- 1Department of Dermatology, Chonnam National University Medical School, Gwangju, South Korea
- 2Department of Hemato-Oncology, Chonnam National University Medical School, Gwangju, South Korea
- 3Department of Anatomy, Chonnam National University Medical School, Gwangju, South Korea
- 4Department of Psychiatry, Chonnam National University Medical School, Gwangju, South Korea
Background: The presence of psychological distress has negatively affected the course and prognosis of melanoma. Psychological distress is influenced by cytokines and gene mutations, particularly in cancer, but no studies have investigated this phenomenon in melanoma patients. This study investigated the correlations of psychological distress, plasma cytokine levels, and gene mutations in melanoma patients, focusing on melanoma sites and TNM stages.
Methods: This study prospectively evaluated melanoma patients who visited Chonnam National University Hwasun Hospital from September 2020 to March 2021. Melanoma sites were divided into acral and non-acral sites. Anxiety and depression were evaluated using the Hospital Anxiety and Depression Scale, and quality of life was evaluated with EuroQol-5 Dimensions. Plasma cytokine levels, and depression- and cytokine-related gene mutations were analyzed.
Results: This study included 151 melanoma patients. Anxiety was found in 14.6% of the patients, and depression in 29.8%. The melanoma sites were not significantly associated with anxiety, depression, or quality of life. However, psychological distress was significantly associated with the plasma cytokines IL-2, IL-4, IL-5, IL-10, IL-12, TNF-α and IFN-γ. COMT, SLC6A4, SLC6A3, and IL-12b gene mutations were also associated with melanoma sites and TNM stage, anxiety, and QOL.
Conclusion: Psychological distress was associated with plasma cytokine levels and depression- and cytokine-related gene mutations. Using psychiatric intervention and emotional support, cytokine levels related to melanoma can be changed, which may have positive effects on the prognosis and treatment of melanoma. More careful follow-up, evaluation, and management are needed for patients with gene mutations.
Introduction
The presence of psychological distress has negative effects on the course and prognosis of melanoma (1). The reported prevalence of anxiety is 32∼40% in melanoma and that of depression is 15∼33% (2, 3), and these patients have a poor quality of life (QOL) (4). Cytokine changes are related to anxiety, depression, and QOL (5–7). These include immune-modulating cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), anti-inflammatory cytokines such as interleukin-4 (IL-4), IL-5, IL-10, and IL-13, and pro-inflammatory cytokines such as IL-2, IL-12, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). Cytokine changes are associated with anxiety, depression, and QOL in patients with various tumors, including breast and colorectal cancers (8–11). However, few studies have investigated this association in patients with melanoma.
Individual differences in cytokine production have genetic origins, affected by the transcriptional activity of cytokine gene mutations (12). Numerous studies have focused on the genetic factors of psychological distress, especially depression (9, 13, 14). Therefore, cytokine- and depression-related genes, which can cause changes in cytokine production associated with cancer progression or treatment, may confer risks of psychological distress in cancer. To our knowledge, however, no studies have tested this hypothesis in melanoma patients.
Therefore, this study investigated the correlations of psychological distress with plasma cytokine levels, and the associations of psychological distress and cytokine- and depression-related gene mutations according to the anatomic sites and TNM stages of melanoma.
Materials and methods
Study population
The study enrolled 151 patients with melanoma who visited Chonnam National University Hwasun Hospital from September 2020 to March 2021. All patients were confirmed to have melanoma clinically and histopathologically. Exclusion criteria included (1) patients who do not consent to study participation; (2) Patients who were not confirmed to have melanoma clinically or histopathologically; and (3) patients who are unable to make their own decisions (e.g., newborns and infants). This study was approved by the Chonnam National University Hospital Institutional Review Board (CNUH 2020-196). All participants provided informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Demographic characteristics
Demographic and clinicopathological characteristics were obtained from the medical records and clinical photographs. Melanoma staging was based on the eighth edition of the American Joint Committee on Cancer melanoma staging system, and the TNM stage was divided into melanoma in situ (stage 0) and stages I to IV (15). The abbreviation ‘TNM’ refers to primary tumor (T), nodes (N), and metastases (M). ‘Nodes’ indicates whether the neighboring regional lymph nodes are affected, and ‘metastases’ describes whether distant metastases have been found. We analyzed the TNM stage at the time of the survey. The anatomic sites of melanoma were classified into acral (palm, sole, and nails) and non-acral (head and neck, trunk and extremities, and genitalia) sites.
Evaluations of psychological symptoms and quality of life
The Hospital Anxiety and Depression Scale (HADS), an instrument used internationally for assessing anxiety and depression, was used (16). This scale comprises two seven-item subscales: the anxiety (HADS-A) and depression (HADS-D) subscales. Each item is scored on 4-point scale (0–3), resulting in a total score of 0–21 for each subscale, with higher scores indicating more severe disease. A score of > 7 suggests clinically relevant anxiety or depression. The EuroQol-5 Dimensions (EQ-5D) is a standardized tool for measuring the health-related QOL (17). It consists of five dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Each dimension has three-point rating scale (1–3), with higher scores indicating a lower QOL. These questionnaires were handed out to patients and self-administered.
Cytokine analyses
Plasma cytokine levels were measured using a multiplex assay on the Luminex 100™ system (Luminex, Austin, TX, USA) according to the manufacturers’ instructions. Immune-modulating (GM-CSF), anti-inflammatory (IL-4, IL-5, IL-10, and IL-13), and pro-inflammatory (IL-2, IL-12, IFN-γ, and TNF-α) cytokines were analyzed.
Cytokine- and depression-related gene mutation analyses
Whole exome sequencing was performed on the participants’ venous blood at Macrogen (Korea), using an Illumina NovaSeq platform, according to manufacturer’s protocols. Cytokine- and depression-related gene mutations were anayzed, including solute carrier family 6 member 4 (SLC6A4), solute carrier family 6 member 3 (SLC6A3), HTR2A (encodes the serotonin receptor), DRD4 (encodes the dopamine receptor), catechol-o-methyl transferase (COMT), tyrosine hydroxylase (TH), and monoamine oxidase A (MAOA).
Statistical analysis
Differences in age, sex, plasma cytokine levels, psychological symptoms, and QOL were evaluated according to melanoma sites and TNM stages using t-tests, chi-square tests, and Mann–Whitney U-tests. Correlations of psychological symptoms and QOL with plasma cytokine levels were evaluated using Spearman’s rank correlation. Associations of cytokine- and depression-related gene mutations with melanoma sites and TNM stages, and psychological distress were evaluated using Mann–Whitney U-tests and Fisher’s exact tests. A P-value < 0.05 was considered significant. Statistical analyses were conducted using SPSS for Windows ver. 21.0 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
Table 1 summarizes the patient demographics and melanoma TNM stages according to anatomic sites. Of the 151 patients, 68 (45.0%) were men and 83 (55.0%) were women. Their mean age was 64.4 ± 13.1 years. Overall, 44 (29.1%) of patients were diagnosed with melanoma in situ, and there were 29 (21.2%), 27 (17.9%), 28 (18.5%), 20 (13.2%) with stage I to IV, respectively. Overall, 98 (64.9%) developed acral melanomas and 53 (35.1%) developed non-acral melanomas. The patients’ baseline characteristics, including age and sex, did not differ significantly between acral and non-acral sites.
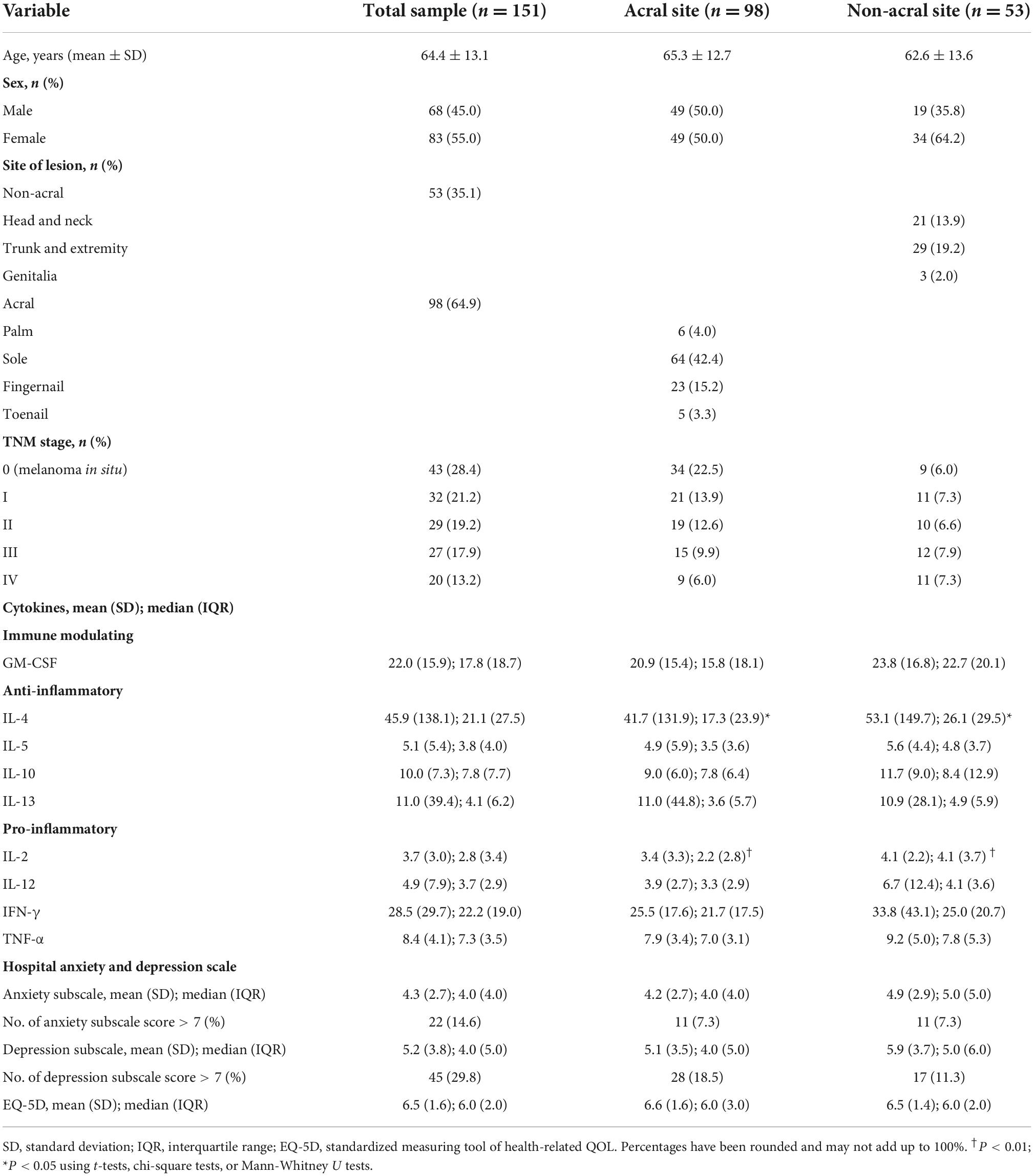
Table 1. Patient demographics, TNM stage of melanoma, plasma cytokine levels, hospital anxiety and depression scale score, and EQ-5D score by melanoma anatomic sites.
Differences of plasma cytokine levels between acral and non-acral melanomas
Plasma cytokine levels of the patients were compared according to anatomic sites of melanoma (Table 1). As a result, IL-2 (P = 0.008) and IL-4 (P = 0.027) were statistically significantly lower in acral melanoma patients than non-acral melanoma patients, and the results showed no statistically significant difference in other cytokines.
Anxiety, depression, and quality of life subscale
The mean anxiety subscale score was 4.3 ± 2.7, and 22 (14.6%) of patients had a score > 7 (Table 1). The mean depression subscale score was 5.2 ± 3.8, and 45 (29.8%) of patients had a score > 7. The mean EQ-5D score was 6.5 ± 1.6. The mean anxiety subscale score of acral melanoma patients was 4.2 ± 2.7, and 7.3% (n = 11) of patients had a score > 7. The mean depression subscale score of acral melanoma patients was 5.1 ± 3.5, and 18.5% (n = 28) of patients had a score > 7. The mean EQ-5D score of acral melanoma patients was 6.6 ± 1.6. The mean anxiety subscale score of non-acral melanoma patients was 4.9 ± 2.9, and 7.3% (n = 11) of patients had a score > 7. The mean depression subscale score of non-acral melanoma patients was 5.9 ± 3.7, and 11.3% (n = 17) of patients had a score > 7. The mean EQ-5D score of non-acral melanoma patients was 6.5 ± 1.4. All subscales were compared between acral site and non-acral site, and there were no significant differences.
Correlations of plasma cytokine levels with psychological distress according to melanoma site and TNM stage
In acral melanoma, the depression was negatively correlated with the IL-2 concentration (P = 0.02, Rho = −0.243), but there were no significant correlations between anxiety and QOL and any of the cytokines (Table 2). In non-acral melanoma, the anxiety was negatively correlated with the IL-4 (P = 0.042, Rho = −0.301) and IL-12 (P = 0.006, Rho = −0.382) concentrations, but there were no significant correlations between depression and QOL and any of the cytokines. In TNM stage 0 or I patients, no significant correlations were found with any cytokine. However, in patients with TNM stages II to IV, the anxiety was negatively correlated with the IL-4 (P = 0.030, Rho = −0.265), IL-12 (P = 0.025, Rho = −0.267), and TNF-α (P = 0.014, Rho = −0.287) concentrations, and the depression (P = 0.046, Rho = 0.231) and low QOL (P = 0.025, Rho = 0.260) were positively correlated with IFN-γ concentration.
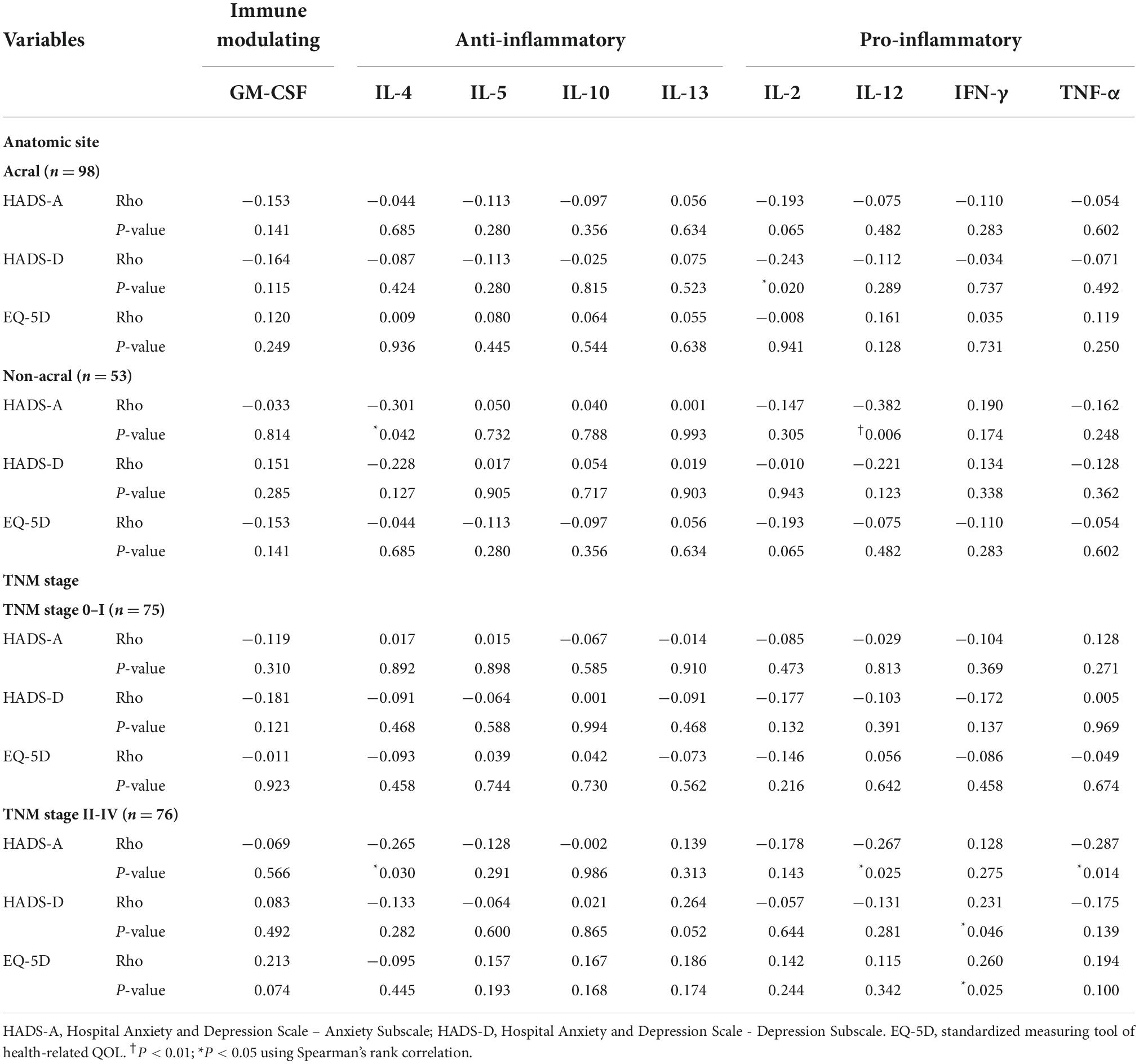
Table 2. Correlations of cytokine levels with anxiety, depression, and QOL by melanoma site and TNM stage.
Correlations of plasma cytokine levels with psychological distress in acral and non-acral melanomas
The correlations of anxiety, depression and QOL with cytokine levels by the acral melanoma TNM stage were not significant for any cytokine (Table 3). However, in TNM stage 0 or I non-acral melanoma, depression was negatively correlated with IL-12 (P = 0.018, Rho = −0.534) concentrations, and low QOL negatively with IL-12 (P = 0.043, Rho = −0.468) and IL-2 (P = 0.023, Rho = −0.519). In stage II to IV non-acral melanoma, anxiety was negatively correlated with the IL-12 concentrations (P = 0.018, Rho = −0.421), depression positively with IFN-γ (P = 0.014, Rho = 0.425), and low QOL positively with IFN-γ (P = 0.008, Rho = 0.457), IL-10 (P = 0.027, Rho = 0.397), and IL-5 (P = 0.044, Rho = 0.363).
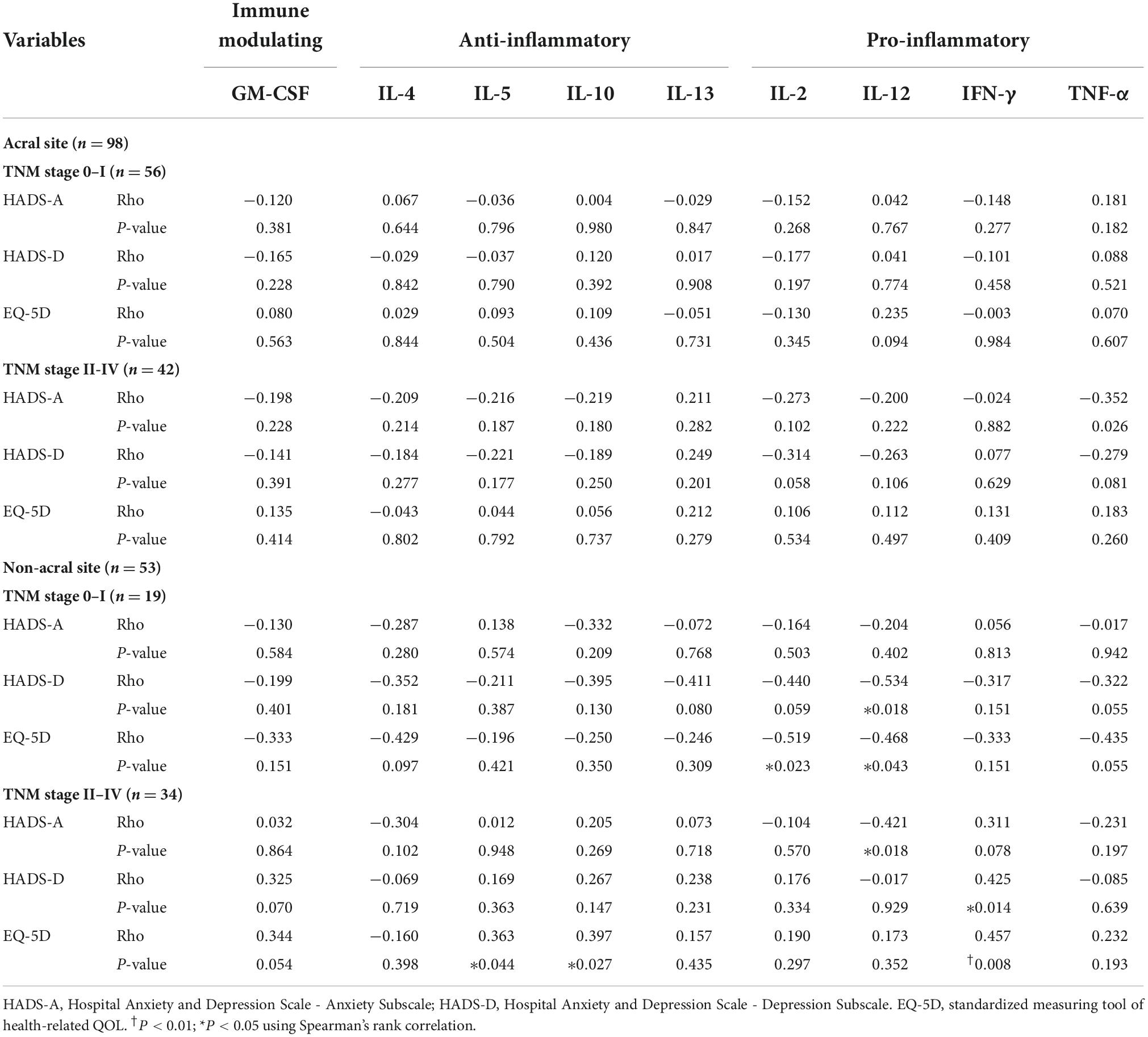
Table 3. Correlations of anxiety, depression, and QOL with cytokine levels by acral and non-acral melanoma TNM stage.
Associations of cytokine- and depression-related gene mutations with anatomic sites, TNM stages, and psychological distress
There were no significant associations of cytokine-related gene mutations with melanoma sites and TNM stages (Table 4). However, the COMT gene mutation showed a significant association in acral melanoma (P = 0.016), and the SLC6A4 gene mutation had a significant association in patients with TNM stage 0 or I (P = 0.008). There were no significant associations of any gene mutations with depression (Table 5). However, the IL-12b gene mutation was significantly associated with anxiety (P = 0.031), and the SLC6A3 gene mutation was significantly associated with low QOL (P = 0.017).
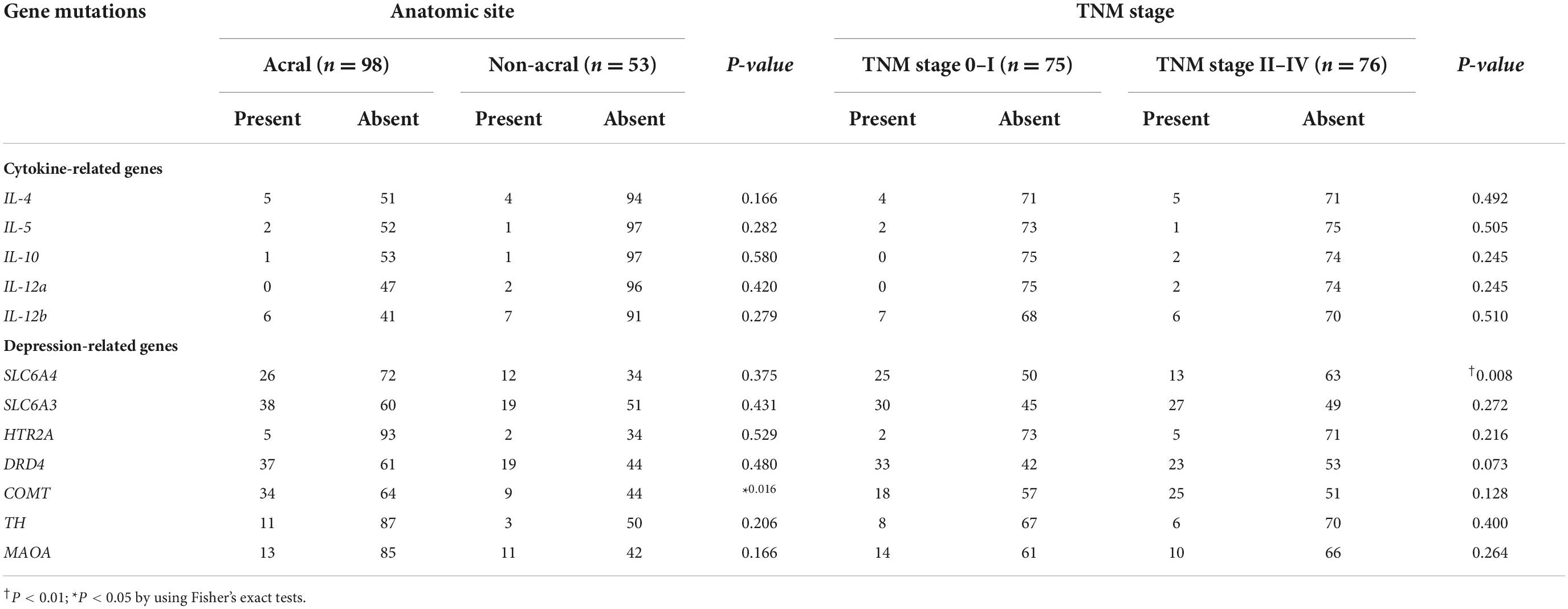
Table 4. Associations of cytokine- and depression-related gene mutations with anatomic sites and TNM stage of melanoma.
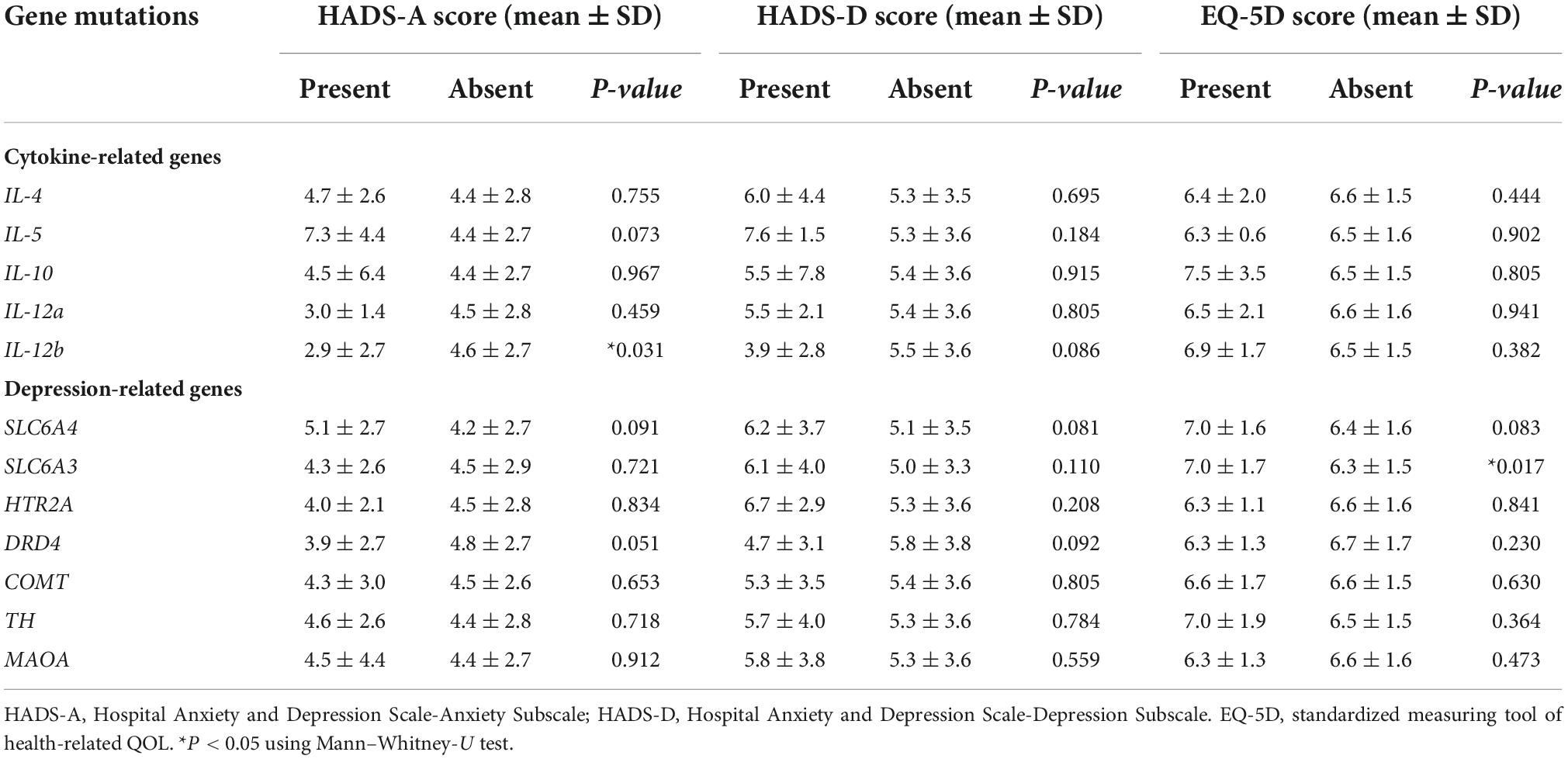
Table 5. Associations of cytokine- and depression-related gene mutations with anxiety, depression, and QOL.
Discussion
A few recent studies have examined prevalence of anxiety and depression in melanoma patients. Tas et al. (2) reported that about one third of Turkish melanoma patients had anxiety and depression, and Beesley et al. (3) reported anxiety in 32% of Australian melanoma patients and depression in 15%, and their emotional QOL was poor compared to the general population. In our study, 14.9% of melanoma patients had clinically relevant anxiety, and 29.8% had depression, similar to slightly lower than previous results (2, 3). No studies have reported associations of psychological distress with anatomic sites of melanoma. Because acral melanoma is the most common subtype in Asian populations (18), we enrolled more patients with acral melanomas. However, the melanoma anatomic sites were not associated with anxiety, depression or QOL. Therefore, we postulate that melanoma itself, not its anatomic sites, causes psychological distress in melanoma patients.
Several studies have examined cytokines in melanoma, including cytokine expression and application to treatments (19, 20). To our knowledge, this is the first investigation to find associations of melanoma anatomic sites and TNM stages with psychological distress by comparing plasma cytokine levels. We found that IL-4 and IL-12 were significantly associated with anxiety. According to Kawakami et al. (21) IL-4 promotes the growth of tumor-infiltrating lymphocytes, which are cytotoxic for human autologous melanoma, and can be used in cellular melanoma immunotherapy. In our study, IL-4 was negatively correlated with anxiety in patients with non-acral melanoma, TNM stages II to IV, and non-acral melanoma with TNM stages II to IV. Our result also supports the finding that IL-4 knock-out mice display anxiety-like behavior, in comparison to wild-type mice (22). In addition, we compared plasma cytokine levels according to anatomic sites, and patients with acral melanoma showed lower levels of IL-4 than patients with non-acral melanoma. It suggests that immunotherapy using IL-4 or immune-checkpoint inhibitors may have lower therapeutic effects on the acral melanoma than non-acral melanoma, however, further research is needed on this issue.
IL-12 is a pro-inflammatory cytokine that stimulates both innate and adaptive immunity (23). In our study, IL-12 was negatively correlated with anxiety in patients with non-acral melanoma and TNM stages II to IV. IL-12 was also negatively correlated with depression in non-acral melanoma with TNM stages 0 and I, and with low QOL in non-acral melanoma TNM stages 0 and I. In addition, the IL-12b gene mutation was significantly associated with a low HADS-A score. Daud et al. (24) reported that plasmid IL-12 electroporation induced disease stabilization and a partial response in patients with metastatic melanoma, along with tumor necrosis and lymphocytic infiltration. Fang et al. (25) reported a significant correlation between the IL-12b gene mutation and serum IL-12 levels in melanoma patients, and the IL-12p40 mutation contributed to melanoma susceptibility and influenced patient outcome.
Due to its antitumor activity, a high-dose bolus of recombinant IL-2 has been used to treat melanoma for decades (26). IL-2, a T-cell growth factor, was negatively correlated with depression in patients with acral melanoma, and with a low QOL in non-acral melanoma with TNM stages 0 and I, in our study. Therefore, the prognosis of melanoma might be improved by increasing IL-2 levels by relieving depression and increasing QOL through psychological support. Also, patients with acral melanoma showed lower levels of IL-2 than non-acral melanoma. It suggests that immunotherapy using IL-2 may have different effects depending on the anatomical site of melanoma, and further studies are needed on this issue.
In our study, TNF-α was negatively correlated with anxiety at TNM stages II to IV. However, IFN-γ was positively correlated with depression at TNM stages II to IV patients overall and in non-acral melanoma, and with a low QOL in TNM stage II to IV patients. TNF-α has potent antitumor activity via endothelial cell activation and vascular damage, resulting in hemorrhagic necrosis (27). The number of TNF-α receptors on malignant cells increases when cultured with IFN-γ (28). Lienard et al. (27) demonstrated that IFN-γ therapy in combination with TNF-α produced good responses in melanoma patients. Thus, the prognosis of melanoma might be improved by increasing the TNF-α levels through reducing anxiety with psychiatric intervention, along with surgical and systemic treatments. Depression and IFN-γ were positively correlated in our study, which is consistent with reports that plasma IFN-γ level increased in patients with depression (5).
IL-5 and IL-10 were positively associated with a low QOL in non-acral melanoma TNM stage II to IV. Although the relationship between melanoma and IL-5 has not been studied, IL-5 facilitates lung metastasis by modulating the immune environment (29). IL-10 also inhibits the production of many cytokines and suppresses tumor growth and metastasis of human melanoma cells by inhibiting macrophage-derived angiogenic factors (30). Based on these findings, the prognosis of melanoma may be improved by reducing the IL-5 levels through improving QOL via sufficient physical and emotional support. Further research on the relationship between the melanoma prognosis and IL-10 according to the QOL is needed.
We also assessed depression-related gene mutations. Several studies have reported that the COMT mutation is associated with the response to anxiety, depression (31), and Alzheimer’s disease (32). Studies have examined the association between melanoma pigmentation and COMT activity (33, 34). In our study, the COMT gene mutation was significant associated in patients with acral melanoma, which usually appear as markedly pigmented lesions, but the association with the COMT gene mutation needs further study.
The SLC6A4 gene mutation showed a significant association only in patients with TNM stage 0 or I; it was not associated with psychological distress. This gene encodes serotonin transporter protein, which is the target of many antidepressants (35), and several studies have reported an association between the SLC6A4 gene mutation and psychiatric disorders (36, 37). Other studies have linked the SLC6A3 gene mutation with psychiatric disorders such as depression, bipolar disorder, and alcoholism (38). In our study, a low QOL was associated with the SLC6A3 mutation. No studies have reported on the relationship between the SLC6A3 gene mutation and melanoma or QOL, and further research in this area is needed.
This study had several limitations. First, due to low prevalence of melanoma, we had no choice but to include almost all melanoma patients who visited our hospital as study subjects. They were included in the study regardless of whether they were first visits to the hospital, before or after surgery, and whether they received chemotherapy, immune checkpoint inhibitor therapy, or radiation therapy, which may had affected the plasma cytokine levels, so we had difficulty equalizing the conditions of study subjects. Second, the number of acral melanoma patients was almost twice that of non-acral melanoma patients, which may have affected the statistical analysis. Third, patients were not assessed for previous psychological disorders. Fourth, we administered an assessment scale (HADS) rather than a formal diagnostic instrument for evaluating anxiety and depression. Therefore, comorbidity of anxiety and depression could not be considered despite of their common simultaneous existence (39). In addition, we used Spearman’s rank correlation to evaluate correlations of psychological symptoms and QOL with plasma cytokine levels. However, Spearman’s rank correlation results can only show the relationship between the two, not the sequence and causality. The data may only suggest correlation, and may not demonstrate causation. Cytokines may be a bystander that are unrelated to melanoma biology and outcome, or the downstream effect of melanoma biology rather than a contributor to melanoma biology.
In conclusion, we found that psychological distress was associated with the cytokines IL-2, IL-4, IL-5, IL-10, IL-12, TNF-α, and IFN-γ. COMT, SLC6A4, SLC6A3, and IL-12b gene mutations were also associated with melanoma sites and TNM stage, anxiety, and QOL. Since this is the first report on supporting the inflammatory hypotheses of psychological symptoms in patients with melanoma, further replication and longitudinal studies are needed to confirm the findings. Moreover, future interventional studies would be helpful to investigate whether psychiatric treatment with emotional support can change cytokine levels related to melanoma, which may have positive effects on the prognosis and treatment of melanoma. More careful follow-up, evaluation, and management are indicated for those with gene mutations.
Data availability statement
The data presented in the study are deposited in the National Library of Medicine repository, at the link: https://dataview.ncbi.nlm.nih.gov/object/ with the accession numbers SAMN31327467, SAMN31327468, SAMN31327469, SAMN31327470 etc. up to SAMN31327617.
Ethics statement
The studies involving human participants were reviewed and approved by the Chonnam National University Hospital Institutional Review Board (CNUH 2020-196). The patients/participants provided their written informed consent to participate in this study.
Author contributions
HK and SY: conceptualization and writing—original draft preparation. HK, HS, J-MK, and SY: formal analysis. J-MK: funding acquisition. HK, CJ, IS, J-MK, and SY: investigation. J-MK and SY: methodology and writing—review and editing. HS, CJ, IS, J-MK, and SY: validation. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Research Foundation of Korea Grant (grant NRF- 2020R1A2C2003472) to J-MK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brandberg Y, Bolund C, Sigurdardottir V, Sjödén PO, Sullivan M. Anxiety and depressive symptoms at different stages of malignant melanoma. Psychooncology. (1992) 1:71–8.
2. Tas F, Karabulut S, Guveli H, Kurul S, Erturk K, Guveli M, et al. Assessment of anxiety and depression status in Turkish cutaneous melanoma patients. Asian Pac J Cancer Prev. (2017) 18:369–73. doi: 10.22034/APJCP.2017.18.2.369
3. Beesley VL, Smithers BM, Khosrotehrani K, Khatun M, O’Rourke P, Hughes MC, et al. Supportive care needs, anxiety, depression and quality of life amongst newly diagnosed patients with localised invasive cutaneous melanoma in Queensland, Australia. Psychooncology. (2015) 24:763–70. doi: 10.1002/pon.3718
4. Beutel ME, Fischbeck S, Binder H, Blettner M, Brähler E, Emrich K, et al. Depression, anxiety and quality of life in long-term survivors of malignant melanoma: a register-based cohort study. PLoS One. (2015) 10:e0116440. doi: 10.1371/journal.pone.0116440
5. Dowlati Y, Hermann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
6. Hou R, Garner M, Holmes C, Osmond C, Teeling J, Lau L, et al. Peripheral inflammatory cytokines and immune balance in generalized anxiety disorder: case-controlled study. Brain Behav Immunol. (2017) 62:212–8. doi: 10.1016/j.bbi.2017.01.021
7. Raymond WD, Eilertsen GØ, Shanmugakumar S, Nossent JC. The impact of cytokines on the health-related quality of life in patients with systemic lupus erythematosus. J Clin Med. (2019) 15:857. doi: 10.3390/jcm8060857
8. Kim SY, Kim JM, Kim SW, Shin IS, Park MH, Yoon JH, et al. Associations between plasma cytokines and depressive mood in patients with breast cancer. Int J Psychiatry Med. (2012) 43:1–17. doi: 10.2190/PM.43.1.a
9. Kim JM, Stewart R, Kim SY, Kang HJ, Jang JE, Kim SW, et al. A one year longitudinal study of cytokine genes and depression in breast cancer. J Affect Disord. (2013) 148:57–65. doi: 10.1016/j.jad.2012.11.048
10. Kim SY, Kim SW, Shin IS, Park MH, Yoon JH, Yoon JS, et al. Changes in depression status during the year after breast cancer surgery and impact on quality of life and functioning. Gen Hosp Psychiatry. (2018) 50:33–7. doi: 10.1016/j.genhosppsych.2017.09.009
11. Miranda DO, Lima TA, Azevedo LR, Feres O, Rocha JJ, Silva GP. Proinflammatory cytokines correlate with depression and anxiety in colorectal cancer patients. Biomed Res Int. (2014) 2014:739650. doi: 10.1155/2014/739650
12. de Craen AJ, Posthuma D, Remarque EJ, van den Biggelaar AH, Westendorp RG, Boomsma DI. Heritability estimates of innate immunity: an extended twin study. Genes Immun. (2005) 6:167–70. doi: 10.1038/sj.gene.6364162
13. Sullivan PF, Neale JM, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. (2000) 157:1552–62. doi: 10.1176/appi.ajp.157.10.1552
14. Shadrina M, Bondarenko EA, Slominsky PA. Genetic factors in major depression disease. Front Psychiatry. (2018) 9:334. doi: 10.3389/fpsyt.2018.00334
15. Keung EZ, Gershenwald JE. The eighth edition American joint committee on cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther. (2018) 18:775–84. doi: 10.1080/14737140.2018.1489246
16. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
17. Rabin R, Charr F. EQ-5D: a measure of health status from EuroQol group. Ann Med. (2001) 33:337–43. doi: 10.3109/07853890109002087
18. Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet. (2018) 392:971–84. doi: 10.1016/S0140-6736(18)31559-9
19. Skalnikova HK, Cizkova J, Cervenka J, Vodicka P. Advances in proteomic techniques for cytokine analysis: focus on melanoma research. Int J Mol Sci. (2017) 18:2697. doi: 10.3390/ijms18122697
20. Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. (1994) 56:853–7. doi: 10.1002/ijc.2910560617
21. Kawakami Y, Rosenberg SA, Lotze MT. Interleukin 4 promotes the growth of tumor-infiltrating lymphocytes cytotoxic for human autologous melanoma. J Exp Med. (1988) 168:183–91. doi: 10.1084/jem.168.6.2183
22. Moon NL, Joesting JJ, Blevins NA, Lawson MA, Gainey SJ, Towers AE, et al. IL-4 knock out mice display anxiety-like behavior. Behav Genet. (2015) 45:451–60. doi: 10.1007/s10519-015-9714-x
23. Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wessa A, Parmiani G, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. (2007) 13:4677–85. doi: 10.1158/1078-0432.CCR-07-0776
24. Daud AI, Deconti RC, Andrews S, Urbas P, Riker AI, Sondak VK, et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol. (2008) 26:5896–903. doi: 10.1200/JCO.2007.15.6794
25. Fang S, Wang Y, Chun Y, Liu H, Ross MI, Gershenwald JE, et al. Association of common genetic polymorphisms with melanoma patient IL-12p40 blood levels, risk, and outcomes. J Invest Dermatol. (2015) 135:2266–72. doi: 10.1038/jid.2015.138
26. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. (1999) 17:2105–16. doi: 10.1200/JCO.1999.17.7.2105
27. Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. (1992) 10:52–60. doi: 10.1200/JCO.1992.10.1.52
28. Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by γ-interferon. Nature. (1985) 318:665–7. doi: 10.1038/318665a0
29. Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. (2015) 75:1624–34. doi: 10.1158/0008-5472
30. Huang S, Xie K, Bucana CD, Ullrich SE, Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin Cancer Res. (1996) 2:1969–79.
31. Wray NR, James MR, Dumenil T, Handoko HY, Lind PA, Montogomery GW, et al. Association study of candidate variants of COMT with neuroticism, anxiety and depression. Am J Med Genet B Neuropsychiatr Genet. (2008) 147B:1314–8. doi: 10.1002/ajmg.b.30744
32. DeMichele-Sweet MA, Sweet RA. Genetics of psychosis in Alzheimer’s disease: a review. J Alzheimers Dis. (2010) 19:761–80. doi: 10.3233/JAD-2010-1274
33. Shibata T, Pavel S, Smit NP, Mishima Y. Differences in subcellular distribution of catechol-O-methyltransferase and tyrosinase in malignant melanoma. J Invest Dermatol. (1993) 100(Suppl. 2):222S–5S.
34. Smit NP, Latter AJ, Naish-Byfield S, Westerhof W, Pavel S, Riley PA. Catechol-O-methyltransferase as a target for melanoma destruction? Biochem Pharmacol. (1994) 48:743–52. doi: 10.1016/0006-2952(94)90052-3
35. Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. (2008) 31:187–99. doi: 10.1111/j.1365-2885.2008.00944.x
36. Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, et al. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. (2008) 17:717–23. doi: 10.1093/hmg/ddm343
37. Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: I. Affective disorders. Mol Psychiatry. (2003) 8:574–91. doi: 10.1038/sj.mp.4001328
38. López-León S, Janssens AC, Ladd AM, Del-Favero J, Claes SJ, Oostra BA, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. (2007) 13:772–85. doi: 10.1038/sj.mp.4002088
Keywords: melanoma, anxiety, depression, quality of life, cytokine, gene mutation
Citation: Kim HE, Shim HJ, Jung C, Shin IS, Kim J-M and Yun SJ (2022) Correlations of psychological distress with plasma cytokine levels and gene mutations in acral and non-acral melanoma. Front. Psychiatry 13:1024206. doi: 10.3389/fpsyt.2022.1024206
Received: 21 August 2022; Accepted: 10 October 2022;
Published: 03 November 2022.
Edited by:
Yong Cheng, Minzu University of China, ChinaReviewed by:
Jiaquan Liang, Foshan Third People’s Hospital, ChinaSeon-Cheol Park, Hanyang University Guri Hospital, South Korea
Copyright © 2022 Kim, Shim, Jung, Shin, Kim and Yun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae-Min Kim, am1raW1AY2hvbm5hbS5hYy5rcg==; Sook Jung Yun, c2p5dW5AY2hvbm5hbS5hYy5rcg==
†These authors have contributed equally to this work
 Hong Euy Kim1
Hong Euy Kim1 Il Seon Shin
Il Seon Shin Jae-Min Kim
Jae-Min Kim