
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 04 October 2022
Sec. Anxiety and Stress Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.898683
 Xue Wang1†
Xue Wang1† Peiran Chen2†
Peiran Chen2† Shenxun Shi3
Shenxun Shi3 Wei Chen4
Wei Chen4 Hongyan Zhang5
Hongyan Zhang5 Ronghua Tang6
Ronghua Tang6 Zhibing Wu7
Zhibing Wu7 Yan Li8
Yan Li8 Jun Wu9
Jun Wu9 Li Zong10
Li Zong10 Lianying Ji10
Lianying Ji10 Ping Feng2*‡
Ping Feng2*‡ Jing Li1*‡
Jing Li1*‡Background: Generalized anxiety disorder (GAD) is a chronic disorder characterized by excessive, pervasive, persistent worrying that is difficult to control. Jiuwei Zhenxin granules may be safer and more effective than non-benzodiazepine anti-anxiety drugs for treating GAD. This study aimed to assess the efficacy and safety of Jiuwei Zhenxin granules alone or in combination with the benzodiazepine alprazolam.
Materials and methods: A total of 710 patients were recruited from outpatient clinics and were randomly divided into two groups to receive Jiuwei Zhenxin granules (single drug group) or Jiuwei Zhenxin granules and alprazolam (combination group). The primary outcome was the response rate, which was defined as a ≥ 50% reduction from the baseline total score on the Hamilton Anxiety Scale (HAMA). Secondary outcome measures included mean changes in HAMA total score, psychological and somatic factors, Hamilton Depression Rating Scale total score, and SF-36 health survey score.
Results: At 4 weeks after treatment, the single and combination treatment groups showed significant improvement in the HAMA total score and they did not differ significantly in response rate (77.58 vs. 79.17%) or rate of adverse drug reactions (16.22 vs. 16.07%).
Conclusion: Jiuwei Zhenxin granules are an effective, safe, and well-tolerated treatment against GAD. Combining them with alprazolam may not significantly improve efficacy.
Clinical trial registration: [www.ClinicalTrials.gov], identifier [CHICTR1800020095].
Generalized anxiety disorder (GAD) is a chronic disorder associated with pervasive and excessive worry that is difficult to control (1). GAD is often accompanied by non-specific physical and psychological symptoms, and the lifetime risk of GAD is about 6% in the general population (2–4). Several drug classes have been evaluated for their therapeutic efficacy in GAD, including benzodiazepines, azapirones, tricyclic antidepressants, selective serotonin reuptake inhibitors, and serotonin-norepinephrine inhibitors (3, 5, 6). Response to treatment is generally defined as a ≥50% reduction from the baseline total score on the Hamilton Anxiety Rating Scale (HAMA) (7, 8), based on which the clinical response rates range between 30 and 68% among GAD patients (6, 9, 10). In addition, several drugs currently used may increase the risk of adverse effects such as drug dependence, withdrawal syndrome, somnolence, gastrointestinal symptoms, and sexual dysfunction (11, 12). These considerations highlight the need for safer, more effective therapeutic approaches (6).
Jiuwei Zhenxin granules are a traditional Chinese remedy consisting of nine kinds of Chinese herbal medicines: Panax ginseng C.A. Mey, Ziziphus jujuba Mil. var spinosa (Bunge) Hu ex H.F. Chou, Schisandra chinensis (Turcz.) Baill, Polygala tenuifolia Willd, Asparagus cochinchinensis (Lour.) Merr, Corydalis yanhusuo W.T. Wang, Paria cocos (Schw.) Wolf, Rehmannia glutinosa Libosch, and Cinnamomum cassia Presl (13). They also contain various active ingredients, such as ginsenosides, Rehmannia-related polysaccharides, jujube seed alcohol, Poria sugar, and deoxyschizandrin, which have demonstrated anti-depressant, anti-anxiety, and neuroprotective properties in animal studies (14–16). Jiuwei Zhenxin granules were approved for the treatment of GAD by the Chinese National Medical Products Administration in 2008 (17). A relatively small phase II clinical trial in GAD patients showed that Jiuwei Zhenxin granules have greater therapeutic efficacy and fewer side effects than buspirone, a non-benzodiazepine anxiolytic drug (18). A later phase III trial showed that Jiuwei Zhenxin granules are similar in efficacy and safety to azapirones (18, 19). To the best of our knowledge, there are rare studies comparing the clinical efficacy and safety of this medication with benzodiazepine-based anti-anxiety drugs.
Therefore, in the present study, we performed a multicenter, randomized, parallel-group, controlled trial to evaluate the efficacy and safety of Jiuwei Zhenxin granules alone or in combination with the most common benzodiazepine, alprazolam, in patients with GAD.
A total of 710 patients were recruited from 12 hospitals across China. Patients were considered eligible for the study if they (1) were between 18 and 70 years old, (2) had been diagnosed with GAD based on the International Classification of Diseases (10th Revision) (20), and (3) had a baseline total HAMA score ≥14 and anxiety subscore ≥2. Patients were excluded if they had any of the following: other mental disorders associated with anxiety disorders, such as depression, terror-induced anxiety, panic disorder, obsessive-compulsive disorder, schizophrenia, or bipolar disorder; a total score ≥17 on the Hamilton Depression Rating Scale (HAMD); significant functional impairment of the heart, kidneys, or liver; or pregnancy or breastfeeding. There was no restriction on whether the subjects were outpatients or inpatients, or whether they were first treated.
Considering a statistical power of 80% and a significance level of 5%, we estimated a minimal sample size of 350 subjects per group, assuming a 10% difference in response rate and a 10–20% dropout rate. Patients were randomly allocated (1:1) to either a single drug group that received Jiuwei Zhenxin granules (6 g in the morning, 6 g at noon, and 6 or 12 g at night) for 4 weeks, or to a combination group that received Jiuwei Zhenxin granules (6 g in the morning, 6 g at noon, and 6 or 12 g at night) for 4 weeks, as well as alprazolam (0.4–0.8 mg bid or tid) for the first 2 weeks. Randomization was performed using the Proc Plan Procedure in SAS 9.2 (SAS Institute, Cary, NC, USA).
The primary outcome of the study was the response rate, which was defined as a ≥50% reduction from the baseline HAMA total score at 4 weeks post-treatment. HAMA is used to assess anxiety symptoms and consists of 14 items scored on a five-point scale, ranging from 0 (absent) to 4 (severe) (21). Higher HAMA total scores indicate greater psychological distress and anxiety.
Secondary outcomes included mean changes in HAMA total score, psychological and somatic factors, HAMD total score, and SF-36 health survey score from baseline to endpoint (22). HAMA examinations were performed at baseline and at 2 and 4 weeks post-treatment. SF-36 health surveys were conducted at baseline and at 4 weeks post-treatment.
Adverse events in both groups were recorded, and their association with Jiuwei Zhenxin granules and alprazolam was classified as related, probably related, possibly related, possibly unrelated, or unrelated. Related, probably related, and possibly related events were considered adverse drug reactions.
Medical history, demographic characteristics, and physical examination results were recorded for all patients at baseline. Follow-up was conducted at 2 and 4 weeks after treatment. At baseline and at 4 weeks after treatment, all patients underwent blood, urine, and stool routine tests, and the levels of serum alanine aminotransferase, blood urea nitrogen, and serum creatinine were determined.
Statistical analyses were performed with the SAS 9.2 software package based on a modified intention-to-treat approach (23). Data were expressed as mean ± standard deviation (SD) for continuous variables and as total number (% frequency) for categorical variables. Continuous variables were checked for the normality of distribution by a Kolmogorov-Smirnov test. If the normality test indicated normal distribution of the data, then a parametric test was used, otherwise, a non-parametric test was used. Paired t-test was used for the comparison of pre- and post-treatment within a group, and two-sample t-test was applied for comparison between two treatment groups in parameter analysis. Wilcoxon signed-rank test for the comparison of pre- and post-treatment within a group and Wilcoxon rank sum test for comparison between two treatment groups in non-parametric test. And differences in categorical variables were assessed using chi-squared or Fisher’s exact tests. Differences associated with P < 0.05 were considered statistically significant.
The study was conducted according to the guidelines of the Declaration of Helsinki and Good Clinical Practice, and the protocol was approved by the local Ethics Committee [(2008 Clinical Trial (Post Marketing) Review (No.13)]. The trial was registered in the Chinese Clinical Trial Registry (registration no. CHICTR1800020095). Written informed consent was obtained from all patients before enrollment. The personnel who made disease diagnosis and performed scale assessments were qualified doctors and they had been uniformly trained at the start of the study.
A total of 710 patients were enrolled in the present study, of whom 353 were assigned to the single drug group and 357 to the combination therapy group. Nine patients from the single drug group and 15 from the combination group were lost during follow-up, while necessary data were missing for 11 patients (Figure 1).
Patients from the two treatment groups were non-significantly different in age, gender, and body weight. Analysis of the clinicodemographic characteristics of the included patients revealed a statistically significant, but clinically unimportant, difference in blood lymphocyte count between the two groups (Table 1). The groups did not show any other significant differences in baseline clinicodemographic features or secondary outcomes (Table 2).
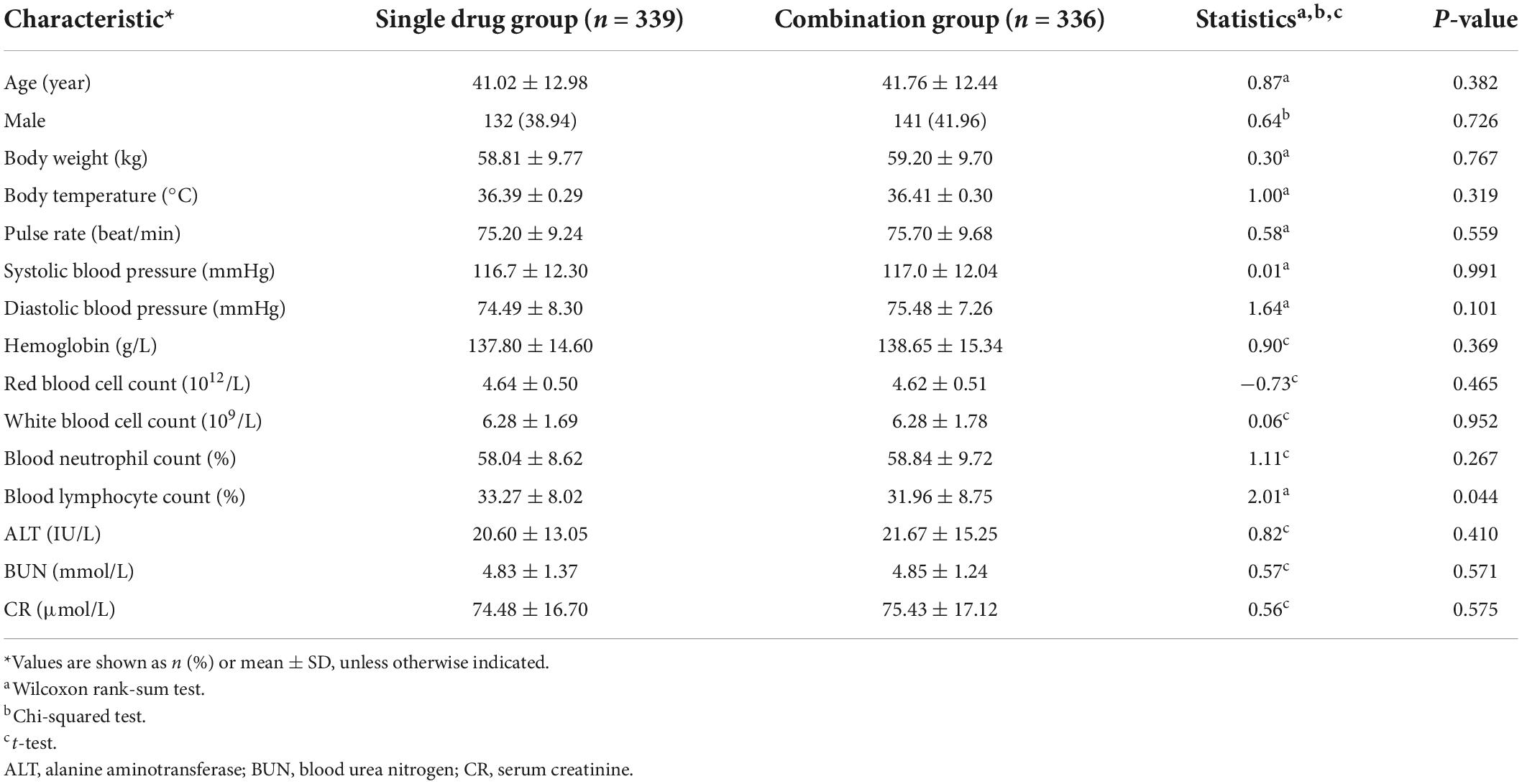
Table 1. Baseline characteristics of patients with generalized anxiety disorder treated with Jiuwei Zhenxin granules alone (single drug group) or in combination with alprazolam (combination group).
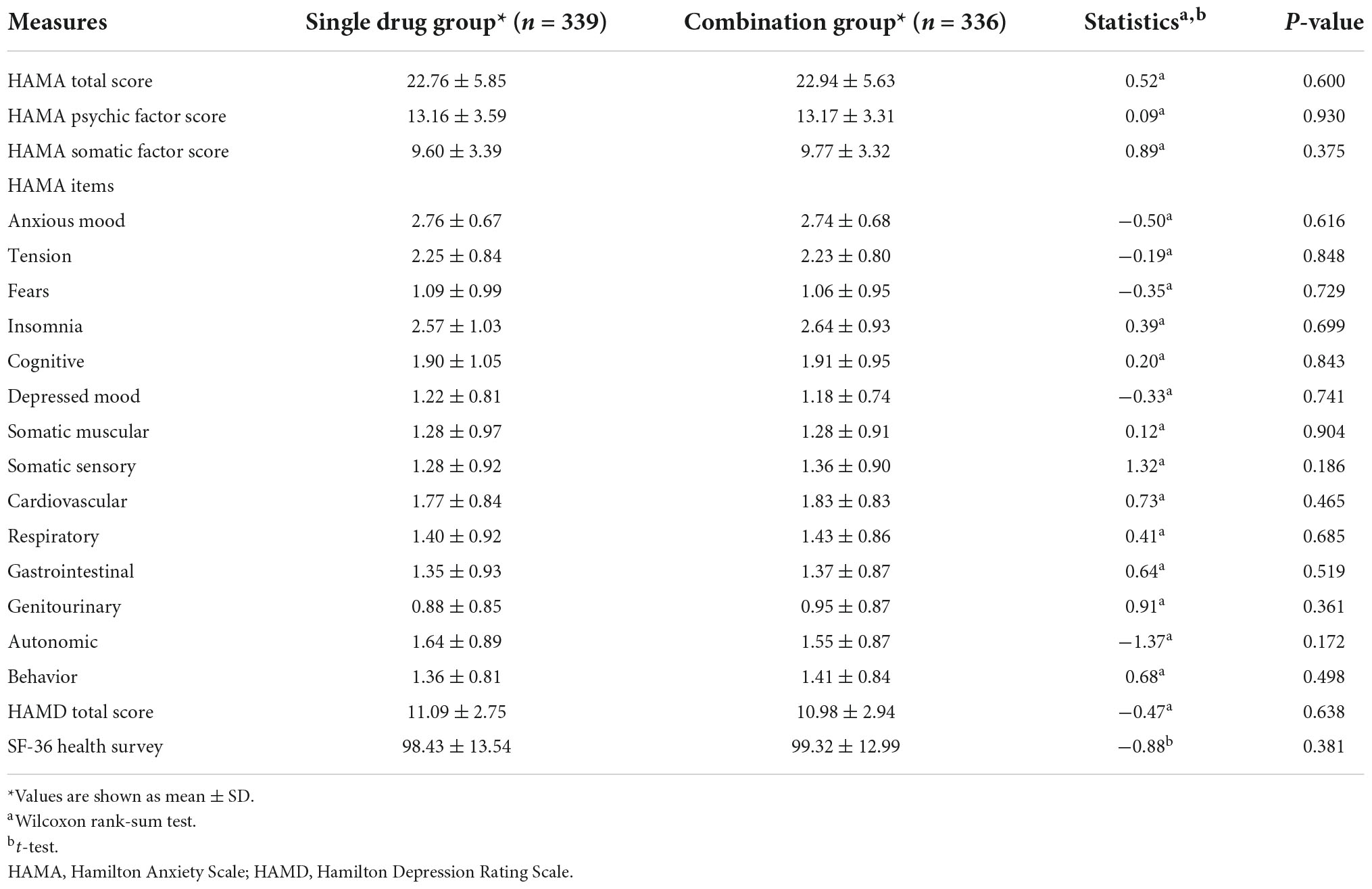
Table 2. Comparison of baseline psychological and somatic factors between patients with generalized anxiety disorder treated with Jiuwei Zhenxin granules alone (single drug group) or in combination with alprazolam (combination group).
Comparison of the HAMA total score at 4 weeks post-treatment indicated a better response in the combination group (77.58%) than in single drug group (79.17%), but the differences did not achieve statistical significance (P = 0.6169, Figure 2). The HAMA was improved continuously during the 4 weeks. Table 3 showed the comparison of HAMA and SF-36 health survey scores at baseline and at 4 weeks post-treatment. The mean change (±SD) for HAMA total score improved 13.09 (±6.52) for single drug group and 13.25 (±5.97) for combination group after 4 weeks treatment, the mean change for HAMA psychic factor score improved 7.17 (±3.77) for single drug group and 7.25 (±3.42) for combination group, and the mean change for HAMA somatic factor score improved 5.92 (±3.46) for single drug group and 6.00 (±3.21) for combination group. The improvements were statistically significant between baseline and post-treatment within each group. However, these changes were not statistically significant between the two groups (Table 3). Similar results were obtained for the SF-36 total score and its eight subscales.
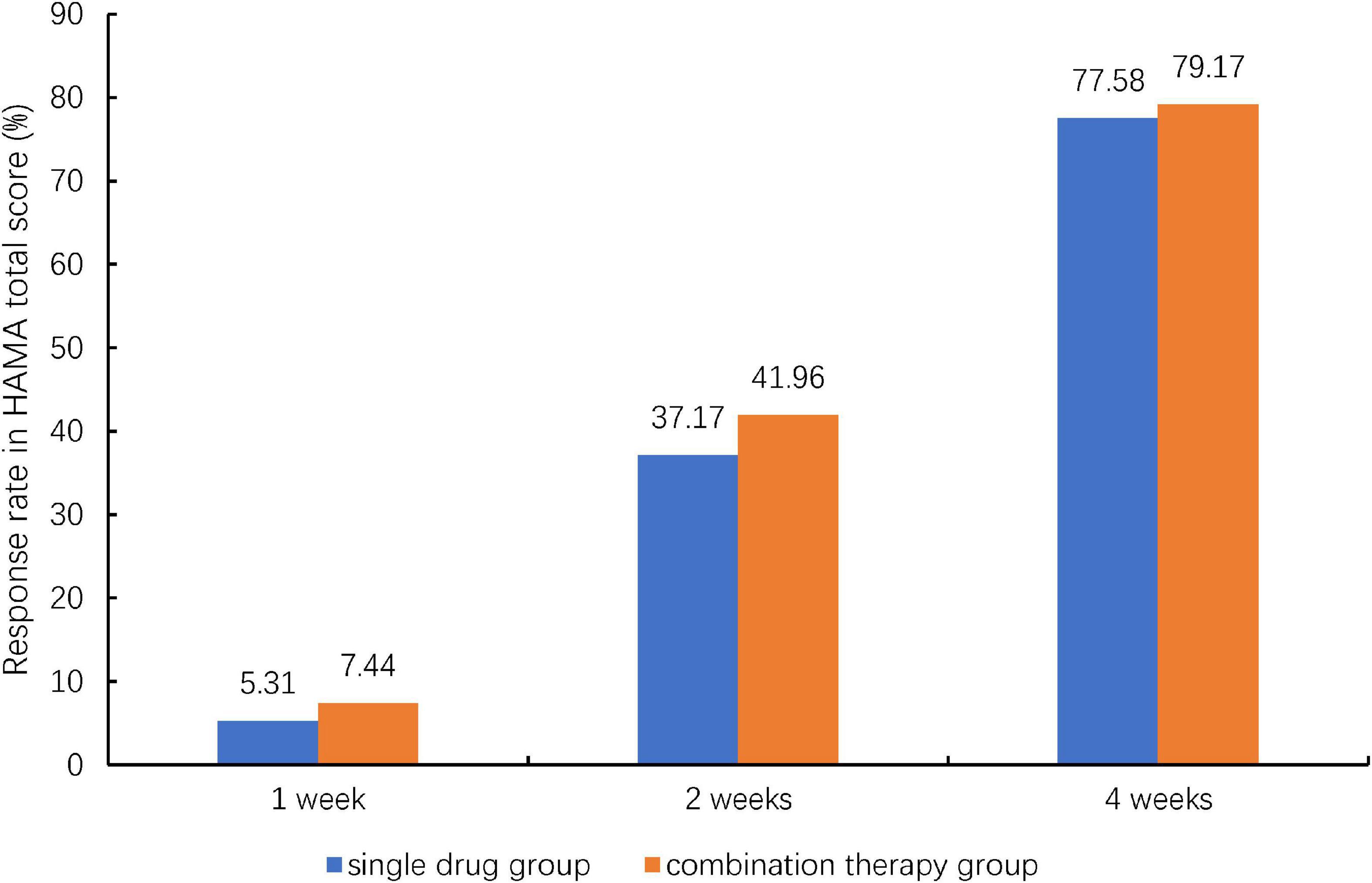
Figure 2. Therapeutic response rate of patients with generalized anxiety disorder to Jiuwei Zhenxin granules alone (single drug group) or in combination with alprazolam (combination therapy group) at 4 weeks post-treatment. HAMA, Hamilton Anxiety Scale; TCM, traditional Chinese medicine. Response was defined as a 50% reduction from the baseline HAMA total score at 4 weeks post-treatment. And Chi-squared test was used for the comparison between the two treatment groups.
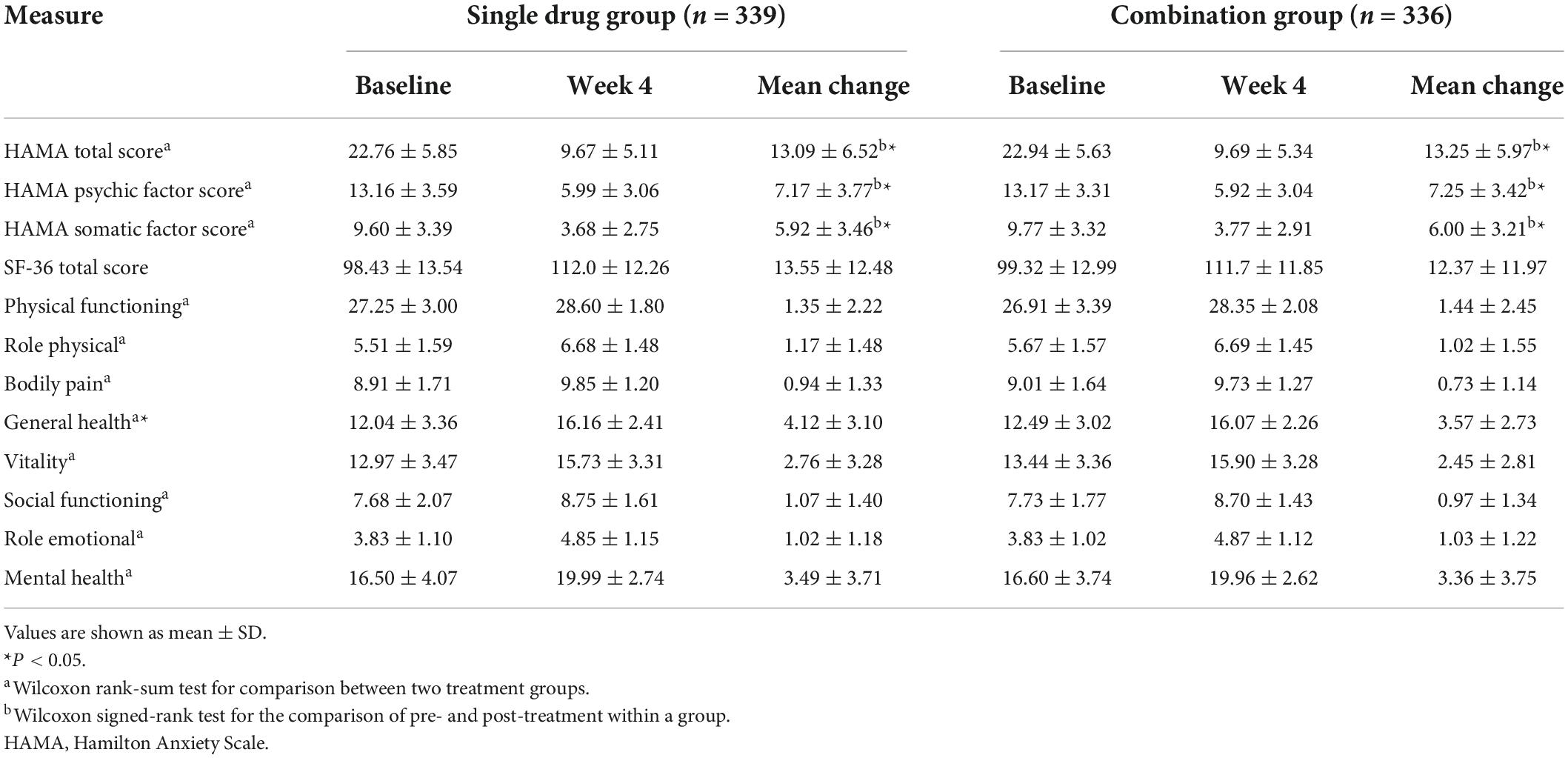
Table 3. Comparison of HAMA and SF-36 health survey scores at baseline and at 4 weeks post-treatment between patients with generalized anxiety disorder treated with Jiuwei Zhenxin granules alone (single drug group) or in combination with alprazolam (combination group).
The rates of adverse events and adverse drug reactions were also similar between the two groups. The adverse event for single drug group was 16.22%, while for combination group was 16.07%. The adverse drug reaction for single drug group was 8.26 and 11.01% for combination group (Table 4). The most common adverse drug reactions were dry mouse (3.24%) and abdominal discomfort (1.77%) in the single drug group; or abdominal discomfort (2.08%), constipation (1.19%), diarrhea (1.49%), and dizziness (1.79%) in the combination group (Table 5).

Table 4. Rates of adverse events and adverse drug reactions in patients with generalized anxiety disorder treated with Jiuwei Zhenxin granules alone (single drug group) or in combination with alprazolam (combination group).
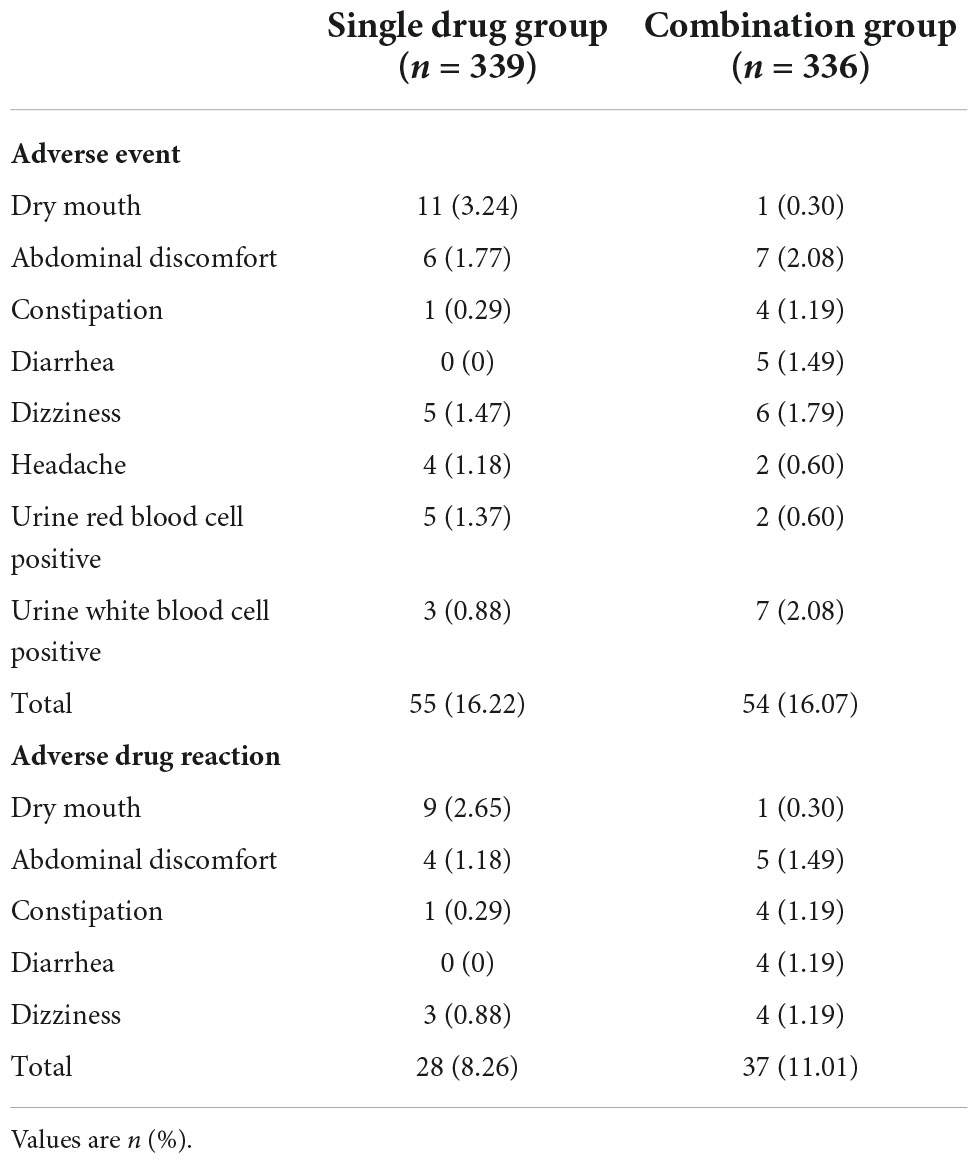
Table 5. Adverse events and adverse drug reactions occurring in >1% of patients with generalized anxiety disorder who were treated with Jiuwei Zhenxin granules alone (single drug group) or in combination with alprazolam (combination group).
Generalized anxiety disorder is a prevalent disorder associated with significant impairments in social, emotional, and physical functioning, and it has received increasing attention in recent years. Jiuwei Zhenxin granules have been approved for the treatment of patients with GAD, but their safety and therapeutic efficacy have been compared mainly to non-benzodiazepine drugs (24, 25). In the present multicenter, randomized controlled study, we evaluated for the first time the safety and efficacy of Jiuwei Zhenxin granules in the presence or absence of the benzodiazepine anxiolytic drug alprazolam. Our results showed that as monotherapy or in combination therapy, Jiuwei Zhenxin granules can effectively relieve GAD without severe adverse events.
Benzodiazepines are considered the primary pharmacological treatment for GAD (26), with alprazolam being the most frequently prescribed agent (27). Jiuwei Zhenxin granules have also been identified as a promising treatment for GAD (24), and their efficacy has been confirmed in phase II and III trials (18, 19). Indeed, Jiuwei Zhenxin granules can significantly reduce HAMA total score in GAD patients (10, 28). Consistent with these results, we found that this traditional medicine, either alone or combined with alprazolam, can greatly improve the HAMA total score. In contrast to our findings, previous trials showed that combining the granules with buspirone (29) or escitalopram (29, 30) was more effective at reducing the HAMA score than the corresponding monotherapies. This discrepancy may be due to differences in the drugs’ mechanism of action, and the fact that we excluded patients with HAMD total score ≥17, which was lower than the threshold used in those previous studies.
The incidence of adverse effects associated with Jiuwei Zhenxin granules was reported to be lower, albeit not significantly so, than the incidence with co-administration of buspirone and granules (30%) or with buspirone alone (25%) (28). Here, the adverse drug reaction rate was 8.26% for the single drug group and 11.01% for the combination group, which was lower than the values previously reported. Further research could explore the safety of benzodiazepine and non-benzodiazepine when combined with the granules.
Our study had certain limitations. One is that we excluded patients with HAMD score ≥17, so whether our findings can be extrapolated to a broader range of GAD patients needs to be confirmed. Another limitation is that the rate of therapeutic response differed by less than 10% between the two groups, suggesting that our results need to be confirmed in a larger sample. In addition, the course of disease before the enrollment was not recorded.
The present study suggests that Jiuwei Zhenxin granules is safe and effective in treating GAD. Nevertheless, our results should be validated in future studies with larger samples and higher HAMD thresholds.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Independent Ethics Committee of West China Hospital. The patients/participants provided their written informed consent to participate in this study.
PF and JL contributed to the conceptualization and design of the study. XW, SS, HZ, WC, RT, ZW, YL, and JW conducted the trial in a separate center. PF analyzed the data. XW and PC wrote the manuscript. LZ and LJ reviewed and revised the manuscript. All authors critically interpreted the results and approved the final version of the manuscript.
This study was funded by Beijing Beilu Pharmaceutical Co., Ltd.
We thank the study participants, LZ and LJ, from Beijing Beilu Pharmaceutical Co., Ltd. for their support with literature review and project management.
Jiuwei Zhenxin granules is being developed by Beijing Beilu Pharmaceutical Co., Ltd. LZ and LJ were employed by Beijing Beilu Pharmaceutical Co., Ltd. and may own stock and/or stock options.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GAD, generalized anxiety disorder; HAMA, Hamilton Anxiety Scale; HAMD, Hamilton Depression Rating Scale; SD, standard deviation; TCM, traditional Chinese medicine.
1. Stein MB, Sareen J. Clinical practice. Generalized anxiety disorder. N Engl J Med. (2015) 373:2059–68. doi: 10.1056/NEJMcp1502514
2. Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Ann Rev Public Health. (2008) 29:115–29. doi: 10.1146/annurev.publhealth.29.020907.090847
3. Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European study of the epidemiology of mental disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. (2004) 420:21–7.
4. Wittchen HU, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jonsson B, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol J Eur College Neuropsychopharmacol. (2011) 21:655–79. doi: 10.1016/j.euroneuro.2011.07.018
5. Chessick CA, Allen MH, Thase M, Batista Miralha da Cunha AB, Kapczinski FF, de Lima MS, et al. Azapirones for generalized anxiety disorder. Cochrane Database Syst Rev. (2006) 3:CD006115. doi: 10.1002/14651858.CD006115
6. Reinhold JA, Mandos LA, Rickels K, Lohoff FW. Pharmacological treatment of generalized anxiety disorder. Exp Opin Pharmacother. (2011) 12:2457–67. doi: 10.1517/14656566.2011.618496
7. Pollack MH, Kornstein SG, Spann ME, Crits-Christoph P, Raskin J, Russell JM. Early improvement during duloxetine treatment of generalized anxiety disorder predicts response and remission at endpoint. J Psychiatric Res. (2008) 42:1176–84. doi: 10.1016/j.jpsychires.2008.02.002
8. Ballenger JC. Remission rates in patients with anxiety disorders treated with paroxetine. J Clin Psychiatry. (2004) 65:1696–707. doi: 10.4088/JCP.v65n1216
9. Rickels K, Rynn M, Iyengar M, Duff D. Remission of generalized anxiety disorder: a review of the paroxetine clinical trials database. J Clin Psychiatry. (2006) 67:41–7. doi: 10.4088/JCP.v67n0107
10. Bereza BG, Machado M, Ravindran AV, Einarson TR. Evidence-based review of clinical outcomes of guideline-recommended pharmacotherapies for generalized anxiety disorder. Can J Psychiatry Rev Canad De Psychiatr. (2012) 57:470–8. doi: 10.1177/070674371205700805
11. Rickels K, Rynn M. Pharmacotherapy of generalized anxiety disorder. J Clin Psychiatry. (2002) 63:9–16.
12. Davidson JR, Feltner DE, Dugar A. Management of generalized anxiety disorder in primary care: identifying the challenges and unmet needs. Primary Care Compan J Clin Psychiatry. (2010) 12:2. doi: 10.4088/PCC.09r00772blu
13. Wang J, Wu X. Traditional chinese medicine jiuwei zhenxin granules in treating depression: an overview. Neuropsychiatr Dis Treat. (2020) 16:2237–55. doi: 10.2147/NDT.S273324
14. Wenlin A, Zhang L, Li Y, Xu Y, Cao J, Lin L. Effects of water extract from poria cocos on mitochondrial impairment in hippocampal neurons induced by sodium azide. Chin Pharm J. (2001) 36:4.
15. Zhu L, Chen D, Li Y, Rong Z. Study of the protection of cells apoptosis after rat serious cerebral trauma by ginsenoside and its mechanism. Chin Arch Tradit Chin Med. (2009) 27:5.
16. Liu D, Zhang H, Gu W, Liu Y, Zhang M. Neuroprotective effects of ginsenoside Rb1 on high glucose-induced neurotoxicity in primary cultured rat hippocampal neurons. PLoS One. (2013) 8:e79399. doi: 10.1371/journal.pone.0079399
17. Pharmaceutical, Beijing Beilu. Jiuwei Zhenxin Granules. Beijing: National Medical Products Administration (2008).
18. Hongwei K, Jin L, Jing L, Xue W, Mingsheng H, Shucheng W, et al. Randomized double-blind controiled trial on efiectiveness and safety of jiu wei lv ping partide in the treatment of generalized anxiety disorder. Chin Evid Based Med. (2004) 4:5.
19. Wang YJ, Wang CY. Efficacy and safety of jiuwei zhenxin keli in treatment of generalized anxiety disorder: a multi center randomized double blind controiled trial. Chin Mental Health J. (2013) 27:6.
21. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:1. doi: 10.1111/j.2044-8341.1959.tb00467.x
22. Ware JE, Gandek B, Kosinski M, Aaronson NK, Apolone G, Brazier J, et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA project. International quality of life assessment. J Clin Epidemiol. (1998) 51:1170. doi: 10.1016/S0895-4356(98)00108-5
23. Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ. (2010) 340:c2697. doi: 10.1136/bmj.c2697
24. Wang S, Zhao LL, Qiu XJ, Wang DS, Tang T, Luo JK, et al. Efficacy and safety of a formulated herbal granula, jiu wei zhen xin, for generalized anxiety disorder: a meta-analysis. Evid Based Complement Alternat Med. (2018) 2018:9090181. doi: 10.1155/2018/9090181
25. Guo Lichun DX, Dezhi Z, Binghua W. Clinical observation on 50 cases of generalized anxiety disorder treated with jiuwei zhenxin granula combining with buspirone. Chin J Tradit Med Sci Technol. (2012) 19:2.
26. Chen P, Zhu H, Ning Y, Yin D, Jia H. Efficacy and safety of shu-gan-qing-re formula for generalized anxiety disorder: study protocol for a multi-center, prospective, double-blind, double-dummy, randomized controlled trial. Trials. (2020) 21:266. doi: 10.1186/s13063-020-4186-6
27. Stahl SM. Don’t ask, don’t tell, but benzodiazepines are still the leading treatments for anxiety disorder. J Clin Psychiatry. (2002) 63:756–7. doi: 10.4088/JCP.v63n0901
28. Hu J, Jun W, Zhijian L, Fenli Z, Kaihua L, Juanjuan C, et al. Phase IV clinical study of jiuwei zhenxin granule in the treatment of generalized anxiety disorder. J Apopl Nervous Dis. (2010) 27:628–30.
29. Jianhong L. Efficacy of jiuwei zhenxin granule combined with escitalopram in the treatment of generalized anxiety disorder. Chin J Int Med Cardio Cerebrov Dis. (2013) 11:2.
Keywords: generalized anxiety disorder, Hamilton Anxiety Scale, traditional Chinese medicine, Jiuwei Zhenxin granules, alprazolam, blood urea nitrogen, serum creatinine
Citation: Wang X, Chen P, Shi S, Chen W, Zhang H, Tang R, Wu Z, Li Y, Wu J, Zong L, Ji L, Feng P and Li J (2022) Effectiveness and safety of Jiuwei Zhenxin granules for treating generalized anxiety disorder: A randomized controlled trial. Front. Psychiatry 13:898683. doi: 10.3389/fpsyt.2022.898683
Received: 17 March 2022; Accepted: 05 September 2022;
Published: 04 October 2022.
Edited by:
Luciana D’Alessio, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Guoping Huang, Mianyang Third People’s Hospital, ChinaCopyright © 2022 Wang, Chen, Shi, Chen, Zhang, Tang, Wu, Li, Wu, Zong, Ji, Feng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Feng, ZmVuZ3BpbmdAd2Noc2N1LmNu; Jing Li, am9hbmEwMjhAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.