appetite and sleep disorders, cognitive bias and cognitive impairment (1, 3). Reports have showed that approximately 5% of adults worldwide suffer from depression and even more than 700, 000 people are subjected to suicide every year. Depression is not only the major contributor to the global disease burden, but also the main cause of disability all over the world (4). At present, the treatment for depression is mainly pharmacological antidepressants, which has been confirmed the rate of non-responsiveness ranging from 30 to 50% (5). The antidepressants are also likely to cause side effects such as headache, insomnia, tachycardia, hypertension, anorexia, or gastrointestinal reactions, which might bring seriously side effects to the depressed patients (6, 7). Currently, the delayed curative effect, and insufficient incidence and remission rate indicate that multi-factors are involved in the etiology of depression (8). Accordingly, there is urgent challenge for us to illustrate the pathological mechanism of depression and explore continuously more comprehensive antidepressant strategies.
Currently, the pathogenesis of depression is considered to be related to heredity and social environment (1, 9). Monoamine neurotransmitter disorder, neuroendocrine changes, neuroplastic injury, or gut microbiota impairment have been investigated to be involved in the etiopathogenesis of depression. However, the pathophysiologic cause of depression is still unknown. And no clinically biological diagnostic markers or biological screening tests are currently available. Notably, studies have shown that the occurrence of depression is closely related to the over-activation of the immune system and the increased secretion of cytokines (5, 10). The pro-inflammatory state has been proved to be an important link in the pathological process of depression (11–13). And it has reported that the inflammatory response is positively correlated with the severity of depression (14). Studies have shown that the stress-induced neuro-inflammation is the key pathological process of depression (2, 14, 15). It has been identified that the activation of microglia is a specific manifestation of the neuroinflammation of the central nervous system (CNS) mediated by stress (2, 12, 14). Studies have indicated that hyperactivation of microglia can induce depression by secreting proinflammatory factors, which might trigger inflammatory response and inhibit neurogenesis in hippocampus (15, 16). In addition, research has shown that insufficient expression of microglia can also induce the degeneration of hippocampus and contribute to depression (17). It has been reported that the stress-mediated activation of microglia could contribute to depression-like behaviors and neuroinflammation in hippocampus. Besides, the increase of inflammatory mediators, including IL-6, IL-18, IL-1 and IL-4, were also observed in the hippocampus of rats exposed to chronic stress (18). Meanwhile, data from the investigation have confirmed that the inhibition of the hippocampal microglia activation and neuroinflammation alleviated depression-like behaviors (16, 19). Hence, the microglia activity at homeostasis may provide a way to target therapy for depression.
High mobility group box-1 (HMGB1), located outside the cell, is considered to be the endogenous risk factor and initiation signal of neuroinflammation (20, 21). Findings from the animal experiment have indicated that activation of HMGB1 could promote depressive-like behaviors in stress models of depression (22, 23). Exposure to chronic unpredictable stress (CUS) has been found to induce depressive-like behaviors, which might be mediated by activating HMGB1-RAGE pathway and upregulation of HMGB1 mRNA in enriched hippocampal microglia (24, 25). It has also been identified the significantly upregulated expression of HMGB1 in the serum and cerebral cortex of chronic unpredictable mild stress (CUMS)-induced depression model (26). HMGB1 has been considered to be the mediator to initiate the microglia sensitization and activation of proinflammatory cytokines (20, 27, 28).
Acupuncture, one part of traditional Chinese medicine with a history of more than 3,000 years, has become one of the treatment methods for depression recommended by the WHO (29, 30). Currently, data from various studies have verified the safety and effectiveness of acupuncture in the treatment of depression by alleviating stress-induced depressive-like behaviors of animal model of depression (31, 32), and improving the symptoms and quality of life of patients with depression (30, 33). It has demonstrated that the antidepressant effect of acupuncture is mainly through increasing hippocampal and network neuroplasticity, and inhibiting the stress-induced inflammation and apoptosis (32, 33). However, whether acupuncture exerts antidepressant effect by regulating the neuroinflammation mediated by hippocampal Iba-1 and HMGB1 has not been fully elucidated.
Therefore, our present study established the depression rat model of CRS and aimed to investigate the antidepressant mechanism of acupuncture of modulating the neuroinflammation induced by HMGB1. The expression of microglial activation and HMGB1 in the hippocampus was detected by immunofluorescence. The expression of hippocampal interleukin-10 was detected by western blot. And the content of serum tumor necrosis factor-α (TNF-α) was detected by enzyme-linked immunosorbent assay (ELISA) method. We aimed to elucidate the potential mechanisms underlying the antidepressant effect of acupuncture, which might provide new experimental evidence for new therapies in the treatment of depression.
Materials and Methods
Animals
Male Sprague-Dawley (SD) rats, weighting 180 ± 20 g, were included in the present experiment. All animals were obtained from Weitong Lihua Experimental Animal Center of Beijing, China. All protocols were approved by the Animal Ethics Committee, Beijing University of Chinese Medicine, China (Permission number: BUCM-4-2020102801-4048). All rats were housed in the cages with free access to water and food, under the circadian of 12-h light/dark, and the ambient temperature and relative humidity were maintained at 23–26°C and 50 ± 2%. After 7 days of adaption, body weight assessment and sucrose preference test were conducted, and a total of 44 rats with the same baseline of behavioral assessment were included in this study. Then, they were randomly divided into control group, model group, escitalopram group, and acupuncture group (see Figure 1), with 11 rats in each group.
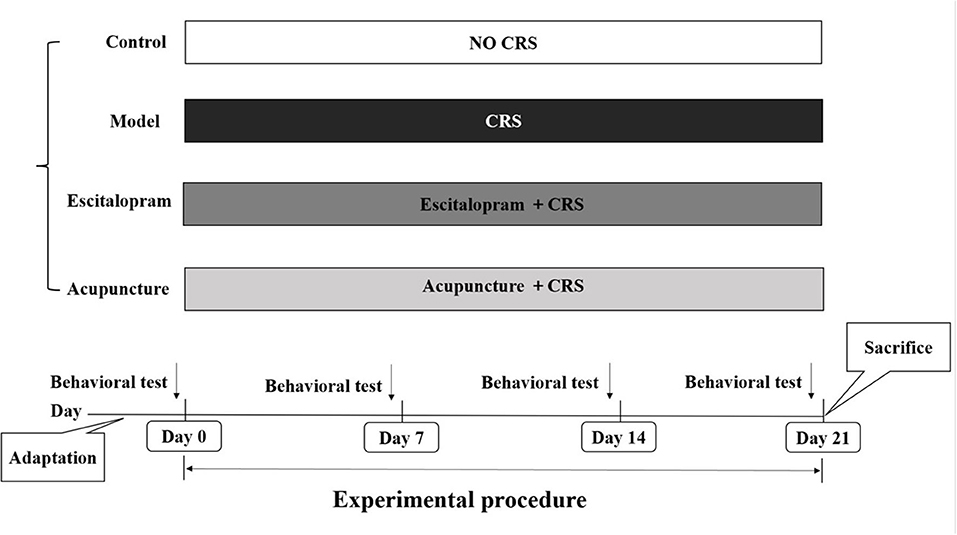
Figure 1. Experimental procedures. CRS, chronic restraint stress; Behavioral tests, including body weight assessment and sucrose preference test.
Establishment of the Animal Model of Depression
Studies have confirmed that CRS procedures can well simulate the pathological process of depression (34). Accordingly, the establishment of the animal model of depression in this study was conducted to be the validation of CRS referring to the previous study (35–37). Except for rats in the control group, all rats were exposed to CRS and social isolation for 21 days continuously. Rats were restrained in a cylinder-shaped wire net (20.5 cm long and 6.5 cm in diameter), fixing both ends with a 64 mm long butterfly clip from 10 a.m. to 4 p.m. The wire net was soft enough to prevent from body impairment of rats. During CRS procedures, rats were subjected to food and water deprivation. After CRS procedures, all rats were put back into the cage and they had free access to food and water.
Escitalopram and Acupuncture Intervention
One hour before CRS procedure, rats in escitalopram group were subjected to the suspension of escitalopram and saline administration (30 mg/100 ml) by gavage (3 mg/kg·d) once per day. The escitalopram oxalate tablets were obtained from Lexapro (H. Lundbeck A/S, 2666079). One hour before CRS procedure, rats in acupuncture group were exposed to acupuncture stimulation, 20 min per session, one session daily for 21 days. The acupoints were selected to be Bai hui (GV20, located at the midline of the head) and Yin tang (GV 29, located at the midpoint between the two eyes). The acupuncture needles were obtained from Suzhou Medical Instrument Co., LTD. (0.25 × 13 mm, No.: 213052). When acupuncture intervention was conducted, rats were placed in separate room under the condition of free activities. Additional stress was strictly avoided during the procedure.
Behavioral Assessment
All observations were conducted under relatively quiet and dark circumstances. Anhedonia and nutritional status were evaluated by sucrose preference test and body weight assessment, respectively.
Body Weight Assessment
The changes of body weight at different time points were observed to evaluate the states of food preference and nutrition status. Body weight assessment was detected at 0 day pre-intervention and at 7, 14, 21 days post-intervention for each rat throughout the experimental procedures.
Sucrose Preference Test
Anhedonia was usually expressed by reduced sucrose consumption in the animal model of depression (38). Rats were trained to adapt to 1% sucrose solution (Sigma-Aldrich, Lot No.513H051) during the acclimation cycle. Before the sucrose preference test (SPT), all rats were deprived of food and water for 23 h. Then all rats had free access to two pre-weighed bottles containing 150 ml 1% sucrose solution and 150 ml pure water for 1 h. At the end of the test, the bottles liquid were re-weighed and recorded to calculate the sucrose preference rate. Sucrose preference rate (%) = sucrose consumption / (sucrose consumption + water consumption) × 100% (35, 36, 38).
Tissue Collection and Processing Procedures
After 21 days of experiment cycle, samples collection were conducted on day 22. No rats died during the experiment. Five rats of each group were anesthetized with 10% chloral hydrate and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde solution. The intact brain was removed after perfusion and fixed in 4% paraformaldehyde solution for the next experimental cycle. After anesthesia, another 6 rats were exposed to blood samples collection taken from the abdominal aorta. After storing at room temperature for 2 h, the blood samples were centrifuged at 3,000 rpm for 10 min (4°C, with a centrifugal radius of 6.6 cm) to separate serum. After the blood samples collection, the hippocampus were isolated from brain on ice quickly and put into cryopreservation tubes, which were quickly placed in liquid nitrogen. Then the serum samples and hippocampus samples were transferred to−80°C for storage for the next experimental process.
Immunofluorescence Staining
For the immunofluorescence staining, brain samples were quickly harvested and fixed in 4% PFA solution, then dehydrated in 10% sucrose solution. Later, the brain samples were embedded in OCT (Tissue-Tek) and serial sectioned with cryostat. The brain samples were cut into 10 μm thickness for the following experimental cycle. Staining was performed on the hippocampal sections fixed on slide glass, air dried for 20 min at room temperature. For permeabilization, slides were fixed with cold acetone for 7 min. Non-specific binding was blocked with 4% donkey serum for 1 h. The slides were washed with 1 × TBS containing 0.1% Tween-20 and then exposed overnight to the following primary antibody mixtures: IBA1 Rabbit Polyclonal antibody (1:500, Proteintech, 10904-1-AP, USA), Anti-HMGB1 antibody - N-terminal (1:500, Abcam, ab228624, USA) at 4°C. Detection of primary antibodies was performed with secondary antibody [Goat Anti-Rabbit IgG H&L (HRP) (1:1000, Abcam, ab6721, USA] for 2 h in the darkness. The sections were then washed five times with PBS. Detection of nucleus was performed with DAPI staining solution (1:1000, Abcam, ab228549, USA) and then incubated at room temperature for 5 min. The slides were washed with PBS for 3 times, air dried for 30 min at room temperature. Finally, the anti-fluorescent extractant was added. Immunofluorescence sections were observed. And the morphology and expression of microglia and HMGB1 were recorded with fluorescence microscope, using excitation wavelengths of 633 nm (helium/neon2, blue Cy5 labeling), 543 nm (helium/neon1, red Cy3 immunofluorescence). Images were captured using a Case Viewer software and analyzed using Fiji (ImageJ) by a condition-blind observer.
Western Blotting
BCA assay was used to determine the protein concentration. Then the sample of hippocampus were homogenized in RIPA buffer, and the final mass concentration of the sample was 4 g/L, the loading amount of the protein sample to be tested was 160 μL. The final mass concentration of 3 samples from separate groups was <4 g/L. Accordingly, 5 samples from each group were used to the detection of western blotting. Protein samples were run on 12% Tris-glycine SDS-PAGE gels, transferred to polyvinylidene difluoride (PVDF) membrane (0.45 μm, Millipore, Massachusetts, USA), and blotted with antibodies against IL-10 (1:1000, Abcam), GAPDH (1:5000, Abcam). Primary antibody incubation was performed overnight at 4 °C. Secondary antibodies (1:1000, Abcam) were incubated for 40 min at room temperature. After the exposure film is scanned, the image is analyzed by Gel Image Ver 4.0 software and the band levels were quantified. The result is the gray value of the target strip/ GAPDH gray value represents the correction error.
ELISA
After the blood samples collection, 2 samples of the blood from separate groups showed hemolysis. Accordingly, 5 samples from each group were used to the detection of ELISA. The content of serum TNF-α was determined by enzyme-linked immunosorbent assay (ELISA). TNF-α ELISA kits (Ray Biotech. Inc., lot: 0611210709, USA) was used according to the manufacturer's protocol. The photometric measurements were performed at 450 nm according to the instruction of the manufacture.
Statistical Analysis
The statistical analysis was performed by SPSS 20.0 (IBM, New York, USA). The results of behavioral tests are expressed as mean ± standard deviation (SD), and analyzed by repeated measures analysis of variance. Data were tested for normal distribution and homogeneity of variance. One-way analysis of variance (ANOVA) with Least-Significant Difference (LSD) post-hoc test was performed for assessing between-group differences. Non-parametric test was used if variance is not uniform or does not conform to normal distribution. A level of P < 0.05 was considered to be significant for analysis.
Results
Chronic Restraint Stress Induces Depressive-Like Behavior in Rats
Effect of Acupuncture on the Changes of Body Weight of CRS Rats
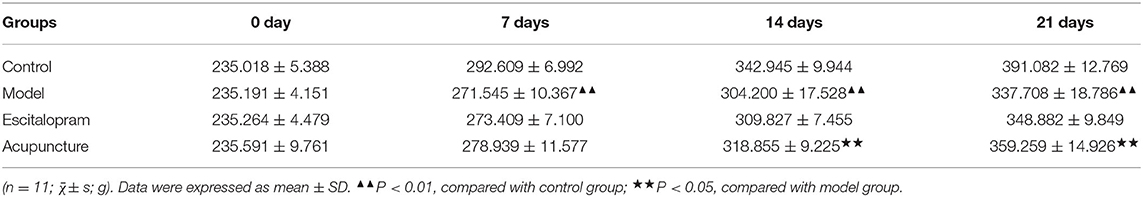
As shown in Figure 2A and Table 1, there was no significance of the body weight of each group before the experiment. After CRS procedures, the body weight of rats in different groups changed (F = 4.488, P = 0.000). Compared with the control group, the body weight of the rats in the model group reduced significantly after 7 days of CRS procedures, even lower than that in the control group significantly until at day 21 (all P < 0.01). Compared with the model group, the body weight of the acupuncture group began to increase significantly on the 14th day, and its mean weight on the 14th and 21st days were significantly higher than that in the model group (all P < 0.05).
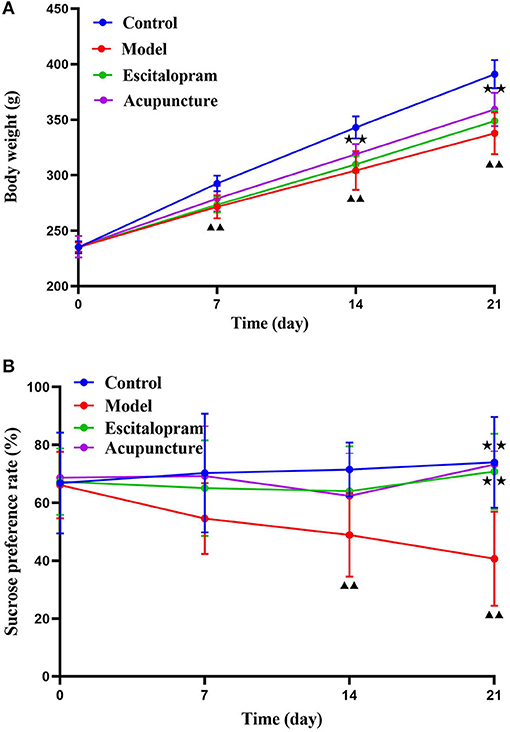
Figure 2. Effects of acupuncture on the CRS-induced depressive-like behaviors in rats. (A) Effect of acupuncture on the changes of body weight of CRS rats; ▴▴P < 0.01, compared with control group; ⋆⋆P < 0.05, compared with model group. (B) Effect of acupuncture on the changes of sucrose preference rate of CRS rats. ▴▴P < 0.01, compared with control group; ⋆⋆P < 0.01, compared with model group. Repeated ANOVA followed by LSD's post-hoc test.
Effects of Acupuncture on the Changes of Sucrose Preference Rate of CRS Rats
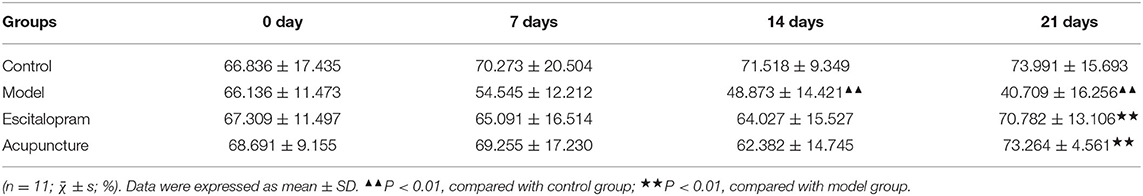
As shown in Figure 2B and Table 2, the sucrose preference rate among each group changed with the change of time points after CRS modeling (F = 2.642, P = 0.008). Compared with the control group, the mean value of sucrose preference rate in the model group decreased significantly at 14 days and 21 days (all P < 0.01), which indicated the stress-induced anhedonia and depressive-like behavior. Compared with the model group, acupuncture and escitalopram intervention had a reversal effect on the 21st day, and the sucrose preference index increased significantly (all P < 0.01), suggesting that acupuncture can significantly alleviate the CRS-induced anhedonia.
Effects of Acupuncture on the Expression of HMGB1 and IBA-1 in the Hippocampus of CRS Rats
As illustrated in Figures 3A–C and Table 3, following 21 days of intervention, there was a significant difference in the expression of HMGB1 (F = 11.863, P < 0.01) and IBA-1 (F = 9.106, P < 0.01) in the hippocampus CA1 region among groups. The expression of HMGB1 and IBA-1 in the hippocampus of the model group was significantly higher than that of the control group (all P < 0.01). Compared with the model group, acupuncture and escitalopram intervention reversed the increase of HMGB1 (all P < 0.05), and acupuncture treatment had down-regulated the expression of IBA-1 in the hippocampus CA1 region of CRS rats (Figures 3A–C; Table 3).
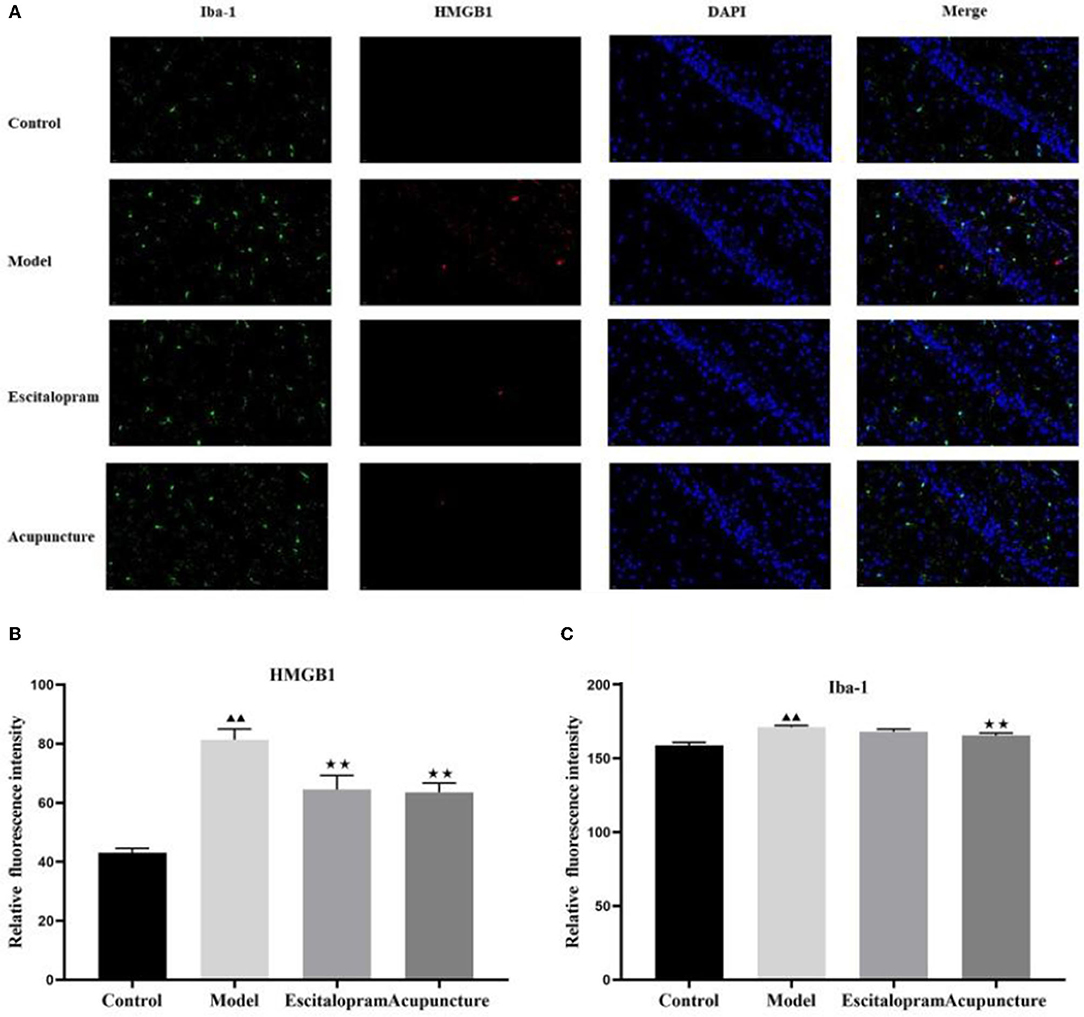
Figure 3. Effect of acupuncture on the expression of HMGB1 and IBA-1 in the hippocampus of CRS rats. (A) Immunofluorescence staining of hippocampal CA1 region in different groups (× 40). IBA-1, green; HMGB1, red; DAPI, blue; scale bar, 20 μm; (B) Relative fluorescence intensity of HMGB1; (C) Relative fluorescence intensity of IBA-1. Data was expressed as means ± SEM (n = 5). ▴▴P < 0.01, compared with control group; ⋆⋆P < 0.05, compared with model group. One-way ANOVA followed by LSD's post-hoc test.
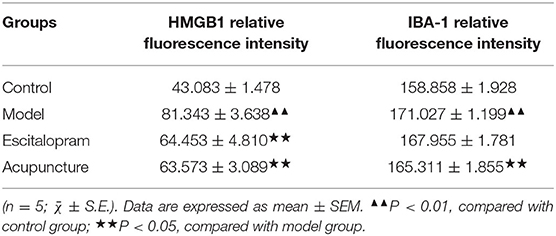
Table 3. Effects of acupuncture on the expression of HMGB1 and IBA-1 in the hippocampus of CRS rats.
Effects of Acupuncture on the Expression of IL-10 Protein in the Hippocampus of CRS Rats
As the results showed in Figures 4A,B and Table 4, compared with the control group, the expression of IL-10 protein in the hippocampus of the model group decreased significantly (P < 0.05). Compared with the model group, escitalopram intervention had an obvious upward trend on the expression of IL-10 protein in the hippocampus of CRS rats (P < 0.01).
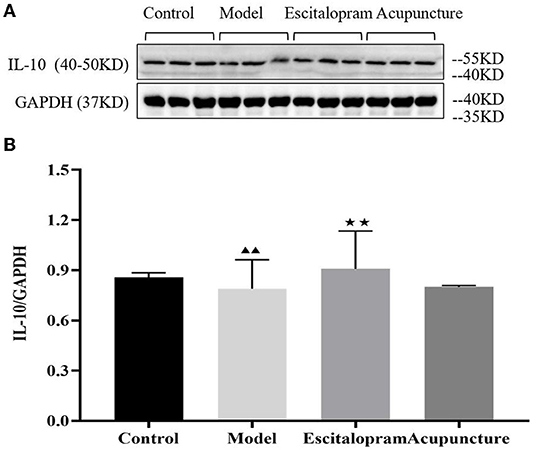
Figure 4. (A) is the protein band diagram of IL-10, and (B) is the bar diagram of IL-10. Effects of acupuncture on the expression of IL-10 protein in the hippocampus of CRS rats. Data are expressed as means ± S.E.M. (n = 5). ▴▴P < 0.05, compared with control group; ⋆⋆P < 0.01, compared with model group. One-way ANOVA followed by LSD's post-hoc test.
Effects of Acupuncture on the Content of Serum TNF-α of CRS Rats
As shown in Figure 5 and Table 5, there was significant differences of serum TNF-α among groups (F = 27.152, P = 0.000). Compared with the control group, serum pro-inflammatory cytokine of TNF-α was significantly increased in the CRS-mediated model group (P < 0.01). Compared with the model group, both acupuncture and escitalopram reversed the high expression of TNF-α in serum-induced by CRS rats (P < 0.01).
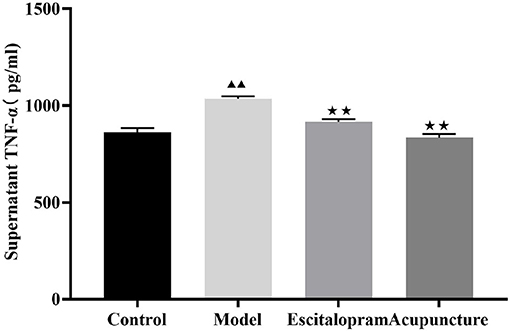
Figure 5. Effects of acupuncture on the content of serum TNF-α of CRS rats. Data are expressed as means ± S.E.M. (n = 5). ▴▴P < 0.01, compared with control group; ⋆⋆P < 0.01, compared with model group. One-way ANOVA followed by LSD's post-hoc test.
Discussion
Studies have confirmed that CRS procedures can well simulate the pathological process of depression. Previous studies have verified that CRS could contribute to depressive-like behaviors in the rat model (35, 36). Accordingly, the establishment of the animal model of depression in this study was conducted to be the validation of CRS procedures. We aimed to elucidate the potential mechanisms underlying the antidepressant effect of acupuncture on modulating the neuroinflammation induced by HMGB1. In the present study, we identified the findings on the antidepressant effect of acupuncture from the microglia and cytokines level alteration. The results have shown that CRS procedures obviously induced depression-like behaviors, which might be triggered by the neuroinflammation through the stress-induced up-regulation of the expression of HMGB1 and IBA-1 in the hippocampus. Notably, acupuncture significantly alleviated the depression-like behaviors and reversed the high expression of HMGB1, Iba-1, and TNF-α in CRS rats, which indicates the antidepressant effect of acupuncture and provides new experimental evidence for new therapies in the treatment of depression.
CRS Induces Depressive-Like Behaviors and Acupuncture Exerts the Antidepressant-Like Effect
In the present study, the changes of behavioral assessment at different time points were observed to evaluate the states of nutrition and anhedonia (39–41). There were consistent baseline values among different groups at 0 day before intervention. After the CRS procedures, a significant comparative difference of behaviors was found. Compared with the control group, the weight of rats was significantly decreased in the model group at 7, 14, and 21 days. Compared with the model group, acupuncture intervention increased the body weight at 14 and 21days, while there was no significant difference from the escitalopram group at four-time points. Our present study manifests that acupuncture has effectively regulated the weight loss of CRS rats and produced positive therapeutic effects. Accordingly, we will focus on relative indicators to elucidate the additional specific mechanism of the significant change in the body weight in our following experiment. Previous studies had found that anhedonia was evaluated the depression-like behavior in rats by detecting the SPT. Compared with the control group, we found that exposure to CRS induced depressive-like behavior in the SPT, which mainly showed reduced sucrose preference rate at 14, 21 days. Acupuncture and escitalopram intervention reversed the decrease of sucrose preference rate at 21 days, which suggested that acupuncture alleviated the CRS-induced depressive-like behaviors.
Acupuncture is used to regulate painful diseases or psychological states, including headaches, arthritis, depression, or anxiety (42). Acupuncture, a traditional Chinese medicine treatment method, has the effect of anti-depression and reducing the severity of depressive symptoms (43). According to the findings from the Resting-State fMRI, acupuncture can induce different brain activity at different points. It has been reported that acupuncture at combined acupoints could activate a wider range of brain areas than single acupoint, and the areas regulated by acupuncture are mostly related to emotion and cognition (44). Previous studies have also shown that acupuncture at Bai hui (GV 20) and Yin tang (GV 29) can regulate the hippocampal injury, and alleviate the depression state of rats and exert antidepressant effects (35). However, single acupuncture at either Bai hui or Yin tang fails to alleviate the state of depression (45). Therefore, the acupoints of Bai hui (GV 20) and Yin tang (GV 29) were selected to be investigated the antidepressant mechanisms of acupuncture. The results of the present study have identified that CRS induced depressive-like behaviors and acupuncture at Bai hui (GV 20) and Yin tang (GV 29) exhibited the antidepressant-like effect.
Acupuncture Reversed CRS-Induced Neuroinflammation Mediated by Hippocampal Iba-1 and HMGB1
Evidence is increasing that psychological and physical stressors could activate immune and inflammation processes, contributing to depressive symptoms. As is common knowledge, the activation of microglia is a specific manifestation of the neuroinflammation of the central nervous system (CNS) mediated by stress (2, 12, 14). The activation of HMGB1 could promote depressive-like behaviors in stress models of depression (22, 23). HMGB1 has been considered to be the mediator to initiate the microglia sensitization and activation of proinflammatory cytokines (20, 27, 28). Ionized calcium binding adaptor molecule 1 (Iba-1) is considered to be the microglia marker. The changes of the concentration of Iba-1 and HMGB1 in hippocampus were considered to be involved in the pathological process of depression. The hippocampus, the regulatory center of the hypothalamic-pituitary-adrenal (HPA) axis, is considered to be involved in the pathological process of depression (46, 47). It has suggested that stress increases the number of microglia in the CA1 and CA3 region of the hippocampus (48), and increases the Iba-1 level (49), significantly increasing relative protein level and fluorescence density of Iba-1 in the hippocampus of CA1 region (50). HMGB1 could induce systemic inflammatory reactions, such as cold, arthritis, anorexia, or weight loss (51). It has also been identified the significantly upregulated expression of HMGB1 in the serum and cerebral cortex of chronic unpredictable mild stress (CUMS)-induced depression mouse model (26). CUMS stress caused concentrations of HMGB1 substantially increased in the hippocampus and serum (52). HMGB1 is involved in depression-like behavior induced by lipopolysaccharide, and mice were used with human Recombinant HMGB1 (rHMGB1) to produce depression-like behavior (23). In our present study, the result indicated that CRS induced the dramatic activation of microglia and up-regulation of HMGB1, which was in accordance with the previous studies indicating the stress-induced neuroinflammation mediated by hippocampal Iba-1 and HMGB1 (53–56). Importantly, Acupuncture intervention reversed the CRS-induced increase of HMGB1 and Iba-1 in the hippocampus of CRS rats, suggesting that acupuncture exhibited the antidepressant effect by regulating the changes of hippocampal microglia and HMGB1 levels, which is consistent with our theoretical hypothesis. At the same time, our results confirmed that it can produce antidepressant effects through the regulation of microglia and HMGB1, which is consistent with the previous results (48–50).
Studies have indicated that hyperactivation of microglia can induce depression by secreting proinflammatory factors (15, 16). The patients suffered from depression generally exhibited an elevated amount of proinflammatory cytokines in the serum, microglia activation, and neuronal deficit in the CNS (54). Depression in the absence of other diseases has been shown to be associated with increased levels of various pro-inflammatory cytokines, including TNF-α and interleukin (57). Data from the clinical study indicated that MDD patients exhibited increased levels of serum IL-1β and TNF-α (58). The animal studies have also identified that stress increased the levels of hippocampal inflammation factors, including IL-1β and TNF-α (49, 50). TNF-α induces similarly depression-like symptoms in mice (6). However, IL-10 can reduce the harmful effects of cytokines on memory and plasticity. Studies have shown that administration of IL-10 can rescue depression-related learning and memory defects and restore the density of hippocampal dendritic spines (49). In the present study, our results showed that rats exposed to CRS exerted decrease of IL-10 in the hippocampus while increase of TNF-α in serum, which is consistent with the reported studies (59, 60). However, it is worth noting that both acupuncture and escitalopram intervention could down-regulate the expression of serum TNF-α, and the escitalopram administration intervention can increase the expression of IL-10 in the hippocampus, indicating that acupuncture and could regulate the CRS-induced proinflammatory factor TNF-α and exhibited antidepressant effect.
Conclusion
Conclusively, all of these results give more support to the hypothesis that the CRS-induced neuroinflammation mediated by hippocampal Iba-1 and HMGB1 was involved in the pathogenesis of depression, and the antidepressant effect of acupuncture might be through modulating the neuroinflammation mediated by hippocampal Iba-1 and HMGB1. Notably, the current study preliminarily highlights the role of acupuncture in alleviating depressive behavior associated with stress-induced neuroinflammation mediated by HMGB1 in the CRS model of depression, which might provide new experimental evidence for new therapies in the treatment of depression. However, there are several limitations of the present study. No specific blocker of HMGB1 was applied to identify whether acupuncture has specificity in the regulation of HMGB1. Meanwhile, our previous studies from clinical investigation has identified the therapeutic effect of acupuncture in the treatment of depression. Accordingly, sham acupuncture was not used in our study. In the future, our team will continue to carry out the study of the antidepressant effect of acupuncture based on the present findings.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Animal Ethics Committee, Beijing University of Chinese Medicine, China (Permission number: BUCM-4-2020102801-4048).
Author Contributions
HJ obtained the funding and designed the research. TB, XR, and HM directed the experiment. LC, YW, SQi, YS, PL, SQu, and WL performed research. LC and HJ analyzed data. HJ and LC wrote the paper and contributed equally to this work.
Funding
This research was supported by grants from the National Natural Science Foundation of China (81904313 and 81973937) and Key Research Program of Beijing University of Chinese Medicine of China (2020-JYB-ZDGG-060).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.903004/full#supplementary-material
References
1. Nobis A, Zalewski D, Waszkiewicz N. Peripheral markers of depression. J Clin Med. (2020) 9:3793. doi: 10.3390/jcm9123793
2. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
3. Han KM, Ham BJ. How inflammation affects the brain in depression: a review of functional and structural MRI studies. J Clin Neurol. (2021) 17:503–15. doi: 10.3988/jcn.2021.17.4.503
4. WHO Depression data fact sheet. Geneva: World Health Organization (2020). Available online at: http://www.who.int/mediacentre/factsheets/fs369/en/ (accessed July 08, 2020).
5. Kopschina Feltes P, Doorduin J, Klein HC, Juárez-Orozco LE, Dierckx RA, Moriguchi-Jeckel CM, et al. Anti-inflammatory treatment for major depressive disorder: implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J Psychopharmacol. (2017) 31:1149–65. doi: 10.1177/0269881117711708
6. Petralia MC, Mazzon E, Fagone P, Basile MS, Lenzo V, Quattropani MC, et al. Close to translation? Autoimmun Rev. (2020) 19:102504. doi: 10.1016/j.autrev.2020.102504
7. Giorgi-Guarnieri D. Clinician liability in prescribing antidepressants. Focus. (2019) 17:372–9. doi: 10.1176/appi.focus.20190024
8. Liu B, Liu J, Wang M, Zhang Y, Li L. From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci. (2017) 11:305. doi: 10.3389/fncel.2017.00305
9. Juruena MF, Gadelrab R, Cleare AJ, Young AH. Epigenetics: a missing link between early life stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 109:110231. doi: 10.1016/j.pnpbp.2020.110231
10. Zou W, Feng R, Yang Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE. (2018) 13:e0197267. doi: 10.1371/journal.pone.0197267
11. Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. (2020) 87:901–9. doi: 10.1016/j.bbi.2020.02.010
12. Enache D, Pariante CM, Mondelli V. Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav Immun. (2019) 81:24–40. doi: 10.1016/j.bbi.2019.06.015
13. Liu CH, Zhang GZ, Li B, Li M, Woelfer M, Walter M, et al. Role of inflammation in depression relapse. J Neuroinflammation. (2019) 16:90. doi: 10.1186/s12974-019-1475-7
14. Maeng SH, Hong H. Inflammation as the potential basis in depression. Int Neurourol J. (2019) 23:S63–71. doi: 10.5213/inj.1938226.113
15. Won E, Kim YK. Neuroinflammation-associated alterations of the brain as potential neural biomarkers in anxiety disorders. Int J Mol Sci. (2020) 21:6546. doi: 10.3390/ijms21186546
16. Bassett B, Subramaniyam S, Fan Y, Varney S, Pan H, Carneiro AMD, et al. Minocycline alleviates depression-like symptoms by rescuing decrease in neurogenesis in dorsal hippocampus via blocking microglia activation/phagocytosis. Brain Behav Immun. (2021) 91:519–30. doi: 10.1016/j.bbi.2020.11.009
17. Tong L, Gong Y, Wang P, Hu W, Wang J, Chen Z, et al. Microglia loss contributes to the development of major depression induced by different types of chronic stresses. Neurochem Res. (2017) 42:2698–711. doi: 10.1007/s11064-017-2270-4
18. Wang YL, Han QQ, Gong WQ, Pan DH, Wang LZ, Hu W, et al. Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation. (2018) 15:21. doi: 10.1186/s12974-018-1054-3
19. Dong SQ, Zhang QP, Zhu JX, Chen M, Li CF, Liu Q, et al. Gypenosides reverses depressive behavior via inhibiting hippocampal neuroinflammation. Biomed Pharmacother. (2018) 106:1153–60. doi: 10.1016/j.biopha.2018.07.040
20. Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci. (2015) 35:316–24. doi: 10.1523/JNEUROSCI.3561-14.2015
21. Zandarashvili L, Sahu D, Lee K, Lee YS, Singh P, Rajarathnam K, et al. Real-time kinetics of high-mobility group box 1 (HMGB1) oxidation in extracellular fluids studied by in situ protein NMR spectroscopy. J Biol Chem. (2013) 288:11621–7. doi: 10.1074/jbc.M113.449942
22. Franklin TC, Xu C, Duman RS. Depression and sterile inflammation: essential role of danger associated molecular patterns. Brain Behav Immun. (2018) 72:2–13. doi: 10.1016/j.bbi.2017.10.025
23. Wu TY, Liu L, Zhang W, Zhang Y, Liu YZ, Shen XL, et al. High-mobility group box-1 was released actively and involved in LPS induced depressive-like behavior. J Psychiatr Res. (2015) 64:99–106. doi: 10.1016/j.jpsychires.2015.02.016
24. Franklin TC, Wohleb ES, Zhang Y, Fogaça M, Hare B, Duman RS. Persistent increase in microglial RAGE contributes to chronic stress-induced priming of depressive-like behavior. Biol Psychiatry. (2018) 83:50–60. doi: 10.1016/j.biopsych.2017.06.034
25. Zhang S, Hu L, Jiang J, Li H, Wu Q, Ooi K, et al. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J Neuroinflammation. (2020) 17:15. doi: 10.1186/s12974-019-1673-3
26. Lian YJ, Gong H, Wu TY, Su WJ, Zhang Y, Yang YY, et al. Ds-HMGB1 and fr-HMGB induce depressive behavior through neuroinflammation in contrast to nonoxid-HMGB1. Brain Behav Immun. (2017) 59:322–32. doi: 10.1016/j.bbi.2016.09.017
27. Zhang H, Ding L, Shen T, Peng D. HMGB1 involved in stress-induced depression and its neuroinflammatory priming role: a systematic review. Gen Psychiatr. (2019) 32:e100084. doi: 10.1136/gpsych-2019-100084
28. Lourbopoulos A, Ertürk A, Hellal F. Microglia in action: how aging and injury can change the brain's guardians. Front Cell Neurosci. (2015) 9:54. doi: 10.3389/fncel.2015.00054
29. Luo D, Liu L, Huang Q, Zhang HM, Yu ZM, Hu M, et al. Crosstalk between acupuncture and NF-κB in inflammatory diseases. Evid Based Complement Alternat Med. (2020) 2020:7924985. doi: 10.1155/2020/7924985
30. Fan L, Fu W, Chen Z, Xu N, Liu J, Lü A, et al. Curative effect of acupuncture on quality of life in patient with depression: a clinical randomized single-blind placebo-controlled study. J Tradit Chin Med. (2016) 36:151–9. doi: 10.1016/S0254-6272(16)30021-8
31. Kou RZ, Chen H, Yu ML, Xu TC, Fu SP, Lu SF. Acupuncture for behavioral changes of experimental depressive disorder: a systematic review and meta-analysis. Sci Rep. (2017) 7:9669. doi: 10.1038/s41598-017-09712-1
32. Lai HC, Chang QY, Hsieh CL. Signal transduction pathways of acupuncture for treating some nervous system diseases. Evid Based Complement Alternat Med. (2019) 2019:2909632. doi: 10.1155/2019/2909632
33. Zhao B, Li Z, Wang Y, Ma X, Wang X, Wang X, et al. Can acupuncture combined with SSRIs improve clinical symptoms and quality of life in patients with depression? secondary outcomes of a pragmatic randomized controlled trial. Complement Ther Med. (2019) 45:295–302. doi: 10.1016/j.ctim.2019.03.015
34. Seewoo BJ, Hennessy LA, Feindel KW, Etherington SJ, Croarkin PE, Rodger J. Validation of chronic restraint stress model in young adult rats for the study of depression using longitudinal multimodal MR imaging. eNeuro. (2020) 7:ENEURO.0113-20.2020. doi: 10.1523/ENEURO.0113-20.2020
35. Sun Y, Tu Y, Guo Y, Jiang HL, Li YH, Wang Y, et al. Acupuncture improved depressive behavior by regulating expression of hippocampal apoptosis-related factors in psychological stress-induced depression rats. Zhen Ci Yan Jiu. (2019) 44:412–8. doi: 10.13702/j.1000-0607.190098
36. Dong S, Jiang HL, Wang Y, Lu J, Chang L, Zhang P, et al. Effect of acupuncture on expression of glial fibrillary acidic protein in hippocampus and prefrontal cortex and serum interleukin-10 in chronic restraint stress depression rats. Zhen Ci Yan Jiu. (2018) 43:209–14. doi: 10.13702/j.1000-0607.170676
37. Wang Y, Jiang H, Meng H, Lu J, Li J, Zhang X, et al. Genome-wide transcriptome analysis of hippocampus in rats indicated that TLR/NLR signaling pathway was involved in the pathogenisis of depressive disorder induced by chronic restraint stress. Brain Res Bull. (2017) 134:195–204. doi: 10.1016/j.brainresbull.2017.07.021
38. Song AQ, Gao B, Fan JJ, Zhu YJ, Zhou J, Wang YL, et al. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J Neuroinflammation. (2020) 17:178. doi: 10.1186/s12974-020-01848-8
39. Liu B, Zhao L, Yue C, Qian M, Xie M. Changes in gonadal function at different stages of chronic restraint stress-induced depression animals. Physiol Behav. (2019) 210:112656. doi: 10.1016/j.physbeh.2019.112656
40. Moreno C, Hermosilla T, Hardy P, Aballai V, Rojas P, Varela D. Cav12 activity and downstream signaling pathways in the hippocampus of an animal model of depression. Cells. (2020) 9:2609. doi: 10.3390/cells9122609
41. Li H, Wang P, Huang L, Li P, Zhang D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol Motil. (2019) 31:e13677. doi: 10.1111/nmo.13677
42. Kaptchuk TJ. Acupuncture: theory, efficacy, and practice. Ann Intern Med. (2002) 136:374–83. doi: 10.7326/0003-4819-136-5-200203050-00010
43. Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. (2018) 3:CD004046. doi: 10.1002/14651858.CD004046.pub4
44. Li X, Cai L, Jiang X, Liu X, Wang J, Yang T, et al. Resting-state fMRI in studies of acupuncture. Evid Based Complement Alternat Med. (2021) 2021:6616060. doi: 10.1155/2021/6616060
45. Takagi K, Tanahashi N, Amagasu N, Mizuno K, Kawanokuchi J, Yi G, et al. Effect of manual acupuncture stimulation at “Bai-Hui” (GV 20) or “Yintáng” (Ex-HN3) on depressed rats. J Acupunct Meridian Stud. (2017) 10:26–32. doi: 10.1016/j.jams.2016.11.006
46. Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci. (2017) 20:1434–47. doi: 10.1038/nn.4661
47. Roddy DW, Farrell C, Doolin K, Roman E, Tozzi L, Frodl T, et al. The hippocampus in depression: more than the sum of its parts? advanced hippocampal substructure segmentation in depression. Biol Psychiatry. (2019) 85:487–97. doi: 10.1016/j.biopsych.2018.08.021
48. Worthen RJ, Garzon Zighelboim SS, Torres Jaramillo CS, Beurel E. Anti-inflammatory IL-10 administration rescues depression-associated learning and memory deficits in mice. J Neuroinflammation. (2020) 17:246. doi: 10.1186/s12974-020-01922-1
49. Zhao D, Xu X, Pan L, Zhu W, Fu X, Guo L, et al. Pharmacologic activation of cholinergic alpha7 nicotinic receptors mitigates depressive-like behavior in a mouse model of chronic stress. J Neuroinflammation. (2017) 14:234. doi: 10.1186/s12974-017-1007-2
50. Zhou S, Chen S, Xie W, Guo X, Zhao J. Microglia polarization of hippocampus is involved in the mechanism of Apelin-13 ameliorating chronic water immersion restraint stress-induced depression-like behavior in rats. Neuropeptides. (2020) 81:102006. doi: 10.1016/j.npep.2020.102006
51. Ding X, Li S, Zhu L. Potential effects of HMGB1 on viral replication and virus infection-induced inflammatory responses: a promising therapeutic target for virus infection-induced inflammatory diseases. Cytokine Growth Factor Rev. (2021) 62:54–61. doi: 10.1016/j.cytogfr.2021.08.003
52. Wang B, Lian YJ, Su WJ, Peng W, Dong X, Liu LL, et al. HMGB1 mediates depressive behavior induced by chronic stress through activating the kynurenine pathway. Brain Behav Immun. (2018) 72:51–60. doi: 10.1016/j.bbi.2017.11.017
53. Wang B, Huang X, Pan X, Zhang T, Hou C, Su WJ, et al. Minocycline prevents the depressive-like behavior through inhibiting the release of HMGB1 from microglia and neurons. Brain Behav Immun. (2020) 88:132–43. doi: 10.1016/j.bbi.2020.06.019
54. Rana T, Behl T, Mehta V, Uddin MS, Bungau S. Molecular insights into the therapeutic promise of targeting HMGB1 in depression. Pharmacol Rep. (2021) 73:31–42. doi: 10.1007/s43440-020-00163-6
55. Jia X, Gao Z, Hu H. Microglia in depression: current perspectives. Sci China Life Sci. (2021) 64:911–25. doi: 10.1007/s11427-020-1815-6
56. Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH, Cui YX, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol. (2020) 177:5224–45. doi: 10.1111/bph.15261
57. Mosiołek A, Pieta A, Jakima S, Zborowska N, Mosiołek J, Szulc A. Effects of antidepressant treatment on peripheral biomarkers in patients with major depressive disorder (MDD). J Clin Med. (2021) 10:1706. doi: 10.3390/jcm10081706
58. Das R, Emon MPZ, Shahriar M, Nahar Z, Islam SMA, Bhuiyan MA, et al. Higher levels of serum IL-1β and TNF-α are associated with an increased probability of major depressive disorder. Psychiatry Res. (2021) 295:113568. doi: 10.1016/j.psychres.2020.113568
59. Parul Mishra A, Singh S, Singh S, Tiwari V, Chaturvedi S, Wahajuddin M, et al. Chronic unpredictable stress negatively regulates hippocampal neurogenesis and promote anxious depression-like behavior via upregulating apoptosis and inflammatory signals in adult rats. Brain Res Bull. (2021) 172:164–79. doi: 10.1016/j.brainresbull.2021.04.017
60. Zhang YX, Zhang XT, Li HJ, Zhou TF, Zhou AC, Zhong ZL, et al. Antidepressant-like effects of helicid on a chronic unpredictable mild stress-induced depression rat model: Inhibiting the IKK/IκBα/NF-κB pathway through NCALD to reduce inflammation. Int Immunopharmacol. (2021) 93:107165. doi: 10.1016/j.intimp.2020.107165
Keywords: acupuncture, depression, microglia, HMGB1, neuroinflammation
Citation: Chen L, Jiang H, Bao T, Wang Y, Meng H, Sun Y, Liu P, Quan S, Li W, Qi S and Ren X (2022) Acupuncture Ameliorates Depressive Behaviors by Modulating the Expression of Hippocampal Iba-1 and HMGB1 in Rats Exposed to Chronic Restraint Stress. Front. Psychiatry 13:903004. doi: 10.3389/fpsyt.2022.903004
Received: 25 March 2022; Accepted: 02 May 2022;
Published: 06 June 2022.
Edited by:
Kai Zhang, Anhui Medical University, ChinaReviewed by:
Zhao Tongtong, Anhui Medical University, ChinaYelei Zhang, Affiliated Kangning Hospital of Wenzhou Medical University, China
Copyright © 2022 Chen, Jiang, Bao, Wang, Meng, Sun, Liu, Quan, Li, Qi and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huili Jiang, MjAxNTA5NDExODhAYnVjbS5lZHUuY24=; Xiujun Ren, cnhpdWp1bkBxcS5jb20=
 Lu Chen
Lu Chen Huili Jiang
Huili Jiang Tuya Bao
Tuya Bao Yu Wang
Yu Wang Hong Meng4
Hong Meng4 Yang Sun
Yang Sun