
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry, 06 May 2022
Sec. Molecular Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.822382
This article is part of the Research TopicTranslational Implications of Immunopsychiatry in Psychiatric Clinical PracticeView all 3 articles
There is evidence that chronic fatigue spectrum disorders (CFAS-Ds), including myalgic encephalomyelitis (ME), chronic fatigue syndrome (CFS), and chronic fatigue with physiosomatic symptoms including when due to comorbid medical disease, are characterized by neuroimmune and neuro-oxidative biomarkers. This study was performed to delineate the protein–protein interaction (PPI) network of CFAS-D and to discover the pathways, molecular patterns, and domains enriched in their PPI network. We performed network, enrichment, and annotation analyses using differentially expressed proteins and metabolics, which were established in patients with CFAS-D. The PPI network analysis revealed that the backbone of the highly connective CFAS-D network comprises NFKB1, CTNNB1, ALB, peroxides, NOS2, tumor necrosis factor (TNF), and interleukin-6 (IL-6) and that the network comprises interconnected immune-oxidative-nitrosative and Wnt/β-catenin subnetworks. Multiomics enrichment analysis shows that the CFAS-D network is highly significantly associated with cellular (antioxidant) detoxification, hydrogen peroxide metabolic process, peroxidase and oxidoreductase activity, interleukin-10 (IL-10) anti-inflammatory signaling and neurodegenerative canonical Wnt, the β-catenin complex, cadherin domains, cell–cell junctions and TLR2/4 pathways, and the transcription factors nuclear factor kappa B (NF-κB) and RELA. The top 10 DOID annotations of the CFAS-D network include four intestinal, three immune system disorders, cancer, and infectious disease. The custom Gene Ontology (GO) term annotation analysis revealed that the CFAS-D network is associated with a response to a toxic substance, lipopolysaccharides, bacterium, or virus. In conclusion, CFAS-D may be triggered by a variety of stimuli and their effects are mediated by aberrations in the cross-talks between redox, NF-κB, and Wnt/β-catenin signaling pathways leading to dysfunctions in multicellular organismal homeostatic processes.
Contemporary study of chronic fatigue spectrum disorders (CFAS-Ds), including myalgic encephalomyelitis (ME), chronic fatigue syndrome (CFS), and chronic fatigue (CF) and associated and associated physiosomatic (psychosomatic) symptoms, is plagued by a cacophony of controversies. These different approaches, sometimes even competing, comprise folk psychology (the culprit of CFAS-D is a psychological problem) and the medical approach (the culprit is one specific virus or different viruses or bacteria). The dominant view, especially in Europe, is that of the cognitive-behavioral and the biopsychosocial schools (1). This view entails that CFAS-Ds, even when due to medical disease (e.g., cancer), are the consequence of psychosocial and biological factors and negative cognitions (1–4). The Wessely model, for example, conceptualizes that the effects of a trigger factor, which may be a virus, are mediated by boom and bust activity and bed rest and the Vercoulen model considers that CFAS-D symptoms are aggravated by causal attributions and reduced physical activity (1). Nevertheless, it appears that the label “biopsychosocial” is more window dressing than the actual approach because, in fact, folk psychology statements abound in their publications, as, for example, “it is in the mind,” “they think themselves ill,” and “it is a disorder of perception, whereby patients think those symptoms are the consequence of a virus” [review: 2]. Nevertheless, this folk psychology approach is embraced by the National Health Service (NHS), the Lancet, and national healthcare system all over Europe (e.g., United Kingdom, France, Sweden, Benelux).
It is incomprehensible that expert committees make consensus diagnostic criteria of ME/CFS (including the 1994 International Research Case Definitions and the 2003 Canadian Consensus Criteria), whereas, in fact, these new diagnostic classes were never validated, for example, by using machine learning techniques and there is no evidence that the case definitions have reliability and replicability validity (2, 4, 5). One diagnostic criterion of both the abovementioned case definitions is that ME and CFS are not secondarily to medical disorders (6). However, even CFS and ME, which are not secondary to any medical disorder, show a high comorbidity with symptoms of irritable bowel syndrome and depression (7), while CFAS-D symptoms are hallmarks of autoimmune, infectious, immune system, and psychiatric disorders, including major depression and schizophrenia and many more medical conditions (1, 3, 7–10). CFAS-D symptoms are reflective manifestation of the symptomatome of, for example, coronavirus disease 2019 (COVID-19), chronic kidney disease with hemodialysis, schizophrenia, and rheumatoid arthritis (9–14), suggesting that CFAS-D symptoms are part of the core of these medical disorders and not the result of some “it is in the mind” process, if there would exist such a process.
It was not until 2010–2013, when Maes et al. (1, 3, 15) launched a new biomedical model that more targeted medical research into CFAS-D began. These biomedical models of Maes et al. conceptualized CFAS-D as medical conditions whereby trigger factors such as immune activation, bacterial and viral infections, and a reduced protectome due to, for example, reduced antioxidant levels may cause multiple adverse outcome pathways (AOPs) leading to the phenome of CFAS-D. The latter comprises CF symptoms, neurocognitive impairments, and depressive, sleep, gastrointestinal, and autonomic symptoms, hyperesthesia, and fibromyalgia-like symptoms, a flu-like malaise including post-exertional malaise (1, 3, 15). Furthermore, we conceptualized physiosomatic symptoms accompanying CFAS-D as being the consequence of different AOPs and, therefore, relabeled those symptoms as “physiosomatic” symptoms (16, 17).
The key AOPs of CFAS-D (including those that are secondary to medical disorders such as COVID-19, chronic kidney disease, rheumatoid arthritis, schizophrenia, and major depression, which we established in these studies between 2001 and 2021) are shown in Table 1. In addition, we also discovered that ME/CFS is characterized by increased immunoglobulin A/immunoglobulin M (IgA/IgM) responses to Gram-negative bacteria indicating increased bacterial or lipopolysaccharide (LPS) translocation and IgM-mediated autoimmune responses to a number of neoantigens including malondialdehyde (MDA), azelaic acid, and nitrosylated proteins, indicating increased nitrosylation (18, 19).
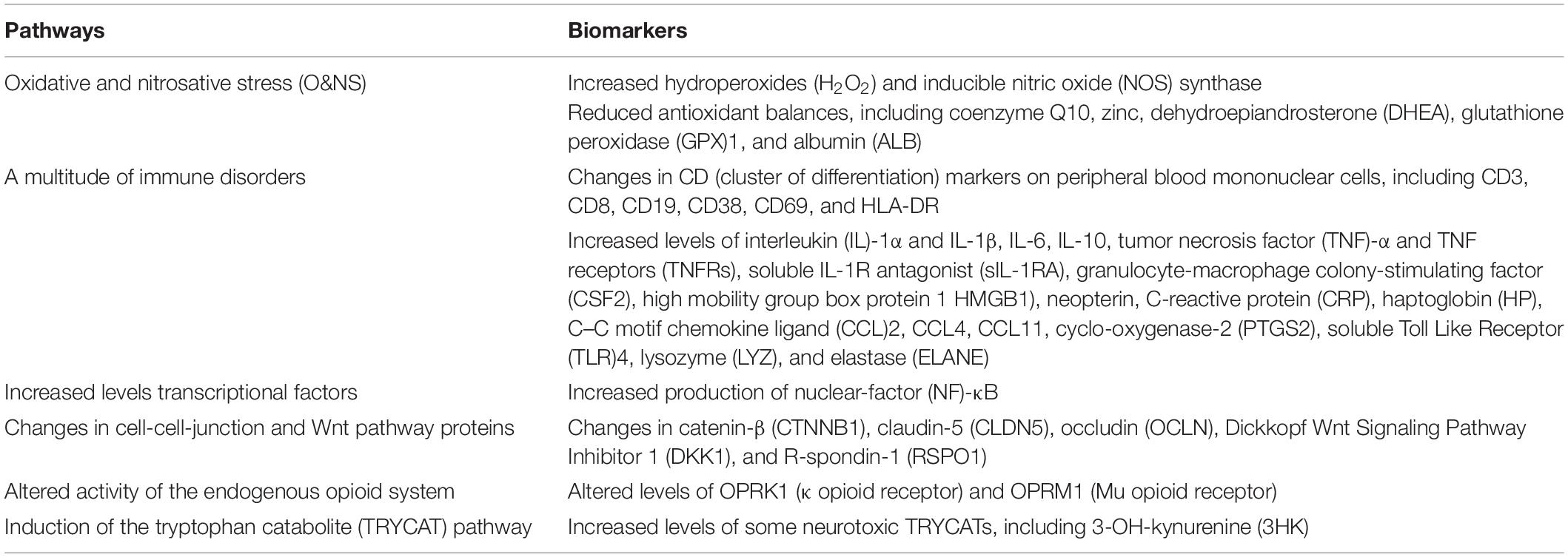
Table 1. The key adverse outcome pathways (AOPs) of the chronic fatigue spectrum disorders (CFAS-Ds), including those that are secondary to medical disorders such as coronavirus disease 2019 (COVID-19), chronic kidney disease, rheumatoid arthritis, schizophrenia, and major depression.
All in all, these findings show that multiple differentially expressed proteins (DEPs) and metabolic pathways are involved in the pathophysiology of CFAS-D. However, no study has delineated the protein–protein interaction (PPI) and metabolic-protein interactions (MPIs) networks of CFAS-D and the biological processes, molecular functions and complexes, cellular components, pathways, transcriptional regulatory relationships, protein domains, and human disease annotations, which are associated with the PPI and MPI networks of CFAS-D.
Hence, we have conducted network, enrichment, and annotation analyses to delineate the hotspots in the CFAS-D network and the top functions and paths enriched in the networks. This is important because the most influential genes, metabolics, and pathways may constitute new drug targets to treat CFAS-D. Moreover, such analyses may disclose the putative trigger factors of the CFAS-D interactome and its associations with comorbid medical disorders. As such, these enrichment and annotation analyses may help to explain the strong comorbidity of CFAS-D with immune, infectious, and neuropsychiatric disorders and the possible shared pathophysiological core, which may underpin CFAS-D.
“This study is a secondary data analysis on existing data using open, deidentified, and non-coded data sets and, therefore, this is non-human subject study, which is not subjected to Institutional Review Board approval” (20). In case–control studies, we have previously identified the metabolic pathways and differentially expressed proteins (DEPs) in CFAS-D, including when due to comorbid medical disease. Almost all the biomarkers included in this study were extracted from our studies on ME/CFS and CF-like symptoms with a duration > 6 months in comorbid disorders, including major chronic kidney disease with hemodialysis, depression, schizophrenia, and rheumatoid arthritis [Electronic Supplementary File (ESF), 1, References]. One study was performed on patients with CF-like symptoms due to acute COVID-19 infection. We were able to include: (a) 42 DEPs, namely, angiotensin-converting enzyme 2 (ACE2), advanced glycosylation end-product specific receptor (AGER), agrin (AGRN), ALB, CCL2, CCL4, CCL11, CD3D, CD8A, CD19, CD38, CD69, HLA-DR, creatine phosphokinase (CKM), CLDN5, CSF2, CRP, CTNNB1, DKK1, ELANE, GPX1, hemoglobin A1 (HbA1), HP, HMGB1, interleukin-1α (IL-1α), interleukin-1β (IL-1β), IL-1RN (IL-1RA), IL-6, IL-10, LYZ, NFKB1 (NF-κB), NOS2, OCLN, opioid receptor Kappa 1 (OPRK1), opioid receptor Mu 1 (OPRM1), proopiomelanocortin (POMC) as precursor of β-endorphins, PTGS2, RSPO1, TLR4, TNF (TNF-α), TNFRSF1A (TNFR60), and TNFRSF1B (TNFR80) and (b) 12 metabolic Kyoto Encyclopedia of Genes and Genomes (KEGG)1 pathways, namely, C01290 (lactosylceramide), C02470 (xanthurenate), C10164 (picolinic acid), C03227 [3-OH-L-kynurenine (3OHK)], C11378 (coenzyme Q10), C00038 (zinc), C00070 (copper), C06428 [eicosapentaenoic acid (EPA)], C00027 [hydrogen peroxides (H2O2)], C19440 [malondialdehyde (MDA)], C004555 (DHEA), and C05926 (neopterin).
The network, enrichment, and annotation analyses were conducted, as reviewed previously (20). In brief, we constructed two network subtypes, the first was constructed using the abovementioned DEPs whereby the physical interactions between the DEPs were visualized using STRING version 11.02 (minimum required interaction score: 0.400; active interaction sources: experiments, textmining, and databases; and set organism: Homo sapiens) and Cytoscape3. The second was constructed using OmicsNet4 using the abovementioned metabolites and examining the MPI based on the KEGG reactions. Consequently, the genes interacting with the metabolics in the different subnetworks were used in STRING to build a giant network based on our seed genes enlarged with the MPI-derived DEPs, which were, consequently, analyzed in OmicsNet to construct a composite network consisting of metabolites and DEPs first entering MPIs and then the PPIs (analyzed with IntAct molecular interaction database)5 with a targeted incorporation of TF protein interactions (TFPIs) (analyzed using TTRUST)6.
Network features were calculated using STRING and the Cytoscape plugin NetworkAnalyzer and comprise number of nodes, number of edges and expected number of edges, average node degree, network diameter and radius, characteristic path length, and network density and heterogeneity. The top hubs (high degree) and bottlenecks (high betweenness centrality) were calculated and used to delineate the backbone of the network, i.e., the top 7 hubs and the top 2 non-hub bottlenecks. We used Markov Clustering (MCL) employing STRING to discover communalities of interconnected nodes, which display similar attributes and/or functions.
We examined the different networks and MCL subnetworks for their enrichment scores and annotated terms and these analyses were also performed on the downregulate seed genes and the hotspots of the enlarged network. Enrichment/annotation analyses were performed using STRING, Enrichr7, OmicsNet, MetaScape8, inBio Discover9, and the R package ClusterProfiler. Heatmaps were produced using Appyter and MetaScape. Functional enrichments were established using the Gene Ontology (GO) biological processes, the GO molecular functions and the GO cellular components, STRING local network clusters, the KEGG pathways, Reactome pathways (the European Bioinformatics Institute Pathway Database)10, PANTHER biological processes (PANTHER---Gene List Analysis)11, TTRUST transcriptional regulatory relationships, InterPro domains (InterPro)12, Wikipathways (WikiPathways - WikiPathways), and DOID annotations of human diseases (Disease Ontology---Institute for Genome Sciences, University of Maryland)13. We also performed Molecular Complex Detection (MCODE) (using Metascape) to delineate small molecular complexes. In this study, all the results are shown as p (or q) values corrected for false discovery rate (FDR).
Figure 1 shows the first-order protein network of CFAS-D. This network comprises 91 nodes and 938 edges, exceeding the expected number of edges (n = 323) with a p-enrichment value of 1.0E-16. The network has an average local clustering coefficient of 0.683 and average node degree of 20.6, with a network diameter of 4, radius of 2, a characteristic path length = 1.978, network density = 0.229, and heterogeneity = 0.682. The top 7 hubs (highest degree) were in descending order of importance: TNF (degree = 63), IL-6 (61), IL-1β (58), ALB (54), TLR4 (47), IL-10 (47), and CTNNB1 (46). The top two non-hub bottlenecks were: EGFR (0.066) and TLR2 (0.0188). EGFR and TLR were the top 5 and 9 in the bottleneck list (top 3 in descending order was ALB, CTNNB1, and TNF). The network of the seed proteins showed 42 nodes and 311 edges, thus, exceeding the expected number of edges (n = 70) with a p-enrichment value of 1.0E-16. The network has an average local clustering coefficient of 0.74 and an average node degree of 14.8.
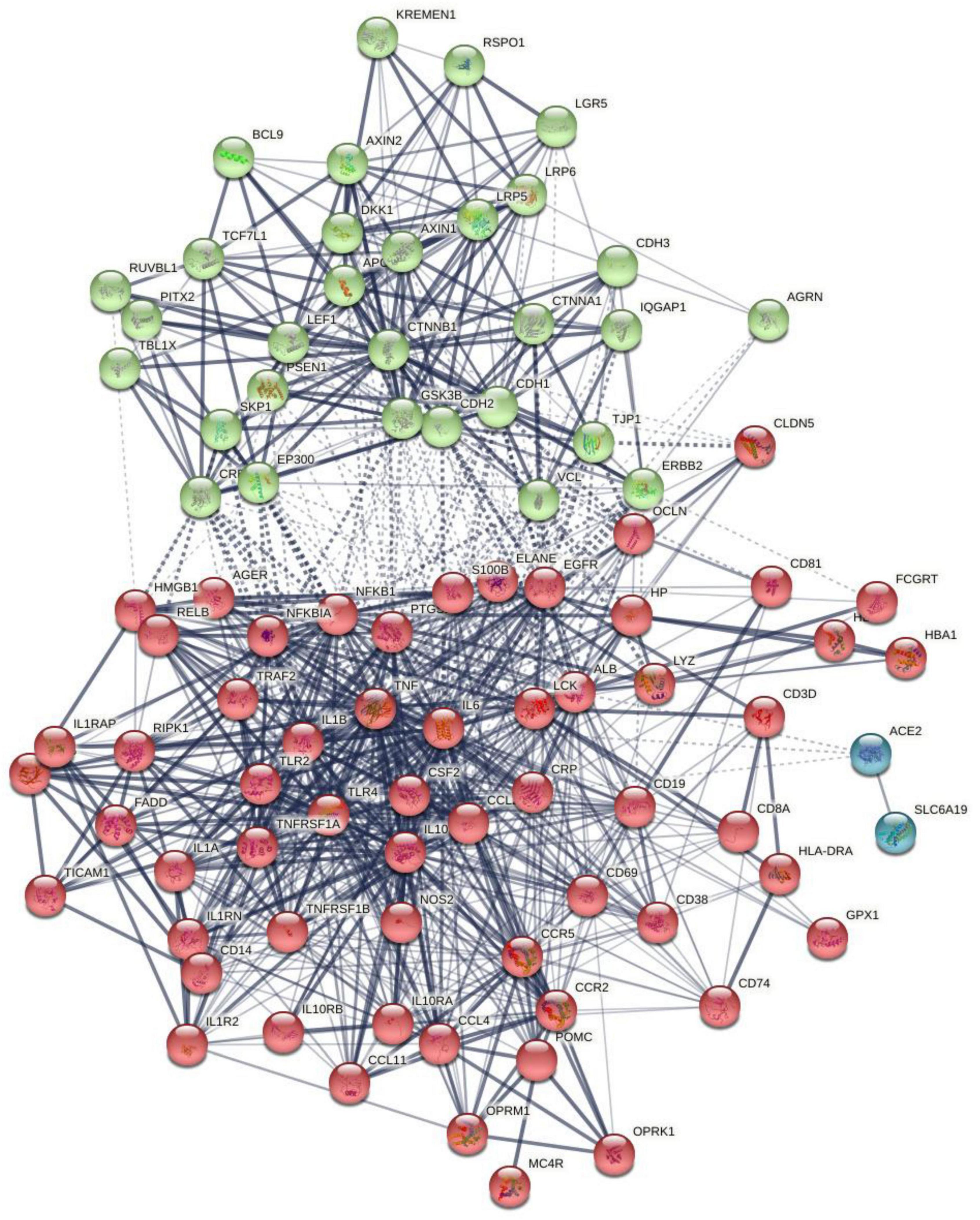
Figure 1. The first-order protein–protein interaction network of chronic fatigue spectrum disorders. Markov Clustering (MCL) cluster analysis found two subnetworks: (1) a first immune subnetwork (red color) was centered around NFKB1, tumor necrosis factor (TNF), interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-1 (IL-1), TLR4, etc., and (2) a second Wnt/β-catenin subnetwork (green nodes) centered around CTNNB1. The solid and dotted lines represent connections inside and between clusters, respectively. Different colors represent the various clusters.
Markov Clustering analysis (inflation parameter of 2) resulted in two protein subnetworks, as shown in Figure 1. The first subnetwork contains predominantly immune genes, such as EGFR, ALB, AGER, HP, GPX1, CD19, etc., and the second subnetwork contains Wnt and cell–cell junction associated genes, including CTNNB1, AGRN, RSPO1, and DKK1. The major connectors (switches) of both the subnetworks were CTNNB1 (belongs to subnetwork 2) and ALB and IL-6 (nodes in subnetwork 1). CTNNB1 showed relevant interactions (confidence level > 0.4) with DKK1 and RSPO1 and with 11 subnetwork 1 seed genes (e.g., NFKB1, ALB, OCLN, TLR4, HMGB1). ALB showed significant interactions with 25 other subnetwork 1 genes and with CTNNB1, AGRN, and DKK1. IL-6 showed interactions with CTNNB1, DKK1, and AGRN and 28 other subnetwork 1 seed nodes. In the first-order non-seed genes, we found that EGFR was connected with 3 subnetwork 2 seed genes (GRN, CTNNB1, and DKK1) and with 19 seed genes in subnetwork 1.
In order to construct a second giant network including proteins interconnecting with the metabolites, we entered the latter in OmicsNet analysis and examined the MPIs (using the KEGG and IntAct and using only the first-order MPIs). We found 5 subnetworks: one centered around hydroperoxides (46 nodes), another centered around EPA (6 nodes), 6 centered around 3OHK, 4 centered around nitric oxide (NO), and 3 centered around DHEA (albeit some overlapping). The 59 nodes coupled with the nodes from the first network (see Figure 2) were consequently examined using STRING and NetworkAnalyzer. Figure 2 shows the network based on this combination of DEPs. This network comprises 147 nodes and 1,407 edges, exceeding the expected number of edges (n = 413) with a p-enrichment value of 1.0E-16. The network has an average local clustering coefficient of 0.624 and average node degree of 19.1, with a network diameter of 5, radius of 3, characteristic path length = 2.278, network density = 0.131, and heterogeneity = 0.799. The top 7 hubs (highest degree) were in descending order of importance: TNF (degree = 82), IL-6 (79), ALB (75), IL-1β (74), catalase (CAT) (59), IL-10 (57), and TLR4 (57). The top 5 bottlenecks were CAT (0.1534), ALB (0.1212), CTNNB1 (0.0918), TNF (0.08867), and IL-6 (0.06755). The first non-hub bottlenecks were EGFR (0.04909) and NOS2 (0.04572).
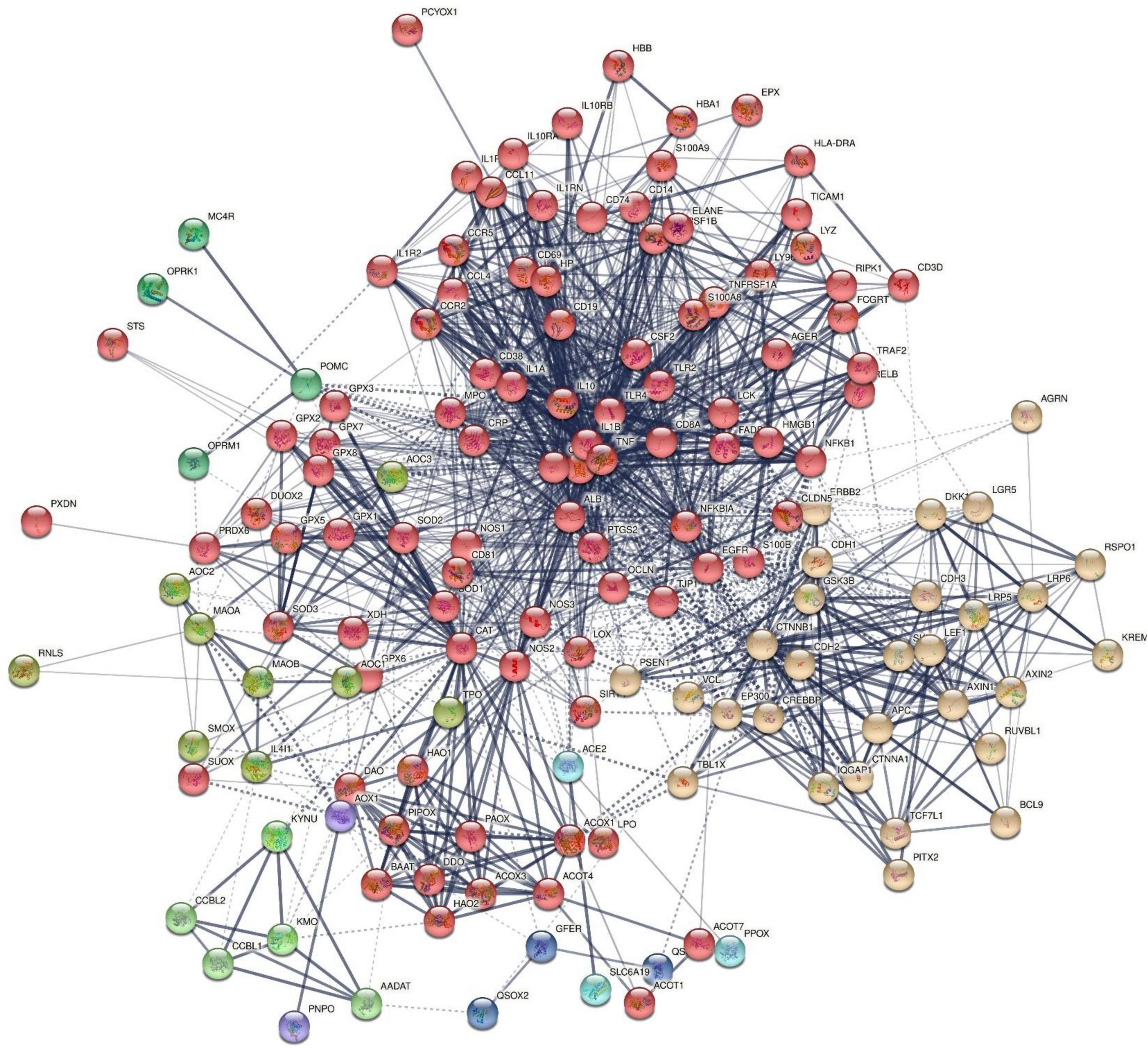
Figure 2. First-order protein network of chronic fatigue spectrum disorders. MCL cluster analysis found two major subnetworks: (1) a first immune, oxidative, and nitrosative subnetwork (IO&NS) (red color) was centered around NOS2, NFKB1, IL-10, etc, and (2) a second Wnt/β-catenin subnetwork (yellow nodes) was centered around CTNNB1. The tryptophan catabolite pathway [kynurenine hydroxylase (KYNU) and kynurenine 3-monooxygenase (KMO)] and opioid [opioid receptor Kappa 1 (OPRK1) and opioid receptor Mu 1 (OPRM1)] genes appear to be spin-offs of the IO&NS subnetworks. The solid and dotted lines represent connections inside and between clusters, respectively. Different colors represent the various clusters.
Figure 2 shows the results of MCL cluster analysis (inflation parameter of 1.7) displaying two significant protein subnetworks, a first comprising immune and nitro-oxidative stress genes [see Figure 1; this cluster is now renamed the immune-inflammatory, oxidative, and nitrosative (IO&NS) cluster] and a second Wnt/β-catenin cluster. There were also some communities with only few nodes, for example, one centered around kynurenic acid (KYNA) and kynurenine 3-monooxygenase (KMO).
Figure 3 shows the results of OmicsNet analysis, which included all the IO&NS/Wnt genes of the network given in Figure 2 with integration of the metabolics and NFKB (as transcriptional factor). This network was constructed using two interaction types, namely, MPI (first rank) and PPI (second rank) and included 574 nodes and 694 edges. The top hubs (including non-seeds) in this network were NOS2 (177), NOS3 (63), SOD2 (57), PRDX6 (47), peroxides (45), SOD1 (41), KYNU (32), and NFKB1 (29). We also examined the hubs of the network built with the PPIs entered in first order and MPI in second order (2,593 nodes and 4,063 edges) and found that the top 3 hubs were in descending order of importance: NFKB1 (307), CTNNB1 (153), and ALB (152). As such, the common backbone of the different networks (top 3 of each network) in this study consists of NFKB1, CTNNB1, ALB, peroxides, NOS2, TNF, and IL-6.
Figure 3. Results of OmicsNet analysis, which included all the genes of the network with integration of the metabolics and NFKB1. Metabolics are shown in yellow color and NFKB1 is shown in green color. 3HK: 3-hydroxykynurenine; NO: nitric oxide; EPA: eicosapentaenoic acid.
Table 2 shows the results of PANTHER functional explorer analyses. The top PANTHER molecular functions, which were enriched in this network, revolved around protein binding, peroxidase, and oxidoreductase activity and the top PANTHER cellular components were the cytosol, protein-containing complex, cytoplasm, and the nucleus.
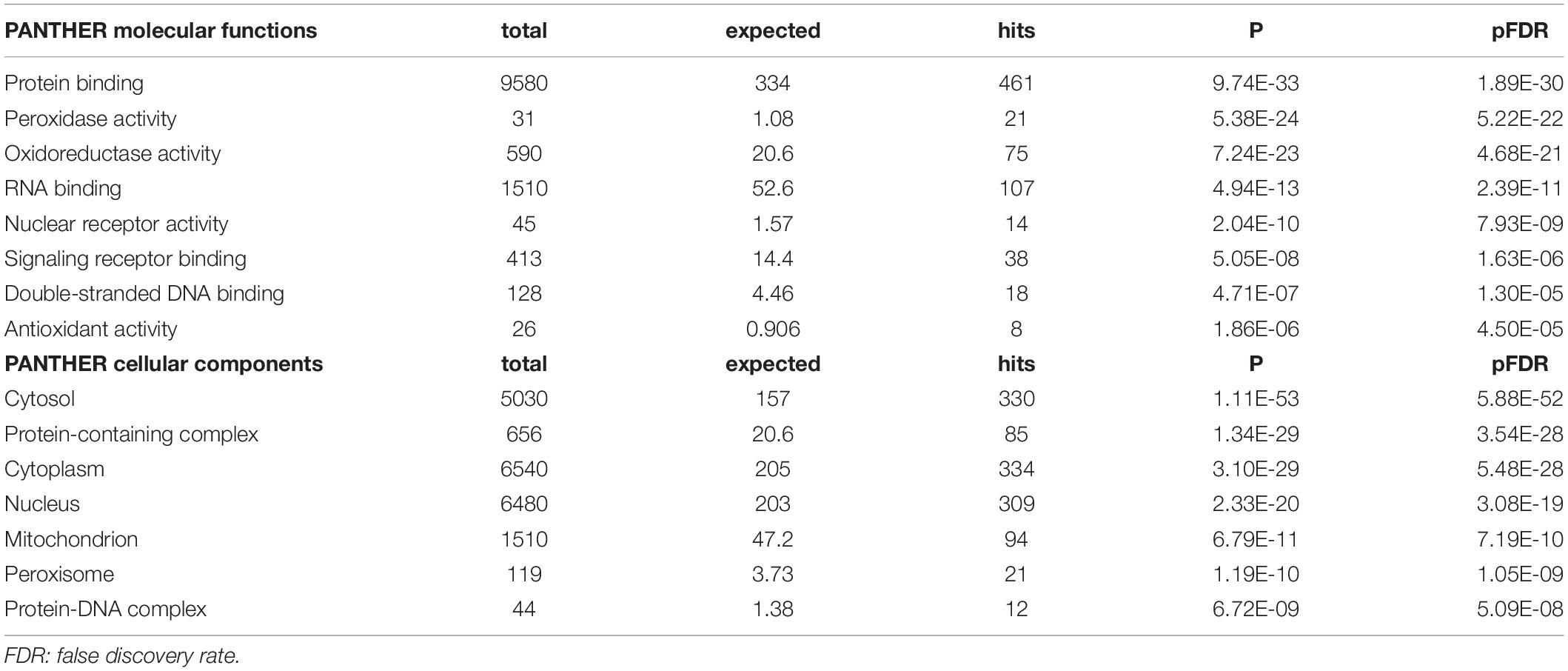
Table 2. PANTHER molecular function and components terms associated with chronic fatigue spectrum disorders.
Table 3 displays the results of MCODE analysis using the KEGG, WikiPaths, the GO biological and molecular, Reactome, and PANTHER performed on the IO&NS/Wnt genes. We found five significant molecular complexes, the first represents cytokine/IL-10 signaling and a response to LPS; the second comprises cellular oxidant detoxification and response to a toxic substance; the third comprises amine oxidase reactions, hydrogen peroxide metabolic process, and degradation of β-catenin; the fourth comprises peroxisomal protein import and a carboxylic acid catabolic response; and the fifth was the same as MCODE2, as given in Table 3.
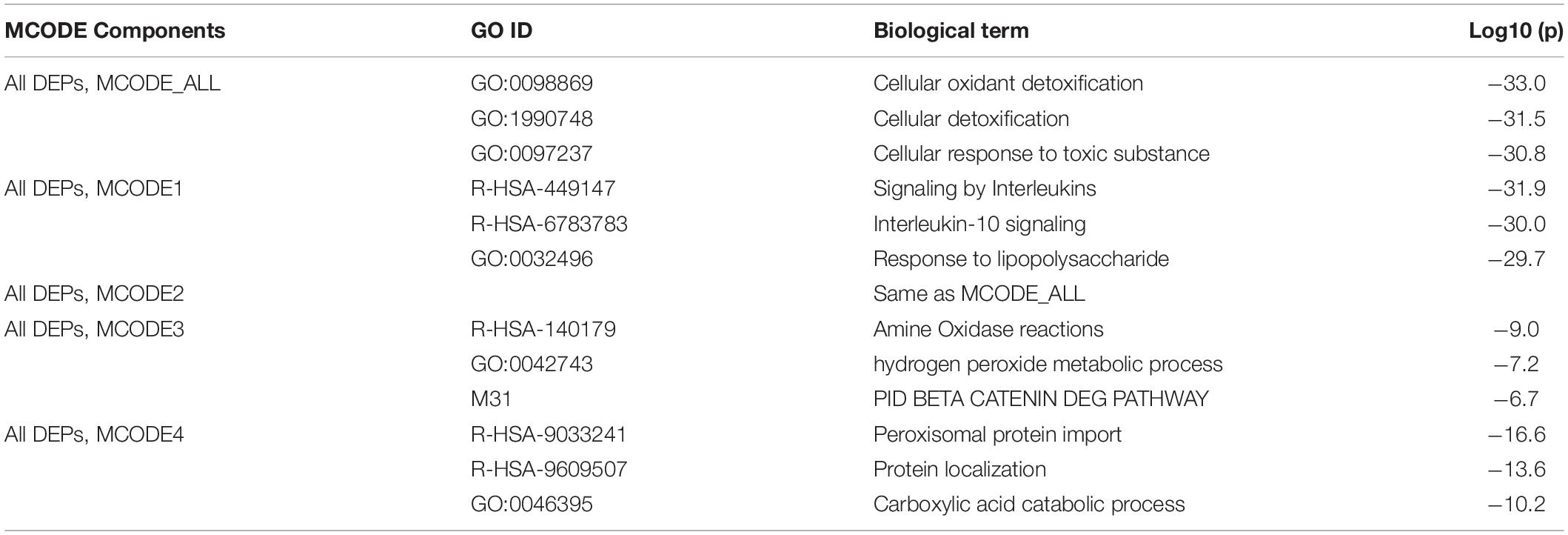
Table 3. Results of molecular complex detection (MCODE) analysis performed on the differently expressed proteins (DEPs) of chronic fatigue spectrum disorders.
Table 4 shows the most important GO biological processes, InterPro domains, and the KEGG pathways enriched in the IO&NS/Wnt network. The GO biological process enrichment analyses showed that this network was significantly associated with oxidoreductase, antioxidant and peroxidase activities, and β-catenin binding. InterPro enrichment analyses showed that heme peroxidase and glutathione peroxidase were the top terms. The KEGG path analysis showed that the network was highly associated with the Wnt signaling and NF-κB pathway, tryptophan metabolism, and illnesses including tuberculosis and Chagas disease.
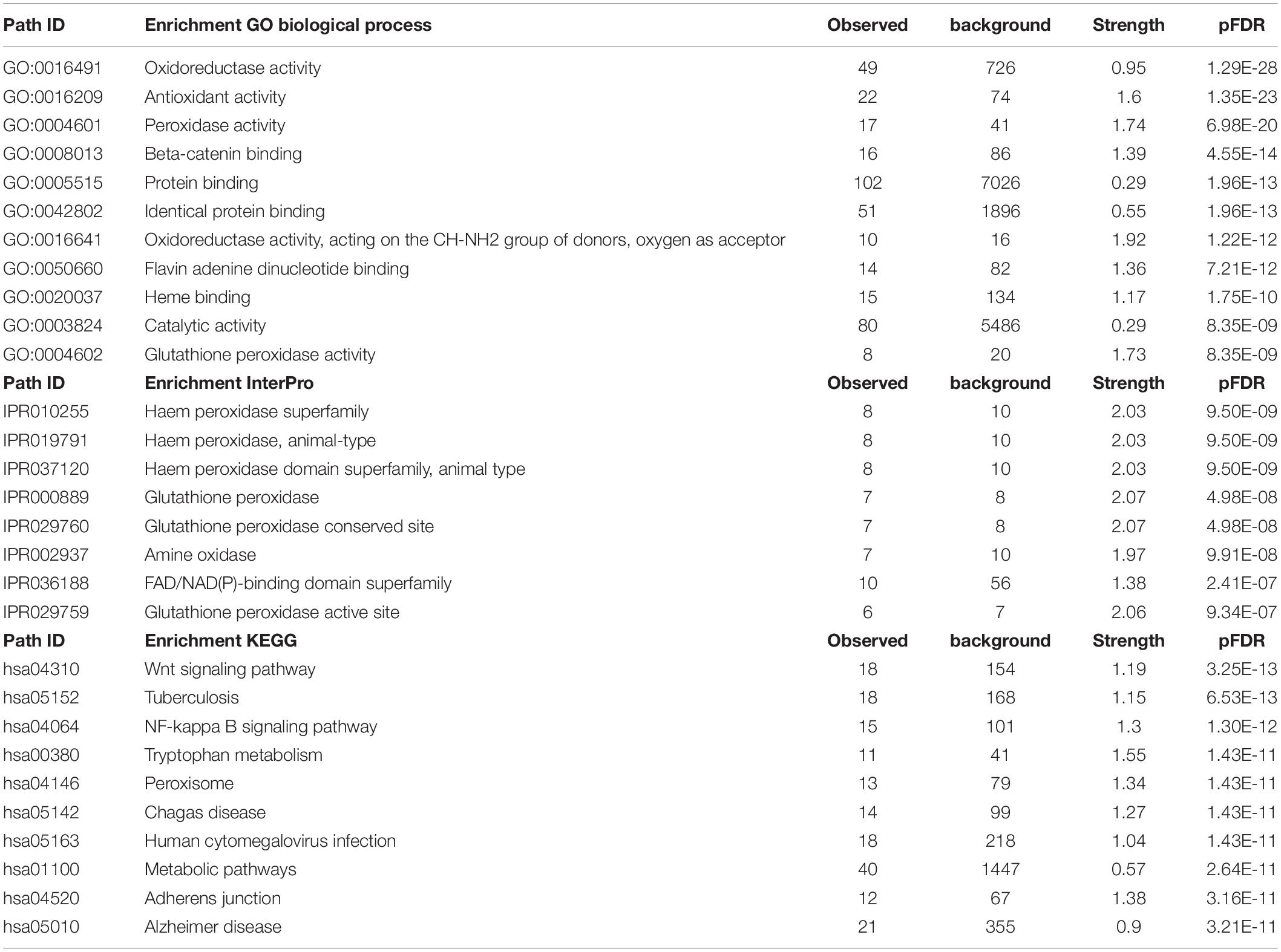
Table 4. The gene ontology (GO) biological process terms, InterPro domains, and the kyoto encyclopedia of genes and genomes (KEGG) pathways associated with chronic fatigue spectrum disorders.
Figure 4 shows the enriched ontology clusters (using MetaScape) in the network with cellular oxidant detoxification, IL-10 signaling, and a response to LPS as the top terms. Figure 5 shows a heatmap (made using Enrichr and Appyter) with the top KEGG pathways that were overrepresented in the IO&NS/Wnt network, namely, pathways of neurodegeneration, Wnt and NF-κB signaling, and peroxisome. Figure 6 shows a heatmap with the PANTHER 2016 pathways, which were overrepresented in the network, namely, Wnt, apoptosis, TLR and cadherin pathways, and the Alzheimer’s disease pre-senilin pathway. TTRUST analysis showed that NF-κB (pFDR = 6.58E-23) and RELA (pFDR = 8.691E-21) were the most important transcriptional factors of the network followed at a large distance by SP1 (pFDR = 7.079E-13).
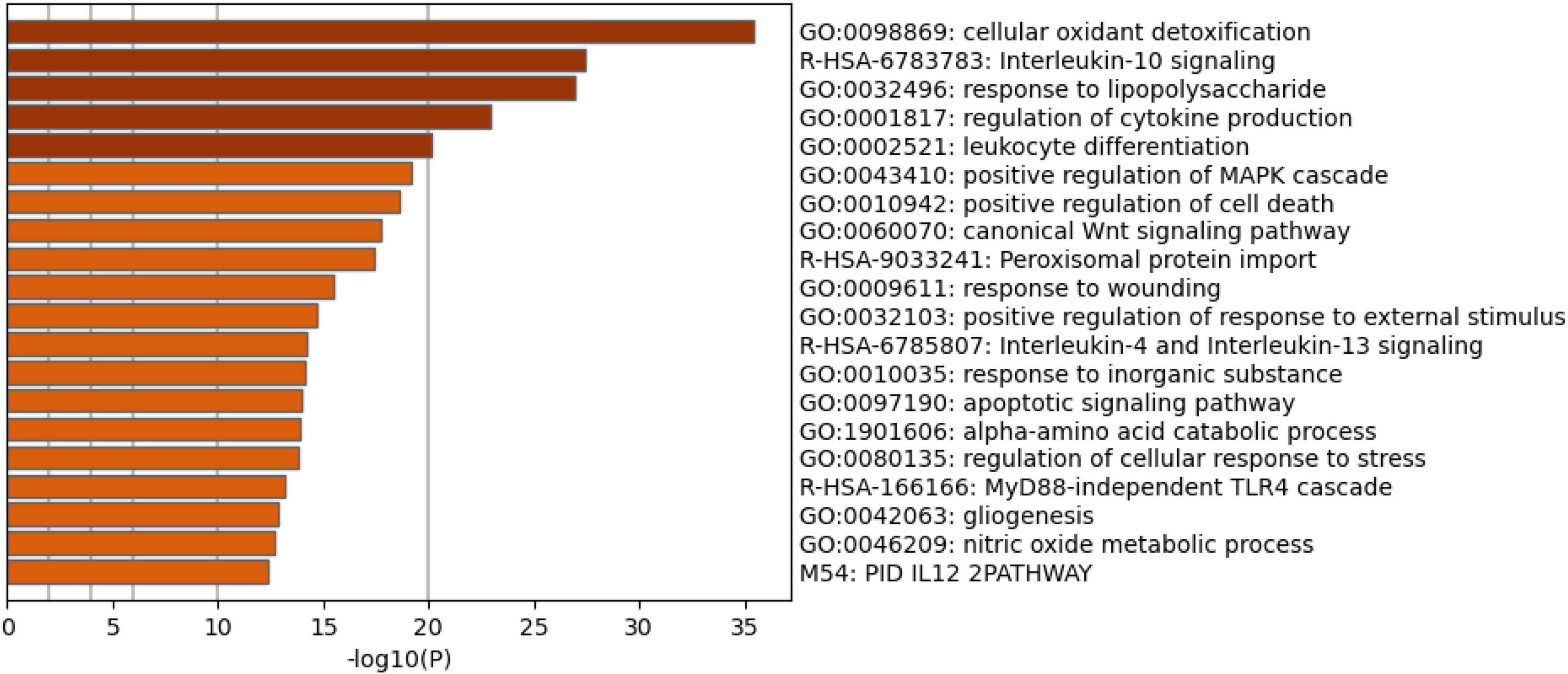
Figure 4. Heatmap of enriched ontology clusters showing the top 20 functions that were overexpressed in the network of patients with chronic fatigue spectrum disorders (accumulative hypergeometric p-values).
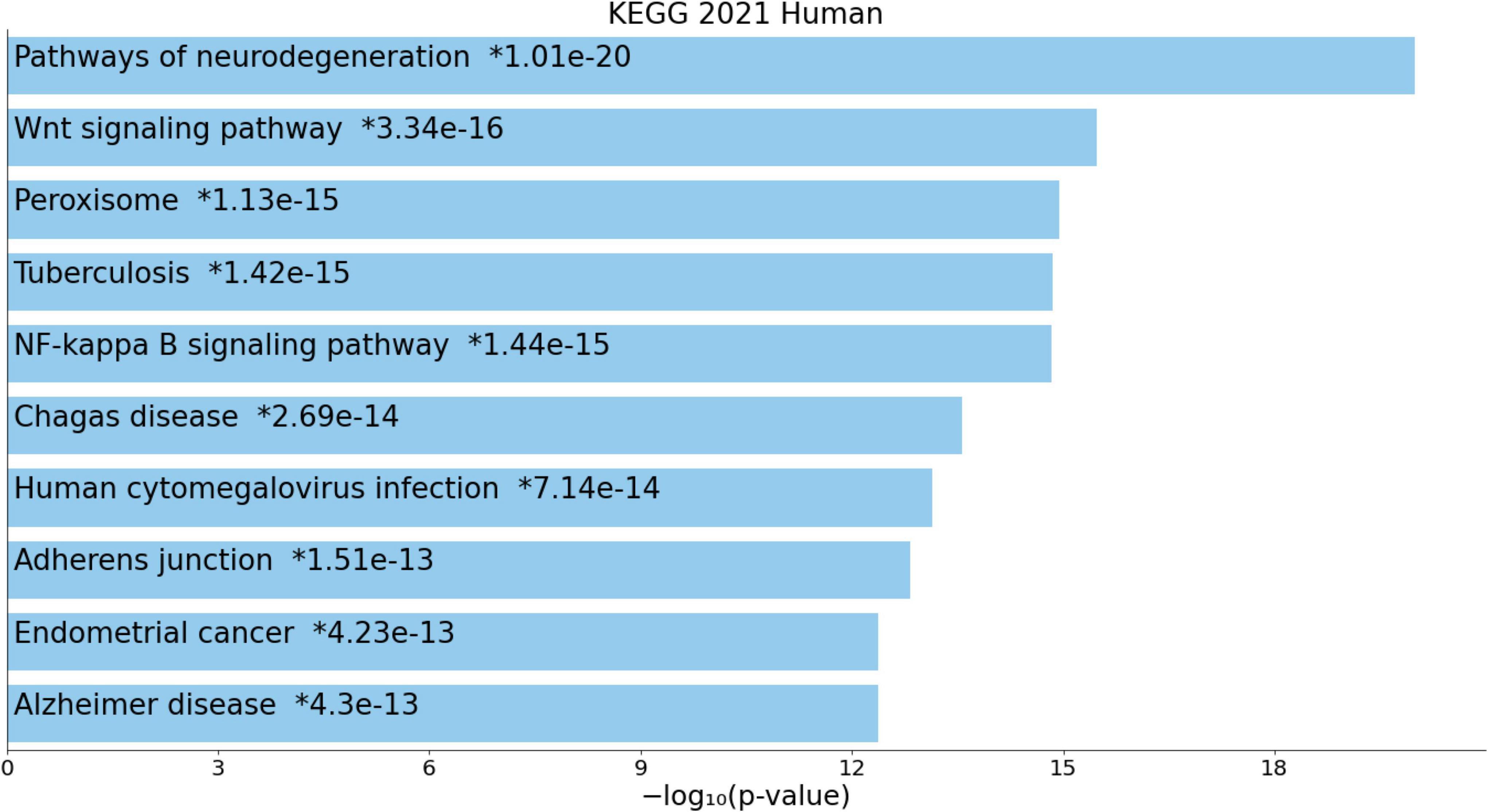
Figure 5. Heatmap (top 10) of the enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) terms accumulated in the differently expressed proteins of the enlarged MultiOmics network of chronic fatigue spectrum disorders.
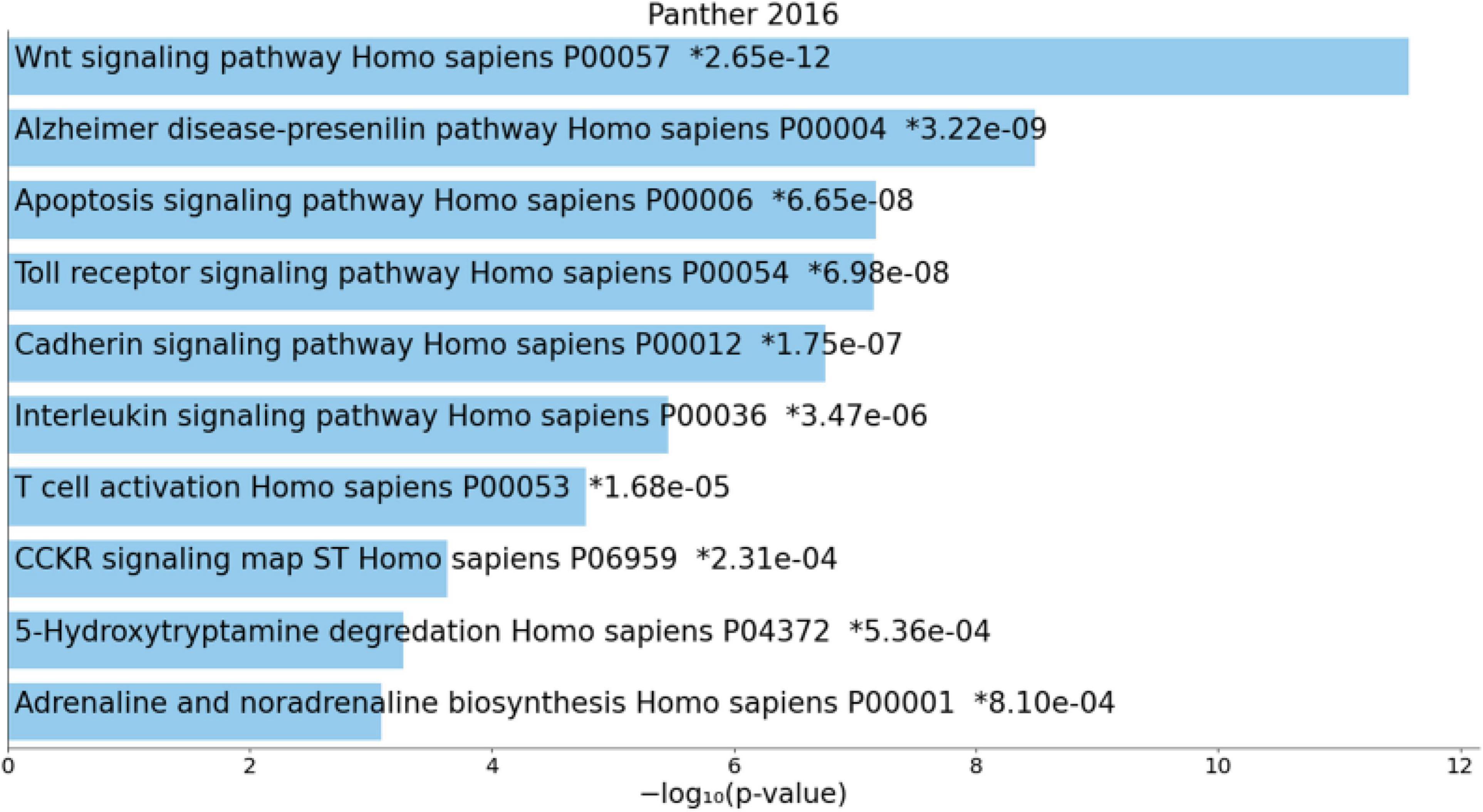
Figure 6. Heatmap (top 10) of enriched PANTHER terms accumulated in the differently expressed proteins of the enlarged MultiOmics network of chronic fatigue spectrum disorders. *significant after FDR p correction.
Table 5 shows the top 10 DOID annotations of an extended IO&NS/Wnt network (inBio Discover) including four intestinal disorders as the top 4 annotations, immune disorders, and cancer.
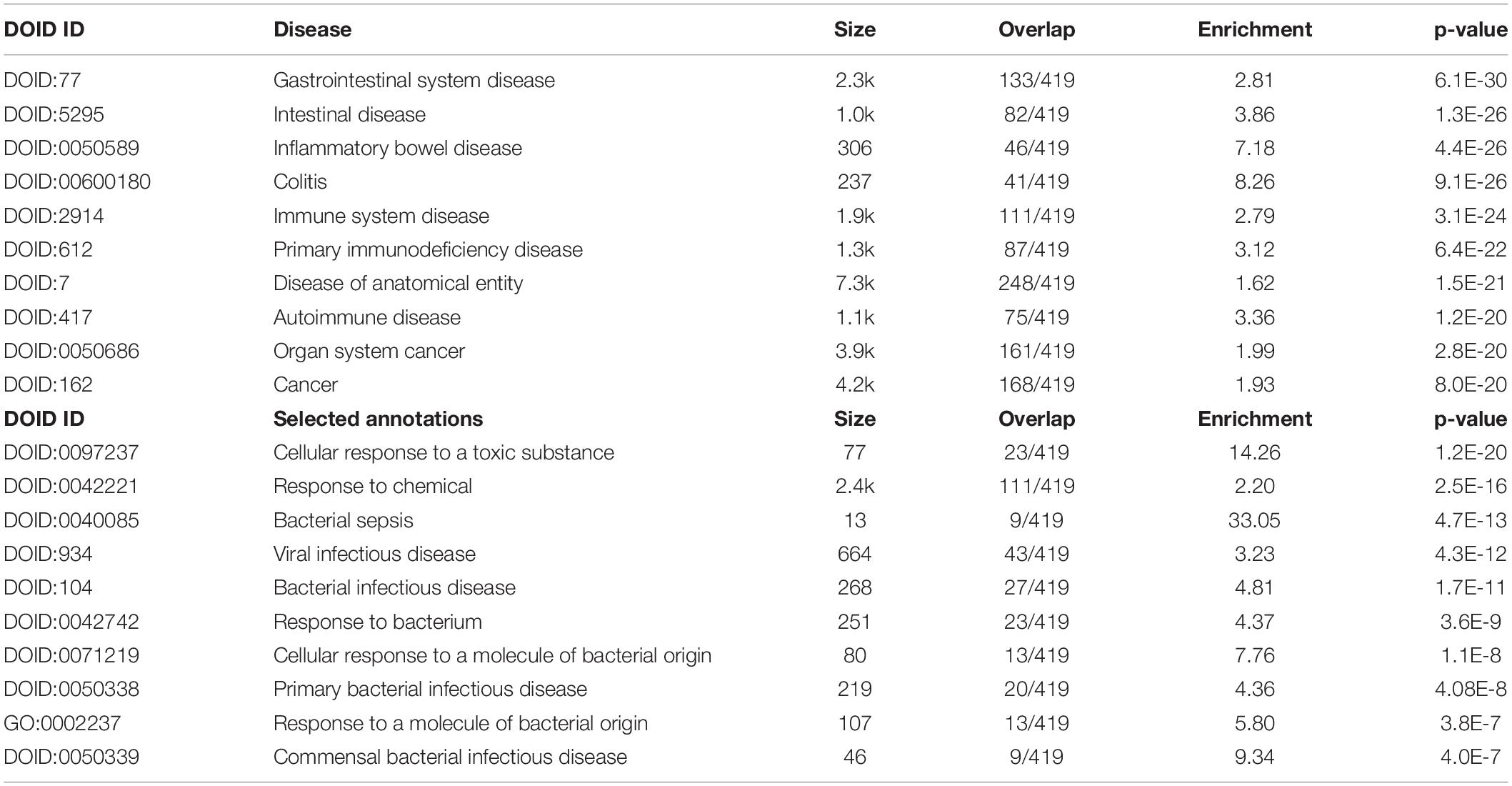
Table 5. Results of inBio discover annotation analysis with the top 10 and custom-made DOID annotations associated with chronic fatigue spectrum disorders.
Electronic Supplementary File (ESF) 1 and ESF 2 show the results of enrichment and annotation analyses performed on the DEPs (or selected DEPs), as given in Figure 1.
The first major finding of this study is that the PPI network of the DEPs and metabolics of CFAS-D show high connectivity and comprises two subnetworks, a first centered around IO&NS genes and a second centered around Wnt/β-catenin genes. The backbone of the master network comprises DEPs/metabolics, including NFKB1, CTNNB1, ALB, peroxides, NOS2, TNF, and IL-6, while in the giant network, many redox-related enzymes were predicted to be hubs, including endothelial NO synthase producing NO relaxing smooth muscle relaxation (NOS3), mitochondrial superoxide dismutase 2 (SOD2), and peroxiredoxin 6 (PRDX6) (catalyzes the reduction of hydrogen peroxides). It is important to note that without the delineation of the MPIs, one would have concluded that especially proinflammatory cytokine genes are the dominant forces in this network, whereas, in fact, the IO&NS subnetwork is more dominated by redox genes, NF-κB and IL-10.
CTNNB1, which belongs to the Wnt subnetwork, was another hotspot and additionally showed many interactions with genes in both the subnetworks indicating that this gene is a relevant switch linking both the subnetworks. Other relevant switches belonging to the immune subnetwork were ALB and IL-6, which showed many interactions with cluster 1 genes, but also with CTNNB1, DKK1, and AGRN. Hotspots and switches are new drugs targets because they govern and control the network and/or link the subnetworks (20). All in all, we may conclude that dysfunctions in the IO&NS and Wnt subnetworks underpin the pathophysiology of CFAS-D.
The second major finding of this study is that the top relevant functions and pathways in the first network revolve primarily around redox mechanisms, namely, cellular oxidant detoxification (and, thus, cellular detoxification and a cellular response to toxic substance), the hydrogen peroxide metabolic process, antioxidant activity, amine oxidase reactions, oxidoreductase activity, peroxidase activity, and heme and glutathione peroxidase. These findings indicate that increased oxidative stress (hydrogen peroxides) probably as a response to a toxic substance coupled with reduced antioxidant defenses (peroxidase, oxidase, oxidoreductase, and ALB) are the major pathways in CFAS-D. Indeed, many of the seed DEPs and metabolics show antioxidant properties, including ALB, GPX1, HP, CoQ10, DHEA, zinc, and EPA and pro-oxidant properties, including NFKB1, NOS2, PTGS2, and hydroperoxides. Moreover, the giant network based on seed DEPs and MPI-derived genes indicates that many redox genes are expected to participate in the CFAS-D network, including CAT, GPX2-9, SOD1, SOD2, LPO, LOX, MPO, NOS1, NOS3, and PRDX6.
Such findings agree with the evidence that CFAS-D is characterized by reduced antioxidant defenses and oxidative damage to lipids, proteins, DNA, and mitochondria as reviewed in (1, 3, 7, 15). Such damage in ME/CFS is accompanied by formation of immunogenic oxidative modified neoepitopes, which, in turn, may cause an IgM-mediated immune response against neoepitopes such as malondialdehyde and azelaic acid (18). The participation of NOS2 in the CFAS-D network is in agreement with our reports that ME/CFS is accompanied by increased nitrosative stress, which is a consequence of increased NO and superoxide production resulting in hypernitrosylation (18). In addition, also more recent articles indicate that increased nitro-oxidative stress and reduced antioxidant defenses contribute to idiopathic chronic fatigue, including increased reactive oxygen species (ROS), malondialdehyde, and F2-isoprostanes and lowered catalase and glutathione levels (21). In patients with CFAS-D, baseline thiobarbituric acid–reactive substances (TBARS) (an indicant of lipid peroxidation) are associated with exercise-induced pain (22). A recent review describes how oxidative stress may cause membranopathies, channelopathies, and mitochondrial dysfunctions, which, in turn, may interfere with intracellular energy processes and imbalances in signal conversion system (23). There are many reports showing that antioxidants may improve CFAS-D in animal models, including carvedilol, melatonin, Withania somnifera, quercetin and Hypericum perforatum L (24), curcumin (25), Sarcodon imbricatus (26), and many more.
The results of our network and enrichment analyses show that NFKB1 was not only one of the most important hotspots in the CFAS-D network, but also that NF-κB (p50–p52 unit) and RELA (NF-κB p65 unit or transcription factor p65) were the most important transcription factors controlling the network and that the NF-κB signaling pathway was one of the most important paths enriched in the network. NF-κB is a major transcriptional factor involved in the response to a vast array of stimuli, including bacterial or viral antigens, cytokines, free radicals, oxidized epitopes, and glutamate and is a transcriptional inducer of many genes, including cellular adhesion molecules, proinflammatory cytokines, chemokines, and growth, apoptosis and coagulation factors, and antioxidants and pro-oxidants (27–29). RELA is involved in the activation of NF-κB and stimulates NF-κB translocation to the nucleoplasm and by forming a RELA-NF-κB complex activates target gene expression (30). NF-κB p50, which is associated with RELA, and NF-κB p52 are major components of the canonical and non-canonical NF-κB signaling pathways, which both lead to target gene activation (29). Both the transcription factors are known to regulate different pathways that we observed to participate in the network, including the mitogen-activated protein kinase (MAPK) pathway (31) and ROS and Wnt/β-catenin pathways.
There are many intersections between reactive oxygen species (ROS) and NF-κB signaling. Indeed, ROS may modulate the NF-κB response leading to transcriptional activation of NF-κB-target antioxidant genes (e.g., SOD, HO1, GPX1, TRX1, and TRX2), which reduce ROS production, thereby promoting cell survival (29) and NF-κB-target pro-ROS genes, including NOX2, COX2, and NOS2 (29, 32). It should be stressed that the effects of ROS on NF-κB are more than complex with stimulatory effects in the cytoplasm and inhibitory effects in the nucleus (33), while ROS may also oxidize NF-κB p50 leading to decreased DNA-binding capacity (34).
All in all, the results of our network and enrichment analysis and Maes et al. (35, 36) indicate that the complex cross-talks between NF-κB and ROS signaling are involved in the pathophysiology of CFAS-D. Moreover, recent studies show that increased NF-κB expression may be associated with central fatigue by modulating central nervous system genes and regulating immune-inflammatory processes, synaptic plasticity, and memory and exerting neurotoxic effects (35–37).
Our MCODE analysis revealed that the IL-10 anti-inflammatory signaling pathway, regulation of cytokine production, signaling by interleukins, and a response to LPS were a relevant molecular complex in the IO&NS subnetwork of CFAS-D. These findings agree with those of our previous studies showing increased IgA/IgM response to LPS of 6 different Gram-negative bacteria in CFAS-D, indicating an increased bacterial translocation (19). Previously, we have argued that part of the effects of translocated LPS on cytokine production could be explained by induction of the TLR signaling pathway, which also signals to NF-κB, which, in turn, may activate cytokine genes (38, 39).
Our enrichment analysis also showed an association between the IO&NS subnetwork and the lactoferrin (LTF) danger signal response pathway. LTF, an iron-binding glycoprotein, activates NF-κB via the TLR2/4 complexes and additionally RAGE and TREM-1 receptors (40). LTF functions as a first-line defense against injuries, either pathogenic or non-pathogenic, and controls cell homeostasis (40). Furthermore, LTF sequesters ROS (thereby attenuating tissue damage due to excessive IO&NS) and maintains intestinal integrity during endotoxemia (40). Our enrichment analysis shows that the proinflammatory TNF-related weak inducer of apoptosis (TWEAK) signaling pathway is another possible link between tissue injury and upregulation of NFKB1-associated DEPs/genes by activating the NF-κB pathways (41).
The NF-κB signaling pathway not only regulates the transcription of proinflammatory genes, including IFNG, IL-1β, and TNF, but also IL-10, which protects against an overzealous inflammatory response (42). IL-10 has negative immunoregulatory activities by inhibiting the production of M1 macrophage and T helper (Th)-1 cytokines, dendritic cells stimulating CD4 + T cells and Th-2 responses, and attenuates CD8 +, M1 macrophage, and Th-1 and Th-2 associated immunopathology (43, 44). It is important to note that our enrichment analysis stresses the importance of anti-inflammatory IL-10 signaling as a response to LPS and regulation of cytokine signaling, suggesting that a predominant IL-10 phenotype may occur in CFAS-D. Presuming that the latter are frequently triggered by a low/moderate pathogen (or LPS) virulence (1), we may expect that IL-10 suppresses the ongoing immune response, thereby contributing to long-term escape of pathogens from immune control and causing persistent or recurrent infections (1, 43). Therefore, immunosuppression, which is another hallmark of CFAS-D, may not only be explained by T-cell exhaustion through increased proinflammatory cytokine production (45), but also by an increased IL-10.
Finally, our network and enrichment analyses showed that the TRYCAT pathway may be involved in CFAS-D, although it is not a key component but rather a spin-off of the IO&NS response. Previously, it was reported that this pathway is highly strongly associated with somatization disorder, a psychiatric disease accompanied by psychosomatic symptoms but not necessarily by fatigue (46). The TRYCAT pathway acts as a redox regulator and is one of the major antioxidant systems, although some TRYCATs have pro-oxidant and neurotoxic activities (47). Thus, 3OHK, one of the metabolics in our MPI network, is one of the neurotoxic TRYCATS produced during activation of this pathway because of IO&NS activation. Kynurenine hydroxylase (KYNU) is, as IDO, an oxygen-consuming enzyme, which catabolizes kynurenine into 3OHK (STRING).
Our enrichment analyses revealed that the CFAS-D network was highly significantly associated with two major interrelated functions/pathways/domains, namely, the canonical Wnt/β-catenin pathway, T-cell factor (TCF)-dependent signaling in response to Wnt, degradation of β-catenin by the destruction complex, the β-catenin complex and DIX domain, and adherens junctions, cadherin prodomain, and cell–cell junctions. The DIX domains and axin, GSK-3, and Disheveled (Dvl) are key players in the β-catenin destruction complex, thereby determining the interaction of β-catenin with the transcription factor TCF and, thus, the expression of the Wnt target genes (48).
The β-catenin/E-cadherin complex is the key component of adherens junctions (AJs), which stabilizes cell–cell junctions, provides cell–cell adhesion, and binds β-catenin with the actin cytoskeleton (9, 49). Moreover, aberrations in the AJs lead to a breakdown of the tight junctions (TJs), another component of the paracellular route (50). β-catenin and occludin are key factors in both the AJs and TJs (paracellular pathway), including those of the blood–brain barrier (BBB) and gut barriers, whereas claudin-5 is more specific to the BBB and E-cadherin to the gut barrier (9).
Therefore, dysfunctions in AJs and TJs may cause aberrations in cell–cell adhesion and the actin cytoskeleton (51). Furthermore, degradation of the E-cadherin/β-catenin complexes may cause aberrations in intracellular signaling, the actin cytoskeleton, and the Wnt/β-catenin signaling pathway, a key component in cell homeostasis (52). Previously, we reported that increased levels of zonulin (prehaptoglobin-2) are strongly associated with CFAS-D in schizophrenia and explain, at least in part, the aberrations in TJs and AJs and consequent breakdown in the paracellular route (9). It should be added that aberrations in the paracellular route of the gut barrier are associated with an increased bacterial or LPS translocation (leaky gut) and, thus, activation of the IO&NS pathways (9). Moreover, increased ROS and NO production and proinflammatory pathways (TNF, IL-6, and IFNG) may damage the epithelial and endothelial TJs (53). Furthermore, hydrogen peroxides may redistribute β-catenin and E-cadherin from the paracellular route into the cell, thereby affecting the TJs and deplete occludin and damage the cytoskeleton as well (53). It should be added that virulence factors of many pathogenenic bacteria may exploit the Wnt/β-catenin pathway to alter the antibacterial immune response, including Pseudomonas aeruginosa, one of the bacteria involved in CFAS-D (19, 54).
Importantly, our MCODE analysis revealed another highly significant molecular complex in the CFAS-D network comprising the hydrogen peroxide metabolic process, amine oxidase reactions, and the β-catenin degradation pathway, indicating that interactions between those factors are involved. Importantly, dysregulated Wnt/β-catenin signaling may cause oxidative stress in pregnant mice with CFS (55). Nucleoredoxin (NRX), a thioredoxin-like protein, strongly inhibits Wnt/β-catenin binding and the expression of early genes, whereas H2O2 treatment stabilizes β-catenin and increases expression of Wnt genes (56). Increased ROS promotes intrinsic apoptosis and the consequent caspase-3 expression inhibits Wnt signaling in association with cleavage of E-cadherin (57). H2O2 not only inhibits Wnt/β-catenin signaling, but may also increase Wnt/β-catenin signaling (58). In fact, peroxides have a biphasic effect on Wnt signaling with an increase 20 min after activation and reduced signaling some hours later (57). Finally, β-catenin is a key regulator of the homeostatic cell response, which helps to repair the damage due to nitro-oxidative stress (59).
Furthermore, the NF-κB and Wnt pathways shows multiple cross-talks and negatively or positively regulate each other, thereby forming a mutual regulatory network (60). Wnt modulates the production of inflammatory cytokines and NF-κB signaling and bridges innate and adaptive immune pathways (61). On the other hand, increased levels of IL-6 and TNF-α may maintain increased Wnt signaling associated with low β-catenin, Disheveled, and axin levels (62). It should be stressed that our network analysis showed that the key seed DEPs of both the IO&NS (NFKB1) and Wnt (CTNBB1) pathways have significant physical interactions based on co-expression, experimental, and biochemical data (STRING), thereby also functioning as an anchor linking the IO&NS and Wnt subnetworks. All in all, it appears that the pathophysiology of CFAS-D may be characterized by aberrations in the intertwined cross-talks between ROS, NF-κB, and Wnt/β-catenin signaling.
Previously, we have explained how IO&NS and NF-κB and their associated pathways may explain the central and peripheral symptoms of CFAS-D (3, 15, 16, 23, 36, 63). This study shows that alterations in the Wnt pathway may contribute to these symptoms because this pathway regulates the homeostasis in self-renewing tissues and has additionally organ-specific effects. For example, the Wnt/β-catenin pathway is involved in BBB integrity, synapse assembly, synapse functions, synaptic plasticity, neurogenesis, white matter lesion remyelination, dopamine neuron neuronal survival protection, and regeneration, whereas aberrations in the Wnt/β-catenin pathway are associated with synaptic loss, BBB breakdown, and neurodegenerative disease, including Alzheimer’s and Parkinson’s disease (64–68).
The Wnt/β-catenin pathway is also involved in: (a) pain and neuropathic pain with Wnt inhibition improving pain (67, 69); (b) skeletal muscle dynamics, the neuromuscular synapse, and musculoskeletal functions, including the electrophysiologic properties of muscle cells (70, 71); and (c) intestinal functions, including epithelial homeostasis and integrity, the physiological proliferation of the transit-amplifying cells and differentiation of Paneth, goblet, and enteroendocrine cells, and the maintenance of mucosa and barrier functions (72–75). The Wnt/β-catenin pathway also plays a key role in autoimmunity as observed in rheumatoid arthritis (76, 77) and in response to bacterial infections and inflammation (78, 79).
Aberrations in the cross-talks among redox, NF-κB, and Wnt signaling are predicted to be key pathways in CFAS-D, which are, therefore, associated with dysfunctions in multicellular organism homeostatic processes (including negative regulation of energy homeostasis and tissue maintenance). Disorders in the intertwined interactions between these systems may explain the broad spectrum of organs and dysfunctions that participate in CFAS-D (brain, musculoskeletal system, immune system, and gastrointestinal system). An important spin-off is increased IL-10 production, which may contribute to immunosuppression and recurrent or protracted infections and increased TRYCAT production may aggravate the neurotoxic effects of oxidative stress. Increased translocation of Gram-negative bacteria with increased LPS load is probably a major trigger factor, but also other bacterial infections, toxoplasmosis, viral infections (e.g., cytomegalovirus), cancer, and gastrointestinal, autoimmune, immune-inflammatory, neuroinflammatory, and neurodegenerative disorders appear to be associated with those pathways via activation of TLR/LTF/TWEAK signaling.
Future study should scrutinize the specific role of the NF-κB, ROS, and Wnt axis in CFAS-D. The cross-talks between these three pathways may also constitute new drug targets to treat CFAS-D. Given that the Wnt pathway shows many complex, negative and positive feedback loops interacting with redox systems and NK-κB signaling, manipulations of Wnt signaling and β-catenin (despite being a hub and master switch) appear to be very challenging. It may be more promising to simultaneously target the cross-talk among redox and NF-κB pathways. New knowledge on the precise aberrations in the Wnt pathway in association with ROS and NF-κB signaling may lead to more effective treatments by specifically targeting β-catenin, the β-catenin destruction complex, or the TCF transcription factor.
There are now many studies showing that targeting redox pathways with selected antioxidants may improve chronic fatigue-like symptoms in animal models of CFS and in chronic fatigue comorbid with neuroinflammatory disease (24, 26, 80, 81). In reserpine-induced zebrafish models of fibromyalgia (which is strongly associated with ME/CFS), treatment with hydroxytyrosol, a phenolic phytochemical that has antioxidant properties, regulates Wnt/β-catenin pathway activation and microglial activation in association with improving the behavioral deficits (82). Administration of Ashwagandha, which has antioxidant properties, to rats with thioacetamide-induced hepatic encephalopathy improves neurocognitive deficits in association with attenuation of NF-κB/MAPK pathways and induction of antioxidant responses via Nrf-2 (83). In addition, as reviewed in the Introduction, many studies indicate that administration of diverse antioxidants improve CFAS-D in animal models (24–26). Since GSK-3B is one of the proteins involved in the PPI network, it could be argued that inhibiting GSK-3B is another possible target to treat the condition and that this could be achieved using different GSK-3 inhibitors including lithium (84). Moreover, some studies showed that GSK-3 inhibition using low-dose lithium may augment muscle force production (85). Nevertheless, GSK-3B is not a key gene in the PPI network and, thus, GSK-3 inhibition will probably yield minimal effects. As such, animal models support that interactions among redox, Wnt/β-catenin and NF-κB pathways participate in CFS-like behaviors and that targeting IO&NS pathways may improve these behaviors.
The new model of CFAS-D proposed here is shown in Figure 7. Disorders in the cross-talks among these three key pathways mediate the effects of a variety of trigger factors in the onset of CFAS-D. These findings also support the theory that once CFAS-D is present, the abovementioned pathways may increase morbidity and even mortality of IO&NS-associated medical disorders through the detrimental effects of disorders in redox, NF-κB, and Wnt axis (24, 26, 86–88). The model also explains that the acute phase of inflammatory conditions may lead to CFAS-D via disorders in this axis, oxidative damage and the neurotoxic effects of LPS, proinflammatory cytokines, and TRYCATs. Since the biomarkers included in this study were extracted from studies on ME/CFS and CF-like symptoms in comorbid disorders with duration > 6 months, we may conclude that after resolution of acute inflammation, CAFS-D symptoms are maintained by continued aberrations in the redox, NF-κB, and Wnt axis, increased IL-10 production, and increasing oxidative damage, including secondary autoimmune responses and nitrosylation.
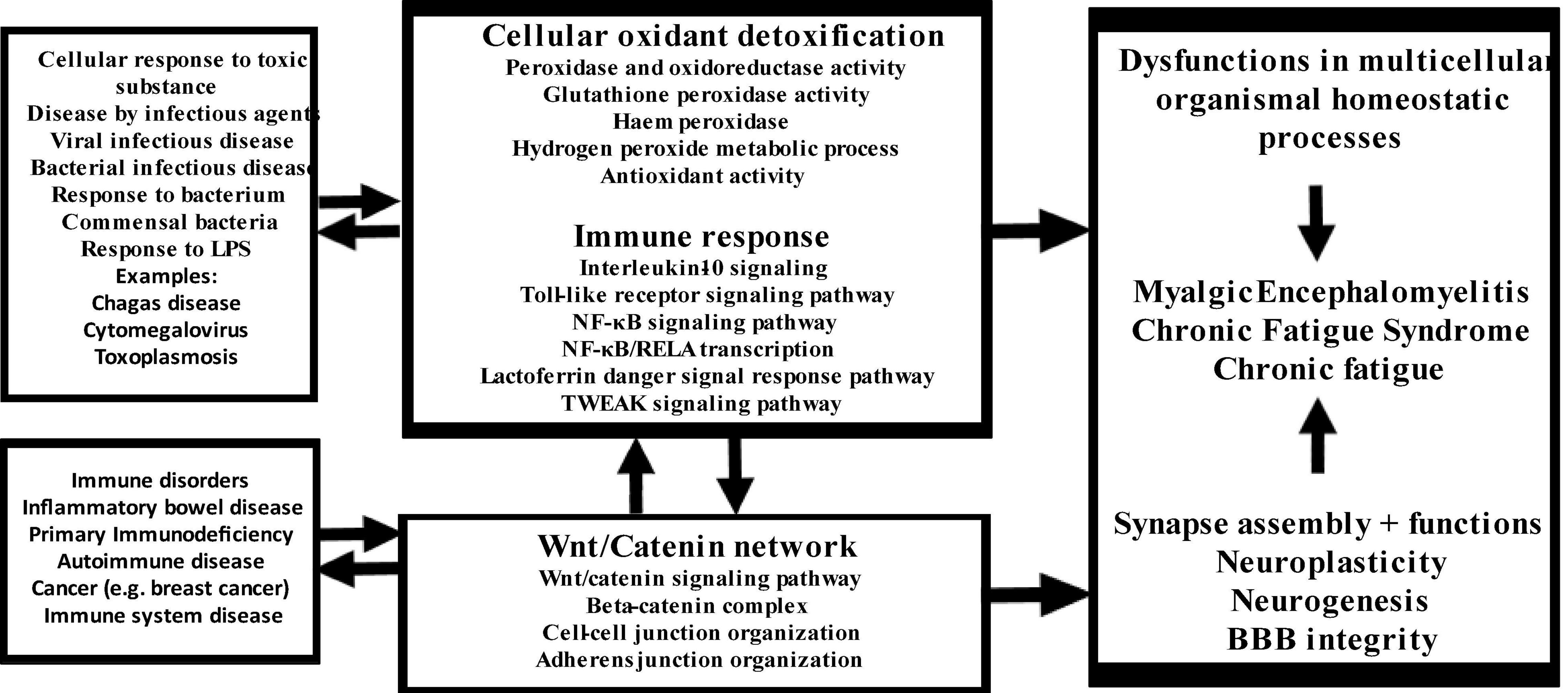
Figure 7. Summary of the findings in this study. LPS: lipopolysaccharides, NF-κB: nuclear factor-κB, RELA: transcription factor p65, TWEAK: TNF-related weak inducer of apoptosis.
MM designed the study and performed the network, enrichment, and annotation analyses. All authors contributed to interpretation of the data, writing of the manuscript, and participated in the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.822382/full#supplementary-material
1. Maes M, Twisk FN. Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Med. (2010) 8:35. doi: 10.1186/1741-7015-8-35
2. Morris G, Maes M. Case definitions and diagnostic criteria for myalgic encephalomyelitis and chronic fatigue syndrome: from clinical-consensus to evidence-based case definitions. Neuro Endocrinol Lett. (2013) 34:185–99.
3. Maes M. Inflammatory and oxidative and nitrosative stress cascades as new drug targets in myalgic encephalomyelitis and chronic fatigue syndrome. Mod Trends Pharmacopsychiatry. (2013) 28:162–74. doi: 10.1159/000343982
4. Maes M, Anderson G, Morris G, Berk M. Diagnosis of myalgic encephalomyelitis: where are we now? Expert Opin Med Diagn. (2013) 7:221–5. doi: 10.1517/17530059.2013.776039
5. Maes M, Rodriguez LA, Morris G. Is a diagnostic blood test for chronic fatigue syndrome on the horizon? Expert Rev Mol Diagn. (2019) 19:1049–51. doi: 10.1080/14737159.2020.1681976
6. Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. (2011) 270:327-38. Erratum J Intern Med. (2017) 282:353. doi: 10.1111/j.1365-2796.2011.02428.x
7. Maes M, Kubera M, Stoyanova K, Leunis JC. The reification of the clinical diagnosis of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) as an immune and oxidative stress disorder: construction of a data-driven nomothethic network and exposure of ME/CFS subgroups. Curr Top Med Chem. (2021) 21:1488–99. doi: 10.2174/1568026621666210727170147
8. Maes M. An intriguing and hitherto unexplained co-occurrence: depression and chronic fatigue syndrome are manifestations of shared inflammatory, oxidative and nitrosative (IO&NS) pathways. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:784–94. doi: 10.1016/j.pnpbp.2010.06.023
9. Maes M, Andrés-Rodríguez L, Vojdani A, Sirivichayakul S, Barbosa D, Kanchanatawan B. In schizophrenia, chronic fatigue syndrome- and fibromyalgia-like symptoms are driven by breakdown of the paracellular pathway with increased zonulin and immune activation-associated neurotoxicity. Preprints. (2021). doi: 10.20944/preprints202105.0182.v1
10. Castro-Marrero J, Faro M, Aliste L, Sáez-Francàs N, Calvo N, Martínez-Martínez A, et al. Comorbidity in chronic fatigue syndrome/myalgic encephalomyelitis: a nationwide population-based cohort study. Psychosomatics. (2017) 58:533–43. doi: 10.1016/j.psym.2017.04.010
11. Mousa RF, Al-Hakeim HK, Alhaideri A, Maes M. Chronic fatigue syndrome and fibromyalgia-like symptoms are an integral component of the phenome of schizophrenia: neuro-immune and opioid system correlates. Metab Brain Dis. (2021) 36:169–83. doi: 10.1007/s11011-020-00619-x
12. Al-Jassas H, Al-Hakeim H, Maes M. Intersections between pneumonia, lowered oxygen saturation percentage and immune activation mediate depression, anxiety and chronic fatigue syndrome-like symptoms due to COVID-19: a nomothetic network approach. Preprints. (2021). doi: 10.20944/preprints202106.0362.v1
13. Asad HN, Al-Hakeim HK, Moustafa SR, Maes M. Causal-pathway phenotype of chronic fatigue syndrome due to hemodialysis in patients with end-stage renal disease. CNS Neurol Disord Drug Targets (2022). doi: 10.2174/1871527321666220401140747 [Epub ahead of print].
14. Al-Hakeim HK, Al-Issa AAR, Maes M. Serum agrin and talin are increased in major depression while agrin and creatine phosphokinase are associated with chronic fatigue and fibromyalgia symptoms in depression. Metab Brain Dis. (2020) 35:225–35. doi: 10.1007/s11011-019-00506-0
15. Morris G, Maes M. Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics. BMC Med. (2013) 11:205. doi: 10.1186/1741-7015-11-205
16. Anderson G, Berk M, Maes M. Biological phenotypes underpin the physio-somatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta Psychiatr Scand. (2014) 129:83–97. doi: 10.1111/acps.12182
17. Anderson G, Maes M, Berk M. Biological underpinnings of the commonalities in depression, somatization, and chronic fatigue syndrome. Med Hypotheses. (2012) 78:752–6. doi: 10.1016/j.mehy.2012.02.023
18. Maes M, Mihaylova I, Leunis JC. Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett. (2006) 27:615–21.
19. Maes M, Mihaylova I, Leunis JC. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): indication for the involvement of gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut-intestinal permeability. J Affect Disord. (2007) 99:237–40. doi: 10.1016/j.jad.2006.08.021
20. Maes M, Plaimas K, Suratanee A, Noto C, Kanchanatawan B. The protein-protein interaction network of first episode psychosis and schizophrenia reveals possible trigger factors and new drug targets among intracellular signal transduction pathways and neurotoxicity processes. Preprints. (2021). doi: 10.20944/preprints202107.0596.v1
21. Lee JS, Kim HG, Lee DS, Son CG. Oxidative stress is a convincing contributor to idiopathic chronic fatigue. Sci Rep. (2018) 8:12890. doi: 10.1038/s41598-018-31270-3
22. Polli A, Van Oosterwijck J, Nijs J, Marusic U, De Wandele I, Paul L, et al. Relationship between exercise-induced oxidative stress changes and parasympathetic activity in chronic fatigue syndrome: an observational study in patients and healthy subjects. Clin Ther. (2019) 41:641–55. doi: 10.1016/j.clinthera.2018.12.012
23. Bjørklund G, Dadar M, Pivina L, Doşa MD, Semenova Y, Maes M. Environmental, neuro-immune, and neuro-oxidative stress interactions in chronic fatigue syndrome. Mol Neurobiol. (2020) 57:4598–607. doi: 10.1007/s12035-020-01939-w
24. Singh A, Naidu PS, Gupta S, Kulkarni SK. Effect of natural and synthetic antioxidants in a mouse model of chronic fatigue syndrome. J Med Food. (2002) 5:211–20. doi: 10.1089/109662002763003366
25. Gupta A, Vij G, Sharma S, Tirkey N, Rishi P, Chopra K. Curcumin, a polyphenolic antioxidant, attenuates chronic fatigue syndrome in murine water immersion stress model. Immunobiology. (2009) 214:33–9. doi: 10.1016/j.imbio.2008.04.003
26. Wang X, Qu Y, Zhang Y, Li S, Sun Y, Chen Z, et al. Antifatigue potential activity of sarcodon imbricatus in acute excise-treated and chronic fatigue syndrome in mice via regulation of Nrf2-mediated oxidative stress. Oxid Med Cell Longev. (2018) 2018:9140896. doi: 10.1155/2018/9140896
27. Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. (2006) 25:6680–4. doi: 10.1038/sj.onc.1209954
28. Brasier AR. The NF-kappaB regulatory network. Cardiovasc Toxicol. (2006) 6:111–30. doi: 10.1385/ct:6:2:111
29. Morgan MJ, Liu ZG. Reactive oxygen species in TNFalpha-induced signaling and cell death. Mol Cells. (2010) 30:1–12. doi: 10.1007/s10059-010-0105-0
30. UniProtB. UniProtKB – Q04206 (TF65_HUMAN). RELA – Transcription Factor p65 – Homo Sapiens (Human) – RELA Gene & Protein. (2021). Available online at: uniprot.org (accessed July 23, 2021).
31. Ma S, Santhosh D, Kumar TP, Huang Z. A brain-region-specific neural pathway regulating germinal matrix angiogenesis. Dev Cell. (2017) 41:366–81.e4. doi: 10.1016/j.devcel.2017.04.014
32. Napolitano M, Zei D, Centonze D, Palermo R, Bernardi G, Vacca A, et al. NF-kB/NOS cross-talk induced by mitochondrial complex II inhibition: implications for Huntington’s disease. Neurosci Lett. (2008) 434:241–6. doi: 10.1016/j.neulet.2007.09.056
33. Kabe Y, Ando K, Hirao S, Yoshida M, Handa H. Redox regulation of NF-kappaB activation: distinct redox regulation between the cytoplasm and the nucleus. Antioxid Redox Signal. (2005) 7:395–403. doi: 10.1089/ars.2005.7.395
34. Toledano MB, Leonard WJ. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci USA. (1991) 88:4328–32. doi: 10.1073/pnas.88.10.4328
35. Maes M, Mihaylova I, Bosmans E. Not in the mind of neurasthenic lazybones but in the cell nucleus: patients with chronic fatigue syndrome have increased production of nuclear factor kappa beta. Neuro Endocrinol Lett. (2007) 28:456–62.
36. Morris G, Maes M. Increased nuclear factor-κB and loss of p53 are key mechanisms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Med Hypotheses. (2012) 79:607–13. doi: 10.1016/j.mehy.2012.07.034
37. Yang X, Li F, Liu Y, Li D, Li J. Study on the correlation between NF-κB and central fatigue. J Mol Neurosci. (2021) 71:1975–86. doi: 10.1007/s12031-021-01803-z
38. Lucas K, Maes M. Role of the toll like receptor (TLR) radical cycle in chronic inflammation: possible treatments targeting the TLR4 pathway. Mol Neurobiol. (2013) 48:190–204. doi: 10.1007/s12035-013-8425-7
39. Lucas K, Morris G, Anderson G, Maes M. The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets. (2015) 14:838–54. doi: 10.2174/1871527314666150317224645
40. Kruzel ML, Zimecki M, Actor JK. Lactoferrin in a context of inflammation-induced pathology. Front Immunol. (2017) 8:1438. doi: 10.3389/fimmu.2017.01438
41. Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. (2011) 21:71–85. doi: 10.1038/cr.2010.177
42. Chen J, Liu XS. Development and function of IL-10 IFN-gamma-secreting CD4(+) T cells. J Leukoc Biol. (2009) 86:1305–10. doi: 10.1189/jlb.0609406
43. Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. (2008) 180:5771–7. doi: 10.4049/jimmunol.180.9.5771
44. Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. (2015) 34:22–7. doi: 10.1016/j.coi.2014.12.009
45. Mihaylova I, DeRuyter M, Rummens JL, Bosmans E, Maes M. Decreased expression of CD69 in chronic fatigue syndrome in relation to inflammatory markers: evidence for a severe disorder in the early activation of T lymphocytes and natural killer cells. Neuro Endocrinol Lett. (2007) 28:477–83.
46. Maes M, Rief W. Diagnostic classifications in depression and somatization should include biomarkers, such as disorders in the tryptophan catabolite (TRYCAT) pathway. Psychiatry Res. (2012) 196:243–9. doi: 10.1016/j.psychres.2011.09.029
47. Mor A, Tankiewicz-Kwedlo A, Krupa A, Pawlak D. Role of kynurenine pathway in oxidative stress during neurodegenerative disorders. Cells. (2021) 10:1603. doi: 10.3390/cells10071603
48. Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, et al. The DIX domain of dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. (2007) 14:484–92. doi: 10.1038/nsmb1247
49. Tietz S, Engelhardt B. Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol. (2015) 209:493–506. doi: 10.1083/jcb.201412147
50. Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem. (2007) 282:11487–98. doi: 10.1074/jbc.M610597200
51. Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function, and connections to the actin cytoskeleton. Biochim Biophys Acta. (2008) 1778:660–9. doi: 10.1016/j.bbamem.2007.07.012
52. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. (2009) 17:9–26. doi: 10.1016/j.devcel.2009.06.016
53. Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. (2008) 13:7210–26. doi: 10.2741/3223
54. Silva-García O, Valdez-Alarcón JJ, Baizabal-Aguirre VM. Wnt/β-catenin signaling as a molecular target by pathogenic bacteria. Front Immunol. (2019) 10:2135. doi: 10.3389/fimmu.2019.02135
55. Zhao H, Zhang J, Qian N, Wu S, Wu Y, Yao G. Oxidative stress caused by a dysregulated Wnt/β-catenin signalling pathway is involved in abnormal placenta formation in pregnant mice with chronic fatigue syndrome. Zygote. (2021) 29:122–9. doi: 10.1017/S096719942000057X
56. Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. (2006) 8:501–8. doi: 10.1038/ncb1405
57. Karimaian A, Majidinia M, Bannazadeh Baghi H, Yousefi B. The crosstalk between Wnt/β-catenin signaling pathway with DNA damage response and oxidative stress: implications in cancer therapy. DNA Repair (Amst). (2017) 51:14–9. doi: 10.1016/j.dnarep.2017.01.003
58. Shin SY, Kim CG, Jho EH, Rho MS, Kim YS, Kim YH, et al. Hydrogen peroxide negatively modulates Wnt signaling through downregulation of beta-catenin. Cancer Lett. (2004) 212:225–31. doi: 10.1016/j.canlet.2004.03.003
59. Korswagen HC. Regulation of the Wnt/beta-catenin pathway by redox signaling. Dev Cell. (2006) 10:687–8. doi: 10.1016/j.devcel.2006.05.007
60. Ma B, Hottiger MO. Crosstalk between Wnt/β-Catenin and NF-κB signaling pathway during inflammation. Front Immunol. (2016) 7:378. doi: 10.3389/fimmu.2016.00378
61. Jridi I, Canté-Barrett K, Pike-Overzet K, Staal FJT. Inflammation and Wnt signaling: target for immunomodulatory therapy? Front Cell Dev Biol. (2021) 8:615131. doi: 10.3389/fcell.2020.615131
62. Gustafson B, Smith U. Cytokines promote Wnt signaling and inflammation and impair the normal differentiation and lipid accumulation in 3T3-L1 preadipocytes. J Biol Chem. (2006) 281:9507–16. doi: 10.1074/jbc.M512077200
63. Morris G, Puri BK, Walker AJ, Maes M, Carvalho AF, Walder K, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: from pathophysiological insights to novel therapeutic opportunities. Pharmacol Res. (2019) 148:104450. doi: 10.1016/j.phrs.2019.104450
64. Laksitorini MD, Yathindranath V, Xiong W, Hombach-Klonisch S, Miller DW. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci Rep. (2019) 9:19718. doi: 10.1038/s41598-019-56075-w
65. Dickins EM, Salinas PC. Wnts in action: from synapse formation to synaptic maintenance. Front Cell Neurosci. (2013) 7:162. doi: 10.3389/fncel.2013.00162
66. Marchetti B. Wnt/β-catenin signaling pathway governs a full program for dopaminergic neuron survival, neurorescue and regeneration in the MPTP mouse model of Parkinson’s disease. Int J Mol Sci. (2018) 19:3743. doi: 10.3390/ijms19123743
67. Manukjan N, Ahmed Z, Fulton D, Blankesteijn WM, Foulquier S. A systematic review of WNT signaling in endothelial cell oligodendrocyte interactions: potential relevance to cerebral small vessel disease. Cells. (2020) 9:1545. doi: 10.3390/cells9061545
68. Varela-Nallar L, Inestrosa NC. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci. (2013) 7:100. doi: 10.3389/fncel.2013.00100
69. Zhao Y, Zhang L, Wang M, Yu J, Yang J, Liu A, et al. Anxiety specific response and contribution of active hippocampal neural stem cells to chronic pain through Wnt/β-catenin signaling in mice. Front Mol Neurosci. (2018) 11:296. doi: 10.3389/fnmol.2018.00296
70. Zhao C, Yu Y, Zhang Y, Shen J, Jiang L, Sheng G, et al. β-catenin controls the electrophysiologic properties of skeletal muscle cells by regulating the α2 isoform of Na+/K+-ATPase. Front Neurosci. (2019) 13:831. doi: 10.3389/fnins.2019.00831
71. Cisternas P, Henriquez JP, Brandan E, Inestrosa NC. Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol. (2014) 49:574–89. doi: 10.1007/s12035-013-8540-5
72. Moparthi L, Koch S. Wnt signaling in intestinal inflammation. Differentiation. (2019) 108:24–32. doi: 10.1016/j.diff.2019.01.002
73. Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. (2005) 19:877–90. doi: 10.1101/gad.1295405
74. Flanagan DJ, Austin CR, Vincan E, Phesse TJ. Wnt signalling in gastrointestinal epithelial stem cells. Genes (Basel). (2018) 9:178. doi: 10.3390/genes9040178
75. Jeon MK, Klaus C, Kaemmerer E, Gassler N. Intestinal barrier: molecular pathways and modifiers. World J Gastrointest Pathophysiol. (2013) 4:94–9. doi: 10.4291/wjgp.v4.i4.94
76. Miao CG, Yang YY, He X, Li XF, Huang C, Huang Y, et al. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell Signal. (2013) 25:2069–78. doi: 10.1016/j.cellsig.2013.04.002
77. Cici D, Corrado A, Rotondo C, Cantatore FP. Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int J Mol Sci. (2019) 20:5552. doi: 10.3390/ijms20225552
78. Rogan MR, Patterson LL, Wang JY, McBride JW. Bacterial manipulation of Wnt signaling: a host-pathogen tug-of-Wnt. Front Immunol. (2019) 10:2390. doi: 10.3389/fimmu.2019.02390
79. Ljungberg JK, Kling JC, Tran TT, Blumenthal A. Functions of the WNT signaling network in shaping host responses to infection. Front Immunol. (2019) 10:2521. doi: 10.3389/fimmu.2019.02521
80. Bjørklund G, Dadar M, Pen JJ, Chirumbolo S, Aaseth J. Chronic fatigue syndrome (CFS): suggestions for a nutritional treatment in the therapeutic approach. Biomed Pharmacother. (2019) 109:1000–7. doi: 10.1016/j.biopha.2018.10.076
81. Ferorelli P, Antonelli F, Shevchenko A, Mischiati C, Doepp M, Lenzi S, et al. Reduction in fatigue symptoms following the administration of nutritional supplements in patients with multiple sclerosis. Med Sci (Basel). (2021) 9:52. doi: 10.3390/medsci9030052
82. D’Amico R, Cordaro M, Siracusa R, Impellizzeri D, Trovato Salinaro A, Scuto M, et al. Wnt/β-catenin pathway in experimental model of fibromyalgia: role of hidrox®. Biomedicines. (2021) 9:1683. doi: 10.3390/biomedicines9111683
83. Khalil HMA, Eliwa HA, El-Shiekh RA, Al-Mokaddem AK, Hassan M, Tawfek AM, et al. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J Ethnopharmacol. (2021) 277:114141. doi: 10.1016/j.jep.2021.114141
84. Maes M, Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates–Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. (2012) 20:127–50. doi: 10.1007/s10787-011-0111-7
85. Whitley KC, Hamstra SI, Baranowski RW, Watson CJF, MacPherson REK, MacNeil AJ, et al. GSK3 inhibition with low dose lithium supplementation augments murine muscle fatigue resistance and specific force production. Physiol Rep. (2020) 8:e14517. doi: 10.14814/phy2.14517
86. Zhao Y, Yang Z. Effect of Wnt signaling pathway on pathogenesis and intervention of neuropathic pain. Exp Ther Med. (2018) 16:3082–8. doi: 10.3892/etm.2018.6512
87. Maes M, Twisk FN. Why myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) may kill you: disorders in the inflammatory and oxidative and nitrosative stress (IO&NS) pathways may explain cardiovascular disorders in ME/CFS. Neuro Endocrinol Lett. (2009) 30:677–93.
Keywords: chronic fatigue syndrome (CFS), inflammation, neuro-immune, oxidative and nitrosative stress, myalgic encephalomyelitis, antioxidants, bacterial translocation
Citation: Maes M, Kubera M and Kotańska M (2022) Aberrations in the Cross-Talks Among Redox, Nuclear Factor-κB, and Wnt/β-Catenin Pathway Signaling Underpin Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. Front. Psychiatry 13:822382. doi: 10.3389/fpsyt.2022.822382
Received: 25 November 2021; Accepted: 23 March 2022;
Published: 06 May 2022.
Edited by:
Milica Milovan Borovcanin, University of Kragujevac, SerbiaReviewed by:
Nemanja Jovicic, University of Kragujevac, SerbiaCopyright © 2022 Maes, Kubera and Kotańska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Maes, ZHIubWljaGFlbG1hZXNAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.