
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 07 April 2022
Sec. Neuroimaging
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.861881
This article is part of the Research TopicThe Neurobiology of Suicide: The 'Suicidal Brain'View all 11 articles
Objective: Bipolar disorder (BD) has a higher lifetime rate of suicide attempts (SA) than other psychiatric disorders. Furthermore, BD patients with SA (BD + S) are prone to a worse quality of life. However, the pathophysiology of BD + S is poorly understood. To further reveal the potential mechanisms of BD + S, abnormalities in peripheral plasma inflammatory cytokines and brain white matter (WM) in BD + S, as well as the correlation between them are investigated.
Methods: We tested the levels of TNF-α, IL-1β, and IL-6 in peripheral plasma and collected the diffusion tensor imaging (DTI) data from 14 BD + S, 24 BD patients without SA (BD-S), and 26 healthy controls (HCs). The three groups were matched by age and gender. The levels of TNF-α, IL-1β, and IL-6 were detected by Luminex multifactor detection technology, and the fractional anisotropy (FA) values were employed to depict the alterations of WM. Partial correlation analyses were conducted to detect correlations between levels of TNF-α, IL-1β, and IL-6 and changes of WM, and the relationships between severity of clinical symptoms, including scores of HAMD-17 and YMRS, and cytokine levels or FA values in all groups.
Results: For plasma inflammatory cytokines, there was no significant difference in their levels except for IL-6 among the three groups. Post-hoc analyses revealed that increased IL-6 level was only detected in BD + S (p < 0.05, Bonferroni correction). For DTI, BD + S showed specifically decreased FA in the bilateral middle cerebellar peduncle and the left superior corona radiata compared to BD-S and HCs (p < 0.05, Bonferroni correction). Additionally, both BD + S and BD-S groups revealed decreased FA in the bilateral body and genu of corpus callosum (CC) compared to HCs (p < 0.05, Bonferroni correction). No significant correlation between plasma inflammatory cytokines and WM integrity was found. In the BD + S group, we found negative correlation between the scores of YMRS and FA values of the left middle cerebellar peduncle (r = −0.74, p = 0.035).
Conclusion: The inflammation and impaired WM integrity may provide a scientific basis to understand the potential mechanisms of BD + S.
Among psychiatric disorders, bipolar disorder (BD) has the highest suicide incidence (1–4). A recent meta-analysis demonstrated that the lifetime prevalence of suicide attempts (SA) in BD was 33.9% (5), which is at least 20 times higher than the general population (6). Epidemiological studies also showed that approximately 20–60% of BD patients had attempted suicide in their lifetime, and approximately 4–19% died due to suicide (7). In addition, BD patients with SA (BD + S) have a worse quality of life (8) and are peculiarly prone to poor functional outcomes (9). However, the pathophysiology of suicide in BD continues to be poorly understood.
Increasing evidence suggests an important role of cell-mediated immune activation and chronic inflammation in the pathophysiology of BD and SA. For example, previous studies consistently showed both higher TNF-α and IL-6 levels were associated with BD (10–12). Similarly, abnormally higher levels of TNF-α, IL-1β, and IL-6 were shown in the SA population (13–15). However, limited research has focused on the immune system impairment of BD + S. To our knowledge, only one study has investigated plasma inflammatory cytokines of BD + S and found increased IL-1β expression (16). The above studies provide a perspective of immunology to understand BD + S.
Another common sign of abnormalities is in white matter (WM). Studies using diffusion tensor imaging (DTI), which is the commonly used neuroimaging method to investigate the impairment of WM (17), found that BD + S exhibited lower fractional anisotropy (FA) in the uncinate fasciculus (UF), the ventral frontal cortex, and the cerebellar regions than BD without SA (BD-S) and healthy controls (HCs) (18, 19). Additionally, lower FA in the orbital frontal cortex, the middle portion of the forceps minor, and the anterior and posterior portion of the right cingulum bundle were also found in BD + S compared to BD-S (20, 21). Furthermore, BD + S showed a smaller WM volume (22) and declined FA values in the corpus callosum (CC), which may be related to suicidality (23). In addition, increased white matter hyperintensities have been consistently reported in BD + S (24–27). In summary, BD + S may have the impairment of WM microstructure.
Moreover, peripheral plasma inflammatory cytokines are closely related to the aberrant WM (28–30). They could pass the blood-brain barrier to activate microglia, which may impair brain cells, including the myelin sheath – an essential component of WM (17). Although evidence has suggested the important roles of plasma inflammatory cytokines and WM impairment in BD + S, the complicated and interwoven relationship between plasma inflammatory cytokines and WM integrity in BD + S has rarely been investigated. Therefore, we combined the plasma levels of inflammatory cytokines with the WM microstructure method to explore the immunologic and neuroimaging changes in BD + S and the relationship between them to further reveal the potential mechanisms of BD + S.
Sixty-four subjects aged from 15 to 47 years old were included in the study. Among them, 14 were BD + S, 24 were BD-S, and 26 were HCs; the three groups were age and gender matched. BD patients were recruited from the Department of Psychiatry, the First Affiliated Hospital of China Medical University. HCs were recruited by advertisement. All subjects were provided with written informed consent after a detailed description of the study. If they were juveniles, further consent was provided by their parents/legal guardians. The study was authorized by the Institutional Review Board of the First Affiliated Hospital of China Medical University.
For adult patients, the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I Disorders (SCID-I) was used to determine whether the patients met the criteria of BD (31). For adolescent and child patients, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) was used to determine whether the patients met the criteria of BD (32). All the BD patients were free of any other Axis I and Axis II disorders. HCs did not have a history of Axis I or Axis II disorders themselves or in their first-degree relatives. The severity of clinical symptoms was evaluated by using the 17-item Hamilton Rating Scale for Depression (HAMD-17) (33) and the Young Mania Rating Scale (YMRS) (34).
In the study, SA was defined as a self-destructive act with the purpose to die at least one attempt in one’s lifetime (35). A self-made scale based on the definition of SA and excluded the self-injurious behavior without suicidal purpose. The reason for exclusion of the self-injurious behavior is that it may be a confounder to obscure the potential mechanism of SA.
Assessment of diagnosis, severity of symptoms, and SA were completed by at least one researcher who had passed a consistency test for clinical assessment.
Subjects were excluded if they were on anti-inflammatory medications or had any somatic diseases which may cause potential brain structural changes such as neurological disorders, uncontrolled hypertension, uncontrolled diabetes, substance or alcohol abuse, a history of head trauma resulting in more than 5 min of unconsciousness, or any magnetic resonance imaging (MRI) contraindications. All the subjects underwent a general physical examination which showed no evidence of ongoing infection.
Blood samples were collected between 10:00 a.m. and 2:00 p.m., with EDTA as an anticoagulant, following standard procedures. After centrifugation at 2,000 rpm for 10 min, the plasma samples were kept at −80°C for further analysis. The plasma inflammatory cytokine levels were measured by the immunoassay (Human Magnetic Luminex Assay, Human Premixed Multi-Analyte Kit, R&D Systems, Inc., Minneapolis, MN, United States). In this process, a Human magnetic premixed microparticle cocktail of antibodies (Kit Lot Number L120614) was used to magnetically label the samples.
MRI data were collected by the GE Signa HDX 3.0T scanner at the Department of Radiology, the First Affiliated Hospital of China Medical University. DTI scanning was performed using the following parameters: TR/TE = 17,000/86 ms, field of view = 24 cm × 24 cm, imaging matrix = 120 × 120, slice number = 65, slice thickness = 2 mm, slice spacing = 2 mm, acquired along 26 directions (25 with b = 1,000 s/mm2 and 1 with b = 0), and voxel size = 2 mm3. Subjects were told to close their eyes and relax while remaining awake throughout scanning.
Pipeline for Analyzing braiN Diffusion imAges software1 was used to process DTI data. For each subject, the voxel-wise diffusion tensor matrix was first constructed in the native space. The eigenvalues and eigenvectors were then yielded by diagonalizing this matrix. Based on these three eigenvalues, each subject’s voxel-wise FA map was calculated. All FA maps were non-linearly registered to the FMRIB58_FA template and normalized to the Montreal Neurological Institute (MNI) space. After that, a mean FA map was generated for all subjects. Finally, FA maps were smoothed using a Gaussian filter kernel of 6 mm full width at half maximum.
Diffusion tensor imaging data were analyzed using SPM8.2 We performed a one-way analysis of covariance (ANCOVA) with age and sex as covariates to examine the statistical differences in WM tracts among three groups. Statistical significance was determined by voxel p < 0.005 and cluster p < 0.05 [Gaussian random field (GRF) correction]. The FA values for each cluster with statistical differences were extracted.
We used IBM SPSS Statistics (version 22.0, Armonk, NY, United States) for Windows to analyze the demographic and clinical characteristics of subjects. The independent-samples t-test was utilized to compare the duration of illness between BD + S and BD-S. One-way analysis of variance (ANOVA) was used to compare age, education years, total scores of HAMD-17 and YMRS, and FA values among BD + S, BD-S, and HCs. Additionally, Chi-square tests were adopted to compare differences in gender, disease state, medication status, and subcategories of BD. We utilized ANCOVA with age and sex as covariates to examine the significant differences in plasma inflammatory cytokine levels across the three groups. Statistical significance was determined by p < 0.05. Partial correlation analyses with age, sex, and medication as covariates were adopted to evaluate correlations between the FA values and levels of TNF-α, IL-1β, and IL-6, and the relationships between severity of clinical symptoms, including scores of HAMD-17 and YMRS, and cytokine levels or FA values in BD + S, BD-S, and HC group, respectively.
There were no significant differences in age, gender, and education years among BD + S, BD-S, and HCs. Duration of illness, medication status, disease state, and subcategories had no significant differences between the two subgroups of BD. The two patient subgroups showed significant higher scores of HAMD-17 and YMRS than HCs, and no significant differences were found between BD + S and BD-S (Table 1).
For plasma inflammatory cytokine levels, there was a significant difference in IL-6 level (F = 6.02, p = 0.01) but no significant difference in IL-1β level (F = 0.81, p = 0.45) or TNF-α level (F = 0.87, p = 0.43) among these three groups. Post-hoc analyses found significantly increased IL-6 level in BD + S compared to BD-S and HCs (p < 0.05, Bonferroni correction) (Figure 1 and Table 1).
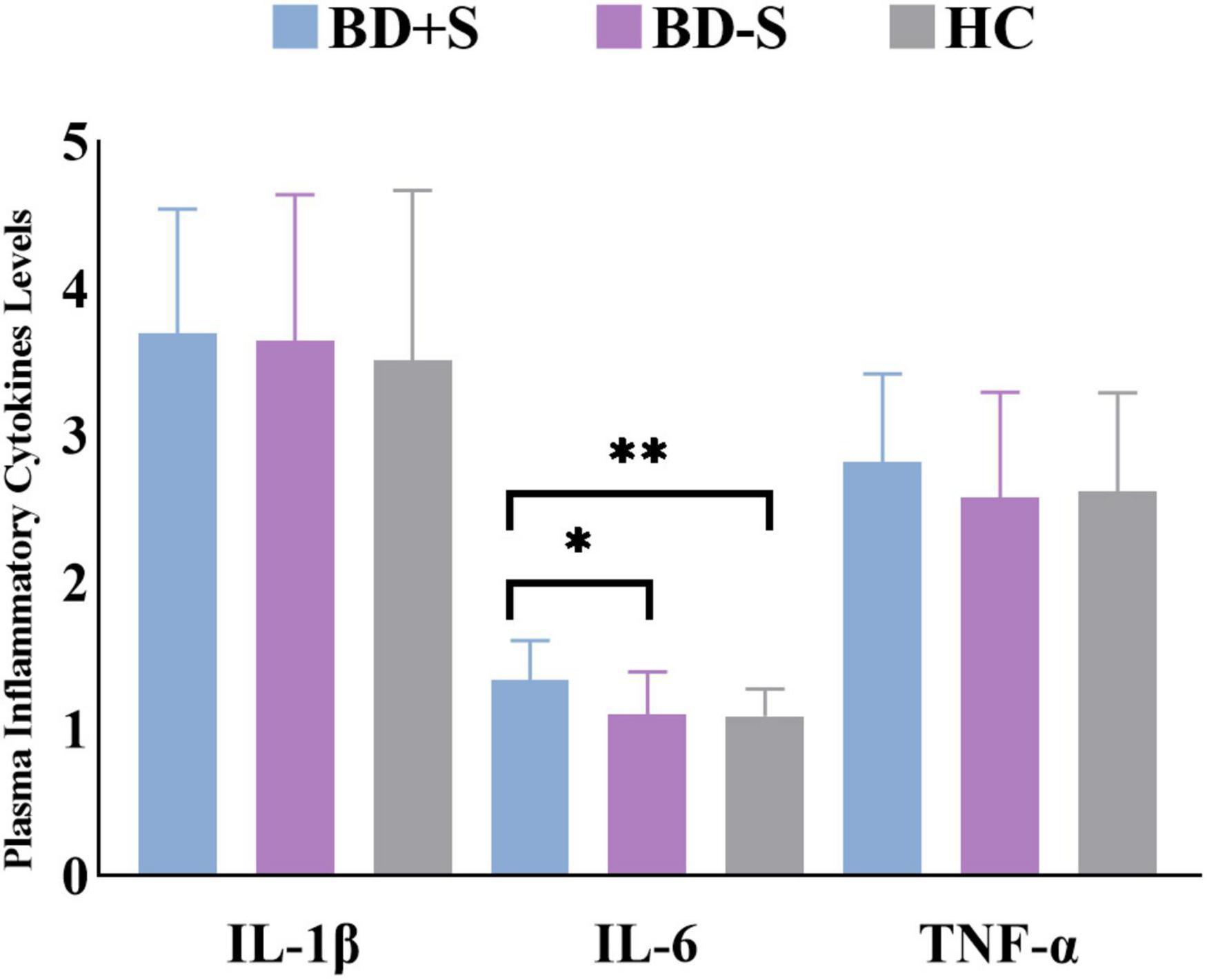
Figure 1. Levels of IL-1β, IL-6, and TNF-α in the BD + S, BD-S, and HC groups. ∗∗p < 0.01; ∗p < 0.05.
Among BD + S, BD-S, and HC groups, significant FA differences were found in the bilateral middle cerebellar peduncle, the bilateral body and genu of CC, and the left superior corona radiata (p < 0.005, GRF correction) (Figure 2 and Table 2). Post-hoc analyses revealed that compared to BD-S and HCs, BD + S showed significantly decreased FA in the bilateral middle cerebellar peduncle and the left superior corona radiata (p < 0.05, Bonferroni correction). Compared to HCs, both BD + S and BD-S groups showed significantly reduced FA in the bilateral body and genu of CC (p < 0.05, Bonferroni correction), and there were no significant differences between the two subgroups of BD (Figure 3).
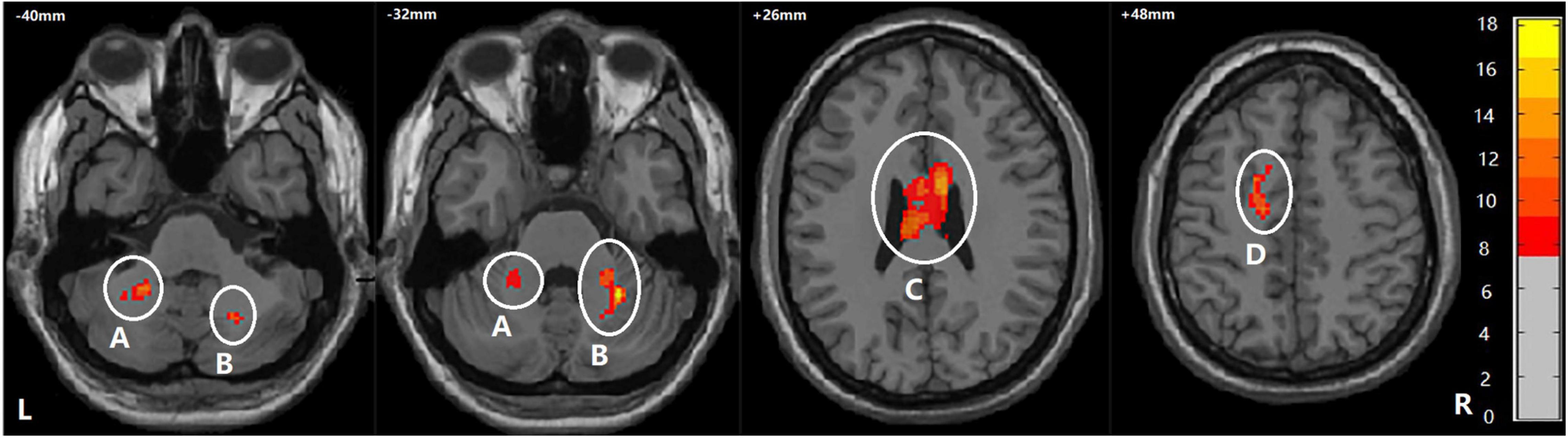
Figure 2. Regions showing WM differences among the BD + S, BD-S, and HC groups. A, the left middle cerebellar peduncle; B, the right middle cerebellar peduncle; C, the bilateral body and genu of corpus callosum; D, the left superior corona radiate. The number is z-coordinate; The color bar is the range of F-values; L, left; R, right.
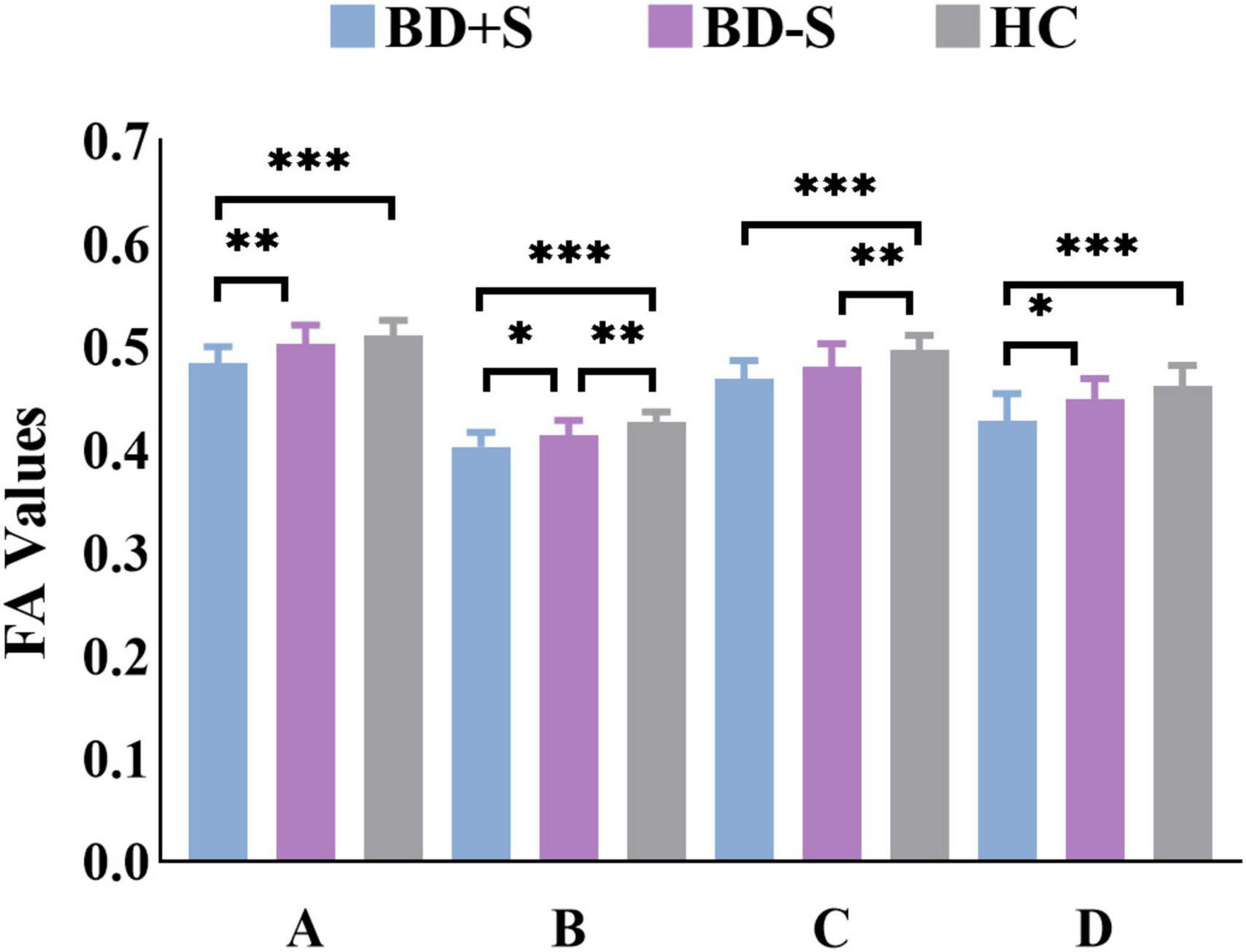
Figure 3. FA values of each cluster in the BD + S, BD-S, and HC groups. A, the left middle cerebellar peduncle; B, the right middle cerebellar peduncle; C, the bilateral body and genu of corpus callosum; D, the left superior corona radiate. ∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05.
No regions showed correlations between the plasma inflammatory cytokine levels and the FA values in all groups.
No correlations between the severity of clinical symptoms and cytokine levels were found in all groups.
In the BD + S group, we found negative correlation between the scores of YMRS and FA values of the left middle cerebellar peduncle (r = −0.74, p = 0.035) (Figure 4). And no correlation between the scores of HAMD-17 and FA values were found in BD + S group.
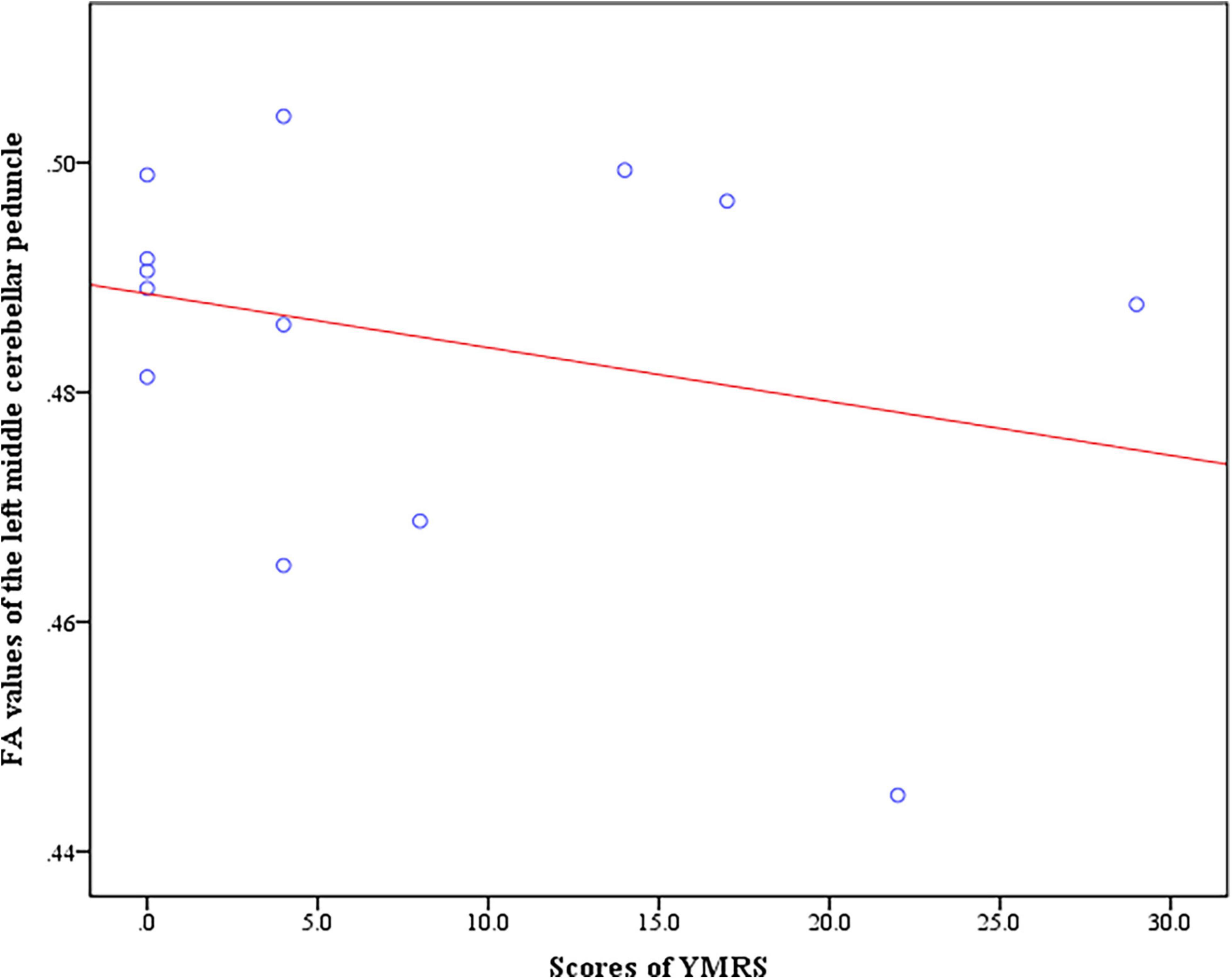
Figure 4. Negative correlation between the scores of YMRS and FA values of the left middle cerebellar peduncle in BD + S group.
In the BD-S group, no correlations between the scores of HAMD-17 or YMRS and FA values were found.
The study explored alterations in the levels of TNF-α, IL-1β, and IL-6, as well as the WM integrity among BD + S, BD-S, and HCs. The correlations between the WM integrity and the levels of TNF-α, IL-1β, and IL-6 were also examined within each group. The findings revealed that BD + S exhibited specifically increased IL-6 levels and lower FA values in the bilateral middle cerebellar peduncle and the left superior corona radiate. Furthermore, both BD + S and BD-S showed decreased FA values in the bilateral body and genu of the corpus callosum, indicating the possible neuroimaging mechanisms of BD. However, no relationship between these plasma inflammatory cytokines and the WM integrity was found in any groups. What is more, we found that the FA values of left middle cerebellar peduncle and the score of YMRS were negatively correlated in BD + S.
Significantly elevated IL-6 level was specific to BD + S in this study. Accumulating evidence suggests that IL-6 is an important proinflammatory cytokine associated with BD (36–40) and suicide (41, 42). One study investigated a similar group found that BD patients with suicide risk had increased IL-1β levels (16). Inconsistent results were predominated in the BD patients and suicide population. For example, BD patients displayed increased levels of TNF-α and IL-1β compared to HCs (12, 16, 38, 40, 43). When comparing suicidal patients with non-suicidal ones and HCs, no significant difference in the levels of IL-6 and TNF-α was found (44). In contrast to the above studies, which focused just on BD or suicide, our research was concerned with SA in BD. Thus, these inconsistent results may be related to the heterogeneous groups. More studies investigating BD + S and BD-S are needed to confirm the results in the future. In addition, BD + S presented higher levels of IL-1β and TNF-α than BD-S and HCs, though it did not reach statistical significance. This may suggest that BD + S has a different immune pattern with BD-S and HCs, and the inflammation of BD + S is more aberrant than BD-S.
Regarding neuroimage, BD + S exhibited specifically impaired WM in the bilateral middle cerebellar peduncle and the left superior corona radiate. It is a pity that we did not find any evidence indicating that the above two brain regions are related to BD + S. The only study of BD + S reported that WM integrity impairments in the right cerebellar regions (19), which is similar with our results. This may indicate that the cerebellum plays a role in the mechanism of suicide in patients with BD. Since we did not find a direct correlation between corona radiata and BD + S, but we found indirect evidence that, internal capsule, which is anatomically connected with the corona radiata (45), is relevant to future SA in adolescents and young adults with BD (46). Although we did not find any alterations in FA values of internal capsule, we infer that the anatomical connection between corona radiata and internal capsule will lead to the functional interaction between them. Thus, corona radiata may be involved to the mechanism of BD + S through its relationship with internal capsule. Furthermore, Reich R. et al. found increased FA values of the corona radiata is positive correlated with greater impulsivity in BD + S (47), which may deepen understanding of our findings. Conversely, decreased FA values of corona radiata in BD + S was revealed in our finding. There are few studies on the WM integrity of corona radiata in BD + S. In the study of other diseases we observed increased FA values of corona radiata in patients with suicide attempts (48, 49). However, the FA values of corona radiata decreased in veterans with suicidal ideation (50). Therefore, the results of the FA values of corona radiata are contradictory in suicidal population, and further study is needed to confirm the role of corona radiata in BD + S.
The FA values of the bilateral body and genu of CC decreased in both BD + S and BD-S groups, indicating that the impaired bilateral body and genu of CC may be involved in the neuroimaging mechanism of BD. CC is the main interhemispheric connector that contains 300 million axons and connects most cortical regions of the brain. It is responsible for integrating motor, cognitive, sensory, and learning information between the two hemispheres (51). A growing body of literature suggests that the CC plays an important role in the pathophysiological mechanism of BD (52–54), and numerous DTI studies have demonstrated that BD patients showed impaired WM integrity in the CC compared to HCs (53, 55–58). Nery-Fernandes et al. found a reduction in the genu and isthmus area of CC in BD patients, but no difference in any subregion of CC between BD + S and BD-S (52), which corresponds to our results. Likewise, similar to the cytokine findings, we also found that the FA values decreased gradually from HC to BD-S to BD + S. Our findings may support the idea that impaired WM occur in a graded manner, i.e., BD + S > BD-S > HCs.
A relationship between the plasma inflammatory cytokines and the WM integrity was not found in the current study. By searching the literature, we found only one study investigating the relationship between WM and cytokines in BD, and the results revealed that the TNF-α level is inversely associated with WM integrity in BD-I patients (28). Nevertheless, the study did not include HCs. On the other hand, it is inconclusive whether the increased cytokine levels in the central nervous system are parallel to those in the peripheral blood (59). As a result, cytokines in peripheral blood may not reflect WM injury. Therefore, the relationship between them may not be determined. Another study, which drew a similar conclusion with ours, showed that the levels of IL-6 and TNF-α did not have a significant increase in BD patients compared to HCs (60). Relatively small number of studies limit our interpretation of the results. Consequently, we should pay attention to BD + S and provide more evidence for the underlying mechanism of the BD + S.
BD + S displayed negative correlation between the scores of YMRS and FA values of the left middle cerebellar peduncle, indicating that the decreased FA values of the left middle cerebellar peduncle is related to manic or hypomanic symptoms of BD + S. Similarly, Olivito G. et al. demonstrated that the cerebellum, which including the middle cerebellar peduncle, plays an important role in mania and hypomania in BD patients (61). The finding is novel and hint subgroup analysis may dig deeper into the relationship between the brain and symptoms.
We recruited BD patients of different ages, different disease states, and different medication use statuses. To explore the potential influence, we compared the FA values and IL-6 levels divided by age, state, and medication use in BD + S and BD-S groups. We found that there were no statistical differences between adolescent and adult groups, between the different states, and between the medication use and medication-free groups (Supplementary Tables 1–6). Therefore, age, state, and medication may have little influence on the results. However, the potential impact of these factors cannot be ignored. In addition, the study was a cross-sectional study with a small sample size, which requires a follow-up and larger sample sizes in the future.
In summary, the results suggest that BD + S may present specific and much more aberrant immunologic and neuroimaging changes compared with BD-S. It may provide a scientific basis to understand the potential mechanisms of BD + S and calls for attention to the suicide attempts of BD patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Medical Scientific Research Ethics Committee of the First Affiliated Hospital of China Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
XJ was responsible for conceptualization, investigation, data curation, writing original draft, and funding acquisition. YG performed the data processing, statistical analyses, visualization, and wrote the original draft. LJ wrote the original draft. YZ validated the results. QS acquired the data. FW and LK recruited the patients, confirmed the diagnosis, and acquired the funding. YT was responsible for conceptualization, project administration, and funding acquisition. All authors reviewed and approved the manuscript.
This work was supported by grants from the National Key R&D Program of China (Grants #2018YFC1311600 and 2016YFC1306900 to YT), Liaoning Revitalization Talents Program (Grant #XLYC1808036 to YT), Natural Science Foundation of Liaoning Province (2020-MS-176 to XJ), National Key R&D Program “Science and Technology Winter Olympics” (2021YFF0306503 to FW), Joint Fund of National Natural Science Foundation of China (U1808204 to FW), and Natural Science Foundation of Liaoning Province (2019-MS-05 to FW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.861881/full#supplementary-material
1. Sani G, Tondo L, Koukopoulos A, Reginaldi D, Kotzalidis GD, Koukopoulos AE, et al. Suicide in a large population of former psychiatric inpatients. Psychiatry Clin Neurosci. (2011) 65:286–95. doi: 10.1111/j.1440-1819.2011.02205.x
2. Osborn D, Levy G, Nazareth I, King M. Suicide and severe mental illnesses. Cohort study within the UK general practice research database. Schizophr Res. (2008) 99:134–8. doi: 10.1016/j.schres.2007.11.025
3. Amercian Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. Am J Psychiatry. (2003) 160:1–60.
4. Brown G, Beck A, Steer R, Grisham J. Risk factors for suicide in psychiatric outpatients: a 20-year prospective study. J Consult Clin Psychol. (2000) 68:371–7. doi: 10.1037/0022-006x.68.3.371
5. Dong M, Lu L, Zhang L, Zhang Q, Ungvari GS, Ng CH, et al. Prevalence of suicide attempts in bipolar disorder: a systematic review and meta-analysis of observational studies. Epidemiol Psychiatr Sci. (2019) 29:e63. doi: 10.1017/s2045796019000593
6. Tondo L, Vazquez GH, Baldessarini RJ. Prevention of suicidal behavior in bipolar disorder. Bipolar Disord. (2021) 23:14–23. doi: 10.1111/bdi.13017
7. Rihmer Z, Gonda X, Döme P. The assessment and management of suicide risk in bipolar disorder. In: AF Carvalho, E Vieta editors. The Treatment of Bipolar Disorder: Integrative Clinical Strategies and Future Directions. Oxford: Oxford University Press (2017).
8. de Abreu L, Nery F, Harkavy-Friedman J, de Almeida K, Gomes B, Oquendo M, et al. Suicide attempts are associated with worse quality of life in patients with bipolar disorder type I. Compr Psychiatry. (2012) 53:125–9. doi: 10.1016/j.comppsych.2011.03.003
9. Almeida VF, Bezerra-Filho S, Studart-Botto P, Leda-Rego G, Silva ITF, Kapczinski F, et al. History of suicide attempts in patients with bipolar disorder type I: socio-demographic and clinical factors, quality of life and functioning. Nord J Psychiatry. (2021) 75:306–13. doi: 10.1080/08039488.2020.1853230
10. Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. (2007) 104:91–5. doi: 10.1016/j.jad.2007.02.018
11. Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. (2013) 74:15–25. doi: 10.1016/j.biopsych.2013.01.007
12. Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. (2013) 47:1119–33. doi: 10.1016/j.jpsychires.2013.05.018
13. Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. (2009) 66:287–92. doi: 10.1016/j.biopsych.2009.01.030
14. Janelidze S, Mattei D, Westrin Å, Träskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. (2011) 25:335–9. doi: 10.1016/j.bbi.2010.10.010
15. Pandey G, Rizavi H, Ren X, Fareed J, Hoppensteadt D, Roberts R, et al. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. (2012) 46:57–63. doi: 10.1016/j.jpsychires.2011.08.006
16. Monfrim X, Gazal M, De Leon P, Quevedo L, Souza L, Jansen K, et al. Immune dysfunction in bipolar disorder and suicide risk: is there an association between peripheral corticotropin-releasing hormone and interleukin-1β? Bipolar Disord. (2014) 16:741–7. doi: 10.1111/bdi.12214
17. Porter A, Leckie R, Verstynen T. White matter pathways as both a target and mediator of health behaviors. Ann N Y Acad Sci. (2018) 1428:71–88. doi: 10.1111/nyas.13708
18. Fan S, Lippard ETC, Sankar A, Wallace A, Johnston JAY, Wang F, et al. Gray and white matter differences in adolescents and young adults with prior suicide attempts across bipolar and major depressive disorders. J Affect Disord. (2019) 245:1089–97. doi: 10.1016/j.jad.2018.11.095
19. Johnston JAY, Wang F, Liu J, Blond BN, Wallace A, Liu J, et al. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry. (2017) 174:667–75. doi: 10.1176/appi.ajp.2016.15050652
20. Mahon K, Burdick KE, Wu J, Ardekani BA, Szeszko PR. Relationship between suicidality and impulsivity in bipolar I disorder: a diffusion tensor imaging study. Bipolar Disord. (2012) 14:80–9. doi: 10.1111/j.1399-5618.2012.00984.x
21. Lima Santos JP, Brent D, Bertocci M, Mailliard S, Bebko G, Goldstein T, et al. White matter correlates of suicidality in adults with bipolar disorder who have been prospectively characterized since childhood. Biol Psychiatry Cogn Neurosci Neuroimaging. (2021) 6:107–16. doi: 10.1016/j.bpsc.2020.07.007
22. Matsuo K, Nielsen N, Nicoletti MA, Hatch JP, Monkul ES, Watanabe Y, et al. Anterior genu corpus callosum and impulsivity in suicidal patients with bipolar disorder. Neurosci Lett. (2010) 469:75–80. doi: 10.1016/j.neulet.2009.11.047
23. Cyprien F, de Champfleur N, Deverdun J, Olié E, Le Bars E, Bonafé A, et al. Corpus callosum integrity is affected by mood disorders and also by the suicide attempt history: a diffusion tensor imaging study. J Affect Disord. (2016) 206:115–24. doi: 10.1016/j.jad.2016.07.026
24. Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, et al. Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1501–7. doi: 10.1016/j.pnpbp.2008.05.009
25. Serafini G, Pompili M, Innamorati M, Fusar-Poli P, Akiskal HS, Rihmer Z, et al. Affective temperamental profiles are associated with white matter hyperintensity and suicidal risk in patients with mood disorders. J Affect Disord. (2011) 129:47–55. doi: 10.1016/j.jad.2010.07.020
26. Pompili M, Ehrlich S, De Pisa E, Mann JJ, Innamorati M, Cittadini A, et al. White matter hyperintensities and their associations with suicidality in patients with major affective disorders. Eur Arch Psychiatry Clin Neurosci. (2007) 257:494–9. doi: 10.1007/s00406-007-0755-x
27. Grangeon MC, Seixas C, Quarantini LC, Miranda-Scippa A, Pompili M, Steffens DC, et al. White matter hyperintensities and their association with suicidality in major affective disorders a meta-analysis of magnetic resonance imaging studies. CNS Spectr. (2010) 15:375–81. doi: 10.1017/s1092852900029242
28. Benedetti F, Poletti S, Hoogenboezem TA, Mazza E, Ambree O, de Wit H, et al. Inflammatory cytokines influence measures of white matter integrity in bipolar disorder. J Affect Disord. (2016) 202:1–9. doi: 10.1016/j.jad.2016.05.047
29. Sugimoto K, Kakeda S, Watanabe K, Katsuki A, Ueda I, Igata N, et al. Relationship between white matter integrity and serum inflammatory cytokine levels in drug-naive patients with major depressive disorder: diffusion tensor imaging study using tract-based spatial statistics. Transl Psychiatry. (2018) 8:141. doi: 10.1038/s41398-018-0174-y
30. Prasad K, Upton C, Nimgaonkar V, Keshavan M. Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: an integrated DTI study. Schizophr Res. (2015) 161:119–25. doi: 10.1016/j.schres.2014.09.043
31. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patients Edition (SCID- I/P,11/2002 revision). New York, NY: New York State Psychiatric Institute (1996).
32. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. (1997) 36:980–8. doi: 10.1097/00004583-199707000-00021
33. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
34. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
35. Oquendo MA, Galfalvy H, Russo S, Ellis SP, Grunebaum MF, Burke A, et al. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. Am J Psychiatry. (2004) 161:1433–41. doi: 10.1176/appi.ajp.161.8.1433
36. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
37. Lu YR, Rao YB, Mou YJ, Chen Y, Lou HF, Zhang Y, et al. High concentrations of serum interleukin-6 and interleukin-8 in patients with bipolar disorder. Medicine (Baltimore). (2019) 98:e14419. doi: 10.1097/MD.0000000000014419
38. Luo Y, He H, Zhang M, Huang X, Fan N. Altered serum levels of TNF-alpha. IL-6 and IL-18 in manic, depressive, mixed state of bipolar disorder patients. Psychiatry Res. (2016) 244:19–23. doi: 10.1016/j.psychres.2016.07.027
39. Munkholm K, Weikop P, Kessing LV, Vinberg M. Elevated levels of IL-6 and IL-18 in manic and hypomanic states in rapid cycling bipolar disorder patients. Brain Behav Immun. (2015) 43:205–13. doi: 10.1016/j.bbi.2014.09.021
40. Rowland T, Perry BI, Upthegrove R, Barnes N, Chatterjee J, Gallacher D, et al. Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: systematic review and meta-analyses. Br J Psychiatry. (2018) 213:514–25. doi: 10.1192/bjp.2018.144
41. Isung J, Aeinehband S, Mobarrez F, Nordstrom P, Runeson B, Asberg M, et al. High interleukin-6 and impulsivity: determining the role of endophenotypes in attempted suicide. Transl Psychiatry. (2014) 4:e470. doi: 10.1038/tp.2014.113
42. Black C, Miller BJ. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry. (2015) 78:28–37. doi: 10.1016/j.biopsych.2014.10.014
43. Munkholm K, Vinberg M, Vedel Kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. (2013) 144:16–27. doi: 10.1016/j.jad.2012.06.010
44. Ducasse D, Olie E, Guillaume S, Artero S, Courtet P. A meta-analysis of cytokines in suicidal behavior. Brain Behav Immun. (2015) 46:203–11. doi: 10.1016/j.bbi.2015.02.004
45. Martini F, Nath JL, Bartholomew EF. Fundamentals of Anatomy & Physiology. San Francisco, CA: Benjamin Cummings (2012).
46. Lippard ETC, Johnston JAY, Spencer L, Quatrano S, Fan S, Sankar A, et al. Preliminary examination of gray and white matter structure and longitudinal structural changes in frontal systems associated with future suicide attempts in adolescents and young adults with mood disorders. J Affect Disord. (2019) 245:1139–48. doi: 10.1016/j.jad.2018.11.097
47. Reich R, Gilbert A, Clari R, Burdick KE, Szeszko PR. A preliminary investigation of impulsivity, aggression and white matter in patients with bipolar disorder and a suicide attempt history. J Affect Disord. (2019) 247:88–96. doi: 10.1016/j.jad.2019.01.001
48. Kim B, Oh J, Kim MK, Lee S, Tae WS, Kim CM, et al. White matter alterations are associated with suicide attempt in patients with panic disorder. J Affect Disord (2015) 175:139–46. doi: 10.1016/j.jad.2015.01.001
49. Lee SJ, Kim B, Oh D, Kim MK, Kim KH, Bang SY, et al. White matter alterations associated with suicide in patients with schizophrenia or schizophreniform disorder. Psychiatry Res Neuroimaging. (2016) 248:23–9. doi: 10.1016/j.pscychresns.2016.01.011
50. Davey DK, Jurick SM, Crocker LD, Hoffman SN, Sanderson-Cimino M, Tate DF, et al. White matter integrity, suicidal ideation, and cognitive dysfunction in combat-exposed Iraq and Afghanistan Veterans. Psychiatry Res Neuroimaging. (2021) 317:111389. doi: 10.1016/j.pscychresns.2021.111389
51. Chao YP, Cho KH, Yeh CH, Chou KH, Chen JH, Lin CP. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp. (2009) 30:3172–87. doi: 10.1002/hbm.20739
52. Nery-Fernandes F, Rocha MV, Jackowski A, Ladeia G, Guimaraes JL, Quarantini LC, et al. Reduced posterior corpus callosum area in suicidal and non-suicidal patients with bipolar disorder. J Affect Disord. (2012) 142:150–5. doi: 10.1016/j.jad.2012.05.001
53. Wang F, Kalmar JH, Edmiston E, Chepenik LG, Bhagwagar Z, Spencer L, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. (2008) 64:730–3. doi: 10.1016/j.biopsych.2008.06.001
54. Atmaca M, Ozdemir H, Yildirim H. Corpus callosum areas in first-episode patients with bipolar disorder. Psychol Med. (2007) 37:699–704. doi: 10.1017/S0033291706009743
55. Wise T, Radua J, Nortje G, Cleare AJ, Young AH, Arnone D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol Psychiatry. (2016) 79:293–302. doi: 10.1016/j.biopsych.2015.03.004
56. Toteja N, Guvenek-Cokol P, Ikuta T, Kafantaris V, Peters BD, Burdick KE, et al. Age-associated alterations in corpus callosum white matter integrity in bipolar disorder assessed using probabilistic tractography. Bipolar Disord. (2015) 17:381–91. doi: 10.1111/bdi.12278
57. Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abe C, et al. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology. (2019) 44:2285–93. doi: 10.1038/s41386-019-0485-6
58. Koshiyama D, Fukunaga M, Okada N, Morita K, Nemoto K, Usui K, et al. White matter microstructural alterations across four major psychiatric disorders: mega-analysis study in 2937 individuals. Mol Psychiatry. (2020) 25:883–95. doi: 10.1038/s41380-019-0553-7
59. Zhu T, Yao Z, Yuan HN, Lu BG, Yang SY. Changes of interleukin-1 beta, tumor necrosis factor alpha and interleukin-6 in brain and plasma after brain injury in rats. Chin J Traumatol. (2004) 7:32–5.
60. Lotrich FE, Butters MA, Aizenstein H, Marron MM, Reynolds CF III, Gildengers AG. The relationship between interleukin-1 receptor antagonist and cognitive function in older adults with bipolar disorder. Int J Geriatr Psychiatry. (2014) 29:635–44. doi: 10.1002/gps.4048
Keywords: bipolar disorder, suicide attempts, inflammatory cytokine, diffusion tensor imaging, TNF-α, IL-1β, IL-6, white matter integrity
Citation: Jiang X, Guo Y, Jia L, Zhu Y, Sun Q, Kong L, Wu F and Tang Y (2022) Altered Levels of Plasma Inflammatory Cytokines and White Matter Integrity in Bipolar Disorder Patients With Suicide Attempts. Front. Psychiatry 13:861881. doi: 10.3389/fpsyt.2022.861881
Received: 25 January 2022; Accepted: 11 March 2022;
Published: 07 April 2022.
Edited by:
Takahiro A. Kato, Kyushu University, JapanReviewed by:
Sota Kyuragi, Kyushu University, JapanCopyright © 2022 Jiang, Guo, Jia, Zhu, Sun, Kong, Wu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanqing Tang, dGFuZ3lhbnFpbmdAY211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.