- 1Department of Anesthesiology, Qingdao Municipal Hospital Affiliated to Qingdao University, Qingdao, China
- 2Department of Anesthesiology, Dalian Medical University, Dalian, China
- 3Department of Anesthesiology, Drum Tower Hospital Affiliated to Nanjing University Medical School, Nanjing, China
Objective: We aimed to investigate the relationship between preoperative lipid level and postoperative delirium (POD) and explore whether lipid’s effect on POD is mediated by POD core protein.
Methods: A total of 635 patients who were planned to undergo knee/hip arthroplasty under combined spinal-epidural anesthesia, regardless of gender, were selected. The patients were aged 40–90 years with American Society of Anesthesiologists physical status I II. The Mini-Mental State Examination (MMSE) was completed 1 day before the operation. Five milliliter elbow venous blood was taken from the patients before anesthesia, and serum levels of total cholesterol (TG), triglyceride (TC), low-density lipoprotein (LDL-C), and high-density lipoprotein (HDL-C) were detected. Cerebrospinal fluid (CSF) was extracted after successful spinal-epidural combined puncture, and amyloid beta40 (Aβ40), amyloid beta42 (Aβ42), total Tau (t-Tau), and phosphorylated Tau (p-Tau) in the CSF were measured by enzyme-linked immunosorbent assays (ELISA). After the operation, the occurrence and severity of POD were assessed using the Confusion Assessment Method and the Memorial Delirium Assessment Scale (MDAS), respectively. Patients were categorized into POD group and NPOD group. Logistic regression was used to analyze the relationship between POD and TC, TG, LDL-C, and HDL-C, and the mediating effect was used to analyze the role of POD core proteins in the relationship between lipid and MDAS. We used the receiver operating characteristic (ROC) and the precision-recall curve (PRC) analysis to assess the ability of TC, TG, LDL-C, and HDL-C ability to predict POD. Finally, we performed a sensitivity analysis to assess the stability of the results.
Results: A total of 562 patients were finally enrolled in this study, and 66 patients developed POD, with an incidence of 11.7%. Logistic regression analysis showed that high concentration of TC (OR = 3.148, 95%CI 1.858∼5.333, P < 0.001), TG (OR = 2.483, 95%CI 1.573∼3.918, P < 0.001), and LDL-C (OR = 2.469, 95%CI 1.310∼4.656, P = 0.005) in serum were risk factors for POD. A high concentration of HDL-C (OR = 0.258, 95%CI 0.112∼0.594, P = 0.001) was a protective factor for POD after adjusted for age, sex, education, and MMSE score. ROC curves showed that HDL-C have the highest sensitivity and specificity in predicting POD. For these four lipid markers, the PRC range from 0.602 to 0.731, respectively. The mediating analysis showed that POD core proteins could partially mediate the relationship between lipid and POD (effect value: 16.19∼91.04%). The results were barely changed in the sensitivity analysis, and the sensitivity analysis has shown that the results were stable.
Conclusion: The increase of serum TG, TC, and LDL-C concentration is a risk factor for POD development, while high HDL-C concentration is a protective factor for POD, and the occurrence of POD is caused by hyperlipidemia may be caused by POD core proteins.
Clinical Trial Registration: [www.ClinicalTrials.gov], identifier [Chictr200033439].
Introduction
Postoperative delirium (POD) is one of the most common complications in patients after surgery. POD may bring about a decline in cognitive ability, impair environmental interpretation, attention-deficit disorder, and dyssomnia (1). Moreover, it can ultimately lead to adverse outcomes such as an increased incidence of postoperative complications, more extended hospital stays, increased medical costs, and increased postoperative mortality (2). In consequence, it is of great importance to explore the effective forecasting methods for POD occurrence.
Metabolic disorder of blood lipids is also familiar in senior citizens. Some researchers found that hemorheology was associated with cognitive decline (3). Hyperlipidemia can lead to abnormal deposition of lipids in vascular endothelium and formation of atherosclerosis, damage the blood-brain barrier, resulting in abnormal accumulation of lipids in the brain, finally leading to the occurrence of neurodegenerative diseases. Changes in lipid-related metabolism and transport levels played a role in the prediction of Alzheimer’s disease (AD) (4), and plasma lipid metabolism levels in patients with cognitive impairment were also apparent differences from those in the normal subjects (5). Furthermore, elevated plasma triglyceride levels precede Amyloid-beta (Aβ) protein deposition (6), the value of triglyceride in predicting the occurrence of AD is not negligible. Hypercholesterolemia can exacerbate Aβ protein deposition in animal models (7), while in humans, lowering cholesterol levels can reduce the Aβ burden and reduce AD occurrence (8). A Retrospective cohort study shows that high cholesterol increases the risk of dementia (9). Aβ abnormal deposition is proportional to neurotoxicity (10, 11), abnormally phosphorylated tau protein deposited in cells to can form neurofibrillary tangles, which all can cause neurodegeneration finally (12). It is a neurodegenerative disease with AD, and delirium pathophysiology is similar to AD (13, 14). For the time being, however, there is still a lack of studies concerning whether Aβ and tau could modulate the relationships of hemorheology with POD.
Thus, we aimed to investigate the relevance between lipid levels and POD, test whether the influences of lipids on delirium were mediated by POD core pathology. All these analyses were conducted based on the Perioperative Neurocognitive Disorder and Biomarker Lifestyle (PNDABLE) study.
Materials and Methods
Participants
A total of 635 Han Chinese patients who were planned to undergo knee or hip arthroplasty under combined spinal-epidural anesthesia were selected from the PNDABLE study. The trial was carried out at Qingdao Municipal Hospital in Shandong Province, China. The PNDABLE study is an ongoing, large-sample cohort study that began in 2018 to explore the pathogenesis, risk factors, and biomarkers of perioperative neurocognitive dysfunction (PND) in the Han Chinese population in northern China for early detection, diagnosis, and intervention of PND. Cerebrospinal fluid (CSF) and blood samples were collected from all enrolled patients after they signed informed consent. The Ethics Committee (Ethical Committee N 2020 PRO FORMA Y number 005) approved this study of Qingdao Municipal Hospital.
We included the following patients: (1) The patients were aged 40 90 years old; (2) American Society of Anesthesiologists physical status(ASA)I∼II; (3) The patients had intact preoperative cognitive function without communication disorders; (4) The patients had sufficient education to complete the preoperative neuropsychological tests. Exclusion criteria included: (1) Mini-Mental State Examination (MMSE) scores of 23 or less; (2) ASA III or higher level; (3) Serious psychological disorders; (4) Severe systemic diseases that may affect related biomarkers in CSF or blood, including but not limited to malignant tumors; (5) Familial genetic diseases; (6) Coagulation dysfunction (possibly due to the long-term use of anticoagulants);
Cognitive Measurements
The MMSE was used to evaluate the basic cognitive level of the patients the day before surgery. The Confusion Assessment Method (CAM) (15) was used to evaluate the postoperative cognitive level at 9:00–10:00 a.m. and at 2:00–3:00 p.m. twice a day on 1–7 days (or before discharge) by an anesthesiologist post-operatively. The diagnostic criteria for POD were as follows: ① acute changes and repeated fluctuations in the state of consciousness; ② lack of attention; ③ disorganized thinking; ④ alterations in the level of consciousness. CAM was determined to be positive if both ① and ② were present on any day, and at the same time either ③ or ④ was met. According to the assessment results, they were divided into the POD group and the NPOD group. Moreover, the POD severity was assessed using the MDAS (16).
Anesthesia and Surgery
All the patients did not need any medication preoperatively. After the patients entered the operating room, peripheral veins were opened, and the same team of surgeons performed knee or hip arthroplasty. ECG, pulse blood oxygen saturation monitoring, and non-invasive arterial pressure measurement were routinely conducted. After the preparation was completed, the spinal and epidural anesthesia was performed in the lateral decubitus under L3∼4 space. After a successful puncture, 0.67% ropivacaine 2.0 ∼ 2.5 ml was injected into the subarachnoid space, and then 3–5 ml 2% lidocaine was added into the epidural catheter according to actual needs to maintain the level of anesthesia at T8 ∼ S5. If the intraoperative systolic blood pressure of the patient was < 90 mmHg, intravenous ephedrine 6 mg was given; If the patient’s heart rate was < 50 bpm, an intravenous injection of atropine 0.5 mg was given. Every patient was treated with a patient-controlled intravenous analgesia pump (Tropisetron 5 mg + Butorphanol Tartrate Injection 10 mg, diluted to 100 ml with normal saline at a rate of 2 ml/h) for 48 h postoperatively. After the operation, the patient was sent to the recovery room, observed for 30 min, and sent back to the ward if there was no abnormality. The duration of surgery, duration of anesthesia, intraoperative blood loss, and fluid input were recorded.
Measurements of Cerebrospinal Fluid Sampling and Blood Sampling
After successful spinal-epidural anesthesia puncture, 2 ml of CSF was taken in 10 mL polypropylene tubes and sent to the laboratory within 2 h. The CSF samples were immediately centrifuged at 2,000 g at room temperature for 10 min and then stored at -80°C for further analysis. The levels of Aβ40, Aβ42, t-Tau and p-Tau in CSF were determined by enzyme-linked immunosorbent assays (ELISAs) on the microplate reader. CSF biomarkers of POD measurements were done with ELISA kits [Aβ42 (BioVendor, Ghent, Belgium Lot: No. 296-64401), P-tau (BioVendor, Ghent, Belgium Lot: QY-PF9092) and T-tau (BioVendor, Ghent, Belgium Lot: No. EK-H12242)]. All CSF samples were randomly distributed on the same batch of plates. All experimental procedures were performed by researchers who were blinded to patient information. All the antibodies and plates were from a single lot to exclude variability between batches. Moreover, the within-batch CV was < 5% and the inter-batch CV was < 15%.
After fasting for at least 8 h, the patient entered the operating room, and 5 ml of medial cubital vein blood was drawn. Venous blood was collected into vacuum tube, which was then measured by the hospital’s laboratory staff. Serum concentrations of total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL-C), and high-density lipoprotein (HDL-C) were measured under standardized research protocols using an automatic biochemical analyzer (DURUI CS-600B, China).
Statistical Analysis
SPSS statistical software, version 25.0 (SPSS, Inc., Chicago, IL, United States), and Medcalc software (version 20.0.1, Ostend, Belgium) were used for data analysis. Continuous variables were expressed as median and interquartile range (M, IQR), and compared using Mann-Whitney U-test. Categorical variables will be tested for baseline comparability with the chi-square test or Fisher’s exact test, expressed in frequency and percentage. To evaluate potential risk factors for POD, we used logistic regression analysis without and with adjustment for age, sex, education, and MMSE score. We also used the receiver operating characteristic (ROC) and the precision-recall curve (PRC) analysis to assess the ability of TG, TC, HDL-C, and LDL-C for predicting POD.
The mediation effect was also evaluated by PROCESS macro Version2.16.3. Statistical significance of the mediating effect was set at zero, which was not encompassed in the 95% CI. where each path of the model was controlled for age, sex, education, and MMSE score.
In addition, a sensitivity analysis was performed to assess the results stability. Sensitivity analysis was carried out as follows: First, we analyzed whether the association would change if only individuals who were aged over 65 at the baseline were selected; Secondly, we added more covariates, including self-reported history of type 2 diabetes (yes or no) and hypertension (yes or no).
The expected sensitivity was 80%, the expected specificity was 50%, and the allowable errors were all 0.05. Bilateral test was required, α was 0.05, and the missed visit ratio was calculated as 20%. The minimum sample size calculated by PASS software was 503.
Results
Participant Characteristics
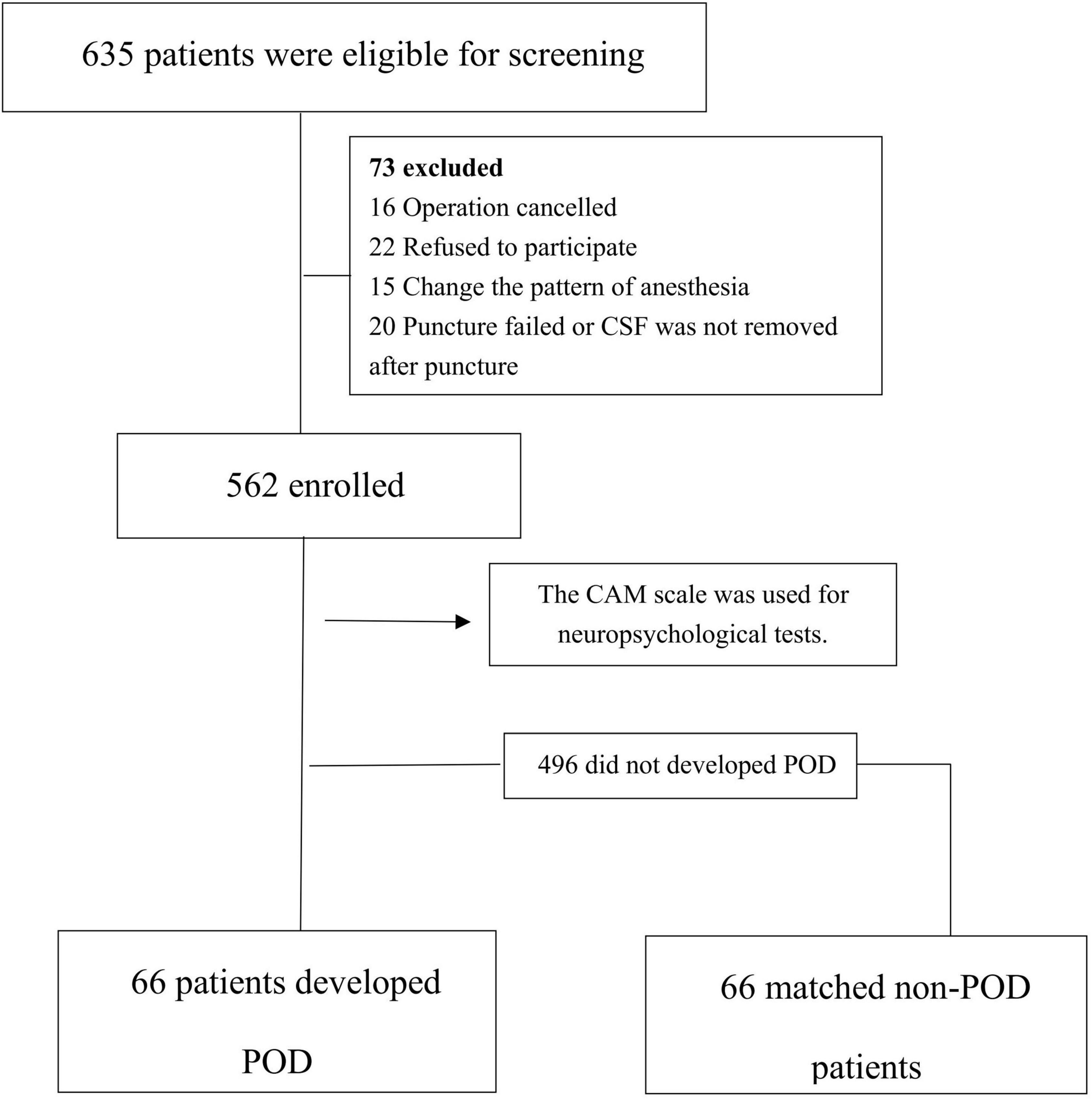
Among the 635 eligible patients, a total of 562 patients were finally included in this study. In the 562 patients, there were 66 POD cases, with an incidence of 11.7%, as shown in Figure 1. The incidence density sampling was used for the comparison between the POD group and the non-POD group, and 1:1 matching was performed on 5 variables, including ASA physical status, duration of surgery, duration of anesthesia, intraoperative blood loss, and fluid input.
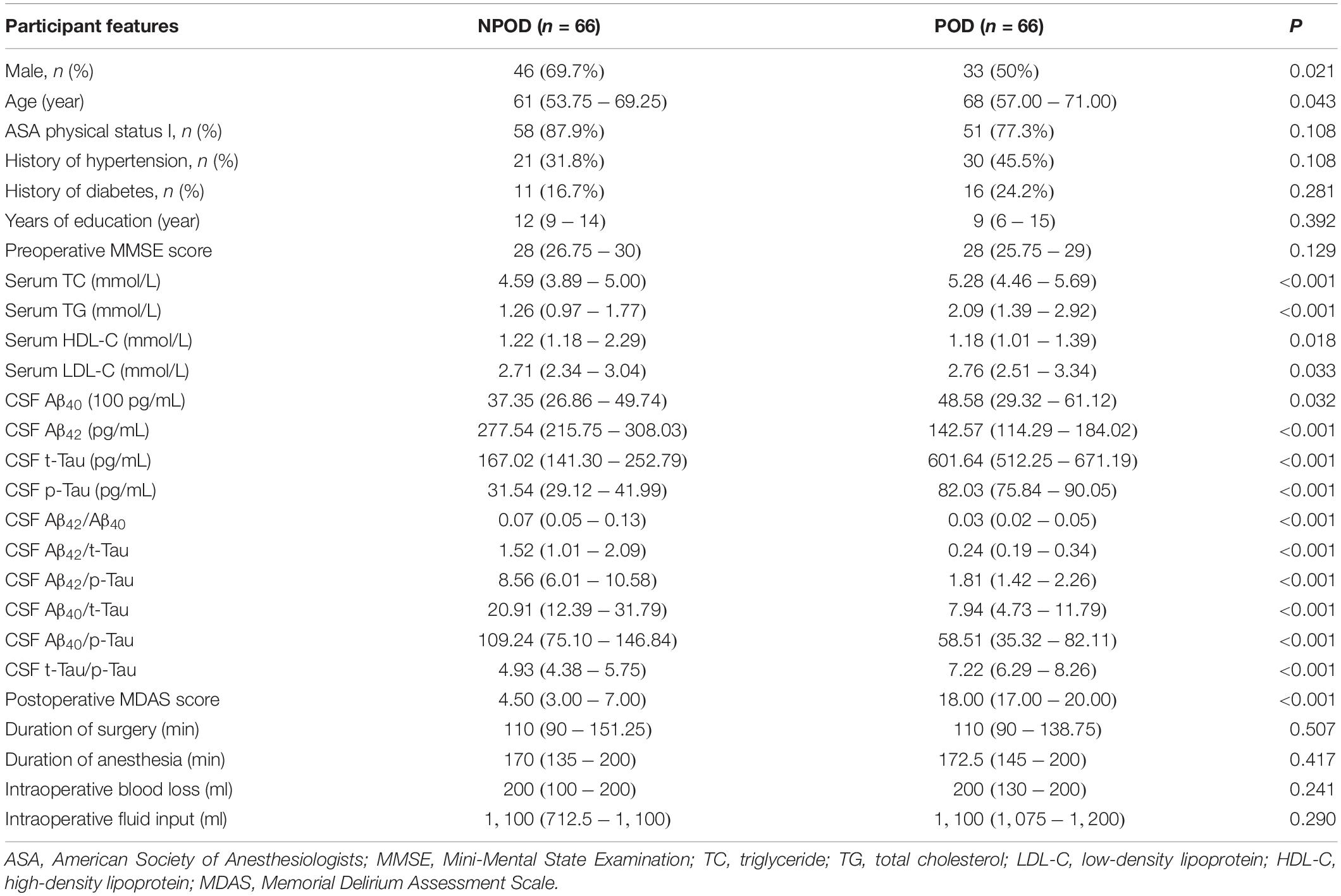
The general conditions of the POD group and the NPOD group were compared (Table 1). There was no statistical significance in years of education, preoperative MMSE score, history of diabetes, or history of hypertension (P > 0.05), while the differences in sex, age, Serum TC, TG, LDL-C, HDL-C, CSF Aβ40, Aβ42, t-Tau, p-Tau, Aβ42/Aβ40, Aβ42/t-Tau, Aβ42/p-Tau, Aβ40/t-Tau, Aβ40/p-Tau, t-Tau/p-Tau, and Postoperative MDAS score were statistically significant (P < 0.05).
Logistic Regression Analysis
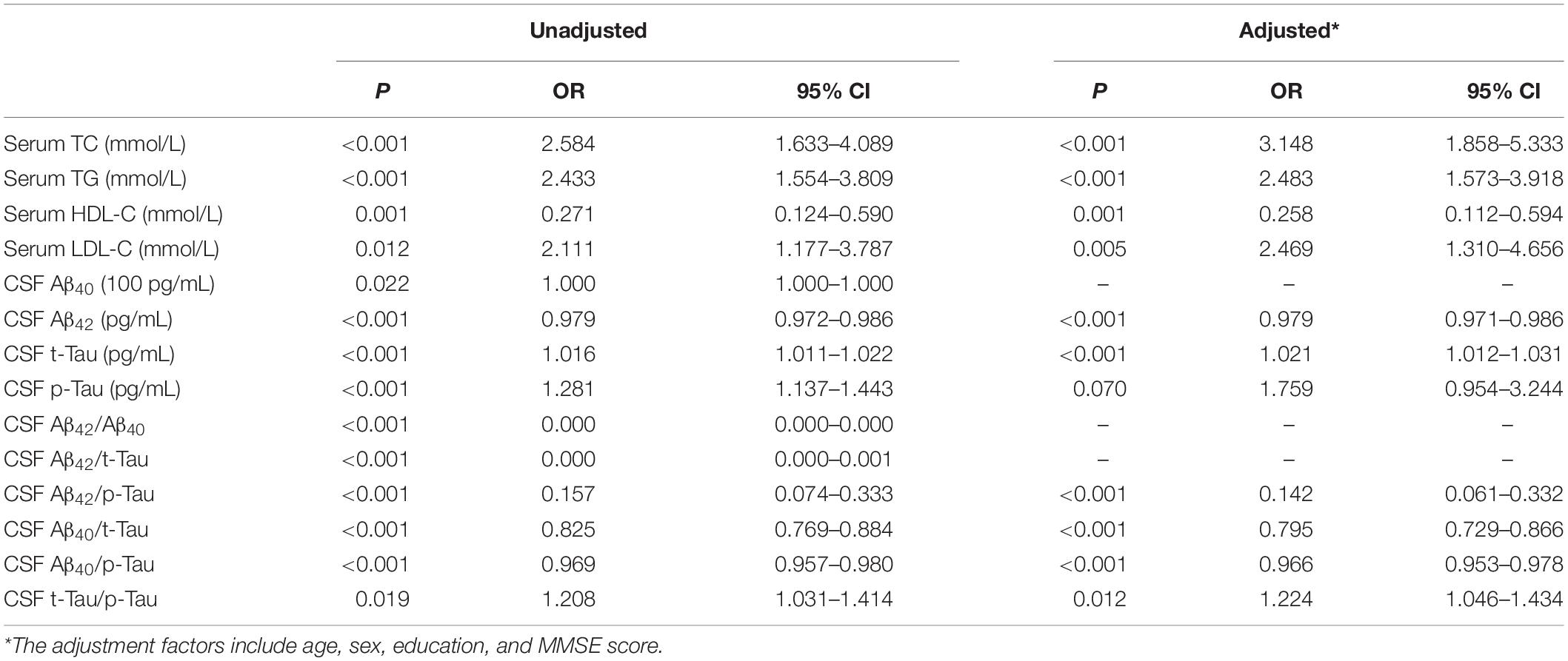
Logistic regression analysis showed that high concentration of TC (OR = 3.148, 95%CI 1.858∼5.333, P < 0.001), TG (OR = 2.483, 95%CI 1.573∼3.918, P < 0.001), and LDL-C (OR = 2.469, 95%CI 1.310∼4.656, P = 0.005) in serum were risk factors for POD. A high concentration of HDL-C (OR = 0.258, 95%CI 0.112∼0.594, P = 0.001) was a protective factor for POD after adjusted for age, sex, education, and MMSE score (Table 2).
We performed two sensitivity analyses. In our first sensitivity analysis, we added more covariates, including self-reported history of type 2 diabetes and hypertension, and the results showed that high concentration of TC (OR = 3.394, 95%CI 1.953∼5.898, P < 0.001), TG (OR = 2.456, 95%CI 1.557∼3.872, P < 0.001) and LDL-C (OR = 2.650, 95%CI 1.376∼5.101, P = 0.004) in serum were remain risk factors for POD. After adjusted for age, sex, education, MMSE score, self-reported history of type 2 diabetes, and hypertension, high concentration of HDL-C (OR = 0.263, 95%CI 0.115∼0.601, P = 0.002) was a protective factor for POD (Supplementary Table 1). In the second sensitivity analysis, we selected patients older than 65 years old. The implication of these results is that high concentration of TC (OR = 3.880, 95%CI 1.653∼9.108, P = 0.002), TG (OR = 2.421, 95%CI 1.218∼4.809, P = 0.012) and LDL-C (OR = 2.639, 95%CI 1.032∼6.743, P = 0.043) in serum were remain risk factors for POD. After adjusted for age, sex, education and MMSE score, high concentration of HDL-C (OR = 0.163, 95%CI 0.040∼0.659, P = 0.011) was a protective factor for POD (Supplementary Table 2). The results were barely changed in the sensitivity analysis, and the sensitivity analysis have showed that the results were stable.
Receiver Operating Characteristic Analysis and Precision-Recall Curve Analysis
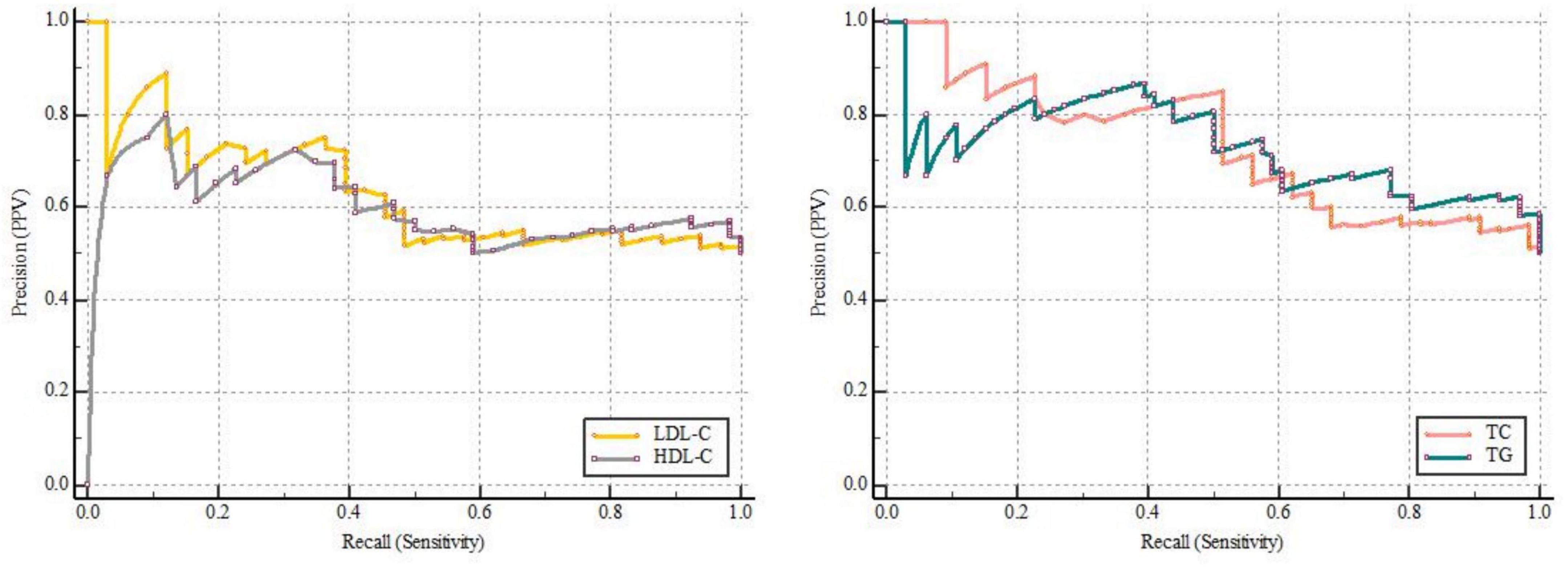
ROC curves showed that LDL-C [0.607 (0.519–0.691)], HDL-C [0.620 (0.531–0.703)], TG [0.761 (0.679–0.831)], and TC [0.708 (0.623–0.784)] can all predict POD (Figure 2 and Supplementary Table 3). Among which, HDL-C had the highest sensitivity and specificity in predicting POD, although AUC was not the largest. We calculated the area under curve and F1 score of TG, TC, LDL-C, and HDL-C in PRC analysis. The results showed that these four lipid markers, the PRC range from 0.602 to 0.731, respectively. The F1 score of TG, TC, LDL-C, and HDL-C were 0.757, 0.714, 0.685, and 0.722, respectively (Figure 3 and Supplementary Table 4).
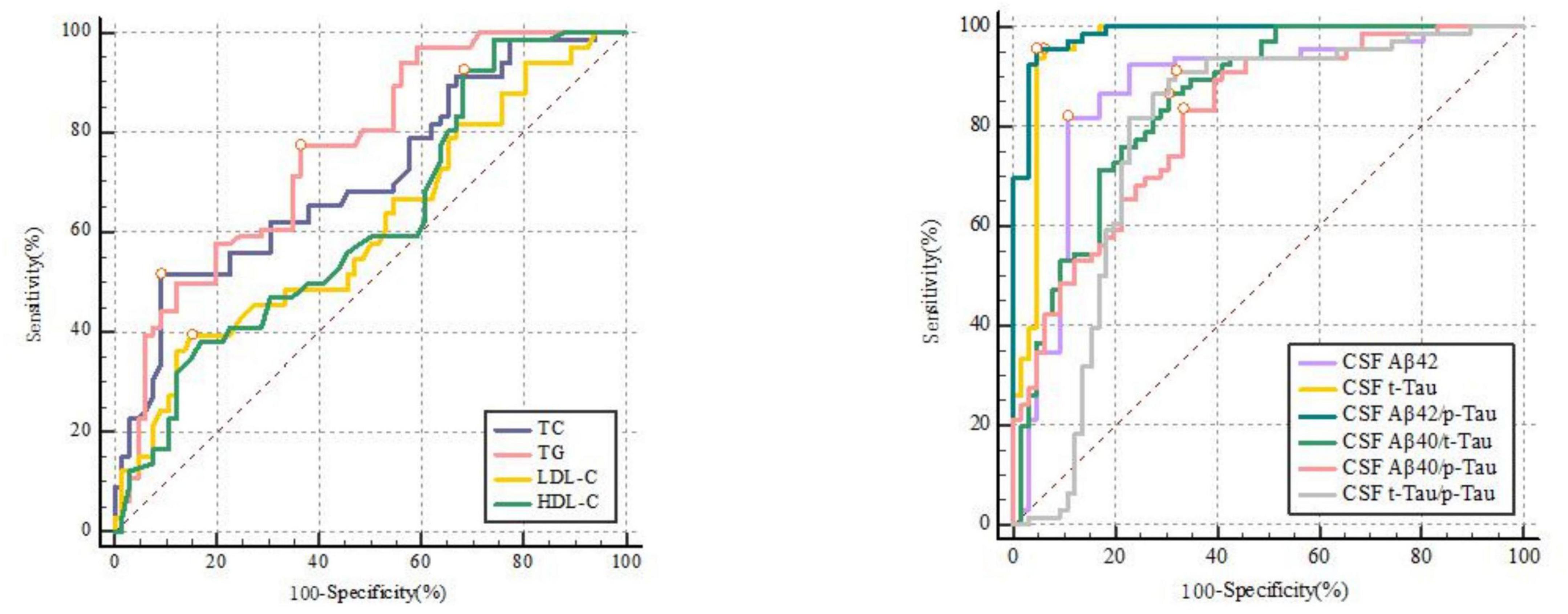
Figure 2. The receiver-operator characteristic analyses for TC [0.708 (0.623–0.784)], TG [0.761 (0.679–0.831)], LDL-C [0.607 (0.519–0.691)], HDL-C [0.620 (0.531–0.703)] and CSF biomarkers in predicting delirium.
Mediation Analyses
In the mediation modeling analysis, we assessed the mediation effects of CSF proteins on the associations of lipid levels with MDAS, after controlling for age, sex, education, and MMSE score. The relationship between TC and POD severity was mediated by amyloid and tau pathology indicated by Aβ42, t-Tau, Aβ42/t-Tau ratios, Aβ42/p-Tau ratios, and Aβ40/p-Tau ratios. While, the relationship between TG and MDAS was mediated by t-Tau, Aβ42/t-Tau ratios, Aβ40/t-Tau ratios, and Aβ40/p-Tau ratios. Aβ42 and t-Tau act as full mediators between LDL-C and MDAS. The result of this study shows that t-Tau, Aβ40/t-Tau ratios, and Aβ40/p-Tau have different performance on HDL-C and MDAS (Figure 4).
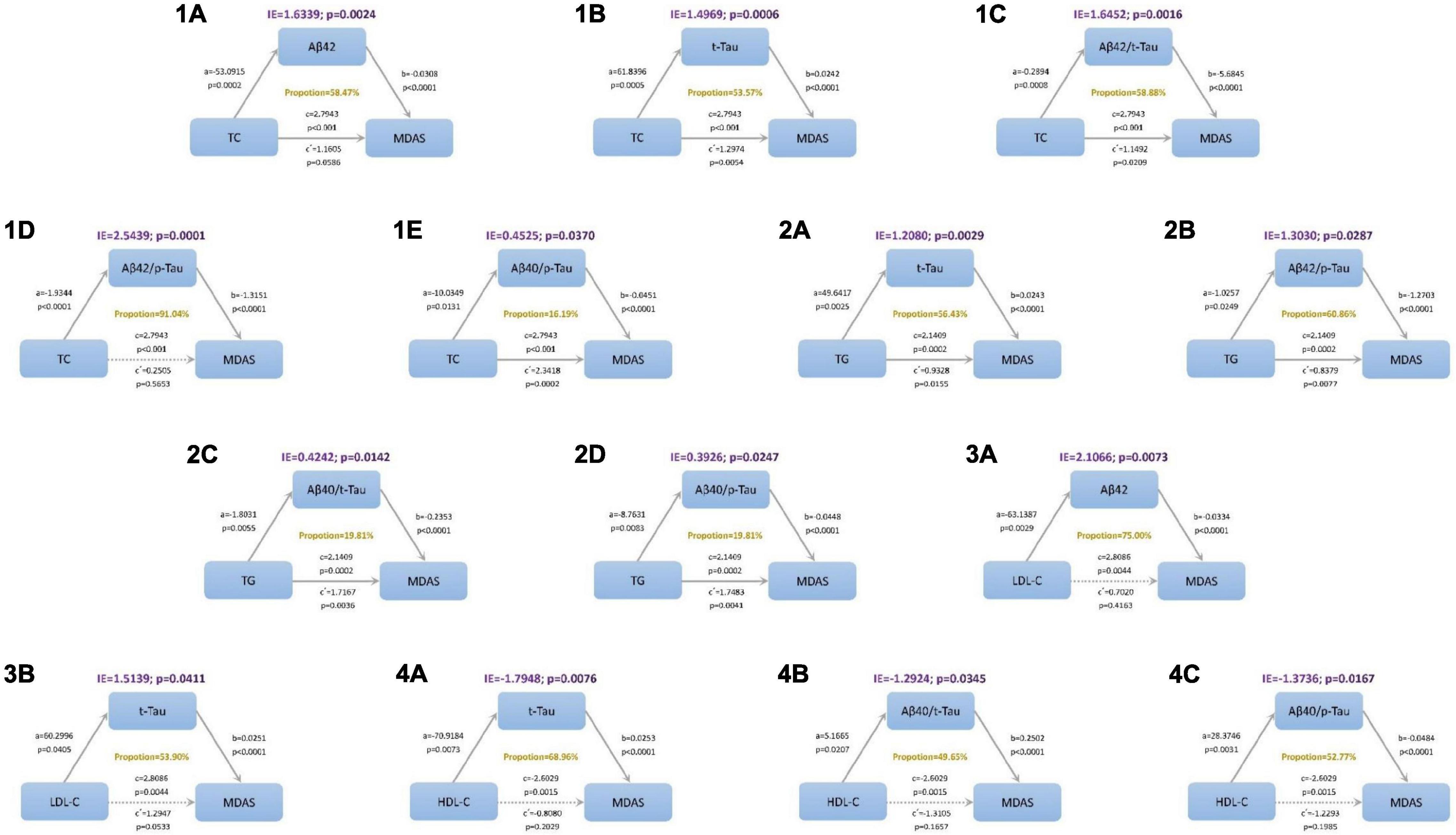
Figure 4. Mediation analyses with Memorial Delirium Assessment Scale (MDAS) as outcome. The relationship between triglyceride (TC) and postoperative delirium severity was mediated by amyloid and tau pathology indicated by (1A) amyloid beta42 (Aβ42), (1B) total Tau (t-Tau), (1C) amyloid beta42/t-Tau (Aβ42/t-Tau) ratios, (1D) Aβ42/p-Tau ratios, (1E) Aβ40/p-Tau ratios. The relationship between total cholesterol (TG) and postoperative delirium severity was mediated by amyloid and tau pathology indicated by (2A) t-Tau, (2B) Aβ42/t-Tau ratios, (2C) Aβ40/t-Tau ratios, (2D) Aβ40/p-Tau ratios. The relationship between low-density lipoprotein (LDL-C) and postoperative delirium severity was mediated by amyloid and tau pathology indicated by (3A) Aβ42 and (3B) t-Tau. The relationship between high-density lipoprotein (HDL-C) and postoperative delirium severity was mediated by amyloid and tau pathology indicated by (4A) t-Tau, (4B) Aβ40/t-Tau ratios and (4C) Aβ40/p-Tau ratios. IE, indirect effect.
Discussion
As far as we are aware, the study is the first that reported the relationship including mediator effects between serum lipid and POD. We mainly screened out several POD core proteins as mediators. Of course, they played different mediating effects in the relationship between different serum lipoprotein and POD.
Cholesterol is an essential component of membranes and plasma lipoprotein, and it also plays an essential part in the accommodation of synaptic function and cell plasticity (17). An independent study (18) found that hypercholesterolemia caused memory impairment, inflammation response, and cholinergic dysfunction. Conversely, taking cholesterol-reducing medications can bring down the risk of neurocognitive-related diseases (19). Our findings indicate that cholesterol amounts altered was concerned with POD, and serum cholesterol was proportional to the severity of POD; that is, it is positively correlated with MDAS scores. Hypercholesterolemia leading to POD partly by Aβ42, t-Tau, Aβ42/t-Tau ratios, Aβ42/p-Tau ratios, and Aβ40/p-Tau ratios, explanation by mediation effects. Likewise, Umeda et al. found that hypercholesterolemia accelerates the accumulation of Aβ oligomers and resulting in memory impairment (20). It is universally recognized that reduced CSF Aβ42 concentration reflects the accumulation of aggregated Aβ42 in amyloid plaques in the brain (21). In patients with hip fracture, this group found lower CSF Aβ42 levels and increased CSF t-Tau levels who developed delirium compared to the control group, the biomarkers remained significant after adjusting for age, gender, and Informant Questionnaire on Cognitive Decline in the Elderly score. This result is consistent with our findings. Some research has found that cholesterol amounts modification altered amyloid precursor protein (APP) and Aβ expression (22, 23). Cholesterol transcellular transportation was altered by Aβ, while inhibition of intracellular transport of cholesterol reduced cleavage of Aβ from APP in neurons (24, 25). Intracellular cholesterol plays a significant role in modulating tau phosphorylation and maintaining microtubule stability, the researchers found (26). Van der et al. (27) found that the effects of cholesterol on tau proteostasis are correlated with APP and Aβ. We also find this relationship by calculating the mediating effect. The interesting thing is that exercise can lower the tau pathology and its pathophysiological consequences (28). Exercise decreased the levels of soluble Aβ40 and Aβ42 (29), also reducing the lipid level in serum. It is tempting to think there is at least a case to be made for exercise to lower cholesterol levels and thus reduce the risk of POD.
Moreover, our results showed that triglyceride levels were higher than the NPOD group in POD patients, and the difference between the two groups has statistical significance. t-Tau, Aβ42/t-Tau ratios, Aβ40/t-Tau ratios, and Aβ40/p-Tau ratios may mediate the effect of triglyceride on POD. Triglyceride components were found to be significantly associated with CSF Aβ42 values (30). A longitudinal cohort study in cognitively healthy individuals concluded that increased levels of triglycerides could even predict CSF Aβ and tau pathology 20 years later (31). Higher serum triglyceride levels are associated with Parkinson’s disease mild cognitive impairment (32) and are one of the risk factors for AD (33). It was proved that triglycerides could cross the blood-brain barrier (BBB), consisting of human CSF, resulting in cognitive impairment (34). Some scholars have argued that the relation between triglycerides and cognition may be mediated by triglyceride regulation of the BBB transport of cognitively active gastrointestinal hormones (35). In animal models, an influential study showed that plasma triglyceride levels increased precede Aβ deposition (6), but total cholesterol levels were not significantly different in this research. In another model of hyperlipidemia-induced age-related neurodegeneration (36), chronic hypertriglyceridemia may lead to impaired neuronal function and neurodegeneration, possibly via hyperphosphorylation of tau protein, and this is similar to our findings.
More importantly, our analysis found that serum HDL level is associated with POD development, and high serum HDL level before surgery is one of the protective factors of POD. HDL-C is known as the “good cholesterol” because of its ability to reverse cholesterol transport. It protects against elevated lipid levels and protects against endothelial dysfunction, oxidative stress, inflammation, thrombosis, and more. Therefore, it is well known that serum HDL-C level is associated with a lower risk of cardiovascular disease. In addition, several studies have shown that individuals with higher levels of serum HDL-C is related to better cognitive function status (37–39), One possible reason is that HDL-C is capable of binding Aβ (40) and prevent Aβ aggregation into amyloid (41), and then improve clearance of Aβ from the brain, which in turn decreases the neurotoxicity of Aβ peptides (42). Another factor may be that serum HDL-C levels are inversely correlated with brain Aβ deposits (43). Our study did not support a significant mediation effect of Aβ deposits in the associations between serum HDL-C and MDAS, while the t-Tau, Aβ40/t-Tau ratios, and Aβ40/p-Tau ratios play full mediators on the relationship between HDL-C and MDAS. A study of older adults in China’s rural area showed that low HDL-C is associated with structural brain aging and cognitive dysfunction, but the association of low HDL-C with cognitive aging is not mediated by brain structure (44). Our data agree with previous research that low HDL-C is associated with cognitive impairment and dementia and is a risk factor for memory deficit and decline (45).
Our data insinuate that preoperative LDL-C levels were positively correlated with POD occurrence. Aβ42 and t-Tau may mediate the effect of LDL-C on POD. In addition, Aβ42 is a complete mediation. Our data support the view that a higher LDL-C level was associated with higher Aβ deposition and lower cognitive function (46, 47). In an Australian study, researchers discovered that higher levels of cholesterol and LDL-C were related to impaired processing speed, recognition memory, and working memory (48). However, in a prospective cohort study in Japan, higher LDL-C levels were associated with higher scores in memory performance after controlling for confounders (49), The Japanese study is broadly similar to the results of a cross-sectional study from China (50). According to the Chinese study, higher LDL-C was significantly negatively related to higher MMSE scores among the oldest old (aged 80 + years). Another Chinese study showed that a high level of LDL-C may be considered a potentially protective factor against cognition decline (51). Still, some research showed that LDL-C level did not influence the incidence of cognitive disorder or global cognitive performance (52, 53). All of the above studies come from different countries and regions, with different living standards and educational levels, so many factors influence the results. Therefore, future large-sample multicenter studies are needed to support the relationship between LDL-C and POD.
There are limitations to this study. As this is an observational cross-sectional design, we only tried to infer the causal relationship, but the specific relationship needs further study. In addition, our study only measured lipid levels at a one-time point before surgery, and more comprehensive monitoring of lipid levels is needed in the future. The research population we included come from the same hospital, which is also the deficiency of our experimental study. If possible, we hope to conduct verification of our experimental model in other independent and comparable hospitals in future studies.
To sum up, the present study indicated that the increase of serum TG, TC, and LDL-C concentration are risk factors for the development of POD, while high HDL-C concentration is a protective factor for POD, and the occurrence of POD caused by hyperlipidemia may be caused by POD core protein. Therefore, we advocate maintaining a healthy lifestyle to reduce lipid levels and thus reduce the incidence of POD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Clinical Trial Ethics Committee of Qingdao Municipal Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YL contributed to the statistical analysis, and manuscript preparation. XD, FL, and HT involved in the data collection and ELISA performance. XP, XL, and RD revised the manuscript. YB and BW conceived the current study. All authors have contributed to the manuscript revising and editing critically for important intellectual content and given final approval of the version and agreed to be accountable for all aspects of the work presented here, reviewed, and approved the final manuscript.
Funding
The current study was funded by National Natural Science Foundation Youth Project (82001132) and B. Braun Anesthesia Research Fund (BBDF-2019-010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.870317/full#supplementary-material
References
1. Lin X, Tang J, Liu C, Li X, Cao X, Wang B, et al. Cerebrospinal fluid cholinergic biomarkers are associated with postoperative delirium in elderly patients undergoing Total hip/knee replacement: a prospective cohort study. BMC Anesthesiol. (2020) 20:246. doi: 10.1186/s12871-020-01166-9
2. Bai J, Liang Y, Zhang P, Liang X, He J, Wang J, et al. Association between postoperative delirium and mortality in elderly patients undergoing hip fractures surgery: a meta-analysis. Osteoporos Int. (2020) 31:317–26. doi: 10.1007/s00198-019-05172-7
3. Ma YH, Shen XN, Xu W, Huang YY, Li HQ, Tan L, et al. A panel of blood lipids associated with cognitive performance, brain atrophy, and Alzheimer’s diagnosis: a longitudinal study of elders without dementia. Alzheimers Dement (Amst). (2020) 12:e12041. doi: 10.1002/dad2.12041
4. Buckley RF, Mormino EC, Amariglio RE, Properzi MJ, Rabin JS, Lim YY, et al. Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer’s disease: findings from three well-characterized cohorts. Alzheimers Dement. (2018) 14:1193–203. doi: 10.1016/j.jalz.2018.04.010
5. Perez-Galvez A, Jaren-Galan M, Garrido-Fernandez J, Calvo MV, Visioli F, Fontecha J. Activities, bioavailability, and metabolism of lipids from structural membranes and oils: promising research on mild cognitive impairment. Pharmacol Res. (2018) 134:299–304. doi: 10.1016/j.phrs.2018.07.013
6. Burgess BL, McIsaac SA, Naus KE, Chan JY, Tansley GHK, Yang J, et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant Aβ in plasma. Neurobiol Dis. (2006) 24:114–27. doi: 10.1016/j.nbd.2006.06.007
7. Shie FS, Jin LW, Cook DG, Leverenz JB, Le Boeuf RC. Diet-induced hypercholesterolemia enhances brain Ab accumulation in transgenic mice. Neuroreport. (2002) 13:455–9.
8. Fassbender K, Bergmann Simons M, Stroick M. Simvastatin strongly reduces levels of Alzheimer’s disease -amyloid peptides A 42 and A 40 in vitro and in vivo. Proc Natl Acad Sci USA. (2001) 98:5856–61.
9. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. (2005) 64:277–81. doi: 10.1212/01.WNL.0000149519.47454.F2
10. Ji MH, Yuan HM, Zhang GF, Li XM, Dong L, Li WY, et al. Changes in plasma and cerebrospinal fluid biomarkers in aged patients with early postoperative cognitive dysfunction following total hip-replacement surgery. J Anesth. (2013) 27:236–42. doi: 10.1007/s00540-012-1506-3
11. Rolandi E, Cavedo E, Pievani M, Galluzzi S, Ribaldi F, Buckley C, et al. Association of postoperative delirium with markers of neurodegeneration and brain amyloidosis: a pilot study. Neurobiol Aging. (2018) 61:93–101. doi: 10.1016/j.neurobiolaging.2017.09.020
12. Wu Z, Zhang M, Zhang Z, Dong W, Wang Q, Ren J. Ratio of beta-amyloid protein (Abeta) and Tau predicts the postoperative cognitive dysfunction on patients undergoing total hip/knee replacement surgery. Exp Ther Med. (2018) 15:878–84. doi: 10.3892/etm.2017.5480
13. Racine AM, Fong TG, Travison TG, Jones RN, Gou Y, Vasunilashorn SM, et al. Alzheimer’s-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol Aging. (2017) 59:55–63. doi: 10.1016/j.neurobiolaging.2017.07.010
14. Fong TG, Vasunilashorn SM, Gou Y, Libermann TA, Dillon S, Schmitt E, et al. Association of CSF Alzheimer’s disease biomarkers with postoperative delirium in older adults. Alzheimers Dement. (2021) 7:e12125. doi: 10.1002/trc2.12125
15. Inouye S, van Dyck C, Alessi C, Balkin S, Siegal A, Horwitz R. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. (1990) 113:941–8. doi: 10.7326/0003-4819-113-12-941
16. Schuurmans MJ, Deschamps PI, Markham SW, Shortridge-Baggett LM, Duursma SA. The measurement of delirium: review of scales. Res Theory Nurs Pract. (2003) 17:207–24. doi: 10.1891/rtnp.17.3.207.53186
17. Eckert G, Kirsch C, Leutz S, Wood W, Müller W. Cholesterol modulates amyloid beta-peptide’s membrane interactions. Pharmacopsychiatry. (2003) 36(Suppl. 2):S136–43.
18. Ullrich C, Pirchl M, Humpel C. Hypercholesterolemia in rats impairs the cholinergic system and leads to memory deficits. Mol Cell Neurosci. (2010) 45:408–17. doi: 10.1016/j.mcn.2010.08.001
19. Gibson Wood W, Eckert GP, Igbavboa U, Müller WE. Amyloid beta-protein interactions with membranes and cholesterol: causes or casualties of Alzheimer’s disease. Biochim Biophys Acta. (2003) 1610:281–90. doi: 10.1016/s0005-2736(03)00025-7
20. Umeda T, Tomiyama T, Kitajima E, Idomoto T, Nomura S, Lambert MP, et al. Hypercholesterolemia accelerates intraneuronal accumulation of Abeta oligomers resulting in memory impairment in Alzheimer’s disease model mice. Life Sci. (2012) 91:1169–76. doi: 10.1016/j.lfs.2011.12.022
21. Idland AV, Wyller TB, Stoen R, Eri LM, Frihagen F, Raeder J, et al. Preclinical Amyloid-beta and axonal degeneration pathology in delirium. J Alzheimers Dis. (2017) 55:371–9. doi: 10.3233/JAD-160461
22. Cho YY, Kwon OH, Park MK, Kim TW, Chung S. Elevated cellular cholesterol in Familial Alzheimer’s presenilin 1 mutation is associated with lipid raft loalization of beta-amyloid precursor protein. PLoS One. (2019) 14:e0210535. doi: 10.1371/journal.pone.0210535
23. Pantelopulos GA, Panahi A, Straub JE. Impact of cholesterol concentration and lipid phase on structure and fluctuation of amyloid precursor protein. J Phys Chem. (2020) 124:10173–85. doi: 10.1021/acs.jpcb.0c07615
24. Avila-Munoz E, Arias C. Cholesterol-induced astrocyte activation is associated with increased amyloid precursor protein expression and processing. Glia. (2015) 63:2010–22. doi: 10.1002/glia.22874
25. Chung J, Phukan G, Vergote D, Mohamed A, Maulik M, Stahn M, et al. Endosomal-lysosomal cholesterol sequestration by U18666A differentially regulates amyloid precursor protein (APP) metabolism in normal and APP-overexpressing cells. Mol Cell Biol. (2018) 38:e529–517. doi: 10.1128/MCB.00529-17
26. Fan QW, Yu W, Senda T, Yanagisawa K, Michikawa M. Cholesterol-dependent modulation of tau phosphorylation in cultured neurons. J Neurochem. (2001) 76:391–400.
27. van der Kant R, Langness VF, Herrera CM, Williams DA, Fong LK, Leestemaker Y, et al. cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-beta in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell. (2019) 24:363–375e9. doi: 10.1016/j.stem.2018.12.013
28. Belarbi K, Burnouf S, Fernandez-Gomez FJ, Laurent C, Lestavel S, Figeac M, et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol Dis. (2011) 43:486–94. doi: 10.1016/j.nbd.2011.04.022
29. Zeng B, Zhao G, Liu HL. The differential effect of treadmill exercise intensity on hippocampal soluble abeta and lipid metabolism in APP/PS1 Mice. Neuroscience. (2020) 430:73–81. doi: 10.1016/j.neuroscience.2020.01.005
30. Bernath MM, Bhattacharyya S, Nho K, Barupal DK, Fiehn O, Baillie R, et al. Serum triglycerides in Alzheimer disease: relation to neuroimaging and CSF biomarkers. Neurology. (2020) 94:e2088–98. doi: 10.1212/WNL.0000000000009436
31. Nagga K, Gustavsson AM, Stomrud E, Lindqvist D, van Westen D, Blennow K, et al. Increased midlife triglycerides predict brain beta-amyloid and tau pathology 20 years later. Neurology. (2018) 90:e73–81. doi: 10.1212/WNL.0000000000004749
32. Huang X, Ng SY, Chia NS, Acharyya S, Setiawan F, Lu Z, et al. Higher serum triglyceride levels are associated with Parkinson’s disease mild cognitive impairment. Mov Disord. (2018) 33:1970–1. doi: 10.1002/mds.27521
33. Proitsi P, Lupton MK, Velayudhan L, Newhouse S, Fogh I, Tsolaki M, et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: a Mendelian randomization analysis. PLoS Med. (2014) 11:e1001713. doi: 10.1371/journal.pmed.1001713
34. Banks WA, Farr SA, Salameh TS, Niehoff ML, Rhea EM, Morley JE, et al. Triglycerides cross the blood-brain barrier and induce central leptin and insulin receptor resistance. Int J Obes (Lond). (2018) 42:391–7. doi: 10.1038/ijo.2017.231
35. Banks WA. Role of the blood-brain barrier in the evolution of feeding and cognition. Ann N Y Acad Sci. (2012) 1264:13–9. doi: 10.1111/j.1749-6632.2012.06568.x
36. Lenart N, Szegedi V, Juhasz G, Kasztner A, Horvath J, Bereczki E, et al. Increased tau phosphorylation and impaired presynaptic function in hypertriglyceridemic ApoB-100 transgenic mice. PLoS One. (2012) 7:e46007. doi: 10.1371/journal.pone.0046007
37. Formiga F, Ferrer A, Chivite D, Pinto X, Cuerpo S, Pujol R. Serum high-density lipoprotein cholesterol levels, their relationship with baseline functional and cognitive status, and their utility in predicting mortality in nonagenarians. Geriatr Gerontol Int. (2011) 11:358–64. doi: 10.1111/j.1447-0594.2010.00681.x
38. Formiga F, Ferrer A, Chivite D, Pinto X, Badia T, Padros G, et al. Serum high-density lipoprotein cholesterol levels correlate well with functional but not with cognitive status in 85-year-old subjects. J Nutr Health Aging. (2012) 16:449–53.
39. Bates KA, Sohrabi HR, Rainey-Smith SR, Weinborn M, Bucks RS, Rodrigues M, et al. Serum high-density lipoprotein is associated with better cognitive function in a cross-sectional study of aging women. Int J Neurosci. (2017) 127:243–52. doi: 10.1080/00207454.2016.1182527
40. Cole G, Beech W, Frautschy S, Sigel J, Glasgow C, Ard M. Lipoprotein effects on Abeta accumulation and degradation by microglia in vitro. J Neurosci Res. (1999) 57:504–20.
41. Olesen OF, Dago L. High density lipoprotein inhibits assembly of amyloid beta-peptides into fibrils. Biochem Biophys Res Commun. (2000) 270:62–6. doi: 10.1006/bbrc.2000.2372
42. Farhangrazi Z, Ying H, Bu G, Dugan L, Fagan A, Choi D, et al. High density lipoprotein decreases beta-amyloid toxicity in cortical cell culture. Neuroreport. (1997) 8:1127–30. doi: 10.1097/00001756-199703240-00013
43. Bates KA, Sohrabi HR, Rodrigues M, Beilby J, Dhaliwal SS, Taddei K, et al. Association of cardiovascular factors and Alzheimer’s disease plasma amyloid-beta protein in subjective memory complainers. J Alzheimers Dis. (2009) 17:305–18. doi: 10.3233/JAD-2009-1050
44. Wang M, Li Y, Cong L, Hou T, Luo Y, Shi L, et al. High-density lipoprotein cholesterol and brain aging amongst rural-dwelling older adults: a population-based magnetic resonance imaging study. Eur J Neurol. (2021) 28:2882–92. doi: 10.1111/ene.14939
45. Singh-Manoux A, Gimeno D, Kivimaki M, Brunner E, Marmot M. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the whitehall II study. Arterioscler Thromb Vasc Biol. (2008) 28:1556–62. doi: 10.1161/atvbaha.108.163998
46. Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. (2014) 71:195–200. doi: 10.1001/jamaneurol.2013.5390
47. Smit RA, Trompet S, Sabayan B, le Cessie S, van der Grond J, van Buchem MA, et al. Higher visit-to-visit low-density lipoprotein cholesterol variability is associated with lower cognitive performance, lower cerebral blood flow, and greater white matter hyperintensity load in older subjects. Circulation. (2016) 134:212–21. doi: 10.1161/CIRCULATIONAHA.115.020627
48. Stough C, Pipingas A, Camfield D, Nolidin K, Savage K, Deleuil S, et al. Increases in total cholesterol and low density lipoprotein associated with decreased cognitive performance in healthy elderly adults. Metab Brain Dis. (2019) 34:477–84. doi: 10.1007/s11011-018-0373-5
49. Katsumata Y, Todoriki H, Higashiuesato Y, Yasura S, Ohya Y, Willcox DC, et al. Very old adults with better memory function have higher low-density lipoprotein cholesterol levels and lower triglyceride to high-density lipoprotein cholesterol ratios: KOCOA Project. J Alzheimers Dis. (2013) 34:273–9. doi: 10.3233/JAD-121138
50. Lv YB, Yin ZX, Chei CL, Brasher MS, Zhang J, Kraus VB, et al. Serum cholesterol levels within the high normal range are associated with better cognitive performance among Chinese elderly. J Nutr Health Aging. (2016) 20:280–7.
51. Zhou F, Deng W, Ding D, Zhao Q, Liang X, Wang F, et al. High low-density lipoprotein cholesterol inversely relates to dementia in community-dwelling older adults: the Shanghai aging study. Front Neurol. (2018) 9:952. doi: 10.3389/fneur.2018.00952
52. Rej S, Saleem M, Herrmann N, Stefatos A, Rau A, Lanctot KL. Serum low-density lipoprotein levels, statin use, and cognition in patients with coronary artery disease. Neuropsychiatr Dis Treat. (2016) 12:2913–20. doi: 10.2147/NDT.S115505
Keywords: delirium, triglycerides (TG), cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), mediation effect
Citation: Lin Y, Peng X, Lin X, Deng X, Liu F, Tao H, Dong R, Wang B and Bi Y (2022) Potential Value of Serum Lipid in the Identication of Postoperative Delirium Undergoing Knee/Hip Arthroplasty: The Perioperative Neurocognitive Disorder and Biomarker Lifestyle Study. Front. Psychiatry 13:870317. doi: 10.3389/fpsyt.2022.870317
Received: 06 February 2022; Accepted: 18 March 2022;
Published: 12 April 2022.
Edited by:
Yiying Zhang, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Wenyu Song, Brigham and Women’s Hospital and Harvard Medical School, United StatesBiyue Zhu, Massachusetts General Hospital and Harvard Medical School, United States
Xiaoyu Yang, University of California, San Francisco, United States
Copyright © 2022 Lin, Peng, Lin, Deng, Liu, Tao, Dong, Wang and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Lin, bGlueHVfZ3JlZW5AMTI2LmNvbQ==; Bin Wang, d2FuZ2JpbjMyOEBzaW5hLmNvbQ==; Yanlin Bi, cG5kYWJsZTIwMjFAc2luYS5jb20=
 Yanan Lin1
Yanan Lin1 Xu Lin
Xu Lin Fanghao Liu
Fanghao Liu Yanlin Bi
Yanlin Bi