
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 15 November 2022
Sec. Psychological Therapy and Psychosomatics
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1040911
This article is part of the Research TopicPsychiatric Comorbidities in Patients with Epilepsy: Diagnosis and treatment.View all 8 articles
A correction has been applied to this article in:
Corrigendum: Toward a possible trauma subtype of functional neurological disorder: impact on symptom severity and physical health
Background: As a group, individuals with functional neurological disorder (FND) report an approximately 3-fold increase in adverse life experiences (ALEs) compared to healthy controls. In patients with FND, studies have identified a positive correlation between symptom severity and the magnitude of ALEs. While not all individuals with FND report ALEs, such findings raise the possibility of a trauma-subtype of FND.
Objective: This study investigated if patients with FND, with or without probable post-traumatic stress disorder (PTSD) and/or significant childhood maltreatment, differed in their symptom severity and physical health.
Materials and methods: Seventy-eight patients with FND were recruited (functional seizures, n = 34; functional movement disorder, n = 56). Participants completed self-report measures of symptom severity [Somatoform Dissociation Questionniare-20 (SDQ-20), Screening for Somatoform Disorders: Conversion Disorder subscale (SOMS:CD), Patient Health Questionniare-15 (PHQ-15)], physical health [Short Form Health Survey-36 (SF36-physical health)], childhood maltreatment [Childhood Trauma Questionnaire (CTQ)], and PTSD [PTSD Checklist-5 (PCL-5)]; a psychometric battery of other common predisposing vulnerabilities was also completed. To adjust for multiple comparisons, a Bonferroni correction was applied to all univariate analyses.
Results: Patients with FND and probable PTSD (n = 33) vs. those without probable PTSD (n = 43) had statistically significant increased scores on all symptom severity measures – as well as decreased physical health scores. In secondary post-hoc regression analyses, these findings remained significant adjusting for age, sex, race, college education, and: pathological dissociation; alexithymia; attachment styles; personality characteristics; resilience scores; functional seizures subtype; or moderate-to-severe childhood abuse and neglect scores; SOMS:CD and SDQ-20 findings also held adjusting for depression and anxiety scores. In a separate set of analyses, patients with FND and moderate-to-severe childhood abuse (n = 46) vs. those without moderate-to-severe childhood abuse (n = 32) showed statistically significant increased SDQ-20 and PHQ-15 scores; in post-hoc regressions, these findings held adjusting for demographic and other variables. Stratification by childhood neglect did not relate to symptom severity or physical health scores.
Conclusion: This study provides support for a possible trauma-subtype of FND. Future research should investigate the neurobiological and treatment relevance of a FND trauma-subtype, as well as continuing to delineate clinical characteristics and mechanisms in individuals with FND that lack a history of ALEs.
Functional neurological disorder (FND) is a prevalent neuropsychiatric condition characterized by distressing motor (e.g., limb weakness, tremors, seizures, etc.), sensory, and/or cognitive symptoms that are diagnosed based on positive features incongruent with other recognized pathology (1). Since early formulations of FND, including outdated terms such as “Hysteria” or “Conversion Disorder,” a range of traumatic events were identified as important predisposing vulnerabilities and/or acute precipitants for developing functional neurological symptoms. In the early 1900’s, Oppenheim described the condition as a “Traumatic Neurosis” that happened to military men, and others thought of the condition as related to post-traumatic stress disorder (PTSD) (2, 3). It is now well-documented that patients with FND compared to healthy controls have an approximately 3-fold greater occurrence of adverse life experiences (ALEs), including childhood maltreatment and other stressful life events across the lifespan (4). However, the 5th Edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) appropriately removed the requirement for a proximal stressor to make the diagnosis of FND—since not all patients endorse ALEs (5). Thus, questions remain regarding how to contextualize the role of ALEs in our modern-day conceptualization of FND (6).
Despite the lack of diagnostic specificity, several independent cohort studies reported positive associations between the magnitude of previously experienced ALEs and functional neurological symptom severity (7–12). For example, in a cohort of 54 patients with mixed FND – childhood physical abuse was associated with a larger number of distinct functional neurological symptoms; furthermore, endorsing multiple childhood traumatic experiences positively correlated with patient-reported symptom severity (9). In individuals with functional seizures (FND-seiz), those reporting childhood sexual abuse had earlier onset and more severe seizures (10). Individuals with FND and concurrent PTSD symptoms also endorsed more somatic symptoms compared to those with FND but without prominent PTSD symptoms (13). Additionally, a large-scale systematic review and meta-analysis identified that childhood abuse was associated with earlier symptom onset across the spectrum of FND (14). There is also some initial data to suggest that patients with FND and childhood maltreatment are associated with worse clinical outcomes (15, 16). Taken together, these findings highlight the clinical relevance of childhood ALEs and PTSD symptoms in FND when present—raising the possibility for a trauma-subtype of FND (17).
A potential trauma subtype—representing a subgroup of patients with the same clinical diagnosis but with additional distinguishing features—is not unique to FND. Emerging data suggests that a range of neuropsychiatric conditions across the spectrum of traditionally conceptualized psychiatric and neurological conditions may potentially show characteristics of a clinically-salient “trauma subtype” (17, 18). For example, literature in individuals with mood, anxiety and substance use disorders with childhood maltreatment highlights a propensity for increased symptom severity, comorbidity, suicidality, and worse treatment response compared to those without childhood maltreatment and the same psychiatric conditions (17). In the neurological literature, there is some evidence to support that the frequency and course of patients with headaches is worse in those who have experienced ALEs—particularly individuals with a history of childhood maltreatment (19–21). In adult men with Tourette Syndrome, a recent study also found an association between adverse childhood experiences and lifetime tic severity (22).
In this cohort study, we aimed to further investigate the possibility of a trauma-subtype of FND—with a focus on potential relationships to symptom severity and physical health. We stratified our mixed FND cohort based on the presence or absence of probable PTSD, and separately based on the presence or absence of endorsed childhood maltreatment (abuse and neglect considered separately) (23). Together, these complementary approaches allowed us to consider the potential implications of childhood maltreatment and/or the ongoing subjective impact of ALE-related psychopathology in potentially delineating FND subgroups (23). We hypothesized that patients with either probable PTSD and/or moderate-to-severe childhood maltreatment scores would demonstrate increased symptom severity and worse physical health scores.
Seventy-eight adults diagnosed with FND (53 females; 25 males; mean age 42.5 ± 13.9 years old; mean illness duration 4.0 ± 4.9 years) based on “rule in” examination signs and semiological features were prospectively recruited for study participation following an outpatient evaluation at the Massachusetts General Hospital FND Unit from 2014 to 2020 (see Supplementary Table 1 for additional details) (1). Importantly, given that patients commonly present with mixed functional neurological symptoms, we took a transdiagnostic approach across the spectrum of functional movement disorder (FND-movt) and FND-seiz (24, 25). Inclusion criteria were individuals 18 years of age or older with clinically-established FND-movt [n = 56; including functional weakness (n = 24), tremor (n = 23), gait (n = 22), jerks (n = 2), dystonia (n = 4), and speech difficulties (n = 5)] and/or FND-seiz [n = 34; composed of probable (n = 2), clinically-established (n = 2), or video-electroencephalography documented functional seizures (n = 30)] (26). FND diagnoses were not mutually exclusive, as 12 individuals had both FND-movt and FND-seiz. Patients with major neurologic comorbidities (n = 11, including 4 with epileptic seizures) were included. Exclusion criteria were illiteracy, history of mania or psychosis, active suicidality, and current illicit drug abuse or alcohol dependence. All participants provided written informed consent as approved by the institutional review board of Mass General Brigham. Note, psychometric findings from this cohort, unrelated to the present set of analyses, have been published elsewhere (7, 27–29).
The PTSD Checklist for DSM-5 (PCL-5) was used as one approach to stratify the FND cohort (30, 31). The PCL-5 has 20-items on the experience of PTSD symptoms in the past month, each rated “Not at all” (scored 0) to “Extremely” (scored 4), with a total score of ≥33 indicative of probable PTSD (30, 31). Individuals meeting current probable PTSD criteria were included in the “FND-PTSD_high” subgroup and all others placed in the “FND-PTSD_low” subgroup. Separately, the abuse and neglect subscale scores of the Childhood Trauma Questionnaire (CTQ) were used as alternative ways of dichotomizing the FND cohort (32). The 25-item CTQ assesses for experiences of abuse and neglect during childhood and adolescence. The cut-off values for moderate-to-severe subscale scores are as follows: ≥13 for emotional abuse, ≥10 for physical abuse, ≥8 for sexual abuse, ≥15 for emotional neglect, and ≥10 for physical neglect (33). To determine our “FND-Abuse_high” and “FND-Neglect_high” subgroups, any of the three abuse subscales or two neglect scales had to be equal to or greater than cut-off scores, respectively. We grouped physical, sexual, and emotional abuse scores together since they were strongly correlated, as were physical and emotional neglect scores.
Participants completed three symptom severity scales: the Somatoform Dissociation Questionnaire-20 (SDQ-20)—a 20-item questionnaire of functional neurological symptoms (e.g., seizures, paralysis, etc.) in the past year with each item rated on a 5-point Likert-type scale from “Not at all” (scored 1) to “Extremely” (scored 5) (34, 35); the Screening for Somatoform Symptoms-7 subscale for Conversion Disorder (SOMS:CD)—a 14-item questionnaire of functional neurological symptoms in the past week, with each item rated on a 5-point Likert scale from “Not at all” (scored 0) to “Severe” (scored 4) (36); and the Patient Health Questionnaire-15 (PHQ-15)—a 15-item measure of functional somatic symptoms (e.g., pain, fatigue) within the past 4 weeks, with each item rated from “Not Bothered at All” (scored 0) to “Bothered A Lot” (scored 2) (37). The physical health subscales of the Short Form Health Survey-36 were used to derive the physical health component score (SF36-physical health)—a measure of overall physical wellness and the extent to which physical symptoms limit an individual’s abilities to work and/or perform regular daily activities, with lower scores indicating greater disability (38).
To allow for post-hoc secondary analyses controlling for other relevant predisposing vulnerabilities, the following psychometric scales were also collected: Beck Depression Inventory-II (BDI)—a 21-item scale measuring depression symptom severity within the past 2 weeks, with each item scored from 0 to 3; the 20-items of the trait anxiety subscale of the Spielberger State-Trait Anxiety Inventory (STAI-trait), with each item scored in a 4-point scale ranging from “Almost Never” to “Almost Always”; the Dissociative Experiences Scale (DES)—a 28-item measure of dissociative experiences in daily life rated from 0 to 100%, with a cut-off mean value of ≥30% for pathological dissociation; Toronto Alexithymia Scale (TAS)—a 20-item measure of difficulty identifying/describing feelings and externally-oriented thinking, with each item scored on a 5-point Likert scale; Relationships Scales Questionnaire (RSQ)—a 30-item dimensional measure of adult attachment styles with each item scored on a 5-point Likert scale; NEO Five Factor Inventory-3 (NEO-FFI-3)—a 60-item measure characterizing the “Big Five” personality traits, with each item scored on a 5-point Likert-type scale from “Strongly Disagree” to “Strongly Agree”; and the Connor-Davidson Resilience Scale (CDRS)—a 25-item item scale accessing stress coping tendencies used in the past month, with each item scored on a 5-point Likert scale.
STATA-17 BE software (StataCorp LLC, College Station, Texas, USA) was used to run all statistical analyses, and missing data (n = 2) were omitted from analyses. We ran the Shapiro–Wilk test (command swilk) to determine the distribution of outcome measurements. A student t-test (command ttest) was used for normally distributed data and the Mann–Whitney Wilcoxon rank sum test (command ranksum) for non-parametric data, to investigate potential statistically significant differences in symptom severity (SDQ-20, SOMS:CD, and PHQ-15) and/or SF36-physical health scores between our stratified FND subgroups: (1) FND-PTSD_high vs. FND-PTSD_low; (2) FND-Abuse_high vs. FND-Abuse_low; (3) FND-Neglect_high vs. FND-Neglect_low. A Bonferroni correction for multiple comparisons was applied to the alpha value of 0.05 across all univariate statistics; only findings that remained significant entered secondary (post-hoc) linear regression analyses.
For all statistically significant univariate findings corrected for multiple comparisons, we conducted separate post-hoc linear regressions with the command regress always adjusting in blocks for the demographic variables of age, sex, white race (yes/no) and college graduate (yes/no), and other variables as follows: (1) only the previously mentioned demographic variables; (2) depression (BDI) and anxiety (STAI-trait) scores; (3) pathological dissociation (DES ≥ 30% yes/no); (4) TAS total score; (5) RSQ subscale scores for dismissing, fearful, preoccupied, and secure attachment styles; (6) NEO agreeableness, conscientiousness, extraversion, neuroticism, and openness personality trait scores; (7) CDRS total score; (8) FND-seiz (yes/no); (9) moderate-to-severe CTQ abuse and neglect scores (yes/no) in FND-PTSD_high analyses, or FND-PTSD_high and moderate-to-severe CTQ neglect (yes/no) if predicting FND-Abuse_high. For all regressions, variance inflation factor (estat vif) was used to look for multicollinearity amongst the independent variables.
See Table 1 for the breakdown of demographic variables and psychometric scores for FND-PTSD_high (n = 33) vs. FND-PTSD_low (n = 43), FND-Abuse_high (n = 46) vs. FND-Abuse_low (n = 32), and FND-Neglect_high (n = 33) vs. FND-Neglect_low (n = 45).
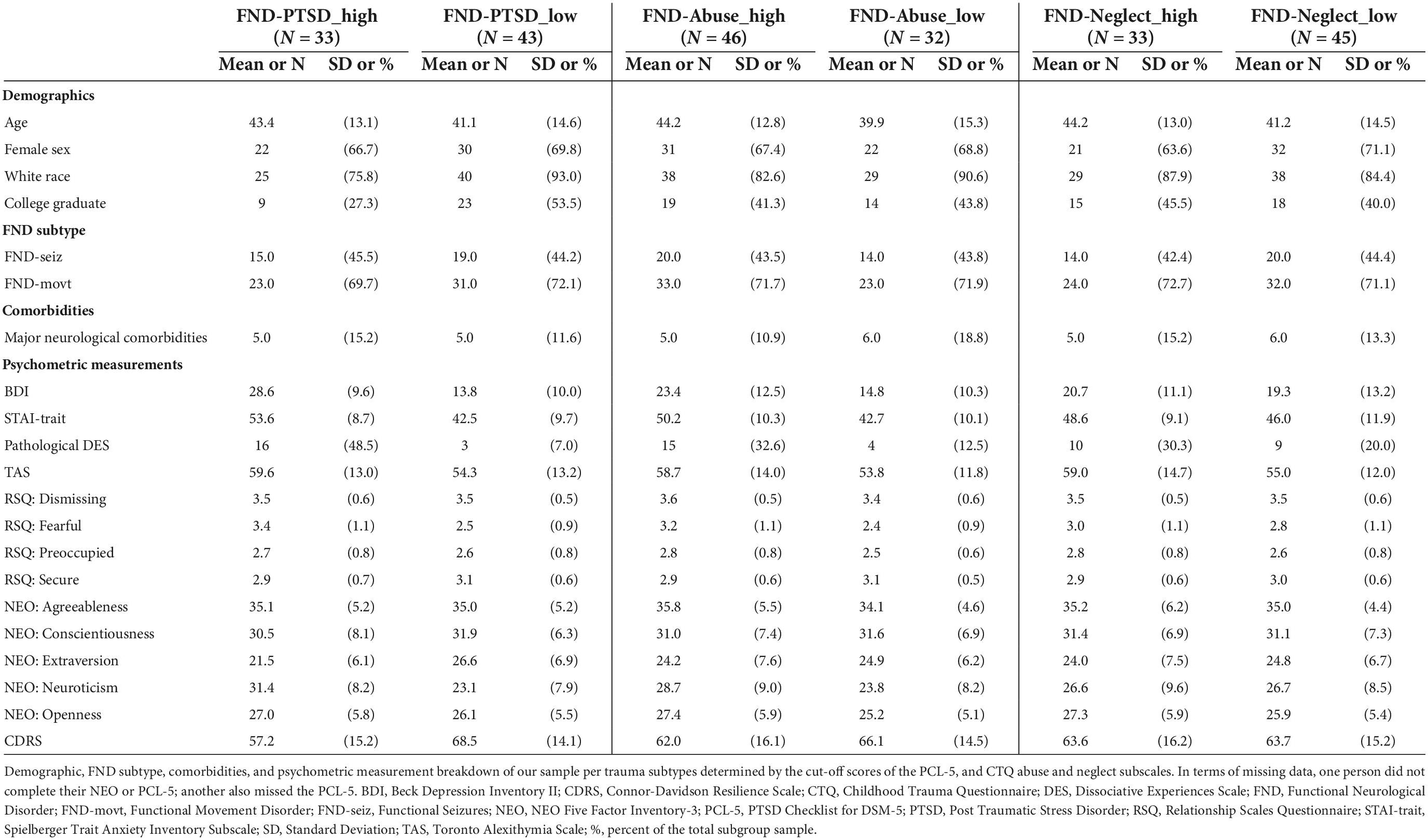
Table 1. Demographic and clinical characteristics of stratified functional neurological disorder subgroups.
The PHQ-15 and SF36-physical health scores were normally distributed, whereas the SOMS:CD and SDQ-20 scores were not. All symptom severity scores were significantly increased, and SF36-physical health scores significantly decreased, in patients with FND-PTSD_high vs. FND-PTSD_low. Additionally, patients with FND-Abuse_high vs. FND-Abuse_low showed statistically significant increases in SDQ-20 and PHQ-15 scores. There were no statistically significant differences in symptom severity or physical health scores in patients with FND-Neglect_high vs. FND-Neglect_low (see Table 2).
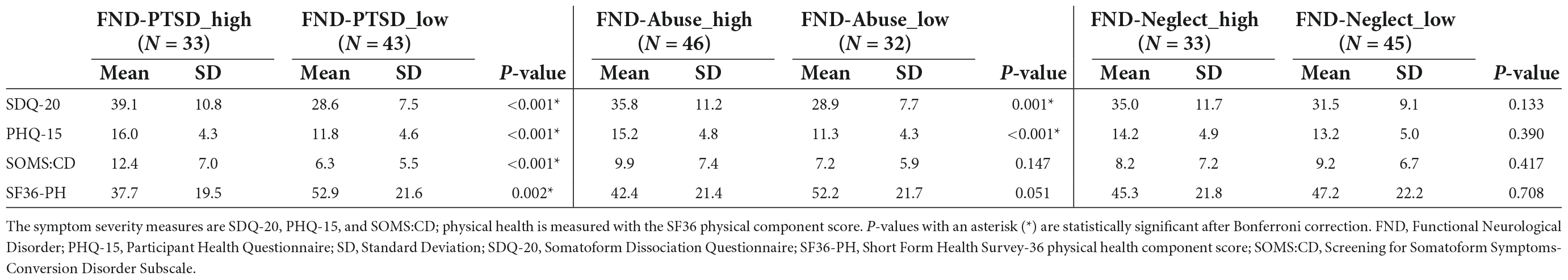
Table 2. Symptom severity and physical health scores in functional neurological disorder stratified by trauma subtypes.
In separate post-hoc linear regression analyses, FND-PTSD_high vs. FND-PTSD_low relationships to increased symptom severity and reduced SF36-physical health scores remained statistically significant adjusting for demographic variables and sequentially correcting for pathological dissociation; alexithymia; attachment styles; personality traits; resilience scores; FND-seiz; and for the other trauma scales. Only relationships between FND-PTSD_high and symptom severity as measured by the SDQ-20 and SOMS:CD also remained significant adjusting for depression and trait anxiety scores.
Additionally, in separate post-hoc linear regression analyses, FND-Abuse_high vs. FND-Abuse_low relationships to symptom severity (i.e., PHQ-15, SDQ-20) remained statistically significant adjusting serially for demographic and all other neuropsychiatric variables. There was no evidence of problematic collinearity across all regression analyses as measured by variance inflation factors. See Supplementary Tables 2–7 for a detailed description of all completed post-hoc analyses.
Our cohort study found that the FND-PTSD_high subgroup reported increased symptom severity and decreased physical health compared to those in the FND-PTSD_low subgroup; this finding remained statistically significant in post-hoc analyses adjusting for other variables except for when controlling for depression and anxiety scores in SF36-physical health or PHQ-15 related analyses. Furthermore, stratification based on the presence of moderate-to-severe childhood abuse also showed statistically significant increases in symptom severity in the FND-Abuse_high subgroup—findings that held for all post-hoc analyses. Stratification per childhood neglect did not relate to either patient-reported symptom severity or physical health scores. This data lends support to the possibility of a clinically-significant trauma subtype of FND (based on high comorbid PTSD symptoms and/or high childhood abuse burden).
Consideration of a trauma subtype of FND is consistent with emerging themes in neurobiological research in this population. In gray matter quantitative analyses in patients with FND, childhood maltreatment and ALEs more broadly are linked to insula, amygdala, hippocampus, putamen, and cerebellum structural alterations (12, 39, 40). Similarly, several resting-state functional magnetic resonance imaging (fMRI) studies identified that the connectivity profiles of nodes implicated in the pathophysiology of FND (i.e., insula, amygdala, temporoparietal junction, motor control areas) are impacted by the magnitude of early life adversity (41–43). For example, one study showed that the magnitude of childhood physical abuse severity related to how closely coupled the amygdala and insula were to the primary motor cortex; this study included a psychiatric control group matched for trauma burden—with the psychiatric control group not showing similar brain-trauma functional connectivity relationships (41). In a neuroimaging—genetics study, the magnitude of endorsed childhood trauma (CTQ total score) in patients with FND with a tryptophan gene polymorphism positively correlated with symptom severity; this genetic variant also impacted the connectivity between the amygdala and middle frontal gyrus (44). In addition to the neuroimaging literature, sexual trauma correlated with increased basal diurnal salivary cortisol in patients with FND-seiz, as well as a decreased cortisol and amylase response to a social stress test (45, 46). Lastly, a study found that a history of sexual trauma in patients with FND-seiz correlated with increased vigilance to a social threat during a Trier Social Stress Task (47). Overall, the literature points toward a clinical and neurobiologically distinct subtype of FND in the context traumatic experiences.
There are treatment implications for a potential framing of a trauma subtype of FND, most notably psychotherapeutic considerations (48). In the largest psychotherapy trial conducted to date, COgnitive behavioral therapy for adults with Dissociative non-Epileptic Seizures (CODES) trial, 368 patients with FND-seiz were randomized to cognitive behavioral therapy (CBT) plus standardized medical care (n = 186) vs. standardized medical care alone (n = 182) (49, 50). Although many secondary benefits were found in the CBT treatment arm, the study did not identify a statistically significant treatment effect on the primary outcome of seizure frequency at 12-months post-randomization. Since the publication of this landmark study, several research groups have advocated for the need to refine the pairing of specific psychotherapy modalities to a given patient with FND based on clinical formulation (e.g., what else is present alongside this patient’s functional neurological symptoms) (51–53). For example, there is evidence to suggest that patients with FND-seiz and PTSD may benefit from prolonged exposure (PE) therapy (54); other psychotherapy treatment modalities such as dialectical behavioral therapy (DBT), eye movement desensitization and reprocessing (EMDR), mindfulness-based psychotherapy (MBT), and acceptance and commitment therapy (ACT) have all been piloted in FND populations (55–58). Operationalizing a trauma-subtype of FND would facilitate increased recognition and research efforts to study how to best pair a given patient with FND to the psychotherapy modality they are most likely to benefit from. Defining this subgroup would also open new areas of therapeutic research inquiry, such as consideration of other forms of trauma-informed psychotherapy (e.g., sensorimotor psychotherapy) and the need for additional clinical trial research in treatment refractory individuals (e.g., testing the potential utility of ketamine infusions) (59, 60).
There are several gaps that remain with regards to the kind of data needed to adequately define a trauma subtype of FND. One of the most critical features to consider further is the nature of traumatic experiences and their impact on a given individual. In this study, the self-report of PTSD symptoms based on the PCL-5 with a cut-off indicative of probable PTSD drew significant results for the comparison of FND-PTSD_high vs. FND-PTSD_low in three symptom severity and one physical health measure. Alternatively, the CTQ abuse subscale measuring reported childhood sexual, physical and emotional abuse was used to divide individuals into FND-Abuse_high vs. FND-Abuse_low subgroups—with this stratification approach also leading to significant associations with two patient-reported symptom severity scores. The endorsement of active PTSD symptoms can be thought of as a proxy for the subjective current impact of past traumatic experiences. CTQ scores help quantify patient-reported childhood maltreatment regardless of concurrent PTSD symptoms. Additional work is needed to further understand if subjective ongoing relevance, subjective occurrence, or both are important factors in how to operationalize a trauma subtype of FND. Notably, large scale research has identified that the subjective occurrence of childhood maltreatment is a strong predictor of the development of psychopathology, irrespective of objective documentation of such experiences (23).
This study has several limitations, including the lack of a DSM-5 structured psychiatric interview and no lifetime measure of ALE severity. We also do not have information on the specific timing of ALEs, and as such we are unable to comment on the potential influence of critical developmental periods in linking childhood abuse to later life FND symptom severity. Other nuanced trauma-related factors such as escape potential require continued inquiry (61, 62), as well as a comprehensive investigation of potential mediators and moderators of relationships between ALEs, PTSD, symptom severity, and physical health in patients with FND. Discrete forms of childhood abuse (physical vs. sexual vs. emotional) may also be important considerations needing more research. Our study found that associations between the FND-PTSD_high subgroup and physical health or somatic symptom scores did not remain significant when adjusting for depression/anxiety scores, underscoring close associations between mood/anxiety, non-motor symptoms and physical health-related quality of life (63, 64). Additionally, while several independent FND cohorts showed positive associations between trauma burden and symptom severity (7–10, 12), one study of individuals with FND-seiz in Iran did not identify this association (65). This suggests that larger scales replication studies are needed, including consideration of sociocultural factors. Furthermore, it will be important to examine similarities and differences between a trauma subtype of FND and other psychological trauma-related clinical populations (66, 67). It also remains unclear what mechanisms link ALEs to FND symptom severity. Some considerations include overlapping brain–trauma and brain–symptom severity relationships, as well as possible shared predictive processing influences. For example, life experiences impact the repertoire of emotion and non-emotion concepts, and differences in emotion granularity and emotion category construction warrant future research as potential bridges between ALEs and the development/severity of FND symptoms (68). Lastly, prospective longitudinal studies are needed to determine if clinical outcomes reliably differ in patients with FND with or without a history of trauma.
In conclusion, this study lends support to a possible trauma-subtype of FND–across the transdiagnostic spectrum of FND-movt and FND-seiz. Additional research is needed to replicate and expand upon the findings of this study, including investigating if neural mechanisms and clinical outcomes are the same or different in patients with FND with or without a history of childhood maltreatment (or concurrently active PTSD symptoms).
The dataset presented in this article are not readily available because sharing of this data requires local IRB approval. However, on reasonable request by other researchers, anonymized raw data related to this research will be made available pending approval by the local IRB. Requests to access the dataset should be directed to DP, ZGxwZXJlekBubXIubWdoLmhhcnZhcmQuZWR1.
The studies involving human participants were reviewed and approved by Mass General Brigham IRB, Massachusetts General Hospital. The patients/participants provided their written informed consent to participate in this study.
All authors were involved in all stages of preparing this manuscript, including the literature review, performance of statistical analyses, drafting and editing the manuscript, and approved the submitted version.
This research was funded by the NIMH K23MH111983 and R01MH125802 grants.
Author DP had received honoraria for continuing medical education lectures on functional neurological disorder, royalties from Springer Nature for a textbook on functional movement disorder, is on the Board of Directors of the Functional Neurological Disorder Society, and on the editorial boards of Epilepsy and Behavior and Brain and Behavior.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1040911/full#supplementary-material
1. Aybek S, Perez DL. Diagnosis and management of functional neurological disorder. BMJ. (2022) 376:o64. doi: 10.1136/bmj.o64
3. Oppenheim H. Diseases of the Nervous System: A Textbook for Student and Practitioner of Medicine. Philadelphia, PA: J.B. Lippincott Company (1900).
4. Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. (2018) 5:307–20. doi: 10.1016/s2215-0366(18)30051-8
5. Stone J, LaFrance WC Jr, Levenson JL, Sharpe M. Issues for Dsm-5: conversion disorder. Am J Psychiatry. (2010) 167:626–7. doi: 10.1176/appi.ajp.2010.09101440
6. Kanaan RAA, Craig TKJ. Conversion disorder and the trouble with trauma. Psychol Med. (2019) 49:1585–8. doi: 10.1017/s0033291719000990
7. Perez DL, Matin N, Barsky A, Costumero-Ramos V, Makaretz SJ, Young SS, et al. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and ptsd in functional neurological disorders. J Neurol Neurosurg Psychiatry. (2017) 88:491–7. doi: 10.1136/jnnp-2016-314998
8. Roelofs K, Spinhoven P, Sandijck P, Moene FC, Hoogduin KA. The impact of early trauma and recent life-events on symptom severity in patients with conversion disorder. J Nerv Ment Dis. (2005) 193:508–14. doi: 10.1097/01.nmd.0000172472.60197.4d
9. Roelofs K, Keijsers GP, Hoogduin KA, Näring GW, Moene FC. Childhood abuse in patients with conversion disorder. Am J Psychiatry. (2002) 159:1908–13. doi: 10.1176/appi.ajp.159.11.1908
10. Selkirk M, Duncan R, Oto M, Pelosi A. Clinical differences between patients with nonepileptic seizures who report antecedent sexual abuse and those who do not. Epilepsia. (2008) 49:1446–50. doi: 10.1111/j.1528-1167.2008.01611.x
11. Spinhoven P, Roelofs K, Moene F, Kuyk J, Nijenhuis E, Hoogduin K, et al. Trauma and dissociation in conversion disorder and chronic pelvic pain. Int J Psychiatry Med. (2004) 34:305–18. doi: 10.2190/ydk2-c66w-cl6l-n5tk
12. Jungilligens J, Popkirov S, Perez DL, Diez I. Linking gene expression patterns and brain morphometry to trauma and symptom severity in patients with functional seizures. Psychiatry Res Neuroimaging. (2022) 326:111533. doi: 10.1016/j.pscychresns.2022.111533
13. Gray C, Calderbank A, Adewusi J, Hughes R, Reuber M. Symptoms of posttraumatic stress disorder in patients with functional neurological symptom disorder. J Psychosom Res. (2020) 129:109907. doi: 10.1016/j.jpsychores.2019.109907
14. Morsy SK, Aybek S, Carson A, Nicholson TR, Stone J, Kamal AM, et al. The relationship between types of life events and the onset of functional neurological (conversion) disorder in adults: a systematic review and meta-analysis. Psychol Med. (2022) 52:401–18. doi: 10.1017/s0033291721004669
15. Tolchin B, Dworetzky BA, Martino S, Blumenfeld H, Hirsch LJ, Baslet G. Adherence with psychotherapy and treatment outcomes for psychogenic nonepileptic seizures. Neurology. (2019) 92:e675–9. doi: 10.1212/wnl.0000000000006848
16. Van der Feltz-Cornelis CM, Allen SF, Van Eck van der Sluijs JF. Childhood sexual abuse predicts treatment outcome in conversion disorder/functional neurological disorder. an observational longitudinal study. Brain Behav. (2020) 10:e01558. doi: 10.1002/brb3.1558
17. Teicher MH, Gordon JB, Nemeroff CB. Recognizing the importance of childhood maltreatment as a critical factor in psychiatric diagnoses, treatment, research, prevention, and education. Mol Psychiatry. (2022) 27:1331–8. doi: 10.1038/s41380-021-01367-9
18. Mendizabal A, Nathan C, Khankhanian P, Young G, Breen L, Dahodwala N. Adverse childhood experiences in patients with neurological disease (5075). Neurology. (2020) 94(15 Suppl.):5075.
19. Anda R, Tietjen G, Schulman E, Felitti V, Croft J. Adverse childhood experiences and frequent headaches in adults. Headache. (2010) 50:1473–81. doi: 10.1111/j.1526-4610.2010.01756.x
20. Anto M, Jaffee S, Tietjen G, Mendizabal A, Szperka C. Adverse childhood experiences and frequent headache by adolescent self-report. Pediatr Neurol. (2021) 121:51–5. doi: 10.1016/j.pediatrneurol.2021.04.004
21. Tietjen GE, Buse DC, Fanning KM, Serrano D, Reed ML, Lipton RB. Recalled maltreatment, migraine, and tension-type headache: results of the ampp study. Neurology. (2015) 84:132–40. doi: 10.1212/wnl.0000000000001120
22. Yang K, Essa A, Noriega D, Yu D, Osiecki L, Gauvin CA, et al. Relationship between adverse childhood experiences and symptom severity in adult men with tourette syndrome. J Psychiatr Res. (2022) 155:252–9. doi: 10.1016/j.jpsychires.2022.08.024
23. Danese A, Widom CS. Objective and subjective experiences of child maltreatment and their relationships with psychopathology. Nat Hum Behav. (2020) 4:811–8. doi: 10.1038/s41562-020-0880-3
24. Matin N, Young SS, Williams B, LaFrance WC Jr, King JN, Caplan D, et al. Neuropsychiatric associations with gender, illness duration, work disability, and motor subtype in a U.S. Functional Neurological Disorders Clinic Population. J Neuropsychiatry Clin Neurosci. (2017) 29:375–82. doi: 10.1176/appi.neuropsych.16110302
25. Tinazzi M, Morgante F, Marcuzzo E, Erro R, Barone P, Ceravolo R, et al. Clinical correlates of functional motor disorders: an italian multicenter study. Mov Disord Clin Pract. (2020) 7:920–9. doi: 10.1002/mdc3.13077
26. LaFrance WC Jr, Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the international league against epilepsy nonepileptic seizures task force. Epilepsia. (2013) 54:2005–18. doi: 10.1111/epi.12356
27. Williams B, Ospina JP, Jalilianhasanpour R, Fricchione GL, Perez DL. Fearful attachment linked to childhood abuse, alexithymia, and depression in motor functional neurological disorders. J Neuropsychiatry Clin Neurosci. (2019) 31:65–9. doi: 10.1176/appi.neuropsych.18040095
28. Ospina JP, Larson AG, Jalilianhasanpour R, Williams B, Diez I, Dhand A, et al. Individual differences in social network size linked to nucleus accumbens and hippocampal volumes in functional neurological disorder: a pilot study. J Affect Disord. (2019) 258:50–4. doi: 10.1016/j.jad.2019.07.061
29. Jalilianhasanpour R, Williams B, Gilman I, Burke MJ, Glass S, Fricchione GL, et al. Resilience linked to personality dimensions, alexithymia and affective symptoms in motor functional neurological disorders. J Psychosom Res. (2018) 107:55–61. doi: 10.1016/j.jpsychores.2018.02.005
30. Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD Checklist for Dsm-5 (Pcl-5) U.S. Department of Veterans Affairs: National Center for PTSD. (2013). Available online at: https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp (accessed July 28, 2022).
31. National Center for PTSD. Using the PTSD Checklist for DSM-5 (Pcl-5). (2020). Available online at: https://www.ptsd.va.gov/professional/assessment/documents/using-PCL5.pdf (accessed September 1, 2022).
32. Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. (1994) 151:1132–6. doi: 10.1176/ajp.151.8.1132
33. Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report: Manual. New York, NY: The Psychological Corporation (1998).
34. Nicholson TR, Carson A, Edwards MJ, Goldstein LH, Hallett M, Mildon B, et al. Outcome measures for functional neurological disorder: a review of the theoretical complexities. J Neuropsychiatry Clin Neurosci. (2020) 32:33–42. doi: 10.1176/appi.neuropsych.19060128
35. Nijenhuis ER, Spinhoven P, Van Dyck R, Van der Hart O, Vanderlinden J. The development and psychometric characteristics of the somatoform dissociation questionnaire (Sdq-20). J Nerv Ment Dis. (1996) 184:688–94. doi: 10.1097/00005053-199611000-00006
36. Rief W, Hiller WA. New approach to the assessment of the treatment effects of somatoform disorders. Psychosomatics. (2003) 44:492–8. doi: 10.1176/appi.psy.44.6.492
37. Kroenke K, Spitzer RL, Williams JB. The Phq-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. (2002) 64:258–66. doi: 10.1097/00006842-200203000-00008
38. Ware JE Jr, Sherbourne CD. The Mos 36-item short-form health survey (Sf-36). I. conceptual framework and item selection. Med Care. (1992) 30:473–83.
39. Maurer CW, LaFaver K, Limachia GS, Capitan G, Ameli R, Sinclair S, et al. Gray matter differences in patients with functional movement disorders. Neurology. (2018) 91:e1870–9. doi: 10.1212/wnl.0000000000006514
40. Perez DL, Williams B, Matin N, LaFrance WC Jr, Costumero-Ramos V, Fricchione GL, et al. Corticolimbic structural alterations linked to health status and trait anxiety in functional neurological disorder. J Neurol Neurosurg Psychiatry. (2017) 88:1052–9. doi: 10.1136/jnnp-2017-316359
41. Diez I, Larson AG, Nakhate V, Dunn EC, Fricchione GL, Nicholson TR, et al. Early-Life trauma endophenotypes and brain circuit-gene expression relationships in functional neurological (conversion) disorder. Mol Psychiatry. (2021) 26:3817–28. doi: 10.1038/s41380-020-0665-0
42. Maurer CW, LaFaver K, Ameli R, Epstein SA, Hallett M, Horovitz SG. Impaired self-agency in functional movement disorders: a resting-state fMRI study. Neurology. (2016) 87:564–70. doi: 10.1212/wnl.0000000000002940
43. Drane DL, Fani N, Hallett M, Khalsa SS, Perez DL, Roberts NA. A framework for understanding the pathophysiology of functional neurological disorder. CNS Spectr. (2020) 26:1–7. doi: 10.1017/s1092852920001789
44. Spagnolo PA, Norato G, Maurer CW, Goldman D, Hodgkinson C, Horovitz S, et al. Effects of Tph2 gene variation and childhood trauma on the clinical and circuit-level phenotype of functional movement disorders. J Neurol Neurosurg Psychiatry. (2020) 91:814–21. doi: 10.1136/jnnp-2019-322636
45. Bakvis P, Spinhoven P, Giltay EJ, Kuyk J, Edelbroek PM, Zitman FG, et al. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia. (2010) 51:752–9. doi: 10.1111/j.1528-1167.2009.02394.x
46. Apazoglou K, Mazzola V, Wegrzyk J, Frasca Polara G, Aybek S. Biological and perceived stress in motor functional neurological disorders. Psychoneuroendocrinology. (2017) 85:142–50. doi: 10.1016/j.psyneuen.2017.08.023
47. Bakvis P, Roelofs K, Kuyk J, Edelbroek PM, Swinkels WA, Spinhoven P. Trauma, stress, and preconscious threat processing in patients with psychogenic nonepileptic seizures. Epilepsia. (2009) 50:1001–11. doi: 10.1111/j.1528-1167.2008.01862.x
48. Gutkin M, McLean L, Brown R, Kanaan RA. Systematic review of psychotherapy for adults with functional neurological disorder. J Neurol Neurosurg Psychiatry. (2020) 92:36–44. doi: 10.1136/jnnp-2019-321926
49. Goldstein LH, Robinson EJ, Mellers JDC, Stone J, Carson A, Reuber M, et al. Cognitive behavioural therapy for adults with dissociative seizures (codes): a pragmatic, multicentre, randomised controlled trial. Lancet Psychiatry. (2020) 7:491–505. doi: 10.1016/s2215-0366(20)30128-0
50. Goldstein LH, Robinson EJ, Chalder T, Stone J, Reuber M, Medford N, et al. Moderators of cognitive behavioural therapy treatment effects and predictors of outcome in the codes randomised controlled trial for adults with dissociative seizures. J Psychosom Res. (2022) 158:110921. doi: 10.1016/j.jpsychores.2022.110921
51. Perez DL. The codes trial for dissociative seizures: a landmark study and inflection point. Lancet Psychiatry. (2020) 7:464–5. doi: 10.1016/S2215-0366(20)30143-7
52. Myers L, Sarudiansky M, Korman G, Baslet G. Using evidence-based psychotherapy to tailor treatment for patients with functional neurological disorders. Epilepsy Behav Rep. (2021) 16:100478. doi: 10.1016/j.ebr.2021.100478
53. Demartini B, Marotta A, Castelnovo A, Del Piccolo L, Nisticò V, Gambini O, et al. Towards a tailored psychotherapy for patients with functional neurological disorders. J Affect Disord. (2022) 313:260–2. doi: 10.1016/j.jad.2022.06.022
54. Myers L, Vaidya-Mathur U, Lancman M. Prolonged exposure therapy for the treatment of patients diagnosed with psychogenic non-epileptic seizures (Pnes) and post-traumatic stress disorder (PTSD). Epilepsy Behav. (2017) 66:86–92. doi: 10.1016/j.yebeh.2016.10.019
55. Barrett-Naylor R, Gresswell DM, Dawson DL. The effectiveness and acceptability of a guided self-help acceptance and commitment therapy (act) intervention for psychogenic nonepileptic seizures. Epilepsy Behav. (2018) 88:332–40. doi: 10.1016/j.yebeh.2018.09.039
56. Bullock KD, Mirza N, Forte C, Trockel M. Group dialectical-behavior therapy skills training for conversion disorder with seizures. J Neuropsychiatry Clin Neurosci. (2015) 27:240–3. doi: 10.1176/appi.neuropsych.13120359
57. Baslet G, Ridlon R, Raynor G, Gonsalvez I, Dworetzky BA. Sustained improvement with mindfulness-based therapy for psychogenic nonepileptic seizures. Epilepsy Behav. (2022) 126:108478. doi: 10.1016/j.yebeh.2021.108478
58. Demirci OO, Sagaltici E. Eye movement desensitization and reprocessing treatment in functional neurological symptom disorder with psychogenic nonepileptic seizures: a study of two cases. Clin Child Psychol Psychiatry. (2021) 26:1196–207. doi: 10.1177/13591045211037276
59. Ogden P, Pain C, Fisher J. A sensorimotor approach to the treatment of trauma and dissociation. Psychiatr Clin North Am. (2006) 29:263–79. doi: 10.1016/j.psc.2005.10.012
60. Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. (2021) 178:193–202. doi: 10.1176/appi.ajp.2020.20050596
61. Aybek S, Nicholson TR, Zelaya F, O’Daly OG, Craig TJ, David AS, et al. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry. (2014) 71:52–60. doi: 10.1001/jamapsychiatry.2013.2842
62. Nicholson TR, Aybek S, Craig T, Harris T, Wojcik W, David AS, et al. Life events and escape in conversion disorder. Psychol Med. (2016) 46:2617–26. doi: 10.1017/s0033291716000714
63. LaFrance WC Jr, Syc S. Depression and symptoms affect quality of life in psychogenic nonepileptic seizures. Neurology. (2009) 73:366–71. doi: 10.1212/WNL.0b013e3181b04c83
64. Vìchetová G, Slovák M, Kemlink D, Hanzlíková Z, Dušek P, Nikolai T, et al. The impact of non-motor symptoms on the health-related quality of life in patients with functional movement disorders. J Psychosom Res. (2018) 115:32–7. doi: 10.1016/j.jpsychores.2018.10.001
65. Asadi-Pooya AA, Bahrami Z. Sexual abuse and psychogenic nonepileptic seizures. Neurol Sci. (2019) 40:1607–10. doi: 10.1007/s10072-019-03887-3
66. Roberts NA, Burleson MH, Weber DJ, Larson A, Sergeant K, Devine MJ, et al. Emotion in psychogenic nonepileptic seizures: responses to affective pictures. Epilepsy Behav. (2012) 24:107–15. doi: 10.1016/j.yebeh.2012.03.018
67. Roberts NA, Burleson MH, Torres DL, Parkhurst DK, Garrett R, Mitchell LB, et al. Emotional reactivity as a vulnerability for psychogenic nonepileptic seizures? responses while reliving specific emotions. J Neuropsychiatry Clin Neurosci. (2020) 32:95–100. doi: 10.1176/appi.neuropsych.19040084
Keywords: functional neurological disorder, functional movement disorder, PTSD, symptom severity, physical health, childhood abuse, functional seizures, trauma
Citation: Paredes-Echeverri S, Guthrie AJ and Perez DL (2022) Toward a possible trauma subtype of functional neurological disorder: impact on symptom severity and physical health. Front. Psychiatry 13:1040911. doi: 10.3389/fpsyt.2022.1040911
Received: 09 September 2022; Accepted: 25 October 2022;
Published: 15 November 2022.
Edited by:
Lorna Myers, Northeast Regional Epilepsy Group, United StatesReviewed by:
Nicole A. Roberts, Arizona State University, United StatesCopyright © 2022 Paredes-Echeverri, Guthrie and Perez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David L. Perez, ZGxwZXJlekBubXIubWdoLmhhcnZhcmQuZWR1
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.