- 1Department of Psychiatry, College of Medicine and Health Science, Bahir Dar University, Bahir Dar, Ethiopia
- 2School of Public Health, College of Medicine and Health Science, Bahir Dar University, Bahir Dar, Ethiopia
Background: Neurocognitive impairment is associated with psychological morbidities, such as depression and anxiety, among people living with HIV. The presence of these comorbidities affects viral load suppression, treatment adherence, quality of life, treatment outcomes, and functionality. Despite this fact, there is a dearth of studies that examined the triple burden of neurocognitive impairment and co-occurring depression and anxiety among antiretroviral therapy attendees in Ethiopia. This study aimed to assess the magnitude of HIV-associated neurocognitive impairment and co-occurring depression and anxiety at the same time among people living with HIV/AIDS.
Method: We conducted an institution-based multicenter cross-sectional study in Bahir Dar, Northwest Ethiopia. A total of 410 study participants were selected using a systematic random sampling technique. Neurocognitive impairment was assessed using the International HIV Dementia Scale. Co-occurring depression and anxiety were assessed using the Hospital Anxiety and Depression Scale. A semi-structured questionnaire was applied to collect data on sociodemographic and clinical-related characteristics. Data were analyzed using descriptive statistics and univariate and multivariable logistic regression.
Results: Two-thirds (66.8%) of the people living with HIV had neurocognitive impairment. The prevalence of co-occurring depression and anxiety was found in 39.8%. Women with HIV, people with comorbid chronic medical illness, and those under a second-line treatment regimen were factors associated with neurocognitive impairment. Furthermore, pill burden, second-line treatment regimen, HIV clinical stages, social support, HIV-perceived stigma, and neurocognitive impairment were associated factors with co-occurring depression and anxiety.
Conclusions: We found a high prevalence of neurocognitive impairment and co-occurring depression and anxiety among people living with HIV/AIDs. Further research is needed to assess the clinical course of neurocognitive impairment and co-occurring depression and anxiety.
Introduction
Despite the decrement in the prevalence of human immunodeficiency virus (HIV), the Sub-Saharan region still accounts for 59% of new HIV infections globally according to the Joint United Nations Program on HIV/AIDS (UNAIDS). In the same report, of among the 38 million people living with HIV, about 21 million (55%) live in Eastern and Southern Africa (1).
Ethiopia is one of the Sub-Saharan nations that have been suffering from HIV/AIDS. In 2019, there were 12,000 AIDS-related deaths and 15,000 new HIV infections, and the total number of people living with HIV was about 670,000. The estimated prevalence had a slight decrement from 1.4% (1–1.8%) in 2010 to.9% (0.7–1.2%) in 2019 (1–3).
Neurocognitive impairment is described as a deficit in cognitive abilities that include learning, attention, concentration, memory, executive function, decision-making skills, and problem-solving ability (4). Because of HIV infection, the Frascati criteria described three categories of HIV-associated neurocognitive disorder (HAND): asymptomatic neurocognitive impairment, mild neurocognitive disorder, and HIV-associated dementia (5).
The HIV-associated neurocognitive disorder (HAND) remains a major problem that lowers the quality of life and increases the public health burden among people living with HIV (6). Currently, the wide use of highly active antiretroviral treatment has decreased the prevalence, in particular, of the severe form of HIV-associated dementia (7).
People living with HIV experience higher rates of mental health disorders, particularly depression and anxiety, than the general population (8). Depression is characterized as the persistence of low mood associated with changes in appetite (weight), sleep pattern, and motor activity. In addition, poor concentrations, excessive tiredness, and a feeling of worthlessness that can even lead to self-harm are accounted for depression. Anxiety is a disorder when a person is unable to control his/her excessive worries, fears, or panic state, and when these symptoms are experienced frequently (9, 10).
HIV/AIDS and mental health have a direct relationship. People living with HIV can develop a mental illness at a higher rate or vice versa (4). For instance, people living with mental illness are at higher risk for HIV (11) due to low access to information, injectable drugs use, having multiple sexual partners, sexual abuse, and unprotected sex (12). Also, the presence of such psychiatric disorders negatively affects treatment adherence, viral load suppression, quality of life, treatment outcomes, and functionality of people living with HIV (13). The worst outcome of comorbid psychiatric disorders is significantly associated with excess mortality among adults (14).
Neurocognitive impairment associated with psychological morbidity (anxiety or depression) among people living with HIV poses complications, worse quality of life, and mortality (15). Therefore, it is very important to undertake a study focusing on neurocognitive impairment, depression, and anxiety among people living with HIV. The main objective of this study was to determine the proportions of neurocognitive impairment and co-occurring depression and anxiety, as well as its associated factors among people living with HIV. It is also in line with the global AIDS strategy (2021–2026) set by the UNAIDS to break down barriers to HIV outcomes. In addition, it can help to plan different intervention packages for people living with HIV.
Methods
Study Design and Period
An institution-based multicenter cross-sectional study was conducted from March to April 2016.
Study Area and Population
This study was conducted in public health institutions in Bahir Dar. Bahir Dar is the capital city of the Amhara regional state, which is located in Northwest Ethiopia and is around 490 km from Addis Ababa, the capital city of Ethiopia. According to the 2007 Census conducted by the central statistical agency of Ethiopia, Bahir Dar Special Zone had a total population of 221,991, of whom 108,456 were men and 113,535 were women. From a total of 8,293,734 screened people from the years 2015 to 2018 in the Amhara region, 57,293 new HIV infections were reported, with an overall incidence rate of 6.9 per 1,000 population, which is the highest. The second highest incidence rate was reported in Bahir Dar (4.27 per 1,000 population) next to Dessie Town (5.74 per 1,000 population) in the Amhara region (16).
During the study period, there were two public hospitals, ten health centers (HCs), and many other private health institutions (clinics, hospitals, and pharmacies). The study was carried out in all public health institutions that provide antiretroviral therapy (ART) services in the city.
Patients with HIV/AIDS who have been on ART follow-up in public institutions in Bahir Dar city were the source population. The study population was composed of people living with HIV who had been attending their treatment in public health institutions in Bahir Dar during the study period.
Inclusion and Exclusion Criteria
All people aged between 18 and 64 years who had follow-up for ART medication were eligible. Patients with severe medical and psychiatric (impaired insight) illnesses at the time of data collection, intellectual disability, and upper limb amputation or defects were excluded from the study.
Sample Size and Sampling Procedures
We used the single population proportion formula n = (zα/2)2 p (1-p)/d2) (17) to determine the sample size with the following assumptions: 95% confidence interval (CI) (Zα/2 = 1.96), 5% margin of error, 36.4% proportion of HIV-associated neurocognitive impairment among HIV/AIDS patients from the previous study (18) and 15% contingency for non-response rate. Based on these assumptions, 410 study participants were selected for the study.
The study participants were selected using a systematic random sampling technique. For each health institution, a proportional allocation was carried out to get the demanded sample size based on the number of people living with HIV/AIDS who attended each health institution. Finally, the required sample in each health institution was enrolled through an exit interview.
Data Collection Tools and Procedures
Data were collected using a semi-structured questionnaire that consisted of sociodemographic characteristics, clinical factors, and psychosocial variables. HIV stages stated by the World Health Organization (WHO), CD4 counts, co-morbid chronic medical diseases, total daily pill burden, and treatment regimen were collected by reviewing patients' charts.
The International HIV Dementia Scale (IHDS) was designed for use in resource-limited settings as a screening tool for HIV-associated dementia under different cultural, linguistic, and educational conditions. The scale evaluates memory, motor, and psychomotor speed without literacy-dependent tests. The maximum score of the tool is 12, with a higher score indicating better functioning (19). The IHDS is the most common HIV-associated neurocognitive disorder (HAND) screening tool used in many African countries including South Africa, Uganda, and Cameroon (20–23). In addition, most recent systematic reviews and meta-analyses show that the IHDS has fair diagnostic accuracy in all forms of HAND (asymptomatic neurocognitive disorder, symptomatic neurocognitive, and HIV-associated dementia) (24). The presence of neurocognitive impairment was explained by the sum of a 3-item IHDS (timed finger tapping test, alternating hand sequence test, and memory-recall test) cutoff score of ≤ 10 in different studies (19, 25). Therefore, this study used the same cutoff score to screen for neurocognitive impairment.
The Hospital Anxiety and Depression Scale (HADS) has been extensively used as a screening tool for depression and anxiety in both community and hospital settings. The advantage of the HADS is focused on psychological symptoms (can assess symptoms of anxiety and depression). The scale comprises 14 items, each is rated from 0 to 3 according to the severity of difficulty experienced (26, 27). It can be separated into two 7-item sub-scales for depression (HAD-D) and anxiety (HAD-A). The scales used a cutoff score of ≥ 8 for both HADS-A and HADS-D (28). In addition, social support was assessed using the Oslo-3 item (29). An 11-item scale was used to assess HIV-perceived stigma (30). Non-adherence was measured using a medication adherence scale that comprises 8 items with a dichotomous patient response (yes/no), and a patient missing at least one or more items on the scale was classified under poor adherence (31, 32).
Data Quality Management
Two days of training were given to 10 data collectors (nurses) and one supervisor (BSc nurse) on how to use tools and approach the participants. The questionnaire was translated into the local language (Amharic) and then translated back into English for consistency. To see the understandability of the questionnaire, a pre-test was conducted on 5% of the sample size 1 week before the actual data collection among people living with HIV/AIDS.
Respondents were interviewed in their local language. Besides, the supervisor, the principal investigator carried out day-to-day strict supervision during the entire period of data collection. The supervisor checked the collected data for completeness, consistency, and accuracy.
Data Management and Analysis
The collected data were entered into a computer using Epidata 3.1 and exported to SPSS software package version 26 for analysis. For all variables, cross-checking and data cleaning were carried out by running frequencies. Relevant variables of the study participants were described using percentage, frequency, and mean. First, cross-tabulation and bivariate logistic regression were conducted for each potentially explanatory variable using crude odds ratio and 95% confidence interval (CI). Variables that satisfied p < 0.2 were taken for further analysis into the multivariate logistic regression model to control confounding effects. Finally, P < 0.05 in the multivariable analysis was considered statistically significant.
Ethical Consideration
Ethical clearance was obtained from the Research Ethical Review Board (IRB) of Bahir Dar University, College of Medicine and Health Sciences. Then, the ethical clearance was submitted to Amhara National Regional State Health Bureau. The regional health bureau wrote permission and a supporting letter for each study site before data collection.
After a clear explanation of the purpose of the study, written informed consent was obtained from each study participant during data collection. The participants were given the right to refuse to participate as well as to withdraw at any time during the study. Refusal to participate did not result in loss of medical care provided or any other benefits. The data collectors linked participants who were screened and were found to have severe anxiety and depression to the psychiatric clinic. They maintained privacy and confidentiality throughout the study by interviewing the patients alone and using a code instead of a name.
Results
Sociodemographic Characteristics
Four hundred people living with HIV/AIDS were involved in the study, with a response rate of 96%. The mean age of the respondents was 36.3 ± 9.7 years. Around 58.3% of the respondents were women, and most (80.5%) of them were from urban areas. About two-thirds (63.1%) of them have educational status below secondary school (Table 1).
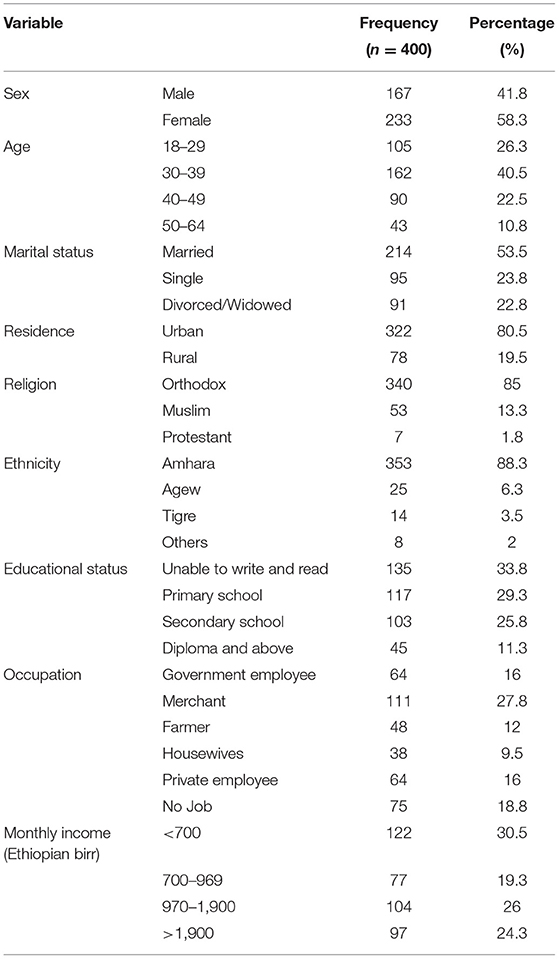
Table 1. Distribution of participants by socio-demographic characteristics of the government health institution in Bahir Dar, 2016.
Clinical and Psychosocial Characteristics
The median duration of illness since the first HIV test was 5 years [interquartile range (IQR) 3–8 years], while the median duration of antiretroviral therapy follow-up was 4 years (IQR 2–7 years). Almost one-fifth (18.3%) of the participants had a comorbid medical illness at the time of data collection: diabetes mellitus (8.3%), tuberculosis (6.3%), epilepsy (1.3%), and other chronic medical diseases (5.5%). Based on the most recent CD4 counts from the chart review, greater than half (56.8%) of the participants had CD4 counts of 500 cells/μl or less; among them, 69.5% had opportunistic infections (Table 2).
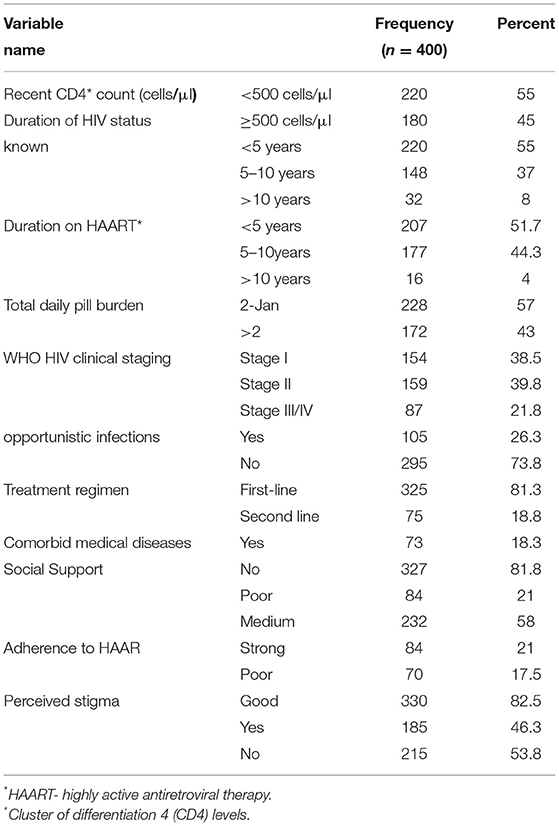
Table 2. Distribution of PLH by their clinical status at government health institution in Bahir Dar, Ethiopia, 2016.
HIV-Associated Neurocognitive Impairment and Co-occurring Anxiety and Depression
Two-thirds (66.8%, 95% CI: 62–71.5%) of the people living with HIV had HIV-associated neurocognitive impairment. The first assessment with the IHDS was a timed finger tapping test, and almost one–third (30.5%) of them scored 4 out of 4. On the other hand, the alternating hand sequence test (psychomotor speed) was assessed, and almost one-fifth (22%) of them scored 4 out of 4. Finally, registration of new things (memory recall) was assessed, and 16.3% of them had recalled all four items without any clue and scored 4 out of 4 (Table 3).
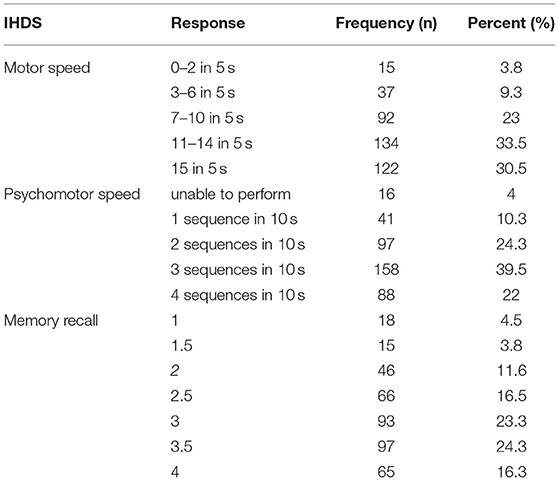
Table 3. The International HIV Dementia Scale (IHDS) Distribution of PLHA at government health institution in Bahir Dar, 2016.
The magnitude of co-occurring anxiety and depression among people living with HIV/AIDS in this study was 39.8% (95% CI: 34.8–44.5). The magnitude of anxiety and depression was 58.5 and 48.8%, respectively. The magnitude of depression (55.4%) and co-occurring anxiety and depression (55.3%) was higher among the female respondents (Figure 1).
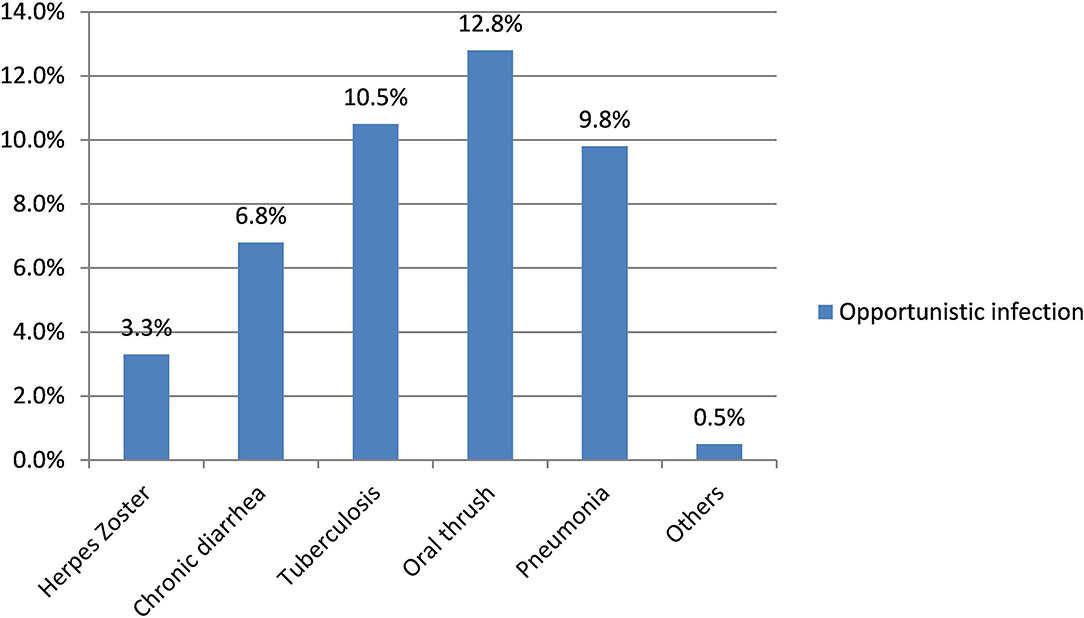
Figure 1. Depression, anxiety, co-occuring anxiety and depression based on sex of the respondents among PLHA at government health institution in Bihar Dar, 2016.
Women, people having comorbid chronic medical diseases, and people under second-line treatment regimens were around two times more likely to have HIV-associated neurocognitive impairment (Table 4).
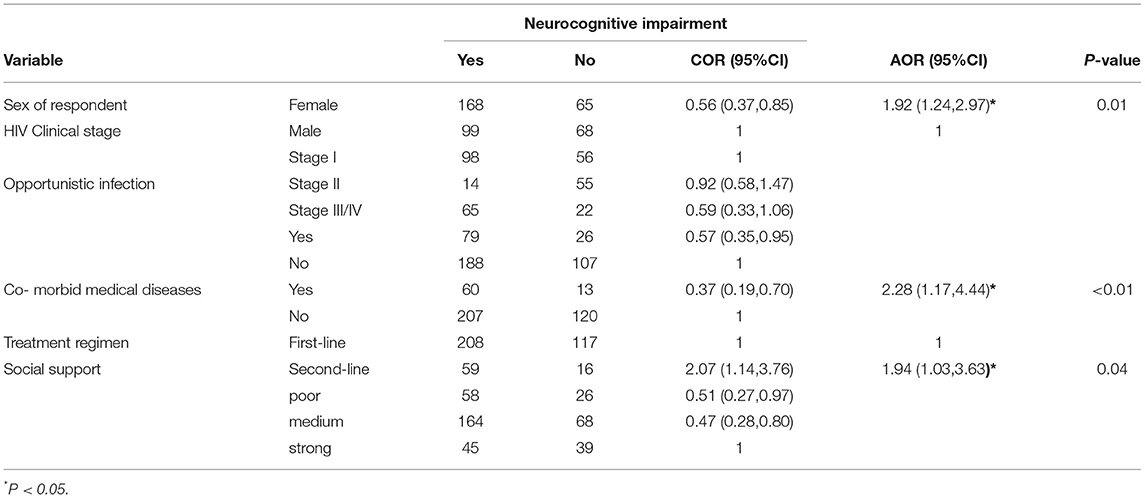
Table 4. Bivariate and multivariate analysis of variables associated with NCI among PLH attending at public health institution in Bahir Dar, Ethiopia, 2016 (n = 400).
People living with HIV/AIDS, who have co-occurring anxiety and depression, were around two times more likely to have perceived stigma, HIV-associated neurocognitive impairment, poor social support, and a second-line treatment regimen. Also, people living with HIV with co-occurring depression and anxiety were three times more likely to be in stage III/IV. Besides, patients who have been taking greater than two pills/day were 57% less likely to develop co-occurring anxiety and depression as compared to patients who were taking less than or equal to two pills per day (Table 5).
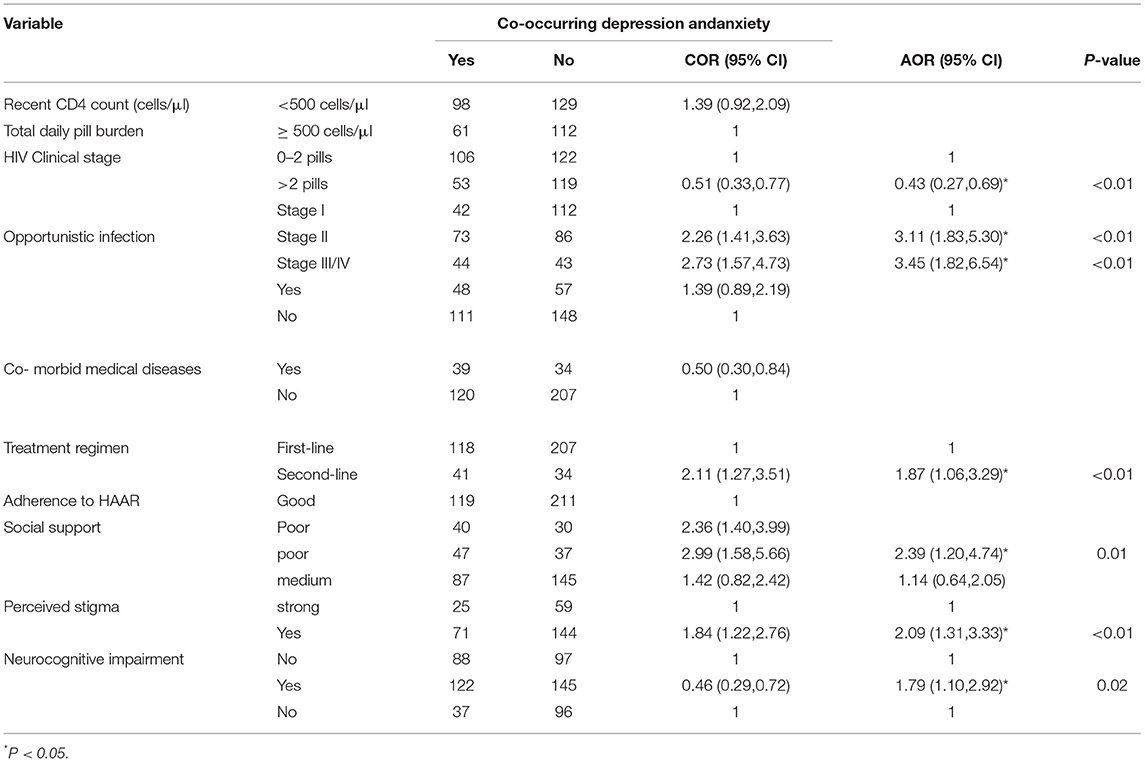
Table 5. Bivariate and Multivariate analysis of variables associated with co-occurrence of depression and anxiety among PLH attending at public health institution in Bahir Dar, Ethiopia, 2016 (n = 400).
Discussion
Among people living with HIV/AIDS who have been screened for neurocognitive impairment, 66.8% (95% CI: 62–71.5) had a neurocognitive impairment, whereas co-occurring anxiety and depression in this study was found in 39.8% (95% CI: 34.8–44.5) of the patients. The prevalence of clinically elevated symptoms of anxiety and depression was 58.5 and 48.8%, respectively.
Women with HIV/AIDS, people having a comorbid chronic medical illness, and those under a second-line treatment regimen were factors associated with neurocognitive impairment. Furthermore, pill burden, second-line treatment regimen, HIV clinical stages, social support, HIV-perceived stigma, and neurocognitive impairment were factors associated with co-occurring depression and anxiety.
The magnitude of neurocognitive impairment in the current study is consistent with the findings done in the semi-urban district of Entebbe, Uganda (64.4%) (33), Kenya (65%) (34), and Southern Ethiopia (67.1%) (35). This study has shown a much higher magnitude of neurocognitive impairment than previous studies conducted in Ethiopia (33.3–39.3%) (18, 36–38). The lower prevalence in previous studies might be due to differences in inclusion criteria, sample size variation, and instrument cutoff point differences for screening neurocognitive impairment. This study focused on the prevalence of neurocognitive impairment among people living with HIV and among those who have been taking combination antiretroviral therapy (cART), unlike others that consume both pre-and on cART medications. Furthermore, we used a large sample size (400) compared to previous studies (244 and 254), which could account for the differences. This study found that a little over half (56.8%) of the people living with HIV had a CD4 count of < 500 cells/μl. In contrast, 44% of residents of Mizan-Aman in Ethiopia who were living with HIV had a CD4 count of < 500 cells/μl during the period of data collection. Low-level CD4 count was strongly associated with severe immunosuppression and increased the prevalence as well as the severity of neurocognitive impairment (37, 39, 40).
This study reported lower neurocognitive impairment than the study done in São Paulo, Brazil (73.6%) (41), Switzerland (84%) (42), at Kenyatta National Hospital in Nairobi (88%) (43), Moi Teaching and Referral Hospital (81.1%) (44), Kenya, and at the Bamenda Regional Hospital AIDS-treatment center, Cameroon (85%) (45). This might be due to differences in sociodemographic and clinical characteristics. For instance, our study participants were somewhat younger (mean age 36.3 years) than the Brazilian (mean age 45.3 years), Kenyan (mean age 42 years), and Cameroonian (41 years) study participants. Older age is among demographic factors that are associated with reduced neurocognitive functioning and increased risk of neurocognitive impairment in people infected with HIV. There are synergistic mechanisms that increase the magnitude of neurocognitive impairment among older adults infected with HIV and accelerate cognitive aging (46). Besides, a Kenyan study found more than a two-fold (46.7%) increase in WHO clinical stage III/IV compared to our finding (21.8% stage III/IV). This is evidenced by late WHO clinical stage III/IV categories that were key predictors of HIV-associated neurocognitive impairment among people living with HIV/AIDS (35, 36).
On the other hand, the prevalence of co-occurring anxiety and depression in this study is much higher than in previous studies conducted in a university hospital, at the Department of Infectious and Tropical Diseases in Conakry, Guinea (8.1%) (47), in South Western Nigeria (21.9%) (48), and in Addis Ababa, Ethiopia (24.5%) (49). Also, the point prevalence of depression (48.8%) and anxiety (58.5%) is higher in this study than in studies conducted in Guinea (13.8 and 16.9%) (47), Nigeria (39.6 and 32.6%) (48), South Ethiopia (32 and 34.4%) (50), and Addis Ababa, Ethiopia (41.2 and 32.4%) (49). This might be due to differences in sociodemographic-related factors, inclusion criteria, different tools to screen anxiety and depression symptoms, and sample size.
However, the prevalence of depression is somewhat lower than in previous studies conducted among patients with HIV/AIDS in Sudan (63.1%) (51), Delhi, India (58.7%) (52), and Kumasi, Ghana (87%) (53). The possible reasons might be sociodemographic variation, inclusion criteria, use of different tools to screen depressive symptoms, and sample size variations. For example, the majority (79%) of the respondents in Ghana were women compared to this study (58.3%). Depression is more prevalent in women, which is supported by different bodies of literature (48, 49, 54).
Regarding clinical factors, co-morbid medical illnesses, daily pill burden, HIV clinical stage, treatment regimen, and neurocognitive impairment were associated with co-occurring anxiety and depression in this study. Depression is highly prevalent among individuals suffering from chronic medical diseases, which is supported by evidence (55, 56). A systematic review and meta-analysis revealed that the risk for a depressive disorder was 2–3 times greater in people with two or more chronic physical conditions than in those without any chronic physical condition. The odds of having a depressive disorder were 45% greater with each additional chronic condition (57).
Concerning pill burden, those who take more than two pills in 1 day were less likely to develop co-occurring anxiety and depression. The possible reason might be an increase in the pill burden when a health professional wants to treat different opportunistic infections. Different bodies of the literature revealed that having an opportunistic infection was a risk factor for developing depression in patients with HIV (56, 58–60). Moreover, immunosuppression (HIV clinical stage III/IV) in people living with HIV was frequently reported as a factor associated with depression (49, 61, 62).
In this study, depression was associated with HIV-associated neurocognitive impairment, which is supported by previous findings in which depression was associated with the risk of neurocognitive impairment (63, 64). Depression is commonly co-morbid with HIV infection, but the association is not clear on which factors may contribute to lower neurocognitive impairment (65).
Furthermore, psychosocial factors (HIV stigma and social support) were associated with co-occurring anxiety and depression in this study. These are supported by many previous studies (49, 50, 60, 62). A systemic review and meta-analysis revealed that poor social support had a statistically significant effect on depression among patients with HIV/AIDS. The odds of having depression among adults living with HIV who had poor social support were 31% higher than among those who had strong social support (66). In addition, people with depression who perceive their social support as poorer have worse outcomes in terms of symptoms, recovery, and social functioning (67). Perceived stigma is found commonly among people living with HIV and at risk for depression, anxiety, and many other psychosocial issues. Stigma has been related to risky sexual practices, delayed health-seeking behavior, low treatment follow-up, and, consequently, the presence of anxiety and depression (68).
The WHO HIV clinical staging, opportunistic infections, and social support were not statistically significant factors in the multivariate logistic regression for HIV-associated neurocognitive impairment. Furthermore, comorbid medical diseases, recent CD4 counts, and opportunistic infections were not statistically significant factors in the multivariate logistic regression for co-occurring anxiety and depression.
Strengths and Limitations of the Study
To the best of our knowledge, this is the first study that has attempted to determine the magnitude of co-occurring depression and anxiety among a sample of people living with HIV, besides neurocognitive impairment in Ethiopia. This study tried to describe various factors associated with this co-occurrence among patients with HIV. The use of validated instruments in Ethiopia to screen depression and anxiety symptoms in patients with somatic diseases was another strength. Despite these strengths, there are a few limitations that should be considered when interpreting the findings. We have not used a standard measure to assess non-adherence to ART. We used a patient self-report that may underestimate or overestimate adherence due to recall bias. In addition, this study is conducted in selected health facilities in Bahir Dar, so the result may not represent all people living with HIV. Some important variables like viral load and substance use-related factors were not included. Furthermore, due to the cross-sectional nature of the study design, we were not able to find a cause-effect relationship.
Conclusions
Our findings suggest that people living with HIV have a high burden of neurocognitive impairment in the era of potent combination antiretroviral therapy. In addition, people living with HIV struggle with co-occurring depression and anxiety symptoms. Women with HIV/AIDS, people having a comorbid chronic medical illness, and people under a second-line treatment regimen were factors associated with neurocognitive impairment. Pill burden, second-line treatment regimen, HIV clinical stages, social support, HIV-perceived stigma, and neurocognitive impairment were factors associated with co-occurring depression and anxiety. We recommend early screening and management of all people in ART clinics. Also, a future prospective study should be carried out to assess the clinical course of neurocognitive impairment and this highly co-occurring depression and anxiety.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethical Review Committee (IRB) of Bahir Dar University College of Medicine and Health Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MT conceived the study design, collected, analyzed and interpreted the data, and drafted the manuscript for important intellectual content. TB interpreted the data and drafted the manuscript for important intellectual content. TE, MTad, MM, and AK contributed to the analysis of the results and writing of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content and approved the final version to be published.
Funding
This study was funded by Bahir Dar University College of Medicine and Health Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to express our gratitude and thanks to Bahir Dar University College of Medicine and Health Sciences for providing research facilities and funding. We are grateful to all the data collectors, supervisors, and study participants for their important contribution to this study.
References
1. UNAIDS End Inequalities End AIDS. Global AIDS Strategy 2021-2026 (2021). Available online at: https://www.unaids.org/en/Global-AIDS-Strategy-2021-2026
2. Federal HIV/AIDS prevention control office. HIV Prevention in Ethiopia National Road Map 2018-2020. (2018). Available online at: https://ethiopia.unfpa.org/sites/default/files/pub-pdf/HIV%20Prevention%20in%20Ethiopia%20National%20Road%20Map%202018%20-%202020%20FINAL_FINAL.pdf.
3. Kibret GD, Ferede A, Leshargie CT, Wagnew F, Ketema DB, Alebel A. Trends and spatial distributions of HIV prevalence in Ethiopia. Infect Dis Poverty. (2019) 8:1–9. doi: 10.1186/s40249-019-0594-9
4. The LID. The challenge of HIV associated neurocognitive disorder. Lancet Infect Dis. (2013) 13:907. doi: 10.1016/S1473-3099(13)70306-2
5. Antinori A, Arendt G, Becker J, Brew B, Byrd D, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. (2007) 69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b
6. Wei J, Hou J, Su B, Jiang T, Guo C, Wang W, et al. The prevalence of Frascati-criteria-based HIV-associated neurocognitive disorder (HAND) in HIV-infected adults: a systematic review and meta-Analysis. Front Neurol. (2020) 11:1613. doi: 10.3389/fneur.2020.581346
7. Sacktor N, Saylor D, Nakigozi G, Nakasujja N, Robertson K, Grabowski MK, et al. Effect of HIV Subtype and Antiretroviral therapy on HIV-associated neurocognitive disorder (HAND) stage in Rakai, Uganda. J Acquir Immune Defic Syndr. (2019) 81:216. doi: 10.1097/QAI.0000000000001992
8. Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, et al. Prevalence of HIV-associated neurocognitive disorders in the multicenter AIDS cohort study. Neurology. (2016) 86:334–40. doi: 10.1212/WNL.0000000000002277
9. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Am Psychiatr Assoc. (2013) 21:591–643. doi: 10.1176/appi.books.9780890425596
10. Stein DJ, Szatmari P, Gaebel W, Berk M, Vieta E, Maj M, et al. Mental, behavioral and neurodevelopmental disorders in the ICD-11: an international perspective on key changes and controversies. BMC Med. (2020) 18:1–24. doi: 10.1186/s12916-020-1495-2
11. Lundberg P, Nakasujja N, Musisi S, Thorson AE, Cantor-Graae E, Allebeck P. HIV prevalence in persons with severe mental illness in Uganda: a cross-sectional hospital-based study. Int J Ment Health Syst. (2013) 7:1–9. doi: 10.1186/1752-4458-7-20
12. Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS (London, England). (2019) 33:1411. doi: 10.1097/QAD.0000000000002227
13. Belayneh Z, Mekuriaw B, Mehare T, Shumy S, Tsehay M. Magnitude and predictors of common mental disorder among people with HIV/AIDS in Ethiopia: a systematic review and meta-analysis. BMC Public Health. (2020) 20:1–11. doi: 10.1186/s12889-020-08800-8
14. Haas AD, Ruffieux Y, van den Heuvel LL, Lund C, Boulle A, Euvrard J, et al. Excess mortality associated with mental illness in people living with HIV in Cape Town, South Africa: a cohort study using linked electronic health records. Lancet Glob Health. (2020) 8:e1326–34. doi: 10.1016/S2214-109X(20)30279-5
15. Osowiecki DM, Cohen RA, Morrow KM, Paul RH, Carpenter CC, Flanigan T, et al. Neurocognitive and psychological contributions to quality of life in HIV-1-infected women. AIDS. (2000) 14:1327–32. doi: 10.1097/00002030-200007070-00004
16. Worku ED, Asemahagn MA, Endalifer ML. Epidemiology of HIV infection in the Amhara region of Ethiopia, 2015 to 2018 surveillance data analysis. HIV AIDS (Auckl). (2020) 12:307. doi: 10.2147/HIV.S253194
17. Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. (2013) 6:14. Available online at: https://pubmed.ncbi.nlm.nih.gov/24834239/
18. Tsegaw M, Andargie G, Alem G, Tareke M. Screening HIV-associated neurocognitive disorders (HAND) among HIV positive patients attending antiretroviral therapy in South Wollo, Ethiopia. J Psychiatr Res. (2017) 85:37–41. doi: 10.1016/j.jpsychires.2016.10.016
19. Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. (2005) 19:1367–74. Available online at: https:/pubmed.ncbi.nlm.nih.gov/16103767/
20. Mwangala PN, Newton CR, Abas M, Abubakar A. Screening tools for HIV-associated neurocognitive disorders among adults living with HIV in sub-Saharan Africa: a scoping review. AAS Open Res. (2019) 1:28. doi: 10.12688/aasopenres.12921.2
21. Joska J, Westgarth-Taylor J, Hoare J, Thomas K, Paul R, Myer L, et al. Validity of the international HIV dementia scale in South Africa. AIDS Patient Care STDS. (2011) 25:95–101. doi: 10.1089/apc.2010.0292
22. Molinaro M, Saylor D, Nakigozi G, Nakasujja N, Robertson K, Kigozi G, et al. The International HIV Dementia Scale as a screening tool for HAND in Uganda. AAN Conference. (2020). doi: 10.13140/RG.2.2.23868.18569
23. Njamnshi A, Djientcheu V, Fonsah jy, Yepnjio F, Njamnshi D, Muna W. The international HIV dementia scale is a useful screening tool for HIV-associated dementia/cognitive impairment in hiv-infected adults in yaoundé-cameroon. J Acquir Immune Defic Syndr. (2008) 49:393–7. doi: 10.1097/QAI.0b013e318183a9df
24. Rosca EC, Tadger P, Cornea A, Tudor R, Oancea C, Simu M. International HIV dementia scale for HIV-associated neurocognitive disorders: a systematic review and meta-analysis. Diagnostics. (2021) 11:1124. doi: 10.3390/diagnostics11061124
25. Molinaro M, Saylor D, Nakigozi G, Nakasujja N, Robertson K, Gray R, et al. The international HIV dementia scale as a screening tool for HAND in rural Rakai, Uganda (P5. 154). In: AAN Enterprises (2018).
26. Cameron IM, Crawford JR, Lawton K, Reid IC. Psychometric comparison of PHQ-9 and HADS for measuring depression severity in primary care. Br J Gen Pract. (2008) 58:32–6. doi: 10.3399/bjgp08X263794
27. Pappin M, Wouters E, Booysen F. Anxiety and depression amongst patients enrolled in a public sector antiretroviral treatment programme in South Africa: a crosssectional study. BMC Public Health. (2012) 12:244. doi: 10.1186/1471-2458-12-244
28. Reda AA. Reliability and validity of the ethiopian version of the hospital anxiety and depression scale (HADS) in HIV infected patients. PLoS ONE. (2011) 6:e16049. doi: 10.1371/journal.pone.0016049
29. Abiola T, Udofia O, Zakari M. Psychometric properties of the 3-item oslo social support scale among clinical students of Bayero University Kano, Nigeria. Malaysian J Psychiatry. (2013) 22:32–41. Available online at: https://www.mjpsychiatry.org/index.php/mjp/article/view/264/0
30. Stangl AL, Lilleston P, Mathema H, Pliakas T, Krishnaratne S, Sievwright K, et al. Development of parallel measures to assess HIV stigma and discrimination among people living with HIV, community members and health workers in the HPTN 071 (PopART) trial in Zambia and South Africa. J Int AIDS Soc. (2019) 22:e25421. doi: 10.1002/jia2.25421
31. Koyra H. Adherence to antiretroviral therapy among adult persons living with HIV/AIDS in Southern Ethiopia. Int J Virol AIDS. (2018) 5:10.23937. doi: 10.23937/2469-567X/1510038
32. Shimels T, Asrat Kassu R, Bogale G, Bekele M, Getnet M, Getachew A, et al. Magnitude and associated factors of poor medication adherence among diabetic and hypertensive patients visiting public health facilities in Ethiopia during the COVID-19 pandemic. PLoS ONE. (2021) 16:e0249222. doi: 10.1371/journal.pone.0249222
33. Nakku J, Kinyanda E, Hoskins S. Prevalence and factors associated with probable HIV dementia in an African population: A cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatry. (2013) 13:126. doi: 10.1186/1471-244X-13-126
34. Tiparo JK. Prevalence Of HIV associated dementia among HIV/AIDS Adults Attending Comprehensive Care Centre-Kapsabet Referral Hospital. Nairobi: University of Nairobi (2017).
35. Debalkie Animut M, Sorrie MB, Birhanu YW, Teshale MY. High prevalence of neurocognitive disorders observed among adult people living with HIV/AIDS in Southern Ethiopia: a cross-sectional study. PLoS ONE. (2019) 14:e0204636. doi: 10.1371/journal.pone.0204636
36. Belete T, Medfu G, Yemiyamrew E. Prevalence of HIV associated neurocognitive deficit among HIV positive people in Ethiopia: a cross sectional study at Ayder Referral Hospital. Ethiop J Health Sci. (2017) 27:67–76. doi: 10.4314/ejhs.v27i1.9
37. Araya T, Abebaw D, Belete A, Derajew H, Umer H. Prevalence and factors associated with neuro cognitive disorders among HIV-positive patients in Ethiopia: a hospital-based cross-sectional study. Ethiopian J Health Dev. (2020) 34:22–9. Available online at: https://www.ajol.info/index.php/ejhd/article/view/201259
38. Salahuddin M, Manzar MD, Hassen HY, Unissa A, Hameed UA, Spence DW, et al. Prevalence and predictors of neurocognitive impairment in ethiopian population living with HIV. HIV AIDS (Auckl). (2020) 12:559. doi: 10.2147/HIV.S260831
39. Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. (2011) 25:1747–51. doi: 10.1097/QAD.0b013e32834a40cd
40. Wang Y, Liu M, Lu Q, Farrell M, Lappin JM, Shi J, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology. (2020) 95:e2610–21. doi: 10.1212/WNL.0000000000010752
41. Gascón MRP, Vidal JE, Mazzaro YM, Smid J, Marcusso RMN, Capitao CG, et al. Neuropsychological assessment of 412 HIV-infected individuals in São Paulo, Brazil. AIDS Patient Care STDS. (2018) 32:1–8. doi: 10.1089/apc.2017.0202
42. Simioni S, Cavassini M, Annoni J-M, Abraham AR, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. (2010) 24:1243–50. doi: 10.1097/QAD.0b013e3283354a7b
43. Mugendi A, Kubo M, Nyamu D, Mwaniki L, Wahome S, Haberer J. Prevalence and correlates of neurocognitive disorders among HIV patients on antiretroviral therapy at a Kenyan Hospital. Neurol Res Int. (2019) 2019:5173289. doi: 10.1155/2019/5173289
44. Mohamed AA, Oduor C, Kinyanjui D. HIV-associated neurocognitive disorders at Moi teaching and referral hospital, Eldoret, Kenya. BMC Neurol. (2020) 20:1–11. doi: 10.1186/s12883-020-01857-3
45. Atashili J, Gaynes BN, Pence BW, Tayong G, Kats D, O'donnell JK, et al. Prevalence, characteristics and correlates of a positive-dementia screen in patients on antiretroviral therapy in Bamenda, cameroon: a cross-sectional study. BMC Neurol. (2013) 13:86. doi: 10.1186/1471-2377-13-86
46. Cohen RA, Seider TR, Navia B. HIV effects on age-associated neurocognitive dysfunction: premature cognitive aging or neurodegenerative disease? Alzheimers Res Therapy. (2015) 7:1–10. doi: 10.1186/s13195-015-0123-4
47. Camara A, Sow M, Touré A, Sako F, Camara I, Soumaoro K, et al. Anxiety and depression among HIV patients of the infectious disease department of Conakry University Hospital in 2018. Epidemiol Infect. (2020) 148:e8. doi: 10.1017/S095026881900222X
48. Adeoti AO, Dada MU, Fadare JO. Prevalence of depression and anxiety disorders in people living with HIV/AIDS in a Tertiary Hospital in South Western Nigeria. Med Rep Case Stud. (2018) 3:1–5.
49. Tesfaw G, Ayano G, Awoke T, Assefa D, Birhanu Z, Miheretie G, et al. Prevalence and correlates of depression and anxiety among patients with HIV on-follow up at Alert Hospital, Addis Ababa, Ethiopia. BMC Psychiatry. (2016) 16:368. doi: 10.1186/s12888-016-1037-9
50. Duko B, Toma A, Asnake S, Abraham Y. Depression, anxiety and their correlates among patients with HIV in South Ethiopia: an institution-based cross-sectional study. Front Psychiatry. (2019) 10:290. doi: 10.3389/fpsyt.2019.00290
51. Elbadawi A, Mirghani H. Depression among HIV/AIDS Sudanese patients: a cross-sectional analytic study. Pan Afr Med J. (2017) 26:10919. doi: 10.11604/pamj.2017.26.43.10919
52. Bhatia M, Munjal S. Prevalence of depression in people living with HIV/AIDS undergoing ART and factors associated with it. J Clin Diagn Res. (2014) 8:WC01. doi: 10.7860/JCDR/2014/7725.4927
53. Kwakye A. Prevalence and impact of depression, anxiety and stress on CD4+ cell counts of HIV/AIDS patients receiving HAART in Ghana. J AIDS Clin Res. (2018) 9:2. doi: 10.4172/2155-6113.1000781
54. Girma A, Tekleselasie W, Yohannes T. Prevalence of depression and associated factors among adults on antiretroviral therapy in public hospitals of kembata tembaro zone, South Ethiopia. Int J Ment Health Syst. (2020) 14:55. doi: 10.21203/rs.3.rs-123623/v1
55. Li H, Ge S, Greene B, Dunbar-Jacob J. Depression in the context of chronic diseases in the United States and China. Int J Nurs Sci. (2019) 6:117–22. doi: 10.1016/j.ijnss.2018.11.007
56. Abadiga M. Depression and its associated factors among HIV/AIDS patients attending ART clinics at Gimbi General hospital, West Ethiopia, 2018. BMC Res Notes. (2019) 12:1–8. doi: 10.1186/s13104-019-4553-0
57. Read JR, Sharpe L, Modini M, Dear BF. Multimorbidity and depression: a systematic review and meta-analysis. J Affect Disord. (2017) 221:36–46. doi: 10.1016/j.jad.2017.06.009
58. Abebe H, Shumet S, Nassir Z, Agidew M, Abebaw D. Prevalence of depressive symptoms and associated factors among HIV-positive youth attending ART follow-up in Addis Ababa, Ethiopia. AIDS Res Treat. (2019) doi: 10.26226/morressier.5d1a037b57558b317a140b36
59. Yeneabat T, Bedaso A, Amare T. Factors associated with depressive symptoms in people living with HIV attending antiretroviral clinic at Fitche Zonal Hospital, Central Ethiopia: cross-sectional study conducted in 2012. Neuropsychiatr Dis Treatment. (2017) 13:2125. doi: 10.2147/NDT.S131722
60. Beyamo A, Bashe T, Wolde Facha TM. Depression and associated factors among adult HIV/AIDS patients attending antiretroviral therapy at wolaita sodo university teaching and referral hospital, Southern Ethiopia. HIV/AIDS (Auckl). (2020) 12:707. doi: 10.2147/HIV.S278794
61. Bernard C, Dabis F, de Rekeneire N. Prevalence and factors associated with depression in people living with HIV in sub-Saharan Africa: a systematic review and meta-analysis. PLoS ONE. (2017) 12:e0181960. doi: 10.1371/journal.pone.0181960
62. Tareke M, Addisu F, Abate A. Depression among patients attending antiretroviral treatment program in public health facilities in Bahir Dar City, Ethiopia. J Affect Disord. (2018) 232:370–4. doi: 10.1016/j.jad.2018.02.078
63. Asiedu N, Kretchy I, Asampong E. Psycho-behavioral factors associated with neurocognitive performance among people living with HIV on antiretroviral therapy in Accra, Ghana. Afr Health Sci. (2020) 20:587–96. doi: 10.4314/ahs.v20i2.6
64. Troncoso FT, Conterno LdO. Prevalence of neurocognitive disorders and depression in a Brazilian HIV population. Rev Soc Bras Med Trop. (2015) 48:390–8. doi: 10.1590/0037-8682-0034-2015
65. Thames AD, Becker BW, Marcotte TD, Hines LJ, Foley JM, Ramezani A, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: An examination of subjective appraisal versus objective performance. Clin Neuropsychol. (2011) 25:224–43. doi: 10.1080/13854046.2010.539577
66. Weldesenbet AB, Kebede SA, Tusa BS. The effect of poor social support on depression among HIV/AIDS patients in ethiopia: a systematic review and meta-analysis. Depress Res Treat. (2020) doi: 10.1155/2020/6633686
67. Wang J, Mann F, Lloyd-Evans B, Ma R, Johnson S. Associations between loneliness and perceived social support and outcomes of mental health problems: a systematic review. BMC Psychiatry. (2018) 18:156. doi: 10.1186/s12888-018-1736-5
Keywords: neurocognitive impairment, people living with HIV, co-occurring, depression, anxiety
Citation: Tareke M, Belete T, Ergetie T, Tadesse M, Menberu M and Ketemaw A (2022) Triple Burden of Neurocognitive Impairment and Co-occurring Depression and Anxiety Among People Living With HIV in Bahir Dar, Ethiopia: A Multicenter Study. Front. Psychiatry 13:732229. doi: 10.3389/fpsyt.2022.732229
Received: 28 June 2021; Accepted: 22 March 2022;
Published: 26 April 2022.
Edited by:
Paul Stokes, King's College London, United KingdomReviewed by:
Kelly Anne Allott, The University of Melbourne, AustraliaSuprakash Chaudhury, Dr. D. Y. Patil Medical College, Hospital and Research Centre, India
Aislinn Joanmarie Williams, The University of Iowa, United States
Copyright © 2022 Tareke, Belete, Ergetie, Tadesse, Menberu and Ketemaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minale Tareke, minale23@gmail.com; minale.tareke@bdu.edu.et
 Minale Tareke
Minale Tareke Tilahun Belete
Tilahun Belete Temesgen Ergetie1
Temesgen Ergetie1 Meseret Tadesse
Meseret Tadesse Melak Menberu
Melak Menberu Asmamaw Ketemaw
Asmamaw Ketemaw