- 1Department of Psychiatry, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 2Department of Nursing, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
- 3Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Background: HIV-associated neurocognitive disorders are common in people living with HIV/AIDS and affect the adherence of patients to prescriptions, activities of daily living, and quality of life of patients. However, there is a lack of summative evidence in the area. The present meta-analysis was therefore addressing this gap.
Methods: We did our electronic search in Psych-Info, EMBASE, Scopus, and PubMed. The retrieved articles were stored with the endnote reference manager and data was extracted using Meta-XL version 5.3. The quality of studies was evaluated with the modified Newcastle–Ottawa Scale (NOS). A random-effect model and STATA-16 were used to compute the average estimate of HAND. Heterogeneity was weighed with I2 statistics. A sensitivity analysis and subgroup analysis were employed. The existence/nonexistence of a publication bias was checked with the Eggers test of publication bias.
Results: The average prevalence of HAND was 50.41% (95% CI: 45.56, 55.26). The average estimate of HAND in Europe was found to be 50.015% whereas in Africa, Asia, and the United States of America (USA) it was 49.566, 52.032, and 50.407% respectively. The prevalence of HAND in studies that used the HIV Dementia Scale (IHDS) was 36.883% and 59.956% at cutoff points of IHDS <9.5 and IHDS <10 respectively. Besides, the estimated average of HAND with the global dementia scale (GDS) was 40.766%. The prevalence of HAND in cross-sectional, cohort, and case-control studies was 49.52, 54.087, and 44.45% in that order. Socio-demographic variables; low level of education and older age, clinical and HIV related variables; the advanced stage of the illness and CD4 count of 500 cells/dl or less and psychological variables such as comorbidity of depression increases the risk of HAND.
Conclusion: The prevalence of HIV-associated neurocognitive disorders was about 50.41%. Low level of education and older age, clinical and HIV related variables such as the advanced stage of the illness and CD4 count of 500 cells/dl or less, and comorbidity of depression were associated with HIV associated neurocognitive disorders. Public health interventions for HIV patients should target these essential problems.
Introduction
HIV/AIDS is a global public health issue with more than 34 million people living with HIV/AIDS(PLHIV) currently (1). Mental, neurological, and substance (MNS) related disorders are very common in People living with HIV/AIDS (2). The latest systematic review and Meta-analysis studies by Necho et al. (3) revealed that 35.8% of HIV/AIDS patients had depressive symptoms (3). Another systematic review and meta-analysis studies reported that the prevalence of post-traumatic stress disorder (PTSD), alcohol use disorder (AUD), and suicidal ideation in individuals living with HIV/AIDS were 32.67% (4), 22.02% (5), and 21.7% (6), respectively.
Since HIV is a neurotropic virus, it affects the cortical and subcortical parts of the brain resulting in cognitive impairment (7). This impact of HIV on the cognitive domain of patients is known as HIV-associated neurocognitive disorder (HAND) (8, 9). The level of HAND ranges from asymptomatic impairment to minor neurocognitive disorder and full-blown dementia (10–13). The HIV-associated neurocognitive disorder affects memory, attention, problem-solving ability, language, higher executive function, and independent activities of daily living (14).
HIV-associated neurocognitive disorders are very common in HIV/AIDS patients (15). A study by Habib et al. (16) reported that the burden of neurocognitive impairment (NCI) among patients on Antiretroviral therapy (ART) attendants was 30.39%. Based on the report of multiple earlier studies the worldwide burden of HIV-associated neurocognitive disorders (HAND) varies from a minimum of 7.3% to a maximum of 85% (8, 10, 12–14, 17–50). Besides, the frequency of HIV-associated neurocognitive disorder (HAND) in developed and developing countries varies between 19–52% (31, 51), and 14–64% (12, 13), respectively.
Different studies reported varieties of socio-demographic and clinical factors associated with HIV-associated neurocognitive disorders in people living with HIV/AIDS. For example studies from, Cameroon, Nigeria, Botswana, Singapore, Malawi, and Dessie Ethiopia reported that socio-demographic variables such as older age, female sex, and lower educational level were risk factors for HIV associated neurocognitive disorder (13, 14, 46, 50, 52, 53). Besides, from Clinical variables CD4 count of <500 cells/mm3 was related to HIV-associated neurocognitive disorder based on reports of studies from Brazil, Singapore, and Northern Nigeria (14, 50, 54). Moreover, advanced stages of AIDS and not being on highly active antiretroviral treatment (HAART) were associated with HIV-associated neurocognitive disorder in South Africa (51–53). In Uganda, behavioral and psychological variables such as depression, Body mass index, and alcohol abuse were associated with HIV-associated neurocognitive disorder (10). Moreover, medication non-adherence and opportunistic infections were associated with HIV-associated neurocognitive disorder (46, 55).
The presence of HIV-associated neurocognitive disorder predisposes people living with HIV/AIDS to substance abuse, poor medication adherence, and unsafe sex so the poor quality of life and loss of follow-up from treatment are outcomes. These conditions speed up the progression of the virus to its advanced stages and the development of severe opportunistic infections and death (11, 12).
Even though a high proportion of the world population has been living with HIV/AIDS and a high prevalence of mental, neurological, and substance use disorders in this population, these problems, especially neurocognitive disorders, are not investigated well. Despite the presence of some studies in the area, they are mostly confined to a small population and a narrow geographical area (8, 10, 12–14, 17–50). Consequently, there arises a need to have aggregate data regarding HIV-associated neurocognitive disorder and its associated factors from the global context.
Therefore, this systematic review and meta-analysis aimed to estimate the prevalence of HIV-associated neurocognitive disorder in people living with HIV AIDS and to analyze the associated factors for HIV-associated neurocognitive disorder in people with HIV AIDS.
Methods
Search Strategy
We have performed our search strategy for this review in different ways. Initially, we did an electronic exploration for eligible articles regarding HIV-associated neurocognitive disorders in people living with HIV AIDS in the databases of Psych-Info, EMBASE, Scopus, and PubMed. As a sample of our search strategy with the PubMed database, we have used the following key terms: [(neurocognitive disorder or HIV-associated neurocognitive disorder or HAND) and (adults)] and (Human immunodeficiency virus or HIV or acquired immunodeficiency syndrome or AIDS). Moreover, Psych-Info, EMBASE, and Scopus databases were investigated in line with the searching guidelines of each database. Besides, the reference lists of included studies were searched manually for additional eligible articles. There was no time restriction to the publication year of the articles during the searching process.
Eligibility Criteria
During our study of a systematic review and meta-analysis on HIV-associated neurocognitive disorder in people living with HIV AIDS, we have set the following inclusion and exclusion criteria based on the: 1) the primary inclusion criteria to analyze where the study should assess prevalence OR associated factors of HIV-associated neurocognitive disorder in people living with HIV AIDS. 2) The HIV-associated neurocognitive disorder had also to be investigated using the International HIV Dementia Scale (IHDS), Frascati criteria, Mini-mental state exam(MMSE), global dementia scale(GDS), Brief Neurocognitive Screen, Neuropsychological battery, Montreal Cognitive Assessment (MoCA), In-depth neuropsychological assessment, Wechsler Adult Intelligence Scale and ADC.
We excluded studies (1) that assessed neurocognitive disorder in samples other than people living with HIV/AIDS. (2) That assessed neurocognitive disorder in individuals taking psychotropic medication. (3) Studies that are letters to the editor with non-original data content, earlier reviews, case studies, studies involving non-human subjects, and articles published in a language other than the English language were also excluded from the analysis. After all relevant articles were searched in the mentioned databases; they were stored in an endnote reference manager. Two of the authors (MN and YZ) individually screened the titles and abstracts of articles stored in an endnote reference manager using the eligibility criteria. Next to that, the above two authors carefully read the full length of articles that passed the initial screening and decided independently articles suitable for inclusion in the final meta-analysis. Any disagreement between them regarding eligibility criteria was resolved by agreement and with a third reviewer (WY).
Data Extraction and Quality Assessment Techniques
The extraction of relevant data from the 40 final included articles was separately done by two authors (MN and YZ) using an identical data extraction form as suggested by PRISMA guidelines (56), using Meta-XL version 5.3 (57) and the result was summarized in a table. Disagreements among these two authors were settled with a discussion. The contents of the data extraction template were author name, year of publication, the country where the study was done, study design, study sample population, an assessment tool for HIV associated neurocognitive disorders, number of cases with HIV associated neurocognitive disorders, prevalence of HIV associated neurocognitive disorders, sampling technique employed to recruit participants, and response rate of the study.
The quality of 40 included studies (8, 10, 12–14, 17–50) had been evaluated using the modified Newcastle–Ottawa Scale (NOS) (58) as the gold standard. Representativeness of sample and sample size, statistical quality, comparability among participants, and ascertainment of cases were the components of this quality assessment scale. Based on this scale studies with a quality score of 7 to 10 were categorized as very good/good, a score of 5 to 6 was categorized as having satisfactory quality, and a score less than 5 was taken as unsatisfactory quality.
Data Analysis and Synthesis
The random-effect model was used to compute the average estimate of HIV-associated neurocognitive disorders and their associated factors with 95% CIs (59). The STATA-16 Meta-prop package (60) was employed to find the average estimate of HIV-associated neurocognitive disorders. Heterogeneity among the 40 involved studies (8, 10, 12–14, 17–50) was weighed with Q and I2 statistics (61). An I2 numerical value of more than 50% implies a significant degree of heterogeneity among 40 studies (61). As there existed a potential heterogeneity during analysis, we further conducted a sensitivity analysis to identify an influential study outweighing the study found. Additionally, we did a subgroup analysis regarding the country of the study, study design, and the assessment tools used to screen HIV-associated neurocognitive disorders. The presence/absence of a publication bias was done with the funnel plot test (62) and eggers test of publication bias.
Results
Identification of Studies
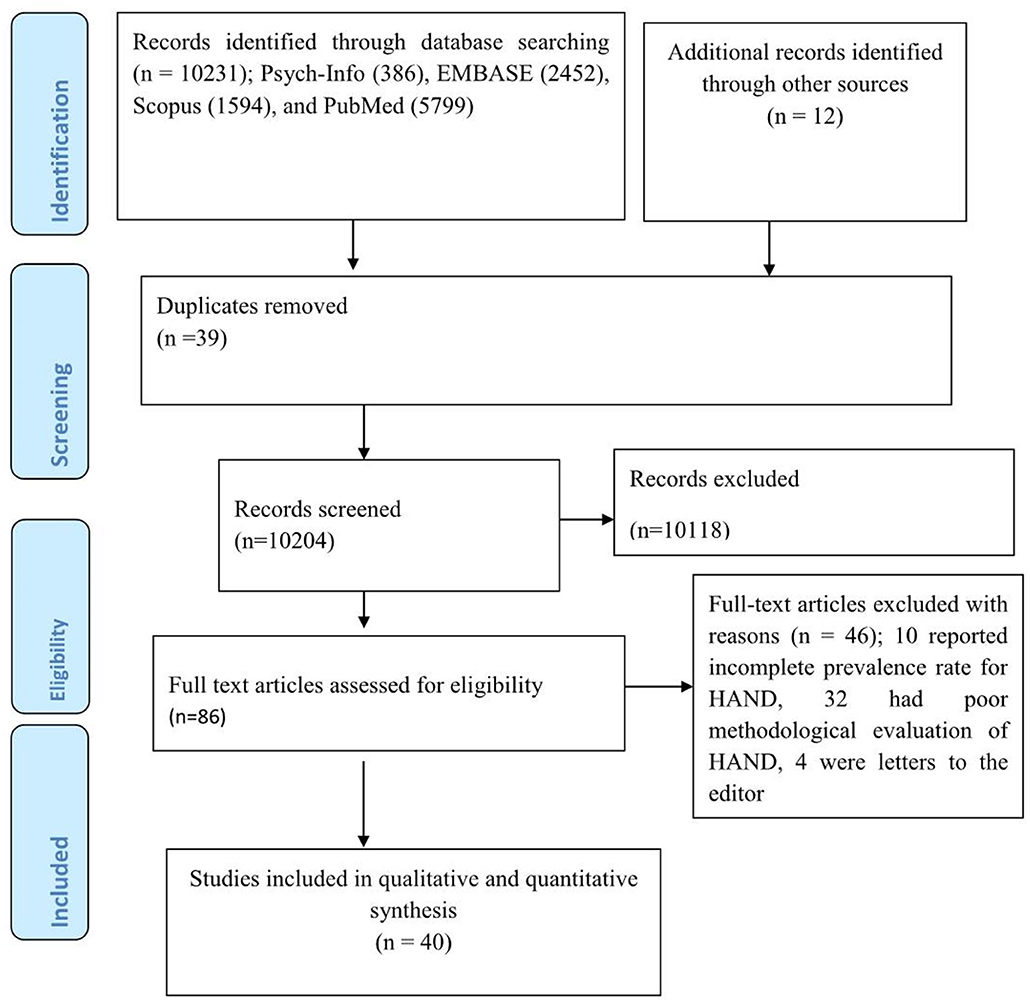
Our electronic search gave a total of 10231 articles; Psych-Info (386), EMBASE (2,452), Scopus (1,594), and PubMed (5,799). Additionally, 12 articles were retrieved by looking for a reference list of earlier articles. Thus, a total of 10,243 articles were retrieved during the overall searching process, of which 39 were removed as they were duplicates. During the initial stage of screening, most of the articles (10,118) were excluded merely by looking at their title or abstract. The remaining 86 articles were completely inspected for suitability for inclusion in the study but only 40 articles were suited for the final meta-analysis as the remaining 46 studies were excluded; 10 reported an incomplete prevalence rate for HAND, 32 had a poor methodological evaluation of HAND, and 4 were letters to the editor (Figure 1).
Characteristics of Included Studies
A total of 40 studies (8, 10, 12–14, 17–50) that surveyed HIV-associated neurocognitive disorders in 14,107 HIV/AIDS patients were integrated into the current systematic review and meta-analysis study. Of the 40 included studies; 11 were from Europe, 21 were from Africa, six were from Asia, and two were from the United States of America (USA). Most of the included studies were cross-sectional in design whereas the remaining 10 and 2 were cohort and case-control, respectively. Regarding tools used for the assessment of HIV-associated neurocognitive disorders, half of the included studies (twenty) used the International HIV Dementia Scale (IHDS). Frascati criteria, global dementia scales (GDS), and Montreal Cognitive Assessment (MoCA) were also used to assess HIV-associated neurocognitive disorders in three, three, and three studies, respectively. The reported prevalence of HIV-associated neurocognitive disorders included in the meta-analysis differs from 7.3% in the United Kingdom (28) to 88% in Kenya (35) (Table 1).
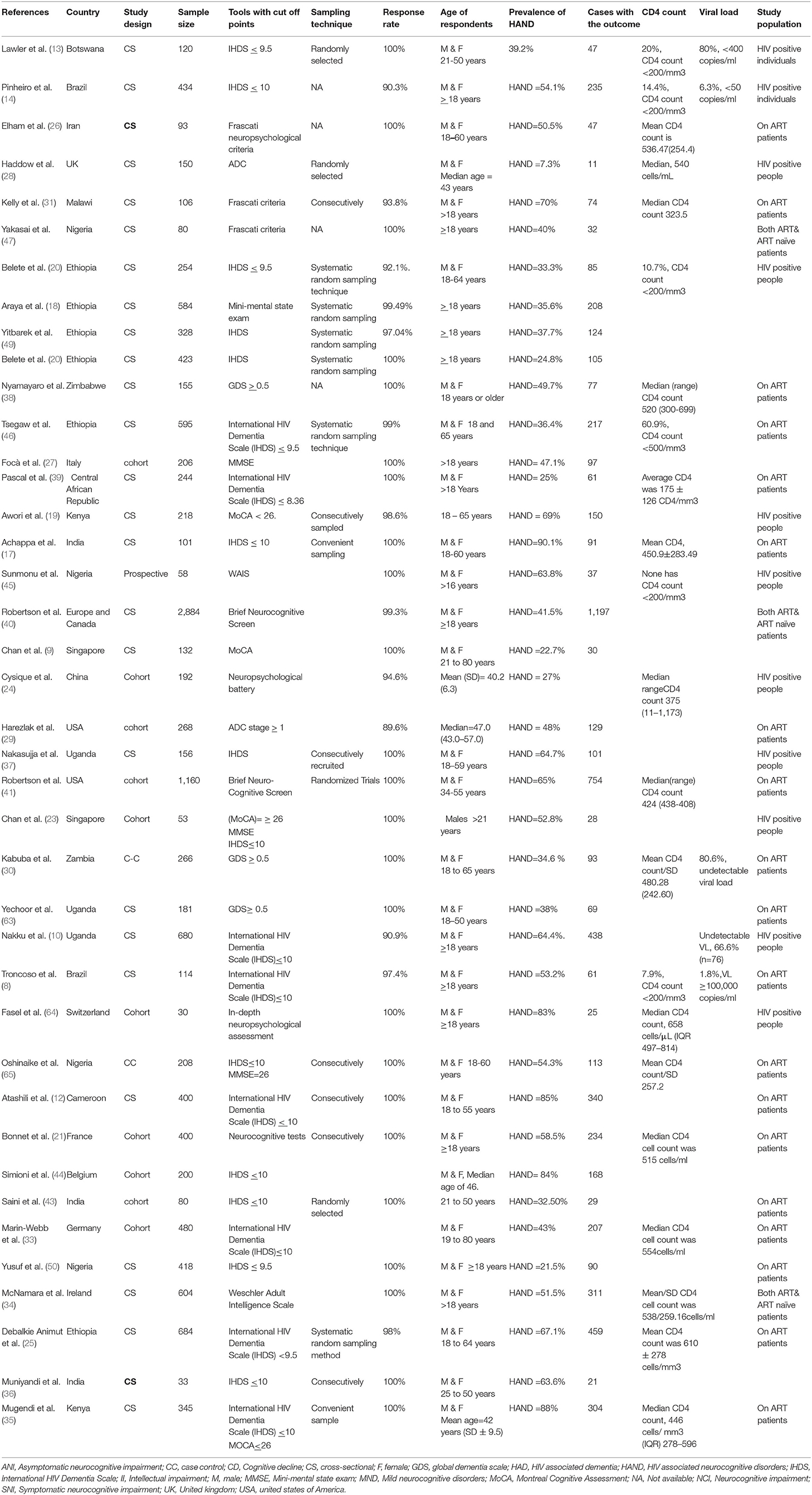
Table 1. Characteristics of studies on HIV associated neurocognitive disorders in HIV/AIDS patients which are incorporated in this meta-analysis.
Quality of Included Studies
Among the 40 included studies; the majority (twenty-nine) had scored from 7 to 10 so good quality scores on the scale. Of the remaining 11 studies, seven had a satisfactory quality, and the remaining four studies had unsatisfactory quality (Additional File 1).
The Prevalence of HIV-Associated Neurocognitive Disorders Among People Living With HIV/AIDS
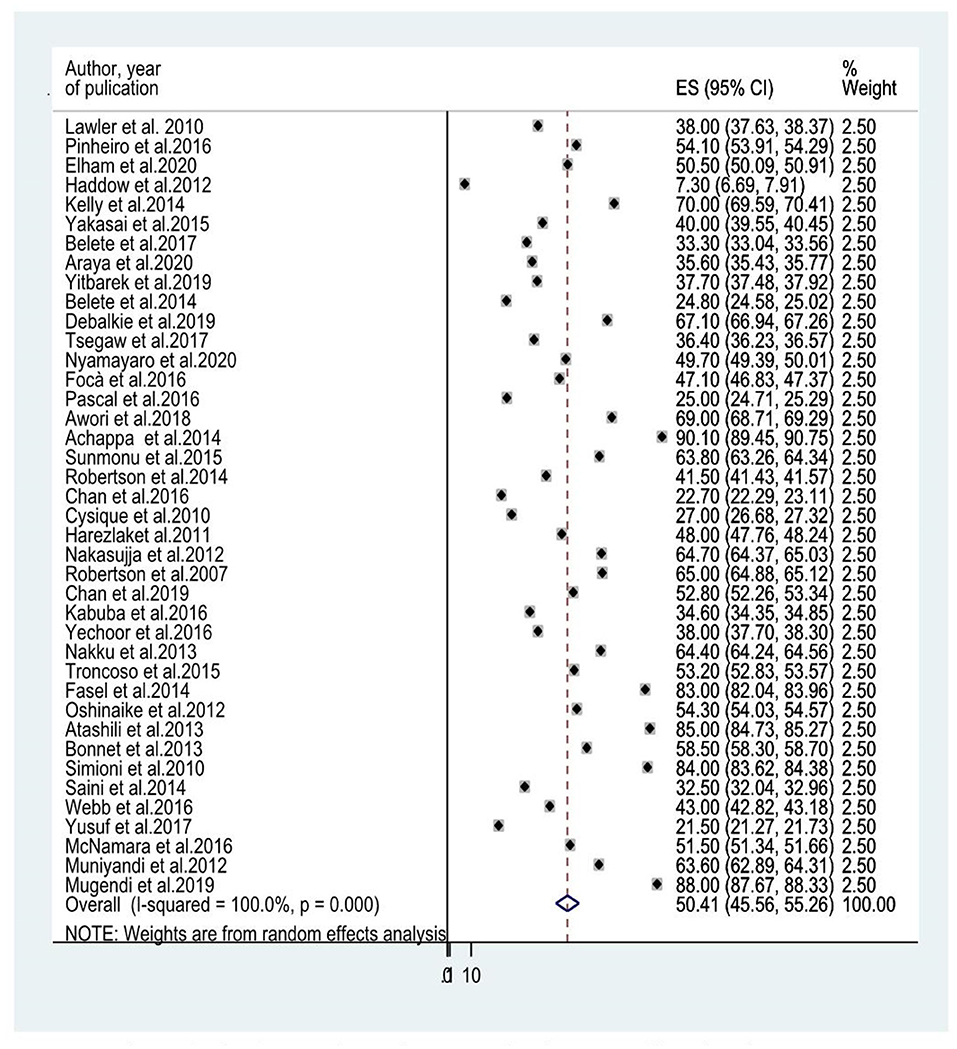
Forty studies that evaluated HIV-associated neurocognitive disorders in HIV/AIDS had been included to determine the average prevalence of HIV-associated neurocognitive disorders. The reported prevalence of HIV-associated neurocognitive disorders included in the meta-analysis differs from 7.3% in the United Kingdom (28) to 88% in Kenya (35). The average prevalence of HIV-associated neurocognitive disorders using the random effect model was 50.41% (95% CI: 45.56, 55.26) (I2 =100%, p ≤ 0.001; Figure 2).
Subgroup Analysis of the Prevalence of HIV Associated Neurocognitive Disorders Among People Living With HIV/AIDS
Since the average estimate of HIV-associated neurocognitive disorders was predisposed to considerable heterogeneity, we employed a subgroup analysis based on the country where the study was done, the assessment tool used to screen HIV-associated neurocognitive disorders, and the study design. The average estimate of HIV associated neurocognitive disorders in Europe (8, 14, 21, 23, 24, 27, 28, 40, 41, 44) was found to be 50.015% (95% CI: 43.339, 56.691) whereas in Africa (10, 13, 18, 20, 25, 30, 31, 35, 37–39, 45–50), Asia (17, 26, 34, 36, 43) and the United States of America (USA) (29, 41) the average prevalence of HAND were 49.566% (95% CI: 41.342, 57.791) with (I2 = 96.6%, p < 0.001), 52.032 % (95% CI: 34.46, 69.604) with (I2 = 98%, p < 0.001) and 50.407% (95%CI: 45.555, 55.258) (I2 =100%, P < 0.001), respectively (Figure 3 and Table 2). The average estimate of HIV associated neurocognitive disorders in studies which used International HIV Dementia Scale (IHDS) (8, 10, 12–14, 17, 20, 25, 35–37, 39, 43, 44, 46, 48–50, 66) was 36.883% (95%CI: 21.196, 52.571) and 59.956% (95%CI: 49.985, 69.928) at a cutoff points of IHDS <9.5 and IHDS <10, respectively (Figure 4). The estimated average of HAND in studies used the global dementia scale (GDS) (30, 38) was 40.766% (95%CI: 31.995, 49.537). The estimated average of HAND in cross-sectional (8, 10, 12–14, 17–20, 23, 25, 26, 28, 30, 31, 34–40, 45–50) cohort (8, 21, 23, 24, 27, 29, 41, 43, 44) and case-control (30, 66) studies was 49.52% (95% CI: 43.490, 55.545) (I2 = 48.6%, P =1.00), 54.087% (95% CI: 45.087, 63.087) (I2 = 96%%, P < 0.001) and 44.45% (95% CI: 25.144, 63.756) (I2 = 94.8%, P < 0.001), respectively (Figure 5).
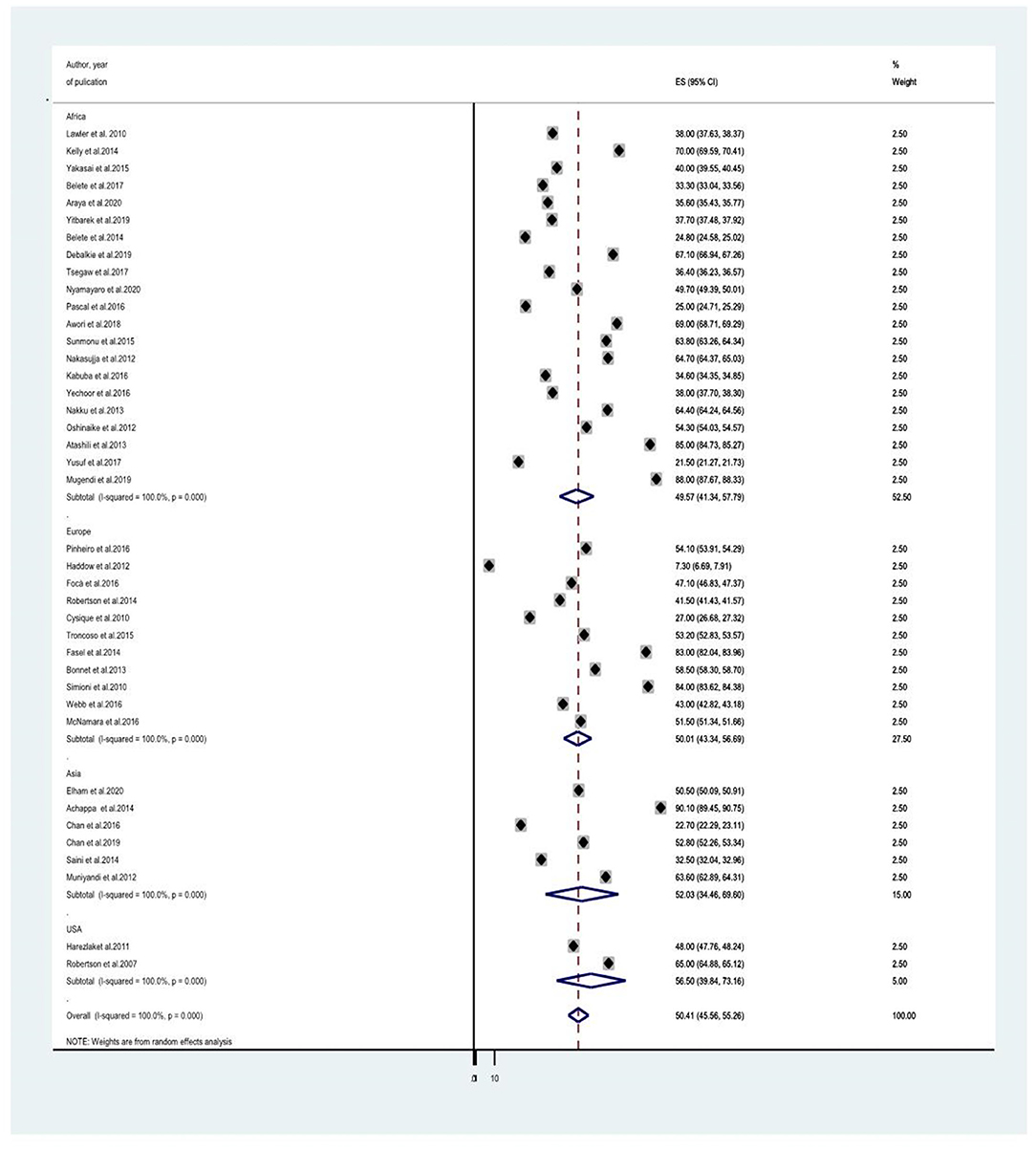
Figure 3. A subgroup analysis for the prevalence of HIV associated neurocognitive disorders based on country of study origin.
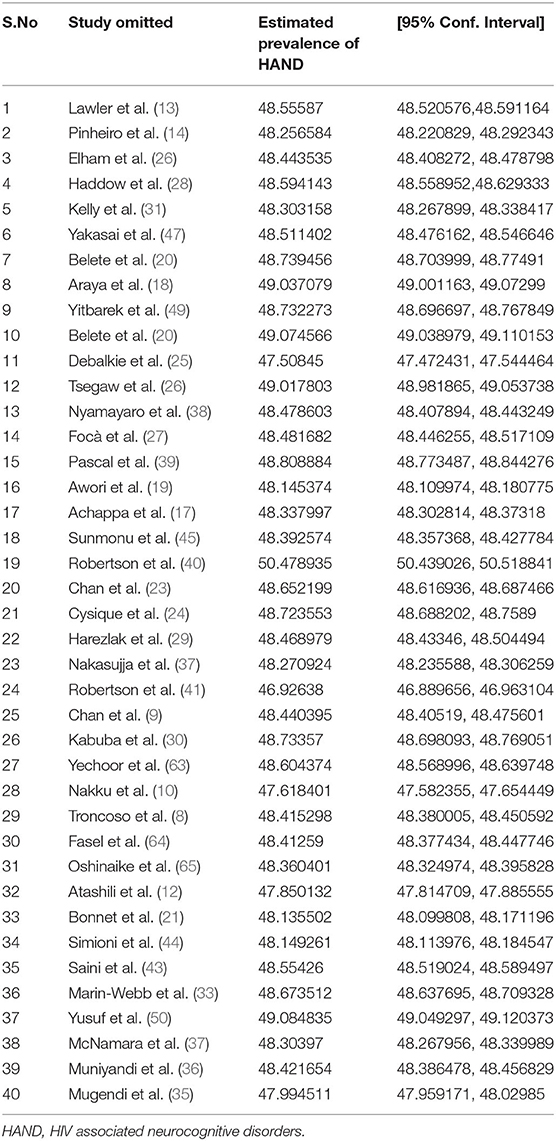
Table 2. A sensitivity analysis of the prevalence of HIV associated neurocognitive disorders in HIV/AIDS patients when each indicated studies are omitted at a time with its 95% confidence interval.

Figure 4. A subgroup analysis for the prevalence of HIV associated neurocognitive disorders based on study tools.
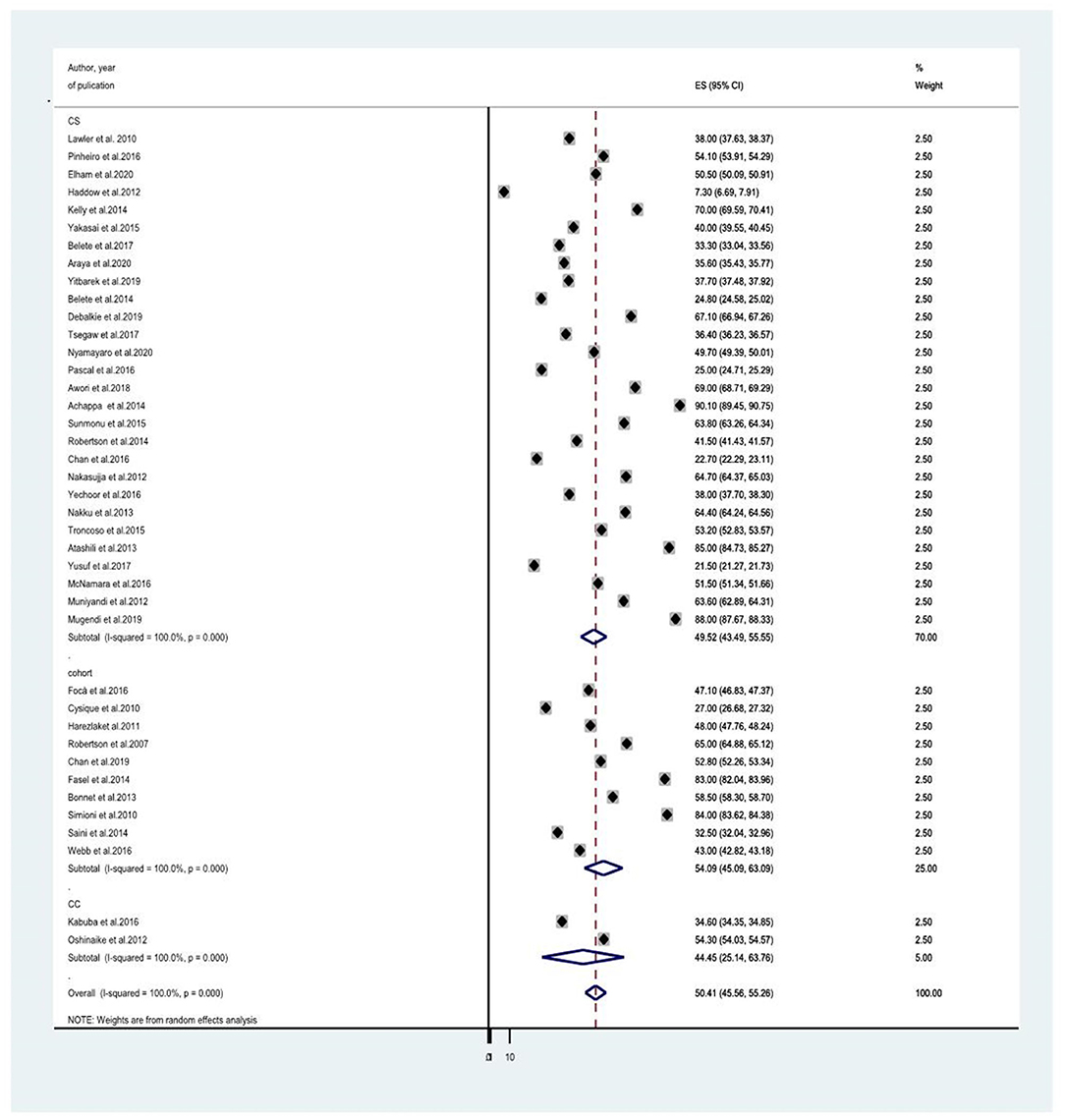
Figure 5. A subgroup analysis for the prevalence of HIV associated neurocognitive disorders based on country of study tools.
Sensitivity Analysis
In addition to subgroup analysis, we did a sensitivity analysis to know whether one or more of the individual studies outweighed the overall estimate of HIV-associated neurocognitive disorders. The result however reported that the average estimate of HIV-associated neurocognitive disorders ranges from 46.92638% (95% CI: 46.889656, 46.963104) to 50.478935% (95% CI: 50.439026, 50.518841) when each study was omitted from the analysis (Table 2). This implies that there was no single influential study outweighing the average estimate.
Publication Bias
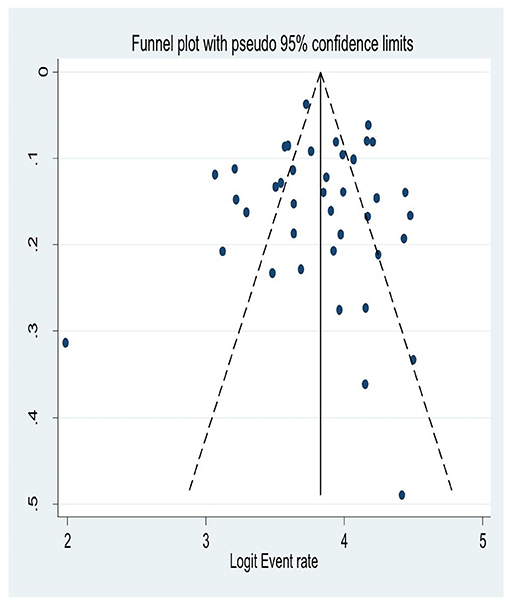
Although a graphical inspection from a funnel plot for a Logit event rate of occurrence of HIV-associated neurocognitive disorders in people living with HIV/AIDS alongside its standard error suggests asymmetrical distribution, the quantitative Eggers test of publication bias had been run and its p-value was not significant; (P = 0.55). This suggests there was no publication bias for the prevalence HIV associated neurocognitive disorders (Figure 6).
Associated Factors of HIV Associated Neurocognitive Disorders Among People Living With HIV/AIDS
Among the 40 studies, only 15 studies described the factors related to HIV-associated neurocognitive disorders (8, 10, 12, 14, 18, 20, 21, 25, 26, 34, 35, 46–49). The most frequently reported sociodemographic variable as the associated factor of HIV-associated neurocognitive disorders were the low level of education (12, 14, 18, 21, 30, 46, 47) and older age (8, 14, 20, 46, 49). Among clinical and HIV-related variables late clinical stage of the illness (20, 21, 25, 49) and a CD4 count of 500 cells/dl or less (8, 18, 46) were the most commonly described factor for HIV associated neurocognitive disorders. Besides, from psychological variables comorbidity of depression increases the risk of HIV-associated neurocognitive disorders (14, 21, 35). Moreover, clinical and HIV related variables such as impairment in the activity of daily living (20), duration of HIV infection > 5 years (26), poor medication adherence (46), co-morbid medical illness, highest prior VL >100,000 copies/ml (8), history of neurological disease (21), body mass index< 16 kg/m2 (25), plasma HIV-1 RNA load between 1.7log10 and 3log10 copies/ml (49), having a co-morbid opportunistic infection (20) and psychological variables like negative life events, high-stress score index (score>10) (10) were related to HIV associated neurocognitive disorders (Table 3).
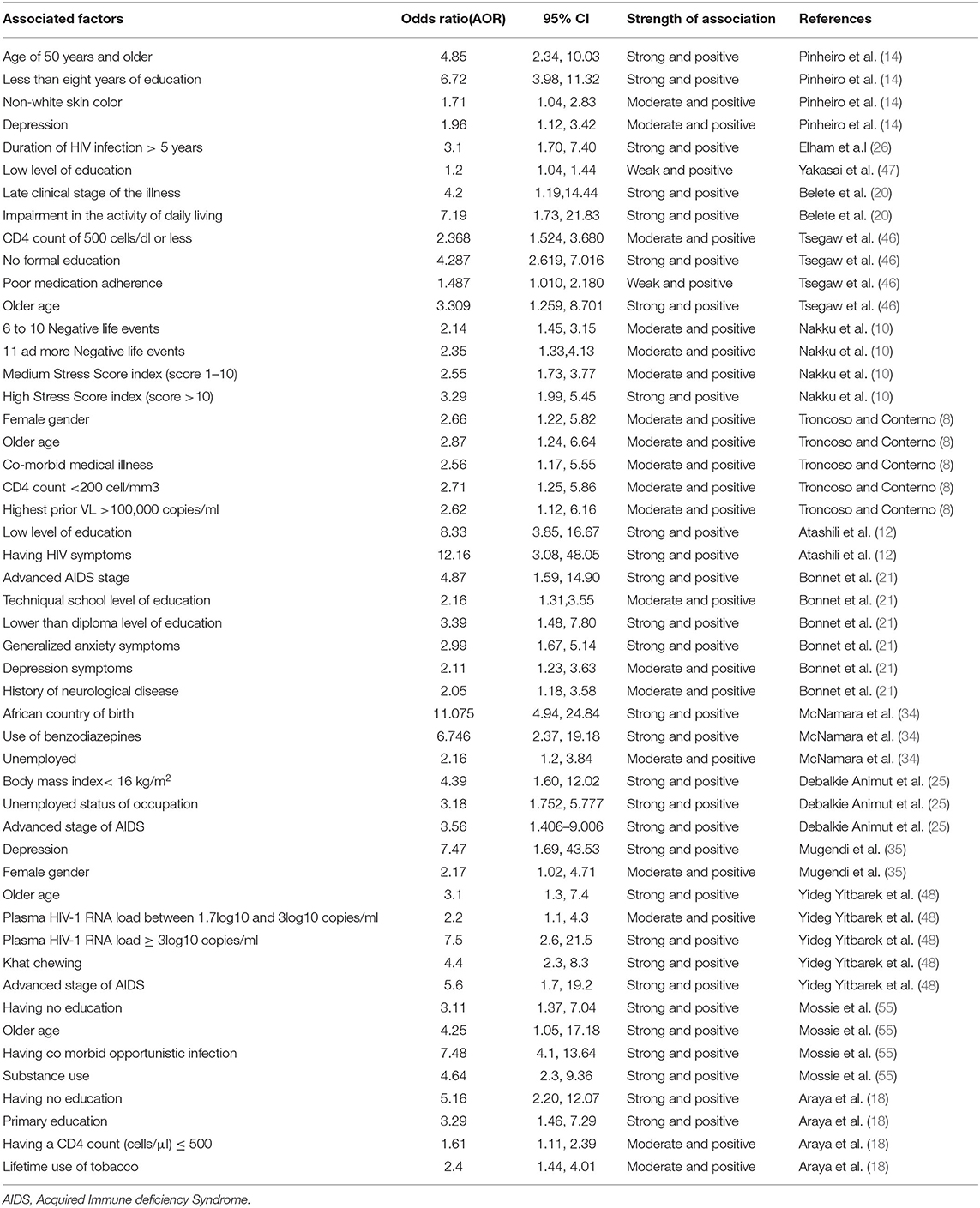
Table 3. Characteristics of associated factors for HIV associated neurocognitive disorders in HIV/AIDS patients by their Odds ratio, Confidence interval, association strength, author and year of publication.
Association Between Old Age and HIV Associated Neurocognitive Disorders Among People Living With HIV/AIDS
Older age was reported as the risk factor for HIV-associated neurocognitive disorders by five studies (8, 14, 20, 46, 49). The pooled odds ratio for the association between old age and HIV-associated neurocognitive disorder among these five studies was found to be 3.68 (95% CI: 2.95, 4.11) (I2 = 98.2%%, P = 0.000; Figure 7).
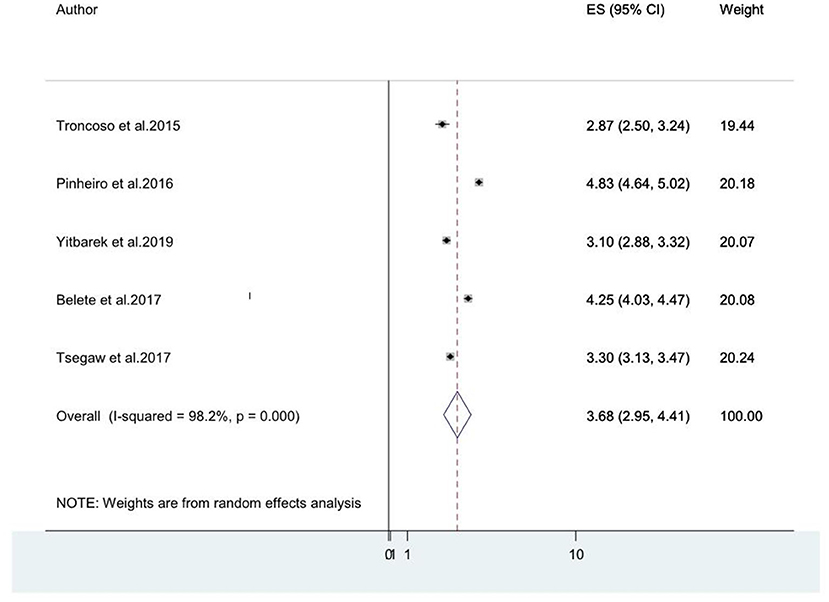
Figure 7. A forest plot for the pooled odds ratio of associated between old age and HIV-associated neurocognitive disorder.
Association Between Depression and HIV Associated Neurocognitive Disorders Among People Living With HIV/AIDS
As reported in three studies (14, 21, 35) that assessed HIV-associated neurocognitive disorders, depression increases the risk of HIV-associated neurocognitive disorders. The pooled odds ratio for the association between depression and HIV-associated neurocognitive disorder among these three studies was found to be 2.87 (95% CI: 0.87, 4.87) (I2 = 99.6%%, P = 0.000).
Association Between Advanced Stages of AIDS and HIV Associated Neurocognitive Disorders Among People Living With HIV/AIDS
Advanced clinical stages of the illness (stage III and stage IV AIDS) (20, 21, 25, 49) were also associated factors for HIV-associated neurocognitive disorders. The pooled odds ratio for the association between advanced stage of AIDs(stage III& IV) and HIV-associated neurocognitive disorder among the four included studies was found to be 5.68 (95% CI: 3.06, 8.29) (I2 = 99.9%%, P< 0.001; Figure 8).
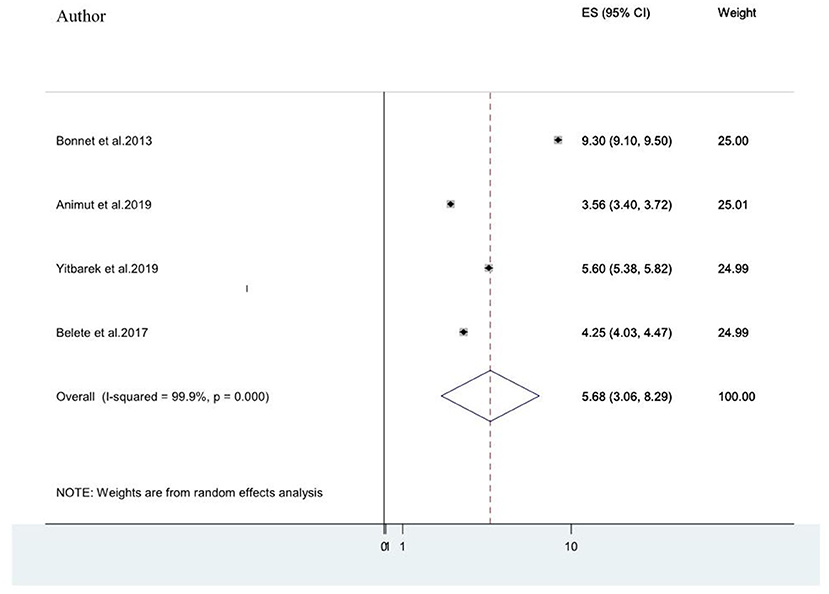
Figure 8. A forest plot for the pooled odds ratio of associated between advanced stage of AIDS and HIV-associated neurocognitive disorder.
Meta-Regression
Firstly, we performed a univariate regression analysis to select the independent variables to incorporate in the final meta-regression model. Then, all variables with P < 0.8 were included in the final regression analysis as recommended by Ferrari et al. 2013 (67). The impacts of the country of origin of the study (Europe, Asia, and Africa), Assessment Tool (IHDS < 9.5, IHDS < 10, Frascati criteria, ADC, MMSE, GDS > 0.5, MoCA, and Other Tools), and study design (case-control, cohort and cross-sectional) were quantified in the meta-regression model. The analysis was conducted for the overall effect on the burden of HIV-associated neurocognitive disorders in HIV/AIDS patients. The overall proportion of variance explained by these covariates in the final model was 11% (R2 = 11%; P = 0.302). All the three covariates such as country of origin of the study, Assessment Tool, and study design were not statistically significant determinants for the observed variation in the association between HIV-associated neurocognitive disorders and HIV/AIDS.
Discussion
To our knowledge, this is the first systematic review and meta-analysis that assessed the global burden of HIV-associated neurocognitive disorders in HIV/AIDS patients. So, the data synthesized will be an important suggestion to varied stakeholders. Overall, 40 studies (8, 10, 12–14, 17–50) that measured the prevalence of HIV associated neurocognitive disorders in 14,107 participants from over 28 different countries and 15 studies that described the factors related HIV associated neurocognitive disorders (8, 10, 12, 14, 18, 20, 21, 25, 26, 34, 35, 46–49) were included.
The result of the present study advocated that more than half of people living with HIV/AIDS were affected with HIV-associated neurocognitive disorders. This suggests that HIV-associated neurocognitive disorders are an important public health problem in people living with HIV/AIDS. The result of HIV-associated neurocognitive disorders is consistent with the prevalence of neurocognitive disorders in patients with chronic kidney disease and diabetes; 48% In the UK (68). However, it was higher than the magnitude of neurocognitive disorders in the general urban population in India (1.5%) (69), and the prevalence of dementia in an urban Turkish population (20%) (70). It was also higher than the prevalence of dementia in Norwegian elders of age 75 and above where dementia was prevalent in 16.3% of the population (71). Besides, the present finding was higher than the prevalence rate of dementia among japans community aged 65 or older; 6.7% (72). The added presence of opportunistic central nervous disease (73–75) in HIV-positive people could be attributing factor to this.
Among studies included in the current review and meta-analysis, the epidemiologic data regarding the prevalence of HIV associated neurocognitive disorders showed a substantial variation across the measurement instrument for HIV associated neurocognitive disorders, and the nature of the study design; however comparable HAND results were found across European, Africa, Asian, US countries. Of the 40 included studies, 20—the majority—were from Africa (10, 13, 18, 20, 25, 30, 31, 35, 37–39, 45–50).
The current study result was higher than the result of a meta-analysis that assessed 16 studies in sub-Sahara Africa where the prevalence of HAND was 30.39% (16). The difference in the study population and significant variance in the number of included studies might cause the variation. Moreover, the risk factors for HIV-associated neurocognitive disorders might be different in Sub-Saharan Africa and the world in general, which may cause the difference in HIV-associated neurocognitive disorders. On the other hand, the current study was higher than the result of the prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder; 43.9% (76). The possible reason for this could be due to the difference in sensitivity and specificity between Frascati-Criteria and other tools to screen HIV-Associated Neurocognitive Disorder.
In a subgroup analysis by type of measurement instrument used for HAND, we found that the prevalence was higher as measured with IHDS < 10 (8, 10, 12–14, 17, 20, 25, 35–37, 39, 43, 44, 46, 48–50, 66) (59.956%) than the result measured with Frascati criteria (53.5%), ADC (27.65%), MMSE (41.349%), and GDS (40.766%) with all these differences at statistically significant level(P < 0.001). The possible differences in the estimated magnitude of HIV associated neurocognitive disorders among measurement instruments will be attributed to the difference in the ability of measurement tools to identify truly those HIV patients who have HIV associated neurocognitive disorders (sensitivity) and to exclude HIV patients who do not have HIV associated neurocognitive disorders (specificity) (33, 77–82). In addition, the lack of country-specific NP norms for the assessment of HAND would have contributed to this variation.
We did also conduct a subgroup analysis with the type of study design. The highest estimated prevalence of HAND (54.087%) was found in cohort studies (8, 21, 23, 24, 27, 29, 41, 43, 44), followed by cross-sectional studies (49.52%) (8, 10, 12–14, 17–20, 23, 25, 26, 28, 30, 31, 34–40, 45–50). The lowest estimated prevalence of HAND (44.45%) was found among case-control studies (30, 66). As noted above, the number of case-control and cohort studies is fewer in number (2 and 10 studies, respectively) compared to the number of cross-sectional studies which is 28. So the smaller number of studies included in cohort and case-control studies might be overestimated and underestimate the prevalence of HAND, respectively.
Even though we conducted a subgroup analysis with a country of origin of the study (categorized as Africa, Europe, Asia, and the USA) as a moderator variable, we found a more or less consistent average estimate of HAND; Europe (50.015%), Africa (49.566%), Asia (52.032%), and United States of America (USA) (50.407%) with the difference of prevalence estimate of HAND being insignificant.
Concerning the associated factors for HIV-associated neurocognitive disorders, our systematic review suggested that older age was among the commonly reported risk factor for HIV-associated neurocognitive disorders (8, 14, 20, 46, 49). Older age group people were 3.68 times more likely to develop HIV-associated neurocognitive disorder than younger age people. This is supported by multiple earlier studies (83). The accumulation of excessive amyloid deposits and various chronic diseases in old age could account for this (84).
Besides, depression also increases the risk of HIV-associated neurocognitive disorders as reported in three studies (14, 21, 35). The HIV-associated neurocognitive disorder was 2.87 times higher among individuals having comorbidity of depression than those not having comorbidity of depression. Multiple supportive findings for this association are reported (85–87). Pseudo-dementia associated with depression could mimic neurocognitive disorders and amplify the prevalence of neurocognitive disorders.
Moreover, advanced clinical stages of the illness (stage III and stage IV AIDS) (20, 21, 25, 49) were also associated factors for HIV-associated neurocognitive disorders. Advanced clinical stages of the illness increase the risk of HIV-associated neurocognitive disorder by 5.68.
Difference Between Studies Incorporated in the Current Review and the Meta-Analysis Study
This meta-analysis study on HIV-associated neurocognitive disorders was subjected to a high degree of heterogeneity from the variation between 40 of the studies integrated with the final analysis. Therefore, the need for a subgroup and sensitivity analysis was mandatory. The country of origin of the study (Africa, Europe, Asia, and the USA), design of the study, and measurement tool were the parameters for subgroup analysis. However, no major difference in the pooled prevalence of HIV-associated neurocognitive disorders was observed between high-income/(Europe, Asia, and the USA) and low-to-middle income/African countries. These should be a focus of future researchers. Results of the subgroup however revealed the design of the study and measurement tool used to screen HIV-associated neurocognitive disorders were contributors to the high heterogeneity between the 40 studies integrated with the analysis. Furthermore, we did a sensitivity analysis to search for additional sources for the high degree of heterogeneity by exploring a particular study outweighing the overall estimate. However, the result showed that none of the 40 studies had outweighed the average estimated prevalence of HIV-associated neurocognitive disorders in HIV patients.
Strength and Limitations of the Study
This systematic review and meta-analysis on HIV-associated neurocognitive disorders have several strengths. The primary strength of the study was the large number of studies included in the review, representing the worldwide spread of HAND in people with HIV. The application of subgroup analysis and sensitivity analysis to identify the source of heterogeneity was another quality of the present study. The study also used a well-designed and pre-determined search approach to lessen the reviewer's bias. The third for this study was the extraction of relevant data and assessment of the quality of 40 studies by self-determining assessors which decrease the reviewer's bias. Quality analysis of all the included studies also increases the study validity. On the other hand, the study has many limitations. First, the definition of HIV-associated neurocognitive disorder could be more explicit. In addition, the reasons for exclusions of 46 identified full texts are not explicitly mentioned in the PRISMA flowchart. The presence of a high degree of heterogeneity that influences the inference of the study outcomes was also another limitation. Moreover, biases and lack of methodological rigor could affect the validity of this finding.
Conclusion and Recommendation
This systematic review and meta-analysis aimed to estimate the prevalence of HIV-associated neurocognitive disorder in people living with HIV AIDS and to analyze the associated factors for HIV-associated neurocognitive disorder in people with HIV AIDS. The prevalence of HIV-associated neurocognitive disorders was about 50.41%. Low level of education and older age, clinical and HIV related variables such as the advanced stage of the illness and CD4 count of 500 cells/dl or less and psychological variables such as comorbidity of depression were associated with HIV associated neurocognitive disorders. Therefore, to increase independent functioning and improve the quality of life of people with HIV/AIDS, much attention has to be given to lessening these neurocognitive disorders and adjusting the allied factors essentially through routine screening and timely intervention of HAND. Moreover, policies and procedures that integrate routine screening and timely intervention of HAND into the routine anti-retroviral therapy should be designed and implemented. Further experimental and follow-up studies with a greater sample population should be done.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
YZ conceived the idea for the study. YZ and MN established the search approach, extracted the relevant data, accomplished the analysis, and wrote the manuscript. MN, YZ, BA, and WY did the quality assessment studies. All authors confirmed the final draft of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AIDS, Acquired Immune-Deficiency Syndrome; ANI, Asymptomatic neurocognitive impairment; ART, Anti-Retroviral Therapy; CC, case-control; CD, Cognitive decline; CS, cross-sectional; F, female; GDS, global dementia scale; HAD, HIV associated dementia; HAND, HIV associated neurocognitive disorders; IHDS, International HIV Dementia Scale; II, Intellectual impairment; M, male; MMSE, Mini-mental state exam; MND, Mild neurocognitive disorders; MoCA, Montreal Cognitive Assessment; NA, Not available; NCI, Neurocognitive impairment; SNI, Symptomatic neurocognitive impairment; UK, United Kingdom; USA, United States of America.
References
1. Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis. (2013) 207:S1–S6. doi: 10.1093/infdis/jis927
2. Chibanda D, Benjamin L, Weiss HA, Abas M. Mental, neurological, and substance use disorders in people living with HIV/AIDS in low-and middle-income countries. JAIDS J Acquir Immune Def Syndr. (2014) 67:S54–67. doi: 10.1097/QAI.0000000000000258
3. Necho M, Belete A, Tsehay M. Depressive symptoms and their determinants in patients who are on antiretroviral therapy in the case of a low-income country, Ethiopia: a systematic review and meta-analysis. Int J Ment Health Syst. (2021) 15:1–14. doi: 10.1186/s13033-020-00430-2
4. Ayano G, Duko B, Bedaso A. The prevalence of post-traumatic stress disorder among people living with HIV/AIDS: a systematic review and meta-analysis. Psychiatr Q. (2020) 91:1317–32. doi: 10.1007/s11126-020-09849-9
5. Necho M, Belete A, Getachew Y. The prevalence and factors associated with alcohol use disorder among people living with HIV/AIDS in Africa: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy. (2020) 15:1–15. doi: 10.1186/s13011-020-00301-6
6. Necho M, Tsehay M, Zenebe Y. Suicidal ideation, attempt, and its associated factors among HIV/AIDS patients in Africa: a systematic review and meta-analysis study. Int J Ment Health Syst. (2021) 15:1–16. doi: 10.1186/s13033-021-00437-3
7. Mahadevan A, Shankar SK, Satishchandra P, Ranga U, Chickabasaviah YT, Santosh V, et al. Characterization of human immunodeficiency virus (HIV)-infected cells in infiltrates associated with CNS opportunistic infections in patients with HIV clade C infection. J Neuropathol Exp Neurol. (2007) 66:799–808. doi: 10.1097/NEN.0b013e3181461d3e
8. Troncoso FT, Conterno LdO. Prevalence of neurocognitive disorders and depression in a Brazilian HIV population. Rev Soc Bras Med Trop. (2015) 48:390–8. doi: 10.1590/0037-8682-0034-2015
9. Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Curr HIV/AIDS Rep. (2014) 11:317–24. doi: 10.1007/s11904-014-0221-0
10. Nakku J, Kinyanda E, Hoskins S. Prevalence and factors associated with probable HIV dementia in an African population: a cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatry. (2013) 13:1–7. doi: 10.1186/1471-244X-13-126
11. Cross HM, Combrinck MI, Joska JA. HIV-associated neurocognitive disorders: antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. South Afr Med J. (2013) 103:758–62. doi: 10.7196/SAMJ.6677
12. Atashili J, Gaynes BN, Pence BW, Tayong G, Kats D, O'donnell JK, et al. Prevalence, characteristics and correlates of a positive-dementia screen in patients on antiretroviral therapy in Bamenda, Cameroon: a cross-sectional study. BMC Neurol. (2013) 13:1–7. doi: 10.1186/1471-2377-13-86
13. Lawler K, Mosepele M, Ratcliffe S, Seloilwe E, Steele K, Nthobatsang R, et al. Neurocognitive impairment among HIV-positive individuals in Botswana: a pilot study. J Int AIDS Soc. (2010) 13:1–9. doi: 10.1186/1758-2652-13-15
14. Pinheiro C, Souza L, Motta J, Kelbert E, Souza M, Martins C, et al. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz J Med Biol Res. (2016) 49:e5344. doi: 10.1590/1414-431x20165344
15. Robbins RN, Scott TM, Gouse H, Marcotte TD, Rourke SB. Screening for HIV-associated neurocognitive disorders: sensitivity and specificity. Curr Top Behav Neurosci. (2019) 50:429–78. doi: 10.1007/7854_2019_117
16. Habib AG, Yakasai AM, Owolabi LF, Ibrahim A, Habib ZG, Gudaji M, et al. Neurocognitive impairment in HIV-1-infected adults in Sub-Saharan Africa: a systematic review and meta-analysis. Int J Infect Dis. (2013) 17:e820–e31. doi: 10.1016/j.ijid.2013.06.011
17. Achappa B, Priyadarshni S, Madi D, Bhaskaran U, Ramapuram JT, Rao S, et al. Neurocognitive dysfunction among HIV positive patients using international HIV dementia scale. Asian J Med Sci. (2014) 5:61–4. doi: 10.3126/ajms.v5i4.8724
18. Araya T, Abebaw D, Belete A, Derajew H, Umer H. Prevalence and factors associated with neuro cognitive disorders among HIV-positive patients in Ethiopia: a hospital-based cross-sectional study. Ethiopian J Health Dev. (2020) 34:22–9.
19. Awori V, Mativo P, Yonga G, Shah R. The association between asymptomatic and mild neurocognitive impairment and adherence to antiretroviral therapy among people living with human immunodeficiency virus. South Afr J HIV Med. (2018) 19:674. doi: 10.4102/sajhivmed.v19i1.674
20. Belete T, Medfu G, Yemiyamrew E. Prevalence of HIV asssociated neurocognitive deficit among HIV positive people in Ethiopia: A cross sectional study at ayder referral hospital. Ethiopian J Health Sci. (2017) 27:67–76. doi: 10.4314/ejhs.v27i1.9
21. Bonnet F, Amieva H, Marquant F, Bernard C, Bruyand M, Dauchy F-A, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? Aids. (2013) 27:391–400. doi: 10.1097/QAD.0b013e32835b1019
22. Carlson RD, Rolfes MA, Birkenkamp KE, Nakasujja N, Rajasingham R, Meya DB, et al. Predictors of neurocognitive outcomes on antiretroviral therapy after cryptococcal meningitis: a prospective cohort study. Metab Brain Dis. (2014) 29:269–79. doi: 10.1007/s11011-013-9476-1
23. Chan LG, Kandiah N, Chua A. HIV-associated neurocognitive disorders (HAND) in a South Asian population-contextual application of the 2007 criteria. BMJ Open. (2012) 2:662. doi: 10.1136/bmjopen-2011-000662
24. Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, et al. Incidence and nature of cognitive decline over one year among HIV-infected former plasma donors in China. AIDS (London, England). (2010) 24:983. doi: 10.1097/QAD.0b013e32833336c8
25. Debalkie Animut M, Sorrie MB, Birhanu YW, Teshale MY. High prevalence of neurocognitive disorders observed among adult people living with HIV/AIDS in Southern Ethiopia: A cross-sectional study. PLoS ONE. (2019) 14:e0204636. doi: 10.1371/journal.pone.0204636
26. Elham M-T, Vahid N, Seyed ASA, Omid D, Cossarizza A, Mussini C, et al. Prevalence of HIV-associated Neurocognitive Disorder (HAND) and its subgroups among HIV-positive persons on anti-retroviral therapy in Iran. Psihologija. (2020) 53:115–27. doi: 10.2298/PSI190414001M
27. Focà E, Magro P, Motta D, Compostella S, Casari S, Bonito A, et al. Screening for neurocognitive impairment in HIV-infected individuals at first contact after HIV diagnosis: the experience of a large clinical center in Northern Italy. Int J Mol Sci. (2016) 17:434. doi: 10.3390/ijms17040434
28. Haddow L, Cpsychoil AA, Cartledge J, Manji H, Benn P, Gilson R. Routine detection and management of neurocognitive impairment in HIV-positive patients in a UK centre. Int J STD AIDS. (2013) 24:217–9. doi: 10.1177/0956462412472452
29. Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, et al. Persistence of hiv– associated cognitive impairment, inflammation and neuronal injury in era of highly active antiretroviral treatment. AIDS (London, England). (2011) 25:625. doi: 10.1097/QAD.0b013e3283427da7
30. Kabuba N, Menon JA, Franklin DR, Heaton RK, Hestad KA. Use of western neuropsychological test battery in detecting HIV-associated neurocognitive disorders (HAND) in Zambia. AIDS Behav. (2017) 21:1717–27. doi: 10.1007/s10461-016-1443-5
31. Kelly CM, van Oosterhout JJ, Ngwalo C, Stewart RC, Benjamin L, Robertson KR, et al. HIV associated neurocognitive disorders (HAND) in Malawian adults and effect on adherence to combination anti-retroviral therapy: a cross sectional study. PLoS ONE. (2014) 9:e98962. doi: 10.1371/journal.pone.0098962
32. Lindayani L, Sudrajat DA, Melnawati C, Anggarini D. Prevalence of neurocognitive impairment and its associated factors among patients with HIV in Indonesia. Br J Neurosci Nurs. (2020) 16:258–64. doi: 10.12968/bjnn.2020.16.6.258
33. Marin-Webb V, Jessen H, Kopp U, Jessen AB, Hahn K. Validation of the international HIV dementia scale as a screening tool for HIV-associated neurocognitive disorders in a German-speaking HIV outpatient clinic. PLoS ONE. (2016) 11:e0168225. doi: 10.1371/journal.pone.0168225
34. McNamara PH, Coen R, Redmond J, Doherty CP, Bergin C, editors. A High Prevalence Rate of a positive Screen for Cognitive Impairment in Patients With Human Immunodeficiency Virus Attending an Irish Clinic. Open Forum Infectious Diseases: Oxford: Oxford University Press US (2017).
35. Mugendi A, Kubo M, Nyamu D, Mwaniki L, Wahome S, Haberer J. Prevalence and correlates of neurocognitive disorders among HIV patients on antiretroviral therapy at a Kenyan hospital. Neurol Res Int. (2019) 2019:5173289. doi: 10.1155/2019/5173289
36. Muniyandi K, Venkatesan J, Arutselvi T, Jayaseelan V. Study to assess the prevalence, nature and extent of cognitive impairment in people living with AIDS. Indian J Psychiatry. (2012) 54:149. doi: 10.4103/0019-5545.99534
37. Nakasujja N, Allebeck P, Agren H, Musisi S, Katabira E. Cognitive dysfunction among HIV positive and HIV negative patients with psychosis in Uganda. PLoS ONE. (2012) 7:e44415. doi: 10.1371/journal.pone.0044415
38. Nyamayaro P, Gouse H, Hakim J, Robbins RN, Chibanda D. Neurocognitive impairment in treatment-experienced adults living with HIV attending primary care clinics in Zimbabwe. BMC Infect Dis. (2020) 20:1–10. doi: 10.1186/s12879-020-05090-8
39. Pascal M, Gaspard T, Philomène N-K, Emmanuel Y, Avilah A-WP, Hortense H. Determinants of neurocognitive impairment in HIV in a cohort of patients on antiretroviral therapy followed in Bangui (Central African Republic). Neurosci. Med. (2016) 7:1. doi: 10.4236/nm.2016.71001
40. Robertson K, Bayon C, Molina J-M, McNamara P, Resch C, Muñoz-Moreno JA, et al. Screening for neurocognitive impairment, depression, and anxiety in HIV-infected patients in Western Europe and Canada. AIDS Care. (2014) 26:1555–61. doi: 10.1080/09540121.2014.936813
41. Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids. (2007) 21:1915–21. doi: 10.1097/QAD.0b013e32828e4e27
42. Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. (2016) 86:334–40. doi: 10.1212/WNL.0000000000002277
43. Saini S, Barar KV. Assessment of neurocognitive functions in HIV/AIDS patients on HAART using the international HIV dementia scale. Int J Nutr Pharmacol Neurol Dis. (2014) 4:252. doi: 10.4103/2231-0738.139408
44. Simioni S, Cavassini M, Annoni J-M, Abraham AR, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. Aids. (2010) 24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b
45. Sunmonu TA, Sellner J, Ogunrin OA, Imarhiagbe FA, Komolafe MA, Afolabi OT, et al. Intellectual impairment in patients with newly diagnosed HIV infection in southwestern Nigeria. Biomed Res Int. (2015) 2015:185891. doi: 10.1155/2015/185891
46. Tsegaw M, Andargie G, Alem G, Tareke M. Screening HIV-associated neurocognitive disorders (HAND) among HIV positive patients attending antiretroviral therapy in South Wollo, Ethiopia. J Psychiatr Res. (2017) 85:37–41. doi: 10.1016/j.jpsychires.2016.10.016
47. Yakasai AM, Gudaji MI, Muhammad H, Ibrahim A, Owolabi LF, Ibrahim DA, et al. Prevalence and correlates of HIV-associated neurocognitive disorders (HAND) in Northwestern Nigeria. Neurol Res Int. (2015) 2015:486960. doi: 10.1155/2015/486960
48. Yideg Yitbarek G, Mossie Ayana A, Bariso Gare M, Garedew Woldeamanuel G. Prevalence of cognitive impairment and its predictors among HIV/AIDS patients on antiretroviral therapy in Jimma University medical center, Southwest Ethiopia. Psychiatry J. (2019) 2019:8306823. doi: 10.1155/2019/8306823
49. Yitbarek GY, Ayehu GW, Ayele BA, Bayih WA, Gebremariam AD, Tiruneh SA. Cognitive impairment and its associated factors among HIV/AIDS patients on anti retro-viral therapy in Sub-Saharan Africa: systematic review and meta-analysis. Neurol Psychiatry Brain Res. (2020) 38:83–91. doi: 10.1016/j.npbr.2020.11.002
50. Yusuf AJ, Hassan A, Mamman AI, Muktar HM, Suleiman AM, Baiyewu O. Prevalence of HIV-associated neurocognitive disorder (HAND) among patients attending a tertiary health facility in Northern Nigeria. J Int Assoc Providers AIDS Care. (2017) 16:48–55. doi: 10.1177/2325957414553839
51. Van Wijk C. Screening for HIV-associated neurocognitive disorders (HANDs) in South Africa: A caution against uncritical use of comparative data from other developing countries. South Afr J HIV Med. (2013) 14:97. doi: 10.4102/sajhivmed.v14i1.97
52. Joska JA, Dreyer AJ, Nightingale S, Combrinck MI, De Jager CA. Prevalence of HIV-1 Infection in an elderly rural population and associations with neurocognitive impairment. Aids. (2019) 33:1765–71. doi: 10.1097/QAD.0000000000002257
53. Patel V, Mungwira R, Tarumbiswa T, Heikinheimo T, Van Oosterhout J. High prevalence of suspected HIV-associated dementia in adult Malawian HIV patients. Int J STD AIDS. (2010) 21:356–8. doi: 10.1258/ijsa.2010.009554
54. Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther. (2014) 11:1–15. doi: 10.1186/1742-6405-11-13
55. Mossie TB, Tegegne MT. HIV dementia among HIV positive people at Debre markos hospital, Northwest Ethiopia. Am J Psychol Neurosci. (2014) 2:18–24. doi: 10.11648/j.ajpn.20140202.11
56. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
58. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
59. Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology. (2008) 19:94–100. doi: 10.1097/EDE.0b013e31815c24e7
60. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. (2014) 72:39. doi: 10.1186/2049-3258-72-39
61. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
62. Liu JL. The role of the funnel plot in detecting publication and related biases in meta-analysis. Evid Based Dent. (2011) 12:121. doi: 10.1038/sj.ebd.6400831
63. Yechoor N, Towe SL, Robertson KR, Westreich D, Nakasujja N, Meade CS. Utility of a brief computerized battery to assess HIV-associated neurocognitive impairment in a resource-limited setting. J Neurovirol. (2016) 22:808–15. doi: 10.1007/s13365-016-0456-1
64. Fasel D, Kunze U, Elzi L, Werder V, Niepmann S, Monsch AU, et al. A short tool to screen HIV-infected patients for mild neurocognitive disorders - a pilot study. BMC Psychol. (2014) 2:5004. doi: 10.1186/2050-7283-2-21
65. Oshinaike OO, Akinbami AA, Ojo OO, Ojini IF, Okubdejo UN, Danesi AM. Comparison of the minimental state examination scale and the international HIV dementia scale in assessing cognitive function in nigerian HIV patients on antiretroviral therapy. AIDS Res Treat. (2012) 2012:581531. doi: 10.1155/2012/581531
66. Gouse H, Masson CJ, Henry M, Marcotte TD, London L, Kew G, et al. Assessing HIV provider knowledge, screening practices, and training needs for HIV-associated neurocognitive disorders. A short report. Aids Care. (2021). 33:468–72.
67. Ayano G, Betts K, Maravilla JC, Alati R. A systematic review and meta-analysis of the risk of disruptive behavioral disorders in the offspring of parents with severe psychiatric disorders. Child Psychiatry Hum Dev. (2021) 52:77–95. doi: 10.1007/s10578-020-00989-4
68. Hobson P, Lewis A, Nair H, Wong S, Kumwenda M. How common are neurocognitive disorders in patients with chronic kidney disease and diabetes? Results from a cross-sectional study in a community cohort of patients in North Wales, UK. BMJ Open. (2018) 8:e023520. doi: 10.1136/bmjopen-2018-023520
69. Vas CJ, Pinto C, Panikker D, Noronha S, Deshpande N, Kulkarni L, et al. Prevalence of dementia in an urban Indian population. Int Psychogeriatr. (2001) 13:439. doi: 10.1017/S1041610201007852
70. Gurvit H, Emre M, Tinaz S, Bilgic B, Hanagasi H, Sahin H, et al. The prevalence of dementia in an urban Turkish population. Am J Alzheimers Dis Other Dement. (2008) 23:67–76. doi: 10.1177/1533317507310570
71. Engedal K, Haugen PK. The prevalence of dementia in a sample of elderly Norwegians. Int J Geriatr Psychiatry. (1993) 8:565–70. doi: 10.1002/gps.930080706
72. Ueda K, Kawano H, Hasuo Y, Fujishima M. Prevalence and etiology of dementia in a Japanese community. Stroke. (1992) 23:798–803. doi: 10.1161/01.STR.23.6.798
73. Tan IL, Smith BR, von Geldern G, Mateen FJ, McArthur JC. HIV-associated opportunistic infections of the CNS. Lancet Neurol. (2012) 11:605–17. doi: 10.1016/S1474-4422(12)70098-4
74. Bowen LN, Smith B, Reich D, Quezado M, Nath A. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol. (2016) 12:662. doi: 10.1038/nrneurol.2016.149
75. Anthony I, Ramage S, Carnie F, Simmonds P, Bell J. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. (2005) 64:529–36. doi: 10.1093/jnen/64.6.529
76. Wei J, Hou J, Su B, Jiang T, Guo C, Wang W, et al. The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Front Neurol. (2020) 11:1613. doi: 10.3389/fneur.2020.581346
77. Phillips NJ, Thomas KG, Myer L, Sacktor N, Zar HJ, Stein DJ, et al. Screening for HIV-associated neurocognitive disorders in perinatally infected adolescents: youth-International HIV Dementia Scale validation. Aids. (2019) 33:815–24. doi: 10.1097/QAD.0000000000002144
78. López E, Steiner AJ, Smith K, Thaler NS, Hardy DJ, Levine AJ, et al. Diagnostic utility of the HIV dementia scale and the international HIV dementia scale in screening for HIV-associated neurocognitive disorders among Spanish-speaking adults. Appl Neuropsychol. (2017) 24:512–21. doi: 10.1080/23279095.2016.1214835
79. Bloch M, Kamminga J, Jayewardene A, Bailey M, Carberry A, Vincent T, et al. A screening strategy for HIV-associated neurocognitive disorders that accurately identifies patients requiring neurological review. Clin Infect Dis. (2016) 63:687–93. doi: 10.1093/cid/ciw399
80. Joska J, Witten J, Thomas K, Robertson C, Casson-Crook M, Roosa H, et al. A comparison of five brief screening tools for HIV-associated neurocognitive disorders in the USA and South Africa. AIDS Behav. (2016) 20:1621–31. doi: 10.1007/s10461-016-1316-y
81. Rosca EC, Albarqouni L, Simu M. Montreal cognitive assessment (MoCA) for HIV-associated neurocognitive disorders. Neuropsychol Rev. (2019) 29:313–27. doi: 10.1007/s11065-019-09412-9
82. Kim WJ, Ku NS, Lee Y-J, Ahn JY, Kim SB, Ahn H-W, et al. Utility of the montreal cognitive assessment (MoCA) and its subset in HIV-associated neurocognitive disorder (HAND) screening. J Psychosom Res. (2016) 80:53–7. doi: 10.1016/j.jpsychores.2015.11.006
83. Mecocci P, Boccardi V. The impact of aging in dementia: it is time to refocus attention on the main risk factor of dementia. Ageing Res Rev. (2020) 2020:101210. doi: 10.1016/j.arr.2020.101210
85. Jorm AF. History of depression as a risk factor for dementia: an updated review. Austr N Z J Psychiatry. (2001) 35:776–81. doi: 10.1046/j.1440-1614.2001.00967.x
86. Cantón-Habas V, Rich-Ruiz M, Romero-Saldaña M, Carrera-González MdP. Depression as a risk factor for dementia and alzheimer's disease. Biomedicines. (2020) 8:457. doi: 10.3390/biomedicines8110457
Keywords: meta-analysis, HIV/AIDS, hand, world, wide
Citation: Zenebe Y, Necho M, Yimam W and Akele B (2022) Worldwide Occurrence of HIV-Associated Neurocognitive Disorders and Its Associated Factors: A Systematic Review and Meta-Analysis. Front. Psychiatry 13:814362. doi: 10.3389/fpsyt.2022.814362
Received: 15 November 2021; Accepted: 05 April 2022;
Published: 31 May 2022.
Edited by:
Yuka Kotozaki, Iwate Medical University, JapanReviewed by:
Tiwabwork Tekalign, Wolaita Sodo University, EthiopiaSiddharth Sarkar, All India Institute of Medical Sciences, India
Copyright © 2022 Zenebe, Necho, Yimam and Akele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mogesie Necho, bmVjaG9tb2dlczIwMTRAZ21haWwuY29t
 Yosef Zenebe
Yosef Zenebe Mogesie Necho
Mogesie Necho Wondwosen Yimam
Wondwosen Yimam Baye Akele3
Baye Akele3