- 1Department of Parasitology and Mycology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Department of Clinic and Medicine, College of Veterinary Medicine, University of Sulaimani, Sulaymaniyah, Iraq
- 3Department of Epidemiology and Biostatistics, School of Health, Torbat Heydariyeh University of Medical Sciences, Torbat Heydariyeh, Iran
- 4Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Background: Psychiatric patients are at increased risk of exposure to Toxoplasma gondii infection, which may be linked to their living facilities and behaviors. Limited knowledge on the prevalence of T. gondii infection and its associated risk factors in psychiatric patients are available to the international medical communities. Thus, the aim of the current study was to assess seroprevalence of T. gondii and its associated risk factors in psychiatric inpatients in Fars Province, southern Iran.
Methods: This cross-sectional study was carried out on psychiatric patients hospitalized in Ibn Sina Hospital affiliated to Shiraz University of Medical Sciences, Fars Province, southern Iran, March to July 2021. Blood samples were collected from 318 psychiatric patients and assessed for the detection of IgG against T. gondii using enzyme-linked immunosorbent assay (ELISA). Moreover, structured questionnaires were completed for the participants at the time of sampling. Logistic regression analysis was used to assess possible associations between the latent toxoplasmosis and the variables.
Results: The overall seroprevalence of anti-T. gondii IgG in psychiatric inpatients was 22.3% (71/318; 95% CI = 17.9–27.3). Multivariate analyses revealed that age > 30 years [adjusted odds ratio (AOR) = 2.24, 95% CI = 1.10–4.60, p = 0.03], contact with cats (AOR = 2.52, 95% CI = 1.14–5.58, p = 0.03), raw vegetable consumption (AOR = 3.65, 95% CI = 1.74–7.65, p = 0.001), raw/undercooked meat consumption (AOR = 4.30, 95% CI = 1.47–12.63, p = 0.008), suicide attempt (AOR = 3.77, 95% CI = 1.58–8.97, p = 0.003) and cigarette smoking history (AOR = 0.38, 95% CI = 0.17–0.83, p = 0.02) were independent risk factors for T. gondii infection.
Conclusion: The current results demonstrated that contact with cats, raw vegetable consumption and raw/undercooked meat consumption were independent risk factors for T. gondii seropositivity. Moreover, the current study showed significant associations between seropositivity of T. gondii and suicide attempts as well as negative associations between seropositivity of T. gondii and cigarette smoking in psychiatric inpatients using multivariate logistic regression.
Introduction
Toxoplasma gondii (T. gondii), the causative agent of toxoplasmosis, is a ubiquitous parasite of warm-blooded animals and is one of the most common parasitic protozoans of humans worldwide. Humans majorly become infected with the parasite via consumption of raw and/or undercooked meats containing tissue cysts or accidental consumption of food and water contaminated with oocysts excreted in cat feces. Although toxoplasmosis is generally asymptomatic in immunocompetent individuals, disease can be complicated and life-threatening in such patients, including organ transplant recipients, cancer patients and those with human immunodeficiency virus (AIDS) (1–3). Primary T. gondii infection in pregnant women can cause severe damages to the fetus and may result in irreversible conditions such as abortion as well as severe neurodevelopmental malformations such as hydrocephaly and microcephaly (4, 5). Latent toxoplasmosis is frequently associated to tissue cysts of T. gondii in the skeletal muscles and brain tissues, leading to increased risks of psychiatric and neurological abnormalities and personality changes (6). It has been well documented that toxoplasmosis may lead to changes in behaviors of humans (7). For example, recent clinical studies have demonstrated that antibodies against T. gondii infection may play roles in pathophysiology of suicide (8). Studies have revealed that increased levels of cytokines are associated to depression and suicide (9).
In Iran, toxoplasmosis has become a serious infection since the first epidemic investigation of the disease in humans was carried out in Caspian Sea region (1978) using indirect fluorescent antibody (IFA) technique (10). Although several studies have been carried out on the seroprevalence of T. gondii infection in Iran, most of these studies have focused on high risk populations, including AIDS and cancer patients as well as pregnant and premarital women (11–14). To date, several studies have been carried out on the seroprevalence of toxoplasmosis in psychiatric inpatients. However, most of these studies have merely been carried out to assess the seroprevalence of T. gondii infection and have not evaluated the possible associated risk factors and contamination routes of T. gondii infection (15). Generally, people with mental illnesses are at increased risk of exposure to T. gondii infection, which may be linked to their living facilities and behaviors. Furthermore, these group of people are generally at increased risks of medical comorbidities due to their decreased resistance to infections. This decreased resistance may occur because of age, underlying medical problems and drug abuse (16–18).
Usually, several factors affect prevalence of toxoplasmosis, including sex, age, residence, education background, and dietary habits (19). Although, seroprevalence of T. gondii antibodies in general adult population in Iran has been 39.3% (95% CI = 33.0–45.7), ranging 12–87.5% with further endemicity in northern provinces (20), limited knowledge are available on the prevalence of T. gondii infection and its associated risk factors in psychiatric patients. Therefore, the current study was carried out to assess seroprevalence of T. gondii infection and identify its associated risk factors as well as assessing possible contamination routes of toxoplasmosis in a population of psychiatric inpatients in Fars Province, southern Iran.
Materials and Methods
Study Design and Participants
This cross-sectional study was carried out on psychiatric patients hospitalized in Ibn Sina Hospital affiliated to Shiraz University of Medical Sciences, Fars Province, southern Iran, March to July 2021. Psychiatric diagnosis included real-world clinical diagnosis by two experienced neuropsychiatrists and neuropsychologists, according to the fifth version of the Guidelines for Diagnosis and Statistics of Mental Disorders (DSM-V) which is routinely used in Iranian psychiatric practices. Psychiatric patients were classified into four major groups based on DSM-V and clinical criteria (21), including anxiety, mood, personality, and psychotic disorders. Anxiety disorders group including panic, obsessive-compulsive, social and specific phobia, posttraumatic stress, generalized anxiety, and posttraumatic stress disorders. Mood disorders group including major depressive, dysthymic, bipolar, and related disorder due to another medical condition, cyclothymic, and substance/medication-induced depressive disorder. Personality disorders group including paranoid, schizoid, schizotypal, borderline, antisocial, histrionic, narcissistic, avoidant, dependent, and obsessive-compulsive personality disorders. Psychotic disorders group including schizophrenia, schizophreniform, schizoaffective, and delusional disorders, as well as substance/medication-induced psychotic disorder, and psychotic disorder due to another medical condition. Inclusion criteria for the participants were being psychiatric inpatient, aging 15 years and older, having full consent to participate in the study and being resident of Fars Province. The exclusion criteria were being non-Iranian citizen and traveler. In total, 16 patients were excluded from the study because they did not accept to participate or fill the questionnaire. Sample size was calculated based on the prevalence of toxoplasmosis in the region using standard statistical formula for the estimation of sample sizes for a proportion with a disease prevalence rate of ∼25% (22), margin of error of 0.05 and 95% confidence interval (CI), resulting in a sample size of 289. To include the non-response rate, sample size was inflated by 10% to create a total sample size of 318.
Serum Collection and Assessment
Samples included 318 sera from psychiatric patients hospitalized in Ibn Sina Hospital. A venous blood sample (up to 3 mL) was collected from each participant and sera were separated by centrifugation at 4,000 rpm for 5 min. Sera were stored at –20°C until use. Specific anti-T. gondii IgG levels were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kit (Vircell, Granada, Spain) with a diagnostic sensitivity of > 98% (95% CI: 88–100) and specificity of 100% (95% CI: 89–100) according to the manufacturer’s instructions. Results were interpreted using the manufacturer’s recommendations as follows: sera with antibody index < 9 were reported as negative, 9–11 as equivocal and > 11 as positive sera (23). Laboratory tests were performed blindly, so that the specialist technician who tested the samples did not know the health status of the people whose serum samples were tested.
Questionnaire
A questionnaire was completed for each participant. Demographic information of the questionnaire included sex, age, residency, education background, and possible risk factors included having cats as pets, contact with cats, raw and/or unwashed vegetable consumption, drinking unsanitary water, raw/undercooked meat consumption, gardening or agricultural activities and blood transfusion. Moreover, further information such as duration of mental illness, suicide attempt, cigarette smoking history and miscarriage history in women were collected at the time of sampling using questionnaires.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) Software v.21.0 (IBM, United States) was used for the analysis of data (24). Data were described using calculation of frequencies (%) and 95% CI. To analyze associations between T. gondii infection and potential risk factors, logistic regression was used followed by multivariate logistic analysis with the full model, including all potential risk factors in analyses to control effects of potential confounding factors. Strength of the associations between the predictors and outcome variables was assessed using adjusted odds ratio (AOR) and 95% CI. Overall number of the participants included in the unadjusted and adjusted regression models varied based on the completeness of the putative confounding variables and all missing data were excluded from the regression modeling. However, proportion of the missing data was relatively small, ranging 0–0.9%. In general, p-values less than 0.05 were reported statistically significant.
Results
General Characteristics of the Participants
Results of sociodemographic characteristics and risk factors linked to seroprevalence of T. gondii in 318 psychiatric inpatients are shown in Table 1. The mean age of the participants was 35.91 y ± 12.23 with minimum and maximum ages of 15 and 76 years, respectively. Of 318 participants, 183 (57.5%, 95% CI: 51.9–60.3) were males (mean age 35.96 ± 12.80) and 135 (42.5%, 95% CI: 37.0–48.1) were females (mean age 35.84 ± 11.48); differences in ages between the two sexes were not significant (t = 0.086, p = 0.93). A majority of the participants were residents of the city (n = 228, 71.7%). Out of 318 psychiatric patients, almost half (n = 144, 45.3%) had mood disorders followed by psychotic disorders (n = 81, 25.5%) and anxiety disorders (n = 68, 21.3%), whereas personality disorders were the least disorders (n = 25, 7.8%) (Table 1).
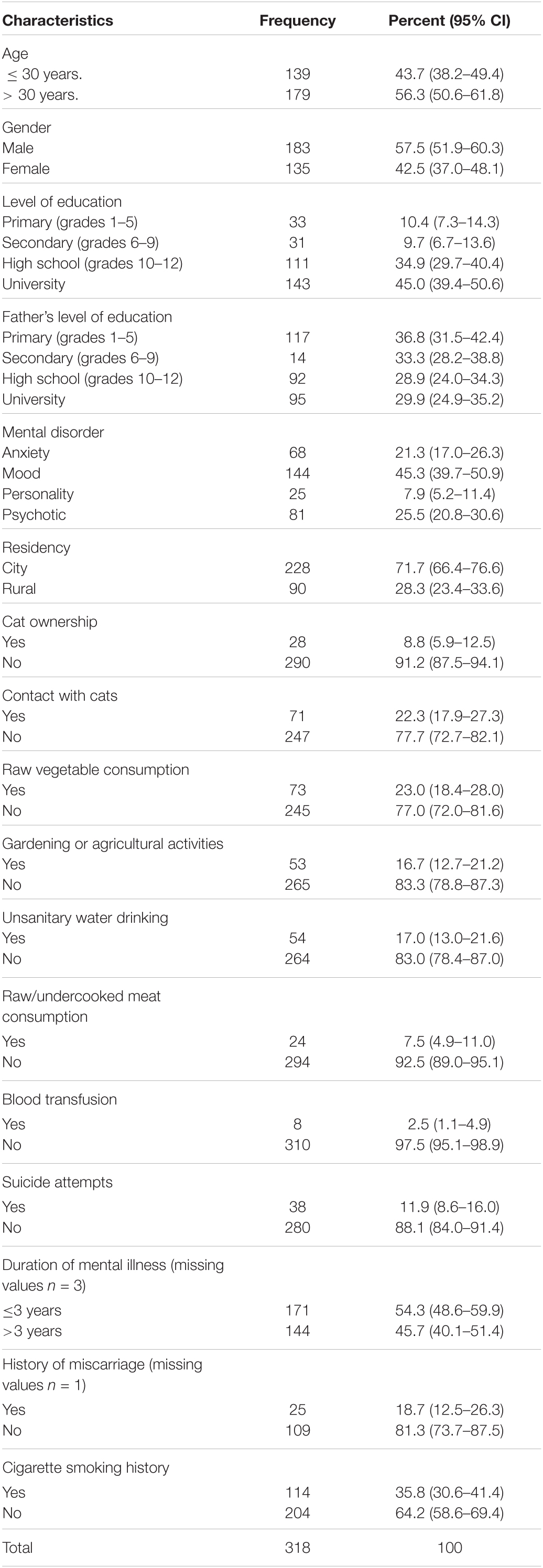
Table 1. Demographic characteristics and risk factors linked to seroprevalence of T. gondii in 318 psychiatric patients.
Seroprevalence and Associated Risk Factors of Toxoplasma gondii in Psychiatric Inpatients
The overall seroprevalence of anti-T. gondii IgG in psychiatric patients was 22.3% (71/318; 95% CI = 17.9–27.3). In univariate analysis, several variables were significantly associated to seropositivity of T. gondii, including age > 30 years [crude odds ratio (COR) = 2.83, 95% CI = 1.57–5.10, p = 0.001], female sex (COR = 1.79, 95% CI = 1.04–3.03, p = 0.03), living in rural areas (COR = 3.14, 95% CI = 1.80–5.44, p < 0.001), having cats in the household (COR = 2.94, 95% CI = 1.32–6.54, p = 0.008), contact with cats (COR = 2.83, 95% CI = 1.59–5.05, p < 0.001), raw vegetable consumption (COR = 4.90, 95% CI = 2.75–8.73, p < 0.001), gardening or agricultural activities (COR = 2.29, 95% CI = 1.21–4.33, p = 0.01), raw/undercooked meat consumption (COR = 5.82, 95% CI = 2.46–13.78, p < 0.001), suicide attempt (COR = 3.39, 95% CI = 1.67–6.86, p = 0.001), duration of mental illness > 3 years (COR = 1.81, 95% CI = 1.06–3.09, p = 0.03) and cigarette smoking history (COR = 0.54, 95% CI = 0.30–0.97, p = 0.04) (Table 2).
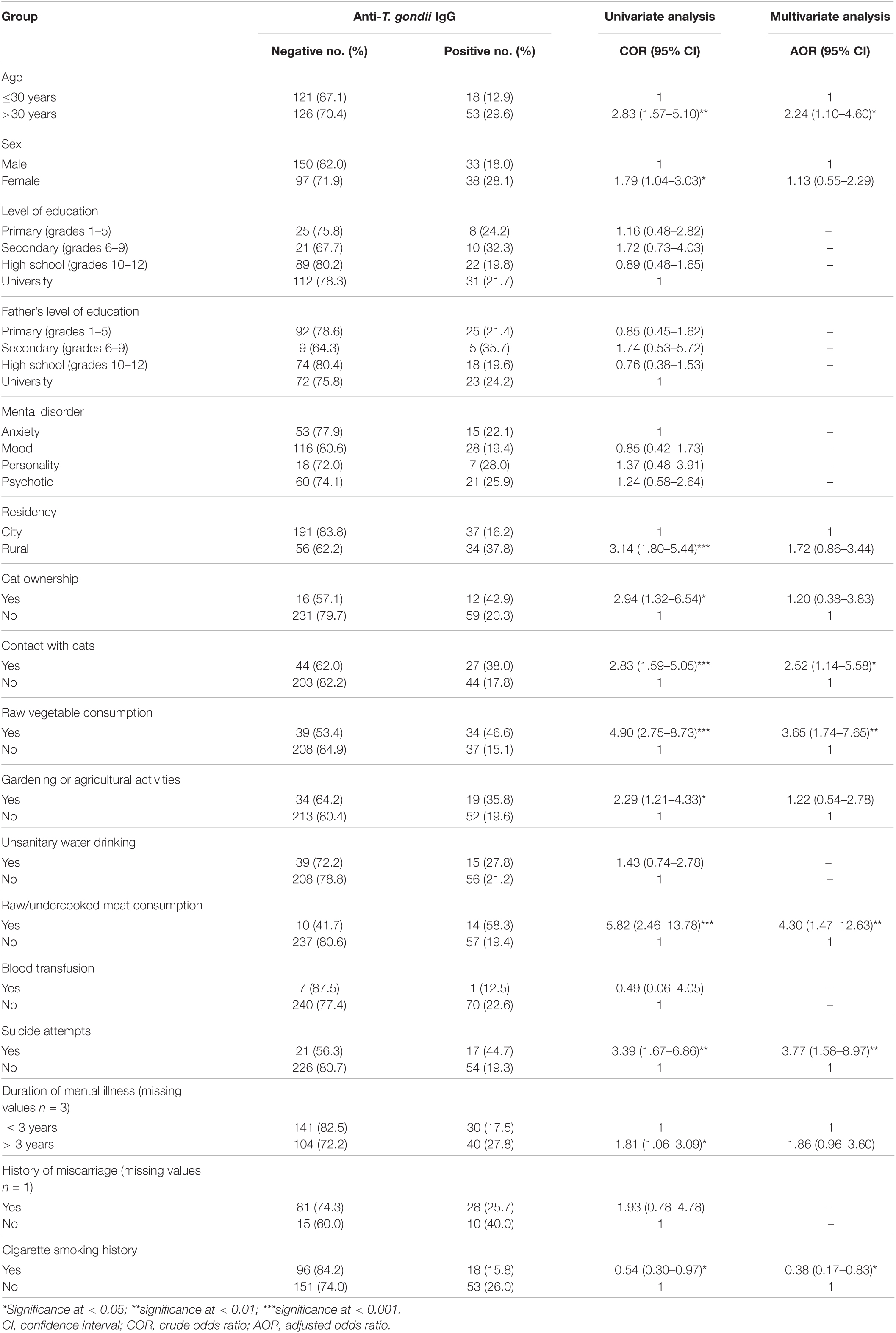
Table 2. Logistic regression analysis of the risk factors associated to anti-T. gondii IgG in 318 psychiatric patients based on univariate and multivariate analyses.
Multivariate analysis revealed that age > 30 years [adjusted odds ratio (AOR) = 2.24, 95% CI = 1.10–4.60, p = 0.03], contact with cats (AOR = 2.52, 95% CI = 1.14–5.58, p = 0.03), raw vegetable consumption (AOR = 3.65, 95% CI = 1.74–7.65, p = 0.001), raw/undercooked meat consumption (AOR = 4.30, 95% CI = 1.47–12.63, p = 0.008), suicide attempt (AOR = 3.77, 95% CI = 1.58–8.97, p = 0.003), and cigarette smoking history (AOR = 0.38, 95% CI = 0.17–0.83, p = 0.02) were independent risk factors for T. gondii infection (Table 2). Results of the association between suicide attempts and T. gondii seropositivity in 318 psychiatric patients are shown in Table 3. T. gondii seropositivity was significantly associated with suicide attempts in anxiety and psychotic disorder groups (Table 3).
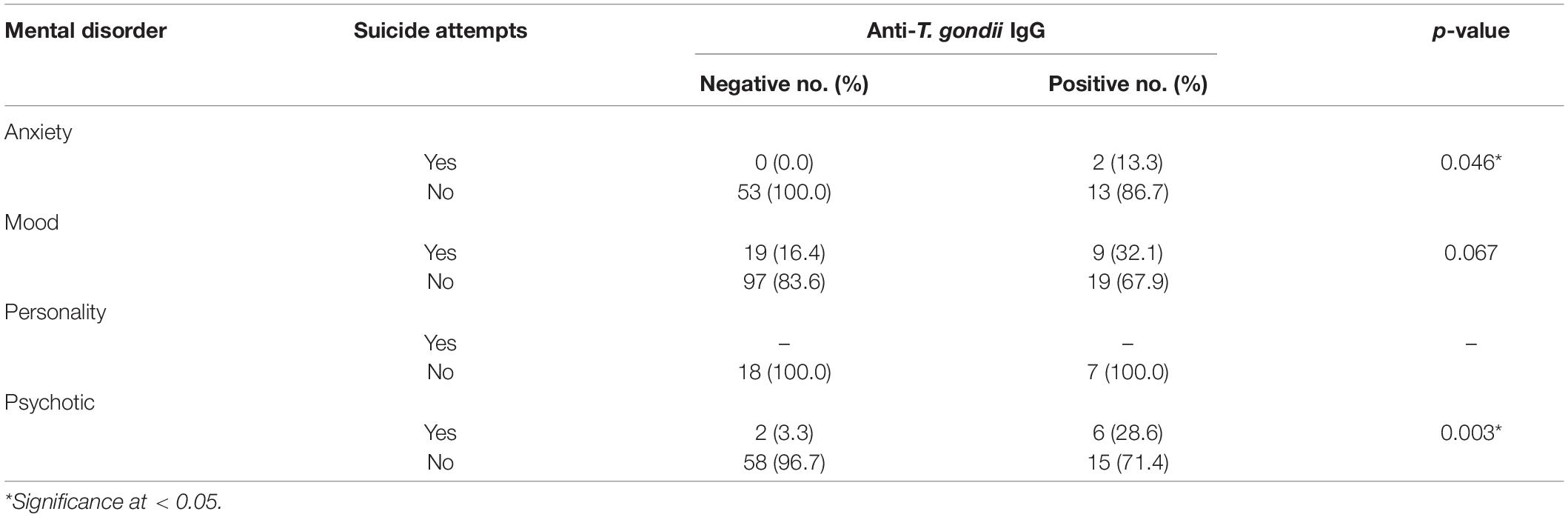
Table 3. Association between suicide attempts and T. gondii seropositivity in 318 psychiatric patients.
Discussion
In this study, the overall seroprevalence of T. gondii infection in psychiatric patients was 22.3%, which was approximately similar to the seroprevalence rate of T. gondii infection in previous studies on the general population and students of Fars Province with an overall prevalence of 21.3% (22) and 27.6% (25), respectively. However, the seroprevalence rate of T. gondii infection in the present study was lower than that in studies on psychiatric patients in Nigeria (32.1%) (26), northwest Ethiopia (33.6%) (27), Libya (41.7%) (28), and western Romania (57.7%) (29). In contrast, prevalence of T. gondii infection in psychiatric patients of the current study was higher than that in studies in eastern China (17.3%) (30) and northern Mexico (18.2%) (31). A possible explanation for the differences might be linked to local cultures, dietary habits, socioeconomic statuses, geographic regions, personal sanitary/hygiene levels, types of the laboratory methods, environmental exposures and spreads of cats in the regions (32).
Results of the present study have shown that T. gondii infection is significantly associated to the age of psychiatric patients. The OR of T. gondii infection increased up to 2.24 (CI = 1.10–4.60, p = 0.03) in participants aged > 30 years, compared to that in participants aged ≤ 30 years. Results were similar to those of previous surveys (33, 34). In a study by Fan et al. (35), a significantly higher seroprevalence (80%) was reported in the age group of ≥ 45 years (35). In a survey by Yang et al. (36), seroprevalence of T. gondii infection showed upward trends with aging and the seroprevalence rate in postgraduate students (2.46%) (≥ 22 years old) was higher than that in undergraduates (1.63%) (≤ 21 years old) (36). Further outdoor activities and longer exposures to the risk factors of infectious sources include possible explanations for further seroprevalences in older people (37, 38). However, in previous studies, T. gondii infection in psychiatric patients was not significantly affected by age (30). This might be resulted from the broad differences in living conditions within the countries as well as lifestyles and study periods.
In the current study, multivariate analysis showed that individuals who contacted with cats and consumed raw vegetables and raw/undercooked meats were, respectively, 2.52, 3.65, and 4.30 times more likely to become infected with T. gondii than those who did not contact with cats and consume raw vegetables and raw/undercooked meats. Constant shedding of oocysts from the infected cats, as the main reservoirs of T. gondii, plays a key role in infection of humans. Moreover, contact with cats has been reported as a risk factor in previous seroepidemiologic studies (39–41). Cats are commonly held as pets in Iran. However, their potential roles in contamination of the environment with T. gondii are neglected. Studies have verified that consumption of infected raw/undercooked meats or infective oocytes via raw vegetables and fruits are independent risk factors in human infection with T. gondii (42–46). It is noteworthy to increase public information of preventive methods against toxoplasmosis with particular emphases on the critical roles of felines in the spread of T. gondii as well as associations between T. gondii infection and behavioral characteristics.
Well-known factors of other studies associated to T. gondii infection, including contact with garden soil (47) and residency in a community (36), were not associated to T. gondii infection in the current study. This is in contrast to previous studies carried out in Gondar (48) and Bench Maji (49) regions of Ethiopia. Yang et al. (36) reported that living in rural areas and gardening or agriculture were independent risk factors for T. gondii seropositivity using multivariate logistic regression analysis; in contrast to the current study (36). In a recent study by Achaw et al. (27), farming (gardening and agriculture) (AOR = 2.058, CI = 1.018–4.163, p = 0.045) was significantly associated to the seroprevalence of anti-T. gondii IgG (27). It could be resulted from the significant survivals of oocysts, remaining infective for up to 24 m under favorable conditions. This could be a potential risk factor for the increased seroprevalence of anti-T. gondii IgG (50). Fars province is located in the south of Iran and has a dry climate with hot sunny summers and relatively cold winters. Environmental conditions and regional climate affect the survival of T. gondii oocysts. Rainfalls, tipping the balance in favor of the survival of the oocysts in the soil. It has been well documented that infection is more prevalent in humid tropical areas than in cold climates and arid areas (19, 20). However, the seroprevalence of human toxoplasmosis depends on several anthropogenic factors such as dietary habits and hygiene practices (19).
In the present study, significant associations were detected between seropositivity to T. gondii and suicide attempt in psychiatric inpatients. This suggests that T. gondii infection increases the risk of suicide with an OR of 3.77. Similar to the present study, several studies have shown that T. gondii infection represents a risk factor for suicidal behaviors (51). Associations of T. gondii seropositivity with suicide attempt, recurrent mood disorders and psychiatric patients have been documented in South Korea (52), United States (8), and Mexico (53). More recently, associations between T. gondii seropositivity and suicidal behaviors have been significant in Mexican patients attending primary health care clinics and those with alcohol consumption (54). In a study by Zhang et al., associations between T. gondii infection and non-fatal suicidal self-directed violence showed that T. gondii infected individuals had 7.12 times more significant risk of non-fatal suicide attempts (55). Ling et al. reported significant upward trends in seropositivity of T. gondii and risks of suicide in women older than 45 years (56). Furthermore, numerous studies on European and Chinese nations highlighted that countries with higher T. gondii prevalence rates included higher suicide rates (57). Interestingly, a case study reported that depressive symptoms were completely improved following treatment of T. gondii infection; however, antidepressant therapy did not resolve patients’ depressive symptoms (58). A recent systematic review and meta-analysis demonstrated that odds of suicide in individuals with T. gondii seropositivity was 43% (OR: 1.43, 95% CI; 1.15–1.78) higher than that in individuals with no infection (51).
Pathophysiological mechanisms of T. gondii in suicide behaviors have not been discovered clearly. Within numerous theories describing possible relationships between T. gondii infection and suicide behavior, direct effects of T. gondii on neuronal function and immune-mediated dopamine and serotonin syntheses is a highlighted hypothesis. In this scenario, host immune response against T. gondii infection produces pro-inflammatory cytokines (IFN- γ, IL-6, and IL-12) through activation of Th cells and macrophages (59). The produced IFN- γ blocks development of T. gondii by inducing activation of enzymes of kynurenine monooxygenase (KMO) and indoleamine 2,3-dioxygenase (IDO), resulting in tryptophan depletion (60, 61). Resultant tryptophan depletion results in decreases in serotonin production in the brain, which may increase human susceptibility to trigger suicide risk factors such as depression, impulsivity and aggression (62). Moreover, the activation of kynurenine pathway in itself as well as the imbalance between neurotoxic and neuroprotective metabolites could trigger the depression (63). The highlighted inflammatory pathways and associated chemical compounds affecting the nervous system can play key roles in behavioral development that triggers suicide attempts (64, 65). In contrast, the current results deny associations between T. gondii infection and suicidal attempts discovered in a study on patients suffering from mental and behavioral disorders due to psychoactive substance use in Mexico (66), adolescents in Turkey (67) and general population study in Finland (68). Further studies with a wide range of sociodemographic, clinical and behavioral variables are necessary to investigate associations between T. gondii infection and suicide attempts.
Another finding of the present study included that T. gondii infection in psychiatric patients was negatively associated to smoking habits (AOR = 0.38, 95% CI = 0.17–0.83, p = 0.02). Infection with T. gondii and smoking are linked to dopamine release. Naturally, dopamine signals pleasure and is considered a mood booster. This pleasure reaction and glee resulting from dopamine is a significant part of nicotine and smoking addictions (69). Regarding possible mechanisms involved in this process, T. gondii seropositivity might affect key dopaminergic pathways of the nervous system and decline the desire or motivation of smoking. Chronic T. gondii infection usually causes specific changes in chemical messengers used by inter-neuronal connections of the brain. Decoding genome of T. gondii has revealed that amino acid hydroxylase, a rate-limiting enzyme in dopamine synthesis, is coded in two loci with high homologies for mammalian genes (70). These homologies demonstrate that the presence of T. gondii in the brain can increase availability of dopamine, leading to the feeling of pleasure and satisfaction (71, 72). Furthermore, experimental studies have verified that T. gondii infection triggers extra releases of dopamine in the brain of infected mice (73). Similarly, pharmaceutical compounds interfering with dopamine metabolism prevent effects of T. gondii on mental manners (74). Associations of tobacco addiction with detrimental health outcomes such as hepatitis C virus (75), postoperative infections after dental implants (76) and spine surgeries (77) have been well-documented. However, mechanisms underlying associations of the infection with tobacco smoking are poorly described. In terms of the limitations, save for the DSM-V guideline, no independent tools were administrated to confirm the clinical diagnosis of patients, and also, it should be noted that IgG assessment in a single serum sample, cannot estimate the exact time of acquiring the T. gondii infection.
Conclusion
The present study showed that the overall seroprevalence of T. gondii infection in psychiatric patients was 22.3%. Multivariate analyses demonstrated that contact with cats, raw vegetable consumption, and raw/undercooked meat consumption were independent risk factors for T. gondii seropositivity. Moreover, the current study revealed significant associations between seropositivity of T. gondii and suicide attempts as well as negative associations between seropositivity of T. gondii and cigarette smoking in psychiatric inpatients using multivariate logistic regression.
Data Availability Statement
Data generated in this study are included in the published article. Data analyzed during the current study are publicly available via Figshare Repository, https://figshare.com/s/111fbbe17cbb6b6c62f3.
Ethics Statement
The studies involving human participants were reviewed and approved by the research Ethics Committee of Shiraz University of Medical Sciences. All participants agreed that their participation was voluntary and was informed that the methodology included no potential risks and that their information was assumed strictly confidential. This study was approved by the research Ethics Committee of Shiraz University of Medical Sciences with an ethical code: IR.SUMS.MED.REC.1399.580. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
AT and QA conceived and designed the study. PH collected the sample from the hospital. MB, AS, and PH contributed in ELISA method. AT, OJN, SM, RA, and QA analyzed and interpreted the data. AT and OJN prepared the original draft manuscript. AT and SM contributed to review and editing of the final version of manuscript. All authors have read and approved the final manuscript.
Funding
This research was financially supported by the office of the Vice-chancellor for research at Shiraz University of Medical Sciences with grant No: 21691.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge all staff from Ibn Sina Hospital, Shiraz, Iran for their useful collaboration.
References
2. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. (2000) 30:1217–58. doi: 10.1016/s0020-7519(00)00124-7
3. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. (2004) 363:1965–76. doi: 10.1016/S0140-6736(04)16412-X
4. Teimouri A, Mohtasebi S, Kazemirad E, Keshavarz H. Role of Toxoplasma gondii IgG avidity testing in discriminating between acute and chronic toxoplasmosis in pregnancy. J Clin Microbiol. (2020) 58:e00505–20. doi: 10.1128/JCM.00505-20
5. Shojaee S, Teimouri A, Keshavarz H, Azami SJ, Nouri S. The relation of secondary sex ratio and miscarriage history with Toxoplasma gondii infection. BMC Infect Dis. (2018) 18:307. doi: 10.1186/s12879-018-3228-0
6. Flegr J, Escudero DQ. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects-an explorative cross-sectional study. Parasitology. (2016) 143:1974–89. doi: 10.1017/S0031182016001785
7. Flegr J, Zitková Š, Kodym P, Frynta D. Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii. Parasitology. (1996) 113:49–54. doi: 10.1017/s0031182000066269
8. Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, et al. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. J Nerv Ment Dis. (2009) 197:905–8. doi: 10.1097/NMD.0b013e3181c29a23
9. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
10. Ghorbani M, Edrissian GH, Assad N. Serological survey of toxoplasmosis in the northern part of Iran, using indirect fluorescent antibody technique. Trans R Soc Trop Med Hyg. (1978) 72:369–71. doi: 10.1016/0035-9203(78)90129-3
11. Foroutan-Rad M, Khademvatan S, Majidiani H, Aryamand S, Rahim F, Malehi AS. Seroprevalence of Toxoplasma gondii in the Iranian pregnant women: a systematic review and meta-analysis. Acta Trop. (2016) 158:160–9.
12. Ahmadpour E, Daryani A, Sharif M, Sarvi S, Aarabi M, Mizani A, et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J Infect Dev Ctries. (2014) 8:1503–10. doi: 10.3855/jidc.4796
13. Anvari D, Sharif M, Sarvi S, Aghayan SA, Gholami S, Pagheh AS, et al. Seroprevalence of Toxoplasma gondii infection in cancer patients: a systematic review and meta-analysis. Microb Pathog. (2019) 129:30–42.
14. Mizani A, Alipour A, Sharif M, Sarvi S, Amouei A, Shokri A, et al. Toxoplasmosis seroprevalence in Iranian women and risk factors of the disease: a systematic review and meta-analysis. Trop Med Health. (2017) 45:7. doi: 10.1186/s41182-017-0048-7
15. Zhu S. Psychosis may be associated with toxoplasmosis. Med Hypotheses. (2009) 73:799–801. doi: 10.1016/j.mehy.2009.04.013
16. Walker ER, Druss BG. Mental and addictive disorders and medical comorbidities. Curr Psych Rep. (2018) 20:86. doi: 10.1007/s11920-018-0956-1
17. Nakamura Y, Koh M, Miyoshi E, Ida O, Morikawa M, Tokuyama A, et al. High prevalence of the hepatitis C virus infection among the inpatients of schizophrenia and psychoactive substance abuse in Japan. Prog Neuropsychopharmacol Biol Psychiatry. (2004) 28:591–7. doi: 10.1016/j.pnpbp.2004.01.018
18. Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax. (2013) 68:171–6. doi: 10.1136/thoraxjnl-2012-202480
19. Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. (2012) 25:264–96. doi: 10.1128/CMR.05013-11
20. Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. (2014) 137:185–94. doi: 10.1016/j.actatropica.2014.05.015
21. Regier DA, Kuhl EA, Kupfer DJ. The DSM-5: classification and criteria changes. World Psychiatry. (2013) 12:92–8. doi: 10.1002/wps.20050
22. Shad-Del F, GhaffariSarvestani R. Seroprevalence of Toxoplasma infection in human and dog population in Shiraz. Iran J Appl Anim Res. (1993) 3:83–9.
23. Teimouri A, Modarressi MH, Shojaee S, Mohebali M, Rezaian M, Keshavarz H. Development, optimization, and validation of an in-house Dot-ELISA rapid test based on SAG1 and GRA7 proteins for serological detection of Toxoplasma gondii infections. Infect Drug Resist. (2019) 12:2657–69. doi: 10.2147/IDR.S219281
25. Razavi SM, Esnaashari HR, Gheisari HR. Seroepidemiological survey of toxoplasmosis by IFA technique in the students of faculty of veterinary medicine, university of Shiraz. J Vet Res. (2003) 58:163–7.
26. James BO, Agbonile IO, Okolo M, Lawani AO, Omoaregba JO. Prevalence of Toxoplasma gondii infection among individuals with severe mental illness in Nigeria: a case control study. Pathog Glob Health. (2013) 107:189–93. doi: 10.1179/2047773213Y.0000000093
27. Achaw B, Tesfa H, Zeleke AJ, Worku L, Addisu A, Yigzaw N, et al. Sero-prevalence of Toxoplasma gondii and associated risk factors among psychiatric outpatients attending University of Gondar Hospital, Northwest Ethiopia. BMC Infect Dis. (2019) 19:581. doi: 10.1186/s12879-019-4234-6
28. Elsaid MMA, Azbedah AG, Dia Eddin E, El-Alem and Alkout A. The prevalence of Toxoplasma gondii infection in psychiatric patients in Tripoli, Libya. J Am Sci. (2014) 10:135–40.
29. Olariu T, Capraru I, Papava I, Romosan R, Dehelean L, Lupu M. Seroprevalence of Toxoplasma gondii in Romanian psychiatric patients. Eur Psychiatry. (2017) 41:S825.
30. Cong W, Dong W, Bai L, Wang XY, Ni XT, Qian AD, et al. Seroprevalence and associated risk factors of Toxoplasma gondii infection in psychiatric patients: a case-control study in eastern China. Epidemiol Infect. (2015) 143:3103–9. doi: 10.1017/S0950268814003835
31. Alvarado-Esquivel C, Alanis-Quiñones OP, Arreola-Valenzuela MÁ, Rodríguez-Briones A, Piedra-Nevarez LJ, Duran-Morales E, et al. Seroepidemiology of Toxoplasma gondii infection in psychiatric inpatients in a northern Mexican city. BMC Infect Dis. (2006) 6:178. doi: 10.1186/1471-2334-6-178
32. Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis. (2009) 49:878–84. doi: 10.1086/605433
33. Nowakowska D, Wujcicka W, Sobala W, Spiewak E, Gaj Z, Wilczyński J. Age-associated prevalence of Toxoplasma gondii in 8281 pregnant women in Poland between 2004 and 2012. Epidemiol Infect. (2014) 142:656–61. doi: 10.1017/S0950268813001179
34. Alvarado-Esquivel C, Estrada-Martınez S, Pizarro-Villalobos H, Arce-Quinones M, Liesenfeld O, Dubey JP. Seroepidemiology of Toxoplasma gondii infection in general population in a Northern Mexican city. J Parasitol. (2011) 97:40–3. doi: 10.1645/GE-2612.1
35. Fan CK, Hung CC, Su KE, Chiou HY, Gil V, dos Reis Ferreira MC, et al. Seroprevalence of Toxoplasma gondii infection among inhabitants in the democratic republic of Sao Tome and Principe. Trans R Soc Trop Med Hyg. (2007) 101:1157–8. doi: 10.1016/j.trstmh.2007.04.010
36. Yang N, Wang D, Xing M, Li C, Li J, Wu A, et al. Seroepidemiology and risk factors of Toxoplasma gondii infection among the newly enrolled undergraduates and postgraduate students in China. Front Microbiol. (2017) 8:2092. doi: 10.3389/fmicb.2017.02092
37. Fan CK, Hung CC, Su KE, Sung FC, Chiou HY, Gil V, et al. Seroprevalence of Toxoplasma gondii infection among pre-schoolchildren aged 1 to 5 years in the Democratic Republic of Sao Tome and Principe, Western Africa. Trans R Soc Trop Med Hyg. (2006) 100:446–9. doi: 10.1016/j.trstmh.2005.07.013
38. Hung CC, Fan CK, Su KE, Sung FC, Chiou HY, Gil V, et al. Serological screening and toxoplasmosis exposure factors among pregnant women in the democratic republic of Sao Tome and Principe, Western Africa. Trans R Soc Trop Med Hyg. (2007) 101:134–9. doi: 10.1016/j.trstmh.2006.04.012
39. Chiang TY, Hsieh HH, Kuo MC, Chiu KT, Lin WC, Fan CK, et al. Seroepidemiology of Toxoplasma gondii infection among healthy blood donors in Taiwan. PLoS One. (2012) 7:e48139. doi: 10.1371/journal.pone.0048139
40. Zemene E, Yewhalaw D, Abera S, Belay T, Samuel A, Zeynudin A. Seroprevalence of Toxoplasma gondii and associated risk factors among pregnant women in Jimma town, Southwestern Ethiopia. BMC Infect Dis. (2012) 12:337. doi: 10.1186/1471-2334-12-337
41. Rodrigues JP, Frei F, Navarro IT, Silva LP, Marcelino MY, Andrade Junior HF, et al. Seroepidemiological analysis of toxoplasmosis in college students. J Venom Anim Toxins Incl Trop Dis. (2015) 21:1. doi: 10.1186/1678-9199-21-1
42. Lopes AP, Dubey JP, Moutinho O, Gargaté MJ, Vilares A, Rodrigues M, et al. Seroepidemiology of Toxoplasma gondii infection in women from the North of Portugal in their childbearing years. Epidemiol Infect. (2012) 140:872–7. doi: 10.1017/S0950268811001658
43. Krueger WS, Hilborn ED, Converse RR, Wade TJ. Drinking water source and human Toxoplasma gondii infection in the United States: a cross-sectional analysis of NHANES data. BMC Public Health. (2014) 14:711. doi: 10.1186/1471-2458-14-711
44. Ertug S, Okyay P, Turkmen M, Yuksel H. Seroprevalence and risk factors for Toxoplasma infection among pregnant women in Aydin province, Turkey. BMC Public Health. (2005) 5:66. doi: 10.1186/1471-2458-5-66
45. Lopez-Castillo CA, Diaz-Ramirez J, Gomez-Marin JE. Risk factors for Toxoplasma gondii infection in pregnant women in Armenia, Colombia. Rev Salud Publica. (2005) 7:180–90.
46. de Moura L, Bahia-Oliveira LM, Wada MY, Jones JL, Tuboi SH, Carmo EH, et al. Waterborne toxoplasmosis, Brazil, from field to gene. Emerg Infect Dis. (2006) 12:326–9. doi: 10.3201/eid1202.041115
47. Spalding SM, Amendoeira MR, Klein CH, Ribeiro LC. Serological screening and toxoplasmosis exposure factors among pregnant women in South of Brazil. Rev Soc Bras Med Trop. (2005) 38:173–7. doi: 10.1590/s0037-86822005000200009
48. Muluye D, Wondimeneh Y, Belyhun Y, Moges F, Endris M, Ferede G, et al. Prevalence of Toxoplasma gondii and associated risk factors among people living with HIV at Gondar University hospital, Northwest Ethiopia. ISRN Trop Med. (2013) 2013:1–15. doi: 10.1155/2013/123858
49. Abamecha F, Awel H. Seroprevalence and risk factors of Toxoplasma gondii infection in pregnant women following antenatal care at Mizan Aman general hospital, bench Maji zone (BMZ), Ethiopia. BMC Infect Dis. (2016) 16:460. doi: 10.1186/s12879-016-1806-6
50. Dubey J. Sources of Toxoplasma gondii infection in pregnancy: until rates of congenital toxoplasmosis fall, control measures are essential. BMJ. (2000) 321:127–8. doi: 10.1136/bmj.321.7254.127
51. Soleymani E, Faizi F, Heidarimoghadam R, Davoodi L, Mohammadi Y. Association of T. gondii infection with suicide: a systematic review and meta-analysis. BMC Public Health. (2020) 20:766. doi: 10.1186/s12889-020-08898-w
52. Bak J, Shim SH, Kwon YJ, Lee HY, Kim JS, Yoon H, et al. The association between suicide attempts and Toxoplasma gondii infection. Clin Psychopharmacol Neurosci. (2018) 16:95–102.
53. Alvarado-Esquivel C, Sánchez-Anguiano LF, Arnaud-Gil CA, López-Longoria JC, Molina-Espinoza LF, Estrada-Martínez S, et al. Toxoplasma gondii infection and suicide attempts: a case-control study in psychiatric outpatients. J Nerv Ment Dis. (2013) 201:948–52. doi: 10.1097/NMD.0000000000000037
54. Alvarado-Esquivel C, Estrada-Martínez S, Ramos-Nevárez A, Pérez-Álamos AR, Beristain-García I, Alvarado-Félix ÁO, et al. Association between Toxoplasma gondii exposure and suicidal behavior in patients attending primary health care clinics. Pathogens. (2021) 10:677. doi: 10.3390/pathogens10060677
55. Zhang Y, Träskman-Bendz L, Janelidze S, Langenberg P, Saleh A, Constantine N, et al. Toxoplasma gondii immunoglobulin G antibodies and nonfatal suicidal self-directed violence. J Clin Psychiatry. (2012) 73:1069–76. doi: 10.4088/JCP.11m07532
56. Ling VJ, Lester D, Mortensen PB, Langenberg PW, Postolache TT. Toxoplasma gondii seropositivity and suicide rates in women. J Nerv Ment Dis. (2011) 199:440–4. doi: 10.1097/NMD.0b013e318221416e
57. Hurley RA, Taber KH. Latent Toxoplasmosis gondii: emerging evidence for influences on neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. (2012) 24:376–83. doi: 10.1176/appi.neuropsych.12100234
58. Kar N, Misra B. Toxoplasma seropositivity and depression: a case report. BMC Psychiatry. (2004) 4:1. doi: 10.1186/1471-244X-4-1
59. Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. (2009) 39:23–39.
60. Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull. (2007) 33:745–51.
61. Webster JP, McConkey GA. Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasitol (Praha). (2010) 57:95–104. doi: 10.14411/fp.2010.012
63. Savitz J. Role of kynurenine metabolism pathway activation in major depressive disorders. Curr Top Behav Neurosci. (2017) 31:249–67. doi: 10.1007/7854_2016_12
64. Khabazghazvini B, Groer M, Fuchs D, Strassle P, Lapidus M, Sleemi A, et al. Psychiatric manifestations of latent toxoplasmosis. Potential mediation by indoleamine 2, 3-dioxygenase. Int J Disabil Hum Dev. (2010) 9:3–10.
65. Amouei A, Moosazadeh M, Sarvi S, Mizani A, Pourasghar M, Teshnizi SH, et al. Evolutionary puzzle of Toxoplasma gondii with suicidal ideation and suicide attempts: an updated systematic review and meta-analysis. Transbound Emerg Dis. (2020). [Epub ahead of print]. doi: 10.1111/tbed.13550
66. Alvarado-Esquivel C, Carrillo-Oropeza D, Pacheco-Vega SJ, Hernández-Tinoco J, Salcedo-Jaquez M, Sánchez-Anguiano LF, et al. Toxoplasma gondii exposure in patients suffering from mental and behavioral disorders due to psychoactive substance use. BMC Infect Dis. (2015) 15:172. doi: 10.1186/s12879-015-0912-1
67. Sari SA, Kara A. Association of suicide attempt with seroprevalence of Toxoplasma gondii in adolescents. J Nerv Ment Dis. (2019) 207:1025–30. doi: 10.1097/NMD.0000000000001046
68. Lindgren M, Holm M, Markkula N, Härkänen T, Dickerson F, Yolken RH, et al. Exposure to common infections and risk of suicide and self-harm: a longitudinal general population study. Eur Arch Psychiatry Clin Neurosci. (2020) 270:829–39. doi: 10.1007/s00406-020-01120-3
70. Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. (2009) 4:e4801. doi: 10.1371/journal.pone.0004801
71. Martin HL, Alsaady I, Howell G, Prandovszky E, Peers C, Robinson P, et al. Effect of parasitic infection on dopamine biosynthesis in dopaminergic cells. Neuroscience. (2015) 306:50–62. doi: 10.1016/j.neuroscience.2015.08.005
72. Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One. (2011) 6:e23866. doi: 10.1371/journal.pone.0023866
73. Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H. Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp Parasitol. (2013) 133:1–7. doi: 10.1016/j.exppara.2012.10.005
74. Webster JP, Lamberton PH, Donnelly CA, Torrey EF. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behaviour. Proc Biol Sci. (2006) 273:1023–30. doi: 10.1098/rspb.2005.3413
75. Waziry R, Jawad M, Ballout RA, Al Akel M, Akl EA. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol. (2017) 46:32–43. doi: 10.1093/ije/dyw021
76. Keenan J, Veitz-Keenan A. The impact of smoking on failure rates, postoperative infection and marginal bone loss of dental implants. Evid Based Dent. (2016) 17:4–5. doi: 10.1038/sj.ebd.6401144
Keywords: psychiatric patients, toxoplasmosis, risk factors, suicide attempts, Iran
Citation: Teimouri A, Nassrullah OJ, Hedayati P, Bahreini MS, Alimi R, Mohtasebi S, Salemi AM and Asgari Q (2022) Prevalence and Predictors of Toxoplasma gondii Infection in Psychiatric Inpatients in Fars Province, Southern Iran. Front. Psychiatry 13:891603. doi: 10.3389/fpsyt.2022.891603
Received: 07 March 2022; Accepted: 18 May 2022;
Published: 14 June 2022.
Edited by:
Maria Vitale, Experimental Zooprophylactic Institute of Sicily (IZSSi), ItalyReviewed by:
Veronica Perez De La Cruz, Manuel Velasco Suárez Instituto Nacional de Neurología y Neurocirugía, MexicoPatryk Piotrowski, Wrocław Medical University, Poland
Copyright © 2022 Teimouri, Nassrullah, Hedayati, Bahreini, Alimi, Mohtasebi, Salemi and Asgari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qasem Asgari, YXNnYXJpZ0BzdW1zLmFjLmly
 Aref Teimouri1
Aref Teimouri1 Qasem Asgari
Qasem Asgari