
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Psychiatry, 20 December 2022
Sec. Psychological Therapy and Psychosomatics
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1076658
This article is part of the Research TopicCase Reports in Psychological Therapy and PsychosomaticsView all articles
Background: Gastrointestinal (GI) complaints are frequently observed in patients who suffer from anorexia nervosa (AN). These symptoms may hamper treatment and weight regain and are often perceived as the cause, not the consequence, of the disease. Since carbohydrate malabsorption also produces these symptoms, this might underly or contribute to these complaints. So far, the role of carbohydrate malabsorption (fructose malabsorption and lactose intolerance) in AN has not yet been investigated.
Methods: For this case series, inpatients with AN of restrictive type (n = 3), purging type (n = 3), and atypical AN (n = 1) conducted hydrogen breath tests with 25 g of fructose and 50 g of lactose to investigate carbohydrate malabsorption. Results were then analyzed in association with body mass index (BMI) and patient-reported outcomes (disordered eating, body image disturbances, anxiety, depressive symptoms, perceived stress, and GI complaints).
Results: Based on the hydrogen breath test results, three of the seven female patients were classified as lactose intolerant and one presented fructose malabsorption. Both hydrogen curves for fructose (r = –0.632, p < 0.001) and lactose (r = –0.704, p < 0.001) showed a negative correlation with BMI. No association was observed between hydrogen values and patient-reported outcomes.
Conclusion: In patients with AN, GI symptoms caused by intolerance of common monosaccharides and disaccharides may be an underestimated burden and should be considered in the diagnosis and therapy of patients with AN. Due to the observed correlation with BMI, GI complaints after ingestion of fructose or lactose likely develop with decreasing body weight and are potentially reversible with weight regain.
Anorexia nervosa (AN) is a serious mental illness affecting the entire body with limited physical performance and impaired cognitive functions due to malnutrition (1). Although AN may be observed in middle-aged individuals and men, this disorder particularly affects female adolescents and young adult women (2). Characteristics of this eating disorder (ED) are defined in the Diagnostic and Statistical Manual of Mental Disorders 5 (DSM-5) and encompass “significantly low body weight” as a “core feature” due to “restriction of energy intake,” an intense “fear of gaining weight” and “disturbance in the way in which one’s body weight or shape is percepted and experienced.” Additionally, “persistent behavior that interferes with weight gain, even though at a significantly low weight,” promotes weight loss such as excessive training sessions or, depending on the existing subtype–restricting or binge-eating/purging–regular “self-induced vomiting or the misuse of laxatives diuretics or enemas” are diagnostic criteria for AN. The aforementioned specifications of AN are coded in the DSM-5 (3) and similarly (although not identically) listed in the ICD-10-CM (4). In addition, several psychological comorbidities (depression, anxiety disorders, and compulsivity) (1, 3) and somatic complications (e.g., fatigue, dizziness, osteoporosis, or constipation) in several organ systems are known (2).
More than 90% of the patients with AN report the presence of gastrointestinal (GI) complaints, such as postprandial fullness (5), bloating, abdominal pain, or diarrhea (6, 7). Furthermore, reduced intestinal motility and/or constipation are frequently reported (8). It is noted that patients with AN indicate the GI disturbances as the cause of the disturbed eating and not as their consequence (9).
The aforementioned symptoms are similar to those reported by subjects who are afflicted by malabsorption of common carbohydrates, such as fructose and lactose (10). These non-absorbed sugars reach the colon where they are fermented by colonic bacteria to gases including H2 (11). Malabsorption of fructose can be explained by reduced expression of the GLUT5 and GLUT2 transporters (12, 13), whereas the reason for impaired absorption of lactose is the enzymatic deficiency of lactase based on different conditions: due to congenital causes, acquired continuous decrease in enzyme activity, or developmental lactase deficiency, which altogether are described as primary lactose intolerance (14). The secondary hypolactasia results from an impaired small intestinal epithelium due to GI pathologies, such as celiac disease (14, 15) or external influences. For instance, lactose intolerance, which can be attributed to transient gut barrier dysfunction was found in patients receiving chemotherapy (16) or following viral gastroenteritis (17). Also, body weight loss might contribute to the development of carbohydrate malabsorption (18).
Studies examining a possible correlation between AN and the presence of malabsorption of specific monosaccharides and disaccharides are still missing (18, 19). One study merely examined the metabolization of glucose with a glucose tolerance test (20) which, however, indicates small intestinal bacterial overgrowth and not lactose intolerance or fructose malabsorption. The lactose and fructose hydrogen (H2) breath tests are the gold standard, which measures H2 content in an end-expiratory breath sample after oral ingestion of fructose or lactose. This can be used as an indicator of malabsorption if a significant H2 increase occurs along with typical GI symptoms (21). The aim of our study was therefore to investigate a case series of patients hospitalized for the treatment of their AN regarding the malabsorption of fructose and lactose.
The inclusion criteria for participation in the study were the diagnosis of AN based on the ICD-10 (F50.0 or F50.1) (4) and the hospitalization for the treatment of the AN. This group consisted of subjects with a restrictive type (F50.00, n = 3, patients 1, 4, and 6), a purging type (F50.01, n = 3, patients 2, 3, and 5), and atypical AN (F50.1, n = 1, patient 7). Patients were excluded if they had an oncological disease, psychotic disorder, existing pregnancy, or they were breastfeeding, and in the case of illegal drug use, medication intake in the last 3 months within a clinical trial (other medication–prescribed and over the counter–is indicated in Table 1) and disease (e.g., exocrine pancreatic insufficiency) or surgical procedure on the digestive tract or hereditary fructose intolerance.
Inpatients (aged 18 years and older) hospitalized in the University Hospital Tübingen, Department for Psychosomatic Medicine and Psychotherapy, were asked at admission to participate in the study with H2 breath testing during the first 3 days after admission and within the last week before discharge. No antibiotics or other drugs that may modify the gut microbiota composition had been administered 7 days before the H2 breath test. All patients provided written informed consent. The study was approved by the Local Ethics Committee (722/2018BO2).
During the first 3 days after admission and (originally intended) within the last week before discharge, patients electronically received validated questionnaires to assess eating behavior, (Eating Disorder Examination Questionnaire (EDE-Q) (22) and Eating Disorder Inventory-2 (EDI-2) (23); EDE-Q: Cronbach’s alpha for the current sample was 0.937; EDI-2: Cronbach’s alpha for the current sample was 0.908), body image (Fragebogen zum Körperbild-20, FKB-20–subdivided into rejecting body image and vital body dynamic (24), FKB-20, body image: Cronbach’s alpha for the current sample was 0.877; FKB-20, body dynamic: Cronbach’s alpha for the current sample was 0.812), anxiety (Generalized Anxiety Disorder Scale-7 (GAD-7) (25), Cronbach’s alpha for the current sample was 0.684), depressive symptoms (Patient Health Questionnaire-9 (PHQ-9) (26), Cronbach’s alpha for the current sample was 0.882), perceived stress (Perceived Stress Questionnaire-20 (PSQ-20) (27), Cronbach’s alpha for the current sample was 0.550), and GI symptoms (Gastrointestinal Symptom Rating Scale (GSRS) (28), Cronbach’s alpha for the current sample was 0.696). The total scores were used for the evaluation of all questionnaires. Only in the case of the GSRS, the subscales and an AN-specific total score (consisting of the GSRS subscales, which—as the study by Riedlinger et al. (7) showed—are most frequently pathological in patients with AN at the beginning of therapy: abdominal pain, indigestion, and constipation) were additionally calculated.
The hydrogen breath tests for fructose (25 g) and lactose (50 g) were performed as described earlier (10). Overnight (at least 12 h)-fasted patients (only water but no consumption of nicotine or caffeine was permitted) underwent the test the following morning at 8 a.m. On the day before the H2 test, the subjects were advised not to eat any foods containing fiber, such as whole grains, beans, or lentils. They should also refrain from intense physical activity before and during the performance of the test. Likewise, intestinal stimulants or antibiotics had to be avoided the week before the hydrogen breath test due to the possible influence on the intestinal microbiota. Sample collection of end-expiratory exhaled air after oral carbohydrate ingestion was performed via a mouthpiece. At first, an H2 baseline value at exhalation was noted, and after this, all subjects received a solution consisting of 300 ml water and the defined amount of the respective carbohydrate (25 g fructose and 50 g lactose) as detailed in a position paper (21). After consumption of the solution (within 5 min), patients recorded and documented H2 concentration in the exhalation air at 15, 30, 60, 90, 120, 150, and 180 min. Reading of these results and documentation was done by the inpatients and no supervision was required by the protocol. At the same time, abdominal complaints were documented. H2 exhalation was assessed using the portable H2 sensor (Gastrolyzer, Bedfont, SpecialMed, Herrsching, Germany; measurement range 0–500 parts per million [ppm], cross-sensitivity <1%, measurement accuracy ± 2%) as performed earlier (10). Hydrogen values starting at ≤ 20 ppm and rising more than 20 ppm above the baseline were determined as pathological. A distinction must be made between the sole increase in H2 and a rising H2 value with simultaneous occurrence of known GI symptoms such as flatulence, diarrhea, nausea, or a feeling of fullness, with varying frequency and intensity as an indication of intolerance (21).
On the day of inpatient admission (and repeatedly as part of the clinical routine), blood was taken to assess blood count, electrolytes, and liver enzymes. In addition, elastase was determined once in the stool at the beginning of the therapy to exclude exocrine pancreatic insufficiency. Body weight was measured in patients wearing light underwear to calculate the BMI.
All participants received the same multimodal psychosomatic therapy, which encompassed individual and group therapy, nutrition counseling, supervised daily eating, joint cooking sessions, body and art therapy, relaxation techniques, and physical therapy. An accompanying drug therapy against tension and depression was indicated for patient 5 (quetiapine 25 mg) and has already been taken by the patient since 2019. Although psychotropics may alter gut microbiota diversity, no data exist so far for low-dose quetiapine (29).
All data (BMI, laboratory results, H2 values, and patient-reported outcomes) were completely assessed at admission. However, against our initial plan, we were able to conduct H2 breath testing only in two patients at discharge (both H2 tests in patient 1 and one H2 test in patient 6). The other patients either denied the conductance of the H2 tests at discharge (patients 2, 4, 6, and 7), were discharged prematurely (patient 3), or were transferred to the psychiatry ward due to increasingly diminished emotional regulation capacity with escalating self-injurious behavior over the course of the inpatient treatment (patient 5). Therefore, we were not able to longitudinally assess these data and report only the data from admission in this case series.
For statistical analyses, the program SPSS version 28.0.1.1 (IBM) was used. Collected data were analyzed descriptively and each item (sociodemographic/somatic data, laboratory results, H2 values, and patient-reported outcomes) was checked for normality using the Shapiro–Wilk test and visually with the QQ plot. Correlations were assessed using Spearman’s rank correlation.
Diagnosed according to standard face-to-face interviews using the national German S3 guidelines for the assessment and therapy of eating disorders (EDs) (30), seven female subjects between 18 and 42 years of age (mean: 30.0 years) and of German origin (Caucasian) were included in the group of test subjects. All of them were admitted as inpatients due to their non-improving ED, and among them, three were with a BMI of <15 mg/m2 (BMI range: 14.3–15.8 15 mg/m2, mean value: 15.1 15 mg/m2). On admission, all inpatients were asked about their physical and mental health over the past 2 weeks (response options ranged from not impaired to severely impaired) and rated their status in both health domains (Table 2). Regarding physical condition, the ED influenced the inpatients’ personal efficiency in their everyday activities (education/work and household), since all of them indicated a medium personal performance level (scale value of 4 or 5 on a Likert scale of 0–10). These scores showed that all female patients were still able to perform their daily tasks and nearly half of the patients felt unable to work. The blood values obtained at the same time did not show any abnormalities (Table 2), and stool elastase was normal in all subjects (> 500 μg/g). Six of the seven inpatients were already undergoing psychotherapeutic treatment at the time of the initial interview.
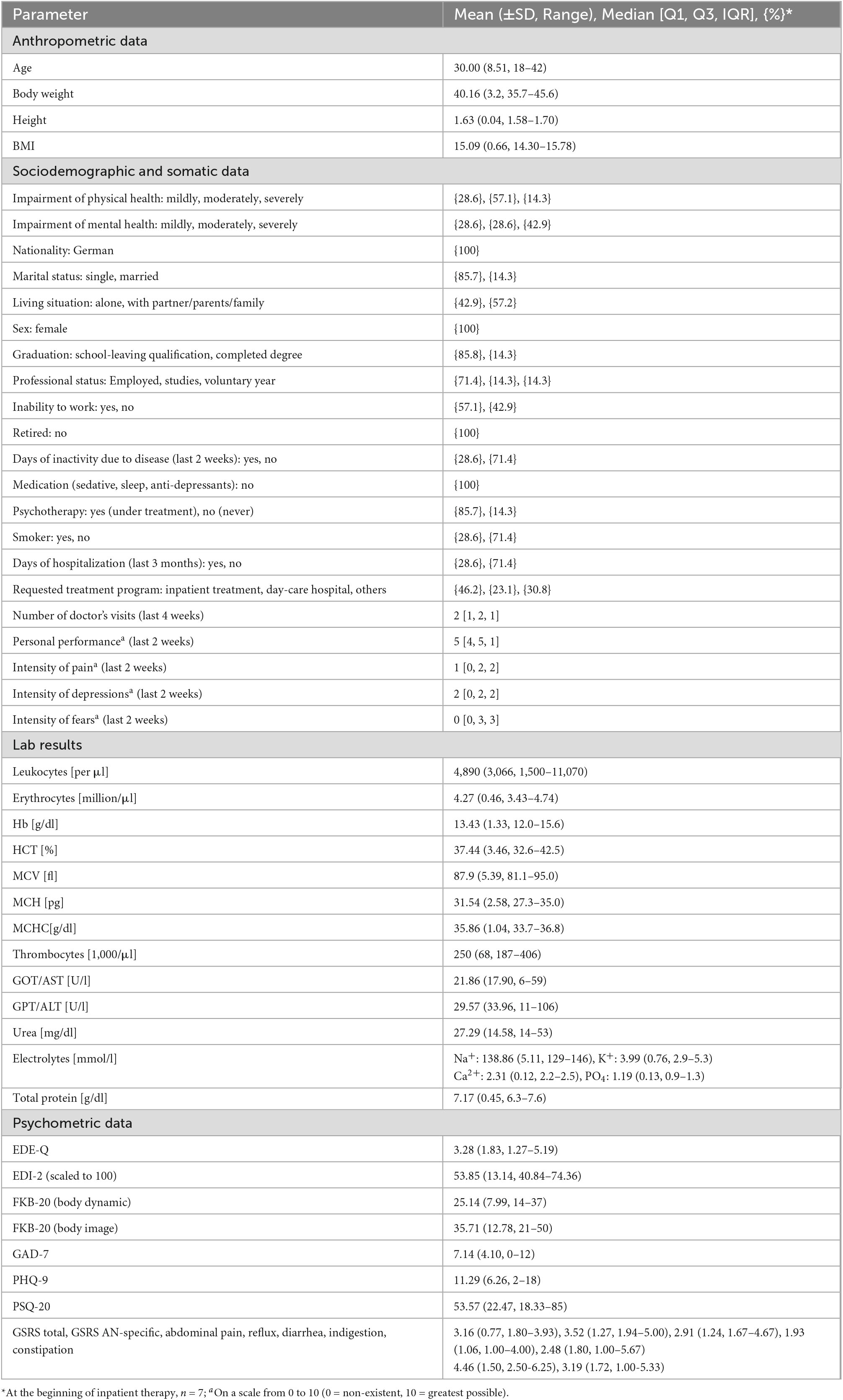
Table 2. Descriptive analysis of anthropometric, sociodemographic, laboratory, and psychometric data.
After the consumption of 25 g fructose, only one patient (patient 3) reported several GI complaints (flatulence, bloating, stomach pains, and diarrhea) in addition to a pronounced (> 20 ppm) rise in breath H2 levels (H2 value range: 2–26), indicative of fructose malabsorption. All other patients were not classified as fructose malabsorbers, and the reported GI symptoms were stomach pain in patient 1 after 60 min (H2 value range: 0–4). In patient 2 directly after fructose ingestion, mild stomach pain was reported (H2 value range: 0–3), patient 4 complained about mild nausea after 90 min (H2 value range: 2–5), patients 5 and 6 reported no GI symptoms, and patient 7 already showed a higher H2 value at the beginning of the fructose test but only increased slightly (H2 value range: 21–36), with the simultaneous existence of only mild stomach pain after 15 min.
After intake of 50 g of lactose, three of the seven patients reported GI and other complaints (flatulence, bloating, stomach pain, diarrhea, nausea, borborygmi, headaches, and discomfort) associated with a significant rise in H2 levels. Therefore, three out of the seven patients (patients 3, 6, and 7) were classified as lactose intolerant. In the other non-lactose intolerant patients, neither rising H2 values nor corresponding GI symptoms were observed: patient 1 reported stomach pain and hunger from the 60th min (H2 value range: 0–2), patient 2 had mild stomach pain after 15 min (H2 value range: 0–2), patient 4 mentioned mild nausea (H2 value range: 2–6), and patient 5 noticed mild rumbling in the stomach at 30 and 90 min (H2 value range: 0–1).
With regard to the established lactose intolerance in patient 3, she reported that she did not tolerate lactose well at low body weights. This observation was made during the eating disorder (diagnosed more than 4 years ago) with the lowest weight of 36 kg (BMI: 14.2). She reported tolerating lactose at higher body weights. The other two inpatients who reacted strongly to lactose intake (patients 6 and 7) had a long history of AN (10–20 years) as well.
GI symptoms due to other causes such as hepatic or pancreatic dysfunction could be excluded for all participants based on the blood test results (Table 2). Only one patient (patient 1) showed slightly elevated levels for GOT and GPT indicating liver damage as a possible consequence of renutrition after a long period of malnutrition.
When investigating the exhaled H2 values and the BMI of each patient, a negative correlation was observed: the hydrogen value increased with decreasing BMI for both fructose (Figure 1) and lactose (Figure 2).
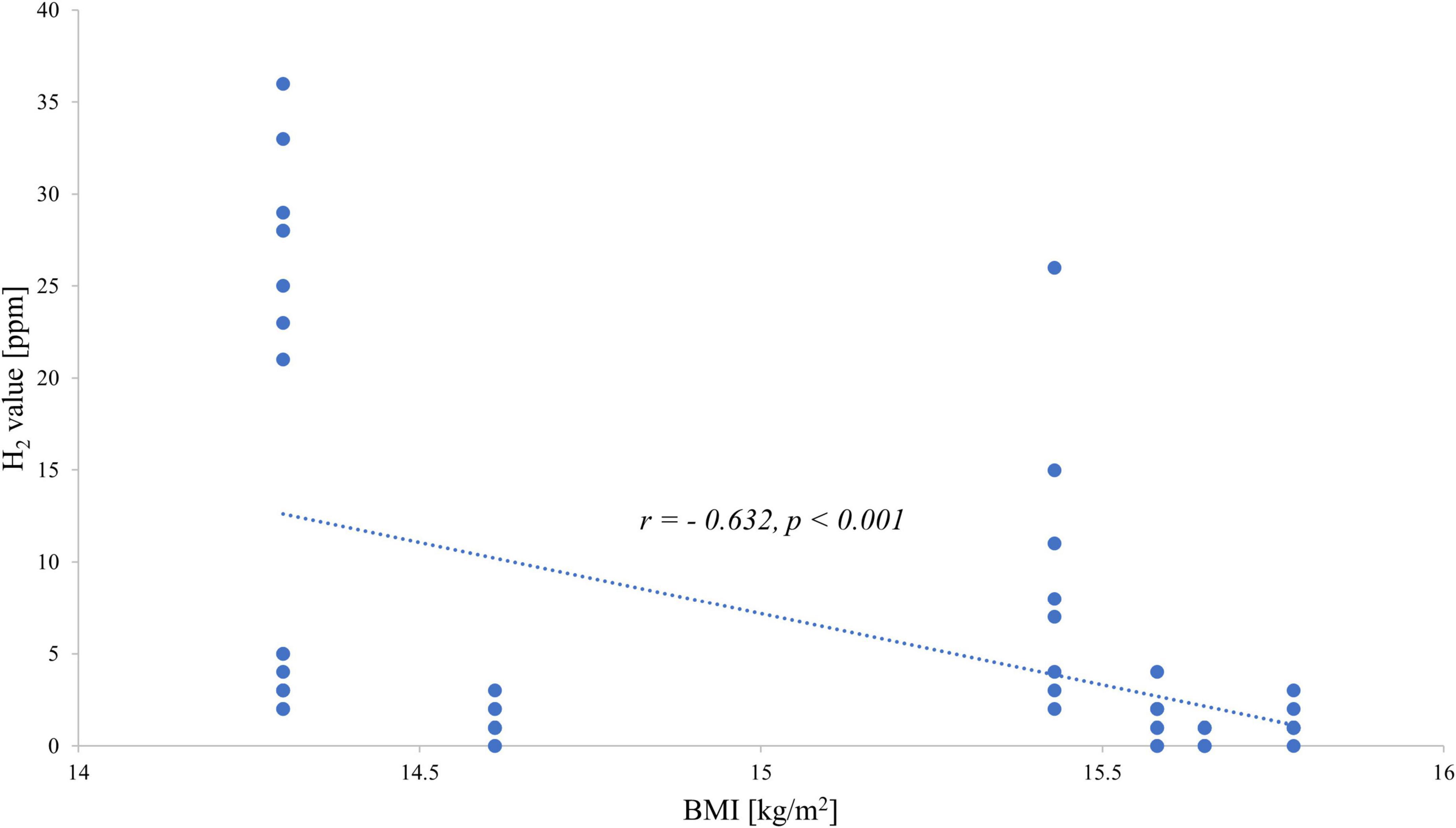
Figure 1. Correlation between end-expiratory hydrogen values during the fructose breath test and body mass index.
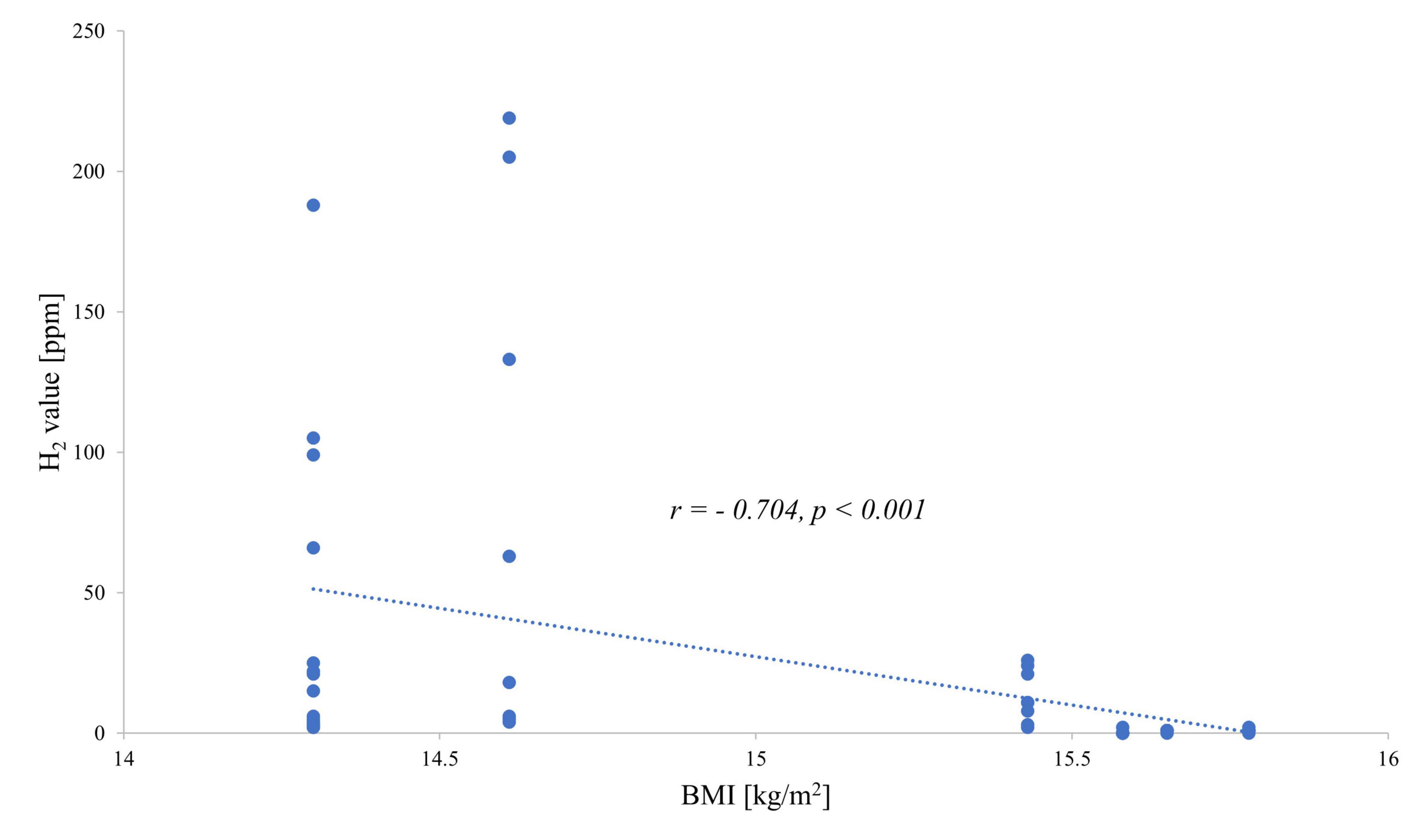
Figure 2. Correlation between end-expiratory hydrogen values during the lactose breath test and body mass index.
Patient-reported outcomes using validated questionnaires at admission are shown in Table 2. The severity level of ED reflected in the EDE-Q (22) was below levels compared with previous studies on patients with EDs (22, 31) with scores of 4 or higher (current mean EDE-Q score: 3.28). The assessment of attitudinal and behavioral features corresponding to AN or bulimia nervosa by EDI-2 (23) was higher than levels reported before in patients with AN (7, 32) (mean EDI-2 score scaled to 100: 53.9). For the assessment of body image, both subscales of the questionnaire FKB-20 were considered. The conception of the energetic aspect was similar to values previously recorded for patients with AN or depressive disorder but below the value calculated for patients with a somatoform disorder (33) (mean FKB-20 body dynamics score: 25.1). The body image score (mean: 35.7) was higher in comparison to the one observed in the mentioned anorexic group and patients with a somatoform disorder (33). Anxiety was reported as mild (mean GAD-7 score: 7.1) (25), and therefore higher compared with the general population (34) but lower than previously reported in patients with AN (7). Depressive symptoms (PHQ-9, score 0–4: minimal, score 5–9: mild, score 10–14: moderate, score 15–19: moderately severe, scale 20–27: severe) (35) were reported to be moderate in the current sample (mean PHQ-9 score: 11.3) consistent with a previous study on patients with AN (7). The perception of stress assessed using the PSQ-20 was above levels reported before for healthy adults and similar to the levels described in psychosomatic outpatients (27) (current mean PSQ-20 score: 53.6).
Frequency and intensity of GI complaints assessed by the GSRS showed increased values compared with previous studies indicating a value of ≥2 in GSRS scores as pathological (36, 37) (current mean GSRS total score: 3.2). The GSRS is composed of five subscales representing GI symptom clusters, such as abdominal pain, constipation, diarrhea, indigestion, and reflux (7). Reported symptoms of the seven female inpatients were indigestion (mean: 4.5) and constipation (mean: 3.2), followed by abdominal pain (mean: 2.9), diarrhea (mean: 2.5), and reflux (mean: 1.9). The first three symptoms are the most prevalent in AN (7). The AN-specific GI symptom scale (mean GSRS AN-typical score: 3.5) was comparable to the results of the study by Riedlinger et al. (7) assessing the prevalence of GI complaints in patients with AN during admission and discharge of therapy. The mean GSRS total score compared with non-psychiatric controls was higher but lower than the levels observed in outpatients with obsessive-compulsive disorder with and without irritable bowel syndrome (38). Comparison of each GSRS subscale with those of patients with functional dyspepsia showed similar levels (39). No associations were observed between exhaled H2 levels and any patient-reported outcome (Table 3).
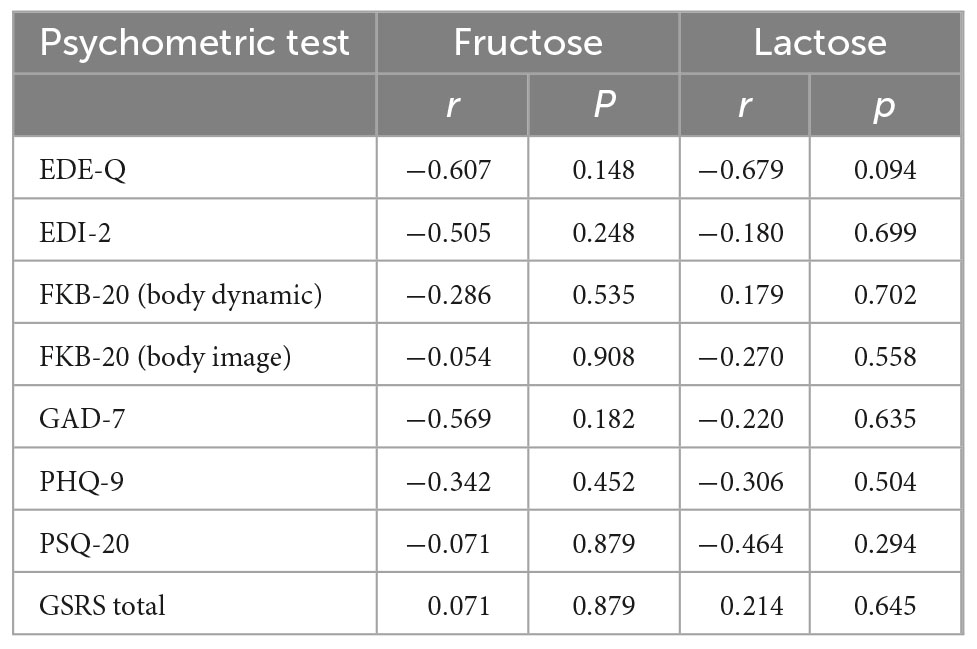
Table 3. Correlation between patient-reported outcomes of inpatients and AUC of H2 values after provocation with fructose or lactose.
In the present study, we conducted hydrogen breath tests with fructose and lactose in female inpatients with AN to examine whether the malabsorption of these carbohydrates might be responsible for the frequently reported GI symptoms. Although malabsorption per se was not particularly frequent in our current sample, both H2 values after fructose and lactose showed a close inverse correlation with BMI giving rise to the possible development of fructose malabsorption and lactose intolerance as a consequence of decreasing body weight. The study of Friesen et al. (19) tested the occurrence of malabsorption after ingestion of a solution made of 25 g fructose (and 5 g sorbitol) in 26 female patients with eating disorders and a BMI of 18.6 ± 3.6 kg/m2; among them, 10 were with AN, in comparison to normal weight female controls. Over a testing period of 3 h, GI complaints were hourly noted in addition to the conduction of a hydrogen breath test: eight inpatients with anorexia reported more frequent and severe GI symptoms (abdominal pain, bloating, nausea, or flatulence) after fructose(-sorbitol) ingestion and 13 of the 26 patients with ED malabsorption was detected based on the hydrogen breath levels. Interestingly, they also indicated greater GI symptom responses in patients with a lower BMI, and therefore more common in patients with AN (19).
The likelihood of developing an intolerance at low body weight or restricted eating behavior also increased for lactose as shown in the study of Täljemark et al. (40). Therein, 12 of 95 children with restrictive eating problems, including three patients with AN, noticed lactose intolerance as one of the most prevalent coexisting GI problems. Low body weight due to severe weight loss in the context of cachexia has also been mentioned earlier as a possible cause of malabsorption entailed by skeletal muscle wasting, leading to the interaction with other tissues/organ systems, including the gut (41). A so-called gut barrier dysfunction might occur which may be responsible for/contribute to malabsorption (42). Another previous study showed that the GI microbiota was perturbed in AN when compared with normal-weight participants reflected by the decreased microbial richness and a shift in bacteria abundance: pro-inflammatory and mucin-degrading bacteria were increased, whereas a decrease of intestinal protective species and carbohydrate-utilizing taxa was observed (43, 44). Especially, Roseburia spp., being a key butyrate producer and important for gut health, appears to be decreased and positively correlated with BMI (45). After weight gain, the GI microbiota composition changes in AN. However, this composition is still distinct from that of healthy normal-weight participants (44). Based on these data, we hypothesize that malabsorption develops with decreasing BMI, which might hamper therapy but may normalize during weight restoration treatment of AN. To further confirm this hypothesis, a larger and longitudinal study is desired.
The assessment of patient-reported outcomes did not indicate an association between eating disorder symptoms and H2 values, further pointing toward malabsorption being most likely not the cause but the consequence of AN.
Patient 3 reacted positively to both fructose and lactose. A significant H2 increase of ≥ 20 ppm over the baseline was found in both the fructose and lactose breath tests after 90 min. Due to this combined H2 increase, small intestinal bacterial overgrowth (SIBO) and celiac disease should be excluded for this patient (10), which was suggested but not performed during her stay. Nonetheless, SIBO in this patient is not likely as the H2 increase in the performed tests was delayed.
Despite the strength of the study adding first data on the yet unexplored field of carbohydrate malabsorption in the context of AN, some limitations should be mentioned as well. First, the study population is very small, mostly because inpatients with severe AN often are unwilling—due to the avoidance of carbohydrates—or unable—due to the severity of the disease—to participate in this type of study. Second, the study was performed—against our initial plan—only cross-sectionally as patients were discharged early, transferred to another ward, or refused to participate in the H2 test at the time of discharge. The rate of discontinuation was 85.7% on performing the second fructose breath test and 71.4% on conducting the second lactose breath test. Third, other exclusion criteria might have been missed, which might confound the results. Finally, SIBO has not been ruled out via endoscopy and aspiration. Therefore, at this point, we can only speculate on the cause and consequence of carbohydrate malabsorption. For this purpose, a larger, possibly also including patients with less severe AN, and a longitudinal study with retesting after weight restoration is warranted to answer this question.
In this study, we showed that H2 levels after fructose and lactose challenges exhibit an inverse association with BMI, and therefore underweight might contribute/lead to reduced capacity of carbohydrate absorption in patients with AN, thereby hampering treatment. This possible cause–consequence relationship should be further investigated in longitudinal studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Local Ethics Committee (722/2018BO2). The patients/participants provided their written informed consent to participate in this study.
AS designed the study, conceived, and planned the experiments in collaboration with MG-S, IM, and SZ. IM supervised the clinical measurements. PB carried out the calculations and wrote the manuscript with input from all the authors.
The authors acknowledge the support of Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tübingen.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AN, anorexia nervosa; BMI, body mass index; ED, eating disorder; GI, gastrointestinal; HBT, hydrogen breath test.
1. Treasure J, Zipfel S, Micali N, Wade T, Stice E, Claudino A, et al. Anorexia nervosa. Nat Rev Dis Primers. (2015) 1:15074. doi: 10.1038/nrdp.2015.74
2. Zipfel S, Giel K, Bulik C, Hay P, Schmidt U. Anorexia nervosa: aetiology, assessment, and treatment. Lancet Psychiatry. (2015) 2:1099–111. doi: 10.1016/S2215-0366(15)00356-9
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, TX: American Psychiatric Association (2013).
4. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (10th Revision). Geneva: WHO (2019).
5. Salvioli B, Pellicciari A, Iero L, Di Pietro E, Moscano F, Gualandi S, et al. Audit of digestive complaints and psychopathological traits in patients with eating disorders: a prospective study. Dig Liver Dis. (2013) 45:639–44. doi: 10.1016/j.dld.2013.02.022
6. Norris M, Harrison M, Isserlin L, Robinson A, Feder S, Sampson M. Gastrointestinal complications associated with anorexia nervosa: a systematic review. Int J Eat Disord. (2016) 49:216–37. doi: 10.1002/eat.22462
7. Riedlinger C, Mazurak N, Schaffeler N, Stengel A, Giel K, Zipfel S, et al. Gastrointestinal complaints in patients with anorexia nervosa in the timecourse of inpatient treatment. Front Psychiatry. (2022) 13:962837. doi: 10.3389/fpsyt.2022.962837
8. Riedlinger C, Schmidt G, Weiland A, Stengel A, Giel K, Zipfel S, et al. Which symptoms, complaints and complications of the gastrointestinal tract occur in patients with eating disorders? A systematic review and quantitative analysis. Front Psychiatry. (2020) 11:195. doi: 10.3389/fpsyt.2020.00195
9. Schalla M, Stengel A. Gastrointestinal alterations in anorexia nervosa – A systematic review. Eur Eat Disord Rev. (2019) 27:447–61. doi: 10.1002/erv.2679
10. Goebel-Stengel M, Stengel A, Schmidtmann M, Voort I, Kobelt P, Monnikes H. Unclear abdominal discomfort: pivotal role of carbohydrate malabsorption. J Neurogastroenterol Motil. (2014) 20:228–35. doi: 10.5056/jnm.2014.20.2.228
11. Simrén M, Stotzer P. Use and abuse of hydrogen breath tests. Gut. (2006) 55:297–303. doi: 10.1136/gut.2005.075127
12. Romagnuolo J, Schiller D, Bailey R. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. (2002) 97:1113–26. doi: 10.1111/j.1572-0241.2002.05664.x
13. Raithel M, Weidenhiller M, Hagel A, Hetterich U, Neurath M, Konturek P. The malabsorption of commonly occurring mono and disaccharides: levels of investigation and differential diagnoses. Dtsch Arztebl Int. (2013) 110:775–82. doi: 10.3238/arztebl.2013.0775
14. Terjung B, Lammert F. Lactose intolerance: new aspects of an old problem. Dtsch Med Wochenschr. (2007) 132:271–5. doi: 10.1055/s-2007-959320
16. Parnes H, Fung E, Schiffer C. Chemotherapy-induced lactose intolerance in adults. Cancer. (1994) 74:1629–33. doi: 10.1002/1097-0142(19940901)74:53.0.co;2-l
17. Lin L, See M, Wang N. Breath hydrogen test for assessment of lactose malabsorption following rotavirus gastroenteritis. J Formos Med Assoc. (1990) 89:1072–6.
18. Sheldon J, Young F. On the carbohydrate metabolism in anorexia nervosa. Lancet. (1938) 231:257–9. doi: 10.1016/S0140-6736(00)93571-2
19. Friesen N, Hansen R, Abraham S, Kellow J. Fructose-sorbitol ingestion provokes gastrointestinal symptoms in patients with eating disorders. World J Gastroenterol. (2009) 15:5295–9. doi: 10.3748/wjg.15.5295
20. Casper R. Carbohydrate metabolism and its regulatory hormones in anorexia nervosa. Psychiatry Res. (1996) 62:85–96. doi: 10.1016/0165-1781(96)02984-8
21. Keller J, Franke A, Storr M, Wiedbrauck F, Schirra J. Clinically relevant breath tests in gastroenterological diagnostics – recommendations of the German society for neurogastroenterology and motility as well as the German society for digestive and metabolic diseases. Z Gastroenterol. (2005) 43:1071–90. doi: 10.1055/s-2005-858479
22. Aardoom J, Dingemans A, Slof Op’t Landt M, Van FE. Norms and discriminative validity of the eating disorder examination questionnaire (EDE-Q). Eat Behav. (2012) 13:305–9. doi: 10.1016/j.eatbeh.2012.09.002
23. Thiel A, Paul T. Test-retest reliability of the eating disorder inventory 2. J Psychosom Res. (2006) 61:567–9. doi: 10.1016/j.jpsychores.2006.02.015
24. Albani C, Blaser G, Geyer M, Daig I, Schmutzer G, Bailer H. Review and standardization of the “Body Image Questionnaire” (FKB-20) by Clement and Löwe (1996) on a representative German population sample. Z Med Psychol. (2006) 15:99–109.
25. Spitzer R, Kroenke K, Williams J, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
26. Gräfe K, Zipfel S, Herzog W, Löwe B. Screening psychischer Störungen mit dem “Gesundheitsfragebogen für Patienten (PHQ-D)”. Diagnostica. (2004) 50:171–81.
27. Fliege H, Rose M, Arck P, Walter O, Kocalevent R, Weber C, et al. The perceived stress questionnaire (PSQ) reconsidered: validation and reference values from different clinical and healthy adult samples. Psychosom Med. (2005) 67:78–88. doi: 10.1097/01.psy.0000151491.80178.78
28. Svedlund J, Sjodin I, Dotevall G. GSRS – a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. (1988) 33:129–34. doi: 10.1007/BF01535722
29. Tomizawa Y, Kurokawa S, Ishii D, Miyaho K, Ishii C, Sanada K, et al. Effects of psychotropics on the microbiome in patients with depression and anxiety: considerations in a naturalistic clinical setting. Int J Neuropsychopharmacol. (2021) 24:97–107. doi: 10.1093/ijnp/pyaa070
30. Herpertz S, Fichter M, Herpertz-Dahlmann B, Hilber A, Tuschen-Caffier B, Vocks S, et al. Diagnosis and Treatment of Eating Disorders. Berlin: DGPM/DKPM (2018).
31. Jennings K, Phillips K. Eating disorder examination-questionnaire (EDE-Q): norms for clinical sample of female adolescents with anorexia nervosa. Arch Psychiatr Nurs. (2017) 31:578–81. doi: 10.1016/j.apnu.2017.08.002
32. Kappel V, Thiel A, Holzhausen M, Jaite C, Schneider N, Pfeiffer E, et al. Eating disorder inventory-2 (EDI-2) normalization on a sample of normal-weight students aged 10 to 20 years and female patients with anorexia nervosa. Diagnostica. (2012) 58:127–44. doi: 10.1026/0012-1924/a000069
33. Löwe B, Clement U. Somatoform disorders and body image – A comparative study. Z Psychosom Med Psychoanal. (1998) 44:268–78.
34. Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. (2008) 46:266–74. doi: 10.1097/MLR.0b013e318160d093
35. Kroenke K, Spitzer R, Williams J. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
36. Laurikka P, Salmi T, Collin P, Huhtala H, Maki M, Kaukinen K, et al. Gastrointestinal symptoms in celiac disease patients on a long-term gluten-free diet. Nutrients. (2016) 8:429. doi: 10.3390/nu8070429
37. Asai S, Takahashi N, Nagai K, Watanabe T, Matsumoto T, Asai N, et al. Influence of gastrointestinal symptoms on patient global assessment in patients with rheumatoid arthritis. SN Compr Clin Med. (2020) 2:619–26. doi: 10.1007/s42399-020-00290-4
38. Turna J, Grosman Kaplan K, Patterson B, Bercik P, Anglin R, Soreni N, et al. Higher prevalence of irritable bowel syndrome and greater gastrointestinal symptoms in obsessive-compulsive disorder. J Psychiatr Res. (2019) 118:1–6. doi: 10.1016/j.jpsychires.2019.08.004
39. Tominaga K, Tsumoto C, Ataka S, Mizuno K, Takahashi K, Yamagami H, et al. Regional brain disorders of serotonin neurotransmission are associated with functional dyspepsia. Life Sci. (2015) 137:150–7. doi: 10.1016/j.lfs.2015.07.023
40. Täljemark J, Råstam M, Lichtenstein P, Anckarsäter H, Kerekes N. The coexistence of psychiatric and gastrointestinal problems in children with restrictive eating in a nationwide Swedish twin study. J Eat Disord. (2017) 5:25. doi: 10.1186/s40337-017-0154-2
41. Rohm M, Zeigerer A, Machado J, Herzig S. Energy metabolism in cachexia. EMBO Rep. (2019) 20:e47258. doi: 10.15252/embr.201847258
42. Argilés J, Stemmler B, Lopéz-Soriano F, Busquets S. Nonmuscle tissues contribution to cancer cachexia. Mediators Inflamm. (2015) 2015:182872. doi: 10.1155/2015/182872
43. Hanachi M, Manichanh C, Schoenenberger A, Pascal V, Levenez F, Cournède N, et al. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: an explicative factor of functional intestinal disorders? Clin Nutr. (2019) 38:2304–10. doi: 10.1016/j.clnu.2018.10.004
44. Mack I, Cuntz U, Gramer C, Niedermaier S, Pohl C, Schwiertz A, et al. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep. (2016) 6:26752. doi: 10.1038/srep26752
Keywords: bloating, complaint, diarrhea, fructose malabsorption, eating disorder, gastrointestinal, hydrogen breath test, lactose intolerance
Citation: Buck P, Goebel-Stengel M, Mack I, Zipfel S and Stengel A (2022) Case report: Carbohydrate malabsorption in inpatients with anorexia nervosa. Front. Psychiatry 13:1076658. doi: 10.3389/fpsyt.2022.1076658
Received: 21 October 2022; Accepted: 24 November 2022;
Published: 20 December 2022.
Edited by:
Veena Kumari, Brunel University London, United KingdomReviewed by:
Pasquale Scognamiglio, ASL Napoli 3 Sud, ItalyCopyright © 2022 Buck, Goebel-Stengel, Mack, Zipfel and Stengel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Stengel, ✉ YW5kcmVhcy5zdGVuZ2VsQG1lZC51bmktdHVlYmluZ2VuLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.