- 1Department of Psychiatry, The First Affiliated Hospital, Zhejiang University School of Medicine, Key Laboratory of Mental Disorder's Management of Zhejiang Province, Zhejiang Engineering Center for Mathematical Mental Health, Hangzhou, China
- 2Faculty of Clinical Medicine, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Clinical Psychology, The Fifth Peoples' Hospital of Lin'an District, Hangzhou, China
- 4College of Biomedical Engineering and Instrument Science, Center for Brain Imaging Science and Technology, Zhejiang University, Hangzhou, China
Background: Anhedonia, as the core endophenotype of major depressive disorder (MDD), is closely related to poor prognosis, but the mechanism of this feature remains to be understood. The aim of this study was to investigate the inflammatory factors and brain structural alterations in MDD patients with anhedonia and evaluate the relationship between these factors.
Methods: We assessed the plasma levels of interleukin-1 beta (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) in MDD patients with anhedonia (n = 22), MDD patients without anhedonia (n = 20), and age- and sex-matched healthy controls (HCs, n = 20) by enzyme-linked immunosorbent assay kits. All participants underwent high-resolution brain magnetic resonance imaging (MRI) scans, and voxel-based morphometry (VBM) was used to evaluate their gray matter volume (GMV). We compared inflammatory factors and GMV among the three groups and explored their relationships in MDD patients with anhedonia.
Results: Compared with those of HCs, plasma levels of IL-1β were increased in patients with MDD independent of anhedonia features, while plasma levels of IL-6 were elevated in MDD patients with anhedonia only. Meanwhile, MDD patients with anhedonia exhibited reduced GMV in the left striatal structures compared to MDD patients without anhedonia and HCs. Moreover, a significant association was observed between increased plasma levels of IL-6 and decreased GMV of the left putamen in MDD patients with anhedonia.
Conclusions: The present research outcomes suggest that anhedonia is associated with increased plasma levels of IL-6 and decreased GMV in the left striatal structures. In addition, this study demonstrates that GMV loss in the left putamen is related to increased plasma levels of IL-6 in MDD with anhedonia, which provides further insights into the possible mechanisms of anhedonia.
Introduction
Major depressive disorder (MDD) is a highly prevalent and debilitating mental condition that causes a significant disease burden to families and world society (1). According to a recent national epidemiological survey of mental disorders conducted in China, the lifetime prevalence of MDD is approximately 3.4% (2). To date, clinical depression is still critical for the diagnosis of MDD (3) as the potential pathogenesis and etiology underlying depression remain unclear (4). In the past few decades, numerous studies have been developed that reveal the pathophysiology of depression. MDD patients, specifically, have been demonstrated to exhibit disturbances of the inflammatory system (5) and volumetric alterations in certain brain structures (6). However, the current findings are inconsistent and controversial because of the different clinical manifestations implicated in MDD (7). In this context, studies to identify clusters of depressive symptoms and corresponding biomarkers have been conducted, which may be beneficial for characterizing depression phenotypes and individualized diagnosis and treatment.
Anhedonia, defined as a loss of interest and/or the capacity to experience pleasure in previously enjoyed activities, is a core feature of MDD. It has been revealed that anhedonia is a strong predictor for poor response to antidepressant treatment (8), increased suicidal behavior (9), and distinct impairments of overall functioning (10) in depressed patients. Recent works have revealed that MDD with anhedonia may be treated as a functional subtype of depression with a unique clinical prognosis and biological alterations (11, 12). Furthermore, convergent lines of research also suggest that anhedonia acts as an endophenotypic marker and as a risk factor for MDD (13, 14). Therefore, clarifying the neural mechanism of anhedonia is both necessary and important in understanding the pathophysiology of MDD.
Previous studies revealed that anhedonia was associated with overactivation of the inflammatory response system and altered levels of inflammatory cytokines. Early animal studies found that acute systemic administration of proinflammatory factors could induce anhedonic-like behaviors in mice (15). A recent study in a rat model of spared nerve injury demonstrated that inflammatory cytokines, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), would promote anhedonia susceptibility (16). Meanwhile, the effects of interleukin-6 neutralizing antibodies have manifested symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman's disease (17). Anhedonia was reported to be associated with inflammatory gene expression in MDD as well as in cocaine use disorder (18, 19). Tang et al. (20) investigated the association between inflammatory characteristics and anhedonia in drug-naïve major depressive disorder and found that MDD patients with anhedonia showed increased levels of IL-6 and complement Factor H compared to patients without anhedonia. This evidence suggests the important role of inflammatory factors in anhedonia.
Meanwhile, anhedonia has been demonstrated to be associated with impairments of the brain reward circuits, including structural abnormalities in reward-related brain regions such as the ventral striatum, nucleus accumbens (NAcc), prefrontal cortex, amygdala, and posterior cingulate. In fact, abnormalities in these brain structures have been shown to reduce the sensitivity of subjective pleasure (21). A recent study with a large population-based sample of 19,592 people reported that reduced total gray matter volume (GMV) in multiple brain structures, including reward-related circuits, were associated with the phenotype of anhedonia and its genetic risk (22). Furthermore, anhedonia has also been found to be associated with reduced caudate (23) and NAcc volumes (24, 25) in nonclinical samples. In MDD, the severity of anhedonic symptoms was associated with reduced bilateral caudate volume (26).
Taken together, these findings suggest that both aberrant inflammation and altered brain structure may be involved in the pathophysiology of anhedonia. Previous animal and clinical studies revealed that brain structure would change after acute and chronic stimulated peripheral infection, and these changes were associated with proinflammatory cytokine levels (27, 28). In addition, increased proinflammatory cytokines were reported to be associated with brain GMV reductions in neuropsychiatric disorders, such as schizophrenia (29), MDD (30), and bipolar disorder (31). Evidence from postmortem human brains of patients with schizophrenia indicated that overexpression of IL-6 and IL-1β mRNA levels were significantly associated with brain volume reduction (32). Tsai et al. (33) reported that persistent inflammation was associated with reduction of hippocampal and GMVs in older patients with bipolar disorder. As mentioned above, although MDD patients with anhedonia were more likely to present increased inflammatory activity, the relationship between inflammation and brain structural alterations in MDD patients with anhedonia was less revealed. In this context, we examined the concentrations of plasma IL-1β, IL-6 and TNF-α, analyzed GMVs using whole-brain voxel-based analyses in outpatients with MDD, and finally explored the interrelationships between these biological determinants in MDD patients with anhedonia. Based on previous evidence, we hypothesized that there could be a link between elevated inflammatory factors and brain structural atrophy in MDD patients with anhedonia.
Methods
Participants
A total of forty-two MDD patients from Department of Psychiatry, The First Affiliated Hospital, Zhejiang University School of Medicine were enrolled. All subjects satisfied the Diagnostic and Statistical Manual of Mental Disorders, IV Edition (DSM-IV) criteria for MDD, as screened with Structured Clinical Interview for DSM-IV (SCID) and met the following criteria: (1) aged 18-45 years; (2) drug-naïve patients with first-episode depression or recurrent depression with continued withdrawal of >3 months; and (3) a total score of 17-item Hamilton Depression Scale (HAMD-17) ≥ 17. In this study, patients with MDD were divided into two groups, MDD with anhedonia and MDD without anhedonia. For assignment to the MDD with anhedonia group, MDD patients must have been experiencing anhedonia according to the Item 2 (loss of interest or pleasure) of the symptom criteria (A) for MDD in the DSM-IV and the threshold of transformed score of Snaith-Hamilton Pleasure Scale (SHAPS). Meanwhile, a total of 20 sex- and age-matched healthy controls (HCs) were recruited from local residents, hospital staffs and students. All HCs were thoroughly interviewed and were free from any current or lifetime history of psychiatric disorders according to the DSM IV criteria. The general exclusion criteria for all subjects were as follows:(1) existence of any major medical disease including cardiovascular, respiratory, endocrine and neurological diseases (e.g., epilepsy, brain trauma and stroke); (2) current use of any medication that might affect the central nervous system, (3) drug or alcohol dependence or abuse; (4) female with pregnancy; (5) with histories of psychotherapy and physical therapy, (6) contraindications to MRI scan, including retractors or braces, metallic implants, and claustrophobia.
This study was approved by the local Medical Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. Prior to commencement of the study, all participants provided written informed consent.
Clinical assessment
Clinical assessments were carried out by two experienced and well-trained psychiatrists from Department of Psychiatry, The First Affiliated Hospital, Zhejiang University School of Medicine. The demographic and clinical data was collected by using a self-designed questionnaire from all the participants. Depression severity was evaluated using the 17-item Hamilton Depression Scale (HAMD-17), which is the most common clinician-rated scale for assessing the severity of depression. The symptoms of anhedonia were assessed by the Snaith–Hamilton Pleasure Scale (SHAPS), which is a self-report questionnaire (34). The Chinese version has been validated (35). A total of 14 items are included in this scale, and the scale covers four categories of pleasurable activities, namely, interests and pastimes, sensory experiences, social interactions, and diet. The responses to each item are rated as either a 0 or a 1, or 0 for “Agree” or “Strongly Agree” and 1 for “Disagree” or “Strongly Disagree”. The total SHAPS score indicates the severity of anhedonia status, and a sum of transformed scores > 5 indicates the presence of severe anhedonia, which is a method that has been used as the anhedonic or nonanhedonic grouping criteria in previous studies (12, 36).
Plasma inflammatory factor measurement
Fasting venous blood was collected from each subject in the morning, and whole blood samples were centrifuged at 3,000 rpm for 20 min. Plasma was separated and stored at −80 °C for simultaneous analysis. The concentrations of IL-1β, IL-6 and TNF-α in plasma were measured by Duoset human ELISA Kits (IL-1β: HSLB00D, R&D Systems, Minneapolis, MN, USA; IL-6: HS60DC, R&D Systems, Minneapolis, MN, USA; TNF-α: HSTA00E, R&D Systems, Minneapolis, MN, USA) according to the manufacturers' instructions. The final results are presented in pg/ml.
MRI acquisition
Imaging data were acquired on a 3.0-T scanner (Signa, HDxt, GE healthcare, USA) with a standard birdcage head coil in the Magnetic Resonance Center at The First Affiliated Hospital, Zhejiang University School of Medicine. All participants were instructed to lie still with their eyes closed and to avoid falling asleep. The protocol in the present study involved 3D T1-weighted structural images, which were acquired by a brain volume (BRAVO) sequence with the following parameters: TR = minimum (7.3 ms), TE = minimum (3.0 ms), TI = 1,100 ms, flip angle = 7, FOV = 256 * 256 mm2, matrix = 256 * 256, slice thickness = 1 mm, bandwidth = 31.25 KHz, NEX = 1, slices = 192.
Voxel-based morphometry (VBM) analysis
To analyze the structural data, we used the voxel-based morphometry (VBM) approach provided in the SPM12 software (http://fil.ion.ucl.ac.uk/spm/) of CAT12 software (http://dbm.neuro.uni-jena.de/vbm/) in the MATLAB environment. For each individual, a 3D T1-weighted image was corrected for bias-field differences and segmented into gray matter, white matter, and CSF (37) before applying DARTEL spatial normalization (38). In addition, segmentation was extended to account for partial volume effects (39) through the use of adaptive maximum a posteriori estimation (40). All scans passed standard quality checks according to an automated protocol after preprocessing, and we assessed sample homogeneity using a box plot and correlation matrix to identify outliers. Artifacts or preprocessing errors were inspected, and poor-quality scans were excluded. In the end, the images were smoothed with an 8-mm full-width Gaussian kernel at half-maximum (FWHM), and an absolute gray-matter threshold of 0.1 was applied to the entire gray/white matter margin to remove artifacts created by, for example, incorrect voxel classification.
Statistical analysis
The analyses of demographic, clinical, and biochemical data were performed with the Statistical Package for the Social Sciences (SPSS) (version 16.0, SPSS Inc., Chicago, IL, USA). Categorical variables are expressed as percentages (m/n), and the differences among the three groups were analyzed using Chi-square tests (χ2). The continuous variables included in this study appeared to be distributed normally based on the normal distribution test and are represented as the mean (standard deviation), and the differences among the three groups were analyzed by one-way analysis of variance (ANOVA). Benjamini-Hochberg method (BH) was used for false discovery rate (FDR) correction to adjust the p-values in multi-comparison. Moreover, the effect size of ANOVA was expressed as . Pearson correlation analysis was used to analyze the associations between the clinical data and those of biochemical factors. In our analysis, a two-sided p < 0.05 significance level was considered significant.
GMVs on the individual imaging maps were analyzed with one-way ANOVA and post hoc analyses in a voxel-by-voxel manner for the intergroup differences. In addition, potential confounders, such as, age, sex, education years, and total internal volume (TIV) were included as covariates. The significance level was set at p < 0.05 and corrected according to Gaussian random field (GRF) theory (voxel significance: p < 0.001, cluster significance: p < 0.05) for multiple comparisons with REST software. Finally, to evaluate any correlations between inflammation data and gray matter structural changes in MDD patients with anhedonia, whole brain multiple regression analyses integrated in SPM basic models were performed at p < 0.05 (GRF corrected).
Results
Demographic and clinical characteristics
The demographic and clinical features of all subjects are summarized in Table 1. No significant differences were observed with respect to age, sex, and education years among three groups (p > 0.05). The two groups of MDD patients did not differ on means of illness duration and HAMD-17 scores (p > 0.05). As we would expect, MDD patients with anhedonia exhibited increased SHAPS scores relative to those without anhedonia (p < 0.05).
Plasma levels of inflammatory factors among the three groups
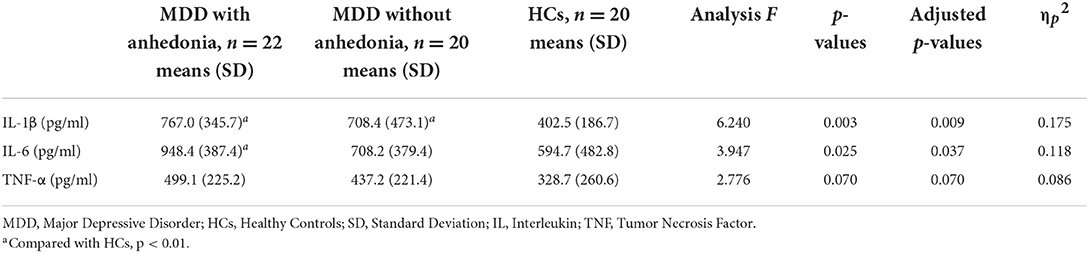
Table 2 presents the plasma levels of inflammatory factors for three groups. Significant differences on plasma levels of IL-1β and IL-6 among three groups were identified (p < 0.05). Compared with HCs, both groups of MDD patients showed higher plasma levels of IL-1β (p < 0.05), regardless of anhedonia, however, there was no significant difference between two groups of MDD patients (p > 0.05). MDD patients with anhedonia showed higher plasma levels of IL-6 than HCs (p < 0.05), while no significant difference was found between MDD patients without anhedonia and HCs, either the two groups of MDD patients. No significant differences were found on plasma levels of TNF-α among three groups (p > 0.05).
Alterations in GMVs
Based on one-way ANOVA, significant GMV difference in the left lateral globus pallidus (LGP) was observed among three groups (Table 3, Figure 1). Post hoc pairwise comparisons revealed that MDD patients with anhedonia exhibited decreased GMV in the left putamen when comparing to HCs (Table 4, Figure 2). Meanwhile, MDD with anhedonia also showed reduced GMV in the left LGP when compared with MDD without anhedonia (Table 4, Figure 3). However, no significant difference was found between MDD patients without anhedonia and HCs.

Table 3. Brain region showing significant structural difference among three groups (p < 0.05, GRF corrected).
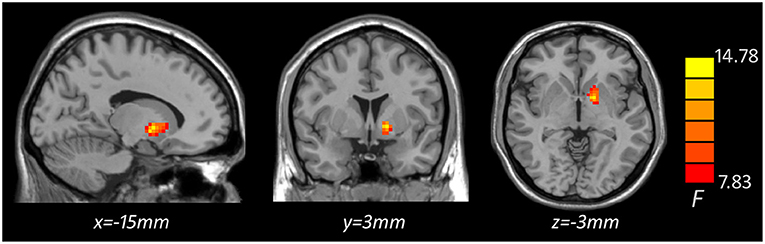
Figure 1. Brain region showing significant structural difference among three groups (p < 0.05, GRF corrected). The color scale represents F values of ANOVA.
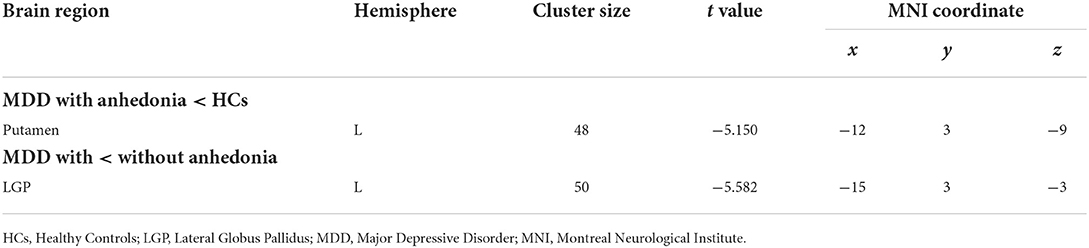
Table 4. Brain region showing inter-group differences based on the results of ANOVA (p < 0.05, GRF corrected).
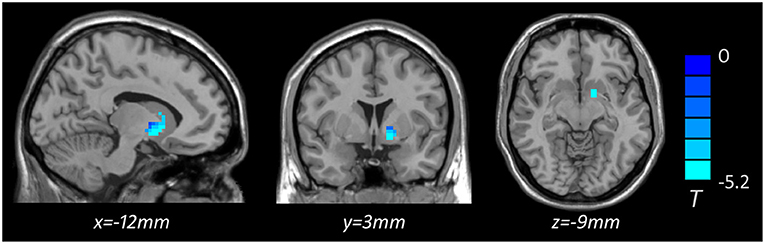
Figure 2. Brain region showing significant structural difference between MDD with anhedonia and HCs (p < 0.05, GRF corrected). The color scale represents t values.
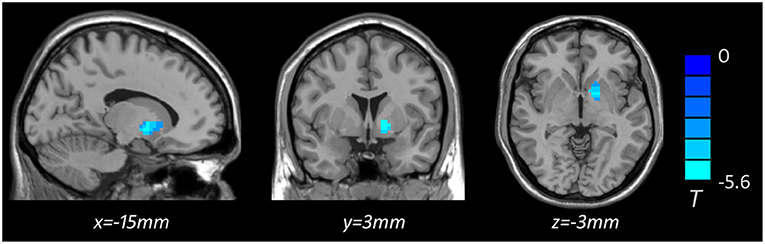
Figure 3. Brain region showing significant structural difference between MDD with and without anhedonia (p < 0.05, GRF corrected). The color scale represents t values.
Correlation analyses
Voxel-based whole-brain correlation analysis showed that GMV in the left putamen was negatively associated with plasma levels of IL-6 in MDD patients with anhedonia (Table 5, Figure 4), while this association was not significant in other two groups. In addition, a positive correlation was observed between plasma levels of IL-6 and total scores of SHAPS (r = 0.421, p = 0.005) in patients with MDD. Nevertheless, there was no significant association of plasma levels of IL-1β or TNF-α with total scores of SHAPS in MDD patients.
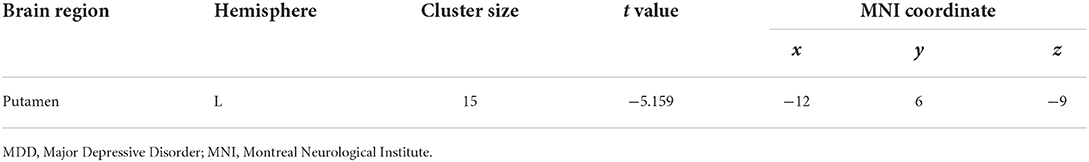
Table 5. Region of decreased gray matter volume in MDD with anhedonia correlated to plasma level of IL-6 (pg/ml), p < 0.05, GRF corrected.
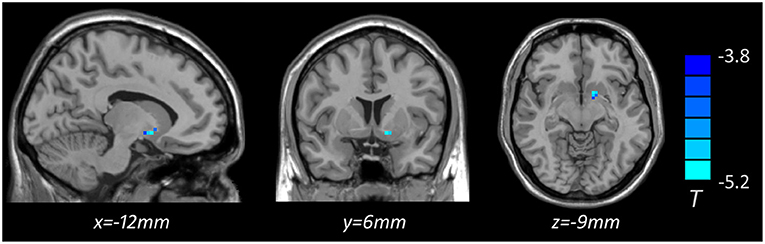
Figure 4. Brain region showing decreased gray matter volume associated with plasma level of IL-6 (pg/ml) in MDD with anhedonia (p < 0.05, GRF corrected). The color scale represents t values.
Discussion
The present study investigated the difference in inflammatory factor levels and GMV between MDD patients with and without anhedonia and explored the association between inflammatory factor concentrations and morphometric alterations in MDD patients with anhedonia. To our knowledge, this is the first study to investigate interrelationships between GMV changes and plasma levels of inflammatory factors in MDD patients with anhedonic symptoms. Our results demonstrated that plasma levels of IL-6 in MDD patients with anhedonia, but not in MDD patients without anhedonia, were higher than those in HCs, which were also positively correlated with SHAPS scores in MDD patients. More importantly, VBM analyses revealed that MDD patients with anhedonia exhibited reduced GMV in the left putamen and LGP and showed a significantly negative association between IL-6 levels and the left LGP GMV. Our results emphasize the importance of focusing on the influence of inflammatory cytokine concentrations and morphometric alterations in MDD patients with anhedonia, which may provide a new perspective for the pathophysiology of anhedonia and depression.
In the present study, significant differences on plasma levels of IL-1β and IL-6 were found among the two groups of MDD patients and HCs, specifically, patients with MDD showed higher plasma levels of IL-1β and IL-6 than HCs, which was consistent with many studies assessing the association between depression and inflammatory markers. More interestingly, we found that the plasma levels of IL-6 were significantly elevated in MDD patients with anhedonia (but not in MDD patients without anhedonia) compared to HCs, and the correlation analysis showed a significantly positive correlation between the IL-6 levels and the severity of anhedonia. Despite the relatively few clinical reports in this area of interest, some previous studies were consistent with and supported our findings. A large-scale cohort study from the UK Biobank and Netherlands Study of Depression and Anxiety revealed an association between IL-6 and anhedonia (41). Similarly, IL-6 was proven to be positively associated with SHAPS scores with an exploratory sensitivity analysis in depressed adolescents (42). Tang et al. (20) also reported that patients with anhedonia showed higher levels of IL-6 than those without anhedonia in drug-naïve major depressive disorder, although they failed to find altered levels of IL-6 between depressive patients and healthy subjects. Meanwhile, some animal studies demonstrated that IL-6 promotes anhedonia susceptibility in spared nerve injury (16) and that IL-6 receptor antagonists attenuate postpartum anhedonia in female rats (43). IL-6 levels play a crucial role in the pathogenesis of depression (44) and have been linked to depression prognosis (45, 46) and treatment outcomes (47, 48). Some scholars supported the idea that the symptoms and subtypes of depression influence IL-6 levels and suggested that using other depression-related cytokines or their receptors as biomarkers may help to classify MDD biological subtypes (49, 50). Rudolf et al. (51) found that peripheral IL-6 levels were elevated in atypical MDD patients but not in typical MDD patients when compared with controls. Hence, we speculated that the altered IL-6 levels may be associated with anhedonic features in MDD. Thus, more investigation is needed in the future.
With respect to neuroimaging findings in the present study, the main influence of anhedonia was discovered in the striatum, with reduced GMV in the left putamen and LGP. The striatum, which is composed of the putamen, globus pallidus and caudate nucleus, is the primary input structure of the basal ganglia and plays a key role in reward-related activities. Similar to emphasizing the prominent role of anhedonia in MDD clinical symptoms, robust gray matter reduction in the striatum was reported in MDD (52). A meta-analysis of a magnetic resonance imaging study revealed that patients with MDD showed reduced GMVs in the putamen relative to healthy individuals (53). Auerbach et al. (24) found that the smaller volume in striatal regions prospectively predicted anhedonia severity in adolescents with MDD. Meanwhile, the hypofunction in the ventral striatal and orbitofrontal regions during unexpected reward receipt was proven to be associated with anhedonia in MDD and schizophrenia, which suggested that abnormal frontostriatal activity may underlie anhedonic symptoms in depression and schizophrenia (54). In addition, some associations between striatum and suicidal risk and poor treatment results in MDD patients were found. A study reported that reduced bilateral putamen and left caudate GMV significantly predicted scores of the death version of the Implicit Association Test (IAT) in adolescents, which suggested that higher suicide risk was associated with limited GMV in the striatum (55). Downar et al. reported that MDD patients with no response to dorsomedial prefrontal repetitive transcranial magnetic stimulation showed aberrant connectivity of the striatum (56), and the effect of electroconvulsive therapy on striatal morphometry was discovered in MDD (57). As anhedonia is regarded as a strong predictor for increased suicidal behavior and poor response to antidepressant treatment, these overlapping findings support the possible role of reduced striatal volume in the pathogenesis of anhedonia in MDD.
Moreover, the present study showed a significant negative relationship between plasma IL-6 levels and GMV in the LGP in MDD patients with anhedonia using whole-brain voxel-based correlation analysis. Previous works demonstrated that acute systemic inflammation could induce decreased striatal reactivity and disturb neural processes involving motivation and loss of interest (58). The potential mechanisms involve the effect of inflammation on dopamine synthesis, release, and reuptake, increased synaptic and additional synaptic glutamate, and activation of kynurenine pathway metabolites., which may influence the reward related neural circuitry and induce the symptom of anhedonia (59–61). Eisenberger et al. (58) reported that low-dose endotoxin was associated with increased depressive mood and reduced ventral striatal response to reward cues in healthy participants. Burrows et al. (62) found that the lower activation of striatal activity was negatively correlated with serum CRP levels in MDD patients in the monetary incentive delay task. Meanwhile, the serum ratio of kynurenine/tryptophan, which presents an enzyme activated by proinflammatory cytokines and is involved in the synthesis of kynurenine from tryptophan, was reported to be negatively correlated with striatal volume (63). These previous findings supported our results and confirmed the complex relationship between the inflammatory state and structural alterations of the brain, and we suspected that IL-6 may be a key biological marker associated with the striatal volume for anhedonia in MDD.
Limitation
The present study had certain limitations. First, the sample size in this study was relatively small, which restricted the statistical power and generalization of the findings. Second, this study was a cross-sectional design and was unable to explore dynamic changes in inflammatory factors and brain structure, precluding causal inferences. Third, although the distinctions of different aspects of anhedonia on brain structure and inflammation have been revealed (64, 65), the present study did not investigate the effects of different aspects of anhedonia due to the modest sample size. Finally, only three inflammatory cytokines, including IL-1β, IL-6, and TNF-α, were measured in this study, consequently, the findings of inflammatory characteristics in MDD patients with anhedonia were quite limited. In this context, future longitudinal studies containing the abovementioned aspects may be helpful to elucidate the detailed pathway underlying inflammation-related brain alterations in MDD patients with anhedonia. Additionally, animal studies should further explore whether inflammatory factors are causally related to neural changes and subsequently anhedonia.
Conclusion
In summary, the present research outcomes suggest that anhedonia is associated with increased plasma levels of IL-6 and decreased GMV in the left striatal structures. In addition, this study demonstrates that GMV loss in the left putamen is related to increased plasma levels of IL-6 in MDD with anhedonia, which provides further insights into the possible mechanisms of anhedonia.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Local Medical Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SL and CW designed the study, conducted the statistical analyses, and wrote the first draft of the manuscript. LJ and ZF finished the clinical assessments. SH recruited the sample. JL and TM tested and stored the blood samples. HH, MH, and YX designed the study and had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to and have approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82071521 to SL) and the Natural Science Foundation of Zhejiang Province (LY19H090017 to SL).
Acknowledgments
All authors would sincerely thank all participants who took part in this study and the experts at the Magnetic Resonance Center of The First Affiliated Hospital, Zhejiang University School of Medicine for providing scan time and technical assistant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhdanava M, Kuvadia H, Joshi K, Daly E, Pilon D, Rossi C, et al. Economic burden of treatment-resistant depression in privately insured us patients with physical conditions. J Manag Care Spec Pharm. (2020) 26:996–1007. doi: 10.18553/jmcp.2020.20017
2. Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in china: a cross-sectional epidemiological study. Lancet Psychiat. (2019) 6:211–24. doi: 10.1016/S2215-0366(18)30511-X
3. Soleimani L, Lapidus KA, Iosifescu DV. Diagnosis and treatment of major depressive disorder. Neurologic Clini. (2011) 29:177–93. doi: 10.1016/j.ncl.2010.10.010
4. Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. (2020) 25:321–38. doi: 10.1038/s41380-019-0585-z
5. Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. (2021) 53:151–71. doi: 10.1111/ejn.14720
6. Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry. (2015) 77:223–35. doi: 10.1016/j.biopsych.2014.08.009
7. Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther. (2018) 24:994–1003. doi: 10.1111/cns.12835
8. Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. (2012) 42:967–80. doi: 10.1017/S0033291711001905
9. Ducasse D, Loas G, Dassa D, Gramaglia C, Zeppegno P, Guillaume S, et al. Anhedonia is associated with suicidal ideation independently of depression: a meta-analysis. Depress Anxiety. (2018) 35:382–92. doi: 10.1002/da.22709
10. Buckner JD, Joiner TE. Jr., Pettit JW, Lewinsohn PM, Schmidt NB. Implications of the Dsm's emphasis on sadness and anhedonia in major depressive disorder. Psychiatry Res. (2008) 159:25–30. doi: 10.1016/j.psychres.2007.05.010
11. Cooper JA, Arulpragasam AR, Treadway MT. Anhedonia in depression: biological mechanisms and computational models. Curr Opin Behav Sci. (2018) 22:128–35. doi: 10.1016/j.cobeha.2018.01.024
12. Vinckier F, Gourion D, Mouchabac S. Anhedonia predicts poor psychosocial functioning: results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur Psychiatry. (2017) 44:1–8. doi: 10.1016/j.eurpsy.2017.02.485
13. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. (2011) 35:537–55. doi: 10.1016/j.neubiorev.2010.06.006
14. Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. (2004) 29:1765–81. doi: 10.1038/sj.npp.1300506
15. Salazar A, Gonzalez-Rivera BL, Redus L, Parrott JM, O'Connor JC. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm Behav. (2012) 62:202–9. doi: 10.1016/j.yhbeh.2012.03.010
16. Fang X, Zhan G, Zhang J, Xu H, Zhu B, Hu Y, et al. Abnormalities in inflammatory cytokines confer susceptible to chronic neuropathic pain-related anhedonia in a rat model of spared nerve injury. Clin Psychopharmacol Neurosci. (2019) 17:189–99. doi: 10.9758/cpn.2019.17.2.189
17. Sun Y, Wang D, Salvadore G, Hsu B, Curran M, Casper C, et al. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric castleman's disease. Brain Behav Immun. (2017) 66:156–64. doi: 10.1016/j.bbi.2017.06.014
18. Bekhbat M, Treadway MT, Goldsmith DR, Woolwine BJ, Haroon E, Miller AH, et al. Gene signatures in peripheral blood immune cells related to insulin resistance and low tyrosine metabolism define a sub-type of depression with high crp and anhedonia. Brain Behav Immun. (2020) 88:161–5. doi: 10.1016/j.bbi.2020.03.015
19. Fries GR, Khan S, Stamatovich S, Dyukova E, Walss-Bass C, Lane SD, et al. Anhedonia in cocaine use disorder is associated with inflammatory gene expression. PLoS ONE. (2018) 13:e0207231. doi: 10.1371/journal.pone.0207231
20. Tang W, Liu H, Chen L, Zhao K, Zhang Y, Zheng K, et al. Inflammatory cytokines, complement factor H and anhedonia in drug-naive major depressive disorder. Brain Behav Immun. (2021) 95:238–44. doi: 10.1016/j.bbi.2021.03.022
21. Martins D, Rademacher L, Gabay AS, Taylor R, Richey JA, Smith DV, et al. Mapping social reward and punishment processing in the human brain: a voxel-based meta-analysis of neuroimaging findings using the social incentive delay task. Neurosci Biobehav Rev. (2021) 122:1–17. doi: 10.1016/j.neubiorev.2020.12.034
22. Zhu X, Ward J, Cullen B, Lyall DM, Strawbridge RJ, Lyall LM, et al. Phenotypic and genetic associations between anhedonia and brain structure in UK biobank. Transl Psychiatry. (2021) 11:395. doi: 10.1038/s41398-021-01522-4
23. Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry. (2007) 12:67–75. doi: 10.1038/sj.mp.4002021
24. Auerbach RP, Pisoni A, Bondy E, Kumar P, Stewart JG, Yendiki A, et al. Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology. (2017) 42:2087–95. doi: 10.1038/npp.2017.28
25. Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, FMRI, and volumetric techniques. Neuroimage. (2009) 46:327–37. doi: 10.1016/j.neuroimage.2009.01.058
26. Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry. (2009) 166:702–10. doi: 10.1176/appi.ajp.2008.08081201
27. Chen J, Yan Y, Yuan F, Cao J, Li S, Eickhoff SB, et al. Brain grey matter volume reduction and anxiety-like behavior in lipopolysaccharide-induced chronic pulmonary inflammation rats: a structural mri study with histological validation. Brain Behav Immun. (2019) 76:182–97. doi: 10.1016/j.bbi.2018.11.020
28. Zheng H, Ford BN, Bergamino M, Kuplicki R, Tulsa I, Hunt PW, et al. A hidden menace? Cytomegalovirus infection is associated with reduced cortical gray matter volume in major depressive disorder. Mol Psychiatry. (2021) 26:4234–44. doi: 10.1038/s41380-020-00932-y
29. Quide Y, Bortolasci CC, Spolding B, Kidnapillai S, Watkeys OJ, Cohen-Woods S, et al. Systemic inflammation and grey matter volume in schizophrenia and bipolar disorder: moderation by childhood trauma severity. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 105:110013. doi: 10.1016/j.pnpbp.2020.110013
30. Han KM, Ham BJ. How inflammation affects the brain in depression: a review of functional and structural MRI studies. J Clin Neurol. (2021) 17:503–15. doi: 10.3988/jcn.2021.17.4.503
31. Chen MH, Kao ZK, Chang WC, Tu PC, Hsu JW, Huang KL, et al. Increased proinflammatory cytokines, executive dysfunction, and reduced gray matter volumes in first-episode bipolar disorder and major depressive disorder. J Affect Disord. (2020) 274:825–31. doi: 10.1016/j.jad.2020.05.158
32. Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry. (2016) 6:e982. doi: 10.1038/tp.2016.238
33. Tsai SY, Gildengers AG, Hsu JL, Chung KH, Chen PH, Huang YJ. Inflammation associated with volume reduction in the gray matter and hippocampus of older patients with bipolar disorder. J Affect Disord. (2019) 244:60–6. doi: 10.1016/j.jad.2018.10.093
34. Nakonezny PA, Morris DW, Greer TL, Byerly MJ, Carmody TJ, Grannemann BD, et al. Evaluation of anhedonia with the snaith-hamilton pleasure scale (Shaps) in adult outpatients with major depressive disorder. J Psychiatr Res. (2015) 65:124–30. doi: 10.1016/j.jpsychires.2015.03.010
35. Liu WH, Wang LZ, Zhu YH Li MH, Chan RC. Clinical utility of the snaith-hamilton-pleasure scale in the chinese settings. BMC Psychiatry. (2012) 12:184. doi: 10.1186/1471-244X-12-184
36. Wu C, Lu J, Lu S, Huang M, Xu Y. Increased ratio of mature BDNF to precursor-bdnf in patients with major depressive disorder with severe anhedonia. J Psychiatr Res. (2020) 126:92–7. doi: 10.1016/j.jpsychires.2020.05.010
37. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. (2005) 26:839–51. doi: 10.1016/j.neuroimage.2005.02.018
38. Ashburner J, A. Fast Diffeomorphic image registration algorithm. Neuroimage. (2007) 38:95–113. doi: 10.1016/j.neuroimage.2007.07.007
39. Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. (2004) 23:84–97. doi: 10.1016/j.neuroimage.2004.05.007
41. Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK biobank and nesda cohorts. Mol Psychiatry. (2021) 26:7393–402. doi: 10.1038/s41380-021-01188-w
42. Rengasamy M, Marsland A, McClain L, Kovats T, Walko T, Pan L, et al. Longitudinal relationships of cytokines, depression and anhedonia in depressed adolescents. Brain Behav Immun. (2021) 91:74–80. doi: 10.1016/j.bbi.2020.09.004
43. Gomez J, Haas NA, Schwarz JM. An Il-6 receptor antagonist attenuates postpartum anhedonia, but has no effect on anhedonia precipitated by subchronic stress in female rats. Psychopharmacology (Berl). (2019) 236:2983–95. doi: 10.1007/s00213-019-05194-3
44. Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. Blockade of interleukin-6 receptor in the periphery promotes rapid and sustained antidepressant actions: a possible role of gut-microbiota-brain axis. Transl Psychiatry. (2017) 7:e1138. doi: 10.1038/tp.2017.112
45. Kelly KM, Smith JA, Mezuk B. Depression and interleukin-6 signaling: a mendelian randomization study. Brain Behav Immun. (2021) 95:106–14. doi: 10.1016/j.bbi.2021.02.019
46. Roohi E, Jaafari N, Hashemian F. On inflammatory hypothesis of depression: what is the role of IL-6 in the middle of the chaos? J Neuroinflammation. (2021) 18:45. doi: 10.1186/s12974-021-02100-7
47. Kang HJ, Bae KY, Kim SW, Kim JT, Park MS, Cho KH, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. (2016) 72:156–60. doi: 10.1016/j.psyneuen.2016.07.001
48. Carboni L, McCarthy DJ, Delafont B, Filosi M, Ivanchenko E, Ratti E, et al. Biomarkers for response in major depression: comparing paroxetine and venlafaxine from two randomised placebo-controlled clinical studies. Transl Psychiatry. (2019) 9:182. doi: 10.1038/s41398-019-0521-7
49. Wang M, Wei J, Yang X, Ni P, Wang Y, Zhao L, et al. The level of Il-6 was associated with sleep disturbances in patients with major depressive disorder. Neuropsychiatr Dis Treat. (2019) 15:1695–700. doi: 10.2147/NDT.S202329
50. Ting EY, Yang AC, Tsai SJ. Role of interleukin-6 in depressive disorder. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21062194
51. Rudolf S, Greggersen W, Kahl KG, Huppe M, Schweiger U. Elevated Il-6 levels in patients with atypical depression but not in patients with typical depression. Psychiatry Res. (2014) 217:34–8. doi: 10.1016/j.psychres.2014.02.016
52. Zheng R, Zhang Y, Yang Z, Han S, Cheng J. Reduced brain gray matter volume in patients with first-episode major depressive disorder: a quantitative meta-analysis. Front Psychiatry. (2021) 12:671348. doi: 10.3389/fpsyt.2021.671348
53. Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. (2009) 30:3719–35. doi: 10.1002/hbm.20801
54. Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Dudas RB, et al. Abnormal frontostriatal activity during unexpected reward receipt in depression and schizophrenia: relationship to anhedonia. Neuropsychopharmacology. (2016) 41:2001–10. doi: 10.1038/npp.2015.370
55. Ho TC, Cichocki AC, Gifuni AJ, Catalina Camacho M, Ordaz SJ, Singh MK, et al. Reduced dorsal striatal gray matter volume predicts implicit suicidal ideation in adolescents. Soc Cogn Affect Neurosci. (2018) 13:1215–24. doi: 10.1093/scan/nsy089
56. Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. (2014) 76:176–85. doi: 10.1016/j.biopsych.2013.10.026
57. Wade BS, Joshi SH, Njau S, Leaver AM, Vasavada M, Woods RP, et al. Effect of Electroconvulsive therapy on striatal morphometry in major depressive disorder. Neuropsychopharmacology. (2016) 41:2481–91. doi: 10.1038/npp.2016.48
58. Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. (2010) 68:748–54. doi: 10.1016/j.biopsych.2010.06.010
59. Byrne ML, Whittle S, Allen NB. The role of brain structure and function in the association between inflammation and depressive symptoms: a systematic review. Psychosom Med. (2016) 78:389–400. doi: 10.1097/PSY.0000000000000311
60. Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, et al. Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology. (2020) 45:998–1007. doi: 10.1038/s41386-020-0607-1
61. Lucido MJ, Bekhbat M, Goldsmith DR, Treadway MT, Haroon E, Felger JC, et al. Aiding and abetting anhedonia: impact of inflammation on the brain and pharmacological implications. Pharmacol Rev. (2021) 73:1084–117. doi: 10.1124/pharmrev.120.000043
62. Burrows K, Stewart JL, Kuplicki R, Figueroa-Hall L, Spechler PA, Zheng H, et al. Elevated peripheral inflammation is associated with attenuated striatal reward anticipation in major depressive disorder. Brain Behav Immun. (2021) 93:214–25. doi: 10.1016/j.bbi.2021.01.016
63. Savitz J, Dantzer R, Meier TB, Wurfel BE, Victor TA, McIntosh SA, et al. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology. (2015) 62:54–8. doi: 10.1016/j.psyneuen.2015.07.609
64. Liu X, Li L, Li M, Ren Z, Ma P. Characterizing the subtype of anhedonia in major depressive disorder: a symptom-specific multimodal MRI study. Psychiatry Res Neuroimaging. (2021) 308:111239. doi: 10.1016/j.pscychresns.2020.111239
Keywords: major depressive disorder, anhedonia, interleukin-6, gray matter volume, striatum
Citation: Lu S, Wu C, Jia L, Fang Z, Lu J, Mou T, Hu S, He H, Huang M and Xu Y (2022) Increased plasma levels of IL-6 are associated with striatal structural atrophy in major depressive disorder patients with anhedonia. Front. Psychiatry 13:1016735. doi: 10.3389/fpsyt.2022.1016735
Received: 11 August 2022; Accepted: 20 October 2022;
Published: 03 November 2022.
Edited by:
Pengfei Xu, Beijing Normal University, ChinaReviewed by:
Fengqiong Yu, Anhui Medical University, ChinaDongsheng Zhou, Ningbo Kangning Hospital, China
Copyright © 2022 Lu, Wu, Jia, Fang, Lu, Mou, Hu, He, Huang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Xu, eHV5aXpqdUB6anUuZWR1LmNu; Manli Huang, aHVhbmdtYW5saUB6anUuZWR1LmNu; Hongjian He, aGhlemp1QHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Shaojia Lu
Shaojia Lu Congchong Wu
Congchong Wu Lili Jia
Lili Jia Zhe Fang
Zhe Fang Jing Lu
Jing Lu Tingting Mou
Tingting Mou Shaohua Hu
Shaohua Hu Hongjian He
Hongjian He Manli Huang
Manli Huang Yi Xu
Yi Xu