
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 14 April 2022
Sec. Molecular Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.858675
This article is part of the Research Topic Role of the Serotonergic System in Pathology of Major Depressive Disorders View all 9 articles
 Zhen Hua Zhu1†
Zhen Hua Zhu1† Xiao Ying Song1†
Xiao Ying Song1† Li Juan Man1†
Li Juan Man1† Peng Chen1
Peng Chen1 Zhen Tang1
Zhen Tang1 Rong Hua Li1
Rong Hua Li1 Cai Fang Ji1
Cai Fang Ji1 Ning Bin Dai2
Ning Bin Dai2 Fang Liu2
Fang Liu2 Jing Wang1
Jing Wang1 Jianping Zhang3
Jianping Zhang3 Qiu Fang Jia1*
Qiu Fang Jia1* Li Hui1*
Li Hui1*
Objective: The interleukin-8 (IL-8) has been reported to play an important role in depression, which might be modulated by the selective serotonin reuptake inhibitors (SSRIs). Thus, the aim of this study was to investigate serum IL-8 levels, depressive symptom, and their associations in drug-free MDD patients, MDD patients with SSRIs, and healthy controls (HCs).
Methods: Fifty-seven drug-free MDD patients (male/female = 35/22, mean age: 39.24 years), 30 MDD patients with SSRIs (male/female = 11/19, mean age: 39.73 years), and 101 HCs (male/female = 52/49, mean age: 37.38 years) were recruited in this cross-sectional study. Serum IL-8 levels and depressive symptom were assessed using the Flow Cytometer and Hamilton Depression Scale (HAMD). The analysis of variance was used for the comparison between groups. The relationship between serum log10 IL-8 levels and HAMD score was analyzed by Pearson correlation.
Results: Serum log10IL-8 levels were lower in all patients than HCs after controlling for covariates (F = 4.86, p = 0.03). There was significant difference in serum Log10IL-8 levels among three groups after controlling for covariates (F = 14.63, p < 0.001). Serum Log10IL-8 levels in drug-free patients were lower compared to HCs (F = 19.38, p < 0.001) or patients with SSRIs (F = 21.89, p < 0.001) after controlling for covariates. However, there was not difference in serum log10IL-8 levels between patients with SSRIs and HCs after controlling for covariates. Moreover, serum Log10IL-8 levels were negatively correlated with HAMD score in all patients (r = −0.37, p = 0.02). Also, serum Log10IL-8 levels were negatively correlated with HAMD score in drug-free patients (r = −0.74, p = 0.01), but not in patients with SSRIs.
Conclusion: Our data supported that the decline in serum IL-8 levels was association with depression. Moreover, the SSRIs might modulate increased serum IL-8 levels of depression.
Major depressive disorder (MDD) is a highly prevalent and severe psychiatric illness influencing psychological function and diminishing life quality (1). The clinical manifestations of MDD are one or more episodes of depressive mood, markedly diminished pleasure or interest, and even recurrent thoughts of death (1). In China, a larger cross-sectional epidemiological study has shown that the lifetime and 12-month prevalence of MDD are approximately 6.8 and 3.6%, respectively (2). In the United States, the lifetime and 12-month prevalence of MDD have been reported to be 20.6 and 10.4% in a sample of 36,309 adults, respectively (3). Globally, it is estimated that the lifetime and annual prevalence of MDD are 11.1 and 5.9%, respectively, in the 18 countries participating in the world mental health surveys (4). In addition, MDD is said to be one of the ten most disabling conditions worldwide (5, 6). The World Health Organization (WHO) has reported that by 2030, MDD will be the first leading cause of global disease burden (7). Thus, MDD has become an important global public health issue. However, the pathogenesis of MDD is still unclear and requires further investigation.
Neuroinflammation may be implicated in the underlying etiology of MDD, and it may be involved in the disruptions to neuroendocrine function, neurotransmitter metabolism, neuroplasticity, and neural circuity of MDD (8–11). The inflammation hypothesis for MDD is further supported by a serious of accumulating evidence, such as serum abnormal concentrations of inflammatory biomarkers (12–14), depression occurrence with the administration of endotoxin or vaccination (15–17), and the associations between aberrant inflammation cytokines and brain abnormalities of MDD (18, 19). Recently, increasing evidence from genetics studies have further indicated the pivotal role of neuroinflammatory cytokines in the MDD pathophysiology by detecting C-reactive protein (CRP) methylation, IL-6 rs1800795, and IL-1β rs16944 as the risk loci of MDD (20–22).
Interleukin 8 (IL-8) is an important inflammatory cytokine synthesized and released mainly by macrophages, microglia and astrocytes (23, 24). Mounting evidence support MDD as a neuroinflammatory disorder that is closely related to abnormal activation of microglia and astrocytes (24–26). Thus, the association between IL-8 levels and MDD has be debated. For example, at the protein level, plasma/serum/cerebrospinal fluid (CSF) levels of IL-8 are significantly increased in patients with MDD in comparison to healthy controls (HCs) (27–31). At the molecular level, higher expression of the IL-8 gene on chromosome 4q is reported in the prefrontal cortex of drug-free MDD (32). The rs4073 (–251T>A) polymorphism in the promotor region of IL-8 gene is a functional locus that further influences the IL-8 production or protein expression both in vivo and vitro (33–36), and this polymorphism has been reported to be significantly associated with the susceptibility to depression (37, 38). However, previous studies also have indicated that the transcripts and translations for the IL-8 gene show significant decline in patients with MDD compared with HCs (12, 39). In addition, several studies have reported that there are no significant differences in the protein and mRNA levels of IL-8 between patients with MDD and HCs (40–42). These discrepant results suggest that the role of IL-8 in depression should deserve further investigation. Moreover, several studies have indicated that the SSRIs might modulate IL-8 levels in patients with MDD and other psychiatric disorder (28, 43, 44).
However, to our best knowledge, few studies have examined the comparisons of serum IL-8 levels in drug-free MDD patients, MDD patients with SSRIs, and HCs in a Han Chinese population. Thus, the main aim of this study was to investigate serum IL-8 levels, depressive symptom, and their associations in three groups.
This study was conducted between August 2017 and May 2021. A research coordinator explained the study protocol and procedure to each participant, then signed informed consent was obtained. The study protocol and informed consent were approved by the Medical Institutional Review Board of Suzhou Guangji Hospital.
Eighty-seven MDD patients (male/female = 46/41) including 57 drug-free MDD patients and 30 MDD patients with SSRIs (the administration of single SSRI) were enrolled from outpatient or inpatient unit of Suzhou Guangji Hospital, a municipal owned psychiatric hospital. The service area of this hospital covered approximately a population of 12.7 million people. All MDD patients met the following inclusion criteria: (1) Han Chinese, aged 18–60 years old; (2) confirmation of unipolar depression according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) by two experienced psychiatrists; (3) had a minimum of 6 years of education; and (4) had the ability to participate in the assessment of depressive scale. Moreover, MDD patient did not take any antidepressants, which was defined as drug-free patient. Patients with SSRIs also did not take other medication.
In total, 101 HCs (male/female = 52/49) were enrolled from the local community in the Suzhou Xiangcheng District. All HCs met the following inclusion criteria: (1) Han Chinese, aged 18–60 years; (2) received education for at least 6 years; (3) had the ability to participate in the assessment of depressive symptom; and (4) had a Zung Self-Rating Depression Scale (SDS) normal score < 50 that was assessed using the SDS Chinese version (45, 46). Current mental status and personal or family history of any mental disorder were assessed using unstructured interviews in HCs. In addition, none of HCs had any history of MDD.
All participants were in good physical health. Any participants with schizophrenia, schizoaffective disorders, dementia, neurodegenerative and neurological disorders, cardiovascular disease, cerebrovascular disease, infections, cancer, diabetes, hypertension, hyperlipidemia, and pregnancy were excluded.
A detailed questionnaire including a complete medical history, physical examination, and medical and psychological conditions was obtained from each participant. Additional information including age, gender, education, body mass index (BMI), smoking, drinking, and suicide status, age of onset was collected from available medical records.
Depressive symptom in all patients were assessed by using the Hamilton Rating Scale for Depression (24-items) (HAMD) (47–49). The consistency/reliability of HAMD (p < 0.01) has been established in MDD patients (50). The HAMD included a total of 24 items. The score range of 10 items was from 0 to 2 that represented: 0 = none, 1 = mild-moderate, and 2 = severe, respectively. In addition, the score range of 14 items was from 0 to 4 that represented: 0 = none, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe, respectively (50). The measurement of HAMD was conducted by a research coordinator after the patient recruitment.
Blood samples with coagulants were collected from all participants between 7 and 9 AM following an overnight fasting. The serum was separated, aliquoted, and stored at −80°C in a refrigerator before laboratory assays. Serum IL-8 levels were measured by the BD™ FACSCanto Flow Cytometer and BD™ Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences, San Jose, CA, United States). The detailed experiment procedure was conducted according to the instruction manuals of the kit and the flow cytometer. The sensitivity for IL-8 measurement was 3.6 pg/ml in this assay, with 4% intra- and inter- assay variation coefficients, respectively. A IL-8 standard curve was established from the BD™ CBA Human Inflammatory Cytokine Standards in triplicate for the experiment per batch. This experiment was conducted by the same technician who was blind to the clinical information and sample’s identity document.
The demographic and clinical data were compared between all MDD patients and HCs using an analysis of variance (ANOVA) for continuous variables and a Chi-squared test for categorical variables. Serum IL-8 levels were not normally distributed and were thus log-transformed. We then compared serum log10IL-8 levels between all MDD patients and HCs using an ANOVA. When the significance was found in the ANOVA, the potential confounding factors (sex, age, education, BMI, smoking, drinking and suicide status) were added as covariates. Serum log10IL-8 levels were further compared among drug-free MDD patients, MDD patients with SSRIs, and HCs using the ANOVA and analysis of covariance (ANCOVA), respectively. Post-hoc comparisons between groups were made using the Fisher’s least significant difference (LSD) procedure. In addition, the comparisons of serum log10IL-8 levels were analyzed in drug-free MDD patients versus HCs, MDD patients with SSRIs versus HCs, and drug-free MDD patients versus MDD patients with SSRIs using the ANCOVA. According to sex grouping, the comparisons of serum log10IL-8 levels were analyzed among drug-free MDD patients, MDD patients with SSRIs and HCs using the ANCOVA. Moreover, the comparisons of serum log10IL-8 levels were analyzed among suicide attempters with drug-free MDD, non-suicide attempters with drug-free MDD and non-suicide attempters of HCs as well as among suicide attempters with SSRI-medicated MDD, non-suicide attempter with SSRI-medicated MDD and non-suicide attempters of HCs using the ANCOVA. The correlations between serum log10IL-8 levels and HAMD score in drug-free MDD patients, MDD patients with SSRIs, and all MDD patients were evaluated with Pearson’s product moment correction coefficients, respectively. Continuous data were presented as the mean and standard deviation (mean ± SD), and all p-values were 2-tailed at a significance level of <0.05.
There were no significant differences in sex, age, BMI, smoking, and drinking between all MDD patients and HCs (Table 1). However, education (F = 58.02, p < 0.001) and suicide status (χ2 = 56.51, p < 0.001) were significantly different between two groups. The mean and SD of age of onset (years), and HAMD score in all MDD patients were 34.69 ± 13.44, and 21.38 ± 6.97, respectively. A total of 87 MDD patients include 57 drug-free MDD patients (65.5%) and 30 MDD patients with SSRIs (34.5%). Moreover, education and suicide status were significantly different in HCs versus drug-free patients versus patients with SSIRs (F = 29.92, p < 0.001; χ2 = 56.65, p < 0.001), HCs versus drug-free patients (F = 55.36, p < 0.001; χ2 = 47.46, p < 0.001), and HCs versus patients with SSRIs (F = 21.87, p < 0.001; χ2 = 42.19, p < 0.001). Sex (F = 3.89, p = 0.04) was significantly different in drug-free patients versus patients with SSRIs. However, the HAMD score (F = 0.98, p = 0.33) was not different in drug-free patients versus patients with SSRIs.
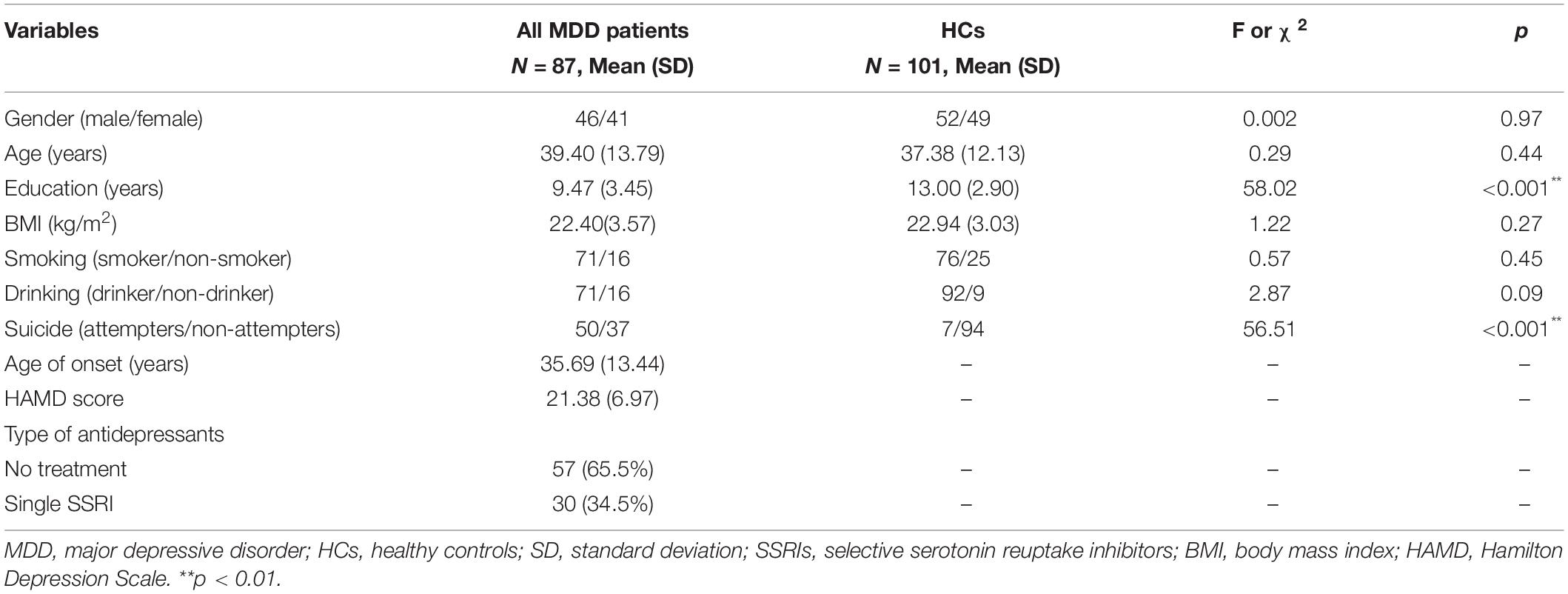
Table 1. Demographic and clinical data between all major depressive disorder (MDD) patients and healthy control (HCs).
There was not significant difference in serum log10IL-8 levels between all MDD patients and HCs in Figure 1A (1.86 ± 0.64 versus 2.03 ± 0.53, F = 3.65, p = 0.06). The significant difference was observed between two groups after adjusting for sex, age, education, BMI, smoking, drinking and suicide status in Figure 1A (F = 4.86, p = 0.03). The confounding factors including education and suicide status did not directly influence the comparative index of serum log10IL-8 levels between two groups (all, p > 0.05). There was significant difference in serum log10IL-8 levels among drug-free MDD patients, MDD patients with SSRIs, and HCs in Figure 1B (1.65 ± 0.56 versus 2.27 ± 0.57 versus 2.03 ± 0.53, F = 14.85, p < 0.001). This difference was still significant after adjusting for covariates (F = 14.63, p < 0.001). Sex (F = 4.90, p = 0.03) and age (F = 4.71, p = 0.03) of covariates directly influenced the comparative index of serum log10IL-8 levels among three groups. Further post-hoc comparisons found the significant differences in serum IL-8 levels in drug-free MDD patients versus HCs (F = 17.68, p < 0.001), MDD patients with SSRIs versus HCs (F = 4.79, p = 0.03), and drug-free MDD patients versus MDD patients with SSRIs (F = 24.00, p < 0.001) using Fisher’s LSD procedure in Figure 1B. The significant differences in drug-free MDD patients versus HCs (F = 19.38, p < 0.001), and drug-free MDD patients versus MDD patients with SSRIs (F = 21.89, p < 0.001) were still observed, but not in MDD patients with SSRIs versus HCs (F = 3.68, p > 0.05) after adjusting for covariates in Figure 1B. The effect of sex on the comparative index of serum log10 IL-8 levels in drug-free patients versus HCs (F = 5.44, P = 0.02), and patients with SSRIs versus HCs (F = 4.35, p = 0.04) was observed as well as the effect of age on the comparative index of serum log10 IL-8 levels in patients with drug-free versus SSRIs (F = 4.91, P = 0.03).
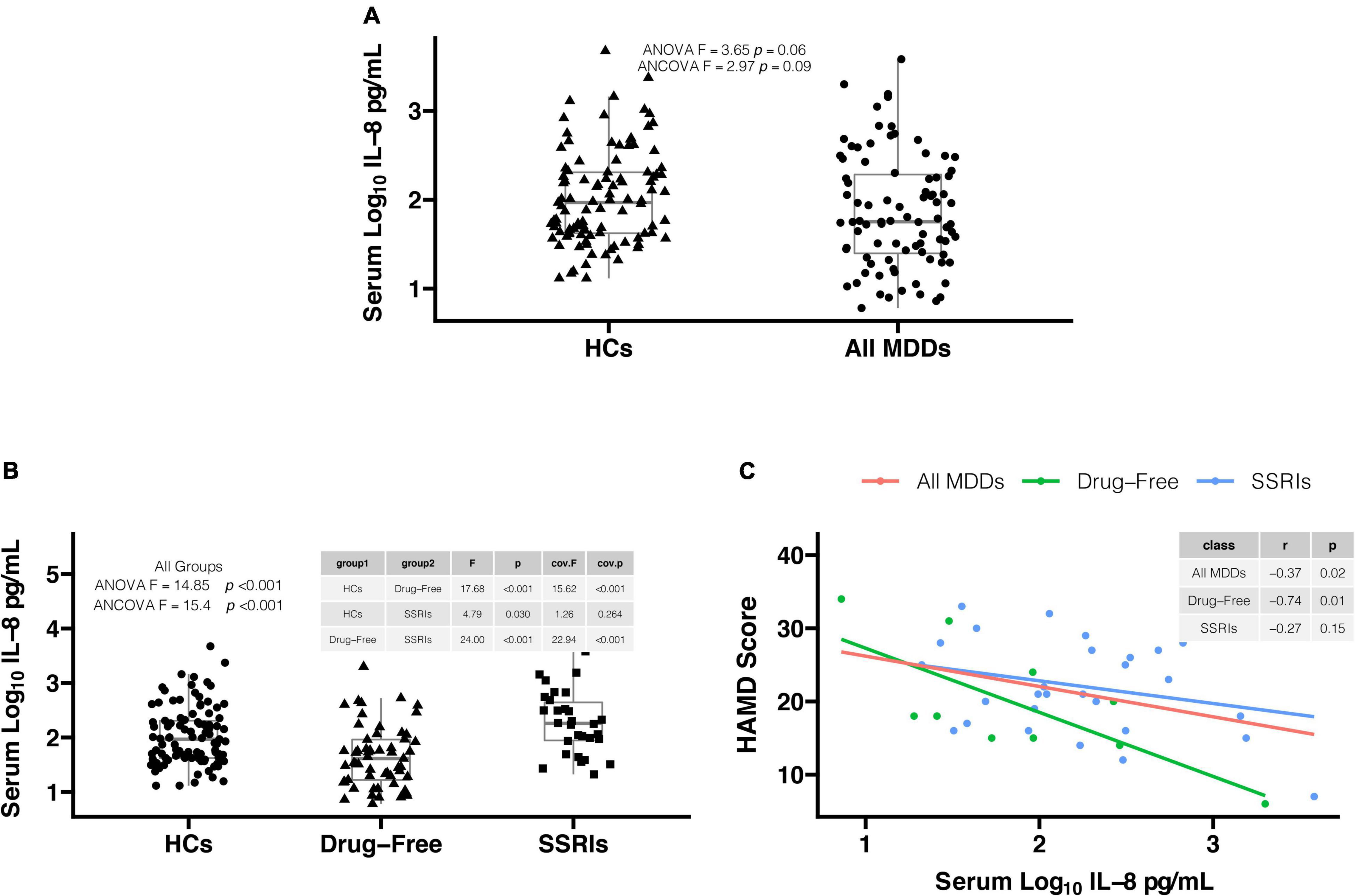
Figure 1. (A) Serum log10IL-8 levels in all major depressive disorder (MDD) patients were lower in comparison to healthy controls (HCs) after adjusting for covariates (1.86 ± 0.64 vs. 2.03 ± 0.53, F = 4.86, p = 0.03). (B) There was significant difference in serum log10IL-8 levels among three groups after adjusting for covariates (1.65 ± 0.56 vs. 2.27 ± 0.57 vs. 2.03 ± 0.53, F = 14.63, p < 0.001). Serum log10IL-8 levels were significantly lower in drug-free MDD patients in comparison to MDD patients with SSRIs (F = 21.89, p < 0.001) and HCs (F = 19.38, p < 0.001) after adjusting for covariates. However, there was no difference in serum log10IL-8 levels between MDD patients with SSRIs and HCs after adjusting for covariates (F = 3.68, p > 0.05). (C) The significant correlations were found in drug-free MDD patients (r = –0.74, p = 0.01) and all MDD patients (r = –0.37, p = 0.02), but not in MDD patients with SSRIs (r = –0.27, p = 0.15). IL-8, interleukin-8; MDD, major depressive disorder; HCs, healthy controls; SSRIs, selective serotonin reuptake inhibitors; HAMD, Hamilton Depression Scale; ANOVA, analysis of variance; ANCOVA, analysis of covariance.
In male, there were significant differences in serum log10IL-8 levels in drug-free patients versus patients with SSRIs versus HCs (F = 7.45, p = 0.001), drug-free patients versus HCs (F = 12.39, p < 0.001), and drug-free patients versus patients with SSRIs (F = 8.97, p = 0.005) rather than patients with SSRIs versus HCs (F = 0.43, p = 0.52) after adjusting for covariates. In female, there were significant differences in serum log10IL-8 levels in drug-free patients versus patients with SSRIs versus HCs (F = 9.53, p < 0.001), drug-free patients versus HCs (F = 8.19, p = 0.006), patients with SSRIs versus HCs (F = 7.68, p = 0.007), and drug-free patients versus patients with SSRIs (F = 12.20, p = 0.001) after adjusting for covariates. However, there were no significant differences in serum log10IL-8 levels between male and female in HCs (p > 0.05), drug-free patients (p > 0.05), and patients with SSRIs (p > 0.05) after adjusting for covariates.
In addition, there was significant difference in serum log10IL-8 levels in suicide attempters with drug-free MDD versus non-suicide attempters with drug-free MDD versus non-suicide attempters of HCs after adjusting for covariates (F = 9.29, p = 0.003). Compared to non-suicide attempters of HCs, serum log10IL-8 levels were significantly lower in suicide attempters with drug-free MDD (F = 14.76, p < 0.001) and non-suicide attempters with drug-free MDD (F = 8.67, p = 0.004) after adjusting for covariates. In addition, serum log10IL-8 levels in suicide attempters with SSRI-medicated MDD were significantly lower than those in non-suicide attempters of HCs after adjusting for covariates (F = 4.01, p < 0.05).
In Figure 1C, the Pearson correlation analysis showed that serum log10IL-8 levels were significantly correlated with HAMD score in all MDD patients (r = −0.37, p = 0.02) and drug-free MDD patients (r = −0.74, p = 0.01), but not in MDD patients with SSRIs (r = −0.27, p = 0.15).
To our best knowledge, it is the first cross-sectional study to investigate serum IL-8 levels, depressive symptom, and their associations in drug-free MDD patients, MDD patients with SSRIs, and HCs in a Han Chinese population. Two main findings were listed as follows: (1) serum log10IL-8 levels were significantly lower in drug-free MDD patients and all MDD patients in comparison to HCs, and serum log10IL-8 levels were negatively associated with depressive symptom score in all MDD patients and drug-free MDD patients, respectively; (2) serum log10IL-8 levels in MDD patients with SSRIs were higher than those in drug-free MDD patients, but did not show in patients with SSRIs and HCs.
Accumulating evidence have indicated that the abnormalities of neuroinflammation might be implicated in the pathogenesis of MDD. IL-8 has been regarded as a crucial inflammatory cytokine produced by macrophages, as well as brain cells such as microglia and astrocytes (23, 24). Thus, the IL-8 abnormality might play an important role in the pathogenesis of MDD. Our data has shown significant decline in serum log10IL-8 levels in all MDD patients and drug-free MDD patients in comparison to HCs, and serum log10IL-8 levels were negatively correlated with HAMD score in all MDD patients and drug-free MDD patients, respectively. The potential mechanism responsible for the association between decreased serum IL-8 levels and depression was that the decline in IL-8 levels might reflect the occurrences of altered microglia-mediated synaptic pruning and/or reduced astrocytes in the brain of MDD (51–56). Previous studies have suggested that the decline in serum IL-8 levels might be involved in the depression. For example, a recent meta-analysis showed that peripheral IL-8 levels were significantly decreased in drug-free first-episode MDD patients in comparison to HCs (12). Another meta-analysis study of small sample including two studies has found that CSF IL-8 levels were significantly lower in 38 MDD patients compared to 114 HCs (31). A study of gene expression profiling in the dorsolateral prefrontal cortex of brain has reported that the transcripts of IL-8 gene were significantly decreased in MDD patients with suicides and non-suicides in comparison to HCs (39), suggesting that the decline in IL-8 expression was implicated in depression neurobiology. In addition, serum IL-8 levels were reported to be inversely related to depressive symptom score in individuals with astrocytoma (57). The above reports supported a notion that the decline in serum IL-8 levels played a vital role in depression. However, previous studies also have found that the transcript and translation levels of IL-8 were significantly higher in MDD patients in comparison to HCs (27–32). Moreover, several studies also reported no significant differences in the IL-8 protein and mRNA levels between MDD patients and HCs (40–42). These discrepant results of the IL-8 comparative index in patients with MDD versus HCs should deserve to be further investigated in future study.
Moreover, previous studies have indicated the effects of different inflammatory processes on different subtypes of depression (58–60). For example, non-melancholic patients exhibited the cytokine production of increased pro-inflammatory, whereas melancholic patients, in contrast, exhibited the cytokine production of reduced pro-inflammatory (61–63). The decline in IL-8 expression in the dorsolateral prefrontal cortex has been reported to be involved in the neurobiology of MDD suicides (39). The dorsolateral prefrontal cortex has been found to be implicated in the regulation of impulsivity, decision-making, cognitive control of mood and other executive function related to suicidal behavior (64, 65), and functional imaging studies further supported the deficits in perfusion and cortical thickness in prefrontal cortex of suicides before death and suicide attempters (66, 67). The data of current study has also shown that the ratio of suicide attempters in all MDD patients was 57.47%, and decreased serum IL-8 levels were observed in suicide attempters with drug-free MDD, non-suicide attempters with drug-free MDD, and suicide attempters with SSRIs-medicated MDD in comparison to non-suicide attempters of HCs, respectively. Thus, suicide attempters with MDD should be regarded as an important subtype that could influence the results of current study based on the relationship between IL-8 and suicidal ideation (68).
Interestingly, our study found that serum IL-8 levels were significantly elevated in MDD patients with SSRIs compared with drug-free MDD patients, but not in MDD patients with SSRIs versus HCs. This result suggested that the SSRIs might modulate increased serum IL-8 levels in patients with MDD. The SSRIs have been reported to modulate microglia and astrocytes in hippocampus to produce the inflammatory cytokines including IL-8 (69–73). Moreover, MDD has been considered a neuroinflammatory disorder that was implicated in the abnormal activities of brain microglia and astrocytes (24–26). A previous study has demonstrated that the monotherapy with escitalopram was found to elevate the IL-8 trend of depression, which further alleviated depressive symptom (74). The above findings supported the notion that the SSRIs were implicated in modulating the microglia and astrocytes, which caused increased IL-8 level of depression. Moreover, two previous studies have found that the electroconvulsive therapy (ECT) treatment might significantly increase serum/CSF IL-8 levels in patients with MDD (75, 76). These findings further suggested that increased IL-8 levels in MDD patients reduced depressive symptom, which further hinted that increased IL-8 levels might have neuroprotective effects for MDD (77, 78). However, the SSRIs were reported to cause the decline in plasma IL-8 levels in patients with MDD (43). In addition, the SSRIs were found to decrease serum IL-8 levels in patients with generalized anxiety disorder (44). These inconsistent findings suggested that future study of a large and independent sample should be carried out to confirm the present findings in first-episode drug-free patients with MDD.
Although previous studies have reported that sex differences influenced inflammatory processes of depression (79–81), the present study did not find the effect of sex differences on serum IL-8 levels in drug-free patients or patients with SSRIs. However, our data showed that serum IL-8 levels in female SSRI-medicated MDD patients were significantly higher than those in female HCs, but did not show in male SSRIs-medicated MDD patients versus male HCs, which further indicated that female might be especially sensitive to the effect of SSRIs on serum IL-8 levels of depression.
The present study had several limitations as follows: (1) A relatively small sample size. The effect size of present study was calculated to be very small (η2 = 0.02). Thus, our results should be regarded as a pilot study. (2) Employing banked samples that were collected from August 2017 to May 2021, and were stored at –80°C in a refrigerator for differing time lengths. The storage time might influence the IL-8 measurement in this study; (3) A cross-sectional design. Thus, it was unclear whether there were causative relationships among serum IL-8 levels, HAMD score, and SSRIs in MDD patients. Thus, further studies with longitudinal and prospective follow-up were necessary to clarify the time course of potential SSRIs effects on IL-8 levels in patients with MDD. (4) Confirmed diagnosis of MDD. Although the patients were confirmed with a diagnosis for unipolar depression rather than bipolar depression at the moment of patient recruitment, a few unipolar depressive patients might convert to bipolar depressive patients during follow-ups. (5) MDD classification. Serum IL-8 levels might be influenced by MDD classification such as first-episode/chronic/recurrence/recovery patients. (6) SSRI types and doses. The effects of different SSRI types and doses on IL-8 levels also had discrepant in patients with MDD (43). (7) Depressive symptom was assessed using the Zung Self-Rating Depression Scale in healthy controls and the Hamilton Depression Scale in participants with MDD. This limited the correlation analysis between depressive symptom severity and IL-8 levels to those with confirmed MDD. (8) the absence of other clinical data and assessments including diet, sleep, and duration of illness. Future studies should be expanded to include those data that might influence serum IL-8 levels and depressive symptom of MDD. Finally, (9) IL-8 levels were analyzed in serum, it is unknown whether the decline in serum IL-8 levels may reflect the decline in IL-8 levels in the brain of MDD in comparison to HCs due to the blood-brain barrier, which should further deserve to be investigated. However, the mRNA expression of IL-8 gene in the dorsolateral prefrontal cortex of depression has been reported to be significantly lower in comparison to HCs (39).
Moreover, the present study had several strengths as follows: (1) It was first cross-sectional study to investigate serum IL-8 levels in drug-free MDD patients versus MDD patients with SSRIs versus HCs in a Han Chinese population. (2) The sensitivity for IL-8 measurement was 3.6 pg/ml in this assay with 4% intra- and inter- assay variation coefficients, which further supported the credibility of present results.
In summary, serum IL-8 levels were lower in all MDD patients and drug-free MDD patients in comparison to HCs, and serum IL-8 levels were negatively correlated with depressive symptom in drug-free MDD patients and all MDD patients. These data supported that the decline in serum IL-8 levels contributed to depression. Serum IL-8 levels were higher in MDD patients with SSRIs in comparison to drug-free MDD patients, but not in MDD patients with SSRIs versus HCs. These data further demonstrated that the SSRIs might modulate increased serum IL-8 levels of MDD. However, the current findings still were preliminary because of the relatively small sample size and absence of a longitudinal follow-up. Therefore, future studies were warranted to verify the current findings in a large and independent cohort of first-episode drug-free patients with MDD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Institutional Review Board of Suzhou Guangji Hospital. The patients/participants provided their written informed consent to participate in this study.
ZZ, XS, LM, and LH were responsible for the study design, statistical analysis, and manuscript preparation. ZZ, XS, LM, PC, ZT, RL, CJ, JW, and QJ were responsible for recruiting the patients, performing the clinical rating, and collecting the samples. ND and FL were involved in evolving the ideas and editing the manuscript. QJ and LH were involved in writing the protocol, providing the funding for the study, and editing the manuscript. JZ commented on the manuscript critically. All authors have contributed and approved the final manuscript.
This study was funded by the National Natural Science Foundation of China (81771439), the Jiangsu Provincial Key Research and Development Program (BE2020661), the Natural Science Foundation of Jiangsu Province (BK20200210), the Jiangsu Provincial Health Commission Science Research Program (H2019056 and LGY2018010), the Jiangsu Provincial Six Talent Peaks Project (WSN-165), the Suzhou Municipal Health Commission Science Research Program (GSWS2020095, SZLCYXZX202109, and SZFCXK202103), the Suzhou Municipal Sci-Tech Bureau Program (SYS2019113, SS2019009, and SKJY2021143), the CAS Key Laboratory of Mental Health, Institute of Psychology (KLMH2019K03), and the Sample Bank of Suzhou Municipal Psychiatric Disorders from the support of Suzhou Municipal Finance Bureau.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank patients with MDD from Suzhou Guangji Hospital for their support and participation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.858675/full#supplementary-material
2. Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. (2019) 6:211–24.
3. Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. (2018) 75:336–46. doi: 10.1001/jamapsychiatry.2017.4602
4. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
6. Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. (2012) 379:1045–55. doi: 10.1016/s0140-6736(11)60602-8
7. WHO. Depression: A Global Crisis. World Mental Health Day. Geneva: World Health Organization (2012).
8. Milenkovic VM, Stanton EH, Nothdurfter C, Rupprecht R, Wetzel CH. The role of Chemokines in the pathophysiology of major depressive disorder. Int J Mol Sci. (2019) 20:2283. doi: 10.3390/ijms20092283
9. Liu CH, Yang MH, Zhang GZ, Wang XX, Li B, Li M, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. (2020) 17:54. doi: 10.1186/s12974-020-01732-5
10. Bai YM, Chen MH, Hsu JW, Huang KL, Tu PC, Chang WC, et al. A comparison study of metabolic profiles, immunity, and brain gray matter volumes between patients with bipolar disorder and depressive disorder. J Neuroinflammation. (2020) 17:42. doi: 10.1186/s12974-020-1724-9
11. Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. (2020) 52:165–80. doi: 10.1016/j.yfrne.2018.12.001
12. Çakici N, Sutterland AL, Penninx BWJH, Dalm VA, de Haan L, van Beveren NJM. Altered peripheral blood compounds in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Brain Behav Immun. (2020) 88:547–58. doi: 10.1016/j.bbi.2020.04.039
13. Mac Giollabhui N, Ng TH, Ellman LM, Alloy LB. The longitudinal associations of inflammatory biomarkers and depression revisited: systematic review, meta-analysis, and meta-regression. Mol Psychiatry. (2021) 26:3302–14. doi: 10.1038/s41380-020-00867-4
14. Ye G, Yin GZ, Tang Z, Fu JL, Chen J, Chen SS, et al. Association between increased serum interleukin-6 levels and sustained attention deficits in patients with major depressive disorder. Psychol Med. (2018) 48:2508–14. doi: 10.1017/S0033291718000090
15. Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
16. Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. (2001) 58:445–52. doi: 10.1001/archpsyc.58.5.445
17. Schedlowski M, Engler H, Grigoleit J-S. Endotoxin-induced experimental systemic inflammation in humans: a model to disentangle immune-to-brain communication. Brain Behav Immun. (2014) 35:1–8. doi: 10.1016/j.bbi.2013.09.015
18. Liu K, Zhao X, Lu X, Zhu X, Chen H, Wang M, et al. Effect of selective serotonin reuptake inhibitor on prefrontal-striatal connectivity is dependent on the level of TNF-α in patients with major depressive disorder. Psychol Med. (2019) 49:2608–16. doi: 10.1017/s0033291718003616
19. Zhou R, Wang F, Zhao G, Xia W, Peng D, Mao R, et al. Effects of tumor necrosis factor-α polymorphism on the brain structural changes of the patients with major depressive disorder. Transl Psychiatry. (2018) 8:217. doi: 10.1038/s41398-018-0256-x
20. Green C, Shen X, Stevenson AJ, Conole ELS, Harris MA, Barbu MC, et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav Immun. (2021) 92:39–48. doi: 10.1016/j.bbi.2020.11.024
21. Tartter M, Hammen C, Bower JE, Brennan PA, Cole S. Effects of chronic interpersonal stress exposure on depressive symptoms are moderated by genetic variation at IL6 and IL1β in youth. Brain Behav Immun. (2015) 46:104–11. doi: 10.1016/j.bbi.2015.01.003
22. Udina M, Moreno-España J, Navinés R, Giménez D, Langohr K, Gratacòs M, et al. Serotonin and interleukin-6: the role of genetic polymorphisms in IFN-induced neuropsychiatric symptoms. Psychoneuroendocrinology. (2013) 38:1803–13. doi: 10.1016/j.psyneuen.2013.03.007
23. Ehrlich LC, Hu S, Sheng WS, Sutton RL, Rockswold GL, Peterson PK, et al. Cytokine regulation of human microglial cell IL-8 production. J Immunol. (1998) 160:1944–8.
25. Khansari PS, Sperlagh B. Inflammation in neurological and psychiatric diseases. Inflammopharmacology. (2012) 20:103–7. doi: 10.1007/s10787-012-0124-x
26. Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. (2015) 300:141–54. doi: 10.1016/j.neuroscience.2015.05.018
27. Baune BT, Smith E, Reppermund S, Air T, Samaras K, Lux O, et al. Inflammatory biomarkers predict depressive, but not anxiety symptoms during aging: the prospective Sydney memory and aging study. Psychoneuroendocrinology. (2012) 37:1521–30. doi: 10.1016/j.psyneuen.2012.02.006
28. Dahl J, Ormstad H, Aass HCD, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. (2014) 45:77–86. doi: 10.1016/j.psyneuen.2014.03.019
29. Kim J-M, Stewart R, Kim J-W, Kang H-J, Bae K-Y, Kim S-W, et al. Changes in pro-inflammatory cytokine levels and late-life depression: a two year population based longitudinal study. Psychoneuroendocrinology. (2018) 90:85–91. doi: 10.1016/j.psyneuen.2018.02.006
30. Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry. (2016) 6:e825. doi: 10.1038/tp.2016.92
31. Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. (2018) 44:75–83. doi: 10.1093/schbul/sbx035
32. Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. (2011) 16:751–62. doi: 10.1038/mp.2010.52
33. Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. (2000) 55:1023–7. doi: 10.1136/yhorax.55.12.1023
34. Taguchi A, Ohmiya N, Shirai K, Mabuchi N, Itoh A, Hirooka Y, et al. Interleukin-8 promotor polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. (2005) 14:2487–93. doi: 10.1158/1055-9965.EPI-05-0326
35. Ohyauchi M, Imatani A, Yonechi M, Asano N, Miura A, Iijima K, et al. The polymorphism interleukin 8 -251 A/T influences the susceptibility of Helicobacter pylori related gastric diseases in the Japanese population. Gut. (2005) 54:330–5. doi: 10.1136/gut.2003.033050
36. Ye BD, Kim SG, Park JH, Kim JS, Jung HC, Song IS. The interleukin-8-251A allele is associated with increased risk of noncardia gastric adenocarcinoma in Helicobacter pylori-infected Koreans. J Clin Gastroenterol. (2009) 43:233–9. doi: 10.1097/MCG.0b013e3181646701
37. Kim J-M, Stewart R, Kim S-W, Kim S-Y, Bae K-Y, Kang H-J, et al. Physical health and incident late-life depression: modification by cytokine genes. Neurobiol Aging. (2013) 34:356.e1–9. doi: 10.1016/j.neurobiolaging.2012.01.111
38. Reyes-Gibby CC, Wang J, Spitz M, Wu X, Yennurajalingam S, Shete S. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients. J Pain Symptom Manage. (2013) 46:161–72. doi: 10.1016/j.jpainsymman.2012.07.019
39. Pantazatos SP, Huang Y-Y, Rosoklija GB, Dwork AJ, Arango V, Mann JJ. Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry. (2017) 22:760–73. doi: 10.1038/mp.2016.130
40. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
41. Eyre HA, Air T, Pradhan A, Johnston J, Lavretsky H, Stuart MJ, et al. A meta-analysis of chemokines in major depression. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 68:1–8. doi: 10.1016/j.pnpbp.2016.02.006
42. Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. (2017) 135:373–87. doi: 10.1111/acps.12698
43. Chen C-Y, Yeh Y-W, Kuo S-C, Liang C-S, Ho P-S, Huang C-C, et al. Differences in immunomodulatory properties between venlafaxine and paroxetine in patients with major depressive disorder. Psychoneuroendocrinology. (2018) 87:108–18. doi: 10.1016/j.psyneuen.2017.10.009
44. Hou WL, Yin XL, Yin XY, Guan LY, Cao JQ, Tang Z, et al. Association between stereopsis deficits and attention decline in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 110:110267. doi: 10.1016/j.pnpbp.2021.110267
45. Gao Y, Wang W, Huang D, Liu H. Analysis of anxiety and depression status and influencing factors among parturient pregnant women. Anhui Med J. (2014) 35:493–6.
46. Hu WM, Yin XY, Yin XL, Zhu ZH, Guan LY, Hou WL, et al. Prevalence, social-demographic and cognitive correlates of depression in Chinese psychiatric medical staff. J Affect Disord. (2020) 263:60–3. doi: 10.1016/j.jad.2019.11.133
47. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. (1967) 6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x
48. Pan S, Liu Z-W, Shi S, Ma X, Song W-Q, Guan G-C, et al. Hamilton rating scale for depression-24 (HAM-D24) as a novel predictor for diabetic microvascular complications in type 2 diabetes mellitus patients. Psychiatry Res. (2017) 258:177–83. doi: 10.1016/j.psychres.2017.07.050
49. Zhu CM. Assessment of the severity of depression. Zhonghua Shen Jing Jing Shen Ke Za Zhi. (1985) 18:295–7.
50. Zhu G, Yin Y, Xiao C-L, Mao R-J, Shi B-H, Jie Y, et al. Serum DHEAS levels are associated with the development of depression. Psychiatry Res. (2015) 229:447–53. doi: 10.1016/j.psychres.2015.05.093
51. Tsai SJ. Role of interleukin 8 in depression and other psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 106:110173. doi: 10.1016/j.pnpbp.2020.110173
52. Remick DG, Kronfol Z. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. (2000) 157:683–94. doi: 10.1176/appi.ajp.157.5.683
53. Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. (2014) 17:400–6. doi: 10.1038/nn.3641
54. Torres-Platas SG, Nagy C, Wakid M, Turecki G, Mechawar N. Glial fibrillary acidic protein is differentially expressed across cortical and subcortical regions in healthy brains and downregulated in the thalamus and caudate nucleus of depressed suicides. Mol Psychiatry. (2015) 21:509–15. doi: 10.1038/mp.2015.65
55. Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. (2013) 14:1225–36. doi: 10.2174/13894501113149990156
56. Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, et al. Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry. (2015) 20:320–8. doi: 10.1038/mp.2014.21
57. Starkweather AR, Sherwood P, Lyon DE, Bovbjerg DH, Broaddus WC, Elswick RK, et al. Depressive symptoms and cytokine levels in serum and tumor tissue in patients with an Astrocytoma: a pilot study. BMC Res Notes. (2014) 7:423. doi: 10.1186/1756-0500-7-423
58. Dunjic-Kostic B, Ivkovic M, Radonjic NV, Petronijevic ND, Pantovic M, Damjanovic A, et al. Melancholic and atypical major depression–connection between cytokines, psychopathology and treatment. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 43:1–6. doi: 10.1016/j.pnpbp.2012.11.009
59. Kaester F, Hettich M, Peters M, Sibrowski W, Hetzel G, Ponath G, et al. Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J Affect Disord. (2005) 87:305–11. doi: 10.1016/j.jad.2005.03.012
60. Karlović D, Serretti A, Vrkić N, Martinac M, Marčinko D. Serum concentrations of CRP, IL-6, TNF-α and cortisol in major depressive disorder with melancholic or atypical features. Psychiatry Res. (2012) 198:74–80. doi: 10.1016/j.psychres.2011.12.007
61. Kronfol Z. Immune dysregulation in major depression: a critical review of existing evidence. Int J Neuropsychopharmacol. (2022) 5:333–43. doi: 10.1017/S1461145702003024
62. Rothermundt M, Arolt V, Fenker J, Gutbrodt H, Peters M, Kirchner H. Different immune patterns in melancholic and non-melancholic mahor depression. Eur Arch Psychiatry Clin Neurosci. (2001) 251:90–7. doi: 10.1007/s004060170058
63. Rothermundt M, Arolt V, Peters M, Gutbrodt H, Fenker J, Kersting A, et al. Inflammtory makers in major depression and melancholia. J Affect Disord. (2001) 63:93–102. doi: 10.1016/s0165-0327(00)00157-9
64. Bredemeier K, Miller IW. Executive function and suicidality: a systematic qualitative review. Clin Psychol Rev. (2015) 40:170–83. doi: 10.1016/j.cpr.2015.06.005
65. Watkins HB, Meyer TD. Is there an empirical link between impulsivity and suicidality in bipolar disorders? A review of the current literature and the potential psychological implications of the relationship. Bipolar Disord. (2013) 15:542–58. doi: 10.1111/bdi.12090
66. Desmyter S, van Heeringen C, Audenaert K. Structural and functional neuroimaging studies of the suicidal brain. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:796–808. doi: 10.1016/j.pnpbp.2010.12.026
67. van Heeringen K, Mann JJ. The neurobiology of suicide. Lancet Psychiatry. (2014) 1:63–72. doi: 10.1016/S2215-0366(14)70220-2
68. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
69. Borroto-Escuela DO, Ambrogini P, Narvaez M, Di Liberto V, Beggiato S, Ferraro L, et al. Serotonin heteroreceptor complexes and their integration of signals in neurons and astroglia-relevance for mental diseases. Cells. (2021) 10:1902. doi: 10.3390/cells10081902
70. Chen B, Zhang M, Ji M, Gong W, Chen B, Zorec R, et al. The association between antidepressant effect of SSRIs and astrocytes: conceptual overview and meta-analysis of the literature. Neurochem Res. (2021) 46:2731–45. doi: 10.1007/s11064-020-03225-6
71. Hashioka S, Klegeris A, Monji A, Kato T, Sawada M, McGeer PL, et al. Antidepressants inhibit interferon-gamma-induced microglial production of IL-6 and nitric oxide. Exp Neurol. (2007) 206:33–42. doi: 10.1016/j.expneurol.2007.03.022
72. Horikawa H, Kato TA, Mizoguchi Y, Monji A, Seki Y, Ohkuri T, et al. Inhibitory effects of SSRIs on IFN-γ induced microglial activation through the regulation of intracellular calcium. Prog Neuropsychopharmacol Biol Psychiatry. (2010) 34:1306–16. doi: 10.1016/j.pnpbp.2010.07.015
73. Hwang J, Zheng LT, Ock J, Lee MG, Kim S-H, Lee H-W, et al. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology. (2008) 55:826–34. doi: 10.1016/j.neuropharm.2008.06.045
74. Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:445–50. doi: 10.1016/j.pnpbp.2007.09.015
75. Mindt S, Neumaier M, Hoyer C, Sartorius A, Kranaster L. Cytokine-mediated cellular immune activation in electroconvulsive therapy: a CSF study in patients with treatment-resistant depression. World J Biol Psychiatry. (2020) 21:139–47. doi: 10.1080/15622975.2019.1618494
76. Stelzhammer V, Guest PC, Rothermundt M, Sondermann C, Michael N, Schwarz E, et al. Electroconvulsive therapy exerts mainly acute molecular changes in serum of major depressive disorder patients. Eur Neuropsychopharmacol. (2013) 23:1199–207. doi: 10.1016/j.euroneuro.2012.10.012
77. Ryu JK, Cho T, Choi HB, Jantaratnotai N, McLarnon JG. Pharmacological antagonism of interleukin-8 receptor CXCR2 inhibits inflammatory reactivity and is neuroprotective in an animal model of Alzheimer’s disease. J Neuroinflammation. (2015) 12:144. doi: 10.1186/s12974-015-0339-z
78. Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab. (2010) 30:459–73. doi: 10.1038/jcbfm.2009.240
79. Kim J, Kim JH, Chang KA. Sex difference in peripheral inflammatory biomarker in drug-naïve patients with major depression in young adulthood. Biomedicines. (2021) 9:708. doi: 10.3390/biomedicines9070708
80. Martinez-Muniz GA, Wood SK. Sex differences in the inflammatory consequences of stress: implications for pharmacotherapy. J Pharmacol Exp Ther. (2020) 75:161–74. doi: 10.1124/jpet.120.266205
81. Mello BSF, Chaves Filho AJM, Custódio CS, Cordeiro RC, Miyajima F, Sousa FCF, et al. Sex influences in behavior and brain inflammatory and oxidative alterations in mice submitted to lipopolysaccharide-induced inflammatory model of depression. J Neuroimmunol. (2018) 320:133–42. doi: 10.1016/j.jneuroim.2018.04.009
Keywords: major depressive disorder, interleukin-8, depressive symptom, SSRIs, association
Citation: Zhu ZH, Song XY, Man LJ, Chen P, Tang Z, Li RH, Ji CF, Dai NB, Liu F, Wang J, Zhang J, Jia QF and Hui L (2022) Comparisons of Serum Interleukin-8 Levels in Major Depressive Patients With Drug-Free Versus SSRIs Versus Healthy Controls. Front. Psychiatry 13:858675. doi: 10.3389/fpsyt.2022.858675
Received: 21 January 2022; Accepted: 14 March 2022;
Published: 14 April 2022.
Edited by:
Stefania Schiavone, University of Foggia, ItalyReviewed by:
Anna Brancato, University of Palermo, ItalyCopyright © 2022 Zhu, Song, Man, Chen, Tang, Li, Ji, Dai, Liu, Wang, Zhang, Jia and Hui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Fang Jia, MTU3NzQzNzQ1NTRAcXEuY29t; Li Hui, aHVpbGkwMDQxMDBAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.