
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry, 17 March 2022
Sec. Addictive Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.829944
This article is part of the Research TopicBroadening the Scope of Addiction Medicine: Integrating Co-Morbid Conditions, Polysubstance Use, and Patient Experiences into Substance Use TreatmentView all 12 articles
Background: Although cannabis use is common in France, it is still criminalized. Cannabidiol (CBD) products, including CBD-rich cannabis, are legally available. Although previous results suggested that CBD may have benefits for people with cannabis use disorder, there is a lack of data on cannabis users who use CBD to reduce their cannabis consumption. We aimed to identify (i) correlates of this motive, and (ii) factors associated with successful attempts to reduce cannabis use.
Methods: A cross-sectional online survey among French-speaking CBD and cannabis users was conducted. Logistic regressions were performed to identify correlates of using CBD to reduce cannabis consumption and correlates of reporting a large reduction.
Results: Eleven percent (n = 105) of our study sample reported they primarily used CBD to reduce cannabis consumption. Associated factors included smoking tobacco cigarettes (adjusted odds ratio (aOR) [95% confidence interval (CI)] 2.17 [1.3–3.62], p = 0.003) and drinking alcohol (aOR [95%CI] 1.8 [1.02–3.18], p = 0.042). Of these 105, 83% used CBD-rich cannabis to smoke, and 58.7% reported a large reduction in cannabis consumption. This large reduction was associated with non-daily cannabis use (aOR [95%CI] 7.14 [2.4–20.0], p < 0.001) and daily CBD use (aOR [95%CI] 5.87 [2.09–16.47], p = 0.001). A reduction in cannabis withdrawal symptoms thanks to CBD use was the most-cited effect at play in self-observed cannabis reduction.
Conclusions: Cannabis use reduction is a reported motive for CBD use—especially CBD-rich cannabis to smoke—in France. More studies are needed to explore practices associated with this motive and to accurately assess CBD effectiveness.
Cannabis use is being increasingly liberalized worldwide (1), and cannabidiol (CBD) products are proliferating (2). Recent trends in Europe and the U.S. suggest an increase in the prevalence of cannabis use disorders (CUD) (3–5), for which there is still no approved pharmaceutical treatment. Preliminary data have highlighted that CBD has benefits in CUD treatment (6). Evidence is also growing that nabiximols—an oromucosal spray providing a balanced mixture of tetrahydrocannabinol (THC) and CBD—brings benefits in CUD treatment (7–11). However, little is known about cannabis users who use CBD to reduce their cannabis use (12).
Cannabis use is still criminalized in France, including for therapeutic purposes. Users may be punished by up to 1 year in prison and a fine of 3,750 € (13); since 2020, an on-the-spot fine of 150 € can replace the normal procedure at the police's discretion (14). Despite this criminalization, France has the highest prevalence of cannabis use among young people and adults in Europe (15), and indicators of cannabis use disorder and treatment for dependence are on the rise (16). The demand for herbal cannabis is also growing, as is its potency (17). A similar trend in increasing potency has been observed internationally (18, 19).
Despite strong development of the CBD market internationally—including in France—in recent years (2), the legal status of CBD products still remains unclear. Recent rulings by the Court of Justice of the European Union (20) and the French Court of Cassation (21) confirmed that CBD products legally produced in the European Union can be sold in France. The legal status of cannabis flowers with <0.2% of THC—which are widely marketed in France—is still unclear.
Given this context, we aimed to investigate whether some French CBD users consume this phytocannabinoid to reduce cannabis consumption, and to identify potential correlates for this motive. We also aimed to document the pattern of CBD use associated with this motive, and to describe the effects at play in reducing cannabis consumption, as reported by users.
An online survey written in French was conducted using a Google form between April 23, 2020 and March 30, 2021. The protocol followed the guidelines of the Declaration of Helsinki, and the INSERM Ethics Committee provided ethical approval (approval #20-677 dated April 23, 2020). A link to the survey was distributed via media outlets specializing in cannabis-based products, CBD user groups on Facebook, and a community of people with chronic health conditions. Inclusion criteria for the present study were: previous-month CBD use and lifetime illegal cannabis use.
In the survey, the acronym “CBD” was used to include all legal products marketed as containing a significant amount of CBD, irrespective of their actual CBD content. This therefore covered legal CBD-rich THC-low (<0.2%) cannabis to smoke (called “CBD-rich cannabis” in this manuscript), as opposed to “regular” high THC cannabis (called “illegal cannabis” here). The survey collected self-reported data on the following: socio-demographic and substance use (cannabis, CBD, alcohol, tobacco) characteristics, preferred mode of CBD use, and primary reason for CBD use. The latter was collected using the question “In the past 30 days, why have you used CBD?”. Only one answer was allowed from a list of options which included “to reduce the use of tobacco or other substances (illegal cannabis, alcohol, etc.)” (Supplementary Table 1). People who ticked this answer were then asked if they used CBD for illegal cannabis use reduction. Those who replied “yes”, were then asked (i) to what extent CBD had an impact on their illegal cannabis use (“large reduction/moderate reduction/no effect/moderate increase/large increase/I do not know”; these answer options were dichotomized into “large reduction” vs. “no large reduction” (i.e., all other answers)), and (ii) which CBD-related effects were involved in reducing their illegal cannabis use (“In your opinion, what CBD-related effects were at play in reducing your illegal cannabis use?”). Participants could choose several responses from the following four pre-determined options: “using less illegal cannabis in a joint,” “longer time between smoking two joints of illegal cannabis.” “reduction in illegal cannabis withdrawal symptoms,” and “longer time before smoking first joint of the day”.
Two principal outcomes were built. The first was “using CBD for illegal cannabis use reduction”, as regarded the whole of the study sample. The second was “reporting a large reduction in cannabis consumption thanks to CBD use”, and regarded only the sub-sample of respondents who answered “yes” to the question for the first outcome.
We characterized users using CBD as a means to reduce their illegal cannabis use by comparing their socio-demographic and socio-behavioral characteristics with the rest of the sample using a Chi-square (categorical variables) or Wilcoxon's (continuous variables) test. We then performed a logistic regression with “having used CBD to reduce illegal cannabis use” as an outcome and socio-demographic and behavioral characteristics as explanatory variables (Figure 1). For the sub-population who reported this reason, we performed a second logistic regression with “reporting a large reduction in illegal cannabis following CBD use” as the outcome, and variables related to CBD use as explanatory variables (Figure 1). For both regressions, only variables with a liberal p-value < 0.20 in the univariable analyses were considered eligible for the multivariable model. The final multivariable model was built using a backward stepwise procedure. The likelihood ratio test (p < 0.05) was used to define the variables to maintain in the final model.
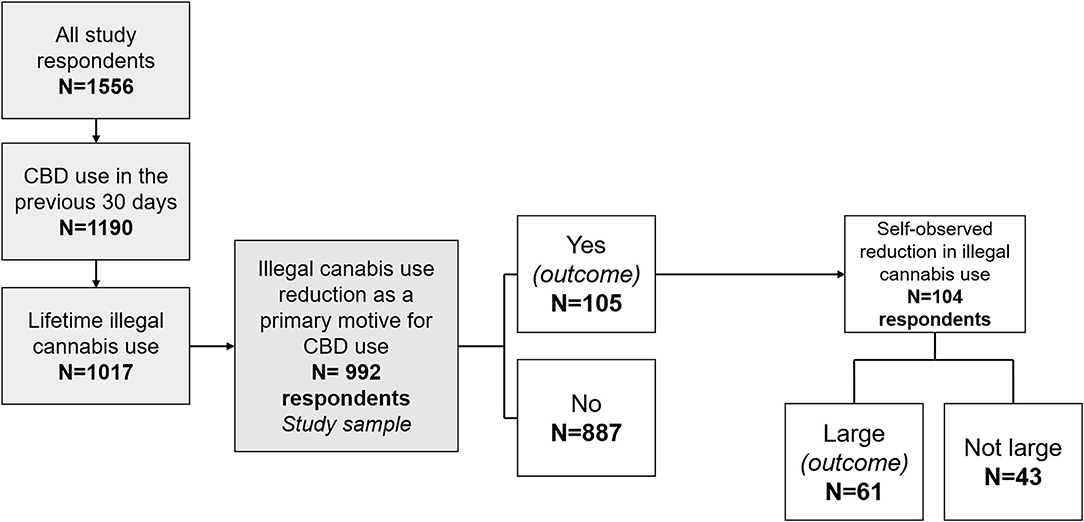
Figure 1. Flow chart of the study, design of the regression outcomes, and distribution of participants according to the two study outcomes. CBD, cannabidiol.
We also provided a description of the self-reported CBD-related effects at play to reduce illegal cannabis consumption, and used Chi-square tests to compare these effects between the group of participants reporting a large reduction in illegal cannabis use and those who did not.
Among the 1,556 respondents, 1,190 participants used CBD in the 30 days before the survey (Figure 1). Of the 1,017 of the latter who reported lifetime illegal cannabis use, 992 answered the question related to the primary reason why they used CBD. Study sample characteristics are provided in Table 1. Our study sample consisted in 992 CBD (and lifetime illegal cannabis) users. Most were men (74.5%), median age was 34 years, and most resided in France (96.5%). Over 10% (10.6% (n = 105)) reported having used CBD in the previous month primarily to reduce their illegal cannabis consumption. The vast majority of the study sample (99.4%) had used illegal cannabis before their first use of CBD. Among those who reported illegal cannabis reduction as their primary reason to use CBD, 66.7 and 8.8% had also used it for tobacco and alcohol use reduction, respectively.
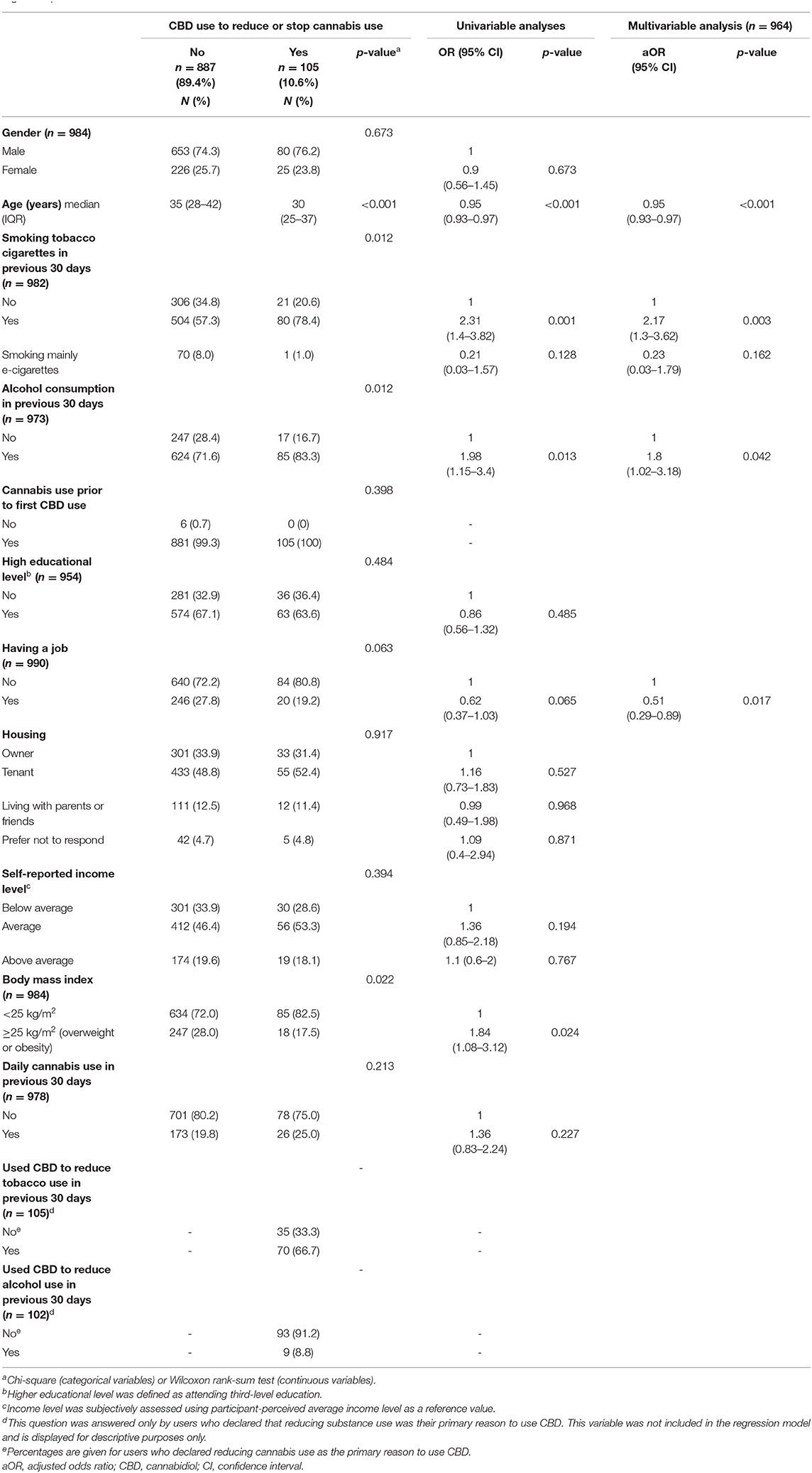
Table 1. Study sample socio-demographic and behavioral characteristics and factors associated with the use of cannabidiol to reduce cannabis consumption (logistic regression).
In multivariable analysis, declaring to use CBD primarily to reduce illegal cannabis consumption was associated with younger age, tobacco cigarette smoking in the previous month, alcohol drinking in the previous month, and not having a job (Table 1).
Among those who declared using CBD to reduce their illegal cannabis, half (51.5%) had used CBD for less than a year, and 38.5% had used it every day in the previous month (Table 2). Sixty-one (58.7%) reported that their CBD use led to a large reduction in illegal cannabis consumption, 36 (34.6%) a moderate reduction, 6 (5.8%) no reduction, and 1 (1.0%) a moderate increase (1 missing value). Most (84.3%) smoked CBD-rich cannabis, while only 7.8% administered it orally (Table 2). A large majority (94.0%) of those who smoked CBD-rich cannabis mixed it into joints (i.e., together with tobacco or illegal cannabis).
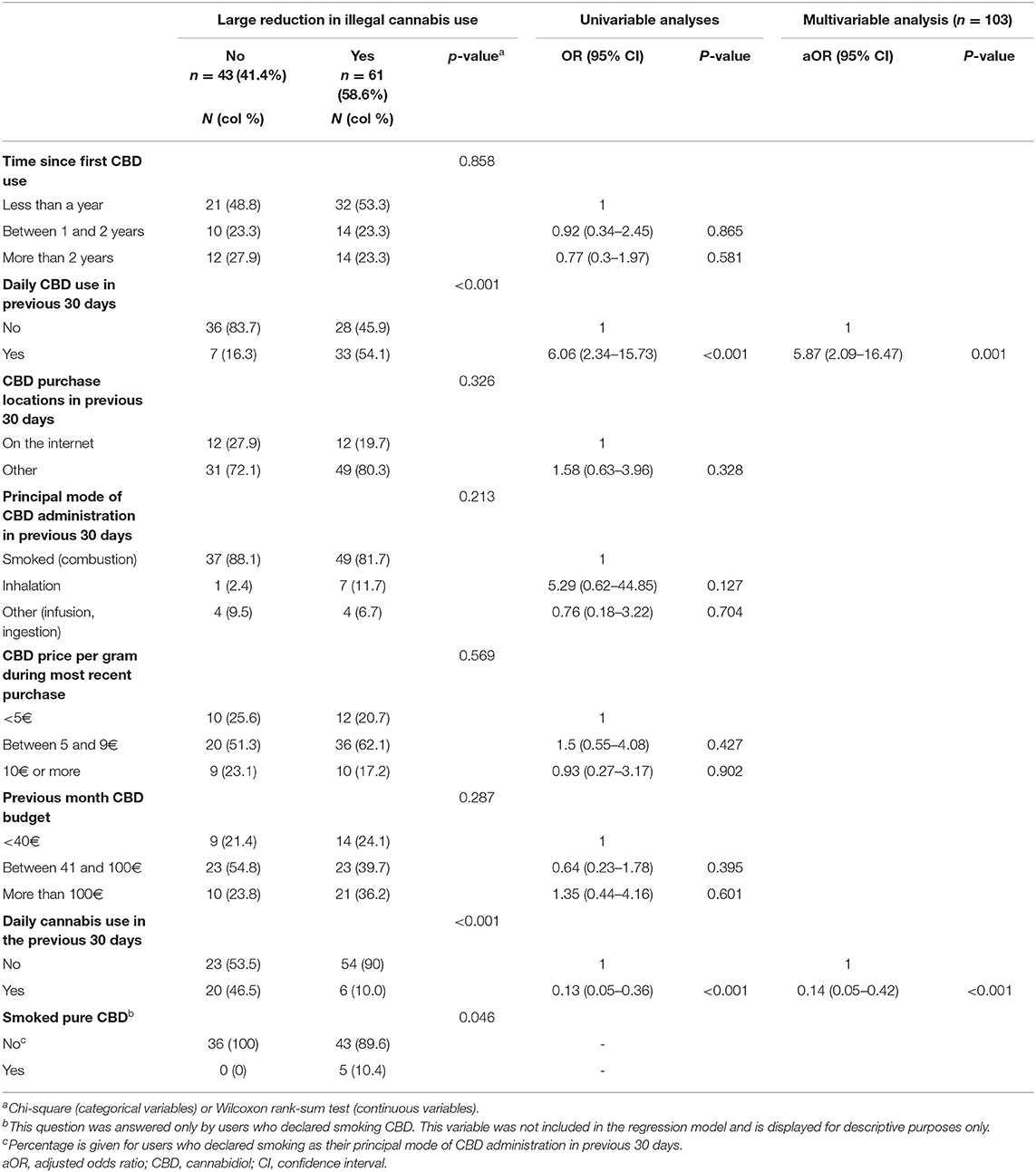
Table 2. Cannabidiol products pattern of use among users who used cannabidiol to reduce illegal cannabis consumption, and factors associated with a large reduction in illegal cannabis consumption.
In multivariable analysis, declaring a large reduction (vs. no large reduction) was associated with daily CBD use in the previous month, and non-daily use of illegal cannabis (Table 2).
The self-reported CBD-related effect involved in illegal cannabis use reduction most frequency cited was “reducing cannabis withdrawal symptoms” (44.2%), followed by “delaying first illegal cannabis joint of the day” (24.0%), “using less illegal cannabis in joints” (21.2%) and “increasing the time between smoking joints” (16.3%) (Table 3). Participants reporting a large reduction in illegal cannabis use were more likely to quote “reducing cannabis withdrawal symptoms” as an effect (p <10−3) but less likely to report “delaying first illegal cannabis joint of the day” (p = 0.008; Table 3).
In a sample of 992 CBD and lifetime illegal cannabis users mostly based in France, we found that using CBD to reduce illegal cannabis use was associated with tobacco smoking, alcohol use and not having a job. Moreover, a large self-reported reduction in illegal cannabis reduction was associated with daily CBD use and non-daily use of illegal cannabis. Finally, in users who used CBD to reduce illegal cannabis consumption, the most common route of CBD administration was smoking (84.3% of all respondents). A reduction in cannabis withdrawal symptoms was the most quoted self-reported CBD-related effect involved in cannabis use reduction (44.2%).
We found that among French CBD and illegal cannabis users, polysubstance use (tobacco and alcohol) is associated with the motivation to reduce illegal cannabis consumption. Interestingly, most of those who reported this motive also reported using CBD to try to cut down or stop tobacco use (few had done so for alcohol use). This would suggest that these CBD users commonly try to reduce their overall smoking (i.e., cannabis and tobacco) behavior. This is very interesting, given that both products are commonly co-consumed (22), and that the continued use of one substance is a barrier to reducing or quitting the other (23) (something already documented for polysubstance use (24, 25)).
The positive association between not having a job and desire to cut down on/stop cannabis consumption through CBD use may seem counter-intuitive given that cannabis is frequently used to cope with stress, and that unemployment is linked with stress. Two hypotheses can be made to explain this association. The first is that the desire to reduce cannabis use may be the result of losing one's job because of cannabis use (26, 27). The second is that unemployed persons may desire to cut down on cannabis-related expenditures because of financial difficulties. Indeed, previous work highlighted that unemployed cannabis buyers were more likely to spend a larger part of their income on cannabis (28). However, as a large majority of the whole study sample was unemployed, this result we found may also be a consequence of biased participant sampling.
We found that a large reduction in illegal cannabis consumption was associated with daily CBD use, which suggests a dose-dependent effect of CBD. This relationship was not observed for high CBD doses (400 and 800 mg) in a phase 2a placebo-controlled randomized trial (6). However, it is possible that having multiple intakes per day enables users to maintain stable CBD plasma levels—and physiological effects—throughout the day. After inhalation, CBD plasma peak is attained within 10 min, with a half-life of ~30 h (29). Moreover, the fact that non-daily illegal cannabis users were more likely to declare a large reduction in cannabis use suggests that the higher the frequency of cannabis use, the more difficult it is to change one's cannabis use pattern; this is probably related to cannabis dependence. In studies elsewhere, the frequency of cannabis flower use was associated with problematic cannabis use (30), the frequency of high-potency cannabis use predicted greater dependence (31), and greater monthly THC exposure was associated with more symptoms of dependence (32).
A few elements in our analysis suggest that CBD-rich cannabis was partially substituted for illegal cannabis in our study sample. First, in the group that used CBD to reduce illegal cannabis use, a majority smoked CBD-rich cannabis. Second, only 6% of the latter smoked “pure” (i.e., non-mixed) CBD-rich cannabis, which means that in almost all cases, it was mixed with either tobacco or illegal cannabis. Third, over 20% of the sub-sample which used CBD to reduce their illegal use declared that it helped them use less illegal cannabis in their joints.
The substitution practice mentioned above should be considered in the context of cannabis use disorder treatment and associated psychiatric outcomes. For example, replacing high-potency cannabis with CBD-rich cannabis would mean a reduction in THC exposure while preserving the gesture and the sensory dimensions of cannabis use. This reduction would likely reduce anxiety and depression in people with cannabis use disorder (33, 34), and be an acceptable and therefore achievable treatment goal for treatment-seeking users (35). Such a reduction in exposure to THC may also lead to cannabis abstinence. These various possibilities need to be clinically tested.
The dominance of smoked CBD-rich cannabis (i.e., as opposed to oral intake) in our study sample is of particular interest, as in France, CBD-rich cannabis is the only cannabis legally available. The global tendency of rising THC levels (i.e., higher potency) and decreasing CBD levels in illegal cannabis (17–19) comes fuel concerns over cannabis use-related harms, as THC is the compound responsible for cannabis use disorder (36, 37). Highly-potent cannabis consumption has been associated with higher risks of cannabis use problems and anxiety disorders (38) as well as psychosis (39, 40). Conversely, CBD seems to attenuate THC-related psychotic-like effects, memory problems (especially in light users), paranoia, anxiety and cannabis-related psychological wellbeing impairment (41–44). This could be due to functional interactions between THC and CBD (45). However, more research is needed to fully elucidate how CBD influences the effects of THC (46). Low-potency cannabis has been described as being one way to reduce cannabis-related health risks (avoiding daily use and combusted cannabis inhalation being two other ways) (47). Accordingly, for cannabis users in France—a population which must choose between illegal high-potency and legal CBD-rich cannabis—mixing both products may be a way for them to create low-risk cannabis, or to move toward creating a “smoking version” of nabiximols. Accordingly, Gibson et al., in the U.S., found that THC + CBD chemovar (9% THC, 10% CBD, from local and legal dispensary) was associated with similar levels of positive subjective effects, but significantly less paranoia and anxiety, as compared to the THC-dominant chemovar (44).
A recent U.S. study also found that CBD and cannabis co-users reported a high proportion of CBD smoking administration (48).
Given that CBD-rich cannabis is sold as the same type of product (i.e., in herbal form) as illegal cannabis, it can be incorporated into one's smoking habits. Moreover, results from previous studies on tobacco smokers suggested that the sensations which smoking creates in the airways contribute to short-term satisfaction, the rewarding effect, and reduced craving (49–51). One can therefore suppose that smoking CBD-rich cannabis may be “beneficial” as part of a strategy to lower exposure to THC: by preserving the smoking-related airway sensation as well as the terpene-related taste (52–54), a minimal reduction in the satisfaction experienced from the act of smoking may be derived from THC-low cannabis as compared to THC-high cannabis (44). In reality, smoking cannabis exposes persons to harmful substances, including carcinogens (55–57). This route of administration is therefore inadvisable, in favor of smoke-free inhalation (58) or oromucosal administration (29).
Our study has several limitations. First, the non-representativeness of our sample of cannabis users in France limits the generalizability of our results, and highlights the need for study duplication. For instance, participants with no job appeared over-represented. Second, we had no data to enable us to detect cannabis use disorder in our sample. However, we did have frequency of use data, which is a good proxy for problematic and low-risk cannabis use (47, 59, 60). Third, we used self-assessed changes (reduction/no change/increase) in cannabis use and had no data on the contextual elements of these changes. Accordingly, we were not able to deduce to what extent CBD was clinically useful in attempts to cut down on cannabis use. Finally, data on the levels of CBD in products consumed by the participants were not available, which limits the solidity of our conclusions. CBD content is highly variable among different products, including cannabis flowers (61–63). For instance, in a large Italian study on THC-low cannabis products, authors found a mean CBD concentration of 4% in the sub-sample (n = 185) of flowers with a THC level under 0.2% (i.e., which would be legal in France), with a strong linear correlation between CBD and THC concentrations (personal communication from (64)). As in the survey “CBD” refers to all CBD-based products irrespective of their actual CBD content, answers given by participants may refer to the use of CBD-low products (e.g., THC-low CBD-low legal cannabis flowers or oil with low CBD concentration). Therefore, the effects we reported should be cautiously attributed to CBD-rich cannabis/products.
The main strength of our study is the explorative and original nature of the data; while the use of CBD and CBD-rich cannabis has previously been reported for opioid and pain medication substitution in people with fibromyalgia (65), and the use of nabiximols clinically investigated elsewhere (7), to the best of our knowledge, the substitution of illegal cannabis with CBD has not been previously investigated.
Our findings have many implications. First, we found that some CBD users in France are using the phytocannabinoid in an “off-label” fashion to reduce their illegal cannabis consumption. Further studies should be implemented to confirm and quantify to what extent CBD or nabiximols can in fact accomplish this task. Second, in countries where cannabis use is criminalized but not CBD-rich cannabis, the latter may represent an acceptable tool for THC-related harm reduction. With this in mind, any ban on smokable CBD products could reduce the number of consumers able to reduce their illegal cannabis consumption through CBD use. Bans could also prevent people who smoke cannabis for therapeutic purposes from adjusting their THC/CBD ratio to optimize benefits (62). Finally, non-smoking (e.g., oromucosal) routes of CBD administration to users who wish to reduce their cannabis consumption should be promoted to reduce health-related risks.
To conclude, CBD is used by some illegal cannabis users in France—especially alcohol and tobacco co-users—who wish to reduce their cannabis consumption. In our study, CBD was mainly smoked (i.e., CBD-rich cannabis), and seemed to contribute to cannabis use reduction by lowering cannabis withdrawal symptoms. More studies are needed to explore practices associated with CBD use to reduce cannabis consumption, and to accurately assess its effectiveness.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was reviewed and approved by INSERM Ethics Committee provided ethical approval (approval #20-677 dated April 23, 2020). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
DF designed the study, interpreted the data, and reviewed the manuscript. VD performed the statistical analyses. SM, YB, and PC designed the study and reviewed the manuscript. TB interpreted the data and wrote the manuscript draft and reviewed it. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all the study participants. We also thank to Jude Sweeney (Milan, Italy) for the English revision and copyediting of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.829944/full#supplementary-material
1. Melchior M, Nakamura A, Bolze C, Hausfater F, Khoury FE, Mary-Krause M, Silva MAD. Does liberalisation of cannabis policy influence levels of use in adolescents and young adults? A systematic review and meta-analysis. BMJ Open. (2019) 9:880. doi: 10.1136/bmjopen-2018-025880
2. Walker LA, Koturbash I, Kingston R, ElSohly MA, Yates CR, Gurley BJ, et al. Cannabidiol (CBD) in dietary supplements: perspectives on science, safety, and potential regulatory approaches. J Diet Suppl. (2020) 17:493–502. doi: 10.1080/19390211.2020.1777244
3. United Nations Office on Drugs Crime. World Drug Report 2020, Second Booklet: Drug Use and Health Consequences. Vienna: UNODC (2020). Available online at: https://wdr.unodc.org/wdr2020/field/WDR20_Booklet_2.pdf (accessed September 14, 2021).
4. Manthey J. Cannabis use in Europe: current trends and public health concerns. Int J Drug Policy. (2019) 68:93–6. doi: 10.1016/j.drugpo.2019.03.006
5. Hasin DS, Shmulewitz D, Sarvet AL. Time trends in US cannabis use and cannabis use disorders overall and by sociodemographic subgroups: a narrative review and new findings. Am J Drug Alcohol Abuse. (2019) 45:623–43. doi: 10.1080/00952990.2019.1569668
6. Freeman TP, Hindocha C, Baio G, Shaban NDC, Thomas EM, Astbury D, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. (2020) 7:865–74. doi: 10.1016/S2215-0366(20)30290-X
7. Lintzeris N, Mills L, Dunlop A, Copeland J, Mcgregor I, Bruno R, et al. Cannabis use in patients 3 months after ceasing nabiximols for the treatment of cannabis dependence: results from a placebo-controlled randomised trial. Drug Alcohol Depend. (2020) 215:108220. doi: 10.1016/j.drugalcdep.2020.108220
8. Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: a pilot randomized clinical trial. PLoS ONE. (2018) 13:e0190768. doi: 10.1371/journal.pone.0190768
9. Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. (2014) 71:281–91. doi: 10.1001/jamapsychiatry.2013.3947
10. Allsop D, Lintzeris N, Copeland J, Dunlop A, McGregor I. Cannabinoid replacement therapy (CRT): nabiximols (Sativex) as a novel treatment for cannabis withdrawal. Clin Pharmacol Therap. (2015) 97:571–4. doi: 10.1002/cpt.109
11. Lintzeris N, Bhardwaj A, Mills L, Dunlop A, Copeland J, McGregor I, et al. Nabiximols for the treatment of cannabis dependence: a randomized clinical trial. JAMA Intern Med. (2019) 179:1242–53. doi: 10.1001/jamainternmed.2019.1993
12. Zobel F, Notari L, Schneider E, Rudmann O. Cannabidiol (CBD): Analyse de Situation. [Rapport de recherche]. Lausanne: Addiction Suisse (2019). Available online at: https://idpc.net/fr/publications/2019/02/cannabidiol-cbd-analyse-de-situation (accessed February 13, 2022).
13. Massin S, Carrieri MP, Roux P, De. jure decriminalisation of cannabis use matters: some recent trends from France. Int J Drug Policy. (2013) 24:634–5. doi: 10.1016/j.drugpo.2013.04.008
14. LOI n° 2019-222 du 23 mars 2019 de programmation 2018-2022 et de réforme pour la justice. (2019). Available online at: https://www.legifrance.gouv.fr/dossierlegislatif/JORFDOLE000036830320/ (accessed July 02, 2021).
15. European Monitoring Centre for Drugs Drug Addiction. European Drug Report 2019: Trends and Developments. Lisbon: EMCDDA (2019). Available online at: https://www.emcdda.europa.eu/publications/edr/trends-developments/2019_en (accessed July 12, 2021).
16. Observatoire Français des Drogues et des Toxicomanies. Drugs, Key Data 2019. OFDT. (2019). Available online at: https://en.ofdt.fr/publications/drugs-key-data/8th-edition-june-2019/ (accessed July 12, 2021).
17. Gandilhon M, Spilka S, Masson C. Les mutations du marché du cannabis en France (transformations of French cannabis market). OFDT (2019). Available online at: https://www.ofdt.fr/publications/collections/rapports/thema/les-mutations-du-marche-du-cannabis-en-france-thema/ (accessed April 27, 2021).
18. Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008-2017). Eur Arch Psychiatry Clin Neurosci. (2019) 269:5–15. doi: 10.1007/s00406-019-00983-5
19. Freeman TP, Craft S, Wilson J, Stylianou S, ElSohly M, Forti MD, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: systematic review and meta-analysis. Addiction. (2021) 116:1000–10. doi: 10.1111/add.15253
20. Court of Justice of the European Union. In Case C-663/18, REQUEST for a Preliminary Ruling Under Article 267 TFEU from the Cour d'appel d'Aix-en-Provence (Court of Appeal, Aix-en-Provence, France), made by Decision of 23 October 2018, received at the Court on 23 October 2018, in the Criminal Proceedings Against BS, CA. Available online at: https://curia.europa.eu/juris/document/document.jsf?text=&docid=233925&pageIndex=0&doclang=EN&mode=lst&dir=&occ=first&part=1&cid=15340648 (accessed April 27, 2021).
21. Cour de cassation, criminelle Chambre, criminelle, 23 juin, 2021, 20-84.212. Publié au bulletin (2021). Available online at: https://www.legifrance.gouv.fr/juri/id/JURITEXT000043711045?isSuggest=true (accessed July 1, 2021).
22. Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review. Addiction. (2012) 107:1221–33. doi: 10.1111/j.1360-0443.2012.03837.x
23. Voci S, Zawertailo L, Baliunas D, Masood Z, Selby P. Is cannabis use associated with tobacco cessation outcome? An observational cohort study in primary care. Drug Alcohol Depend. (2020) 206:107756. doi: 10.1016/j.drugalcdep.2019.107756
24. McCabe SE, West BT. The three-year course of multiple substance use disorders in the united states: a national longitudinal study. J Clin Psychiatry. (2017) 78:e537–44. doi: 10.4088/JCP.16m10657
25. Crummy EA, O'Neal TJ, Baskin BM, Ferguson SM. One is not enough: understanding and modeling polysubstance use. Front Neurosci. (2020) 14:e00569. doi: 10.3389/fnins.2020.00569
26. Airagnes G, Lemogne C, Meneton P, Plessz M, Goldberg M, Hoertel N, et al. Alcohol, tobacco and cannabis use are associated with job loss at follow-up: findings from the CONSTANCES cohort. PLoS ONE. (2019) 14:e0222361. doi: 10.1371/journal.pone.0222361
27. Okechukwu CA, Molino J, Soh Y. Associations between marijuana use and involuntary job loss in US-representative longitudinal and cross-sectional samples. J Occup Environ Med. (2019) 61:21–8. doi: 10.1097/JOM.0000000000001463
28. Wilkins C, Sweetsur P. Individual dollar expenditure and earnings from cannabis in the New Zealand population. Int J Drug Policy. (2007) 18:187–93. doi: 10.1016/j.drugpo.2006.11.002
29. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. (2018) 84:2477–82. doi: 10.1111/bcp.13710
30. Steeger CM, Hitchcock LN, Bryan AD, Hutchison KE, Hill KG, Bidwell LC. Associations between self-reported cannabis use frequency, potency, and cannabis/health metrics. Int J Drug Policy. (2021) 97:103278. doi: 10.1016/j.drugpo.2021.103278
31. Freeman TP, Winstock AR. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol Med. (2015) 45:3181–9. doi: 10.1017/S0033291715001178
32. van der Pol P, Liebregts N, Brunt T, van Amsterdam J, de Graaf R, Korf DJ, et al. Cross-sectional and prospective relation of cannabis potency, dosing and smoking behaviour with cannabis dependence: an ecological study. Addiction. (2014) 109:1101–9. doi: 10.1111/add.12508
33. Mooney LJ, Zhu Y, Yoo C, Valdez J, Moino K, Liao J-Y, et al. Reduction in cannabis use and functional status in physical health, mental health, and cognition. J Neuroimmune Pharmacol. (2018) 13:479–87. doi: 10.1007/s11481-018-9813-6
34. Hser Y-I, Mooney LJ, Huang D, Zhu Y, Tomko RL, McClure E, et al. Reductions in cannabis use are associated with improvements in anxiety, depression, and sleep quality, but not quality of life. J Subst Abuse Treat. (2017) 81:53–8. doi: 10.1016/j.jsat.2017.07.012
35. Lozano BE, Stephens RS, Roffman RA. Abstinence and moderate use goals in the treatment of marijuana dependence. Addiction. (2006) 101:1589–97. doi: 10.1111/j.1360-0443.2006.01609.x
36. Kroon E, Kuhns L, Hoch E, Cousijn J. Heavy cannabis use, dependence and the brain: a clinical perspective. Addiction. (2020) 115:559–72. doi: 10.1111/add.14776
37. Zehra A, Burns J, Liu CK, Manza P, Wiers CE, Volkow ND, et al. Cannabis addiction and the brain: a review. J Neuroimmune Pharmacol. (2018) 13:438–52. doi: 10.1007/s11481-018-9782-9
38. Hines LA, Freeman TP, Gage SH, Zammit S, Hickman M, Cannon M, et al. Association of high-potency cannabis use with mental health and substance use in adolescence. JAMA Psychiatry. (2020) 77:1044–51. doi: 10.1001/jamapsychiatry.2020.1035
39. Forti MD, Quattrone D, Freeman TP, Tripoli G, Gayer-Anderson C, Quigley H, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry. (2019) 6:427–36. doi: 10.1016/S2215-0366(19)30048-3
40. Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. (2015) 2:233–8. doi: 10.1016/S2215-0366(14)00117-5
41. Morgan CJA, Gardener C, Schafer G, Swan S, Demarchi C, Freeman TP, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med. (2012) 42:391–400. doi: 10.1017/S0033291711001322
42. Morgan CJA, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry. (2010) 197:285–90. doi: 10.1192/bjp.bp.110.077503
43. Morgan CJA, Freeman TP, Hindocha C, Schafer G, Gardner C, Curran HV. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry. (2018) 8:181. doi: 10.1038/s41398-018-0191-x
44. Gibson LP, Karoly HC, Ellingson JM, Klawitter J, Sempio C, Squeri JE, et al. Effects of cannabidiol in cannabis flower: implications for harm reduction. Addict Biol. (2022) 27:e13092. doi: 10.1111/adb.13092
45. Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and preclinical evidence for functional interactions of cannabidiol and Δ9-Tetrahydrocannabinol. Neuropsychopharmacology. (2018) 43:142–54. doi: 10.1038/npp.2017.209
46. Freeman AM, Petrilli K, Lees R, Hindocha C, Mokrysz C, Curran HV, et al. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci Biobehav Rev. (2019) 107:696–712. doi: 10.1016/j.neubiorev.2019.09.036
47. Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, et al. Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am J Public Health. (2017) 107:e1–e12. doi: 10.2105/AJPH.2017.303818
48. Vilches JR, Taylor MB, Filbey FM. A multiple correspondence analysis of patterns of CBD use in hemp and marijuana users. Front Psychiatry. (2021) 11:1583. doi: 10.3389/fpsyt.2020.624012
49. Westman EC, Behm FM, Rose JE. Airway sensory replacement as a treatment for smoking cessation. Drug Dev Res. (1996) 38:257–262. doi: 10.1002/(SICI)1098-2299(199607/08)38:3/4lt257::AID-DDR14>3.0.CO;2-W
50. Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. (1996) 53:309–15. doi: 10.1016/0091-3057(95)02027-6
51. Naqvi NH, Bechara A. The airway sensory impact of nicotine contributes to the conditioned reinforcing effects of individual puffs from cigarettes. Pharmacol Biochem Behav. (2005) 81:821–9. doi: 10.1016/j.pbb.2005.06.005
52. Fischedick JT, Hazekamp A, Erkelens T, Choi YH, Verpoorte R. Metabolic fingerprinting of Cannabis sativa L, cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. (2010) 71:2058–73. doi: 10.1016/j.phytochem.2010.10.001
53. Booth JK, Bohlmann J. Terpenes in Cannabis sativa- From plant genome to humans. Plant Sci. (2019) 284:67–72. doi: 10.1016/j.plantsci.2019.03.022
54. Casano S, Grassi G, Martini V, Michelozzi M. Variations in terpene profiles of different strains of Cannabis sativa L. Acta Hortic. (2011) 925:115–21. doi: 10.17660/ActaHortic.2011.925.15
55. Tashkin DP, Roth MD. Pulmonary effects of inhaled cannabis smoke. Am J Drug Alcohol Abuse. (2019) 45:596–609. doi: 10.1080/00952990.2019.1627366
56. Wei B, Smith D, O'Connor R, Travers MJ, Hyland A. Examining the association between body burdens of harmful chemicals and heaviness of marijuana smoking. Chem Res Toxicol. (2018) 31:643–5. doi: 10.1021/acs.chemrestox.8b00160
57. Graves BM, Johnson TJ, Nishida RT, Dias RP, Savareear B, Harynuk JJ, et al. Comprehensive characterization of mainstream marijuana and tobacco smoke. Sci Rep. (2020) 10:7160. doi: 10.1038/s41598-020-63120-6
58. Lanz C, Mattsson J, Soydaner U, Brenneisen R. Medicinal cannabis: in vitro validation of vaporizers for the smoke-free inhalation of cannabis. PLoS ONE. (2016) 11:e0147286. doi: 10.1371/journal.pone.0147286
59. Brezing CA, Choi CJ, Pavlicova M, Brooks D, Mahony AL, Mariani JJ, et al. Abstinence and reduced frequency of use are associated with improvements in quality of life among treatment-seekers with cannabis use disorder. Am J Addict. (2018) 27:101–7. doi: 10.1111/ajad.12660
60. Liao J-Y, Mooney LJ, Zhu Y, Valdez J, Yoo C, Hser Y-I. Relationships between marijuana use, severity of marijuana-related problems, and health-related quality of life. Psychiatry Res. (2019) 279:237–43. doi: 10.1016/j.psychres.2019.03.010
61. Vergara D, Bidwell LC, Gaudino R, Torres A, Du G, Ruthenburg TC, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Sci Rep. (2017) 7:46528. doi: 10.1038/srep46528
62. Zeyl V, Sawyer K, Wightman RS. What do you know about maryjane? A systematic review of the current data on the THC:CBD ratio. Subst Use Misuse. (2020) 55:1223–7. doi: 10.1080/10826084.2020.1731547
63. Jikomes N, Zoorob M. The cannabinoid content of legal cannabis in washington state varies systematically across testing facilities and popular consumer products. Sci Rep. (2018) 8:4519. doi: 10.1038/s41598-018-22755-2
64. Dei Cas M, Casagni E, Saccardo A, Arnoldi S, Young C, Scotti S, et al. The Italian panorama of cannabis light preparation: determination of cannabinoids by LC-UV. Foren Sci Int. (2020) 307:110113. doi: 10.1016/j.forsciint.2019.110113
Keywords: cannabidiol (CBD), cannabis (marijuana), cannabis use disorder (CUD), smoking, France, harm reduction
Citation: Fortin D, Di Beo V, Massin S, Bisiou Y, Carrieri P and Barré T (2022) A “Good” Smoke? The Off-Label Use of Cannabidiol to Reduce Cannabis Use. Front. Psychiatry 13:829944. doi: 10.3389/fpsyt.2022.829944
Received: 06 December 2021; Accepted: 18 February 2022;
Published: 17 March 2022.
Edited by:
Matthew S. Ellis, Washington University School of Medicine in St. Louis, United StatesReviewed by:
Gentian Vyshka, University of Medicine, Tirana, AlbaniaCopyright © 2022 Fortin, Di Beo, Massin, Bisiou, Carrieri and Barré. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tangui Barré, dGFuZ3VpLmJhcnJlQGluc2VybS5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.