
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Psychiatry, 14 November 2022
Sec. Sleep Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.866951
This article is part of the Research TopicSleep, Vigilance & Disruptive BehaviorsView all 15 articles
The bi-directional relationship between sleep and wake is recognized as important for all children. It is particularly consequential for children who have neurodevelopmental disorders (NDDs) or health conditions which challenge their sleep and biological rhythms, and their ability to maintain rhythms of participation in everyday activities. There are many studies which report the diverse reasons for disruption to sleep in these populations. Predominantly, there is focus on respiratory, pharmaceutical, and behavioral approaches to management. There is, however, little exploration and explanation of the important effects of body thermoregulation on children’s sleep-wake patterns, and associated behaviors. Circadian patterns of sleep-wake are dependent on patterns of body temperature change, large enough to induce sleep preparedness but remaining within a range to avoid sleep disturbances when active thermoregulatory responses against heat or cold are elicited (to maintain thermoneutrality). Additionally, the subjective notion of thermal comfort (which coincides with the objective concept of thermoneutrality) is of interest as part of general comfort and associated behavioral responses for sleep onset and maintenance. Children’s thermoregulation and thermal comfort are affected by diverse biological functions, as well as their participation in everyday activities, within their everyday environments. Hence, the aforementioned populations are additionally vulnerable to disruption of their thermoregulatory system and their capacity for balance of sleep and wakefulness. The purpose of this paper is to present hitherto overlooked information, for consideration by researchers and clinicians toward determining assessment and intervention approaches to support children’s thermoregulation functions and promote their subjective thermal comfort, for improved regulation of their sleep and wake functions.
Sleep and thermoregulation are critical biological functions. Through dynamic physiological mechanisms, the functions of both are affected by behavioral, social, and environmental factors, including circadian rhythms of meals, exercise, bathing, indoor and outdoor activity, and associated exposure to ambient temperatures and light (1). They are tightly related, and have direct impacts on each other (2–4). The association between these functions and the impact of biological, behavioral, social and environmental factors is well understood in the general adult population, allowing for strategies to alter thermoregulation and improve sleep onset, maintenance and quality, and to optimize the patterns of sleep, wakefulness and daytime performance (5–8). However, thermoregulation and sleep is less well understood in children, especially those with neurodevelopmental disorders (NDDs) or chronic health conditions (CHCs).
All children need the appropriate quality and duration of sleep, for optimal physical and mental health (9, 10), learning and behavior (11–13), and meaningful participation in their daily lives (14). Sleep is especially important for children with NDDs and CHCs who, along with their caregivers, are vulnerable to additional challenges to their health, participation and wellbeing (15, 16). Indeed, sleep has valuable therapeutic potential for these children (17, 18). Unfortunately, it is common for these children to have difficulties with sleep, and with their patterns of sleep and wake (19–22). Furthermore, they are more susceptible than their peers to thermoregulation difficulties. Thermoregulatory dysfunction is discussed by Svedberg et al. (23) who found a higher incidence of cold extremities in children with severe neurological impairment, compared to their peers, with skin temperatures in the feet significantly lower in the non-ambulant children than those who walked. More specifically, difficulties with thermoregulation during sleep are a concern for these children (24, 25). Indeed, a recent study of 33 children with cerebral palsy (CP), found that 37.5% of children had sleep hyperhydrosis, compared to 4.2% of the control group of typically developing children (26). Similarly, a retrospective study of sleep concerns in 154 children with cerebral palsy, aged 1–18, found that approximately 33% reported temperature and perspiration as major concerns affecting their sleep (27). This was the case across all age groups (aged 1–6, 7–12, 13–18 years). Despite these known difficulties, and the extensive knowledge on the interaction between sleep and thermoregulation, there is no published evidence to guide research and practice for management of sleep and wake in children who have NDDs and CHCs and thermoregulation difficulties.
The dynamic and multi-directional relationships between the biological, behavioral, social, and environmental factors that affect sleep and thermoregulation can be understood when viewed within the framework of the International Classification for Functioning, Disability and Health for Children and Youth [ICF-CY; (28)]. This well-established framework represents the dynamic interaction of biopsychosocial components (body functions and structures, activity and participation, environments and other contextual factors) which influence the determinants of health and wellbeing and the long-term consequences of living with a CHCs or disability. Most importantly, it guides the focus of research and clinical practice toward promoting children’s participation (their active engagement in the important and meaningful aspects of their lives) by illustrating the variability of functioning within everyday settings and noting effects of environments and personal factors such as gender, age, ethnicity, social and educational background, behavior patterns, and life events (29).
This paper is aimed at bringing attention to clinicians and researchers who work to support sleep of children with NDDs and CHCs, of the functional relationships between sleep and thermoregulation, and to postulate pathways to optimize this important aspect of participation and quality of life in these children.
Children and adults are homeotherms. Through dynamic physiological and behavioral thermoregulation functions they typically maintain a constant resting core body temperature between 36.5°C and 37.5°C despite changes in the surrounding environment. Thermoregulation is a critical biological function, for maintenance of vital physiological conditions for cell function, systems function and life itself (4). When conditions or environments challenge the thermoneutral state, biological, and behavioral responses are elicited, with effects on other homeostatic systems (hormonal, digestive, cardio-vascular, respiratory) and alterations to behavioral states (sleep, appetite, psychological stress, vigilance, performance) and wellbeing (30).
The body system for thermoregulation is described by two compartments: a core (including organs such as the lungs, heart, abdominal organs and brain) and a peripheral “shell,” corresponding to skin layers and associated musculo-skeletal, nervous and circulatory systems. The core system has a relatively stable temperature, and is regulated and maintained by a combination of feedforward and feedback mechanisms (4). Feedback responses occur in response to changes in internal temperatures which are detected by thermoreceptors in the core organs, and are triggered when the core temperature deviates from the homeostatic range. The peripheral “shell” is responsible for feedforward mechanisms—pre-emptive responses to anticipated thermal challenges, which are triggered prior to change in core temperatures, primarily through functions of cold and warm thermoreceptors in the skin. These dynamic interactions are heavily controlled by the autonomic nervous system (ANS), with integration mainly at the suprachiasmatic nuclei (SCN) in the pre-optic area of the anterior hypothalamus [see (31) for analysis of skin temperatures as feedforward or feedback systems].
Further to this, thermoregulatory responses can be described through a model which includes a passive system (represented by heat exchanges between the body and the environment) and an active controlled system of thermosensors, central controller and effector mechanisms for thermogenesis (heat production), sudomotion (activity of sweat glands), behavior (changes in posture and movement, and adjusting the environment, clothing or bedding), and vasomotion (vasodilation and vasoconstriction) (Figure 1). In environments with ambient temperature ranges that are “thermoneutral” an almost constant and normal core body temperature is maintained, through autonomic changes in the peripheral skin blood flow (influencing the thickness of the shell compartment), with minimal metabolic heat production. Both core and skin temperatures are controlled via these homeostatic responses, with skin temperature involved in the regulating of core temperature (4). Outside the boundaries of the thermoneutral zone, active thermoregulatory responses are required to maintain homeothermia. Thermoregulatory responses to cold environments involve vasoconstriction, shivering and increased body activity, whereas in warm environments vasodilation and sweating occur. When these responses are not sufficient to compensate for the external thermal challenge, core body temperature decreases or increases and homeothermia is lost. Thus, constant core body temperature is a process of homeostasis which results from the body’s balance between heat production and heat losses in association with behavioral and environmental factors.
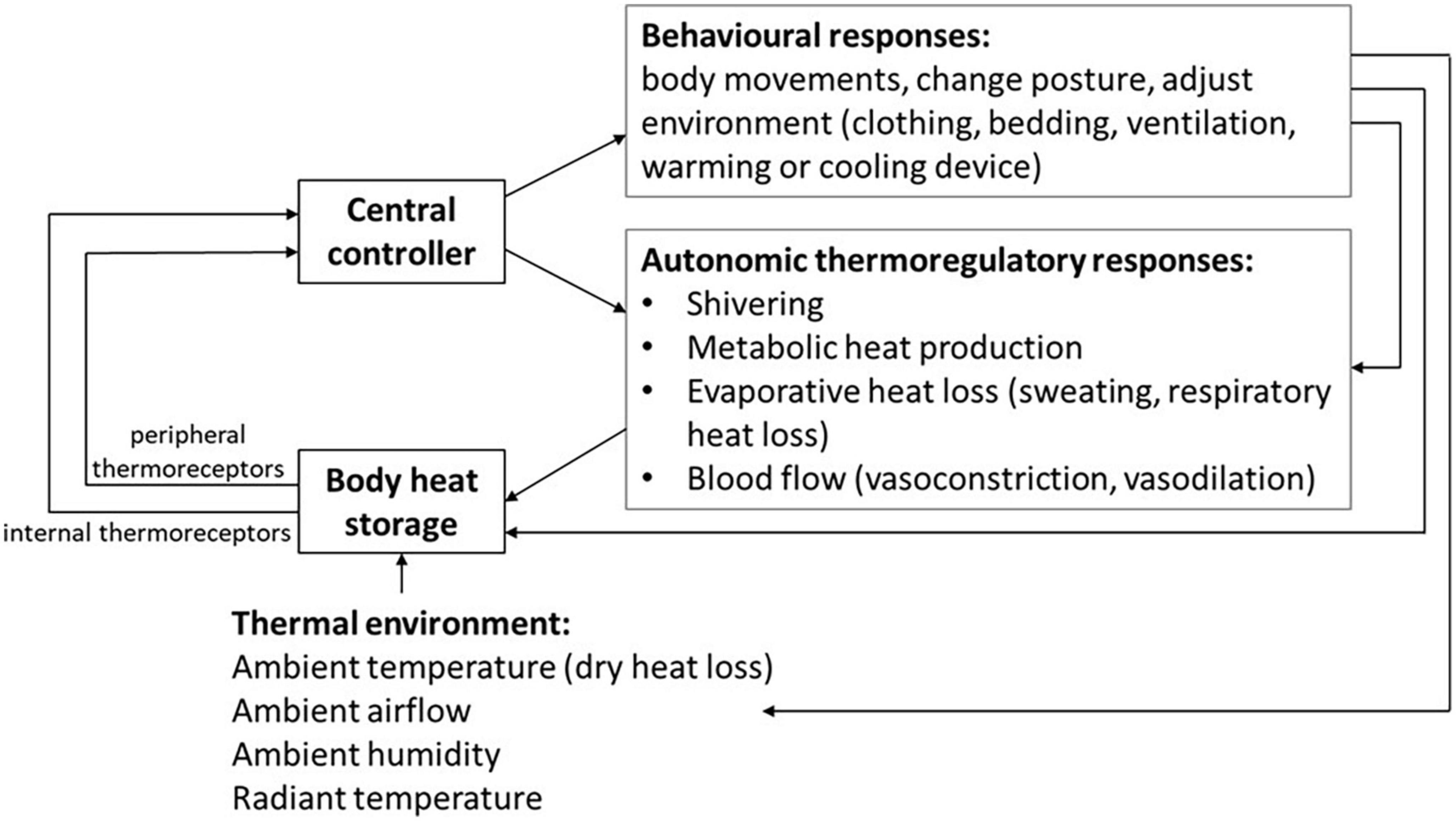
Figure 1. Autonomic and behavioral thermoregulatory response systems for homeostasis in varied thermal environments.
Such is the complexity of thermoregulation, and the dynamic interaction between the body and environments, it can be viewed through specific models of function of the nervous system (4, 32), or through multi-element models as reviewed by Katic et al. (33), who report 22 different thermophysiological models through the years 1970–2016. Additional complexity emerges when we consider the notion of thermal comfort, and the influence of age, and sex.
Thermal comfort is described as the result of combination and adaptation of factors of the body in context with the environment (34) and can be measured through parameters of subjective comfort sensations, as well as measures of skin temperatures and sweat rate as functions of metabolic rate. It can be used to determine optimal (or otherwise) factors for function in occupational and domestic settings, and accounts for effects of ambient temperatures and airflow, and effects of various types of materials used in buildings, bedding and clothing (35). Additional factors are important to thermal comfort, including acclimatization to natural environments, habituation to different climate zones (for example time spent indoors in air-conditioning vs. the dynamic conditions of naturally ventilated environments), seasonal adaptations, diurnal rhythms and, interestingly, the effects of perceived personal control, light intensity, and personality traits (36).
Thermoregulation and thermal comfort vary according to age and gender. The control of environmental temperature is particularly important in children, since their body surface relative to body mass is typically greater than adults. Thin body segments (arms and legs), and lean body shape are subjected to larger and more rapid heat exchanges with the environment than thicker body segments or larger body shape (37). Interestingly, Inoue et al. (38) found that when the air temperature is lower than skin temperature, prepubertal children can thermoregulate as efficiently as young adults, due to their greater surface area-to-mass ratio and relatively greater heat loss from cutaneous vasodilation on the head and trunk. In contrast, in their study comparing thermoregulatory responses of pre-pubertal boys to young men to exposure to linear increases in air temperature, Inoue et al. (39) found that when heat stress was increased the mean body temperature at the onset of sweating was significantly greater in the boys than in the men. They concluded that compared with young men, prepubertal boys manifest greater physiological and perceptual strain under heat stress when air temperature exceeded skin temperature. Reflecting this, in their review of the literature on human thermal comfort in the built environment, Rupp et al. (40) report that young children are shown to have preference for lower temperatures than adults, with apparently greater sensitivity to changes in their metabolism. In the same review, the authors also reported on the gender differences in thermal sensation between school aged girls (more sensitive to low temperatures) and boys (more sensitive to high temperatures).
The fundamental relationship between the circadian patterns of body temperature change and sleep-wake is well known, with clear patterns of change in the sensitivity and functions of the thermoregulatory system across the sleep-wake cycle and across stages of sleep. Sleep propensity is associated with an observed drop in core body temperature (Tcore). Correspondingly, readiness for wake is accompanied by a rise in Tcore. Interestingly, this pattern of rise and fall of core body temperature across the 24 h sleep-wake period is the inverse of patterns of change in melatonin (41). The drop in Tcore that triggers sleep onset is precipitated by rise in distal skin temperatures (Tdistal), namely at the extremities (hands and feet). This commences approximately 100 min before sleep onset and is known as “vegetative sleep preparedness,” first described by Magnussen in 1938 [cited in van den Heuvel et al. (42)] The rise in Tdistal is caused by peripheral vasodilation, allowing inflow of heated blood from the core to the shell, and facilitating heat loss to the environment through the small peripheral blood vessels, the arterio-venous anastomoses (AVAs) which are particularly abundant in the glabrous (non-hairy) palms of the hands, soles of the feet, surface of the ears, and certain facial sites (31, 43). The degree of peripheral vasodilation can be estimated by the difference between Tdistal and proximal skin temperatures (Tproximal, variously measured at thighs, abdomen, shoulders and back). Importantly, this difference, Tdistal minus Tproximal, the distal-proximal gradient (DPG), is regarded as the most significant thermal marker and mechanism of sleep readiness. Indeed, Kräuchi et al. [(44), p.36] wrote that “the degree of dilation of blood vessels in the skin of the hands and feet, which increases heat loss at these extremities, is the best physiological predictor for the rapid onset of sleep.”
The relationship between distal skin vasodilation (with rapid rise in Tdistal and DPG) and sleep onset is understood to be causal, with several studies performed in adults showing that experimentally induced distal vasodilation by subtle skin warming promotes sleep. Accordingly, skin warming for passive body heating (e.g., shower or bath before bedtime) or non-thermal manipulations capable of promoting distal vasodilation (e.g., lights off, lying down, a spicy meal, physical exercise) may increase sleepiness, accelerate the process of falling asleep and improve sleep maintenance in adults (45–48). Interestingly, a recent study of the effects of manipulation of periocular skin temperatures of 19 healthy males showed that a warming eye mask, used prior to sleep, significantly increased the temperatures of hands, feet and the rise of DPG, with significant increase in self-reported sleepiness (49). Most recently, in their randomized controlled study of 11 young healthy males, Haghayegh et al. (50) found that selective thermal stimulation, consisting of a heated pillow that provided mild heating to the cervical spinal skin, in combination with a cool central and warm peripheral temperature-controlled mattress, induced significantly greater distal vasodilation, increased rate of change of DPG, shorter sleep onset latency and significantly better subjective sleep quality in the treatment than the control nights.
Behaviors that lead to pre-sleep relaxation and reduced anxiety when retiring for bed also promote skin vasodilation prior to sleep onset by decreasing sympathetic nervous system activity (51). Additionally, the hypnotic effects of medications such as benzodiazepine and temazepam, and the sleep readiness effect of melatonin is associated with their effects on distal skin vasodilation (52, 53). In contrast, adults with conditions or behaviors which cause attenuated rise in Tdistal take longer to fall asleep (8, 48). This important relationship between rapid rise in Tdistal and DPG and sleep onset has also been shown for preterm neonates, infants, and school-aged children (54–56). Logically, the propensity for morning wake correlates with core temperature rise in the morning, as a result of distal skin vasoconstriction which is evident in lowering distal skin temperatures and DPG. Consistent with this, manipulations that induce distal vasoconstriction promote awakening and vigilance in adults (57). Similar studies have not been reported for children.
Consideration of Tproximal is also important to understanding sleep, especially in relation to sleep quality and maintenance. This may be especially important for young children. In their study of sleep and skin temperatures of pre-school children and their mothers, Okamoto-Mizuno et al. (58) found that children’s proximal temperatures increased more than distal temperatures, and that heat dissipation in this group was dependent more on increase in Tproximal than Tdistal. They noted cardiovascular differences in this younger group, and surmised that Tproximal is nearer to the body core and possibly of more benefit for heat loss and decreased cardiovascular strain than the more distal sites. Interestingly, this group of young children were noted to predominantly sleep without bed coverings, and to move about the bed surface during sleep, enabling a greater dry-heat exchange during sleep and indicating a greater dependence on behavioral thermoregulation than for adults. While many studies report Tabdomen as a measure of Tproximal, we have recently demonstrated that Tback was a good indicator of Tproximal during sleep of school aged children (59). This corresponds with studies of adults, which report that conductive heat loss through the proximal back is critical for slow wave sleep and subjective sleep quality (60, 61). Similarly, Lan et al. (62) found that local cooling of Tback was most effective in alleviating thermal stress in a warm environment. Less frequently reported than Tdistal and Tproximal, forehead skin temperature (Tforehead) is also considered to be an important region of thermoregulation (63, 64). It is reported to be affected by the temperature of the underlying brain, and to have a high rate of heat transfer with ambient temperature due to venous as well as arterial systems, making it a unique site of Tskin measurement (31). In studies of adults and elderly, Tforehead has been shown to be particularly affected by seasonal ambient temperatures (65), and may be an important factor in sleep maintenance within this context.
While there is a wealth of research examining the association between body temperature and sleep in adults, it is not clear if such findings can be directly translatable to children, thus warranting further research in this population.
The notion of thermal comfort is fundamental to understanding the interactions between thermoregulation and sleep. Warm or cool challenges (beyond thermoneutral) before and during sleep affect the timing of sleep onset, the duration of sleep stages and the overall efficiency of sleep (66, 67). They also affect subjective perceptions of “thermal comfort” and elicit associated behavioral thermoregulation strategies such as adjusting bedding and changing body position, affecting sleep depth and propensity to wakefulness (8, 48, 68, 69). While autonomic responses to hot or cold stimuli are reduced during REM compared with NREM sleep, REM sleep is more vulnerable to thermal discomfort than the other sleep stages (70).
Thermal comfort during sleep is underpinned by physiological and behavioral responses to environmental factors: seasonal, household and bedroom temperature and humidity, and, more specifically, the “microclimate” of the bed which is influenced by interactions of bedding and clothing. These factors are affected by building design and the use of heaters, air-conditioners, fans and ventilation (70). In their review of the environmental parameters for optimal sleep, Caddick et al. (71) recommended ambient bedroom temperatures between 17°C and 28°C, depending on effects of bedding and with relative humidity between 40 and 60%. The effects of environmental temperatures have been found to vary across sleep periods. Whilst humid heat is reported to particularly affect slow wave sleep in the earlier phase of sleep period, cold exposure is found to impact on quality of sleep in later segments of sleep (69). Furthermore, in their review study, Lan et al. (62) reported that a cooler sleep environment at the beginning of the sleep period caused delayed sleep onset, while in a warm setting, local cooling to neck and back improved thermal comfort and sleep efficiency during the sleep period. Associated with this, the microclimate of the bed is particularly important for thermoregulation and sleep, for sleep onset and protection of sleep stage structure (70–72). In their review of thermal environment and sleep quality, Lan et al. (70) found that an in-bed microclimate of around 30°C was most consistently associated with thermoneutrality for the sleeping human body, with relatively small variation across seasons and change in ambient temperatures. Various studies in adults have shown that the microclimate can be determined and indeed manipulated by types of mattresses (60, 61, 73–75), bed sheets (76), personal heating or cooling devices such as electric blankets or airflow devices (77–80) and clothing worn during sleep (46, 81). While environment conditions, bedding and the microclimate clearly influence body temperature and perspiration, there is limited research examining the thermal comfort of children within their normal sleeping environments.
The complex and interactive functions and effects of thermoregulation and sleep are especially important when considered in the contexts of everyday living. This is relevant for people of all ages, in all environments, with varied daily occupations, and with various health and medical conditions. For children with NDDs and CHCs, the dynamic functional relationships between these factors can be best understood when viewed in context of the framework of the ICF-CY. The following sections will discuss the interactions between thermoregulation and sleep in relation to body structures and functions, activity and participation, environments and personal factors, as illustrated within the adapted model of the framework of the ICF-CY (Figure 2).
With the understanding that central and peripheral interactions of the ANS involve all body structures and functions, and knowledge of the potent interaction of behavioral and autonomic thermoregulatory responses for thermal homeostasis and for sleep, it is clear that disturbance to structures (anatomy) or the functions (physiology) of body systems can have important implications for thermoregulation and sleep in children with NDDs and CHCs.
For children with NDDs, neurological impairment may occur at cortical and subcortical levels, with direct impact on thermoregulatory and sleep system functions. Children with neuro-motor conditions such as cerebral palsy commonly experience pain, circadian dysregulation and hyper-arousal in association with co-morbid epilepsy, and impairment of their respiratory, cardio-vascular, endocrine, gastro-intestinal, integumentary, musculo-skeletal and sensory functions, with impact on their ANS and associated thermoregulatory and sleep system functions (82, 83). Additionally, vision impairment is also common in this group (84, 85), impacting the SCN pathway of effects of light and dark on circadian functions of thermoregulation, and sleep and arousal. The functional relationships between hormonal, sympathetic and parasympathetic pathways mean that children with developmental conditions such as autism spectrum disorder (ASD), attention-deficit/hyperactivity disorder (ADHD) and fetal alcohol spectrum disorders (FASD) also have ANS dysfunction (86–88). Furthermore, pain and discomfort are common for these children, related to co-morbid anxiety and gastro-intestinal, musculo-skeletal or sensory regulation impairment, compounding disruption to the functional relationship between their thermoregulation and sleep (89).
Sleep disruption is also widely reported in children with CHCs, due to impairment of their body structures and functions, and, associated pain and discomfort, with effects on ANS function and rhythms of thermoregulation, thermal comfort and sleep. Given the importance of skin in thermoregulation, conditions which involve impairment of skin function are of particular interest. Sleep disruptions in children with eczema are commonly reported (90, 91), and the association between skin conditions, thermoregulation and sleep is well described by Gupta and Gupta (92). Sleep disturbance is also commonly reported in people with other conditions which affect skin and peripheral vascular functions, such as burns injury (93, 94), diabetes (95), obesity (96) and connective tissue disorders such as Ehlers Danlos syndrome (97). Individuals with childhood cancer are also reported to experience sleep difficulties (98, 99), with hyperarousal due to pain or anxiety. There is a logical relationship between the effects of these conditions on ANS, thermoregulatory functions and sleep.
There is potential for remediation of sleep and thermoregulation difficulties through intervention at the level of body structures and functions. Children who have NDDs and CHCs and need medications, procedures and hospitalization are likely to experience various levels of pain and anxiety, with effects on ANS and vasomotor functions, and subsequent impact on the patterns of core and peripheral temperature changes that are essential to circadian patterns of sleep and wake. With consideration of the individual responses to interventions, these effects could be mitigated by consideration of the timing and dosage of medications (effects on pain and anxiety; effects on core and proximal temperatures; effects on distal vasodilation) and consideration of timing of medical appointments and procedures. Furthermore, changes in environmental conditions, and types and timing of activities may moderate the physiological functions and responses at the level of the ANS, with impact on thermoregulatory effects on sleep, as discussed below.
The timing and rhythms of activities and behaviors play an important part in circadian biological function, with interactions that affect and are affected by thermoregulation and sleep. van den Heuvel et al. (42) confirmed the importance of regularity in bedtime time and demonstrated that physiological sleep preparedness (i.e., distal vasodilation, with rise in Tdistal, rise in DPG, drop in Tcore) was associated with habitual activities for sleep onset. In contrast, when activities associated with sleep onset varied, distal vasodilation was attenuated. Supporting this, Martinez-Nicolas et al. (100) showed that people with the highest contrast between their day and night factors of activity, body position and light exposure had more marked circadian rhythms of Tdistal and sleep. The authors postulated that intensifying the contrast between day and night lifestyle factors may enhance the rhythm of the circadian system. It is notable that pre-sleep activities (anticipating sleep, assuming supine position, cognitive and physical relaxation, switching off the light) can enhance distal vasodilation and promote sleep onset. It is common for children with NDDs and CHCs to experience disruptions to pre-sleep activities, due to the need for complex personal and medical care prior to sleep, as well as communication, cognitive, sensory or behavioral difficulties which impact their ability to anticipate sleep and readily engage in suitable pre-sleep activities (101). There is a likely compounding effect to this, with a bidirectional relationship between parenting stress and inconsistent bedtime routines (102).
More specifically, circadian changes in motor activity promote temperature changes that are associated with patterns of sleep and vigilance (103). This is important when considering the effects of movement impairment on children with neuro-motor conditions, who may have consistently limited or disrupted activity level across any 24-h period (104–106), as well as those with developmental conditions and associated dysregulation of their levels of activity and arousal behaviors (107, 108). For sleep onset, there is an additional important effect of postural changes on ANS and thermoregulatory responses when lying down, unloading the cardiovascular system and reducing sympathetic tone (8). Children with neuro-motor conditions may need to be supported in an upright position during and following their feeding regime, or to assist with breathing throughout the sleep period (109, 110) creating an additional impediment to the vasomotor process that is essential to sleep onset and maintenance.
The timing and temperature of bathing or showering have been shown to influence the rhythms of thermoregulation and sleep. In particular, studies have shown that warm bath, shower or foot bath in the hour before sleep enhance distal vasodilation, with increased rate of change in DPG and reduced sleep onset latency (45). This is significant for families of children with NDDs and CHCs. Some may need to provide bathing in the mornings, for necessary morning hygiene, or need for a simpler evening routine or because of behavioral or sensory responses which cause them to be increasingly agitated or excited during bath or shower, and thus less able to calm and settled for sleep. Some may simply be unaware of the potentially powerful physiological effects of warm bath or shower before sleep and thus miss the benefits of this routine practice.
The type timing, volume, and stimulant consumption and micronutrient intake food and drink intake is known to directly influence sleep (111). Additionally, diet may also influence sleep though effects on body temperature regulation. Indeed, it is known that various foods have vasomotion effects, such as spicy foods, and those high in nitrates or caffeine (112, 113). Additionally, Fronczek et al. (6) showed that the temperature of food or drink may directly affect thermoregulation and vigilance. Children with NDDs and CHCs may have significant disruption to their intake of food and drink. Those with neuro-motor conditions such as cerebral palsy may have disruption to their oral-motor and gastro-intestinal functions, needing modified diets, or overnight tube feeds (oro-gastric, naso-gastric or percutaneous endoscopic gastrostomy), with disturbance to the usual rhythms and timing of oral intake (114). Those with neurodevelopmental conditions such as autism, ADHD or intellectual disabilities may have sensitivities or aversion to specific food types, being selective about or averse to tastes, textures and temperatures of food and drink (115). The type, amount, temperature and timing of food and drink intake may also be compromised for children with CHCs such as diabetes, and intolerances or allergies (116–118).
The seasonal effects of temperature, humidity and light on sleep onset, maintenance and architecture are widely reported, with variations according to geographical locations, age, occupations, gender, and health conditions. These factors interact with building design and the use of heaters, air-conditioners, fans and ventilation, with varied impact on temperature and humidity of the ambient sleep setting (70). More specifically, the creation of an approximately thermoneutral microclimate of the bed is increasingly recognized as particularly important for thermoregulation and sleep, for sleep onset and protection of sleep stage structure, as described above. With the interactive effect of the ambient room environment, the microclimate of the bed is affected by materials used in the mattress, pillows, bed linen and clothing, and by presence or absence of other bodies (people or pets) in the bed (54, 76, 119, 120). The increasingly diverse and sophisticated range of types and materials used for bedding and clothing, such as blankets or throws with timers and varied heating zones, and the development of “smart” materials such as phase change materials, reflects the prevailing knowledge about the importance of thermal comfort for sleep (121). Clothing insulation is a relevant component of behavioral thermoregulation for management of the sleep microclimate (120, 122). Depending on the season, cultural preferences and age, children may or may not wear clothing (pajamas) in bed. Clothing may be especially effective in reducing heat loss, but when used with bedcovers and in warm settings, can increase the risk of body overheating. The ability to choose and adjust bedding and clothing is an important, dynamic behavioral response toward thermoneutrality and thermal comfort, however, children with impairment of their movement, sensory or cognitive functions may have reduced or no capacity for this.
In addition to the above factors, it is important to consider the impact of timing, type and intensity of ambient light on the rhythms of sleep and thermoregulation. There is synchrony between the strength of circadian rhythmicity and the timing of evening dim light and morning bright light. Evening exposure to bright light will reduce evening sleep propensity, while morning exposure to bright light will increase evening sleep propensity (123), and fragmentation in the rhythms of light and dark exposure is associated with more fragmented sleep (124). Even low light levels during sleep, with eyes closed, can disrupt circadian responses (125). Through interactions at the SCN, light in the evening can reduce melatonin secretion, with associated slowing of rise in Tdistal and delay in decline in Tcore (126). In children, the deleterious effects on sleep of portable electronic devices in the bedroom setting are particularly notable (127). The disruptive effects of light on circadian functions of sleep and thermoregulation varies with children’s age, as described in the review by Logan and McClung (128), of circadian changes across the lifespan.
The impacts of environmental factors on sleep and thermoregulation in children with NDDs and CHCs is important for many reasons. Children with mobility impairment may be unable to manage their environment (e.g., open or close windows, or adjust their bedding or clothing) as needed. Those with sensory and cognitive impairment may not register the sensation of thermal discomfort, and be unable to make the necessary behavioral adjustments to promote their thermal comfort. Moreover, those with conditions such as autism or ADHD may have sensory preferences which cause them to actively eschew the use of footwear before bedtime, with resulting cold feet and attenuation of the distal vasodilation which is essential to sleep onset. Those with communication impairment may be unable to communicate their comfort needs to their care providers so that the environment is adjusted to best suit them. Additionally, those with communication impairment may rely on electronic devices and use of screens for communication function at bedtime and possibly during occasions of night waking. Furthermore, the child’s condition may require instruments or actions which compromise the optimal thermal environment. For some, it is necessary to maintain bedroom lighting, for safe provision of nighttime care and use of technology to support sleep (129). Children with movement or postural impairments require positioning equipment such as padded brackets or customized cushions to support their body shape (130), with the encompassing effect of foam and padding materials likely to cause a warmer microclimate in the bed. Those with incontinence may need moisture proof bedding for hygiene purposes (27), compromising attempts to use thermobalancing bedding and clothing materials for optimal management of the bed microclimate.
There are numerous reports of the effects of personal factors on children’s sleep. Variously, these factors can be understood to also impact on thermoregulation, although the connection is rarely made clear. In a recent study of the impact of gender differences on the sleep of adolescents (131), it was shown that sleep quality and daytime dysfunction was significantly worse in girls than in boys. For girls, reduced sleep duration was particularly associated with consumption of hot drinks before bedtime while for boys the key factor was time spent on technology. Body mass index (BMI) is also an important factor, with U-shaped relationships reported between BMI z-scores and poor sleep quality. These factors are found to be related to family and household function, with household “chaos” reported to be associated with less physical activity, less sleep and more screen time in households that have elevated stimulation, lack of structure and reduced predictability in activities and routines (132–134).
Children with NDDs and CHCs, and their families, are additionally vulnerable to the effects of personal factors, with impact on related factors which affect their sleep and thermoregulation. A particular concern is the effect of caring for a child with high support needs on caregiver health and wellbeing. Poor child sleep has a strong association with poor parental sleep, with associated impairment in physical and mental health, including an increased risk of cardiovascular and metabolic disease and a weakened immune system (135, 136).
The important interaction between sleep and thermoregulation is well known, as is the fact that children with NDDs and CHCs are more vulnerable than their peers to sleep disturbance and the associated deleterious effects. There is a dearth of studies reporting on thermoregulation and sleep in these groups. It is likely that this is because the questions have not been asked and studies not been done, rather than through absence of an important relationship. Despite the prevailing knowledge about the importance of distal temperatures in relation to sleep onset and maintenance, to our knowledge, there are no standardized or validated pediatric sleep questionnaires which ask about children’s temperatures before and during the sleep period. In their recent overview of 70 pediatric sleep tools, Sen and Spruyt (137), describe an extensive range of conditions which are considered, including sleep disordered breathing, morning symptoms, nighttime awakenings, insomnia, excessive daytime sleepiness, daytime behavior, sleep habits and irregular/delayed sleep phase sleep routine, bedtime anxiety, morning tiredness, night arousals, sleep disordered breathing, and restlessness before and during sleep. They note that there is an emerging need for further research into children’s sleep, with tools which assess sleep ecology, routines and hygiene, regularity, and treatment. Concerning such research, it is notable that the Children’s Sleep Habits Questionnaire [CSHQ; (138)], one of the most widely used questionnaires to assess sleep problems in children, has one question which could pertain to body temperature regulation (“awakens screaming, sweating”), as part of the subscale of parasomnias. It does not include questions about children’s observed patterns of body temperature or thermal comfort before and during the sleep period. Similarly, another widely used pediatric sleep questionnaire, the Sleep Disorders Scale for Children [SDSC; (139, 140)] includes 2 questions, specifically about perspiration before sleep and during sleep, to give a score relating to functions of sweating (reported as the domain SHY, sleep hyperhydrosis), with no option to provide information about patterns of skin temperatures or thermal comfort before or during sleep. Further to this, we note, with interest, one recent study (examining sleep hygiene factors in young children with and without ASD) which includes thermal comfort variables. For this study, Richdale and Schreck (141) developed a questionnaire which included a section on Thermal Sleep Environment description, with 16 items which asked about the child’s typical bedding, sleep wear and sleep environment. Parents were asked about the use of extra stimuli to keep warm (e.g., hot water bottle, hat, socks), and if the child was too cold or hot at night in relation to warm-hot/cool-cold weather or summer/winter.
Given the clear relationship between sleep and thermoregulation, the vulnerability of children with NDDs and CHCs to difficulties with sleep and thermoregulation, and the known interplay between domains of body structure and function, activities and environments on sleep and thermoregulation, it is clear that there is broad scope for further relevant research, to guide possibly valuable clinical applications (Table 1).
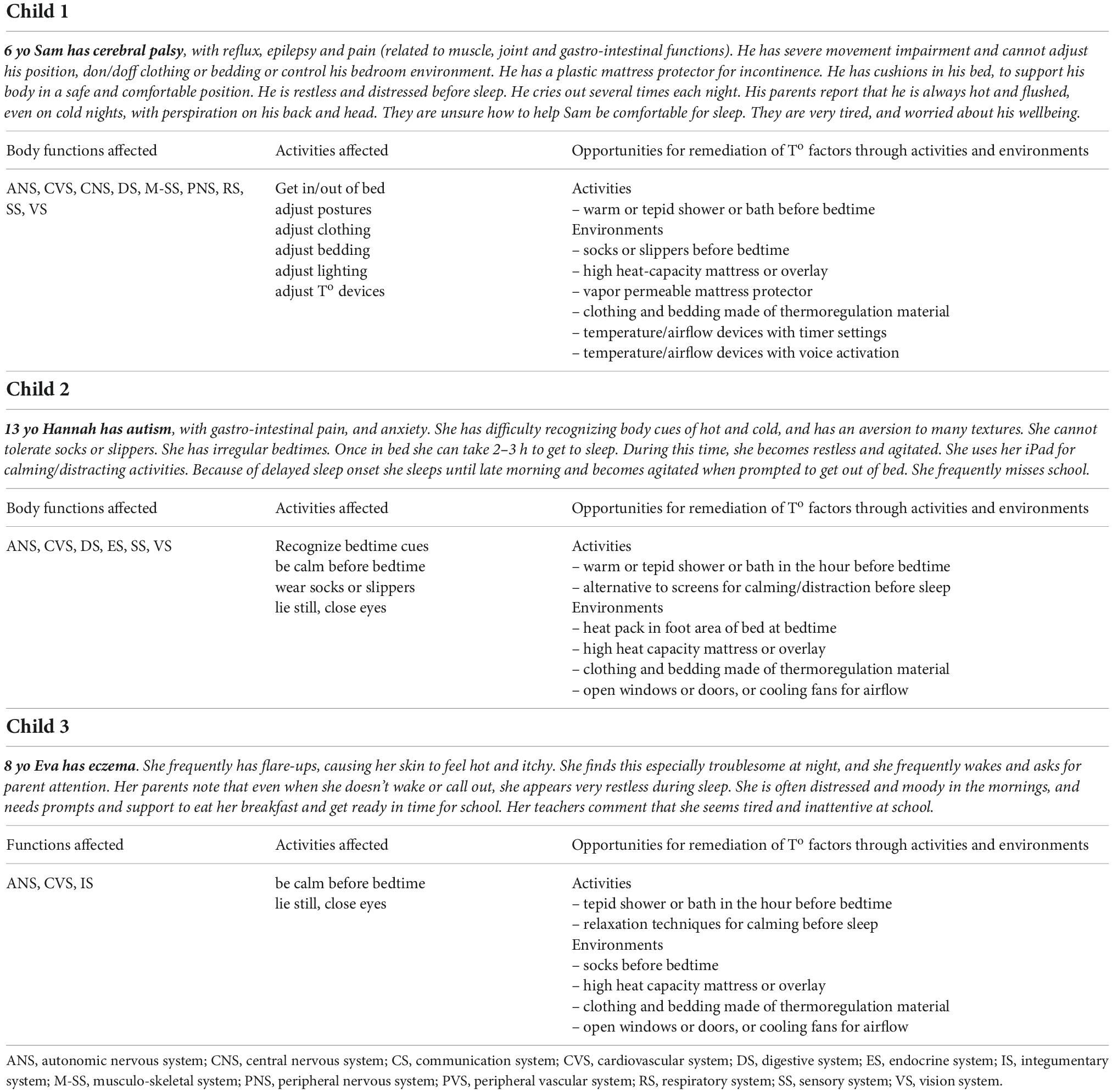
Table 1. Case descriptions of children with neuromotor, developmental and chronic health conditions, illustrating the interactions of body structures and function for thermoregulation and sleep, with opportunities for therapeutic modification to activities and environments.
The purpose of this translational review is to draw attention to the functional links between sleep and thermoregulation, and to highlight the important implications for the health and wellbeing of children with neurodevelopmental and CHCs. Currently, there are missing links between the knowledge that exists regarding the importance of various aspects of sleep (onset, maintenance, architecture, rhythms, subjective quality, daytime sleepiness) for diverse pediatric populations, and the knowledge that exists regarding thermoregulation and thermal comfort in relation to these same aspects of sleep. When viewed in context of body structures and functions, activities and participation, and environment, there is seemingly boundless scope for targeted research, to promote understanding about practical, ecological assessment of thermoregulatory functions, and related interventions to support good sleep in these vulnerable populations.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
SM and VB conceptualized, drafted, contributed, modeled the manuscript, and developed the models. CA and J-PL contributed to the manuscript. All authors have reviewed and approved the final draft.
The authors wish to acknowledge the financial support of University of Picardie Jules Verne (S2R program) and Developmental Occupational Therapy Western Australia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Atkinson G, Reilly T, Waterhouse J. Chronobiological aspects of the sleep-wake cycle and thermoregulation. Physiol Behav. (2007) 90:189. doi: 10.1016/j.physbeh.2006.09.011
2. Cerri M, Amici R. Thermoregulation and sleep: functional interaction and central nervous control. Compr Physiol. (2021) 11:1591–604.
3. Krauchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Front Biosci. (2010) 15:604–25. doi: 10.2741/3636
4. Morrison SF, Nakamura K. Central mechanisms for thermoregulation. Annu Rev Physiol. (2019) 81:285–308. doi: 10.1146/annurev-physiol-020518-114546
5. Ebben MR, Spielman AJ. The effects of distal limb warming on sleep latency. Int J Behav Med. (2006) 13:221–8. doi: 10.1207/s15327558ijbm1303_5
6. Fronczek R, Raymann R, Romeijn N, Overeem S, Fischer M, van Dijk J, et al. Manipulation of core body and skin temperature improves vigilance and maintenance of wakefulness in narcolepsy. Sleep. (2008) 31:233–40.
7. Reid KJ, Kräuchi K, Grimaldi D, Sbarboro J, Attarian H, Malkani R, et al. Effects of manipulating body temperature on sleep in postmenopausal women. Sleep Med. (2021) 81:109–15. doi: 10.1016/j.sleep.2021.01.064
8. Romeijn N, Raymann R, Møst E, Te Lindert B, Van Der Meijden WP, Fronczek R, et al. Sleep, vigilance, and thermosensitivity. Eur J Physiol. (2012) 463:169–76. doi: 10.1007/s00424-011-1042-2
9. Combs D, Goodwin JL, Quan SF, Morgan WJ, Shetty S. Insomnia, health-related quality of life and health outcomes in children: a seven year longitudinal cohort. Sci Rep. (2016) 6:27921. doi: 10.1038/srep27921
10. Short MA, Gradisar M, Lack LC, Wright HR. The impact of sleep on adolescent depressed mood, alertness and academic performance. J Adolesc. (2013) 36:1025–33. doi: 10.1016/j.adolescence.2013.08.007
11. Hill C, Hogan A, Karmiloff-Smith A. To sleep, perchance to enrich learning?. Arch Dis Child. (2007) 92:637–43. doi: 10.1136/adc.2006.096156
12. Holdaway AS, Becker SP. Children’s sleep problems are associated with poorer student–teacher relationship quality. Sleep Med. (2018) 47:100–5. doi: 10.1016/j.sleep.2017.12.001
13. Holley S, Hill CM, Stevenson J. An hour less sleep is a risk factor for childhood conduct problems. Child Care Health Dev. (2011) 37:563–70. doi: 10.1111/j.1365-2214.2010.01203.x
14. Spruyt K. A review of developmental consequences of poor sleep in childhood. Sleep Med. (2018) 60:3–12. doi: 10.1016/j.sleep.2018.11.021
15. Bourke-Taylor H, Pallant JF, Law M, Howie L. Relationships between sleep disruptions, health and care responsibilities among mothers of school-aged children with disabilities. J Paediatr Child Health. (2013) 49:775–82. doi: 10.1111/jpc.12254
16. Chu J, Richdale AL. Sleep quality and psychological wellbeing in mothers of children with developmental disabilities. Res Dev Disabil. (2009) 30:1512–22. doi: 10.1016/j.ridd.2009.07.007
17. Dan B. Sleep as therapy in neurodevelopmental disorders. Dev Med Child Neurol. (2018) 60:434. doi: 10.1111/dmcn.13728
18. Verschuren O, Gorter JW, Pritchard-Wiart L. Sleep: an underemphasized aspect of health and development in neurorehabilitation. Early Hum Dev. (2017) 113:120–8. doi: 10.1016/j.earlhumdev.2017.07.006
19. Horwood L, Li P, Mok E, Oskoui M, Shevell M, Constantin E. Behavioral difficulties, sleep problems, and nighttime pain in children with cerebral palsy. Res Dev Disabil. (2019) 95:103500. doi: 10.1016/j.ridd.2019.103500
20. Hulst RY, Gorter JW, Voorman JM, Kolk E, Van Der Vossen S, Visser-Meily JMA, et al. Sleep problems in children with cerebral palsy and their parents. Dev Med Child Neurol. (2021) 63:1344–50. doi: 10.1111/dmcn.14920
21. Schreck KA, Richdale AL. Sleep problems, behavior, and psychopathology in autism: inter-relationships across the lifespan. Curr Opin Psychol. (2020) 34:105–11. doi: 10.1016/j.copsyc.2019.12.003
22. Tietze A, Blankenburg M, Hechler T, Michel E, Koh M, Schlüter B, et al. Sleep disturbances in children with multiple disabilities. Sleep Med Rev. (2012) 16:117–27. doi: 10.1016/j.smrv.2011.03.006
23. Svedberg LE, Stener-Victorin E, Nordahl G, Lundeberg T. Skin temperature in the extremities of healthy and neurologically impaired children. Eur J Paediatr Neurol. (2005) 9:347–54. doi: 10.1016/j.ejpn.2005.06.001
24. Atmawidjaja RW, Wong SW, Yang WW, Ong LC. Sleep disturbances in Malaysian children with cerebral palsy. Dev Med Child Neurol. (2014) 56:681–5. doi: 10.1111/dmcn.12399
25. Zuculo GM, Knap CCF, Pinato L. Correlation between sleep and quality of life in cerebral palsy. Codas. (2014) 26:447–56. doi: 10.1590/2317-1782/20140201435
26. Santos JS, Giacheti CM, Dornelas LS, Silva NC, Souza ALDM, Guissoni Campos LM, et al. Day/night melatonin content in cerebral palsy. Neurosci Lett. (2018) 686:23–7. doi: 10.1016/j.neulet.2018.08.045
27. McCabe S, Blackmore AM, Abbiss C, Langdon K, Elliott C. Sleep concerns in children and young people with cerebral palsy in their home setting. J Paediatr Child Health. (2015) 51:1188–94. doi: 10.1111/jpc.12933
28. World Health Organization [WHO]. The International Classification of Functioning: An Overview. Geneva: World Health Organization (2010).
29. Schiariti V, Longo E, Shoshmin A, Kozhushko L, Besstrashnova Y, Król M, et al. Implementation of the International classification of functioning, disability, and health (ICF) core sets for children and youth with cerebral palsy: global initiatives promoting optimal functioning. Int J Environ Res Public Health. (2018) 15:1899. doi: 10.3390/ijerph15091899
30. Mishra AK, Loomans MG, Hensen JL. Thermal comfort of heterogeneous and dynamic indoor conditions – An overview. Build Environ. (2016) 109:82–100. doi: 10.1016/j.buildenv.2016.09.016
31. Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol. (2014) 210:498–507. doi: 10.1111/apha.12231
32. Tan CL, Knight ZA. Regulation of Body Temperature by the Nervous System. Neuron. (2018) 98:31–48. doi: 10.1016/j.neuron.2018.02.022
33. Katic K, Li R, Zeiler W. Thermophysiological models and their applications?: a review. Build Environ. (2016) 106:286–300. doi: 10.1016/j.buildenv.2016.06.031
34. Peeters L, de Dear R. Thermal comfort in residential buildings?: comfort values and scales for building energy simulation. Appl Energy. (2009) 86:772–80. doi: 10.1016/j.apenergy.2008.07.011
35. Schweiker M, Huebner GM, Kingma BRM, Kramer R, Pallubinsky H. Drivers of diversity in human thermal perception – A review for holistic comfort models. Temperature. (2018) 5:308–42. doi: 10.1080/23328940.2018.1534490
36. Schweiker M, Ampatzi E, Andargie MS, Andersen RK, Azar E, Barthelmes VM, et al. Review of multi-domain approaches to indoor environmental perception and behaviour. Build Environ. (2020) 176:106804. doi: 10.1016/j.buildenv.2020.106804
37. Bach V, Telliez F, Chardon K, Tourneux P, Cardot V, Libert J-P. Thermoregulation in wakefulness and sleep in humans. Handb Clin Neurol. (2011) 98:215–27.
38. Inoue Y, Kuwahara T, Araki T. Maturation- and aging-related changes in heat loss effector function. J Physiol Anthropol Appl Hum Sci. (2004) 23:289–94. doi: 10.2114/jpa.23.289
39. Inoue Y, Ichinose-Kuwahara T, Nakamura S, Ueda H, Yasumatsu H, Kondo N, et al. Cutaneous vasodilation response to a linear increase in air temperature from 28°C to 40°C in prepubertal boys and young men. J Physiol Anthropol. (2009) 28:137–44. doi: 10.2114/jpa2.28.137
40. Rupp R, Vasquez N, Lamberts R. A review of human thermal comfort in the built environment. Energy Build. (2015) 105:178–205. doi: 10.1016/j.enbuild.2015.07.047
41. Lack L, Gradisar M, Van Someren E, Wright H, Lushington K. The relationship between insomnia and body temperatures. Sleep Med Rev. (2008) 12:307–17.
42. van den Heuvel C, Noone J, Lushington K, Dawson D. Changes in sleepiness and body temperature precede nocturnal sleep onset?: evidence from a polysomnographic study in young men. J Sleep Res. (1998) 7:159–66.
43. Walløe L. Arterio-venous anastomoses in the human skin and their role in temperature control. Temperature. (2016) 3:92–103. doi: 10.1080/23328940.2015.1088502
44. Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Warm feet promote the rapid onset of sleep. Nature. (1999) 5477:36–7.
45. Haghayegh S, Khoshnevis S, Smolensky MH, Diller KR, Castriotta RJ. Before-bedtime passive body heating by warm shower or bath to improve sleep: a systematic review and meta-analysis. Sleep Med Rev. (2019) 46:124–35. doi: 10.1016/j.smrv.2019.04.008
46. Ko Y, Lee J. Effects of feet warming using bed socks on sleep quality and thermoregulatory responses in a cool environment. J Physiol Anthropol. (2018) 37:13. doi: 10.1186/s40101-018-0172-z
47. Kräuchi K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol Behav. (2007) 90:236–45. doi: 10.1016/j.physbeh.2006.09.005
48. Raymann R, Swaab D, Van Someren E. Skin deep: enhanced sleep depth by cutaneous temperature manipulation. Brain. (2008) 131:500–13. doi: 10.1093/brain/awm315
49. Ichiba T, Suzuki M, Aritake-Okada S, Uchiyama M. Periocular skin warming elevates the distal skin temperature without affecting the proximal or core body temperature. Sci Rep. (2019) 9:5743. doi: 10.1038/s41598-019-42116-x
50. Haghayegh S, Khoshnevis S, Smolensky MH, Hermida RC, Castriotta RJ, Schernhammer E, et al. Novel temperature-controlled sleep system to improve sleep: a proof-of-concept study. J Sleep Res. (2022). [Epub ahead of print]. doi: 10.1111/jsr.13662
51. Edwards SJ, Montgomery IM, Colquhoun EQ, Jordan JE, Clark MG. Spicy meal disturbs sleep: an effect of thermoregulation? Int J Psychophysiol. (1992) 13:97–100.
52. Bach V, Libert J-P. Thermoregulation and Metabolism. In: Gozal D, Kheirandish-Gozal L editors. Pediatric Sleep Medecine. Cham: Springer (2021). doi: 10.1007/978-3-030-65574-7_6
53. Kräuchi K, Cajochen C, Pache M, Flammer J, Wirz-Justice A. Thermoregulatory effects of melatonin in relation to sleepiness. Chronobiol Int. (2006) 23:475–84. doi: 10.1080/07420520500545854
54. Abe N, Kodama H. Distal-proximal skin temperature gradient prior to sleep onset in infants for clinical use. Pediatr Int. (2015) 57:227–33. doi: 10.1111/ped.12473
55. Barcat L, Decima P, Bodin E, Delanaud S, Stephan-Blanchard E, Bach V. Distal skin vasodilation promotes rapid sleep onset in preterm neonates. J Sleep Res. (2017) 26:572–7. doi: 10.1111/jsr.12514
56. McCabe S, Elliott C, Langdon K, Abbiss C. Patterns and reliability of children’s skin temperature prior to and during sleep in the home setting. Physiol Behav. (2018) 194:292–301. doi: 10.1016/j.physbeh.2018.06.005
57. Romeijn N, Van Someren EJW. Correlated fluctuations of daytime skin temperature and vigilance. J Biol Rhythms. (2011) 26:68–77. doi: 10.1177/0748730410391894
58. Okamoto-Mizuno K, Mizuno K, Shirakawa S. Sleep and skin temperature in preschool children and their mothers. Behav Sleep Med. (2016) 16:64–78. doi: 10.1080/15402002.2016.1173552
59. Bach V, Abbiss CR, Libert J-P, McCabe SM. Skin temperatures of back or neck are better than abdomen for indication of average proximal skin temperature during sleep of school- aged children. Front Psychiatry. (2020) 11:494528. doi: 10.3389/fpsyt.2020.494528
60. Herberger S, Kräuchi K, Glos M, Lederer K, Assmus L, Hein J, et al. Effects of sleep on a high-heat capacity mattress on sleep stages, EEG power spectra, cardiac interbeat intervals and body temperatures in healthy middle-aged men. Sleep. (2020) 43:zsz271. doi: 10.1093/sleep/zsz271
61. Kräuchi K, Fattori E, Giordano A, Falbo M, Iadarola A, Aglì F, et al. Sleep on a high heat capacity mattress increases conductive body heat loss and slow wave sleep. Physiol Behav. (2018) 185:23–30. doi: 10.1016/j.physbeh.2017.12.014
62. Lan L, Qian X, Lian Z, Lin Y. Local body cooling to improve sleep quality and thermal comfort in a hot environment. Indoor Air. (2017) 28:135–45. doi: 10.1111/ina.12428
63. Kräuchi K, Wirz-Justice A. Circadian clues to sleep onset mechanisms. Neuropsychopharmacology. (2001) 25:S92–5.
64. Park S-J, Waterhouse J. A comparison between rhythms in forehead skin and rectal (core) temperature in sedentary subjects living in a thermally neutral environment. Biol Rhythm Res. (2013) 45:415–28. doi: 10.1080/09291016.2013.830849
65. Okamoto-Mizuno K, Tsuzuki K. Effects of season on sleep and skin temperature in the elderly. Int J Biometeorol. (2010) 54:401–9. doi: 10.1007/s00484-009-0291-7
66. Bach V, Telliez F, Libert J-P. The interaction between sleep and thermoregulation in adults and neonates. Sleep Med Rev. (2002) 6:481–92.
68. Batinga H, Martinez-Nicolas A, Zornoza-Moreno M, Sánchez-Solis M, Larqué E, Mondéjar MT, et al. Ontogeny and aging of the distal skin temperature rhythm in humans. Age. (2015) 37:29. doi: 10.1007/s11357-015-9768-y
69. Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J Physiol Anthropol. (2012) 31:14.
70. Lan L, Tsuzuki K, Liu YF, Lian ZW. Thermal environment and sleep quality: a review. Energy Build. (2017) 149:101–13. doi: 10.1016/j.enbuild.2017.05.043
71. Caddick ZA, Gregory K, Arsintescu L, Flynn-Evans EE. A review of the environmental parameters necessary for an optimal sleep environment. Build Environ. (2018) 132:11–20. doi: 10.1016/j.buildenv.2018.01.020
72. Wang Y, Liu Y, Song C, Liu J. Appropriate indoor operative temperature and bedding micro climate temperature that satisfies the requirements of sleep thermal comfort. Build Environ. (2015) 92:20–9. doi: 10.1016/j.buildenv.2015.04.015
73. Califano R, Naddeo A, Vink P. The effect of human-mattress interface’s temperature on perceived thermal comfort. Appl Ergon. (2017) 58:334–41. doi: 10.1016/j.apergo.2016.07.012
74. Chiba S, Yagi T, Ozone M, Matsumura M, Sekiguchi H, Ganeko M, et al. High rebound mattress toppers facilitate core body temperature drop and enhance deep sleep in the initial phase of nocturnal sleep. PLoS One. (2018) 13:e0197521. doi: 10.1371/journal.pone.0197521
75. Li X, Shen L, Califano R. The comparative study of thermal comfort and sleep quality for innovative designed mattress in hot weather. Sci Technol Built Environ. (2020) 26:643–57. doi: 10.1080/23744731.2020.1720445
76. Ikeda R, Fukai K. Effects of different bed sheets on bed climate and thermal response. Jpn J Nurs Sci. (2005) 2:51–5.
77. Fletcher A, van den Heuvel C, Dawson D. Sleeping with an electric blanket: effects on core temperature, sleep, and melatonin in young adults. Sleep. (1999) 22:313–8.
78. Okamoto-Mizuno K, Tsuzuki K, Ohshiro Y, Mizuno K. Effects of an electric blanket on sleep stages and body temperature in young men. Ergonomics. (2005) 48:749–57. doi: 10.1080/00140130500120874
79. Shen L, Chen Y, Guo Y, Zhong S, Fang F, Zhao J, et al. Research on the relationship between the structural properties of bedding layer in spring mattress and sleep quality. Work. (2012) 41:1268–73. doi: 10.3233/WOR-2012-0312-1268
80. Worsley PR, Bader DL. A modified evaluation of spacer fabric and airflow technologies for controlling the microclimate at the loaded support interface. Text Res J. (2019) 89:2154–62. doi: 10.1177/0040517518786279
81. Park S-J. Effects of two types of clothing offering different thermal insulation to the extremities upon nocturnal secretion of urinary 6-sulfatoxymelatonin and sleep propensity. Biol Rhythm Res. (2013) 44:885–96. doi: 10.1080/09291016.2013.780699
82. Angriman M, Caravale B, Novelli L, Ferri R, Bruni O. Sleep in children with neurodevelopmental disabilities. Neuropediatrics. (2015) 46:199–210.
83. Heussler H, Hiscock H. Sleep in children with neurodevelopmental disabilities. J Paediatr Child Health. (2018) 54:1142–7.
84. Allen J, Zareen Z, Doyle S, Whitla L, Afzal Z, Stack M, et al. Multi-organ dysfunction in cerebral palsy. Front Pediatr. (2021) 9:668544. doi: 10.3389/fped.2021.668544
85. Salt A, Sargent J. Common visual problems in children with disability. Arch Dis Child Educ Pract Ed. (2014) 99:1163–8. doi: 10.1136/archdischild-2013-305267
86. Jan JE, Asante KO, Conry JL, Fast DK, Bax MCO, Ipsiroglu OS, et al. Sleep health issues for children with FASD: clinical considerations. Int J Pediatr. (2010) 2010:639048. doi: 10.1155/2010/639048
87. Kushki A, Drumm E, Pla Mobarak M, Tanel N, Dupuis A, Chau T, et al. Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS One. (2013) 8:e59730. doi: 10.1371/journal.pone.0059730
88. Spruyt K, Gozal D. Sleep disturbances in children with attention-deficit/hyperactivity disorder. Expert Rev Neurother. (2011) 11:565–77. doi: 10.1586/ern.11.7
89. Gagnon K, Godbout R. Melatonin and Comorbidities in Children with Autism Spectrum Disorder. Curr Dev Disord Rep. (2018) 5:197–206. doi: 10.1007/s40474-018-0147-0
90. Camfferman D, Short MA, Kennedy JD, Gold M, Kohler M, Lushington K. Thermoregulation, scratch, itch and sleep deficits in children with eczema. Sleep Med. (2016) 25:145–50. doi: 10.1016/j.sleep.2016.06.011
91. Meltzer L, Moore M. Sleep disruptions in parents of children and adolescents with chronic illnesses: prevalence, causes, and consequences. J Pediatr Psychol. (2008) 33:279–91. doi: 10.1093/jpepsy/jsm118
92. Gupta MA, Gupta AK. Sleep-wake disorders and dermatology. Clin Dermatol. (2013) 31:118–26. doi: 10.1016/j.clindermatol.2011.11.016
93. Mayes T, Gottschlich MM, Khoury J, McCall J, Simakajornboon N, Kagan RJ. A pilot review of the long-term impact of burn injury on sleep architecture in children. J Burn Care Res. (2013) 34:15–21. doi: 10.1097/BCR.0b013e318272178e
94. Rose M, Sanford A, Thomas C, Opp MR. Factors altering the sleep of burned children. Sleep. (2001) 24:45–51. doi: 10.1093/sleep/24.1.45
95. Rutkove S, Veves A, Mitsa T, Nie R, Fogerson P, Garmirian L, et al. Impaired distal thermoregulation in diabetes and diabetic polyneuropathy. Diabetes Care. (2009) 32:671–6. doi: 10.2337/dc08-1844
96. Calhoun S, Vgontzas AN, Fernandez-Mendoza J, Mayes SD, Tsaoussoglou M, Basta M, et al. Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: the role of obesity, asthma, anxiety/depression, and sleep. Sleep. (2011) 34:503–7.
97. Kamara D, Beauchaine T. A review of sleep disturbances among infants and children with neurodevelopmental disorders. Rev J Autism Dev Disord. (2020) 7:278–94. doi: 10.1007/s40489-019-00193-8.A
98. Kawada T. Accuracy of wrist accelerometer during nap sleep in subjects with a variety of sleep efficiencies. J Pain Symptom Manag. (2011) 42:e9–10. doi: 10.1016/j.jpainsymman.2011.04.002
99. Silva-Rodrigues FM, de Lucca M, Leite ACAB, de Andrade Alvarenga W, Nunes MDR, Nascimento LC. Management of chemotherapy-related symptoms in children and adolescents: family caregivers’ perspectives. Rev Esc Enferm. (2021) 55:e20200484. doi: 10.1590/1980-220X-REEUSP-2020-0484
100. Martinez-Nicolas A, Madrid JA, Rol MA. Day-night contrast as source of health for the human circadian system. Chronobiol Int. (2014) 31:382–93. doi: 10.3109/07420528.2013.861845
101. Martin CA, Papadopoulos N, Chellew T, Rinehart NJ, Sciberras E. Associations between parenting stress, parent mental health and child sleep problems for children with ADHD and ASD: systematic review. Res Dev Disabil. (2019) 93:103463. doi: 10.1016/j.ridd.2019.103463
102. Larsen KL, Erp LA, Jordan M, Jordan SS. Bedtime routines of young children, parenting stress, and bedtime resistance: mediation models. Child Psychiatry Hum Dev. (2021). [Epub ahead of print]. doi: 10.1007/s10578-021-01275-7
103. Atkinson G, Davenne D. Relationships between sleep, physical activity and human health. Physiol Behav. (2007) 90:229–35. doi: 10.1016/j.physbeh.2006.09.015
104. Hulst RY, Willem J, Obeid J, Voorman JM, Van Rijssen IM, Gerritsen A, et al. Accelerometer measured physical activity, sedentary behavior, and sleep in children with cerebral palsy and their adherence to the hour activity guidelines. Dev Med Child Neurol. (2022). [Epub ahead of print]. doi: 10.1111/dmcn.15338
105. Sato H, Iwasaki T, Yokoyama M, Inoue T. Monitoring of body position and motion in children with severe cerebral palsy for 24 hours. Disabil Rehabil. (2014) 36:1156–60. doi: 10.3109/09638288.2013.833308
106. Smit DJM, Zwinkels M, Takken T, Hulst RY, de Groot JF, Lankhorst K, et al. Sleep quantity and its relation with physical activity in children with cerebral palsy; insights using actigraphy. J Paediatr Child Health. (2020) 56:1618–22. doi: 10.1111/jpc.15055
107. van der Heijden KB, Stoffelsen RJ, Popma A, Swaab H. Sleep, chronotype, and sleep hygiene in children with attention-deficit/hyperactivity disorder, autism spectrum disorder, and controls. Eur Child Adolesc Psychiatry. (2018) 27:99–111. doi: 10.1007/s00787-017-1025-8
108. Wiebe S, Carrier J, Frenette S, Gruber R. Sleep and sleepiness in children with attention deficit / hyperactivity disorder and controls. J Sleep Res. (2013) 22:41–9. doi: 10.1111/j.1365-2869.2012.01033.x
109. Fitzgerald D, Follett J, Van Asperen P. Assessing and managing lung disease and sleep disordered breathing in children with cerebral palsy. Paediatr Respir Rev. (2009) 10:18–24. doi: 10.1016/j.prrv.2008.10.003
110. Mahant S, Friedman JN, Connolly B, Goia C, Macarthur C. Tube feeding and quality of life in children with severe neurological impairment. Arch Dis Child. (2009) 94:668–73. doi: 10.1136/adc.2008.149542
111. Frank S, Gonzalez K, Lee-Ang L, Young M, Tamez M, Mattei J. Diet and sleep physiology: public health and clinical implications. Front Neurol. (2017) 8:393. doi: 10.3389/fneur.2017.00393
112. Lundberg J, Carlstrom M, Weitzberg E. Metabolic effects of eietary nitrate in health and disease. Cell Metab Rev. (2018) 28:9–22.
113. McHill AW, Smith BJ, Wright KP. Effects of caffeine on skin and core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythms. (2014) 29:131–43. doi: 10.1177/0748730414523078
114. Penagini F, Mameli C, Fabiano V, Brunetti D, Dilillo D, Zuccotti GV. Dietary intakes and nutritional issues in neurologically impaired children. Nutrients. (2015) 7:9400–15. doi: 10.3390/nu7115469
115. Smith B, Rogers SL, Blissett J, Ludlow AK. The relationship between sensory sensitivity, food fussiness and food preferences in children with neurodevelopmental disorders. Appetite. (2020) 150:104643. doi: 10.1016/j.appet.2020.104643
116. de Bock M, Lobley K, Anderson D, Davis E, Donaghue K, Pappas M, et al. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: an illustrative case series. Pediatr Diabetes. (2018) 19:129–37. doi: 10.1111/pedi.12527
117. Filiz S, Keleş S, Akbulut UE, Işık A, Kara MZ. Sleep disturbances and affecting factors in young children with food allergy and their mothers. Allergol Immunopathol. (2020) 48:158–64. doi: 10.1016/j.aller.2019.06.014
118. Salah NY, Abido AY, Rashed HR. Relationship of glycaemic derangement using continuous glucose monitoring system with sleep pattern among children with type 1 diabetes. Diabetes Metab Res Rev. (2021) 37:e3407. doi: 10.1002/dmrr.3407
119. Baddock SA, Galland BC, Beckers MG, Taylor BJ, Bolton DP. Bed-sharing and the infant’s thermal environment in the home setting. Arch Dis Child. (2004) 89:1111–6. doi: 10.1136/adc.2003.048082
120. Shin M, Halaki M, Swan P, Ireland A, Chow C. The effects of fabric for sleepwear and bedding on sleep at ambient temperatures of 17°C and 22°C. Nat Sci Sleep. (2016) 8:121–31.
121. Mondal S. Phase change materials for smart textiles – an overview. Appl Therm Eng. (2008) 28:1536–50. doi: 10.1016/j.applthermaleng.2007.08.009
122. Mahar TJ, Halaki M, Ireland A. The impact of sleepwear fiber type on sleep quality under warm ambient conditions. Nat Sci Sleep. (2019) 11:167–78.
123. Carrier J, Dumont M. Sleep propensity and sleep architecture after bright light exposure at three different times of day. J Sleep Res. (1995) 4:202–11.
124. Martinez-Nicolas A, Ortiz-Tudela E, Madrid JA, Rol MA. Crosstalk between environmental light and internal time in humans. Chronobiol Int. (2011) 28:617–29. doi: 10.3109/07420528.2011.593278
125. Tähkämö L, Partonen T, Pesonen AK. Systematic review of light exposure impact on human circadian rhythm. Chronobiol Int. (2019) 36:151–70. doi: 10.1080/07420528.2018.1527773
126. te Kulve M, Schellen L, Schlangen L, van Marken Lichtenbelt W. The influence of light on thermal responses. Acta Physiol. (2016) 216:163–85. doi: 10.1111/apha.12552
127. Twenge JM, Hisler GC, Krizan Z. Associations between screen time and sleep duration are primarily driven by portable electronic devices: evidence from a population-based study of U.S. children ages 0–17. Sleep Med. (2019) 56:211–8. doi: 10.1016/j.sleep.2018.11.009
128. Logan RW, McClung CA. Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat Rev Neurosci. (2019) 20:49–65. doi: 10.1038/s41583-018-0088-y
129. Heaton J, Noyes J, Sloper P. The experiences of sleep disruption in families of technology-dependent children living at home. Child Soc. (2006) 20:196–208. doi: 10.1002/CHI.881
130. Humphreys G, King T, Jex J, Rogers M, Blake S, Thompson-Coon J, et al. Sleep positioning systems for children and adults with a neurodisability: a systematic review. Br J Occup Ther. (2019) 82:5–14. doi: 10.1177/0308022618778254
131. Galland B, Gray A, Penno J, Smith C, Lobb C, Taylor R. Gender differences in sleep hygiene practices and sleep quality in New Zealand adolescents aged 15 to 17 years. Sleep Health. (2017) 3:77–83. doi: 10.1016/j.sleh.2017.02.001
132. Kracht CL, Katzmarzyk PT, Staiano AE. Household chaos, family routines, and young child movement behaviors in the U.S. during the COVID-19 outbreak: a cross-sectional study. BMC Public Health. (2021) 21:860. doi: 10.1186/s12889-021-10909-3
133. Krupsky KL, Parrott A, Andridge R, Zvara BJ, Keim SA, Anderson SE. A mixed methods analysis of environmental and household chaos: considerations for early-childhood obesity research. BMC Public Health. (2021) 21:1867. doi: 10.1186/s12889-021-11936-w
134. Spilsbury JC, Patel SR, Morris N, Ehayaei A, Intille SS. Household chaos and sleep-disturbing behavior of family members: results of a pilot study of African American early adolescents. Sleep Health. (2017) 3:84–9. doi: 10.1016/j.sleh.2016.12.006
135. Bin Eid W, Lim M, Gabrieli G, Kölbel M, Halstead E, Esposito G, et al. Alterations in cortisol profiles among mothers of children with ASD related to poor child sleep quality. Healthcare. (2022) 10:666. doi: 10.3390/healthcare10040666
136. Halstead EJ, Jones A, Esposito G, Dimitriou D. The moderating role of parental sleep knowledge on children with developmental disabilities and their parents’ sleep. Int J Environ Res Public Health. (2021) 18:746. doi: 10.3390/ijerph18020746
137. Sen T, Spruyt K. Pediatric sleep tools: an updated literature review. Front Psychiatry. (2020) 11:317. doi: 10.3389/fpsyt.2020.00317
138. Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. (2000) 23:1043–51. doi: 10.1111/j.1469-8749.2001.tb00204.x
139. Bruni O, Ottaviano S, Guidetti V, Romoli M, Innocenzi M, Cortesi F, et al. The Sleep Disturbance Scale for Children (SDSC): construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. (1996) 5:251–61.
140. Romeo D, Bruni O, Brogna C, Ferri R, Galluccio C, De Clemente V, et al. Application of the Sleep Disturbance Scale for Children (SDSC) in preschool age. Eur J Paediatr Neurol. (2013) 17:374–82. doi: 10.1016/j.ejpn.2012.12.009
Keywords: thermoregulation, sleep, vigilance, children, disability, chronic health conditions
Citation: McCabe SM, Abbiss CR, Libert J-P and Bach V (2022) Functional links between thermoregulation and sleep in children with neurodevelopmental and chronic health conditions. Front. Psychiatry 13:866951. doi: 10.3389/fpsyt.2022.866951
Received: 31 January 2022; Accepted: 24 October 2022;
Published: 14 November 2022.
Edited by:
Linda J. Larson-Prior, University of Arkansas for Medical Sciences, United StatesReviewed by:
Matteo Cerri, University of Bologna, ItalyCopyright © 2022 McCabe, Abbiss, Libert and Bach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susan M. McCabe, c3VlLm1jY2FiZTdAZ21haWwuY29t; Véronique Bach, dmVyb25pcXVlLmJhY2hAdS1waWNhcmRpZS5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.