- 1Department of Physiology, College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman
- 2Department of Physiology, Oman College of Health Sciences, Muscat, Oman
- 3Department of Biochemistry, College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman
Introduction: Sleep has different patterns followed worldwide and can be influenced by social, cultural, and environmental factors. Daytime napping is commonly practiced in different parts of the world with controversial results of its effect on glucose metabolism. The current study aims to examine the association of afternoon napping and night sleep duration with metabolic derangements.
Methods: This is a cross-sectional study involving young adults and middle-aged subjects. Anthropometric measurements were taken for height and weight and hip and waist ratio. Consented subjects were asked to wear actigraphy for 1 week and run their usual daily activities. Home sleep apnea testing was performed to exclude obstructive sleep apnea. Subjects had been asked to come fasting on day seven for blood collection to test for fasting glucose, glycated hemoglobin, lipid profile, and insulin.
Results: A total of 405 subjects were involved to complete the study (52% male, 48% female). The mean age of participants was 32.8 ± 11.5 years. The study indicated that the duration of afternoon napping was significantly associated with abnormal glycated hemoglobin (HbA1c > 5.7%) (p = 0.01) and body mass index (p = 0.046) independent of age, gender, and nocturnal sleep duration. Nocturnal sleep duration was associated with increased insulin level (p = 0.04).
Conclusion: Afternoon napping is associated with an increased level of glycated hemoglobin and obesity and that may predispose to the development of type 2 diabetes mellitus.
Introduction
Sleep is an imperative physiological aspect that maintains the circadian rhythm and hence a healthy life. Sleep patterns are identified according to individual satisfaction, specific sleep timing, sufficient duration, high efficiency, and continuous alertness throughout one's waking hours. Reduced sleep duration and quality are becoming endemic in many societies because of lifestyle changes and modernization and chronic insufficient sleep is considered a public health problem (1). Obesity and cardiometabolic risk are on the rise worldwide (2, 3) and the underlying etiology of this pandemic is multifactorial, with well-recognized contributions from the diet (large portion sizes, nutrient-dense foods, and others) and physical inactivity (3, 4). However, such factors alone do not explain the significant epidemic of obesity and cardiometabolic diseases and sleep duration has a strong confounding factor.
The link between sleep and metabolism is well established for a long time and that includes mainly the association between sleep duration and timing and cardiometabolic diseases (5, 6). Studies indicated the association between chronic sleep restriction and poor sleep quality and the increased risk of obesity, insulin resistance, hypertension, and lipid abnormalities in different age populations. Nevertheless, several epidemiologic studies have reported that diabetes risk is elevated in both short (6, 7) and long (8) sleepers after adjusting for confounding risk factors, including waist girth. Therefore, it is a U-shaped relationship that shows an association between sleep duration and cardiometabolic risk, independent of sleep pattern. Afternoon napping also known as “siesta” is a common practice in some parts of the world, particularly in hot areas, such as the middle east (8). Relationships between napping and other health outcomes, such as diabetes, have been even less well characterized by debatable results about the duration of the napping and the metabolic effect (9–12). A study in Mediterranean islands indicated that daytime napping is associated with increasing metabolic syndrome (MetS) and frequent “siesta” was positively linked to the odds of MetS presence in women but not in men (13). Short daytime napping duration (<1 h) is associated with a reduced rate of metabolism-related diseases and it may have a protective effect on people from negative health conditions, whereas long daytime napping duration is associated with a higher prevalence of diabetes, and therefore may predispose to unfavorable health outcomes (14).
We have earlier reported that people may adapt to different sleep patterns, which could be multiple episodes per 24 h with variable durations (15). Moreover, we found that the majority of participants practiced afternoon napping (85%) with a mean duration of 45 ± 43 min (15). Earlier reports found that the prevalence of napping reported in surveys and in diaries ranges from ~10–65% (16, 17).
Despite conflicting literature on the associations between sleep duration and timing with obesity and metabolic risk, normal sleep is being explored as a possible factor to minimize the risk of cardiovascular and metabolic morbidities. To address these issues, we measured sleep duration using actigraphy, anthropometry, and markers of metabolic syndrome. Specifically, we aimed to determine the link between long afternoon napping (>1 h) and metabolic abnormalities. We also aimed to determine if associations existed between night sleep duration and traits of metabolic components: lipid profile, blood pressure, hyperglycemia, insulin resistance, and adiposity. In the present study, we hypothesized that long afternoon napping of more than 1 h and in presence of a normal night, sleep duration is associated with an increased risk of metabolic diseases.
Methods
Study design and sample size
This was a cross-sectional descriptive community-based study. The Medical Research Ethics Committee at Sultan Qaboos University approved the study design and methods with code MREC # 1078 and the study was conducted in accordance with the declaration of Helsinki. All participants were asked to sign a written consent before enrolling in the study.
The sample size was determined based on previous literature reviews that study the cardiometabolic risks in siesta vs. nonsiesta sleep patterns. The mean, standard deviation, and mean difference of five quantitative parameters were collected including, glycated hemoglobin, fasting blood glucose (FG), total cholesterol (TC), triglycerides (TG), and BMI (BMI). The target group was healthy local adults' men and women, aged 18–64 years. An adequate sample size of 400 subjects was estimated based on the prevalence of event of interest (50%), with a margin of error of 10% on either side of the estimate with 95% confidence intervals with a prior estimate that 90% of participants would have the siesta habit, the calculated sample size would achieve a power of 80%.
Participants were recruited through a variety of methods, including press releases, emails, brochure distribution to local schools, government establishments, and community events. The study was conducted in Muscat, the capital city and the largest metropolitan area in Oman from January 2016 to February 2018. Subjects were excluded if they met any of the following criteria: (1) past/present medical history including cancer, any psychiatric illness affecting his sleep behavior including anxiety and depression, (2) shift workers, (3) subjects with moderate and severe obstructive sleep apnea (OSA) are defined by apnea-hypopnea index (AHI) > 15 (18, 19), (4) Pregnant and breast-feeding women were also excluded as well as mothers who have children below 1 year of age, (5) The participants who had not traveled across the time zone for at least 1 month before enrolling in study, (6) Smokers and regular alcohol drinkers, and (7) people with an established diagnosis of type 2 diabetes mellitus (T2DM) and/or hypertension. The Muslim's holy month of Ramadan and the following month in the lunar calendar were excluded because of changes in people's rituals, sleep, and eating habits.
Measurement of sleep patterns
An actigraphy watch (SOMNOWATCH TM Plus, SOMNO medics, Germany, 2014) was used in this study. The participants were requested to wear the actigraphy watch on the dominant arm for 7 days 24 h a day except during showering. They were also given an activity diary at the same time for confirmation. The diary includes working, eating and resting time, and physical activities. The participants were asked to run their daily activities as usual and avoid traveling across time zone during the period of the study. The variation in night sleep duration and afternoon napping was identified and the average of 1 week was considered for the statistical analysis. Subjects were also provided with home sleep apnea testing (HSAT) (AASM level III) (Somnotouch, Somnomedics, Germany, 2015) for one night to exclude obstructive sleep apnea (20). Actigraphy recordings were downloaded and analyzed by the researcher and the timing of sleep and wake up and afternoon napping was obtained (21). Nocturnal sleep duration and afternoon napping were also calculated. Afternoon siesta was categorized into two groups: short siesta (<60 min) and long siesta (22).
All potential confounders were also identified a priori and that include age, gender, diet, level of exercise, level of education, and socioeconomic status, all of which are known to be risk factors for cardiometabolic diseases and it is already reported in previously published articles (15, 23). All participants reported a carbohydrate-rich diet and no or mild (one episode of walking/ week) exercise.
Anthropometric measurements and fasting blood collection
The participants were informed to come on day 8 early morning fasting for blood collection. After ensuring 12 h of overnight fasting, 15 ml of blood (butterfly blue needle of 23 gauge, BD, USA) was collected from each participant between 6 and 10 am. The biochemical investigations were measured in a clinical laboratory (The analysis is performed on the Roche COBAS INTEGRA 400 analyzer, Germany) and that includes fasting glucose (FG), HbA1c, serum insulin level, total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG).
Before blood collection, anthropometric measurements were taken, which included weight (kg), height (cm), hip and waist circumferences during the first visit (day 1) and the second visit (day 8). The weight was measured by a digital weighing scale (Seca, Equip Medical, Singapore). Blood pressure was measured in a sitting position using an appropriate-sized cuff with a blood pressure unit (SureSigns VM8, Philips, Germany), and it was measured two times in two different visits and the average of the two readings was taken for the statistical analysis. All participants were asked to fill in the Arabic version of the Epworth sleepiness questionnaire (ESS) to assess for daytime sleepiness (24).
Outcome ascertainment
There was a total of six metabolic outcomes in the present study and that include, obesity, elevated fasting glucose, HbA1C, HDL, LDL, and TG. Metabolic syndrome was defined according to the Joint Interim Statement of the International Diabetes Federation (IDF) (25). Obesity was defined as hip/waist and waist circumference over 80 cm in women and over 90 cm in men and body mass index (BMI) was classified according to the world health organization (WHO) classifications (26).
Participants were classified as normal HbA1c <5.7%, pre-diabetes 5.8–6.4% and type 2 diabetes > 6.5%. (FPG < 5.6 mmol/L), IFG (5.6 ≤ FPG < 7.0 mmol/L), and diabetes (FPG ≥ 7.0 mmol/L) based on local laboratory guidelines. Lipid profile that includes HDL, LDL, and TG were classified according to the latest guidelines of lipid treatment guidelines in Oman (27).
Statistical analysis
Descriptive analysis for continuous variables was used to calculate mean, median, and standard deviation (SD). Prevalence and frequencies were expressed as percentages. The t-test was used to find the mean difference between the two groups (short and long siesta). Regression analysis was performed to correlate sleep parameters (night sleep durations in hours and siesta duration in minutes) and metabolic components. A p-value (two-tailed) of <0.05 was considered statistically significant. All data were coded and analyzed with the Statistical Package for Social Sciences Software (SPSS Version 22.0, Inc., Chicago, IL, USA). Participants who fully completed the study were included in the data analysis. Non-responders or those with incomplete actigraphy data (he/she used the watch for <1 week) were excluded. Additionally, subjects with AHI>15 are also excluded.
Results
Demographics of the study population
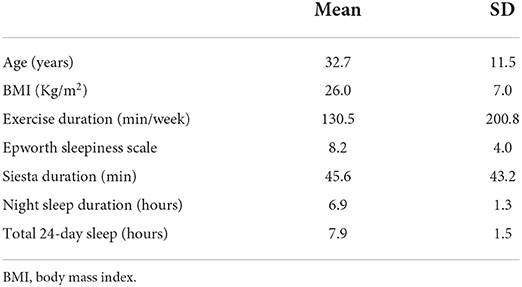
Around 800 people were approached and only 405 subjects completed the study (52% male, 48% female) with a response rate of 50.6%. The mean age of the subjects was 32.82 ± SD11.5years and BMI 26.0 ± 7.0 kg/m2. The mean night sleep duration is 6.9 ± SD 1.33 h and the mean afternoon napping was 45.56 ± SD 43.18 min. The most frequent bed time of the study sample was between 11:00 pm and 12:00 am and the frequent final wake up time was between 6 and 7:00 am. More demographic data are listed in Table 1.
Afternoon napping and metabolic components
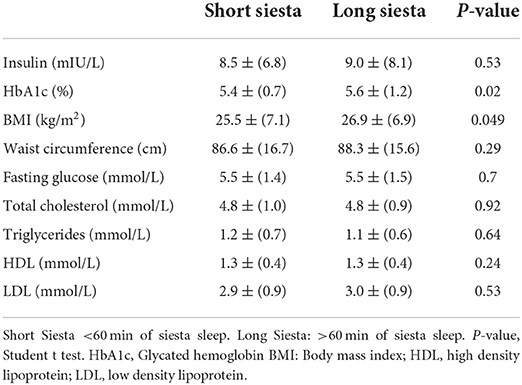
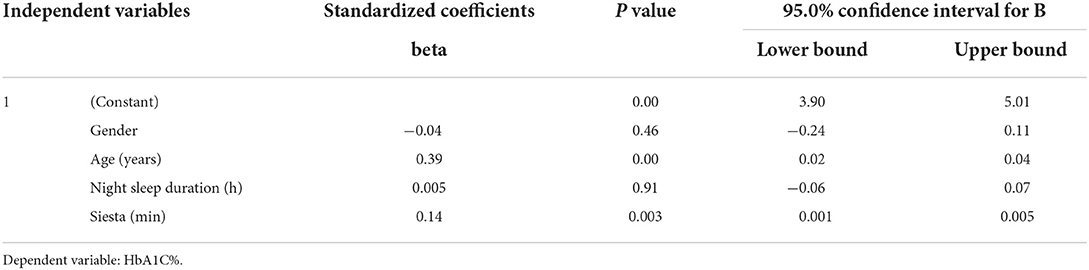
Table 2 shows a comparison between short and long afternoon napping with different metabolic syndrome traits. Results showed significant difference in HbA1c between short nap (mean = 5.38 ± 0.71%) and long afternoon nap (mean = 5.63 ± 1.19%) (p = 0.02) and in BMI; short nap (mean = 25.47 ± 7.08 kg/m2) and long afternoon nap (mean = 29.90 ± 6.87 kg/m2) (p = 0.049). Other biochemical parameters did not show any significant association with an afternoon nap. We further adjusted the statistics for the following confounding variables: age, exercise durations, night sleep duration, and total 24-h sleep duration. Afternoon nap duration was significantly correlated with HbA1c (p = 0.01) (Table 3), and BMI (p = 0.046) (Table 4) after adjusting to night sleep duration, age and gender. Other glycemic parameters did not show any significant association with an afternoon nap (p > 0.05) (Table 5).
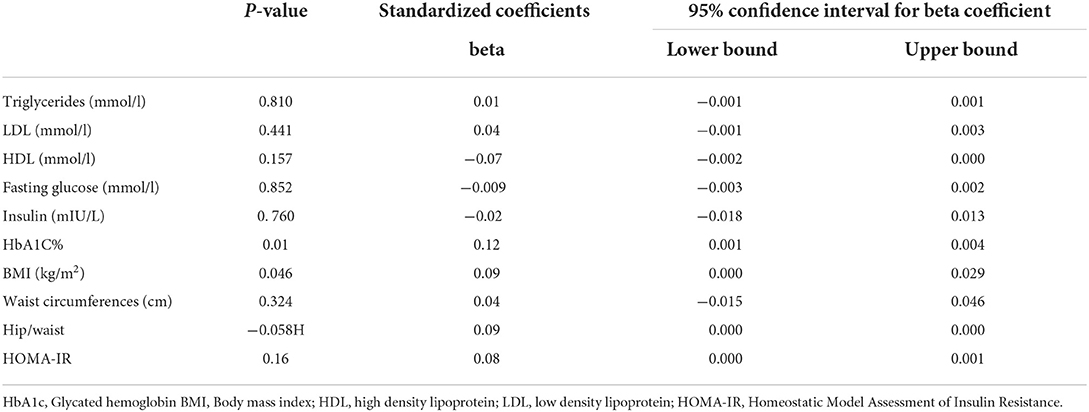
Table 5. Regression analysis of afternoon napping (min) with metabolic components after adjustment for age, exercise and night sleep duration.
Night sleep duration and metabolic components
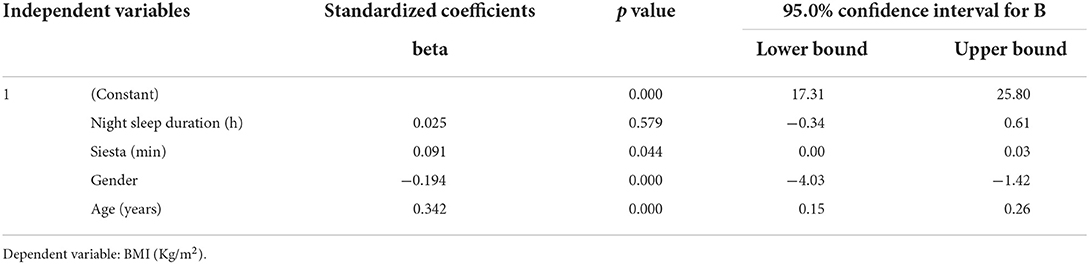
We then assessed the association of night sleep duration with metabolic traits. Regression analysis (Table 6) indicates that night sleep duration was associated with increased insulin level (p = 0.04) and hip/wait (p = 0.03). Lipid profile parameters of TG (p = 0.62) and LDL (p = 0.50) did not show any correlation except HDL (p = 0.04). Furthermore, HbA1c, fasting glucose, and BMI did not show any significant association with night sleep duration (p > 0.05) after adjusting for an afternoon siesta, age, and total 24-h day sleep.
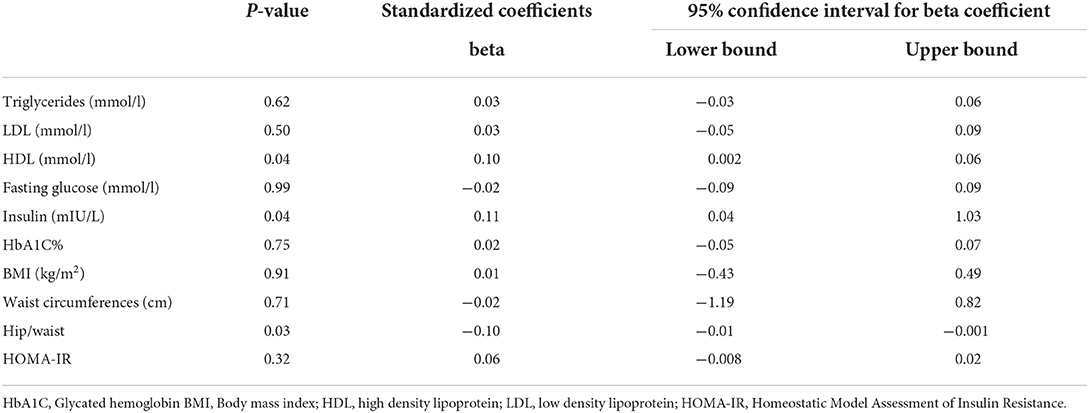
Table 6. Regression analysis of night sleep duration with metabolic components after adjustment for age and afternoon siesta (napping).
Total 24-h sleep and metabolic components
Table 7 shows that total 24-h sleep was significantly associated with TG (p = 0.04) but not with other biochemical parameters (p > 0.5) nor with obesity parameters (BMI, h/w, and waist circumference, p > 0.05).
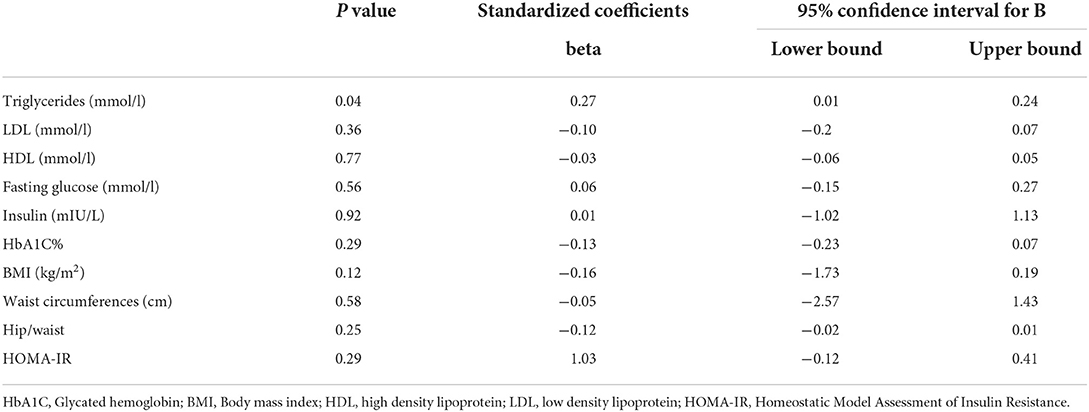
Table 7. Regression analysis of total 24-h sleep duration with metabolic components after adjustment for age and afternoon siesta (napping) and night sleep duration.
Discussion
This cross-sectional descriptive study was conducted among the Arab population in Oman using an objective method of actigraphy to measure sleep duration and timing. We have indicated earlier that people in Oman and other similar communities have different sleep patterns which involve habitually long afternoon napping that may extend to more than 1 h (15, 23, 28). The current study indicated that long afternoon napping of more than 60 min is associated with increased glycated hemoglobin (HbA1C) and obesity in subjects with normal nocturnal sleep duration.
Our findings support the hypothesis that a long afternoon siesta is associated with abnormal glycemic control and adapting certain sleep patterns that involved long afternoon napping may predispose to metabolic abnormalities. The evidence for daytime napping and metabolic abnormalities from previous studies was inconclusive with variable results about the duration and frequency of the nap. Although this study is cross-sectional, it objectively measured the sleep duration using actigraphy, unlike previous studies that used questionnaire-based surveys which are subjected to re-call bias (29). Previous studies concerning the health impact of napping examined the relationship with T2DM. One study found that afternoon nap duration was associated with the risk for T2DM, and longer nap duration (60–90 min) was related to an increased risk for impaired fasting plasma glucose (11). Nevertheless, they used self-report sleep habits and the average age of their cohort was 50 years. In our study, we used a more objective method and we ruled out moderate and severe OSA as a confounder to draw our conclusion. Moreover, we studied a relatively younger population with an average age of 32 years. Another study by the Guangzhou group found that napping for more than 30 min in a population older than 50 years is associated with T2DM (30). A prospective study reported that daytime napping of more than 30 min and after an average of 4.5 years of follow-up, was significantly associated with an increased risk of elevated HbA1c level (>6.5%) in men and women in the Chinese population (31). Our sample did not reveal nocturnal sleep deprivation with an average night sleep duration at almost 7 h (32) but almost all participants practiced afternoon siesta with variable duration. Therefore, our findings are in agreement with the prospective study and in absence of nocturnal sleep deprivation.
Our findings could be explained by the fact that long afternoon napping of more than an hour may alter the circadian rhythm and that would indirectly alter the link between healthy sleep and normal metabolism of carbohydrates (33). Long afternoon napping would shift night bed and rise timing toward late phase sleep and this may cause circadian misalignment (34).
It is important to mention that night sleep duration is correlated with insulin level and obesity by hip/waist and that may add to the burden of metabolic derangement if the subject goes for afternoon napping. Nevertheless, the total 24-h day of sleep did not correlate significantly with any metabolic parameters except for TGA and that might be attributed that the majority of young people in Oman tend to have late dinner. We could assume at this point that afternoon napping with normal night sleep duration is independently associated with glycemia abnormalities.
A major limitation of this study is the nature of the study itself as with cross-sectional analysis you cannot infer causality; indeed, afternoon napping may be a marker of unfavorable health outcomes and lifestyle traits, which is accounted for to some extent by adjusting for measured confounders. Nevertheless, doing a prospective long-term study with objective methods of actigraphy and home sleep study would be cumbersome and incur a bigger cost. The strength of our study is the objective method of measuring sleep and all participants were screened for OSA using HSAT. Another limitation of the study is the possible impact of diet which is not fully addressed in this study, particularly the quantity of carbohydrate consumption which is a major dietary component among the local population. Furthermore, the confounding factor of exercise should also be fully investigated in terms of duration and frequency and that would add more strength to any study associating afternoon siesta and metabolism.
Conclusion
Afternoon napping of more than 1 h is correlated with increased glycated hemoglobin and obesity in participants with normal night sleep duration in young and middle-aged adults. Night sleep duration is correlating with insulin level but total 24-h day sleep did not correlate with any glycemia parameters or obesity. Therefore, long napping of more than 1 h could be considered a risk for developing T2DM. It would be recommended for public health workers and physicians to educate people to avoid long napping duration, especially in societies with a high prevalence of obesity and diabetes (3, 35).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee, College of Medicine and Health Sciences, Sultan Qaboos University, Muscat, Oman. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was funded by the research council of Oman Project No. RC/MED/PHYS/15/01.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. (2015) 38:1161–83. doi: 10.5665/sleep.4886
2. Kelishadi R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol Rev. (2007) 29:62–76. doi: 10.1093/epirev/mxm003
3. Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metabol. (2008) 93(11 Suppl 1):S9–30. doi: 10.1210/jc.2008-1595
4. Erlichman J, Kerbey AL, James WPT. Physical activity and its impact on health outcomes. Paper 1: the impact of physical activity on cardiovascular disease and all-cause mortality: an historical perspective. Obes Rev. (2002) 3:257–71. doi: 10.1046/j.1467-789X.2002.00077.x
5. Archer SN, Oster H. How sleep and wakefulness influence circadian rhythmicity: Effects of insufficient and mistimed sleep on the animal and human transcriptome. J Sleep Res. (2015) 24:476–93. doi: 10.1111/jsr.12307
6. Al-Abri MA, Jaju D, Al-Sinani S, Al-Mamari A, Albarwani S, Al-Resadi K, et al. Habitual sleep deprivation is associated with type 2 diabetes: a case-control study. Oman Med J. (2016) 31:399–403. doi: 10.5001/omj.2016.81
7. Buscemi D, Kumar A, Nugent R, Nugent K. Short sleep times predict obesity in internal medicine clinic patients. J Clin Sleep Med. (2007) 3:681–8. doi: 10.5664/jcsm.27023
8. Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: a review of possible mechanisms. Sleep Med Rev. (2018) 40:127–34. doi: 10.1016/j.smrv.2017.11.001
9. Lam KBH, Jiang CQ, Thomas GN, Arora T, Zhang W, Sen Taheri S, et al. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank Cohort Study. Sleep. (2010) 33:402–7. doi: 10.1093/sleep/33.3.402
10. Owens JF, Buysse DJ, Hall M, Kamarck TW, Lee L, Strollo PJ, et al. Napping, nighttime sleep, and cardiovascular risk factors in mid-life adults. J Clin Sleep Med. (2010) 6:330–5. doi: 10.5664/jcsm.27873
11. Fang W, Li Z, Wu L, Cao Z, Liang Y, Yang H, et al. Longer habitual afternoon napping is associated with a higher risk for impaired fasting plasma glucose and diabetes mellitus in older adults: results from the Dongfeng-Tongji cohort of retired workers. Sleep Med. (2013) 14:950–4. doi: 10.1016/j.sleep.2013.04.015
12. Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia. (2020) 63:2359–71. doi: 10.1007/s00125-020-05253-x
13. Georgousopoulou EN, Naumovski N, Mellor DD, Tyrovolas S, Piscopo S, Valacchi G, et al. Association between Siesta (Daytime Sleep), dietary patterns and the presence of metabolic syndrome in elderly living in mediterranean area (Medis Study): the moderating effect of gender. J Nutr Health Aging. (2017) 21:1118–24. doi: 10.1007/s12603-016-0865-0
14. Zhao X, Cheng L, Zhu C, Cen S, Lin W, Zheng W, et al. A double-edged sword: the association of daytime napping duration and metabolism related diseases in a Chinese population. Eur J Clin Nutr. (2021) 75:291–8. doi: 10.1038/s41430-020-00777-2
15. Al-Abri MA, Al Lawati I, Zadjali F, Ganguly S. Sleep patterns and quality in omani adults. Nat Sci Sleep. (2020) 12:231–7. doi: 10.2147/NSS.S233912
16. Guilleminault C. Sleep and alertness: chronobiological, behavioral, and medical aspects of napping. JAMA. (1990) 263:2255. doi: 10.1001/jama.1990.03440160117048
17. Rosenberg C. Sleep and alertness: chronobiological, behavioral, and medical aspects of napping. Neurology. (1990) 15:335–43.
18. Epstein LJ, Kristo D, Strollo PJ, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. (2009) 5:263–76. doi: 10.5664/jcsm.27497
19. Patil SP, Ayappa IA, Caples SM, John Kimoff R, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2019) 15:335–43. doi: 10.5664/jcsm.7640
20. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: An American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:479–504. doi: 10.5664/jcsm.6506
21. Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. (2018) 14:1231–7. doi: 10.5664/jcsm.7230
22. Bursztyn M, Ginsberg G, Stessman J. The siesta and mortality in the elderly: effect of rest without sleep and daytime sleep duration. Sleep. (2002) 25:187–91. doi: 10.1093/sleep/25.2.187
23. Al Lawati I, Zadjali F, Al-Abri MA. Seasonal variation and sleep patterns in a hot climate Arab Region. Sleep Breath. (2022). doi: 10.1007/s11325-022-02620-3. [Epub ahead of print].
24. Al-Abri M, Al-Hamhami A, Al-Nabhani H, Al-Zakwani I. Validation of the Arabic version of the epworth sleepiness scale in Oman. Oman Med J. (2013) 28:454–6. doi: 10.5001/omj.2013.126
25. IDF. IDF Worldwide Definition of the Metabolic Syndrome, Federation, Internal Diabetes. (2014). Available online at: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionofthe-metabolic-syndrome.html
26. World Health Organization. WHO global database on body mass index (BMI): An interactive surveillance tool for monitoring Nutrition transition. Public Health Nutr. (2006) 9:658–60. Available online at: http://www.journals.cambridge.org/abstract_S1368980006001078
27. Al-Zakwani I, Sulaiman K, Al-Rasadi K, Mikhailidis DP. Prevalence of low high-density lipoprotein cholesterol (HDL-C) as a marker of residual cardiovascular risk among acute coronary syndrome patients from Oman. Curr Med Res Opin. (2011) 27:879–85. doi: 10.1185/03007995.2011.559537
28. Sayón-Orea C, Bes-Rastrollo M, Carlos S, Beunza JJ, Basterra-Gortari FJ, Martínez-González MA. Association between sleeping hours and siesta and the risk of obesity: The SUN mediterranean cohort. Obes Facts. (2013) 6:337–47. doi: 10.1159/000354746
29. Al Lawati I, Zadjali F, Al-Abri MA. Agreement analysis of sleep patterns between self-reported questionnaires and actigraphy in adults. Sleep Breath. (2021) 25:1885–91. doi: 10.1007/s11325-021-02296-1
30. Cheng KK, Adab P, Taheri S, Zhang WS, Arora T, Thomas GN, et al. Napping is associated with increased risk of type 2 diabetes: the Guangzhou Biobank cohort study. Sleep. (2010) 33:402–7.
31. Li X, Pang X, Zhang Q, Qu Q, Hou Z, Liu Z, et al. Long-term single and joint effects of excessive daytime napping on the homa-ir index and glycosylated hemoglobin. Medicine. (2016) 95:e2734. doi: 10.1097/MD.0000000000002734
32. Panel CC, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med. (2015) 11:931–52. doi: 10.5664/jcsm.4950
33. Bailey SM, Udoh US, Young ME. Circadian regulation of metabolism. J Endocrinol. (2014) 222:R75–96. doi: 10.1530/JOE-14-0200
34. Akacem LD, Simpkin CT, Carskadon MA, Wright KP, LeBourgeois MK. Circadian phase and sleep timing differ between napping and non-napping toddlers. Sleep. (2014) 10:e0125181. doi: 10.1371/journal.pone.0125181
Keywords: napping, sleep, diabetes mellitus, glycated hemoglobin, obesity
Citation: Al-Abri MA, Al Lawati I and Al Zadjali F (2022) Association of elevated glycated hemoglobin and obesity with afternoon napping for more than 1 h in young and middle-aged healthy adults. Front. Psychiatry 13:869464. doi: 10.3389/fpsyt.2022.869464
Received: 04 February 2022; Accepted: 05 September 2022;
Published: 10 October 2022.
Edited by:
Octavio Luiz Franco, Catholic University of Brasilia (UCB), BrazilReviewed by:
Haitham Jahrami, Arabian Gulf University, BahrainRosane Harter Griep, Oswaldo Cruz Foundation (FIOCRUZ), Brazil
Copyright © 2022 Al-Abri, Al Lawati and Al Zadjali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed A. Al-Abri, bWFsYWJyaUBzcXUuZWR1Lm9t
†ORCID: Mohammed A. Al-Abri orcid.org/0000-0002-2841-3478
Ibtisam Al Lawati orcid.org/0000-0001-9916-7692
Fahad Al Zadjali orcid.org/0000-0001-5891-4700
 Mohammed A. Al-Abri
Mohammed A. Al-Abri Ibtisam Al Lawati
Ibtisam Al Lawati Fahad Al Zadjali
Fahad Al Zadjali