- Department of Psychiatry, Wuhan Mental Health Center, Wuhan Hospital for Psychotherapy, Wuhan, China
Background: Attention deficit hyperactivity disorder (ADHD) is a common neurobehavioral disorder in childhood. Brain-derived neurotrophic factor (BDNF) is widely distributed in the central nervous system and plays an important role in neural development. Despite several previous studies have examined the association between the Val66Met polymorphism BDNF and ADHD, the results are conflicting.
Objective: This study aimed to evaluate the association between Val66Met polymorphism and ADHD in case-control and transmission disequilibrium test (TDT) studies using a meta-analysis.
Methods: Keywords “rs6265” or “Val66Met” and “Attention deficit hyperactivity disorder” were used to search in the PubMed, Embase, Web of Science, Wanfang, and China National Knowledge Infrastructure databases before April 2021. Genotype data were extracted to calculate odds ratios (ORs) and 95% confidence intervals (CIs).
Results: Fifteen studies, comprising of 8,692 samples (containing 4,364 cases, 4,328 controls) and 1,578 families were included and results demonstrated that rs6265 was not associated with susceptibility to ADHD (OR = 0.95, 95% CI: 0.87–1.04, P = 0.291). Stratified analyses by study design, ethnicity, and sample size further supported that rs6265 was not associated with ADHD.
Conclusion: The present study shows that the polymorphism of the BDNF Val66Met gene is not associated with susceptibility to ADHD.
Introduction
Attention deficit hyperactivity disorder (ADHD) is a common neurobehavioral disorder in childhood (1), characterized by inattention, impulsivity, and hyperactivity, and usually accompanied by cognitive impairment, conduct disorder, and other mental disorders. According to recent meta-analysis including 175 eligible worldwide studies, the prevalence of ADHD is approximately 7.2% (2). Recent research has indicted that ADHD is not limited to childhood, but can also occur during adolescence and adulthood. For adolescents, ADHD symptoms often include internet addiction, increased smoking rates, drug abuse, alcohol-related problems, and high-risk sexual behaviors (3), whereas adult patients often face conduct-related problems and criminality (4). Given diverse and continuous negative effects on patients and the serious economic burden on families and society (5), ADHD is a major public health problem. Despite the etiology of ADHD is not fully understood, it is thought to be caused by both genetic and environmental factors. Decades of research on case–control, twin, and family studies have identified the genetic factors influencing the development of ADHD, and reported the mean heritability of ADHD to be up to 74% (6).
The brain-derived neurotrophic factor (BDNF) gene maps to human chromosome 11p14.1 (7). It contains four exons (I–IV) associated with distinct promoters located in the 5’ terminus, and only one (exon V) located in the 3’ terminus, which encodes the mature BDNF protein (8). Both mRNA and protein of BDNF are widely distributed in the central nervous system, particularly in the hippocampus and cerebral cortex (9). BDNF is a key protein that regulates neuronal survival and growth, and plays an important role in neural development and the maintenance of normal brain function (10). Several polymorphisms located in BDNF have been identified to be associated with ADHD, of which the polymorphism rs6265 is the most widely studied. Rs6265, also called Val66Met or G196A, leads to a Val to Met substitution at position 66 in the BDNF pro-domain that disrupts its transport and secretion, resulting in reduced cell surface expression (11, 12). Despite numerous studies that have examined the relationship between rs6265 and ADHD risk, the results are controversial and inconclusive. Whereas some studies have found that the Val allele is significantly associated with ADHD (13–16), other studies could not replicate these positive results (17–27), controversial results may be due to population stratification, false-positive results, and insufficient statistical power.
To overcome limitations of inadequate sample sizes, improve statistical power, and reduce false-positive results, we performed a meta-analysis to integrate results from both case-control (CC) and transmission disequilibrium test (TDT) studies for the association of rs6265 in BDNF with ADHD risk.
Methods
Literature Search
We performed a comprehensive search in the PubMed, Embase, Web of Science, Wanfang, and China National Knowledge Infrastructure databases to identify all potentially relevant studies using the keywords “rs6265,” “Val66Met,” and “attention deficit hyperactivity disorder.” The literature search was updated on April 30, 2021, and articles in English and Chinese were included for further analysis.
Inclusion Criteria
The inclusion criteria for the studies were as follows: (1) published in English or Chinese; (2) CC or TDT studies; (3) evaluation of the association between rs6265 in BDNF and ADHD risk, (4) clear definition of ADHD, and (5) availability of data necessary for calculating odds ratios (ORs) with 95% confidence intervals (CIs). Reviews, case reports, case-only studies, animal studies, simple commentaries, and studies without sufficient data were excluded. When more than one study had overlapping data, only the study with the largest sample size was selected.
Data Extraction
All data were extracted from the original studies by two independent investigators, and disagreements were resolved by discussion. The following information was extracted from the selected studies: authors, publication year, ethnicity and country of the study population, study design, diagnostic criteria for ADHD, sample size, counts of alleles in case and control groups in CC studies, and number of transmitted alleles from heterozygous parents to affected offspring in TDT studies.
Statistical Analysis
The association between rs6265 and the risk of ADHD was evaluated using integrated ORs and 95% CIs. The between-study heterogeneity was measured using a Q-statistic test (28). When the P-value of the Q statistic test was smaller than 0.10, indicating significant heterogeneity across studies, a random-effects model was used (29). Otherwise, fixed effects model was applied (30). Stratified analysis was carried out according to study design (CC study and TDT study), ethnicity (Asian and European populations), and sample size (large sample-size subgroup: number of cases in CC studies or number of families in family based studies > 200; small sample-size: subgroup: number of cases in CC study or number of families in family based study < 200). Sensitivity analysis was performed by recalculating the ORs after removing each study to evaluate the stability of the results. Publication bias was investigated using a funnel plot and Egger’s test using the “catmap” and “metaphor” package in R software (31). A P-value of < 0.05 was considered statistically significant.
Results
Characteristics of Included Studies
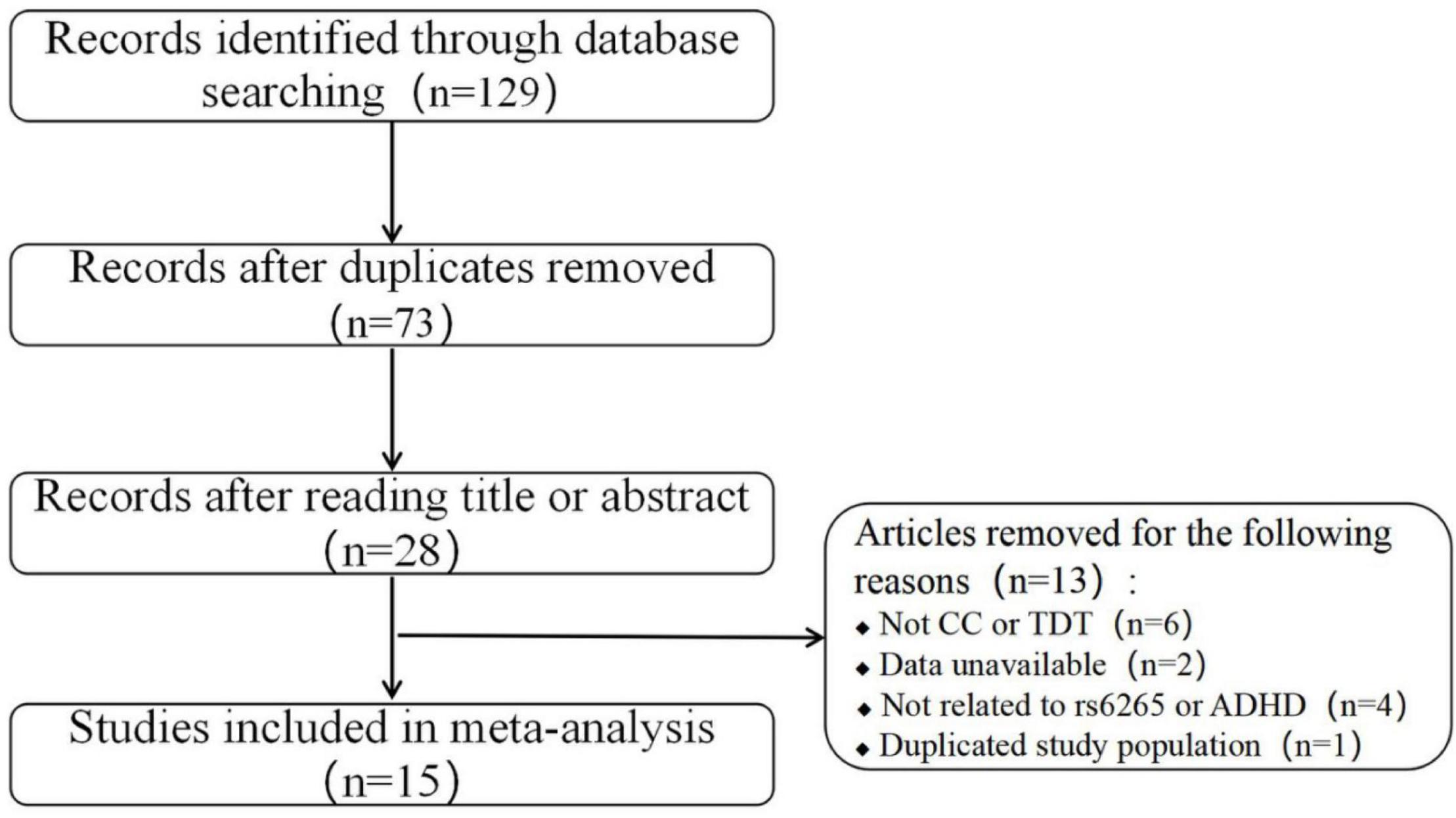
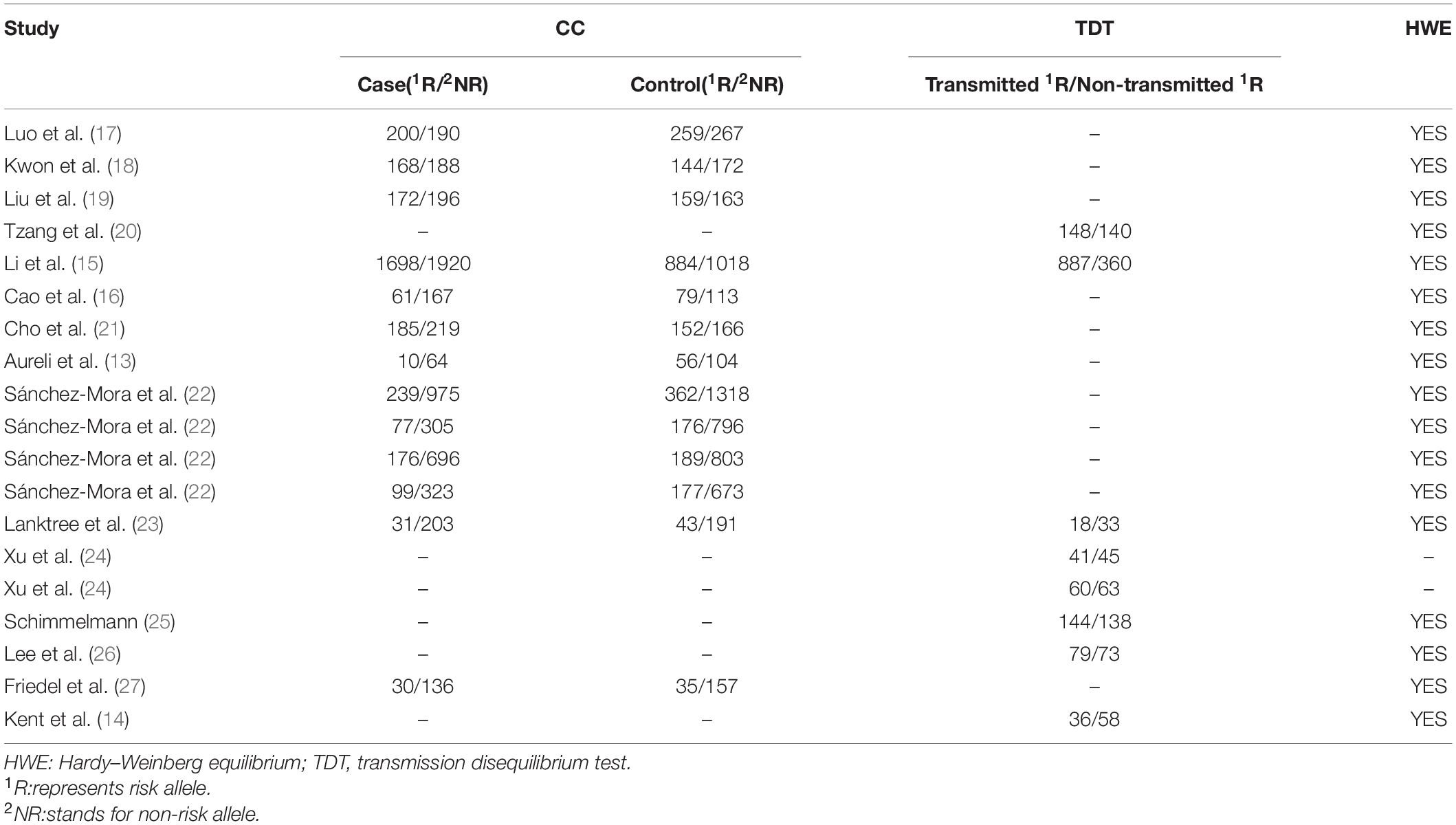
The literature search and study selection procedures are presented in a flow chart in Figure 1. A total of 129 records were identified through the above search strategy, and 28 records remained after removing the duplications and scanning the titles and abstracts. Of the remaining 28 records, 13 were further excluded according to the selection criteria: not CC or TDT studies (six studies), lack of sufficient data (two studies), not related to rs6265 or ADHD (four studies), and data duplication (one study). In addition, two records (15, 23) applied both CC and TDT designs in overlapping subjects. In these two instances, only CC studies were included in the overall meta-analysis because of the larger population, but the data from TDT designs were still used for the stratified analysis. After applying these inclusion criteria, 15 studies (seven conducted in Asian populations, seven in European populations, and one in a mixed population) containing 4,364 cases, 4,328 controls, and 1,578 families were retained for the meta-analysis (13–19, 21–23, 25–27). The detailed characteristics and allele information of the included studies are shown in Tables 1, 2.
Combined Results of Case-Control Transmission Disequilibrium Test Studies
Figure 2 shows the combined results of CC and TDT studies for the study of rs6265 association with ADHD risk. A random-effects model was applied because of significant heterogeneity (χ2 = 33.19, Pheterogeneity = 0.016). In the overall meta-analysis, no significant association was observed between rs6265 and ADHD (OR = 0.95, 95% CI: 0.87–1.04, P = 0.291).
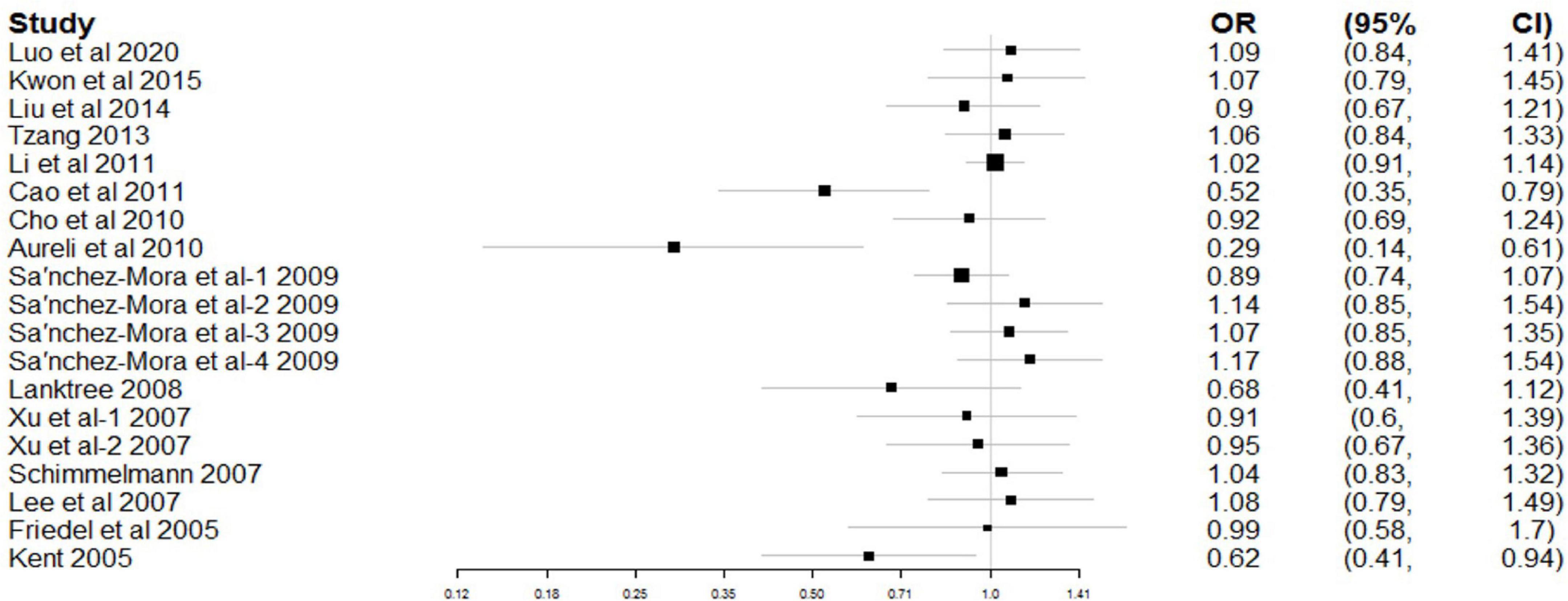
Figure 2. Forest plot for the association between rs6265 and attention deficit hyperactivity disorder (ADHD) risk.
Stratified Analysis
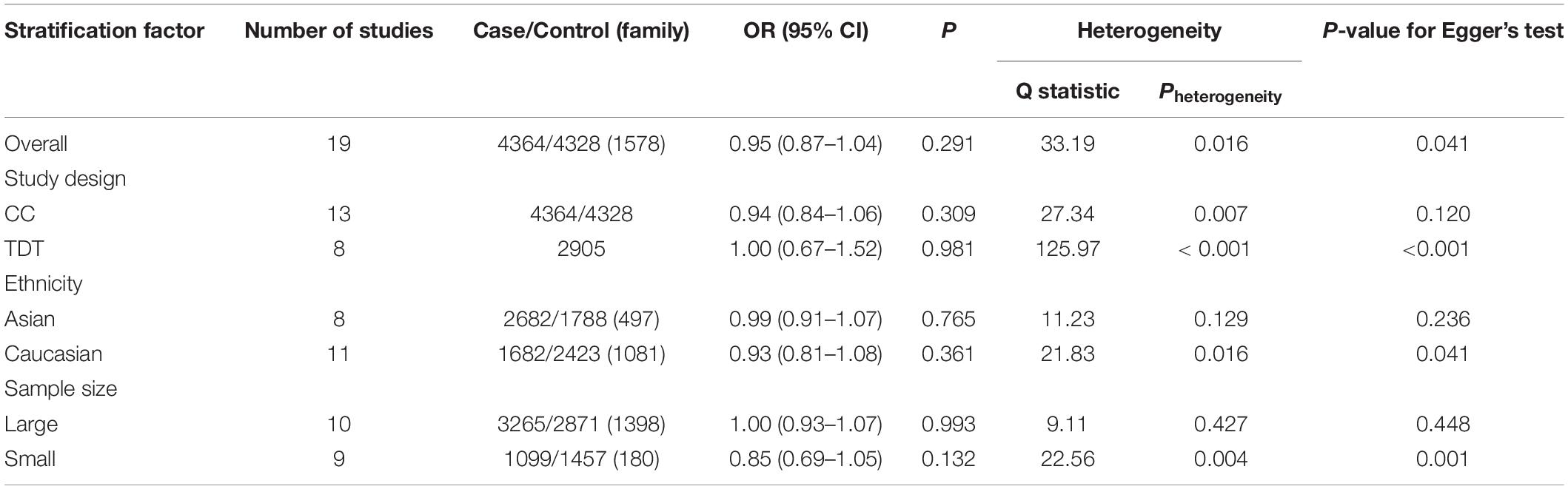
As shown in Table 3, a stratified analysis was first performed by grouping the included studies by study design. However, both the CC and TDT subgroups were significantly heterogeneous (CC: χ2 = 27.34, Pheterogeneity = 0.007; TDT: χ2 = 125.97, Pheterogeneity < 0.001). No significant association was observed between rs6265 and ADHD in either subgroup (CC: OR = 0.94, 95% CI: 0.84–1.06, P = 0.309; TDT: OR = 1.00, 95% CI: 0.67–1.52, P = 0.981).
Then, the data were stratified according to ethnicity. No heterogeneity was found the Asian population (χ2 = 11.23, Pheterogeneity = 0.129), but still existed in the Caucasian population (χ2 = 21.83, Pheterogeneity = 0.016). Ethnicity-specific meta-analysis indicated no association between rs6265 and ADHD in either the Asian (OR = 0.99, 95% CI: 0.91–1.07, P = 0.765) or Caucasian (OR = 0.93, 95% CI: 0.81–1.08, P = 0.361) populations.
Finally, the data were stratified according to sample size. The large sample-size subgroup showed no heterogeneity (χ2 = 9.11, Pheterogeneity = 0.427), however the small-sample-size subgroup still demonstrated significant heterogeneity (χ2 = 22.56, Pheterogeneity = 0.004). No significant association was observed between rs6265 and ADHD in either subgroup (large sample-size: OR = 1.00, 95% CI: 0.93–1.07, P = 0.993; small sample size: OR = 0.85, 95% CI: 0.69–1.05, P = 0.132).
Sensitivity Publication Bias Analyses
Sensitivity analysis was performed by systematically removing a study from the analysis to assess the influence of each study on the pooled OR. This demonstrated relatively robust results for rs6265, with no reverse outcomes. As reflected by the funnel plot (Figure 3) and Egger’s test, significant publication bias was detected for rs6265 (P = 0.041), which may be related to the fact that negative results are not commonly published.
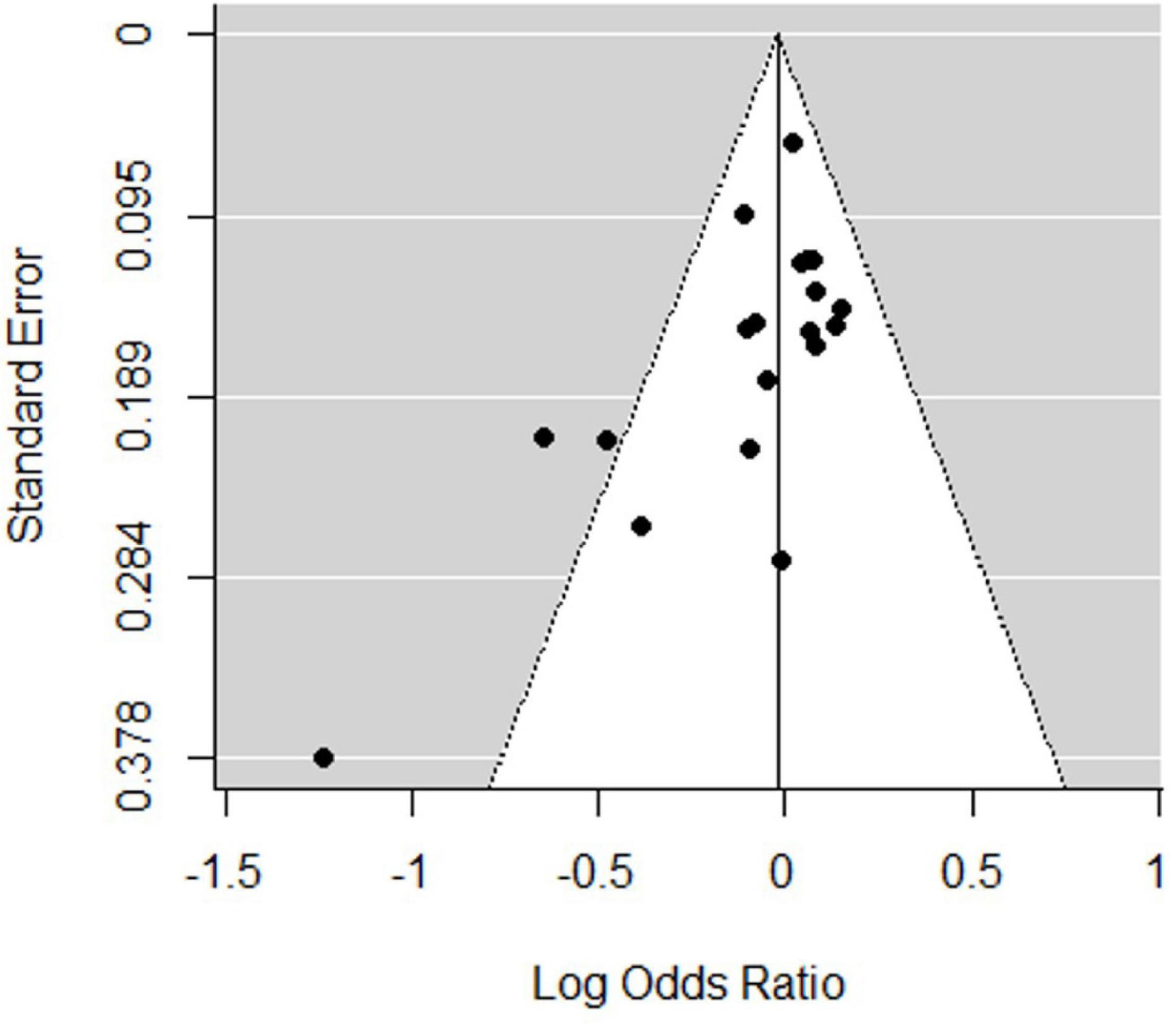
Figure 3. Funnel plot of the association between rs6265 and attention deficit hyperactivity disorder (ADHD) risk.
Discussion
A previous meta-analysis reported a negative association between the rs6265 polymorphism of the BDNF gene and ADHD in European and Asian populations (22, 32). However, subsequent studies failed to replicate this result (13, 16–20). This meta-analysis aimed to investigate this controversy, including CC and TDT studies. The results of this meta-analysis indicated null associations between BDNF rs6265 polymorphism and ADHD risk in all populations. Further analyses revealed that BDNF rs6265 polymorphism was not associated with CC and TDT study design, Asian and European populations, and large and small sample-size populations.
As the most widely distributed and most important neurotrophic factor in the brain (33), the role of BDNF in brain neurodevelopment has received widespread attention. BDNF has been reported to regulate the plasticity of neurons and play a protective role in the regeneration and repair of neurons, through binding to tyrosine kinases B (TrkB), and mediating three signal transduction pathways, including phospholipase, mitogen-activated protein kinase/extracellular signal-regulated protein kinase, and phosphoinositol 3 kinase (34–36). Although the underlying mechanism of ADHD is unclear, the most studied models of pathophysiology of ADHD involved frontal-striatal-cerebellar circuits and ventral striatal-limbic circuits (37). Dopamine is an important neurotransmitter in the central nervous system, and its content in brain is one of the pathogenesis of ADHD. Inhibition of AC/cAMP/PKA pathway leads to feedback reduction of DA, resulting in cognitive impairment (38). Dopamine transporter is essential to maintaining Dopamine balance, Dopamine transporter knockout rats altered the BDNF system in the dorsolateral striatum (39). In addition, studies using patients serum, animal models, and genetic studies have suggested that BDNF is closely related to ADHD (40–42). BDNF serum concentration has been reported to correlate with the clinical symptoms of neurodevelopmental disorders such as reduced intelligence, behavioral problems, and intellectual impairment in preschoolers (43). A recent study uncovered that the level of serum BDNF in ADHD children was significantly higher than that in healthy controls, and that it had a positive relation with the severity of symptoms (33). However, another study reported the opposite result among adults with ADHD (44). In line with this result, BDNF knockout mice showed aggressive behavior similar to that of ADHD compared with wild littermates (45). Although these studies indicate that BDNF plays a role in the development of ADHD, the underlying mechanism remains unclear.
A common exonic Val66Met substitution within the pro-region of BDNF has been widely implicated in ADHD by affecting intracellular transport and activity-dependent BDNF secretion (12, 46). Val66Met was detected in the hippocampus and prefrontal cortex, which are the regions with the most abundant expression of BDNF (47), the central regions of neural plasticity and adaptation related to learning and memory (48), as well as important brain regions in the development of ADHD (12). Therefore, due to the role of BDNF Val66Met in these processes, its relationship with ADHD has attracted increasing attention, but the results are not completely consistent. The results of this meta-analysis do not support the role of the Val66Met polymorphism in susceptibility to ADHD, which was not consistent with the results of epidemiological and functional studies (23, 49–51). TDT studies, such as those conducted by Xu et al. (24) and Tzang et al. (20) did not observe significant associations in the Taiwanese population. Similarly, other studies have reported negative results in the Caucasian population (25, 26). In contrast, Kent et al. (14) and Lanktree et al. (23) reported excessive transmission of allele G of BDNF with a strong paternal effect. In line with this, Li et al. (15) found excessive transmission of allele G in female Chinese ADHD patients. Finally, the CC studies proceeded by Friedel et al. (27), Cho et al. (21), and Luo et al. (17) were unable to demonstrate the effect of Val66Met on susceptibility to ADHD, but Aureli et al. (13) reported a positive result.
This study aimed to explore the relationship between BDNF Val66Met polymorphism and ADHD, including eight CC studies, five TDT studies, and two mixed trials. Our results suggest that the BDNF rs6265 polymorphism may not be a genetic factor for ADHD. The reasons underlying discrepancies regarding the role of rs6265 in ADHD susceptibility are unclear. Stratified analysis revealed that the heterogeneities in the Caucasian group and in the small sample group were increased in comparison with the other groups, which might be related to the fact that negative results are not easy to publish. One potential explanation for discrepancies in the different association studies is the variable distribution of the assessed phenotype in each sample. In this study, we assessed the proportions of ADHD-C (44.4–100%), ADHD-I (7–50.4%), ADHD-HI (0–27%). In Chinese population, the proportion of ADHD-C, ADHD-I, and ADHD-HI were 44.4–79.49%, 15.38–50.4%, 4.8–5.31%, respectively; whereas they were 54.0–100%, 7–24.0%, 0–27.0%, respectively, in the European population. Clinical heterogeneity between samples may mask a real association. Although it is currently unclear how clinical heterogeneity may relate to these differences, additional studies on BDNF Val66Met polymorphism on the different subtypes of response to ADHD are still warranted. Another possible underlying explanation relies on the gender imbalance of the samples. The prevalence of ADHD in men is significantly higher than that in women; however, only one study in the Chinese Han population was evaluated the association considering the gender of patients. The role of gender in ADHD susceptibility needs to be investigated in future research.
Despite these limitations, this updated meta-analysis integrating CC and TDT studies helped to clarify the association between BDNF Val66Met polymorphism and susceptibility to ADHD and suggested that rs6265 may not contribute to the risk of ADHD.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
SM and WC designed the study and did the literature review. SM, SC, YH, and XD did the statistical analysis and prepared the manuscript draft. XL and WC did quality control and contributed to the revisions in depth for the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kieling C, Goncalves RRF, Tannock R, Castellanos FX. Neurobiology of attention-deficit hyperactivity disorder. Child Adolesc Psychiatr Clin North Am. (2008) 17:285–307.
2. Thomas R, Sanders S, Doust J, Beller E, Glasziou P. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. (2015) 135:e994–1001. doi: 10.1542/peds.2014-3482
3. Nugent K, Smart W. Attention-deficit/hyperactivity disorder in postsecondary students. Neuropsychiatr Dis Treat. (2014) 10:1781–91. doi: 10.2147/ndt.s64136
4. Rosler M. Adult Attention-Deficit Hyperactivity Disorder – Functional Impairment, Conduct Problems and Criminality. Basel: Karger (2009).
5. American Academy of Child and Adolescent Psychiatry. National institutes of health consensus development conference statement: diagnosis and treatment of attention-deficit/hyperactivity disorder (ADHD). J Am Acad Child Adolesc Psychiatry. ed. JH, Ferguson (2000) 39:182–93. doi: 10.1097/00004583-200002000-00018
6. Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. (2019) 24:562–75.
7. Hanson IM, Seawright A, Van Heyningen VJG. The human BDNF gene maps between FSHB and HVBS1 at the boundary of 11p13-p14. Genomics. (1992) 13:1331–3. doi: 10.1016/0888-7543(92)90060-6
8. Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. (1993) 10:475–89. doi: 10.1016/0896-6273(93)90335-o
9. Maisonpierre PC, Belluscio L, Friedman B, Alderson RF, Wiegand SJ, Furth ME, et al. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. (1990) 5:501–9. doi: 10.1016/0896-6273(90)90089-x
10. Cattaneo A, Cattane N, Begni V, Pariante CM, Riva MA. The human BDNF gene: peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl Psychiatry. (2016) 6:e958. doi: 10.1038/tp.2016.214
11. Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. (2004) 24:4401–11. doi: 10.1523/JNEUROSCI.0348-04.2004
12. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. (2003) 112:257–69. doi: 10.1016/s0092-8674(03)00035-7
13. Aureli A, Del Beato T, Sebastiani P, Marimpietri A, Melillo CV, Sechi E, et al. Attention-deficit hyperactivity disorder and intellectual disability: a study of association with brain-derived neurotrophic factor gene polymorphisms. Int J Immunopathol Pharmacol. (2010) 23:873–80. doi: 10.1177/039463201002300323
14. Kent L, Green E, Hawi Z, Kirley A, Dudbridge F, Lowe N, et al. Association of the paternally transmitted copy of common valine allele of the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene with susceptibility to ADHD. Mol Psychiatry. (2005) 10:939–43. doi: 10.1038/sj.mp.4001696
15. Li HM, Qian QJ, Wang YF, Yang L, Chen Y, Ning JI, et al. Association between single nucleotide polymorphisms of brain-derived neurotrophic factor gene and attention deficit hyperactivity disorder. J Appl Clin Pediatr. (2011) 26:277–82.
16. Cao Yl, Tang CH, Li SJ, Chang X, Qin TC. Association between gene polymorphism of brain-derived neurotrophic factor and attention deficit hyperactivity disorder. Progr Mod Biomed. (2011) 11: 317–9.
17. Luo L, Jiang X, Cao G, Xiong P, Yang R, Zhang J, et al. Association between BDNF gene polymorphisms and attention deficit hyperactivity disorder in school-aged children in Wuhan, China. J Affect Disord. (2020) 264:304–9. doi: 10.1016/j.jad.2020.01.017
18. Kwon HJ, Ha M, Jin HJ, Hyun JK, Shim SH, Paik KC, et al. Association between BDNF gene polymorphisms and attention deficit hyperactivity disorder in Korean children. Genet Test Mol Biomarkers. (2015) 19:366–71. doi: 10.1089/gtmb.2015.0029
19. Liu DY, Shen XM, Yuan FF, Guo OY, Zhong Y, Chen JG, et al. The physiology of BDNF and its relationship with ADHD. Mol Neurobiol. (2015) 52:1467–76. doi: 10.1007/s12035-014-8956-6
20. Tzang RF, Hsu CD, Liou YJ, Hong CJ, Tsai SJ. Family-based association of the brain-derived neurotrophic factor gene in attention-deficit hyperactivity disorder. Psychiatr Genet. (2013) 23:177–8. doi: 10.1097/ypg.0b013e328360c8a9
21. Cho SC, Kim HW, Kim BN, Kim JW, Shin MS, Chung S, et al. Gender-specific association of the brain-derived neurotrophic factor gene with attention-deficit/hyperactivity disorder. Psychiatry Investig. (2010) 7:285–90. doi: 10.4306/pi.2010.7.4.285
22. Sánchez-Mora C, Ribasés M, Ramos-Quiroga JA, Casas M, Bosch R, Boreatti-Hümmer A, et al. Meta-analysis of brain-derived neurotrophic factor p.Val66Met in adult ADHD in four European populations. Am J Med Genet Part B Neuropsychiatr Genet. (2010) 153b:512–23. doi: 10.1002/ajmg.b.31008
23. Lanktree M, Squassina A, Krinsky M, Strauss J, Jain U, Macciardi F, et al. Association study of brain-derived neurotrophic factor (BDNF) and LIN-7 homolog (LIN-7) genes with adult attention-deficit/hyperactivity disorder. Am J Med Genet Part B Neuropsychiatr Genet. (2008) 147b:945–51. doi: 10.1002/ajmg.b.30723
24. Xu X, Mill J, Zhou K, Brookes K, Chen CK, Asherson P. Family-based association study between brain-derived neurotrophic factor gene polymorphisms and attention deficit hyperactivity disorder in UK and Taiwanese samples. Am J Med Genet Part B Neuropsychiatr Genet. (2007) 144b:83–6. doi: 10.1002/ajmg.b.30406
25. Schimmelmann BG, Friedel S, Dempfle A, Warnke A, Lesch KP, Walitza S, et al. No evidence for preferential transmission of common valine allele of the Val66Met polymorphism of the brain-derived neurotrophic factor gene (BDNF) in ADHD. J Neural Trans. (2007) 114:523–6. doi: 10.1007/s00702-006-0616-1
26. Lee J, Laurin N, Crosbie J, Ickowicz A, Pathare T, Malone M, et al. Association study of the brain-derived neurotropic factor (BDNF) gene in attention deficit hyperactivity disorder. Am J Med Genet Part B Neuropsychiatr Genet. (2007) 144b:976–81. doi: 10.1002/ajmg.b.30437
27. Friedel S, Horro FF, Wermter AK, Geller F, Dempfle A, Reichwald K, et al. Mutation screen of the brain derived neurotrophic factor gene (BDNF): identification of several genetic variants and association studies in patients with obesity, eating disorders, and attention-deficit/hyperactivity disorder. Am J Med Genet Part B Neuropsychiatr Genet. (2005) 132b:96–9. doi: 10.1002/ajmg.b.30090
28. Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. (2005) 21:3672–3. doi: 10.1093/bioinformatics/bti536
29. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Instit. (1959) 22:719–48.
30. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controll Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
32. Lee YH, Song GG. BDNF 196 G/A and COMT Val158Met polymorphisms and susceptibility to ADHD: a meta-analysis. J Atten Disord. (2015) 22:872–77. doi: 10.1007/s10072-014-1645-4
33. Shim SH, Hwangbo Y, Kwon YJ, Jeong HY, Lee BH, Lee HJ, et al. Increased levels of plasma brain-derived neurotrophic factor (BDNF) in children with attention deficit-hyperactivity disorder (ADHD). Progr Neuro Psychopharmacol Biol Psychiatry. (2008) 32:1824–8. doi: 10.1016/j.pnpbp.2008.08.005
34. Lipsky RH, Marini AM. Brain-derived neurotrophic factor in neuronal survival and behavior-related plasticity. Ann N Y Acad Sci. (2007) 1122:130–43. doi: 10.1196/annals.1403.009
35. Wang QC, Lu L, Zhou HJ. Relationship between the MAPK/ERK pathway and neurocyte apoptosis after cerebral infarction in rats. Eur Rev Med Pharmacol Sci. (2019) 23:5374–81. doi: 10.26355/eurrev_201906_18206
36. Botsakis K, Mourtzi T, Panagiotakopoulou V, Vreka M, Stathopoulos GT, Pediaditakis I, et al. BNN-20, a synthetic microneurotrophin, strongly protects dopaminergic neurons in the “weaver mouse, a genetic model of dopamine-denervation, acting through the TrkB neurotrophin receptor. Neuropharmacology. (2017) 121:140–57. doi: 10.1016/j.neuropharm.2017.04.043
37. Baroni A, Castellanos FX. Neuroanatomic and cognitive abnormalities in attention-deficit/hyperactivity disorder in the era of ‘high definition’ neuroimaging. Curr Opin Neurobiol. (2015) 30:1–8. doi: 10.1016/j.conb.2014.08.005
38. Kitagishi Y, Minami A, Nakanishi A, Ogura Y, Matsuda S. Neuron membrane trafficking and protein kinases involved in autism and ADHD. Int J Mol Sci. (2015) 16:3095–115. doi: 10.3390/ijms16023095
39. Leo D, Sukhanov I, Zoratto F, Illiano P, Caffino L, Sanna F, et al. Pronounced hyperactivity, cognitive dysfunctions, and BDNF dysregulation in dopamine transporter knock-out rats. J Neurosci. (2018) 38:1959–72. doi: 10.1523/JNEUROSCI.1931-17.2018
40. Wolraich M, Hagan J, Allan C, Chan E, Davison D, Earls M, et al. Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. (2019) 144:e20192528.
41. Meredith G, Callen S, Scheuer D. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. (2002) 949:218–27. doi: 10.1016/s0006-8993(02)03160-8
42. Heal DJ, Smith SL, Findling RL. ADHD: current and future therapeutics. Curr Top Behav Neurosci. (2012) 9:361. doi: 10.1007/7854_2011_125
43. Yeom CW, Park YJ, Choi SW, Bhang SY. Association of peripheral BDNF level with cognition, attention and behavior in preschool children. Child Adolesc Psychiatry Ment Health. (2016) 10:1–10.
44. Margarida CR, Ramos-Quiroga JA, Marta R, Cristina SM, Gloria P, Sergi V, et al. Decreased serum levels of brain-derived neurotrophic factor in adults with attention-deficit hyperactivity disorder. Int J Neuropsychopharmacol. (2013) 16:1267–75. doi: 10.1017/S1461145712001629
45. Ito W, Chehab M, Thakur S, Li J, Morozov A. BDNF-restricted knockout mice as an animal model for aggression. Genes Brain Behav. (2011) 10:365–74. doi: 10.1111/j.1601-183X.2010.00676.x
46. Hawi Z, Cummins T, Tong J, Arcos-Burgos M, Zhao Q, Matthews N, et al. Rare DNA variants in the brain-derived neurotrophic factor gene increase risk for attention-deficit hyperactivity disorder: a next-generation sequencing study. Mol Psychiatry. (2017) 22:580–4. doi: 10.1038/mp.2016.117
47. Bergman O, Westberg L, Lichtenstein P, Eriksson E, Larsson H. Study on the possible association of brain-derived neurotrophic factor polymorphism with the developmental course of symptoms of attention deficit and hyperactivity. Int J Neuropsychopharmacol. (2011) 14:1367–76. doi: 10.1017/S1461145711000502
48. Bachmann V, Klein C, Bodenmann S, Schäfer N, Berger W, Brugger P, et al. The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity. Sleep. (2012) 35:335–44. doi: 10.5665/sleep.1690
49. Jeong HI, Ji ES, Kim SH, Kim TW, Baek SB, Choi SW. Treadmill exercise improves spatial learning ability by enhancing brain-derived neurotrophic factor expression in the attention-deficit/hyperactivity disorder rats. J Exerc Rehabil. (2014) 10:162–7. doi: 10.12965/jer.140111
50. Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell Mol Neurobiol. (2002) 22:663–74. doi: 10.1023/a:1021848706159
Keywords: BDNF, Val66Met, polymorphism, attention deficit hyperactivity disorder, meta-analysis, risk
Citation: Mei S, Chen W, Chen S, Hu Y, Dai X and Liu X (2022) Evaluation of the Relationship Between BDNF Val66Met Gene Polymorphism and Attention Deficit Hyperactivity Disorder: A Meta-Analysis. Front. Psychiatry 13:888774. doi: 10.3389/fpsyt.2022.888774
Received: 03 March 2022; Accepted: 23 March 2022;
Published: 28 April 2022.
Edited by:
Yi-lang Tang, Emory University, United StatesReviewed by:
Reiji Yoshimura, University of Occupational and Environmental Health Japan, JapanNela Pivac, Rudjer Boskovic Institute, Croatia
Copyright © 2022 Mei, Chen, Chen, Hu, Dai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiujun Liu, whmhpd@163.com
†These authors have contributed equally to this work and share first authorship
 Shufang Mei
Shufang Mei Wencai Chen†
Wencai Chen†