
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 25 April 2022
Sec. Behavioral and Psychiatric Genetics
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.788677
Preclinical and clinical studies have suggested that fibroblast growth factor (FGF) system contributed to the onset and development of schizophrenia (SCZ). However, there was no strong clinical evidence to link an individual FGF with SCZ. In this study, we aim to measure blood FGF9 levels in the patients with SCZ with and/or without medication, and test whether FGF9 has a potential to be a biomarker for SCZ. We recruited 130 patients with SCZ and 111 healthy individuals, and the ELISA and qRT-PCR assays were used to measure serum FGF9 levels in the participants. ELISA assay demonstrated that serum FGF9 protein levels were dramatically reduced in first-episode, drug-free patients, but not in chronically medicated patients when compared to healthy control subjects. Further analysis showed that treatment of the first-episode, drug-free SCZ patients with antipsychotics for 8 weeks significantly increased the serum FGF9 levels. In addition, we found that blood FGF9 mRNA levels were significantly lower in first-onset SCZ patients than controls. Under the receiver operating characteristic curve, the optimal cutoff values for FGF9 protein level as an indicator for diagnosis of drug-free SCZ patients was projected to be 166.4 pg/ml, which yielded a sensitivity of 0.955 and specificity of 0.86, and the area under the curve was 0.973 (95% CI, 0.954-0.993). Furthermore, FGF9 had good performance to discriminate between drug-free SCZ patients and chronically medicated patients, the optimal cutoff value for FGF9 concentration was projected to be 165.035 pg/ml with a sensitivity of 0.86 and specificity of 0.919, and the AUC was 0.968 (95% CI, 0.944, 0.991). Taken together, our results for the first time demonstrated the dysregulation of FGF9 in SCZ, and FGF9 has the potential to be served as a biomarker for SCZ.
Schizophrenia (SCZ) is a chronic, serious psychosis with an incidence of approximately 1% (1). The clinical manifestations are mainly emotional reactions characterized by paranoia, hallucinations and inappropriate social misconduct (2). The mechanism underlying the devastating disease is very complex and still poorly understood, although it is considered that the occurrence of the disease has a very strong relationship with genetic and the environment background (3). More recently, there is increased awareness of the critical role of epigenetics in the onset and/or development of SCZ (4, 5). The typical treatments for SCZ are antipsychotics which target the neurotransmitter system in the central nervous system (6). However, long-term use of these drugs had side effects, and failed to improve the negative symptom and cognitive impairment of the patients (7, 8). In addition, it is often difficult to distinguish SCZ with other neuropsychiatric diseases due to the lack of the specific biomarkers. Therefore, there is an urgent need to better understand the etiology of SCZ and subsequently develop biomarkers for the diagnosis and prognosis of the disease.
The neurotrophic factor hypothesis of schizophrenia has generated great interest over the last decade. It postulated that the disturbances of developing processes involving neurotrophic factors result in the changes in the brain of patients with SCZ (9). This hypothesis was mainly supported by the large number of preclinical and clinical studies suggesting the involvement of brain-derived neurotrophic factor and nerve growth factor in the pathogenesis of SCZ (10–13). More recent studies suggested that fibroblast growth factor system is involved in the pathogenesis of SCZ. FGF2 is the most studied FGFs in the literature, which has been demonstrated to play crucial roles in neurodevelopment and maintenance of nervous system in adult (14, 15). The clinical data from Hashimoto et al. showed that blood FGF2 levels were significantly increased in the medicated patients with SCZ when compared with healthy controls, but they did not show a statistically significant difference between non-medicated patients and controls for FGF2 levels (16). In contrast, results from Li et al. indicated non-medicated SCZ patients had higher serum FGF2 levels than healthy individuals (17). In addition to FGF2, FGF9 has been proposed as a novel modulator for mood disorder, as both preclinical and clinical data indicated a role of FGF9 in depression (18, 19). Moreover, FGF9 has been suggested to be involved in the development of SCZ, this is supported by the impaired social discrimination and heightened acoustic startle reactivity in FGF9 mutant mice (20). However, there is no clinical data to support a role of FGF9 in SCZ.
In this study, we recruited 130 SCZ patients and 111 healthy control (HC) subjects to determine whether FGF9 levels were dysregulated in the peripheral blood of SCZ patients. Our results indicated that the levels of FGF9 in peripheral blood of SCZ patients were significantly reduced, and treatments with the antipsychotics restored the FGF9 levels in the patients. Our analyses also suggested that serum FGF9 has the potential to be served as a biomarker to inform the diagnosis and/or treatment response for SCZ.
We recruited 130 SCZ patients from The Third People’s Hospital of Foshan. SCZ Patients diagnosed by experienced psychiatrists according to Structured Clinical Interview for DSM-IV (SCID) and International Classification of Diseases 10 (ICD-10) were included, including 57 patients who were first-episode, drug-free SCZ patients, and 73 patients with chronic medication. It should be noted that 19 patients from the group of drug-free SCZ patients had two evaluations: baseline and after 8-week antipsychotics treatments. Positive and negative syndrome scale (PANSS) was used to assess the patients’ psychiatric symptoms. All patients with intellectual disability, severe physical illnesses, substance abuse, and serious infectious diseases were excluded. At the same time, 111 healthy volunteers without mental and physical illnesses were recruited as controls.
All subjects included in this study have signed an informed consent form (or by family members for some patients). The research plan was reviewed and approved by the Ethics Committee of the Third People’s Hospital of Foshan, Guangdong, China. And the experiments were conducted in accordance with the Helsinki Declaration.
In total, 10 ml of peripheral blood from the patients or healthy individuals were collected in the morning after overnight fasting. The blood samples were allowed to clot at room temperature for 1 h, and then we obtained the serum by centrifugation at 3000 × g for 10 min. The serum was then stored in a −80 refrigerator for subsequent experiments. We used the enzyme-linked immunosorbent assay (ELISA) kit to measure FGF9 levels in serum. The specific protocol was carried out according to the instructions of the kit (Cloud-Clone, Wuhan, China; Catalog No: SEA036Hu). The sensitivity of the human serum FGF9 kit was 6.6 pg/ml, and the concentration of the protein was expressed as pg/ml serum.
Blood FGF9 mRNA levels of 48 first-onset SCZ patients and 48 HC subjects were measured by quantitative real time PCR (qRT-PCR). RNA extraction and reverse transcription were performed according to the manufacturer’s instruction (Beijing Zhuangmeng International Biological Gene Technology Co., Ltd, Beijing, China). qRT-PCR was performed as previously described (21), and the cycling conditions were: preincubation at 95°C for 600 s, followed by 45 cycles of synthesis at 95°C for 15 s, 59°C for 20 s and 72°C for 40 s. The primer sequences for FGF9 forward: 5′-ATGGCTCCCTTAGGTGAAGTT-3,′ reverse: 5′-CC CAGGTGGTCACTTAACAAAAC-3′; for GAPDH forward: 5′-CTGGGCTACACTGAGCACC-3,′ reverse: 5′-AAGTGGTCG TTGAGGGCAATG-3.′
All data were presented in the form of mean ± standard deviation (SD). We used GraphPad Prism 8.0 to calculate and graph the statistics for FGF9 expression analysis. The Kolmogorov-Smirnov test (normality test) was applied to determine whether using parametric test (student’s t-test) or non-parametric test (Mann–Whitney U test) to compare FGF9 expression changes between different groups. The differences between cases and controls for FGF9 mRNA/protein expressions were analyzed by the Mann-Whitney U test (Kolmogorov–Smirnov test, p < 0.05), whereas the difference between drug-free SCZ patients at baseline and after 8-week antipsychotics treatment for FGF9 protein levels was analyzed by the student’s t-test (Kolmogorov–Smirnov test, p > 0.05). To analyze the relationship between age, disease severity and FGF9, we used the Kendell test and Spearman test. The receiver operating characteristic (ROC) curve method was used to assess whether serum FGF9 levels could be served as a biomarker for the diagnosis and/or drug treatment response in SCZ, the accuracy of the ROC test was calculated by area under the curve (AUC). P < 0.05 was considered statistically significant in this study.
In this study, 57 first-episode, drug-free SCZ patients, 73 chronically medicated SCZ patients, and 111 healthy volunteers were recruited. The demographic information and clinical characteristics of all volunteers are shown in Table 1.
The results demonstrated that serum FGF9 levels were significantly reduced in 57 first-episode, drug-free SCZ patients as compared to 111 HC subjects (Figure 1A, Mann–Whitney U = 169, P < 0.001), whereas no significant difference was found between 73 chronically medicated patients and 111 controls for FGF9 levels (Figure 1A, Mann–Whitney U = 3637, P > 0.05). Furthermore, serum FGF9 levels were significantly increased in 73 chronically medicated SCZ patients relative to 57 first-episode, drug-free SCZ patients (Figure 1A, Mann–Whitney U = 134, P < 0.001). Of these SCZ patients, 19 patients from the group of drug-free SCZ patients had two evaluations: baseline and after 8-week antipsychotics treatments. The PANSS total score, PANSS positive score, and PANSS negative score the 19 patients were assessed, and the results indicated the drug treatments were effective (Table 1). The ELISA assay suggested that the serum FGF9 levels were also significantly increased in these patients after 8-week antipsychotics treatments (Figure 1B, t = 4.362, P < 0.001), suggesting that the antipsychotics up-regulated the serum FGF9 levels in the SCZ patients. In addition, we did not find gender significantly affect the dysregulation of serum FGF9 levels in the SCZ patients (Figures 1C,D).
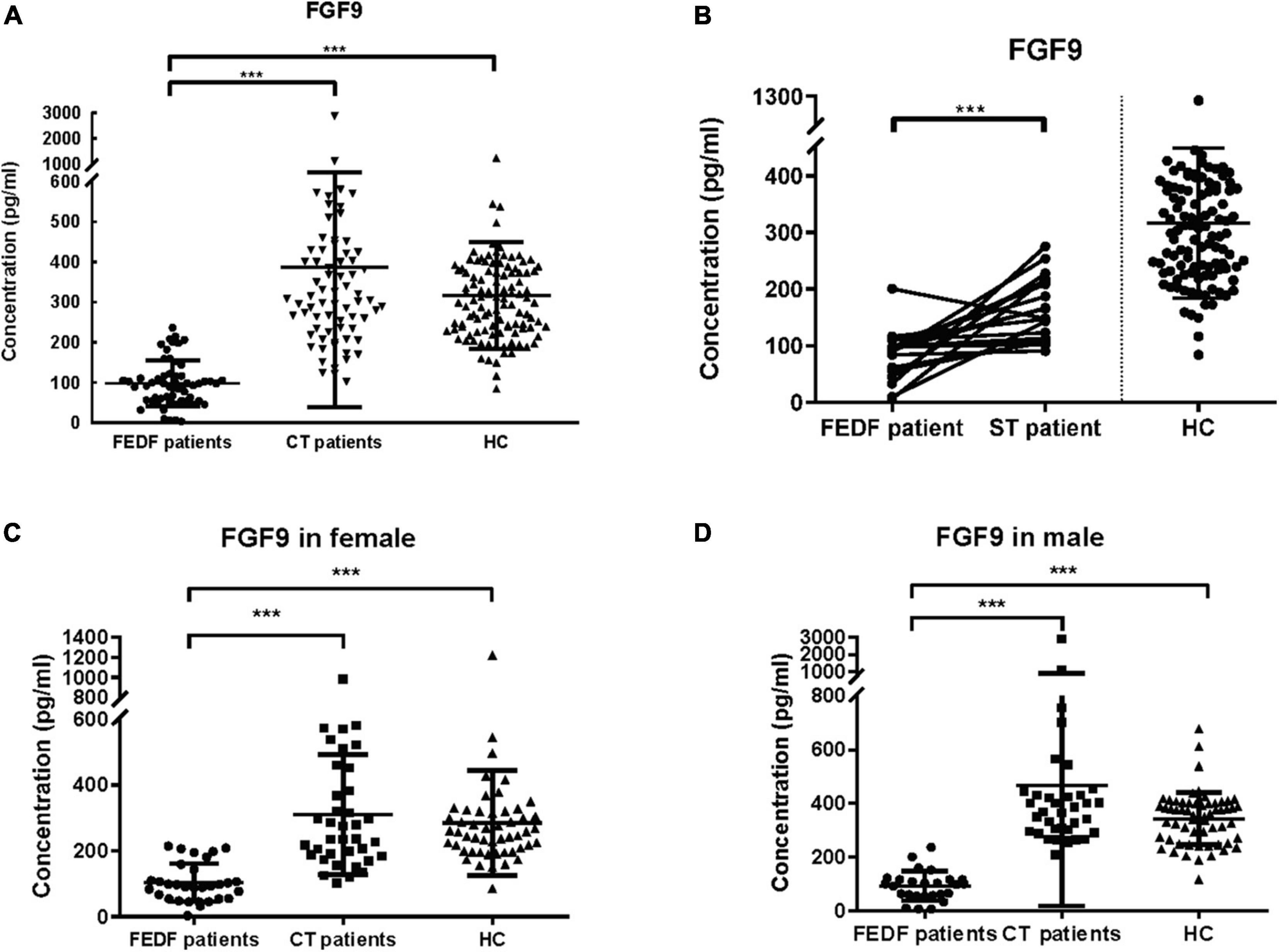
Figure 1. (A) Serum FGF9 levels were reduced in 57 first-episode, drug-free SCZ patients (Mann–Whitney U = 169, P < 0.001), but not 73 chronically treated SCZ patients (Mann–Whitney U = 3637, P > 0.05) when compared with 111 HC subjects. (B) Serum FGF9 levels in 19 first-episode, drug-free SCZ patients at baseline and at 8-week follow up (t = 4.362, P < 0.001). (C) FGF9 levels were reduced in the first-episode, drug-free SCZ female patients (Mann–Whitney U = 84, P < 0.001), but not chronically treated female SCZ patients when compared with female controls (Mann–Whitney U = 941, P > 0.05). (D) FGF9 levels were reduced in the first-episode, drug-free SCZ male patients (Mann–Whitney U = 16, P < 0.001), but not chronically treated male SCZ patients (Mann–Whitney U = 880, P > 0.05) when compared with male controls. FEDF, first-episode, drug-free; CT, chronically treated (long term treated); ST, short term treated (8-week); SCZ, schizophrenia; HC, healthy control. ***p < 0.001. For mean ± SD, HC: 316.3 ± 132.4; FEDF patients: 98.11 ± 56.56; CT patients: 387.7 ± 348.6; ST patients: 163.6 ± 56.08.
Next, we examined blood FGF9 mRNA levels in the patients with SCZ, and the result from qRT-PCR showed that FGF9 mRNA levels were significantly reduced in 44 first-episode, drug-free SCZ patients as compared with 44 controls (Figure 2, Mann–Whitney U = 578, P = 0.001).
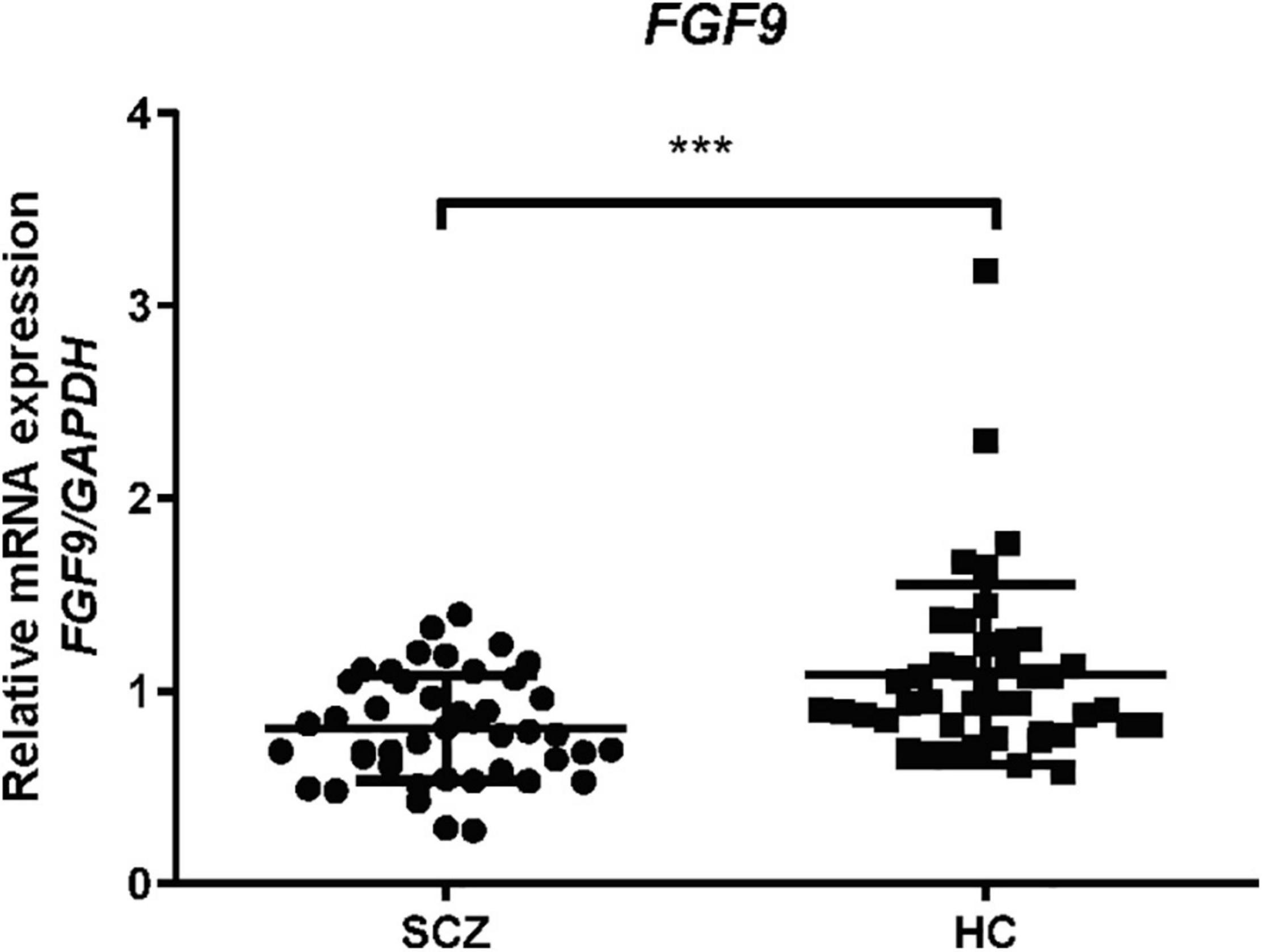
Figure 2. Blood FGF9 mRNA levels were reduced in 44 drug-free SCZ patients when compared with 44 HC subjects (Mann-Whitney U = 578, P = 0.001). HC, healthy control; SCZ, schizophrenia. ***p < 0.001. For mean ± SD, HC: 1.08 ± 0.47, SCZ patients: 0.81 ± 0.28.
We then investigated whether serum FGF9 levels were correlated with age and disease severity, which analyzed 130 SCZ patients. The results showed age (Figure 3A) and PANSS total score (Figure 3B) did not significantly associated with FGF9. However, there was a significantly positive correlation between serum FGF9 level and PANSS positive score in the patients (Figure 3C, Kendall r = 0.18, p = 0.0013; Spearman r = 0.28, p = 0.005). In addition, a highly negative correlation between serum FGF9 level and PANSS negative score in the patients was found (Figure 3D, Kendall r = −0.232, p < 0.001; Spearman r = −0.351, p < 0.001).
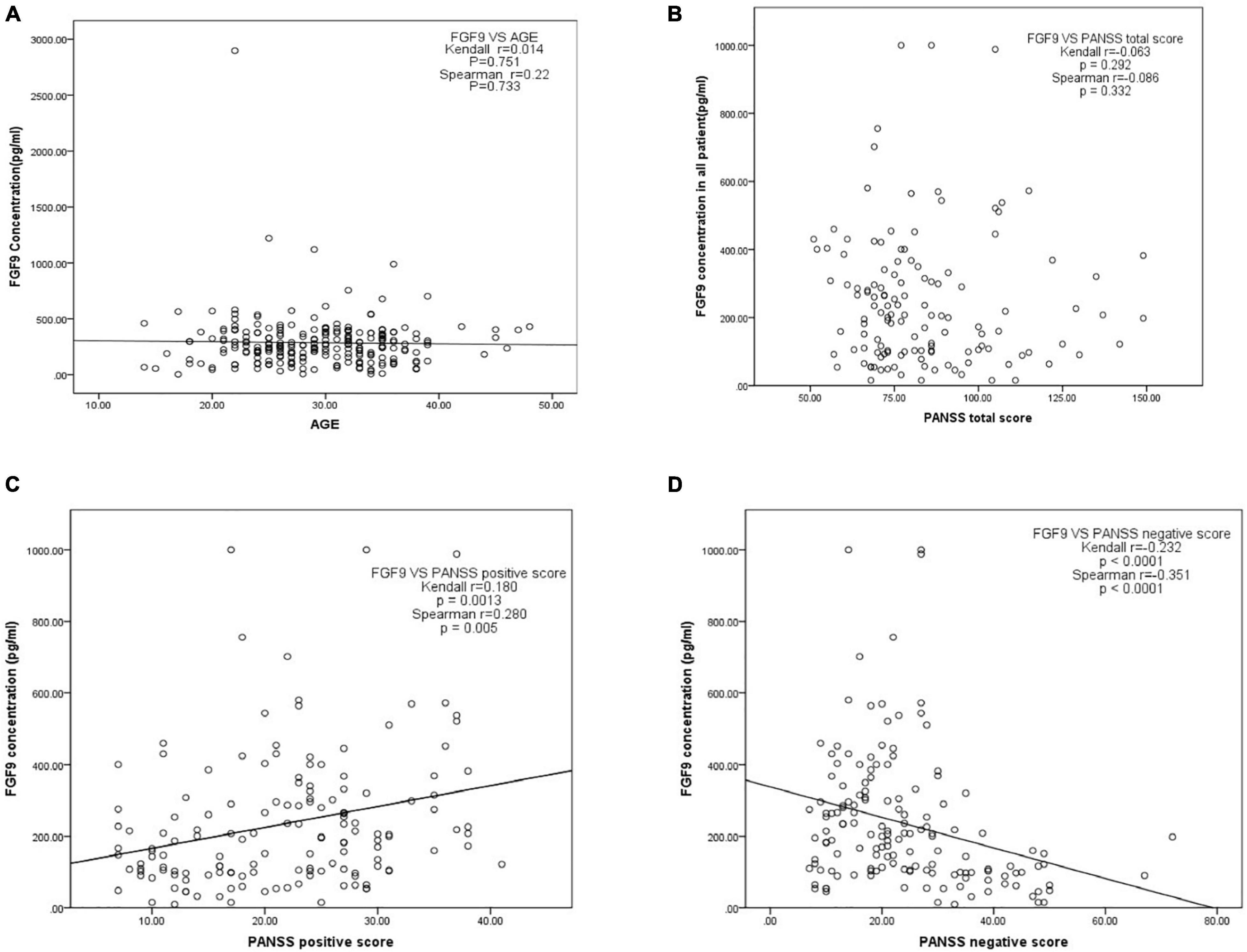
Figure 3. (A) Correlation between serum FGF9 concentration and age. (B) Correlation between serum FGF9 concentration and PANSS total score. (C) Correlation between serum FGF9 concentration and PANSS positive score. (D) Correlation between serum FGF9 concentration and PANSS negative score. N = 130.
Given that the serum FGF9 levels were highly down-regulated in the patients with SCZ when compared with healthy individuals, we therefore tested whether serum FGF9 has the potential be a biomarker for SCZ. On the basis of ROC curve, the optimal cutoff value of serum FGF9 level to diagnose 57 first-episode, drug-free SCZ patients from 111 healthy individuals was projected to be 166.4 pg/ml, which yielded a sensitivity of 0.955 and specificity of 0.86 (Figure 4A and Supplementary Table 1), and the area under curve (AUC) was 0.973 (95% CI, 0.954–0.993). In addition, analyses from ROC suggested that peripheral blood FGF9 had good performance to discriminate between 57 drug-free SCZ patients and 73 chronically medicated patients, the optimal cutoff value for FGF concentration was projected to be 165.035 pg/ml with a sensitivity of 0.86 and specificity of 0.919 (Figure 4B and Supplementary Table 1), and the AUC was 0.968 (95% CI, 0.944, 0.991).
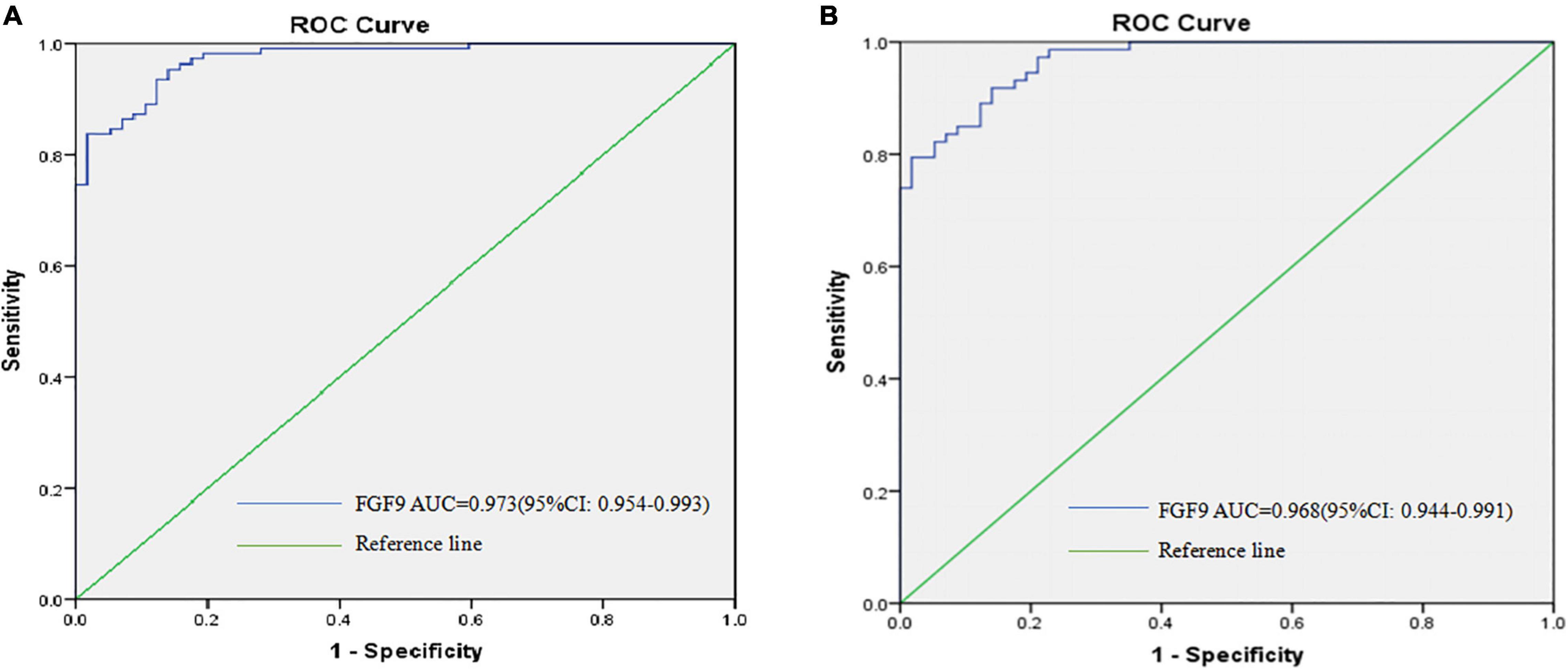
Figure 4. ROC curves were utilized to evaluate the accuracy of serum FGF9 concentrations to diagnose 57 first-episode, drug-free SCZ patients (A), and to discriminate between 57 first-episode, drug-free SCZ patients and 73 chronically medicated SCZ patients (B).
In this case-control study, we have included 130 patients with SCZ and 111 healthy volunteers, and demonstrated that serum FGF9 levels were highly down-regulated in the first-episode, drug-free SCZ patients. In contrast, the serum FGF9 levels in the long-term medicated SCZ patients were not significantly different when compared with healthy controls. We also performed a longitudinal study with 19 SCZ patients, and the data suggested that short-term antipsychotics treatment may increase FGF9 levels in the blood of the patients. Further analysis indicated that blood FGF9 mRNA levels in the first-onset SCZ patients were also down-regulated. Additionally, our study indicated that gender did not significantly affect the differences of FGF9 levels between SCZ patients and controls, whereas disease severity is a confounding factor for FGF9 levels in SCZ patients. Furthermore, data from this study suggested declined PANSS positive score in SCZ patients with 8-week antipsychotics treatment, but not chronically medicated patients. It is well known that antipsychotics mainly target positive symptoms of SCZ patients, and therefore it is reasonable that PANSS positive score declined after 8-week antipsychotics treatment in the patients. However, the chronically medicated patients that we recruited were inpatients, implying that they were partially or non-response to antipsychotics for positive symptoms, and therefore it is not surprising that these patients still had high PANSS positive score. Taken together, our data provided strong clinical evidence that the onset of SCZ was accompanied by reduced blood FGF9 levels, offers a novel insight into a potential molecular pathway that confer vulnerability to the development of SCZ.
Although the data from our study revealed that the onset of SCZ was accompanied by reduced blood FGF9 levels, whether insufficiency of this growth factor in SCZ contribute to the onset of the disease is unclear. Here we hypothesize that the lack of FGF9 in SCZ contributes to the disease onset, and this is supported by the increasing evidence showing the crucial roles of FGF system in neurodevelopment and mood disorders (15). In fact, the physiological and pathological roles of FGF2 in the nervous system have been extensively studied (15). More recent in vitro and in vivo studies suggested that FGF9 was involved in the neurodevelopment and mood disorder. Results from Seuntjens et al. showed that the fate of uncommitted precursors and generation of appropriate numbers of different neurons and glia in the neocortex were regulated by neuron-to-progenitor feedback signaling, and the mechanism involved the regulation of the expression of FGF9 in the post-mitotic neurons (22). In addition, Falcone et al. indicated that FGF9 may promote astrogenesis, since they showed that the Emx2 homeobox gene-dependent repression of FGF9 in cortico-cerebral stem cells caused the shrinkage of the astrogenic pool (23). Beside the potential role of FGF9 in neurodevelopment, FGF9 has been proposed as a novel modulator for mood disorder. This was supported by studies showing that FGF9 expression was significantly up-regulated in frontal cortex (19) and locus coeruleus (24) of patients with major depression. Consistently, animals subjected to chronic social defeat stress showed significant increase of hippocampal FGF9 expression, and administration of FGF9 increased anxiety- and depression-like behaviors in animals, whereas knocking down FGF9 expression in the dentate gyrus of the hippocampus ameliorated anxiety-like behavior (18). These results indicated that FGF9 was a modulator of negative affect for affective disorder, FGF9 thus has provided a novel therapeutic target for treatment of depression. More recently, Garrett et al. generated FGF9Y162C mutant mice to analyze the role of FGF9 in brain functions, they discovered that the mutant mice had heightened acoustic startle reactivity and impaired social discrimination (20), therefore evidence from in vivo animal study suggested lacking of FGF9 activity may contribute to the etiology of SCZ. More interestingly, our results showed that antipsychotics increased serum FGF9 levels in SCZ patients. Although the molecular mechanism underlying this regulation has not been addressed in the study, one possible explanation is the involvement of brain-derived neurotrophic factor. This speculation is due to the findings that antipsychotics significantly increased brain-derived neurotrophic factor levels in SCZ patients (11), and brain-derived neurotrophic factor has been reported increasing growth factor expression in vitro (25). Nevertheless, future studies are necessary to explore the exact roles of FGF9 in the onset and development of SCZ.
Biomarker has been defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological response” (26). Therefore, great efforts have been made over the last several decades to identify biomarkers for the major psychiatric diseases including SCZ (27), in hope of better understanding the etiologies of these devastating diseases, providing objective measurements for the diagnosis, prognosis and treatment response of the diseases. Although no reliable and validated biomarkers have been established for the major psychiatric diseases, potential biomarkers from blood have been extensively studied since it is easily accessible and cost-effective, and these efforts have led to some promising biomarkers for the diseases. As an example is a test called VeriPsych, which measured 51 blood analytes (small molecules and proteins), was available in 2010 from a company. This panel of biomarkers reported a specificity and sensitivity of 83% to distinguish SCZ patients from controls, and the ROC-AUC was 89% (28). However, this assay had been withdrawn from the market partly due to the specificity issue and the high cost (2500 US dollars) (27). In this study we have used a simple ELISA assay and demonstrated that blood FGF9 differentiated SCZ patients from control subjects with a sensitivity of 95.5% and specificity of 86%, and ROC-AUC of 97.3%, suggesting that FGF9 has the potential to provide excellent diagnostic accuracy for SCZ. In addition, data from our study also demonstrated that peripheral blood FGF9 had good performance to discriminate between first-onset SCZ patients and chronically medicated patients with a sensitivity of 0.86 and specificity of 0.919, suggesting FGF9 has the potential to be used as a biomarker for the treatment response of SCZ patients. However, one limitation of our study is the limited sample size of SCZ patients with 8-week antipsychotics treatment, although our analysis suggested that blood FGF9 had a reasonable performance in discriminating between first-onset SCZ patients and short-term (8-week) treated SCZ patients (data not shown). Therefore, further investigations into the peripheral blood FGF9 levels in SCZ patients are necessary to translate the potential blood biomarker into practical clinical use.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of The Third People’s Hospital of Foshan. The patients/participants provided their written informed consent to participate in this study.
X-SL and YC conceived and designed this study. X-LL, YY, and H-TW performed the experiments. X-LL, YY, YH, H-TW, and G-YC analyzed and interpreted the data. YC drafted the manuscript with critical revisions from X-LL and X-SL. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (82071676), High-Level Hospital Development Program for Foshan “Climbing” Project, Foshan Science and Technology Innovation Project (2020001005608), and the Natural Science Foundation of Guangdong Province (2114050002827).
YC, YY, and YH have a patent related to this manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.788677/full#supplementary-material
1. Keller WR, Kum LM, Wehring HJ, Koola MM, Buchanan RW, Kelly DL. A review of anti-inflammatory agents for symptoms of schizophrenia. J Psychopharmacol. (2013) 27:337–42. doi: 10.1177/0269881112467089
2. Akbarian S. Epigenetic mechanisms in schizophrenia. Dial Clin Neurosci. (2014) 16:405–17. doi: 10.31887/dcns.2014.16.3/sakbarian
3. Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HW, Rutten BP. An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiat. (2012) 17:1194–205. doi: 10.1038/mp.2011.183
4. Du Y, Tan WL, Chen L, Yang ZM, Li XS, Xue X, et al. Exosome transplantation from patients with schizophrenia causes schizophrenia-relevant behaviors in mice: an integrative multi-omics data analysis. Schizophrenia Bull. (2021) 47:1288–99. doi: 10.1093/schbul/sbab039
5. Du Y, Yu Y, Hu Y, Li XW, Wei ZX, Pan RY, et al. Genome-Wide, integrative analysis implicates exosome-derived MicroRNA dysregulation in schizophrenia. Schizophrenia Bull. (2019) 45:1257–66. doi: 10.1093/schbul/sby191
6. Cassoli JS, Guest PC, Santana AG, Martins-de-Souza D. Employing proteomics to unravel the molecular effects of antipsychotics and their role in schizophrenia. Proteomics Clin Appl. (2016) 10:442–55. doi: 10.1002/prca.201500109
7. Smith T, Weston C, Lieberman J. Schizophrenia (maintenance treatment). Am Fam Physician. (2010) 82:338–9.
8. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophrenia Res. (2008) 100:4–19. doi: 10.1016/j.schres.2008.01.022
9. Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiat. (2005) 10:345–52. doi: 10.1038/sj.mp.4001637
10. Aloe L, Iannitelli A, Angelucci F, Bersani G, Fiore M. Studies in animal models and humans suggesting a role of nerve growth factor in schizophrenia-like disorders. Behav Pharmacol. (2000) 11:235–42. doi: 10.1097/00008877-200006000-00007
11. Fernandes BS, Steiner J, Berk M, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiat. (2015) 20:1108–19. doi: 10.1038/mp.2014.117
12. Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiat. (2011) 16:960–72. doi: 10.1038/mp.2010.88
13. Qin XY, Wu HT, Cao C, Loh YP, Cheng Y. A meta-analysis of peripheral blood nerve growth factor levels in patients with schizophrenia. Mol Psychiat. (2017) 22:1306–12. doi: 10.1038/mp.2016.235
14. Terwisscha van Scheltinga AF, Bakker SC, Kahn RS. Fibroblast growth factors in schizophrenia. Schizophrenia Bull. (2010) 2010:1157–66. doi: 10.1093/schbul/sbp033
15. Terwisscha van Scheltinga AF, Bakker SC, Kahn RS, Kas MJ. Fibroblast growth factors in neurodevelopment and psychopathology. Neuroscientist. (2013) 19:479–94. doi: 10.1177/1073858412472399
16. Hashimoto K, Shimizu E, Komatsu N, Nakazato M, Okamura N, Watanabe H, et al. Increased levels of serum basic fibroblast growth factor in schizophrenia. Psychiat Res. (2003) 120:211–8. doi: 10.1016/s0165-1781(03)00186-0
17. Li XS, Wu HT, Yu Y, Chen GY, Qin XY, Zheng GE, et al. Increased serum FGF2 levels in first-episode, drug-free patients with schizophrenia. Neurosci Lett. (2018) 686:28–32. doi: 10.1016/j.neulet.2018.08.046
18. Aurbach EL, Inui EG, Turner CA, Hagenauer MH, Prater KE, Li JZ, et al. Fibroblast growth factor 9 is a novel modulator of negative affect. Proc Natl Acad Sci USA. (2015) 112:11953–8. doi: 10.1073/pnas.1510456112
19. Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA. (2004) 101:15506–11. doi: 10.1073/pnas.0406788101
20. Garrett L, Becker L, Rozman J, Puk O, Stoeger T, Yildirim AÖ, et al. Fgf9 (Y162C) Mutation Alters Information Processing and Social Memory in Mice. Mol Neurobiol. (2017) 55:4580–95. doi: 10.1007/s12035-017-0659-3
21. Wei ZX, Xie GJ, Mao X, Zou XP, Liao YJ, Liu QS, et al. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology. (2020) 45:1050–8. doi: 10.1038/s41386-020-0622-2
22. Seuntjens E, Nityanandam A, Miquelajauregui A, Debruyn J, Stryjewska A, Goebbels S, et al. Sip1 regulates sequential fate decisions by feedback signaling from postmitotic neurons to progenitors. Nat Neurosci. (2009) 12:1373–80. doi: 10.1038/nn.2409
23. Falcone C, Filippis C, Granzotto M, Mallamaci A. Emx2 expression levels in NSCs modulate astrogenesis rates by regulating EgfR and Fgf9. Glia. (2015) 63:412–22. doi: 10.1002/glia.22761
24. Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiat. (2011) 16:634–46. doi: 10.1038/mp.2010.44
25. Lin CY, Hung SY, Chen HT, Tsou HK, Fong YC, Wang SW, et al. Brain-derived neurotrophic factor increases vascular endothelial growth factor expression and enhances angiogenesis in human chondrosarcoma cells. Biochem Pharmacol. (2014) 91:522–33. doi: 10.1016/j.bcp.2014.08.008
26. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Therapeut. (2001) 69:89–95. doi: 10.1067/mcp.2001.113989
27. Weickert CS, Weickert TW, Pillai A, Buckley PF. Biomarkers in schizophrenia: a brief conceptual consideration. Dis Mark. (2013) 35:3–9. doi: 10.1155/2013/510402
Keywords: fibroblast growth factor 9, schizophrenia, antipsychotics, biomarker, first-episode, drug-free
Citation: Li X-L, Yu Y, Hu Y, Wu H-T, Li X-S, Chen G-Y and Cheng Y (2022) Fibroblast Growth Factor 9 as a Potential Biomarker for Schizophrenia. Front. Psychiatry 13:788677. doi: 10.3389/fpsyt.2022.788677
Received: 11 October 2021; Accepted: 14 March 2022;
Published: 25 April 2022.
Edited by:
Gary Donohoe, National University of Ireland Galway, IrelandReviewed by:
Mi Mi Tang, Central South University, ChinaCopyright © 2022 Li, Yu, Hu, Wu, Li, Chen and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-Song Li, ZnM4MzMxNTAwNkAxNjMuY29t; Yong Cheng, eW9uZ2NoZW5nQG11Yy5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.