- 1Department of Psychiatry, First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Psychiatry, Banan People’s Hospital of Chongqing, Chongqing, China
- 3Chongqing Hospital of Traditional Chinese Medicine, Chongqing, China
Introduction: Bipolar disorder (BD) is a common and debilitating mental illness that affects about 400 million people worldwide, decreasing their functionality and quality of life. Medication and psychotherapy are recommended for treatment of BD, while some evidence indicates that exercise could improve the clinical outcome of BD. This study aims to investigate whether exercise intervention could reduce the mood symptoms and inflammation level of BD.
Methods: This is a longitudinal, interventional, randomized, and single-blind trial. We plan to recruit 94 patients diagnosed with BD in depression episode. Patients will be randomly assigned to treatment as usual + aerobic exercise group (intervention group) and treatment as usual (TAU) only group, at a ratio of 1:1. The intervention group will undergo 40-min aerobic exercise training twice a week for eight weeks. The primary outcome of this study is the mean change of Hamilton Depression Rating Scale 17 (HAMD 17) scores from baseline to week 8. The Young Manic Rating Scale (YMRS), Self-Rating Depression Scale (SDS), and Interleukin-6 (IL-6), Tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP) levels will also be measured. The measurements will be performed at baseline, immediately after intervention and two months after intervention.
Discussion: Aerobic exercise training + treatment is expected to bring more benefits to BD patients than TAU only. This trial might provide stronger evidence of physical exercise efficacy for BD treatment.
Clinical trial registration: This study was approved by the Chinese Clinical Trial Registry (Registration Code: ChiCTR2200057159). Registered on 1 March 2022.
Introduction
Bipolar disorder (BD) is a chronic, recurrent, psychiatric disorder characterized by alternating episodes of depression and mania/hypomania, affecting about 2.4% of the population worldwide across diverse cultures and ethnic groups (1), leading to substantial medical burden. Serious mental illness (SMI) includes BD, major depression disorder (MDD) and schizophrenia-spectrum disorders. BD patients have high rates of suicide, disability, and low social functioning, placing a significant burden on families and society (2). BD is also the seventeenth leading cause of disability worldwide (3).
Medication and psychotherapy are commonly used to treat BD (4), but their efficacy remains unsatisfactory. Compared to manic episodes, depression episodes in BD are more incapacitating, prolonged and frequent (5). Hence, mood stabilizers, antipsychotics, and antidepressants are often used as pharmacotherapy. Lithium is commonly used and found to be effective in some patients (6), but is also more likely to cause safety events compared to placebo (7). Additionally, there is significant controversy over the use of antidepressants (8–10), which may cause mania and the treatment for mania may rebound as depressive episodes (11). Although psychosocial treatments are effective for BD (6, 12), psychotherapy is not easily accessible and expensive. Therefore, the treatment for BD remains a huge challenge, especially in depression episodes (8–10).
In this context, exercise is recommended as an adjunctive therapy for BD. Exercise has been considered useful in major depression and schizophrenia, and is recommended by the European Psychiatric Association (EPA). Exercise is considered important for people with SMI, including schizophrenia-spectrum disorders, MDD and BD, because these patients typically engage in significantly more sedentary behavior and less physical activity (13, 14), which also puts them at higher risk of developing cardiovascular and metabolic diseases (13, 15, 16). Significant evidence supports the use of exercise interventions in the treatment of MDD (17) and schizophrenia-spectrum disorders. For example, exercise could improve mental and physical heath among general population, while physical activity could reduce recurrence, relieve depressive symptoms and improve cardiorespiratory fitness and quality of life for MDD patients (18–21). The EPA suggested moderate-vigorous intensity aerobic exercise 2–3 times per week for MDD and schizophrenia-spectrum disorders, which is similar to the recommendation of the American College of Sports Medicine (ACSM) for normal population (22).
However, current evidence is insufficient to include exercise as daily recommendation for BD population since exercise-based clinical trials in this population have been consistently poor. Previous systematic reviews concluded that although exercise treatment is promising for BD, existing studies have limitations (23–26).
For example, a pilot study found that individuals with BD who participated in 40-min walking group, five times a week for 24 months reported lower depression symptoms than those who did not, without a control group (27). Another pilot study found that lifestyle intervention including exercise program showed improvement in overall functioning assessed by The Longitudinal Interval Follow-Up Evaluation-Range of Impaired Functioning Tool, but no differences in mood symptoms between groups (28). A randomized controlled trial found that 30-h aerobic exercise intervention did not augment cognition compared to the control group (29). A recent randomized trial, examining effects of N-acetylcysteine for BD, found that patients with BD who engaged more physical activity may reduce more scores on the Bipolar Depression Rating Scale. Physical activity which could enhance mitochondrial may be a novel treatment for BD (30). The possible mechanism of exercise for treating BD includes augmenting the synthesis of monoamine neurotransmitters (30–34), neurotrophin types (35, 36), and anti-inflammation. Inflammation has been shown to decrease after aerobic exercise in the general population (37) and patients with depression (38–40). Also, increasing evidence indicates that inflammatory factors are potential biomarkers of BD in evaluating severity of BD mood symptoms. Recently, a large meta-analysis indicated that C-reactive protein (CRP) and Tumor necrosis factor- α (TNF-α) might be considered as “mood episode” markers of BD, while Interleukin-6 (IL-6) was speculated to be a trait marker of BD (41). The above-mentioned data implies that CRP, TNF-α, and IL-6 could be used to examine the exercise efficacy in BD treatment.
Several researches have documented the reduction of Brain-derived Neurotrophin (BDNF) among BD patients (42, 43). At the biological level, physical exercise increases the levels of neurotransmitters, cortisol, β-endorphins, and neurotrophins such as BDNF (44, 45). BDNF could enhance neuronal ATP production in several ways (46). Also, BDNF plays an important role in brain plasticity, neuron differences and survival, neuronal function and stimulates neurogenesis by activating TrkB. There are two forms of BDNF receptors: active TrkB-FL and inactiveTrkB-T1 (47). It has been shown that physical exercise increases the levels of neurotransmitters, cortisol, β –endorphins, and neurotrophins such as BDNF, through a PGC-1α/FNDS5 pathway (48). Thus, BDNF signaling system could play a role in the pathophysiology of BD (49).
The main hypothesis of this trial is that aerobic exercise is an effective therapy to improve depressive symptoms among bipolar depression patients.
Method and analysis
Study objective
The primary objective of this study is to assess whether 8-week aerobic exercise will improve depressive symptoms among BD patients compared to treatment as usual. The secondary objective is to explore the effect of aerobic exercise training on inflammatory biomarkers such as CRP, IL-6, and TNF-a, manic symptoms, and cardio-respiratory fitness compared to treatment as usual. The exploratory objective is to evaluate the effects of aerobic exercise on BDNF level, emotional symptoms, quality of life, and other physical parameters, 8 weeks after intervention. The results may provide evidence for exercise therapy benefits among BD patients.
Study setting
This trial is a longitudinal, interventional, randomized, single-blind trial to be conducted at the First Affiliated Hospital of Chongqing Medical University, China. BD patients will be randomly divided into two groups: (1) Treatment as usual + aerobic exercise group (intervention group); (2) Treatment as usual group (control group).
The treatment as usual for participants in this study would be decided by a professional psychiatrist. Patients continue to be seen for medication visit every 2–4 weeks. There is no prohibition about medication changes during the trial. All pharmacotherapy including any changes in dose or medication will be recorded in detail.
Eligibility criteria
The participants will be recruited from the Psychiatry Outpatient Department in the First Affiliated Hospital of Chongqing Medical University, China. Research assistants will use the Mini-International Neuropsychiatric Interview (MINI), a structured diagnostic interview, to confirm the diagnosis of DSM-5 bipolar disorder depression episode before inclusion.
Inclusion criteria
1. Aged 18–50 years old, female or male.
2. A diagnosis of BD depression episode according to the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).
3. The score of HAMD ≥ 17, the score of YRMS < 6.
4. Adequate audiovisual skills to complete the necessary checks for the study.
5. Willing to participate in this trial, with signed written informed consent.
Exclusion criteria
1. BMI ≥ 40 kg/m2.
2. Systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg.
3. Perinatal or lactation period.
4. Exercise time > 40 min per week over the last 6 months.
5. Serious physical illness (such as heart disease, diabetes, hyperthyroidism, etc.).
6. Infectious diseases, autoimmune diseases, use of anti-inflammatory drugs, cortisol hormones or antibiotics over the last 6 months.
7. Severe suicidal ideation or attempt in last 30 days or need to be hospitalized immediately due to suicidal ideation, attempt, or behaviors.
Outcomes
The primary outcome of this study is the HAMD 17 score mean change from baseline to week 8. Participants will be assessed with HAMD 17 at baseline and week 8. The secondary outcomes are the changes of CRP, TNF-α, and IL-6 from baseline to week 8.
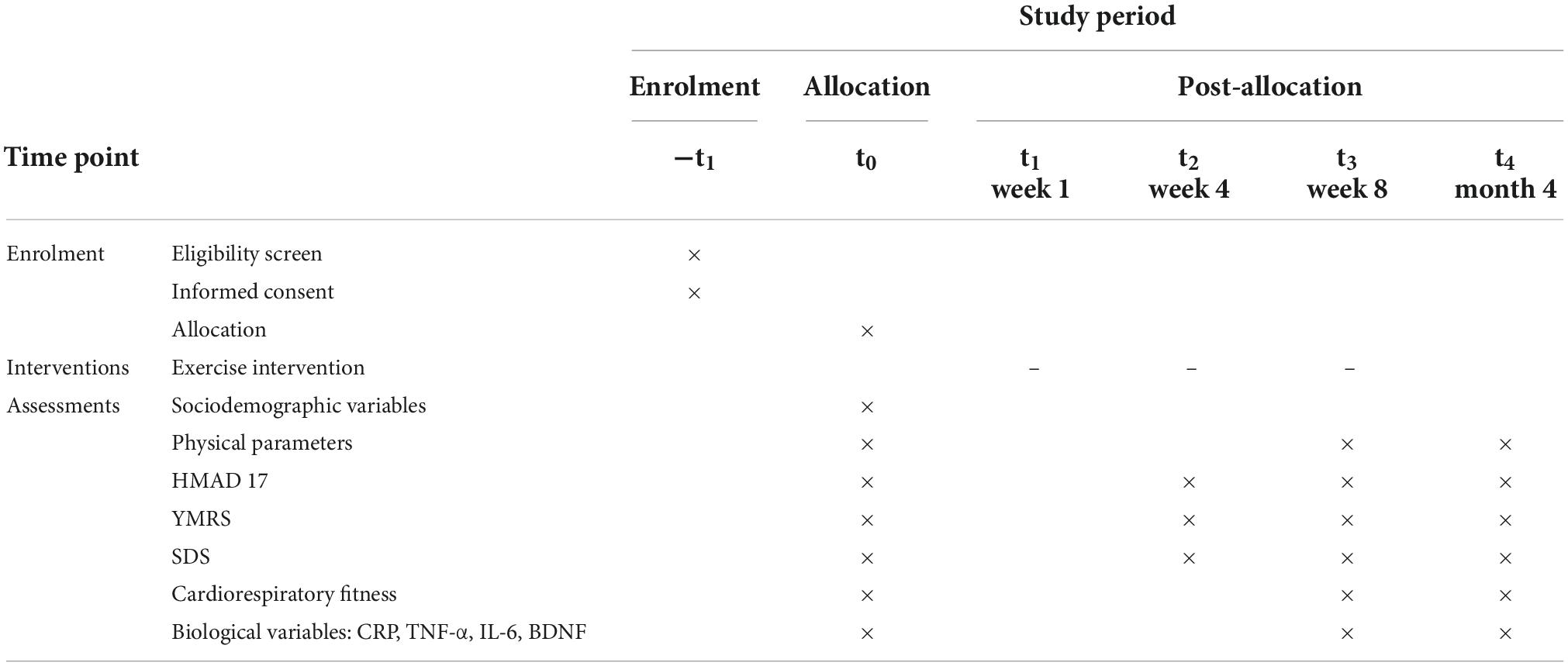
Participant timeline
Figure 1 shows a clear and concise timeline of the study visits, enrollment process, interventions, and assessments to be performed on participants.
Sample size
The sample size was calculated based on the primary hypothesis. To calculate the sample size, we reviewed some literature related to the main topic and used the PASS.15 software. Power was determined as 0.09 with α = 0.05. According to related study (50), the mean value among Chinese BD patients is 13, and standard deviation is 6. It was determined that permissible error was 1/3 of the mean value, δ = 4.3, population standard deviation σ = 6. It’s necessary to have 42 samples per group. According to similar study (51, 52), it’s possible to have 20% drop-out, and we won’t replace the subjects if participants drop out. So, it is necessary to have 53 patients per group (Supplementary material).
Recruitment
Potential participants will be recruited from the Psychiatry Outpatient Department of the First Affiliated Hospital of Chongqing Medical University, China, which is one of the largest hospitals in southwest China. A post will be put in the Psychiatry Outpatient Department to advertise this trial and patients interested in the trial can contact us for information and participation. Researchers will also call potential participants to invite them to join the study. The recruitment will be conducted from March 2022 to December 2022 (Table 1).
Screening failures
Screening failures are defined as ineligible participants based on screening assessments. We will record the reason for screening failure, date of screening, and participant identification number.
Participant withdrawal or discontinuation
All participants will be free to quit the study for any reason at any time, and we will record reasons and date of dropout. Subjects will not be replaced if discontinued.
Allocation/Randomization
Patients will be randomized in the ratio 1:1. Random sequences will be generated by a research associate using Microsoft Office Excel 2017, and stored by hospital nurses in sequentially numbered, sealed and opaque envelopes. Researchers will open the corresponding numbered envelope after obtaining consent from each patient.
Blinding
In this single-blinded trial, participants and main assessors will be aware of treatment allocation but not aware of the study hypothesis. Research assistants who are responsible for assessment and statistical analysis will be blinded to participant randomization assignments. Research assistants for assessment will be blinded to the treatment allocation throughout the study. An employee who is not involved in the study will enter the data into the computer so that the statistician will be unaware of the allocation.
Assessments
Sociodemographic variables
Sociodemographic variables will be collected at baseline, including age, gender, ethnicity, education level, family history of mental illness, economic condition, and marital status.
Physical parameters
Physical parameters will be collected at baseline and immediately after 8 weeks intervention. The Ultrasonic Health Examination Scale made by Sonka Medical Co., limited (SK-X80/TCS-160D-w/h) will be used to measure the height (m) and body mass (kg). Body Mass Index (BMI) will be calculated by formula2: BMI = weight/height2.
Cardiorespiratory fitness
As maximum oxygen uptake (VO2max) is the gold standard of exercise physiology for evaluating cardiorespiratory fitness, Chest Step Test will be hold to assess VO2max at baseline, with the recommendation of test manual (53).
Clinical variables
Individuals will be diagnosed by two psychiatrists according to DSM-5 diagnostic criteria. The Mini-International Neuropsychiatric Interview (MINI), a short structured diagnostic interview, will be used to confirm the diagnosis (54).
Depression symptoms will be measured by HMRD 17, which was designed by Hamilton in 1960 (55). The reliability of Chinese version of HMRD-17 is 0.88–0.99 and the validity is 0.92 (50). Some items are defined by increasing intensity, and others are defined by number of equal valued terms. According to Davis JM, the scores of HMRD 17 ≤ 7 indicate that individuals do not have depressive symptoms; the scores >7 and ≤17 indicate that individuals have minor or moderate depression; the score >24 indicate that individuals have major depression. Two trained psychiatrists will independently conduct the assessments. This is the most widely used clinical scale and contains 17 variables.
Depression symptoms will also be measured by the Self-Rating Depression Scale (SDS), which was designed in 1965 by Zung and recommended by the United States Department of Education, Health and Welfare in psychopharmacological research (56). SDS includes 20 items, of which 10 items are positive and 10 items are negative. The form will be directly filled by patients. The row score will be derived by adding up 20 items, with maximum possible score being 80. Index score is equal to the row score × 1.24, expressed as decimal. The standard score in China is 53 and the cutoff value of total row score is 41, which is similar to the results of a study in the USA (57).
Manic symptoms will be measured by the Young Manic Rating Scale (YMRS), which contains 11 items (58). YMRS was designed by Young in 1978, and is the most widely used manic scale worldwide. YRMS contained 11 items. Five choices are included in items 1–4, 7, 10, 11, ranging from scores 0–4, while the items 5, 6, 8, 9 have five choices scored in double points (score of 0, 2, 4, 6, 8). The score ranges from 0 to 40. When the total score ≥5 and ≤10, individuals have manic symptoms and when the score ≥22, individuals have severe manic symptoms. The reliability and validity of YRMS have been examined in a previous study.
Biological variables
Blood samples will be collected using EDTA anticoagulant tubes at baseline and after eight weeks of intervention, and stored at 4°C for less than 1 h before being sent to the laboratory at The First Affiliated Hospital of Chongqing Medical University. Plasma level of CRP will be measured by emulsion method with Roche Cobase 701. The levels of IL-6 and TNF-α will be measured by chemiluminescence with Siemens ZMM1000. In vitro enzyme-linked immunosorbent assay will be used to quantify the plasma of human BDNF (Table 1).
Intervention
The intervention group will participate in online exercise program on two non-consecutive days per week for eight weeks via Tencent Conference Software. Each exercise session will include no more than eight participants. Participants will be guided and supervised by a professional coach during the entire online training. Each session will include a 10-min warm-up, a 25-min high-intensity or moderate-intensity interval training and a 10-min cool-down stretching. The training protocol is created by the first author who got fitness coach qualification certificate as well. Participants are expected to reach 70% of their age-predicted maximum heart rate (HR), which equals to 220 beats per minute-0.7 × age as an indicator of moderate intensity of aerobic exercise (59). Warm-up and cool-down exercises will include walking at low intensity (e.g., jogging in place) and stretching. Wearable devices (Huami amazfit neo smart bracelet) will be used during intervention to collect VO2 max.
Participants will be allowed to withdraw at any time during the trial. Some strategies will be used for adherence and compliance. For example, we will provide free follow-up service and online consulting service after program completion to encourage participants to complete the intervention. The smart bracelet will be sent to the participants for free. Completion of this program will be defined as finishing at least 50% of the exercise at each session and reaching at least 70% adherence rate of the whole intervention. The 70% adherence rate is defined as completing 11 sessions out of 16 sessions (Table 1).
Data collection methods
Two permanent research assistants, majoring in psychology with uniform training will perform all assessments at baseline and after eight weeks of intervention using standard procedure. Blood sampling for the analysis of biological parameters will be performed during the same visit.
Statistical analysis
SPSS 19.0 statistical software will be used for statistical analysis. Measurements will be expressed as mean ± standard deviation or median + quartiles, using t-test or rank sum test. Small sample composition ratios between groups will be expressed as frequency (f), composition ratio (P), and compared using Fisher’s exact probability method or chi-square test.
The Hamilton Depression Inventory 17-item (HAMD-17) score at eight weeks will be used as the primary outcome indicator. Differences between groups in HAMD-17 scores will be compared first by chi-square Levene test, and if the variances are chi-square, t-test will be used, and if the variances are not chi-square, differences within groups in HAMD-17 scores will be compared by paired t-test. Analysis of covariance will be used to control for baseline confounders and to further explore the differences in scores between the two groups before and after the intervention. Eight-week scores on other scales and biological indicators will be used as secondary outcomes, using similar methods as described above. Missing data will be subjected to multiple fill method, and sensitivity analysis before and after filling. An alpha = 0.05 will be taken. Intentional analysis (ITT), consistent with scenario set analysis (PP) as sensitivity analysis, will be mainly used in this study.
Anticipated results
We anticipate that the intervention group will benefit from the aerobic exercise at the end of intervention compared to the TAU group, with less severe depressive symptoms. The intervention may increase treatment compliance of patients as well. More evidence on exercise treatment for BD is needed to prove this hypothesis. In addition to the clinical outcome, we will also investigate whether exercise could improve the quality of life and physical fitness of patients.
Dissemination
This protocol and the study results will be shared through publication in scientific journal, and national and international conferences.
Discussion
This randomized controlled trial is designed to investigate whether exercise therapy is effective for patients with BD. To the best of our knowledge, this is the first randomized trial investigating the direct effect of aerobic exercise in patients with BD. This trial is expected to provide current evidence for adjunct exercise therapy for BD patients with depressive symptoms.
One of the most important aims of treatment is improving the clinical outcome of patients. The treatment of BD, especially treatment of depression episode in BD, remains a major challenge. Exercise as a psychosocial adjunct for BD should be assessed with rigorous randomized clinical trials.
Many studies have been conducted to certify that aerobic exercise should be used as an adjunct therapy for depression. However, a few randomized controlled trials have been conducted to investigate whether exercise treatment is useful for BD patients. As mentioned above, there is insufficient clinical trial evidence on exercise intervention for BD. The limitations of studies on exercise intervention among BD patients included small sample sizes, no distinction between different types of exercise (lifestyle physical activity or structured intervention program), definitions of the exercise intensity, duration, and frequency of exercise. Therefore, more high-quality evidence from randomized controlled trials are essential to investigate if exercise treatment is efficacious and safe for BD. If this trial can establish that exercise treatment is beneficial for clinical outcome of BD, people suffering from this illness may achieve better clinical outcome. Aerobic exercise can be easily performed, without any major adverse events. The results of this relatively small trial may be useful for the development of future exercise regimens for BD.
This trial also has some limitation. Firstly, it is not a large sample sized trial, and the recruitment may be difficult due to some reasons, such as the 8-week intervention duration, decreased motivation, busy schedule, sedentary habits. Additionally, this trial will be supervised by professional coach and psychotherapist, which may add bias during the trial. Also, the drop out of participants may be higher than expected. To address these problems, the researchers will keep in touch with participants and their doctors. Some inexpensive gifts and free physical examination will be provided to encourage participants to finish this intervention.
Conclusion
This is a strictly designed randomized controlled trial aimed to provide high quality evidence for adjunct treatment of bipolar disorder. Our findings may provide additional evidence for better prognosis of patients with bipolar disorder. Larger sample should be included in future studies.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (March 23rd, 2022, Certificate No. 2022-065). The patients/participants provided their written informed consent to participate in this study.
Author contributions
XW and HL contributed to the conception and design of the study and wrote sections of the manuscript. YZ and CJ organized the database. ZZ, MM, and YL performed the statistical analysis. XW wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was funded by the Chongqing Science and Health Joint Medical Scientific Research Project. The project number is 2020GDRC02.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1011978/full#supplementary-material
References
1. Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. (2016) 387:1561–72. doi: 10.1016/s0140-6736(15)00241-x
2. Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. (2011) 68:241–51. doi: 10.1001/archgenpsychiatry.2011.12
3. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. (2016) 3:171–8. doi: 10.1016/s2215-0366(15)00505-
4. Haggarty SJ, Karmacharya R, Perlis RH. Advances toward precision medicine for bipolar disorder: Mechanisms & molecules. Mol Psychiatry. (2021) 26:168–85. doi: 10.1038/s41380-020-0831-4
5. Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. (2002) 59:530–7. doi: 10.1001/archpsyc.59.6.530
6. Sole B, Jimenez E, Torrent C, Reinares M, Bonnin CDM, Torres I, et al. Cognitive impairment in bipolar disorder: Treatment and prevention strategies. Int J Neuropsychopharmacol. (2017) 20:670–80. doi: 10.1093/ijnp/pyx032
7. McKnight RF, de La Motte de Broons de Vauvert S, Chesney E, Amit BH, Geddes J, Cipriani A. Lithium for acute mania. Cochrane Database Syst Rev. (2019) 6:CD004048. doi: 10.1002/14651858.CD004048.pub4
8. Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, et al. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: Update 2013. Bipolar Disord. (2013) 15:1–44. doi: 10.1111/bdi.12025
9. Frye MA, Ha K, Kanba S, Kato T, McElroy SL, Ozerdem A, et al. International consensus group on depression prevention in bipolar disorder. J Clin Psychiatry. (2011) 72:1295–310. doi: 10.4088/JCP.10123co1c
10. Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM. Antidepressants for bipolar depression: A systematic review of randomized, controlled trials. Am J Psychiatry. (2004) 161:1537–47. doi: 10.1176/appi.ajp.161.9.1537
11. Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. (2013) 381:1672–82. doi: 10.1016/s0140-6736(13)60857-0
12. Miklowitz DJ, Scott J. Psychosocial treatments for bipolar disorder: Cost-effectiveness, mediating mechanisms, and future directions. Bipolar Disord. (2009) 11(Suppl. 2):110–22. doi: 10.1111/j.1399-5618.2009.00715.x
13. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: A systematic review and meta-analysis. World Psychiatry. (2015) 14:339–47. doi: 10.1002/wps.20252
14. Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: A global systematic review and meta-analysis. World Psychiatry. (2017) 16:308–15. doi: 10.1002/wps.20458
15. De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Moller HJ. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European psychiatric association (EPA), supported by the European association for the study of diabetes (EASD) and the European society of cardiology (ESC). Eur Psychiatry. (2009) 24:412–24. doi: 10.1016/j.eurpsy.2009.01.005
16. Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. (2021) 18:136–45. doi: 10.1038/s41569-020-00463
17. Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR, et al. Exercise for depression. Cochrane Database Syst Rev. (2013):CD004366. doi: 10.1002/14651858.CD004366
18. Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, et al. EPA guidance on physical activity as a treatment for severe mental illness: A meta-review of the evidence and Position Statement from the European psychiatric association (EPA), supported by the international organization of physical therapists in mental health (IOPTMH). Eur Psychiatry. (2018) 54:124–44. doi: 10.1016/j.eurpsy.2018.07.004
19. Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life Span. Neurosci Biobehav Rev. (2013) 37(9 Pt B):2268–95. doi: 10.1016/j.neubiorev.2013.01.028
20. Hayashino Y, Jackson JL, Hirata T, Fukumori N, Nakamura F, Fukuhara S, et al. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Metabolism. (2014) 63:431–40. doi: 10.1016/j.metabol.2013.08.018
21. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA. (2009) 301:2024–35. doi: 10.1001/jama.2009.681
22. Thompson PD, Arena R, Riebe D, Pescatello LS, American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. (2013) 12:215–7. doi: 10.1249/JSR.0b013e31829a68cf
23. Thomson D, Turner A, Lauder S, Gigler ME, Berk L, Singh AB, et al. A brief review of exercise, bipolar disorder, and mechanistic pathways. Front Psychol. (2015) 6:147. doi: 10.3389/fpsyg.2015.00147
24. de Sá Filho AS, de Souza Moura AM, Lamego MK, Ferreira Rocha NB, Paes F, Oliveira AC, et al. Potential therapeutic effects of physical exercise for bipolar disorder. CNS Neurol Disord Drug Targets. (2015) 14:1255–9. doi: 10.2174/1871527315666151111122219
25. Sylvia LG, Ametrano RM, Nierenberg AA. Exercise treatment for bipolar disorder: Potential mechanisms of action mediated through increased neurogenesis and decreased allostatic load. Psychother Psychosom. (2010) 79:87–96. doi: 10.1159/000270916
26. Sa Filho AS, Cheniaux E, de Paula CC, Murillo-Rodriguez E, Teixeira D, Monteiro D, et al. Exercise is medicine: A new perspective for health promotion in bipolar disorder. Expert Rev Neurother. (2020) 20:1099–107. doi: 10.1080/14737175.2020.1807329
27. Ng F, Dodd S, Berk M. The effects of physical activity in the acute treatment of bipolar disorder: A pilot study. J Affect Disord. (2007) 101:259–62. doi: 10.1016/j.jad.2006.11.014
28. Sylvia LG, Pegg SL, Dufour SC, Janos JA, Bernstein EE, Chang WC, et al. Pilot study of a lifestyle intervention for bipolar disorder: Nutrition exercise wellness treatment (NEW Tx). J Affect Disord. (2019) 250:278–83. doi: 10.1016/j.jad.2019.03.033
29. McGurk SR, Otto MW, Fulford D, Cutler Z, Mulcahy LP, Talluri SS, et al. A randomized controlled trial of exercise on augmenting the effects of cognitive remediation in persons with severe mental illness. J Psychiatr Res. (2021) 139:38–46. doi: 10.1016/j.jpsychires.2021.04.033
30. Ashton MM, Mohebbi M, Turner A, Marx W, Berk M, Malhi GS, et al. Physical activity as a predictor of clinical trial outcomes in bipolar depression: A subanalysis of a mitochondrial-enhancing nutraceutical randomized controlled trial. Can J Psychiatry. (2020) 65:306–18. doi: 10.1177/0706743719889547
31. Meeusen R, Thorré K, Chaouloff F, Sarre S, De Meirleir K, Ebinger G, et al. Effects of tryptophan and/or acute running on extracellular 5-HT and 5-HIAA levels in the hippocampus of food-deprived rats. Brain Res. (1996) 740:245–52. doi: 10.1016/s0006-8993(96)00872-4
32. Ouchi Y, Yoshikawa E, Futatsubashi M, Okada H, Torizuka T, Sakamoto M. Effect of simple motor performance on regional dopamine release in the striatum in Parkinson disease patients and healthy subjects: A positron emission tomography study. J Cereb Blood Flow Metab. (2002) 22:746–52. doi: 10.1097/00004647-200206000-00013
33. Rogers PJ, Tyce GM, Weinshilboum RM, O’Connor DT, Bailey KR, Bove AA. Catecholamine metabolic pathways and exercise training. Plasma and urine catecholamines, metabolic enzymes, and chromogranin-A. Circulation. (1991) 84:2346–56. doi: 10.1161/01.cir.84.6.2346
34. Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: Relation to the speed of running. Brain Res Bull. (1994) 35:41–9. doi: 10.1016/0361-9230(94)90214-3
35. Barbosa IG, Huguet RB, Sousa LP, Abreu MN, Rocha NP, Bauer ME, et al. Circulating levels of GDNF in bipolar disorder. Neurosci Lett. (2011) 502:103–6. doi: 10.1016/j.neulet.2011.07.031
36. Barbosa IG, Rocha NP, Miranda AS, Huguet RB, Bauer ME, Reis HJ, et al. Increased BDNF levels in long-term bipolar disorder patients. Braz J Psychiatry. (2013) 35:67–9. doi: 10.1016/j.rbp.2012.05.011
37. Vijay A, Kouraki A, Gohir S, Turnbull J, Kelly A, Chapman V, et al. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes. (2021) 13:1997559. doi: 10.1080/19490976.2021.1997559
38. Paolucci EM, Loukov D, Bowdish DME, Heisz JJ. Exercise reduces depression and inflammation but intensity matters. Biol Psychol. (2018) 133:79–84. doi: 10.1016/j.biopsycho.2018.01.015
39. Handschin C, Spiegelman BM. The role of exercise and PGC1α in inflammation and chronic disease. Nature. (2008) 454:463–9. doi: 10.1038/nature07206
40. Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. (2015) 300:141–54. doi: 10.1016/j.neuroscience.2015.05.018
41. Solmi M, Suresh Sharma M, Osimo EF, Fornaro M, Bortolato B, Croatto G, et al. Peripheral levels of C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and interleukin-1beta across the mood spectrum in bipolar disorder: A meta-analysis of mean differences and variability. Brain Behav Immun. (2021) 97:193–203. doi: 10.1016/j.bbi.2021.07.014
42. Fernandes BS, Molendijk ML, Köhler CA, Soares JC, Leite CM, Machado-Vieira R, et al. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker in bipolar disorder: A meta-analysis of 52 studies. BMC Med. (2015) 13:289. doi: 10.1186/s12916-015-0529-7
43. Soares AT, Andreazza AC, Rej S, Rajji TK, Gildengers AG, Lafer B, et al. Decreased brain-derived neurotrophic factor in older adults with bipolar disorder. Am J Geriatr Psychiatry. (2016) 24:596–601. doi: 10.1016/j.jagp.2016.02.052
44. Phillips C. Physical activity modulates common neuroplasticity substrates in major depressive and bipolar disorder. Neural Plast. (2017) 2017:7014146. doi: 10.1155/2017/7014146
45. Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U.S.A. (2011) 108:3017–22. doi: 10.1073/pnas.1015950108
46. Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. (2014) 25:89–98. doi: 10.1016/j.tem.2013.10.006
47. Martinez-Cengotitabengoa M, MacDowell KS, Alberich S, Diaz FJ, Garcia-Bueno B, Rodriguez-Jimenez R, et al. BDNF and NGF signalling in early phases of psychosis: Relationship with inflammation and response to antipsychotics after 1 year. Schizophr Bull. (2016) 42:142–51. doi: 10.1093/schbul/sbv078
48. Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. (2013) 18:649–59. doi: 10.1016/j.cmet.2013.09.008
49. Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. (2006) 398:215–9. doi: 10.1016/j.neulet.2005.12.085
50. Huang J, Yuan CM, Xu XR, Wang Y, Hong W, Wang ZW, et al. The relationship between lifestyle factors and clinical symptoms of bipolar disorder patients in a Chinese population. Psychiatry Res. (2018) 266:97–102. doi: 10.1016/j.psychres.2018.04.059
51. Kwok JYY, Kwan JCY, Auyeung M, Mok VCT, Lau CKY, Choi KC, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: A randomized clinical trial. JAMA Neurol. (2019) 76:755–63. doi: 10.1001/jamaneurol.2019.0534
52. Hallgren M, Kraepelien M, Öjehagen A, Lindefors N, Zeebari Z, Kaldo V, et al. Physical exercise and internet-based cognitive-behavioural therapy in the treatment of depression: Randomised controlled trial. Br J Psychiatry. (2015) 207:227–34. doi: 10.1192/bjp.bp.114.160101
54. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59(Suppl. 20):22–57.
55. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
56. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
57. Leung KK, Lue BH, Lee MB, Tang LY. Screening of depression in patients with chronic medical diseases in a primary care setting. Fam Pract. (1998) 15:67–75. doi: 10.1093/fampra/15.1.67
58. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. (1978) 133:429–35. doi: 10.1192/bjp.133.5.429
Keywords: bipolar depression, aerobic exercise, online intervention program, physical activity, pro-inflammatory cytokines
Citation: Wang X, Luo H, Zhang Y, Mao M, Lu Y, Zhang Z, Jiang C and Luo Q (2022) Effect of online aerobic exercise training in patients with bipolar depression: Protocol of a randomized clinical trial. Front. Psychiatry 13:1011978. doi: 10.3389/fpsyt.2022.1011978
Received: 04 August 2022; Accepted: 21 October 2022;
Published: 09 November 2022.
Edited by:
Qingzhong Wang, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Birgit Ludwig, Medical University of Vienna, AustriaArron Metcalfe, University of Toronto, Canada
Copyright © 2022 Wang, Luo, Zhang, Mao, Lu, Zhang, Jiang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Luo, bHVvcWluZ2h1YUBob3NwaXRhbC5jcW11LmVkdS5jbg==
 Xueqian Wang
Xueqian Wang Huirong Luo
Huirong Luo Yinlin Zhang1
Yinlin Zhang1 Qinghua Luo
Qinghua Luo