- 1Department of Public Health, University of Illinois at Chicago, Chicago, IL, United States
- 2Department of Public Health, Benedictine University, Lisle, IL, United States
- 3Department of Pharmacy, University of Illinois, Chicago, IL, United States
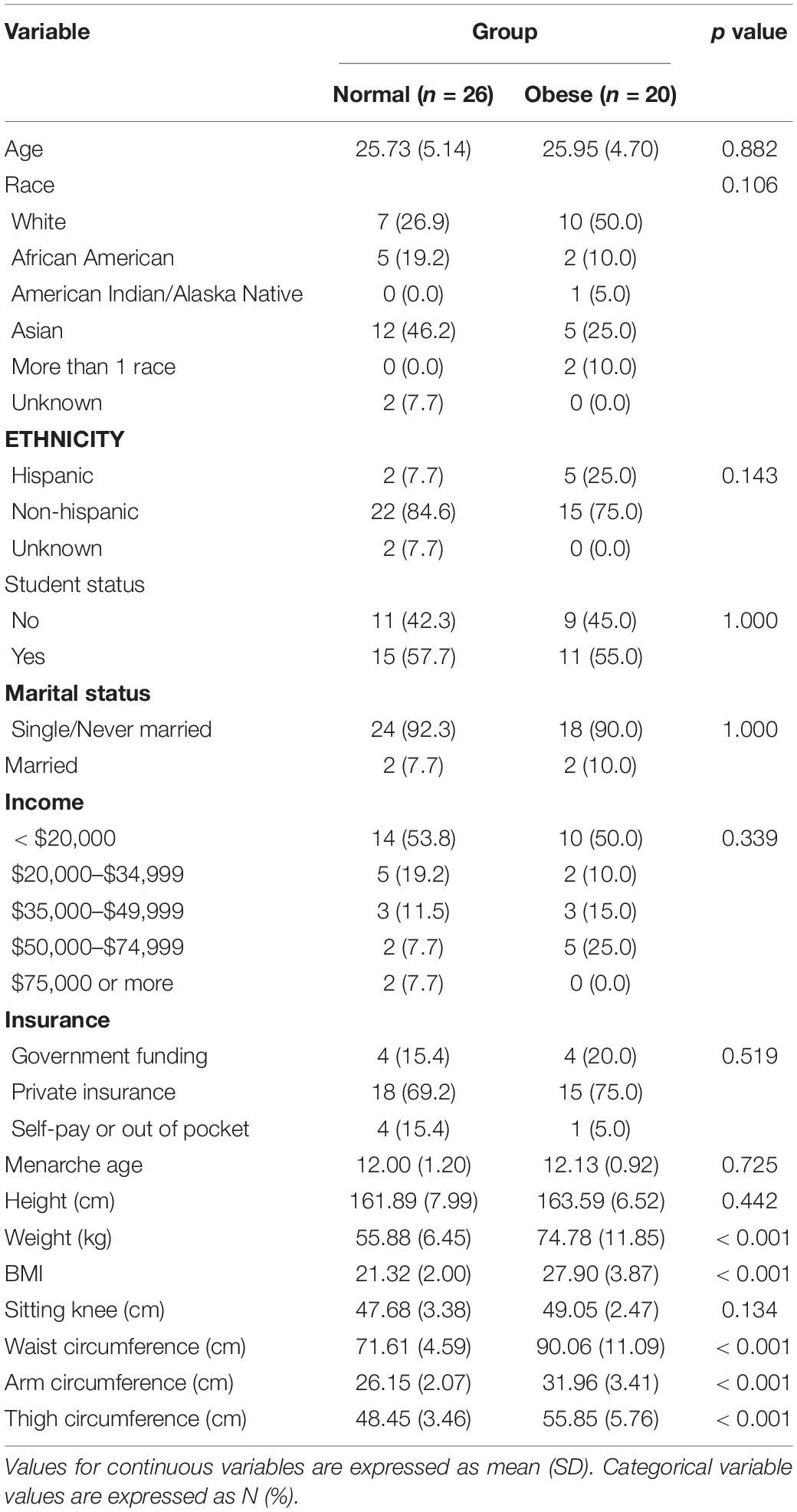
Visceral adiposity is a significant marker of all-cause mortality. Reproductive age women are at a considerable risk for developing visceral adiposity; however, the associated factors are poorly understood. The proposed study evaluated whether food craving experienced during the premenstrual period is associated with waist circumference. Forty-six women (mean BMI = 24.36) prospectively provided daily ratings of food craving across two-three menstrual cycles (122 cycles total). Their premenstrual rating of food craving was contrasted against food craving in the follicular phase to derive a corrected summary score of the premenstrual food craving increase. Study groups were divided into normal (n = 26) and obese (n = 20) based on the 80 cm waist circumference cutoff signifying an increase in risk. Waist circumference category was significantly associated with premenstrual food cravings [F(1,44) = 5.12, p = 0.028]. Post hoc comparisons using the Tukey HSD test (95% family-wise confidence level) showed that the mean score for the food craving effect size was 0.35 higher for the abdominally obese vs. normal study groups (95% CI: 0.039 to 0.67). The result was statistically significant even following inclusion of BMI in the model, pointing to a particularly dangerous process of central fat accumulation. The present study establishes an association between temporal vulnerability to an increased food-related behavior and a marker of metabolic abnormality risk (i.e., waist circumference), thereby forming a basis for integrating the premenstruum as a viable intervention target for this at-risk sex and age group.
Introduction
Obesity is considered a 21st century epidemic and reliance on the measurement of body mass index (BMI) alone has been shown to be inadequate in assessing and managing obesity-related cardiometabolic risk (1, 2). Waist circumference—a marker of visceral adiposity—is strongly associated with all-cause (3, 4) and cardiovascular (5, 6) mortality. Visceral fat accumulates when adipose tissue has a limited ability to expand. This process is indicative of a hyperlipolytic state; marked by resistance to insulin and an enhanced adipokine output.
There are large differences in body composition between men and women. With an overall weight gain, waist circumference also increases in young adults; however, men have larger increases in waist circumference naturally accompanying their overall greater weight gain compared to women. Hence, a universally acceptable approach is to set a higher circumference cutoff value for abdominal obesity in men compared to women (7). For example, the International Diabetes Federation sets the threshold for abdominal obesity for men at 90 cm (or 94 cm for White men), and 80 cm for women across ethnic backgrounds. Metabolic syndrome—providing clinicians with a simple screening tool to identify individuals who are likely to have insulin resistance and related metabolic abnormalities—lists waist circumference (≥ 80 cm in women) as one of the five possible criteria for the syndrome, highlighting the importance of central adiposity on disease probability (8, 9).
The premenstruum is marked by an increase in food craving in a significant number of reproductive age women, who are particularly vulnerable to weight gain (10–13). A study by Hartlage et al. (14) administered a questionnaire listing 50 premenstrual symptoms to women from the community and showed that food craving ranked second in magnitude, immediately after bloating. Indeed, food craving, defined as an “intense desire to eat a specific food or food type” (15, 16) is endorsed by women in the premenstruum both retrospectively (17, 18) and prospectively (14, 19, 20). Functional magnetic resonance tomography studies show higher brain responses to food stimuli in the luteal vs. follicular phase, particularly in the corticolimbic areas involved in homeostasis and reward (21–24). Combined, the results of this research characterize functional and self-reported indices of increased food craving in the premenstruum.
No study to date has established an association between either the PMS/PMDD diagnosis, or a particular premenstrual symptomatology, and central adiposity. Data from the Nurses’ Health Study 2 (NHS2) was analyzed for central adiposity (waist circumference); however, only an association between PMS and BMI was found. PMS cases were identified by asking women whether they ever received a diagnosis for PMS—an approach which may have diminished the power to isolate an association. PMS diagnosis is based on various symptom types (see Section “Diagnostic Criteria”), some of which may be distal to central adiposity. As only one symptom must be met (and up to the total of four), a woman would meet the PMS diagnosis if she reported, for example, increased difficulty concentrating in the premenstruum. An association with the PMS diagnosis in this case may be more difficult to capture than if the diagnosis was based on food craving. Moreover, PMS/PMDD diagnosis is rarely established clinically using prospective recordings across two menstrual cycles, as required in DSM-5; hence, it is possible that there were diagnostic misclassifications of the ever/never diagnostic status in the Bertone-Johnson et al. (25) analysis because the diagnosed participants were likely not followed prospectively at the time of the diagnosis determination.
Our analysis is based on a stringent prospective symptom data collection of premenstrual food craving symptomatology. Importantly, it is adjusted for BMI, which is necessary as the full strength of the association between waist circumference with morbidity and mortality is realized only after adjustment for BMI (3, 26, 27). Based on the literature showing that women’s food craving increases premenstrually (14), and that there is an association between food craving and waist circumference in women even independent of the reproductive status (28), we hypothesized that there would be a positive association between premenstrual food craving and waist circumference. Our study identified the premenstruum as a window of opportunity for the development of targeted interventions, which is of critical importance for health promotion in reproductive age women, who are especially vulnerable to developing obesity.
Materials and Methods
Study Design
Premenstrual Hormonal and Affective State Evaluation (PHASE) is a single-cohort longitudinal design study with a nested human laboratory experiment. The study enrolls women with regular menstrual cycles to chart their symptoms using the Daily Record of Severity of Problems (DRSP) (29), menstruation timing and urinary luteinizing hormone (LH) levels during three menstrual cycles. In the third menstrual cycle, in addition to DRSP collection and LH testing, study participants complete: (1) blood and salivary sample collection at eight different times of the menstrual cycle (early follicular, mid-follicular, periovulatory 1, periovulatory 2, periovulatory 3, early luteal, mid-luteal, and late luteal), and (2) psychosocial stress testing in the late luteal phase. Knowledge gained from PHASE is expected to increase our understanding of PMS/PMDD etiology. PHASE is a registered clinicaltrials.gov study (NCT03862469).
Study Sample
Women between the ages of 18 and 35, with regular menstrual cycles lasting 21 to 35 days (30–33), were recruited from the general population using electronic media (Instagram, and Facebook) and flyers. The advertisement specified that the objective of the study is to evaluate “what causes” PMS.
Study participants first completed an online survey, following which they were scheduled to complete an in-person screening. Study exclusion criteria were: (a) lifetime DSM-5 Axis I disorder, except anxiety and depression [based on the Structured Clinical Interview for DSM Disorders (SCID)], (b) current (i.e., within the past 12 months) DSM-5 Major Depressive Disorder or an anxiety disorder (based on SCID), (c) positive urine drug screen test, (d) breath alcohol concentration > 0.00%, (e) Alcohol Use Disorders Identification Test (AUDIT) score > 7, (e) self-reported smoker or carbon monoxide concentration ≥ 6 ppm, (f) irregular menstrual cycle, (g) current pregnancy (urine test-verified) or lactation, or a plan to become pregnant, (h) moderate or high suicide risk, (i) Shipley IQ (vocabulary standard score) < 80, (j) prescription medications, and (k) hormonal contraception.
At the screening visit, study participants’ anthropomorphic measures were taken. Once enrolled and begun their subsequent menstrual cycle, study participants completed DRSP, as described in the next section.
Study Measures
Food Cravings. The Daily Record of Severity of Problems (DRSP) (29) is a validated questionnaire which measures 24 symptoms of PMS/PMDD (affective, psychological, behavioral, and functional). The symptoms are rated on a scale of 1 (not at all) to 6 (extreme). The present analysis evaluated ratings of food craving (“Had cravings for specific foods”). Once enrolled, study participants notified the research coordinator when their next menstrual cycle started. Upon notification, the research coordinator set up the DRSP surveys to be sent out daily for the total of 2 menstrual cycles (3 menstrual cycles if the participant chose to complete the third menstrual cycle procedures). Study participants received a new survey link every day and were asked to complete it between 7 PM and midnight. They were also asked to use the day’s link and not any previous links to minimize retrospective reporting. In case a participant missed more than two DRSP entries in a row, or four or more in a month, the research coordinator contacted the participant to complete the survey daily.
Anthropomorphic Measures. Participants removed their shoes, hats, or any head gear when their height was measured using a standard measuring board with a moveable stadiometer. They removed outer layer of clothing and shoes prior to stepping on the digital scale for weight measurement. When measuring waist circumferences, participants were asked to cross their arms and place them on opposite shoulders. Waist circumference was measured by palpating the hip area to locate the right ilium of pelvis and positioning the tape in a horizontal place at the level of the measurement mark. The measurements were made with the tape held snugly, but not constricting, and at a level parallel to the floor.
Diagnostic Criteria
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) lists food craving as one of the symptoms (out of the total 11) for premenstrual dysphoric disorder (PMDD) and premenstrual syndrome (PMS) diagnoses. The 11 symptoms may be affective, cognitive, behavioral, or physical in nature. Premenstrual Dysphoric Disorder (PMDD) requires presence of at least one affective symptom to reach the total of five required symptoms, which must be present in most cycles from the past year and confirmed in a prospective manner for at least two menstrual cycles. In addition, the symptoms have to be associated with clinically significant distress or interference with work, school, usual social activities, or relationship with others. Premenstrual syndrome requires presence of 1–4 symptoms, without the requirement that one must be affective in nature.
Statistical Analysis
All analyses were performed in R software (version 4.0.2) (34). To compute premenstrual increase in food cravings, we averaged food craving ratings from the fifth to the tenth day from the start of the menstrual cycle and subtracted it from the average food craving ratings 5 days prior to thru and including the first day of the menstrual cycle. This value was then divided by a participant-specific variance across the entire menstrual cycle, yielding the participant-specific effect size (14). An effect size greater than 1.0 reflects presence of a particular PMS symptom (14).
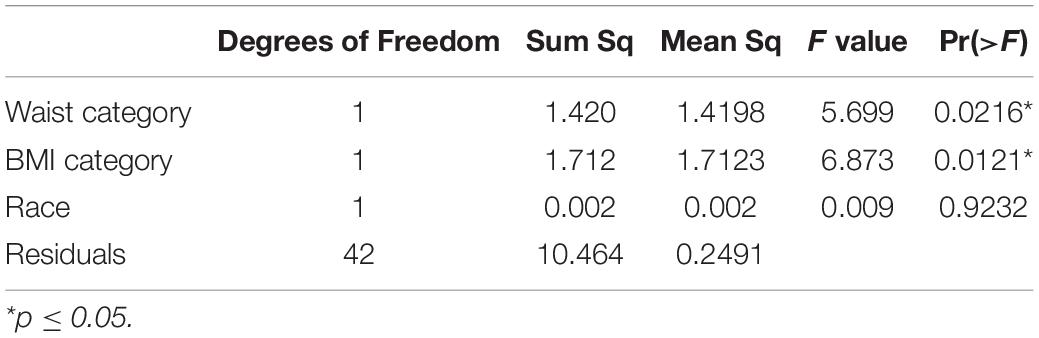
We first assessed premenstrual food craving score for normality using the Shapiro–Wilk normality test. We categorized waist circumference as “normal” vs. “obese,” based on the 80-centimeter cutoff according to the International Diabetes Federation consensus guideline (35). BMI was analyzed as a categorical variable (≤ 24.9 “underweight/normal” and (≥ 24.9 “overweight/obese”). Race was analyzed as “White” vs. non-White.
The groups were compared on demographic characteristics using chi square tests for categorical, and independent 2-group t-test for continuous variables. We then assessed whether the waist circumference groups differed with respect to premenstrual food cravings. For this, we used the Analysis of Variance, with premenstrual food craving as the outcome and waist circumference (normal vs. obese) as the predictor followed by Tukey Honestly Significant Differences for the post hoc comparisons. The model was checked for homogeneity of variance (using the Levene Test) and normality of residuals’ distribution (using the Shapiro Test). In the next step (Analysis of Covariance), additional variables were added: BMI and race (White vs. non-White). The significance value was set at ≤ 0.05. For descriptive purposes, we also reported percent increases in premenstrual food cravings for abdominally obese vs. normal study groups. Additionally, for interpretability, we report analysis of variance and analysis of covariance results on premenstrual increase (5 days prior to thru and including the first day of the menstrual cycle) relative to the fifth to the tenth day from the start of the menstrual cycle, without adjustment for variance across the menstrual cycle.
Results
Study Participants
Forty-six participants, approximately 26 years old on average, contributed data for the present study. Based on the prospective rating of symptoms using the DRSP, twenty-six participants were in the normal group and twenty participants were in the obese group. Seven study participants met the diagnosis for PMDD, 19 for PMS, and 20 were healthy. The effect size and standard deviation for food craving ratings were 0.63 and 0.55. The participant sample was mostly White and Asian of non-Hispanic ethnicity. Approximately half of the participants were students. The average age of menarche was 12 years old. Table 1 lists demographic and anthropomorphic characteristics of study participants according to the group category. Study groups did not differ on any demographic characteristics or age of menarche.
Association Between Food Craving and Waist Circumference
The effect size means and standard deviations for food cravings were 0.47 (0.51) and 0.82 (0.53) for the abdominally normal and obese groups, respectively. Waist circumference category was significantly associated with premenstrual food cravings [F(1,44) = 5.12, p = 0.028]. Post hoc comparisons using the Tukey HSD test (95% family-wise confidence level) indicated that the mean score for the food craving effect size was 0.35 higher for the abdominally obese vs. normal study groups (95% CI: 0.039 to 0.67, p-adjusted = 0.028) (Figure 1). Table 2 lists the ANOVA model details. The Levene’s Test for Homogeneity of Variance (center = median) showed that the study groups had similar variance [F(1,43) = 0.07, p = 0.79]. The Shapiro–Wilk normality test showed that residuals had normal distribution (W = 0.99, p = 0.96).
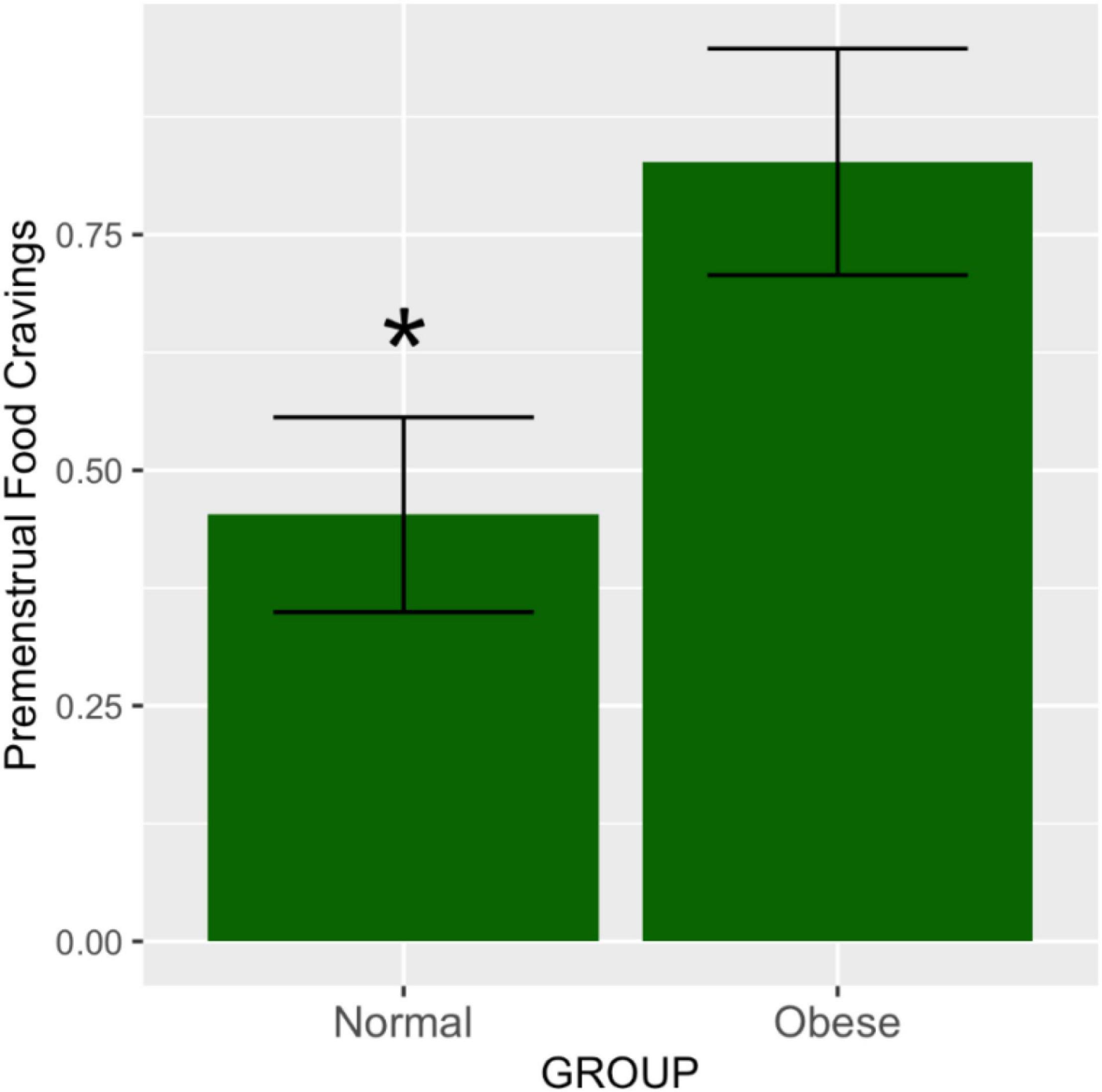
Figure 1. Premenstrual food cravings according to abdominal obesity groups. The means and SEM represent food cravings scores in the premenstrual vs. the mid-follicular subphase, adjusted for total variance across the entire menstrual cycle. As demonstrated by the ANOVA test, the obese group had significantly higher ratings of food cravings in the premenstruum compared to the non-obese group (p = 0.02). *p ≤ 0.05.
In the ANCOVA model, waist circumference category was significantly associated with premenstrual food cravings [F(1,41) = 5.10, p = 0.029]. Post hoc comparisons using the Tukey HSD test (95% family-wise confidence level) indicated that the mean score for the food craving effect size was 0.35 higher for the abdominally obese vs. normal study groups (95% CI: 0.05 to 0.65; p-adjusted = 0.021). Participants’ BMI was also associated with premenstrual food cravings [F(1,41) = 6.87, p = 0.012], however, this significance did not pass the adjustment (p-adjusted = 0.085). Table 3 lists the ANCOVA model details. The Shapiro–Wilk normality test showed that residuals had normal distribution (W = 0.99, p = 0.98).
In relative terms, the mean and standard deviation percent change in premenstrual food cravings was 37.33 (41.43) for the normal group and 80.16 (84.47) for the abdominally obese group. Table 4 shows results of analysis of variance (and analysis of covariance) evaluating mean scores for the two groups.
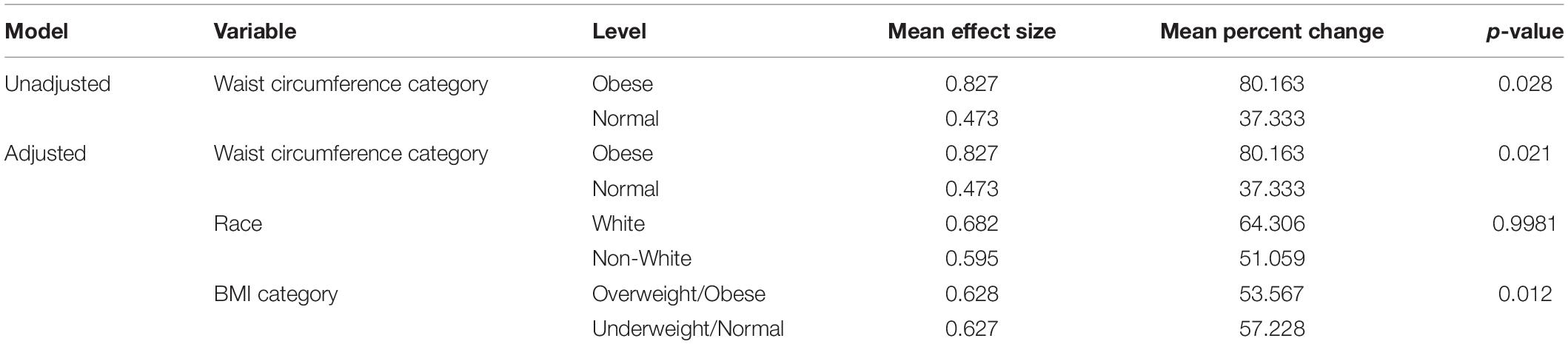
Table 4. Mean Effect Size and Percent Change of Premenstrual Food Craving Increase for the Normal and Obese Study Groups (Unadjusted and Adjusted Models).
Discussion
In accordance with our hypothesis, the present study shows a positive association between premenstrual food craving and waist circumference. This association remained significant even after adjusting for BMI, suggesting a particularly dangerous central fat accumulation. Though preliminary in nature, this is the first study to link food craving in the premenstruum with central adiposity.
Changing sex hormones in the luteal phase are associated with decreased levels of amino acids and lipid species, presenting a physiological milieu of heightened energy requirement (36–41) and indicative of an anabolic state (41, 42). The amino acid decrease likely reflects the effect of rising progesterone levels in the luteal phase. Progesterone upregulates cell cycle and growth, resulting in protein biosynthesis for endometrial thickening (43). Therefore, women may feel an increase in food craving during this time to satisfy this underlying energy expenditure. In one study (41), as many as 39 amino acids were suppressed in the luteal phase. A potential relationship between premenstrual amino acid levels, food craving, eating patterns and central adiposity should be investigated in future studies. Understanding which amino acid suppression is potentially linked to heightened premenstrual food craving may provide a basis for a targeted intervention development.
Alternatively, the relationship observed in the present study is driven by increased food intake as a means of affect regulation in the premenstruum, though this hypothesis should be tested. Reward-driven behaviors are goal-oriented changes and are based on past experiences as well as positive associations we may make with food (44, 45). If eating provides a euphoric experience, the behavior to consume this food will be reinforced and repeated. Therefore, craving, operationalized as appetitive motivation, can be portrayed as the amount of work and effort an individual is willing to perform to obtain a type of food. Our finding possibly reflects deficiency in reward-driven behaviors in a segment of women with PMS/PMDD, who score high on reward dependence (46), show low positive affect (47, 48) as well as low frontal electroencephalography (49–51), which are associated with deficient reward processing (52, 53).
Temporal behavioral interventions should be developed in the event subsequent studies demonstrate that the association observed in the present study is mediated via an increase in food intake. Reducing central adiposity in reproductive age women would have a population-level impact, as interventions would improve not only their health and well-being, but also that of their offspring (54). However, unless carefully designed, such efforts may fail. Generic interventions are generally not successful in reproductive age women, as evidenced by lower recruitment and retention rates, inferior attendance and compliance, and worse weight loss outcomes in comparison to older adult participants (55).
There is some, though limited, evidence that individualized treatment in reproductive age women is effective. For example, Eiben et al. (56) targeted diet, exercise, and behavior change utilizing face-to-face contact followed by ongoing regular telephone and e-mail contact for 1 year (56), and showed reduced central adiposity. Levitskey and colleagues (57) showed that a minimal intervention, where young women weighed themselves daily for 10 weeks and were provided with feedback on energy needs for weight maintenance, resulted in weight stability compared with controls who gained weight. However, long-term evaluation of these or other behavioral interventions in reproductive age women is not known (58) and no studies to date incorporated premenstrual behavioral changes related to food cravings. Results of our study provide compelling evidence that an intervention designed to specifically target premenstrual cravings may result in an efficacious reduction of central adiposity given that food cravings may be conditioned by interventions (59–64).
By calculating effect size for premenstrual food craving, our goal was to emphasize its importance as a clinical problem due to its link to central adiposity. We show that nutrient craving is affected by changing sex hormones between menstrual phases, which may predispose a segment of women to high nutritional needs. The mean effect size for premenstrual food craving in our study was 0.69, while in the study by Hartlage et al. (14), it was 0.29 for the community sample and 0.96 for the PMDD clinical sample. The effect size difference in our study is likely due to our advertising strategy, which asked “Do you have PMS?,” drawing a sample of participants with PMS/PMDD symptomatology from the community pool.
The present study is the first to identify a segment of reproductive age women who have an adverse health marker (waist circumference) related to premenstrual food craving. The findings of our study are preliminary in nature, and future evaluations should be completed in larger participant samples. If replicated, the finding of the present study provides a platform for further novel nutrition interventions, with an emphasis on improved health and well-being in this at-risk population.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
This study protocol was reviewed and approved by the Office for the Protection of Research Subjects (OPRS) at the University of Illinois at Chicago, approval number 2018-1533. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
ND analyzed the study data. DK, AN, and FS assisted with the manuscript preparation and submission. JS provided the statistical consulting. AH designed the study, analyzed the study data, and wrote the initial and final manuscript versions. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Institute of Mental Health/National Institutes of Health, grant number R21MH119642.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank all the individuals who provided their expertise and assistance throughout our study. We would also like to thank the subjects who provided the data for this research study.
References
1. Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19 2 million participants. Lancet. (2016) 387:1377–96. doi: 10.1016/S0140-6736(16)30054-X
2. Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. (2020) 16:177–89. doi: 10.1038/s41574-019-0310-7
3. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. New Engl J Med. (2008) 359:2105–20. doi: 10.1056/NEJMoa0801891
4. Cerhan JR, Moore SC, Jacobs EJ, Kitahara CM, Rosenberg PS, Adami HO, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. (2014) 89:335–45. doi: 10.1016/j.mayocp.2013.11.011
5. Zhang C, Rexrode KM, Van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality – sixteen years of follow-up in US women. Circulation. (2008) 117:1658–67. doi: 10.1161/CIRCULATIONAHA.107.739714
6. Song X, Jousilahti P, Stehouwer CDA, Soderberg S, Onat A, Laatikainen T, et al. Comparison of various surrogate obesity indicators as predictors of cardiovascular mortality in four European populations. Eur J Clin Nutr. (2013) 67:1298–302. doi: 10.1038/ejcn.2013.203
7. Lear S, James P, Ko G, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. (2010) 64:42–61. doi: 10.1038/ejcn.2009.70
8. Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, Loos RJF, et al. Obesity. Nat Rev Dis Primers. (2017) 3:17034.
9. Bijari M, Jangjoo S, Emami N, Raji S, Mottaghi M, Moallem R, et al. The accuracy of visceral adiposity index for the screening of metabolic syndrome: a systematic review and meta-analysis. Int J Endocrinol. (2021) 2021:6684627. doi: 10.1155/2021/6684627
10. Dutton GR, Kim Y, Jacobs DR Jr., Li X, Loria CM, Reis JP, et al. 25-year weight gain in a racially balanced sample of US adults: the CARDIA study. Obesity. (2016) 24:1962–8. doi: 10.1002/oby.21573
11. Norman JE, Bild D, Lewis CE, Liu K, West DS. The impact of weight change on cardiovascular disease risk factors in young black and white adults: the CARDIA study. Int J Obes. (2003) 27:369–76. doi: 10.1038/sj.ijo.0802243
12. Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity. (2010) 18:1801–4. doi: 10.1038/oby.2009.451
13. Yen JY, Liu TL, Chen IJ, Chen SY, Ko CH. Premenstrual appetite and emotional responses to foods among women with premenstrual dysphoric disorder. Appetite. (2018) 125:18–23. doi: 10.1016/j.appet.2018.01.029
14. Hartlage SA, Freels S, Gotman N, Yonkers K. Criteria for premenstrual dysphoric disorder secondary analyses of relevant data sets. Arch Gen Psychiatry. (2012) 69:300–5. doi: 10.1001/archgenpsychiatry.2011.1368
15. Hill AJ. Symposium on ‘molecular mechanisms and psychology of food intake’ – the psychology of food craving. Proc Nutr Soc. (2007) 66:277–85. doi: 10.1017/s0029665107005502
16. Weingarten HP, Elston D. The phenomenology of food cravings. Appetite. (1990) 15:231–46. doi: 10.1016/0195-6663(90)90023-2
17. McVay MA, Copeland AL, Geiselman PJ. Eating disorder pathology and menstrual cycle fluctuations in eating variables in oral contraceptive users and non-users. Eat Behav. (2011) 12:49–55. doi: 10.1016/j.eatbeh.2010.11.005
18. Rozin P, Levine E, Stoess C. Chocolate craving and liking. Appetite. (1991) 17:199–212. doi: 10.1016/0195-6663(91)90022-k
19. Hill AJ, Heatonbrown L. The experience of food craving – a prospective investigation in healthy women. J Psychosom Res. (1994) 38:801–14. doi: 10.1016/0022-3999(94)90068-x
20. Zellner DA, Garriga-Trillo A, Centeno S, Wadsworth E. Chocolate craving and the menstrual cycle. Appetite. (2004) 42:119–21. doi: 10.1016/j.appet.2003.11.004
21. Alonso-Alonso M, Ziemke F, Magkos F, Barrios FA, Brinkoetter M, Boyd I, et al. Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: relation to fasting and feeding. Am J Clin Nutr. (2011) 94:377–84. doi: 10.3945/ajcn.110.010736
22. Arnoni-Bauer Y, Bick A, Raz N, Imbar T, Amos S, Agmon O, et al. Is it me or my hormones? Neuroendocrine activation profiles to visual food stimuli across the menstrual cycle. J Clin Endocrinol Metab. (2017) 102:3406–14. doi: 10.1210/jc.2016-3921
23. van Vugt DA. Brain imaging studies of appetite in the context of obesity and the menstrual cycle. Hum Reprod Update. (2010) 16:276–92. doi: 10.1093/humupd/dmp051
24. Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res. (2010) 1363:81–92. doi: 10.1016/j.brainres.2010.09.071
25. Bertone-Johnson ER, Hankinson SE, Willett WC, Johnson SR, Manson JE Adiposity and the development of premenstrual syndrome. J Womens Health (Larchmt). 2010, 19:1955–62. doi: 10.1089/jwh.2010.2128
26. Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CDA, Kostense PJ, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the hoorn study. Am J Clin Nutr. (2003) 77:1192–7. doi: 10.1093/ajcn/77.5.1192
27. Jacobs EJ, Newton CC, Wang Y, Patel AV, McCullough ML, Campbell PT, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. (2010) 170:1293–301. doi: 10.1001/archinternmed.2010.201
28. Taetzsch A, Roberts SB, Gilhooly CH, Lichtenstein AH, Krauss AJ, Bukhari A, et al. Food cravings: associations with dietary intake and metabolic health. Appetite. (2020) 152:104711. doi: 10.1016/j.appet.2020.104711
29. Endicott J, Nee J, Harrison W. Daily record of severity of problems (DRSP): reliability and validity. Arch Womens Ment Health. (2006) 9:41–9. doi: 10.1007/s00737-005-0103-y
30. Gingnell M, Bannbers E, Wikstrom J, Fredrikson M, Sundstrom-Poromaa I. Premenstrual dysphoric disorder and prefrontal reactivity during anticipation of emotional stimuli. Eur Neuropsychopharmacol. (2013) 23:1474–83. doi: 10.1016/j.euroneuro.2013.08.002
31. Deng D, Pang Y, Duan G, Liu H, Liao H, Liu P, et al. Larger volume and different functional connectivity of the amygdala in women with premenstrual syndrome. Eur Radiol. (2018) 28:1900–8. doi: 10.1007/s00330-017-5206-0
32. Solis-Ortiz S, Corsi-Cabrera M. Sustained attention is favored by progesterone during early luteal phase and visuo-spatial memory by estrogens during ovulatory phase in young women. Psychoneuroendocrinology. (2008) 33:989–98. doi: 10.1016/j.psyneuen.2008.04.003
33. Sohda S, Suzuki K, Igari I. Relationship between the menstrual cycle and timing of ovulation revealed by new protocols: analysis of data from a self-tracking health app. J Med Internet Res. (2017) 19:e391. doi: 10.2196/jmir.7468
34. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2020).
35. International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels: IDF (2006). p. 1–23.
36. Wallace M, Hashim YZHY, Wingfield M, Culliton M, McAuliffe F, Gibney MJ, et al. Effects of menstrual cycle phase on metabolomic profiles in premenopausal women. Hum Reprod. (2010) 25:949–56. doi: 10.1093/humrep/deq011
37. Fong AKH, Kretsch MJ. Changes in dietary-intake, urinary nitrogen, and urinary volume across the menstrual-cycle. Am J Clin Nutr. (1993) 57:43–6. doi: 10.1093/ajcn/57.1.43
38. Lariviere F, Moussalli R, Garrel DR. Increased leucine flux and leucine oxidation during the luteal-phase of the menstrual-cycle in women. Am J Physiol. (1994) 267:E422–8. doi: 10.1152/ajpendo.1994.267.3.E422
39. Moller SE, MaachMoller B, Olesen M, Fjalland B. Effects of oral contraceptives on plasma neutral amino acids and cholesterol during a menstrual cycle. Eur J Clin Pharmacol. (1996) 50:179–84. doi: 10.1007/s002280050089
40. Kriengsinyos W, Wykes LJ, Goonewardene LA, Ball RO, Pencharz PB. Phase of menstrual cycle affects lysine requirement in healthy women. Am J Physiol Endocrinol Metab. (2004) 287:E489–96. doi: 10.1152/ajpendo.00262.2003
41. Draper CF, Duisters K, Weger B, Chakrabarti A, Harms AC, Brennan L, et al. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. (2018) 8:14568.
42. Faustmann G, Meinitzer A, Magnes C, Tiran B, Obermayer-Pietsch B, Gruber HJ, et al. Progesterone-associated arginine decline at luteal phase of menstrual cycle and associations with related amino acids and nuclear factor kB activation. PLoS One. (2018) 13:e0200489. doi: 10.1371/journal.pone.0200489
43. Strauss J, Barbieri R. Yen & Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. 7th ed. Amsterdam: Elsevier (2014).
44. Rogers PJ, Smit HJ. Food craving and food “addiction”: a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav. (2000) 66:3–14. doi: 10.1016/s0091-3057(00)00197-0
45. Avitia GC. Cravings, Sugar and fat Consumption as Determinant Factors of Obesity in Young Adults in Juarez City. Juárez: Instituto de Ciencias Sociales y Administración (2018).
46. Freeman EW, Schweizer E, Rickels K. Personality-factors in women with premenstrual-syndrome. Psychosom Med. (1995) 57:453–9. doi: 10.1097/00006842-199509000-00007
47. Rubinow DR, Roybyrne P, Hoban MC, Grover GN, Stambler N, Post RM. Premenstrual mood changes – characteristic patterns in women with and without premenstrual-syndrome. J Affect Disord. (1986) 10:85–90.
48. Metcalf MG, Livesey JH. Distribution of positive moods in women with the premenstrual-syndrome and in normal women. J Psychosom Res. (1995) 39:609–18. doi: 10.1016/0022-3999(94)00167-7
49. Liu P, Yu Y, Gao S, Sun J, Yang X, Liu PA, et al. Structural integrity in the genu of corpus callosum predicts conflict-induced functional connectivity between medial frontal cortex and right posterior parietal cortex. Neuroscience. (2017) 366:162–71. doi: 10.1016/j.neuroscience.2017.10.017
50. Sutton SK, Davidson RJ. Prefrontal brain electrical asymmetry predicts the evaluation of affective stimuli. Neuropsychologia. (2000) 38:1723–33. doi: 10.1016/s0028-3932(00)00076-2
51. Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness – a source-localization study. Psychol Sci. (2005) 16:805–13. doi: 10.1111/j.1467-9280.2005.01618.x
52. Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. (2003) 26:507–13. doi: 10.1016/s0166-2236(03)00233-9
53. Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal detection approach. Biological Psychiatry. (2005) 57:319–27. doi: 10.1016/j.biopsych.2004.11.026
54. Mccaffery J, Jablonski KA, Pan Q, Astrup A, Christiansen MR, Corso LM, et al. 250-OR: higher genetic risk score predicts smaller waist circumference change over one year across five lifestyle intervention clinical trials. Am Diabetes Assoc. (2021) 70:250–OR.
55. Gokee-LaRose J, Gorin AA, Wing RR. Behavioral self-regulation for weight loss in young adults: a randomized controlled trial. Int J Behav Nutr Phys Act. (2009) 6:10. doi: 10.1186/1479-5868-6-10
56. Eiben G, Lissner L. Health Hunters – an intervention to prevent overweight and obesity in young high-risk women. Int J Obes. (2006) 30:691–6. doi: 10.1038/sj.ijo.0803167
57. Levitsky DA, Garay J, Nausbaum M, Neighbors L, DellaValle DM. Monitoring weight daily blocks the freshman weight gain: a model for combating the epidemic of obesity. Int J Obes. (2006) 30:1003–10. doi: 10.1038/sj.ijo.0803221
58. Hutchesson MJ, Hulst J, Collins CE. Weight management interventions targeting young women: a systematic review. J Acad Nutr Diet. (2013) 113:795–802. doi: 10.1016/j.jand.2013.01.015
59. Anton SD, Gallagher J, Carey VJ, Laranjo N, Cheng J, Champagne CM, et al. Diet type and changes in food cravings following weight loss: findings from the pounds lost trial. Eat Weight Disord. (2012) 17:E101–8. doi: 10.1007/BF03325333
60. Batra P, Das SK, Salinardi T, Robinson L, Saltzman E, Scott T, et al. Relationship of cravings with weight loss and hunger. Results from a 6 month worksite weight loss intervention. Appetite. (2013) 69:1–7. doi: 10.1016/j.appet.2013.05.002
61. Buscemi J, Rybak TM, Berlin KS, Murphy JG, Raynor HA. Impact of food craving and calorie intake on body mass index (BMI) changes during an 18-month behavioral weight loss trial. J Behav Med. (2017) 40:565–73. doi: 10.1007/s10865-017-9824-4
62. Gilhooly CH, Das SK, Golden JK, McCrory MA, Dallal GE, Saltzman E, et al. Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes. (2007) 31:1849–58. doi: 10.1038/sj.ijo.0803672
63. Martin CK, O’Neil PM, Pawlow L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity. (2006) 14:115–21. doi: 10.1038/oby.2006.14
Keywords: central obesity, food craving, premenstrual dysphoric disorder (PMDD), premenstrual syndrome (PMS), body mass index (BMI)
Citation: Dang N, Khalil D, Sun J, Naveed A, Soumare F and Hamidovic A (2022) Waist Circumference and Its Association With Premenstrual Food Craving: The PHASE Longitudinal Study. Front. Psychiatry 13:784316. doi: 10.3389/fpsyt.2022.784316
Received: 28 September 2021; Accepted: 04 April 2022;
Published: 27 April 2022.
Edited by:
Ahmed El-Sohemy, University of Toronto, CanadaReviewed by:
Gaoxiong Duan, Guangxi University, ChinaBibiana Garcia-Bailo, University of Toronto, Canada
Copyright © 2022 Dang, Khalil, Sun, Naveed, Soumare and Hamidovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ajna Hamidovic, YWhhbWlkb3ZAdWljLmVkdQ==
 Nhan Dang1
Nhan Dang1 Dina Khalil
Dina Khalil Jiehuan Sun
Jiehuan Sun Ajna Hamidovic
Ajna Hamidovic