
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 17 October 2022
Sec. Molecular Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.1003034
Background/objectives: Immune-inflammatory changes have been found in all types of suicidal ideation and behavior (SIB), independently of associated mental disorders. Since several Single Nucleotide Polymorphisms (SNPs) affect the function of inflammation-related genes, we searched the literature for genetic variations potentially altering inflammatory processes in SIB.
Methods: We included studies that looked for associations between SIB and SNPs in genes related to inflammatory processes. Case reports, literature reviews, and animal studies were excluded. Articles were retrieved from PubMed and PsycINFO databases, Google Scholar and GreySource Index until September 17th, 2022. Quality was assessed using Q-Genie.
Results: We analyzed 32 studies. SIB has been associated with eighteen SNPs located in genes encoding for interleukin-8 (rs4073), C-reactive protein (rs1130864), tumor necrosis factor α (rs1800629, rs361525, and rs1099724), tumor necrosis factor receptor 2 (rs1061622), transforming growth factor β-1 (rs1982073), acid phosphatase 1 (rs7419262, rs300774), interleukin-10 (rs1800896), interferon γ (rs2430561), amino-carboxy muconate semialdehyde decarboxylase (rs2121337), interleukin 7 (rs10448044, rs10448042), macrophage migration inhibitory factor (rs755622), interleukin 1-α (rs1800587), and interleukin 1-β (rs1143634 and rs16944. A genome-wide association study reported one association at the threshold of significance with the rs300774 SNP, located in the 2p25 region containing ACP1 gene.
Discussion: The studies included were methodologically and clinically diverse and of moderate quality. Their findings suggest that some inflammation-related SNPs could increase the likelihood of SIB but the evidence to date is insufficient. Further research using gene-gene (GxG) and gene-environment (GxE) approaches is warranted.
Systematic review registration: [https://www.crd.york.ac.uk], identifier [CRD42022296310].
Suicidal ideation and behavior (SIB) is a global phenomenon and a major public health problem. A conservative estimate suggests that at least 700,000 people die by suicide annually (1). Despite increasing research in recent years, the pathophysiological mechanisms leading to SIB remain poorly understood due to the complex interactions between multiple risk factors at different levels. Most studies are based on a stress-diathesis model involving distal-vulnerability factors (such as a family history of suicide, early-life adversity, and genetics), and proximal-stressful factors (such as psychiatric disorders, hopelessness, or acute substance abuse) (2).
Biological factors for SIB are generally studied as a distal vulnerability that increases the risk of attempting suicide under stressful circumstances (3). Indeed, SIB could be considered a clinical entity associated with a biologically impaired response to stress in three major systems: the hypothalamic pituitary adrenal axis (4), the serotoninergic system (5), and the immune-inflammatory system (6). Although more recent, the evidence linking the immune-inflammatory system with SIB is already compelling. Changes in the inflammatory response appear in many psychiatric disorders such as major depression (7), bipolar disorder (BD) (8) or schizophrenia (9), as well as in SIB (10). The extent to which inflammatory changes are specifically related to SIB beyond other psychiatric conditions is yet unclear (11).
The first biological measurement of inflammation associated with SIB was an increased concentration of soluble interleukin 2 receptors (IL-2R) in the plasma of suicide attempters (SAs) compared to healthy controls (12). An imbalance between the population of type 1 and type 2 T-helper cells and their corresponding cytokines was also observed in the blood of depressed subjects and those with suicidal ideation (SI) [Mendlovic et al. (13)]. But it was not until the last decades that evidence of a dysregulated immune system in SIB began to build up. Kim et al. (14) showed that interleukin 2 (IL-2) concentration in the blood of depressed SAs was lower than in depressed subjects who had never attempted suicide and healthy subjects. Indeed, most published studies associate SIB with changes in the levels of inflammatory-related molecules, but there are some exceptions. Interleukin 6 (Il-6) levels in the blood or cerebrospinal fluid did not differ between SAs and controls (15, 16). Plasma interleukin 4 (IL-4) levels were similar in suicide ideators compared to a control group (17) and suicide completers (SCs) compared to other SAs that did not die by suicide (15). Other studies have also failed to find significant associations between SIB and tumor necrosis factor α (TNFα) (18, 19) or interleukin 1-beta (IL-1β) (20).
Despite these conflicting findings, two meta-analyses and a systematic review confirm that SIB is associated with changes in the levels of proteins and cytokines that have pro-inflammatory proprieties such as C-reactive protein (CRP), interferon γ (IFNγ), TNFα, IL-1β, IL-6, and transforming growth factor β1 (TGF-β1), as well as anti-inflammatory IL-4 (10, 21, 22). Accordingly, the neutrophil-to-lymphocyte ratio (NLR), an inflammatory marker, is higher in SAs compared to non-attempters (23) and an excess of activated microglia, the primary immune cells of the central nervous system, is found in the brains of SCs, independently of psychiatric diagnoses (24).
Some studies have analyzed the gene expression profile in recent years. They report abnormalities of gene expression related to inflammation mostly in postmortem brain tissues but also in isolated blood monocytes of subjects with SIB. The messenger ribonucleic acid (mRNA) expression of IL-4 and interleukin 13 (IL-13) was elevated in the orbitofrontal area of suicide decedents compared with controls who died from other causes (25). Pandey et al. (26) compared depressed suicide victims with non-psychiatric controls in a cross-sectional case-control study. They found that mRNA and protein levels of pro-inflammatory cytokines (e.g., TNF-α IL-6, IL-1β, lymphotoxin A) were significantly increased and those of the anti-inflammatory cytokine interleukin 10 (IL-10) were reduced in the prefrontal cortex of suicide decedents (26). These findings are consistent with those reported by Schiweck et al. (27) using monocyte mRNA analyses of 32 inflammation-related genes. They found an association between suicide risk in major depressive disorder (MDD) and the upregulation of IL1α, IL1β, and IL-6 gene expression in monocytes (27). More recently, mRNA expression profile analysis of various chemokines showed significant downregulation of CXCL1 (CXC chemokine ligand 1), CXCL2, CXCL3, and CCL2 in the prefrontal cortex of depressed subjects compared to non-psychiatric controls (28).
All these observations suggest a deregulation in gene expression and protein production of several inflammatory-related molecules in people with SIB, but this deregulation still remains poorly understood. This article reviews studies examining the relationship between single nucleotide polymorphisms (SNPs) in inflammation-related genes and SIB. A better knowledge of the role of these genes could clarify the mechanisms linking inflammatory abnormalities with SIB.
The protocol for this systematic review is registered on PROSPERO (CRD42022296310).
Eligible studies were included using the following inclusion criteria: (1) participants presented SIB: namely suicidal ideation (SI) or suicidal behavior (SB) (attempt, suicide); (2) the study examined the association between SIB and SNPs within genes coding for proteins directly implicated in inflammation [association studies or genome-wide association studies (GWAS)]. The exclusion criteria were: (1) case reports, reviews of the literature and commentaries; (2) studies using animal models.
The search terms used for database searching were “suicide,” “inflammation” and “genetic,” using Medical Subject Headings (MeSH) terms with no time limit until 1st June, 2022 (see Supplementary material for exact search terms). Documents were sought in two databases (PubMed, PsycINFO) and in the Google Scholar and GreySource Index search engines. We also used a backward and forward snowball searching strategy by exploring the reference lists as well as the citations of the selected papers to identify additional studies.
Two authors (RT and JL-C) independently screened and selected the studies. In case of disagreement, they searched for consensus through discussion. Ruling by a third party was not necessary.
From the total list of papers, duplicates were first eliminated and then the titles and summaries of the articles were read to also eliminate those not fulfilling the eligibility criteria. The full text of articles meeting eligibility criteria was read. Two authors (RT and JL-C) independently extracted the data from the selected articles using data collection forms. The following information was gathered from the studies when available: polymorphisms investigated, main outcomes, genotype and allelic frequencies, haplotype, age, sex, name of the first author, publication year, location or/and ethnicity, number of cases and controls, and population type.
Risk of bias was assessed using the Q-Genie tool (29). The Q-Genie Tool contains 11 items rated on a scale of 1–7 that assess the following domains: (1) Rationale for study; (2) Selection and definition of the outcome of interest; (3) Selection and comparability of comparison groups; (4) Technical classification of the exposure; (5) Non-technical classification of the exposure; (6) Other sources of bias; (7) Sample size and power; (8) A priori planning of analyses; (9) Statistical methods and control for confounding; (10) Testing of assumptions and inferences for genetic analyses; and (11) Appropriateness of inferences drawn from results. Two authors (RT and JL-C) independently assessed studies. An overall score was given to classify studies as poor, moderate, or good quality.
The results are presented according to the affected proteins: pro- and anti-inflammatory cytokines, CRP, and kynurenine pathway enzymes. All presented results are statistically significant unless specified otherwise. The following genes were studied: TNFα, tumor necrosis factor receptor 2 (TNF-RII), IL-6, IL-8, interleukin 8 receptor-alpha (IL8RA), IFNγ, interleukin 18 (IL-18), monocyte chemoattractant protein 1 (MCP-1), macrophage migration inhibitory factor (MIF), IL-10, interleukin 1-α (IL-1α), IL-1β, interleukin 7 (IL-7), TGF-β1, CRP, aminocarboxy muconate semialdehyde decarboxylase (ACMSD), hydroxyanthranilate 3,4-dioxygenase (HAAO), interleukin 28 Receptor-Alpha (IL28RA), acid phosphatase 1 (ACP1), cluster of differentiation (CD44), ADAM metallopeptidase with thrombospondin type 1 motif 14 (ADAMTS14), and proteasome activator complex subunit 2 (PSME2). The most frequently mentioned genes were TNFα (9 studies) followed by ACP1 (4), IL-1β (3), IL-8 (2), IFNγ (2), MIF (2), IL-10 (2), IL-1α (2), IL-7 (2), TGF-β1 (2). All other genes were mentioned only in a single study.
Initially, 397 studies were identified. After duplicates were excluded, the remaining 294 abstracts were read and 44 were selected for full text retrieval. Twelve full-text articles were excluded (reviews, genes not directly involved in inflammation, not association studies). In total, 32 studies met the inclusion criteria (Figure 1).
The studies have been conducted in different regions of the world. USA (n = 7), Iran (n = 4), Sweden (n = 3), Spain (n = 2), Korea (n = 4), China (n = 3), Turkey (2), Brazil (2), Canada, Italy, Japan, India, and Poland. Five studies were GWAS, and all others were case-control studies (Table 1). The quality assessment classified only six studies as having good quality, fifteen with moderate quality, and eleven with poor quality (Supplementary Table 1).
The associations between SIB and SNPs are shown in (Table 2).
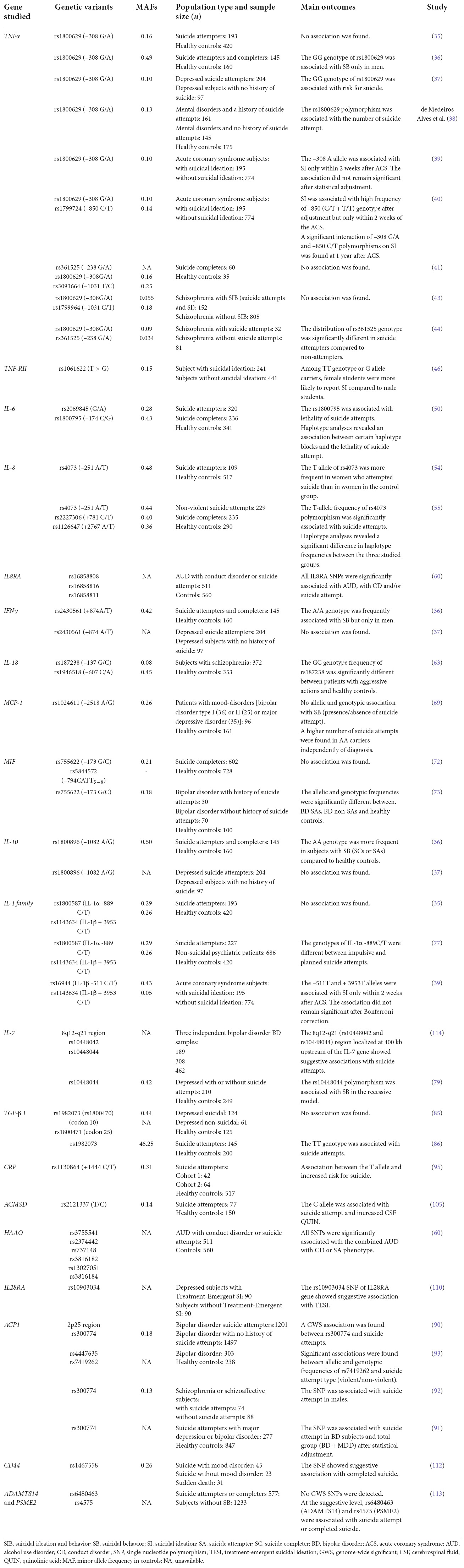
Table 2. Association between suicidal behavior and SNPs in genes encoding molecules involved in inflammation.
TNFα is a pro-inflammatory cytokine (30). First discovered in 1975 (31), it belongs to the superfamily of TNFs comprising 19 members (32). It is mainly produced by macrophages (33), but also by a large range of cell types such as natural killer cells (NK), T and B cell lymphocytes (34).
The first association study compared SAs to healthy controls. No significant differences were observed in genotype or allelic frequencies of rs1800629 (–308 G/A) polymorphism (35). Omrani et al. (36) then compared SCs or SAs to healthy controls. They found that the frequency of the GG genotype of rs1800629 polymorphism was higher in those with SB. However, this result was only significant for men. In an independent sample of patients with MDD (major depressive disorder), SAs were also more likely to present the GG genotype compared to non-attempters (37). Another study compared mental-disordered patients with and without a history of SAs to healthy controls. They reported that the G allele was more frequent among SAs having made a single attempt compared to controls (38).
Kang et al. (39, 40) conducted two studies on the same cohort to investigate the relationship of SI to TNFα polymorphisms 2 weeks and 1 year after an acute coronary syndrome (ACS). In the first study, a significant association between SI and the –308A allele was found during the acute phase (2 weeks) but it did not persist at the end of follow-up or after Bonferroni correction (39). In the second study, SI was associated with the –850 (C/T + T/T) genotype of rs1799724 polymorphism only within the first 2 weeks. The authors also found a significant interaction of –308 G/A and –850 C/T polymorphisms on SI at 1 year after ACS (40). Wang et al. (41) studied three polymorphisms, rs361525, rs1800629, and rs1799964, in a post-mortem sample (SCs and controls). They found no rare allele for rs361525 in the TNFα encoding gene. The allelic and genotypic frequencies of the two other SNPs rs1800629 and rs1799964 were not different between SCs with any psychiatric disorder (including MDD) and healthy control subjects (42).
A Chinese study compared suicidal patients (SAs and/or SI) to non-suicidal patients, all of them affected by schizophrenia. The genotypic and allelic distributions of rs1800629 (–308 G/A) and rs1799964 (–1031 C/T) polymorphisms did not differ between the groups (43).
Lastly, rs1800629 and rs361525 (–238 G/A) polymorphisms have been the subject of one study in patients with schizophrenia with or without a history of SA. The distribution of rs361525 genotype, but not that of rs1800629, was significantly different in SAs compared to individuals with no history of SB (44).
TNF-RII is a protein found mainly on the surface of immune cells that mediates the biological effects of TNF (45). One study on young students after the 2008 Wenchuan earthquake in China investigated, among other things, the role of the TNF-RII gene at position + 676 (rs1061622) regarding the susceptibility to SI. Among TT genotype or G allele carriers, women were more likely to report SI than men (46).
IL-6 is a pro-inflammatory cytokine with a pleiotropic activity on the immune response and inflammation (47). It is produced by T and B cell lymphocytes, macrophages, and glial cells (48) and is one of the cytokines most strongly associated with SIB. Indeed, several post-mortem studies mentioned in meta-analyses and systematic reviews, have shown an abnormally high rate of this cytokine in the blood and brains of SAs or SCs (10, 21, 49).
One study compared the frequencies of two SNPs within the IL-6 encoding-gene, rs2069845 (3329 G/A) and rs1800795 (–174 C/G), between SCs, SAs, and healthy controls (50). The C allele of rs1800795 was significantly more common in SCs than in SAs. Haplotype analysis of these two SNPs revealed that the haplotype AG was more common in SAs compared to healthy controls. The haplotype AC was more common among SCs compared to SAs, and less frequent among SAs compared to controls. The allelic and genotypic frequencies of rs2069845 were not significantly different between the groups.
Originally known as neutrophil chemotactic factor (NCF), it was later given its current title, IL-8, in 1989 (51). IL-8 plays a role in inflammation (52) and in the development of several cancers (53). Monocytes, neutrophils, fibroblasts, and endothelial cells can release IL-8 (52).
Two studies explored the relationship between IL-8 polymorphisms and SB. In the first of them, female SAs were more likely to present the T allele of rs4073 (–251 A/T) polymorphism than female controls (54). No significant difference was observed among males or in the whole sample. The relationship of this polymorphism with anxiety and depressive symptoms, and with IL-8 plasma levels, was also studied among SAs. The only significant differences concerned anxiety scores, which were lower in subjects with the AA genotype compared with AT and TT.
Another study comparing SCs, SAs and healthy controls found that the T allele of the rs4073 polymorphism was significantly more frequent among SAs than in the other groups (55). The TCA haplotype (rs4073, rs2227306, and rs1126647, respectively) was also more prevalent in SAs compared to SCs. No allelic or genotype differences were found between groups for the rs2227306 and rs1126647 polymorphisms.
IL8RA, also known as CXCR1, is the chemokine receptor for the IL-8/CXCL8 (56). This molecule is expressed in a wide variety of cell types: NK cells, mast cells, basophil cells, CD8 + T cells, dendritic cells, and endothelial cells (57). IL8RA plays an essential role in the immune system and inflammation (58, 59). It transfers the signal into the immune cells including neutrophils, lymphocytes, and monocytes.
A follow-up investigation of the Collaborative Study on the Genetics of Alcoholism (COGA) data provided evidence for the involvement of 23 genes, including the IL8RA gene, on alcohol use disorder (AUD) with conduct disorder (CD) and/or SA. Three polymorphisms in this gene (rs16858808, rs16858816, and rs16858811) were associated with the combined phenotype after permutation testing (60).
IFNγ is a pro-inflammatory cytokine mainly produced by NK cells, T and B cells, macrophages, and dendritic cells (61). Two studies investigated rs2430561 (+874 A/T) polymorphism and SB. The first one compared allelic and genotypic frequencies of the polymorphism between suicidal subjects (SCs or SAs) and healthy controls. The A/A genotype frequency was significantly higher in males with SB compared to male controls (36). The second study did not find any difference in allelic and genotypic frequencies between MDD subjects with a history of suicide attempts and MDD without such a history (37).
IL-18, initially called interferon γ inducing factor, is a proinflammatory cytokine that has been implicated in neuroinflammatory and neurodegenerative pathways (62). One study investigated the relationship of two IL-18 promoter polymorphisms, rs187238 (–137 G/C) and rs1946518 (–607 C/A), with suicidal acts and non-suicidal aggression in schizophrenia. The only association concerned rs187238, the frequency of the GC genotype was higher in subjects with schizophrenia and a history of aggression than in healthy controls (63).
MCP-1 is a potent inflammatory cytokine. It has a chemoattractant effect on the immune cells. It belongs to the human CC-chemokines family (64, 65). MCP-1 has been associated with several inflammatory disorders like multiple sclerosis (66), rheumatoid polyarthritis (67), and inflammatory bowel disease (68).
One study of Italian outpatients investigated the rs1024611 (–2518 A/G) polymorphism of the MCP-1 encoding-gene in mood disorders (MDD and Type 1 and Type 2 BD. The number of SA was higher in AA genotype carriers compared to AG genotype carriers, independently of diagnosis. The authors showed an association between BD and a history of attempted suicide. Moreover, in the BD group, a higher number of SAs was found in A carriers (69).
Migration inhibitory factor is a pro-inflammatory cytokine that plays a major role in the regulation of the immune response and inflammation (70). Multiple cell types produce MIF, including T and B cells, monocytes/macrophages, eosinophils, endothelial cells, epithelial cells, fibroblasts, and muscle cells (71). A Japanese study investigated two functional polymorphisms of the MIF gene promoter, rs5844572 (794CATT5–8 microsatellite) and rs755622 (–173 G/C), in SCs. No significant differences were found in the allelic and genotypic frequency distribution of the rs5844572 and rs755622 polymorphisms between SCs and healthy controls. Haplotype analysis of these polymorphisms also showed no association with suicide (72).
Aytac et al. (73) also investigated rs755622 polymorphism in subjects with BD. The allelic and genotypic frequencies of the SNP were significantly different between SAs, non-SAs and healthy controls (73).
IL-10 is an immunoregulatory cytokine with anti-inflammatory properties (74). Several cell types synthesize IL-10 such as Th2 cells, monocytes, macrophages, B cells, eosinophils, mast cells and keratinocytes. Three studies examined the relationship between polymorphisms affecting the IL-10 gene and SB. In the first one, the genotype AA of the rs1800896 (–1082 A/G) SNP was more frequent in SCs or SAs compared to healthy controls (36). In the second study, the allele and genotypic frequencies of rs1800871 (–819 C/T) were not statistically different between mental-disordered patients with and without a history of SAs and healthy controls (38). Finally, in the study by Kim et al. (37), the genotypic and allelic distribution of the rs1800896 polymorphism did not differ between two groups of MDD patients: those who had attempted suicide and those who had not (37).
The IL-1 family comprises five members: IL-1α, IL-1β, IL-1ra, IL-18, IL-33 (75), with both pro- and anti-inflammatory properties. IL-1 α, IL-1 β are potent pro-inflammatory cytokines while IL-1ra has anti-inflammatory properties (76). Saiz et al. (35, 77) investigated the relationship between two functional polymorphisms: rs1800587 (IL-1α -889 C/T) and rs1143634 (IL-1β + 3953 C/T) with SB. No significant differences in the genotypic frequency were found between SAs, non-suicidal psychiatric patients, and healthy controls (35, 77). However, the IL-1α -889 TT genotype frequency was higher in SAs that had planned their attempt(s), and the C/T genotype was more common in SAs who made impulsive attempts (77). The aforementioned study by Kang et al. (39) with ACS patients investigated also IL-1β polymorphisms. Two IL-1β alleles, –511T (rs16944) and +3953T, showed a significant association with SI during the acute phase that disappeared after applying Bonferroni correction (39).
IL-7 is an important cytokine for the development of both B and T lymphocytes [(78), p. 15]. A study conducted on suggestive significant SNPs for SIB reported in GWAS studies found that seven SNPs were significant in different genetic models, particularly, the IL-7 rs10448044 polymorphism in the recessive model (79).
TGF-β1 is a pleiotropic cytokine with pro- and anti-inflammatory activities (80) that is considered a major player in the regulation of the immune response and is produced by many cell types (81). Many studies showed an association between TGF-β1 encoding-gene and inflammatory disorders, asthma (82), systemic sclerosis (83), and rheumatoid polyarthritis (84).
Two studies on SB have been performed on two polymorphisms: rs1800471 (codon 25) and rs1982073 within codon 10, which now bears the number rs1800470 (dbSNP). One study compared depressed SAs and depressed non-attempters to healthy controls, the authors reported the absence of the rare allele in codon 25, but found all three genotypes C/C, C/T, and TT in codon 10. No significant differences in genotypic distribution and allele frequency emerged between the three groups. In vitro, TGF-β1 production was higher in depressed patients (suicidal and non-suicidal) compared to healthy controls subjects, but it did not differ between the TGF-β1 genotypes (C/C, C/T, TT) (85). In the second study, SAs were more likely to present the TGF-β1 codon 10 T/T genotype of rs1982073 than healthy controls (86).
The ACP1 gene encodes the LMW-PTP (low molecular weight phosphotyrosine protein phosphatase), a tyrosine phosphatase (87) involved in signal transduction pathways required in immune responses (88). This enzyme appears to have a role in the pathophysiology of inflammatory diseases (89).
The rs300774 SNP of ACP1 gene has been associated with SA in a GWAS by Willour et al. (90), and the finding was later replicated by two independent studies. In subjects with BD or MDD with or without SA compared with healthy controls (91), and in males suffering from schizophrenia or schizo-affective disorder with or without history of SA (92). Two different SNPs, rs4447635 and rs7419262, were investigated in a sample of patients with BD and a history of SA. The allelic and genotypic frequencies of rs7419262 were associated with the violence of the suicide attempt (93).
C-reactive protein is an acute-phase inflammatory protein (94). Several SNPs have been identified on the CRP gene and the polymorphism rs1130864 (+1444 C/T) has been associated with several diseases. One study compared SAs with various psychiatric diagnoses to healthy controls. An increased risk of SB was found, showing that the T allele was more prevalent among SAs. In addition, the analysis of personality traits in the SA cohort showed that the T allele carriers (CT + TT) had a significantly higher impulsivity score compared with CC carriers (95).
The kynurenine pathway (KP) is the principal route of tryptophan (TRP) catabolism (96, 97). It is implicated in a wide range of psychiatric (98) and central nervous system disorders (99), but also in SB (100). Several enzymes are involved in the production of KP metabolites, such as quinolinic acid (QUIN) (101, 102), and Picolinic acid (PA) (103). Their products can regulate inflammation and cytokine release (104).
The ACMSD enzyme modulates the levels of protective picolinic acid (PA) and toxic quinolinic acid (QUIN). Reduced activity of ACMSD can lead to increased QUIN production. Increased QUIN has been associated with SB (105–107). One study examined the genetic variants of the ACMSD gene related to SB. They showed that the C allele of rs2121337 (T/C) polymorphism was more common in SAs compared to healthy controls (105). The C allele was also associated with increased cerebrospinal fluid levels of QUIN (105). The findings suggest that the rs2121337 polymorphism may influence the activity or expression of ACMSD, and could be linked to SB.
The HAAO enzyme catalyzes the conversion of 3-HAA acid to acroleyl aminofumarate, which further converts to QUIN and PA. Analysis of the COGA data showed that the HAAO gene SNPs: rs3755541, rs2374442, rs737148, rs3816182, rs13027051, and rs3816184 were significantly associated with the combined AD + CD or SA phenotype after permutation testing (60).
Genome-wide association studies have identified novel associations between genetic variants and phenotypes, but they have several limitations: population stratification, missing heritability, epistasis, ultra-rare mutations, and causal variants (108), especially for complex phenotypes like SB.
Although there are few significant associations between SB and SNPs in inflammatory-related genes, some have been reported at the genome-wide significant (GWS) level (p-values < 5 × 10–8), but some suggestive associations (5 × 10–8 ≤ p-values < 1 × 10–5) also deserve mention.
The rs10903034 SNP, located in the IL28RA gene encodes for a transmembrane protein that heterodimerizes with another subunit to form a type II cytokine receptor (109). This SNP showed a suggestive association with antidepressant-emergent SI [Laje et al., (110)].
Another GWAS study comparing BD subjects with and without a history of SAs, reported an association signal on chromosomal region 2p25 (rs300774) at the threshold of GWS with p = 5.07 × 10–8 (90). The rs300774 is within a large linkage disequilibrium block containing the acid phosphatase 1 gene (ACP1).
The first GWAS study on suicides and non-suicidal deaths found suggestive associations between 22 SNPs of 19 genes and suicide, independently of psychiatric diagnoses. Among these SNPs, the rs1467558 of the CD44 gene is known to be involved in inflammation (111, 112). The CD44 gene was also under-expressed in the SCs group compared to the control group. The same team later performed another GWAS comparing SCs, SAs, psychiatric controls, and healthy volunteers. No GWS SNPs were detected, but two SNPs (rs6480463 and rs4575), within the ADAMTS14 and PSME2 genes which are known to have a regulatory role in inflammatory responses, showed a suggestive association with SB (including SA and SC) (113).
Finally, a GWAS of SA was performed on three BD samples. Two regions showed suggestive associations with SA. One of them was localized to 8q12-q21 (containing rs10448042 and rs10448044), 400 kb upstream of the IL-7 gene (114).
This systematic review found several genetic variants involved in the inflammatory process that could be potentially associated with SIB. Specifically, polymorphisms in IL-8 (rs4073), CRP (rs1130864), TNFα (rs1800629, rs361525, and rs1099724), TNF-RII (rs1061622), TGFβ1 (rs1982073), ACP1 (rs7419262 and rs300774), IL-10 (rs1800896), IFNγ (rs2430561), ACMSD (rs2121337), IL-7 (rs10448044, rs10448042), MIF (rs755622), IL-1α (rs1800587), and IL-1 β (rs1143634 and rs16944). In addition, a GWAS reported one significant association at the threshold of genome-wide significance (GWS) level with the rs300774 SNP, located at the 2p25 region containing the ACP1 gene. Some suggestive associations were also found with the rs10903034 (IL28RA), rs1467558 (CD44), rs6480463 (ADAMTS14) and rs4575 (PSME2), rs10448042 and rs10448044 (Interleukin-7) polymorphisms.
Five of the SNPs mentioned above have shown a particularly consistent association with the production and/or transcription of inflammatory-related molecules: rs1130864 with CRP, rs1800629 with TNF-α, rs4073 with IL-8, rs1982073 (rs1800470) with TGF-β1, and rs1800896 with IL-10. Below, we briefly describe the changes that have been related to these five polymorphisms:
1) The CRP + 1444T allele (rs1130864) was associated with higher CRP concentrations in a cross-sectional study of 562 European adolescents (115). In another study, homozygosity for the same allele was associated with an increased level of CRP following an inflammatory stimulus (116).
2) Higher TNF-α serum level was found in A carriers (AA/AG) of the rs1800629 polymorphism compared to GG carriers in different clinical samples (117, 118). Also, reporter gene assays demonstrated that the A allele had higher transcriptional activity than the G allele in the human B cell line [Wilson et al. (119)].
3) IL-8 production was significantly higher in A carriers of the rs4073 polymorphism, compared to TT carriers, in blood samples from healthy individuals stimulated with lipopolysaccharide (120). Similarly, in gingival biopsies of chronic periodontitis patients, a higher expression of the IL-8 gene was found in patients with rs4073 AT genotype compared to those with TT genotype (121).
4) Concerning rs1982073, the TGF-β1 serum level was significantly higher in CC/CT carriers than TT carriers in healthy individuals (122) and in peripheral blood mononuclear cells of hypertensive and normotensive individuals, the TGF-β1 mRNA level was higher in CC or TC carriers compared to TT carriers (123).
5) The serum (124) and mRNA level (125) of IL-10 have been associated with the rs1800896.
Overall, the results of this review suggest that genetic variations affecting the inflammatory-related genes may have an influence on the risk of SB. To date, separating the role of inflammatory abnormalities in SB and mood disorders is particularly difficult since the mechanisms overlap and many studies are conducted in mood-disordered samples with no unaffected group (11, 49). However, a recent report based on the NESDA study in the Netherlands suggests the existence of a dose-effect response between inflammation and suicidal risk. This study was based on a large sample of nearly 2000 patients with depressive and/or anxiety disorders and found high levels of CRP and IL-6 in suicide ideators and attempters after controlling for potential confounders and multiple testing (126). A genetic propensity to a more intense inflammatory response might help to identify individuals prone to SIB.
The results should also be considered in light of several limitations. First, we identified a small number of eligible studies. Some studies were flawed by a small sample size. Within the case-control studies that were selected, case samples averaged 260.6 ± 253.5 and ranged from 30 to 1201 samples, while control samples averaged 390.0 ± 352.6 and ranged from 31 to 1479. Testing a single SNP for a relatively uncommon condition (such as SIB) would require 248 cases and ideally 1:4 case-control ratio according to a commonly cited estimation (127). Besides, there is evidence of a publication bias toward positive results in polymorphism studies. Among the studies selected for this review 21 analyzed a low number of polymorphisms (1–3), thus increasing the risk of publication bias. These studies should be considered as providing a low level of evidence (128).
Another selection bias is the fact of using clinical samples with patients affected by diverse psychiatric disorders. This point is important since some of the above-mentioned SNPs have been associated with specific disorders: rs1130864 with anxiety (129), rs1800629 with depression (130, 131), rs2430561 with an increased risk of developing BD (132) and IFN-alpha-induced depression (133).
Also, important confounding factors related to SIB are frequently not considered such as the severity of the suicide attempts or personality traits such as impulsive aggression levels. Low-lethality SAs are phenotypically different from those making violent or severe suicide attempts (15, 134, 135). Most studies included low-lethality attempters (i.e., drug-poisoning) or did not consider this factor (35, 77, 85, 95). The inclusion of high-lethality attempters could yield different results. Another factor for which the analyses are often not adjusted is impulsive aggression, a mediator between genetic vulnerability and the risk of SIB (2, 136, 137). On the other hand, most studies recruit ethnically homogeneous samples or do not control for ethnicity limiting the generalizability of genetic findings.
To date, genetic association studies addressing the involvement of inflammation-related genes in SIB have been performed without considering potential interactions between these SNPs and other factors, such as gene-gene (GxG), gene-environment (GxE), and epigenetic interactions. This severely limits the understanding of genetic mechanisms in SIB. For instance, in patients with schizophrenia, a GxG interaction between the rs16940665 of the corticotrophin-releasing hormone receptor type 1 (CRHR1) and the rs1875999 in CRH binding protein increases the risk of SB (138). One GxE study found that the interaction between childhood trauma and FK506-binding protein 5 (FKBP5) SNPs and haplotypes may influence SB (139, 140). Epigenetic mechanisms, such as DNA methylation (141), microRNAs (41), and histone modifications (142) could also increase the risk of SB.
In summary, several SNPs that could affect the production or expression of inflammatory-related molecules and modify the intensity of the inflammatory response have been associated with SIB. However, the extant studies presenting noteworthy limitations, more comprehensive and larger studies, as well as novel approaches based on GxG or GxE, are warranted.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
RT and JL-C conceptualized the study. RT conducted the literature search and drafted the manuscript. All authors reviewed the manuscript and approved the submitted version.
This study was supported by the Nimes University Hospital (NIMAO/2019-2/JLC-01). The organization cited above had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We are grateful to Sarah Kabani for editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.1003034/full#supplementary-material
1. WHO. Suicide Worldwide in 2019. (2019). Available online at: https://www.who.int/publications/i/item/9789240026643
2. Turecki G, Brent DA. Suicide and suicidal behaviour. Lancet. (2016) 387:1227–39. doi: 10.1016/S0140-6736(15)00234-2
3. Calati R, Nemeroff CB, Lopez-Castroman J, Cohen LJ, Galynker I. Candidate biomarkers of suicide crisis syndrome: what to test next? A concept paper. Int J Neuropsychopharmacol. (2020) 23:192–205. doi: 10.1093/ijnp/pyz063
4. Berardelli I, Serafini G, Cortese N, Fiaschè F, O’Connor RC, Pompili M. The involvement of hypothalamus–pituitary–adrenal (HPA) axis in suicide risk. Brain Sci. (2020) 10:653. doi: 10.3390/brainsci10090653
5. Sadkowski M, Dennis B, Clayden RC, ElSheikh W, Rangarajan S, DeJesus J, et al. The role of the serotonergic system in suicidal behavior. Neuropsychiatr Dis Treat. (2013) 9:1699–716. doi: 10.2147/NDT.S50300
6. Brundin L, Erhardt S, Bryleva EY, Achtyes ED, Postolache TT. The role of inflammation in suicidal behaviour. Acta Psychiatr Scand. (2015) 132:192–203. doi: 10.1111/acps.12458
7. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. (2010) 67:446–57. doi: 10.1016/j.biopsych.2009.09.033
8. Munkholm K, Braüner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. (2013) 47:1119–33. doi: 10.1016/j.jpsychires.2013.05.018
9. Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. (2011) 70:663–71. doi: 10.1016/j.biopsych.2011.04.013
10. Ganança MA, Tyrka AR, Cisneros-Trujillo S, Mann JJ, Sublette ME. The role of cytokines in the pathophysiology of suicidal behavior. Psychoneuroendocrinology. (2016) 63:296–310. doi: 10.1016/j.psyneuen.2015.10.008
11. Serafini G, Parisi VM, Aguglia A, Amerio A, Sampogna G, Fiorillo A, et al. A specific inflammatory profile underlying suicide risk? Systematic review of the main literature findings. Int J Environ Res Public Health. (2020) 17:E2393. doi: 10.3390/ijerph17072393
12. Nässberger L, Träskman-Bendz L. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta Psychiatr Scand. (1993) 88:48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x
13. Mendlovic S, Mozes E, Eilat E, Doron A, Lereya J, Zakuth V, et al. Immune activation in non-treated suicidal major depression. Immunol Lett. (1999) 67:105–8. doi: 10.1016/S0165-2478(98)00145-X
14. Kim YK, Lee S-W, Kim S-H, Shim S-H, Han S-W, Choi S-H, et al. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:356–61. doi: 10.1016/j.pnpbp.2007.08.041
15. Isung J. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J Biol Psychiatry. (2012) 13:468–73. doi: 10.3109/15622975.2011.624549
16. Vargas HO, Nunes SO, Pizzo de Castro M, Bortolasci CC, Sabbatini Barbosa D, Kaminami Morimoto H, et al. Oxidative stress and lowered total antioxidant status are associated with a history of suicide attempts. J Affect Disord. (2013) 150:923–30. doi: 10.1016/j.jad.2013.05.016
17. Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, et al. A Preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol. (2009) 19:423. doi: 10.1089/cap.2008.0140
18. Hoyo-Becerra C, Huebener A, Trippler M, Lutterbeck M, Liu ZJ, Truebner K, et al. Concomitant interferon alpha stimulation and TLR3 activation induces neuronal expression of depression-related genes that are elevated in the brain of suicidal persons. PLoS One. (2013) 8:e83149. doi: 10.1371/journal.pone.0083149
19. O’Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. (2013) 30:307–14. doi: 10.1002/da.22087
20. Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. (2009) 66:287–92. doi: 10.1016/j.biopsych.2009.01.030
21. Black C, Miller BJ. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol Psychiatry. (2015) 78:28–37. doi: 10.1016/j.biopsych.2014.10.014
22. Ducasse D, Olié E, Guillaume S, Artéro S, Courtet P. A meta-analysis of cytokines in suicidal behavior. Brain Behav Immun. (2015) 46:203–11. doi: 10.1016/j.bbi.2015.02.004
23. Ekinci O, Ekinci A. The connections among suicidal behavior, lipid profile and low-grade inflammation in patients with major depressive disorder: a specific relationship with the neutrophil-to-lymphocyte ratio. Nord J Psychiatry. (2017) 71:574–80. doi: 10.1080/08039488.2017.1363285
24. Suzuki H, Ohgidani M, Kuwano N, Chrétien F, Lorin de la Grandmaison G, Onaya M, et al. Suicide and microglia: recent findings and future perspectives based on human studies. Front Cell Neurosci. (2019) 13:31. doi: 10.3389/fncel.2019.00031
25. Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr Scand. (2008) 117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x
26. Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J Psychiatry Neurosci. (2018) 43:376–85. doi: 10.1503/jpn.170192
27. Schiweck C, Claes S, Van Oudenhove L, Lafit G, Vaessen T, de Beeck GO, et al. Childhood trauma, suicide risk and inflammatory phenotypes of depression: insights from monocyte gene expression. Transl Psychiatry. (2020) 10:296. doi: 10.1038/s41398-020-00979-z
28. Pandey GN, Rizavi HS, Bhaumik R, Zhang H. Chemokines gene expression in the prefrontal cortex of depressed suicide victims and normal control subjects. Brain Behav Immun. (2021) 94:266–73. doi: 10.1016/j.bbi.2021.01.033
29. Sohani ZN, Meyre D, de Souza RJ, Joseph PG, Gandhi M, Dennis BB, et al. Assessing the quality of published genetic association studies in meta-analyses: the quality of genetic studies (Q-Genie) tool. BMC Genet. (2015) 16:50. doi: 10.1186/s12863-015-0211-2
30. Sedger LM, McDermott MF. TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants – past, present and future. Cytokine Growth Factor Rev. (2014) 25:453–72. doi: 10.1016/j.cytogfr.2014.07.016
31. Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. (1975) 72:3666–70.
32. Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. (2003) 3:745–56. doi: 10.1038/nri1184
33. Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. (2010) 20:87–103.
34. Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. (2003) 66:1403–8. doi: 10.1016/S0006-2952(03)00490-8
35. Saiz PA, García-Portilla P, Paredes B, Arango C, Morales B, Alvarez V, et al. Association study of the interleukin-1 gene complex and tumor necrosis factor alpha gene with suicide attempts. Psychiatr Genet. (2008) 18:147–50. doi: 10.1097/YPG.0b013e3282fb002a
36. Omrani MD, Bushehri B, Bagheri M, Salari-Lak S, Alipour A, Anoshae M-R, et al. Role of IL-10 -1082, IFN-γ +874, and TNF-α -308 genes polymorphisms in suicidal behavior. Arch Suicide Res. (2009) 13:330–9. doi: 10.1080/13811110903266418
37. Kim YK, Hong J-P, Hwang J-A, Lee H-J, Yoon H-K, Lee B-H, et al. TNF-alpha-308G>A polymorphism is associated with suicide attempts in major depressive disorder. J Affect Disord. (2013) 150:668–72. doi: 10.1016/j.jad.2013.03.019
38. de Medeiros Alves V, E Silva ACP, de Souza EVM, de Lima Francisco LCF, de Moura EL, de-Melo-Neto VL, et al. Suicide attempt in mental disorders (MeDi): association with 5-HTT, IL-10 and TNF-alpha polymorphisms. J Psychiatr Res. (2017) 91:36–46. doi: 10.1016/j.jpsychires.2017.02.022
39. Kang H-J, Bae K-Y, Kim S-W, Shin I-S, Hong YJ, Ahn Y, et al. Genetic predisposition toward suicidal ideation in patients with acute coronary syndrome. Oncotarget. (2017) 8:94951–8. doi: 10.18632/oncotarget.21661
40. Kang H-J, Kim J-W, Lee J-Y, Kim S-W, Shin I-S, Hong YJ, et al. Time-specific associations of tumor necrosis factor-α levels and polymorphisms (–850 C/T or –308 G/A) with suicidal ideation in acute coronary syndrome patients. Front Psychiatry. (2021) 12:739823. doi: 10.3389/fpsyt.2021.739823
41. Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y. A complex epigenetic switching is critical for TNF-α upregulation in prefrontal cortex of suicide subjects. Am J Psychiatry. (2018) 175:262–74.
42. Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y. Role of complex epigenetic switching in tumor necrosis factor-α upregulation in the prefrontal cortex of suicide subjects. Am J Psychiatry. (2018) 175:262–74. doi: 10.1176/appi.ajp.2017.16070759
43. Lang X, Trihn TH, Wu HE, Tong Y, Xiu M, Zhang XY. Association between TNF-alpha polymorphism and the age of first suicide attempt in chronic patients with schizophrenia. Aging (Albany NY). (2020) 12:1433–45. doi: 10.18632/aging.102692
44. Aytac HM, Ozdilli K, Tuncel FC, Pehlivan M, Pehlivan S. Tumor necrosis factor-alpha (TNF-α) -238 G/A polymorphism is associated with the treatment resistance and attempted suicide in schizophrenia. Immunol Invest. (2022) 51:368–80. doi: 10.1080/08820139.2020.1832115
45. Chen X, Oppenheim JJ. The phenotypic and functional consequences of tumour necrosis factor receptor type 2 expression on CD4+ FoxP3+ regulatory T cells. Immunology. (2011) 133:426–33. doi: 10.1111/j.1365-2567.2011.03460.x
46. Si YJ, Guo QW, Chen X, Yang M, Lin J, Fang DZ. Increased TG/HDL-C in female G allele carriers of rs1061622 at gene of tumour necrosis factor receptor 2 with suicidal ideation. Eur J Clin Invest. (2020) 50:e13322. doi: 10.1111/eci.13322
47. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
48. Kishimoto T. IL-6: from laboratory to bedside. Clin Rev Allergy Immunol. (2005) 28:177–85. doi: 10.1385/CRIAI:28:3:177
49. Brundin L, Bryleva EY, Thirtamara Rajamani K. Role of inflammation in suicide: from mechanisms to treatment. Neuropsychopharmacology. (2017) 42:271–83. doi: 10.1038/npp.2016.116
50. Eftekharian MM, Noroozi R, Omrani MD, Sharifi Z, Komaki A, Taheri M, et al. Single-nucleotide polymorphisms in interleukin 6 (IL-6) gene are associated with suicide behavior in an Iranian population. J Mol Neurosci. (2018) 66:414–9. doi: 10.1007/s12031-018-1190-3
51. Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. (1989) 84:1045–9.
52. Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. (1989) 1:2–13. doi: 10.1016/1043-4666(89)91043-0
53. Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. (2001) 12:375–91. doi: 10.1016/S1359-6101(01)00016-8
54. Janelidze S, Suchankova P, Ekman A, Erhardt S, Sellgren C, Samuelsson M, et al. Low IL-8 is associated with anxiety in suicidal patients: genetic variation and decreased protein levels. Acta Psychiatr Scand. (2015) 131:269–78. doi: 10.1111/acps.12339
55. Noroozi R, Omrani MD, Ayatollahi SA, Sayad A, Ata A, Fallah H, et al. Interleukin (IL)-8 polymorphisms contribute in suicide behavior. Cytokine. (2018) 111:28–32. doi: 10.1016/j.cyto.2018.07.036
56. Weathington NM, Blalock JE. The Biology of CXC Chemokines and Their Receptors. In Current Topics in Membranes. (Vol. 55). Cambridge, MA: Academic Press (2005). p. 49–71. doi: 10.1016/S1063-5823(04)55002-0
57. Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, et al. International union of pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. (2014) 66:1–79. doi: 10.1124/pr.113.007724
58. Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. (2017) 7:1543–88. doi: 10.7150/thno.15625
59. Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. (2015) 7:a016303. doi: 10.1101/cshperspect.a016303
60. Dick DM, Meyers J, Aliev F, Nurnberger J, Kramer J, Kuperman S, et al. Evidence for genes on chromosome 2 contributing to alcohol dependence with conduct disorder and suicide attempts. Am J Med Genet B Neuropsychiatr Genet. (2010) 153B:1179–88. doi: 10.1002/ajmg.b.31089
61. Kak G, Raza M, Tiwari BK. Interferon-gamma (IFN-γ): exploring its implications in infectious diseases. Biomol Concepts. (2018) 9:64–79. doi: 10.1515/bmc-2018-0007
62. Alboni S, Cervia D, Sugama S, Conti B. Interleukin 18 in the CNS. J Neuroinflammation. (2010) 7:9. doi: 10.1186/1742-2094-7-9
63. Liu J, Liu J, Zhou Y, Li S, Li Y, Song X, et al. Association between promoter variants of interleukin-18 and schizophrenia in a Han Chinese population. DNA Cell Biol. (2011) 30:913–7. doi: 10.1089/dna.2011.1221
64. Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. (2003) 10:247–57. doi: 10.1038/sj.mn.7800190
65. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. (2009) 29:313–26. doi: 10.1089/jir.2008.0027
66. Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. (2006) 112:195–204. doi: 10.1007/s00401-006-0083-7
67. Rantapää-Dahlqvist S, Boman K, Tarkowski A, Hallmans G. Up regulation of monocyte chemoattractant protein-1 expression in anti-citrulline antibody and immunoglobulin M rheumatoid factor positive subjects precedes onset of inflammatory response and development of overt rheumatoid arthritis. Ann Rheum Dis. (2007) 66:121–3. doi: 10.1136/ard.2006.057331
68. Spoettl T, Hausmann M, Herlyn M, Gunckel M, Dirmeier A, Falk W, et al. Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes. Clin Exp Immunol. (2006) 145:190–9. doi: 10.1111/j.1365-2249.2006.03113.x
69. Altamura AC, Mundo E, Cattaneo E, Pozzoli S, Dell’osso B, Gennarelli M, et al. The MCP-1 gene (SCYA2) and mood disorders: preliminary results of a case-control association study. Neuroimmunomodulation. (2010) 17:126–31. doi: 10.1159/000258696
70. Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. (2003) 3:791–800. doi: 10.1038/nri1200
72. Shimmyo N, Hishimoto A, Otsuka I, Okazaki S, Boku S, Mouri K, et al. Association study of MIF promoter polymorphisms with suicide completers in the Japanese population. Neuropsychiatr Dis Treat. (2017) 13:899–908. doi: 10.2147/NDT.S130855
73. Aytac HM, Oyaci Y, Yazar MS, Erol A, Pehlivan S. Association of MIF and MBL2 gene polymorphisms with attempted suicide in patients diagnosed with schizophrenia or bipolar disorder. J Clin Neurosci. (2020) 78:264–8. doi: 10.1016/j.jocn.2020.04.001
74. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. (2003) 55:241–69. doi: 10.1124/pr.55.2.4
75. Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol. (2010) 125:S53–72. doi: 10.1016/j.jaci.2009.07.008
76. Banerjee M, Saxena M. Interleukin-1 (IL-1) family of cytokines: role in Type 2 Diabetes. Clin Chim Acta. (2012) 413:1163–70. doi: 10.1016/j.cca.2012.03.021
77. Saiz PA, García-Portilla P, Paredes B, Corcoran P, Arango C, Morales B, et al. Role of serotonergic-related systems in suicidal behavior: data from a case-control association study. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:1518–24. doi: 10.1016/j.pnpbp.2011.04.011
78. Alpdogan Ö, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. (2005) 26:56–64. doi: 10.1016/j.it.2004.11.002
79. Gupta G, Deval R, Mishra A, Upadhyay S, Singh PK, Rao VR. Re-testing reported significant SNPs related to suicide in a historical high -risk isolated population from north east India. Hereditas. (2020) 157:31. doi: 10.1186/s41065-020-00144-y
80. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti- and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. (2009) 9:447–53. doi: 10.1016/j.coph.2009.04.008
81. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. (2006) 24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737
82. Chiang C-H, Chuang C-H, Liu S-L, Shen H-D. Genetic polymorphism of transforming growth factor β1 and tumor necrosis factor α is associated with asthma and modulates the severity of asthma. Respir Care. (2013) 58:1343–50. doi: 10.4187/respcare.02187
83. Ohtsuka T, Yamakage A, Yamazaki S. The polymorphism of transforming growth factor-β1 gene in Japanese patients with systemic sclerosis. Br J Dermatol. (2002) 147:458–63. doi: 10.1046/j.1365-2133.2002.04947.x
84. Zhou T-B, Zhao H-L, Fang S-L, Drummen GPC. Association of transforming growth factor-β1 T869C, G915C, and C509T gene polymorphisms with rheumatoid arthritis risk. J Recept Signal Transduct Res. (2014) 34:469–75. doi: 10.3109/10799893.2014.919594
85. Lee H-Y, Kim Y-K. Transforming growth factor-β1 and major depressive disorder with and without attempted suicide: preliminary study. Psychiatry Res. (2010) 178:92–6. doi: 10.1016/j.psychres.2009.03.023
86. Omrani MD, Bagheri M, Bushehri B, Azizi F, Anoshae M-R. The association of TGF-β1 codon 10 polymorphism with suicide behavior. Am J Med Genet B Neuropsychiatr Genet. (2012) 159B:772–5. doi: 10.1002/ajmg.b.32082
87. Mitchell Bryson GL, Massa H, Trask BJ, Van Etten RL. Gene structure, sequence, and chromosomal localization of the human red cell-type low-molecular-weight acid phosphotyrosyl phosphatase gene, ACP1. Genomics. (1995) 30:133–40. doi: 10.1006/geno.1995.9893
88. Caselli A, Paoli P, Santi A, Mugnaioni C, Toti A, Camici G, et al. Low molecular weight protein tyrosine phosphatase: multifaceted functions of an evolutionarily conserved enzyme. Biochim Biophys Actas. (2016) 1864:1339–55. doi: 10.1016/j.bbapap.2016.07.001
89. Bottini N, Bottini E, Gloria-Bottini F, Mustelin T. Low-molecular-weight protein tyrosine phosphatase and human disease: in search of biochemical mechanisms. Arch Immunol Ther Exp (Warsz). (2002) 50:95–104.
90. Willour VL, Seifuddin F, Mahon PB, Jancic D, Pirooznia M, Steele J, et al. A genome-wide association study of attempted suicide. Mol Psychiatry. (2012) 17:433–44. doi: 10.1038/mp.2011.4
91. Pawlak J, Dmitrzak-Weglarz M, Wilkosc M, Szczepankiewicz A, Leszczynska-Rodziewicz A, Zaremba D, et al. Suicide behavior as a quantitative trait and its genetic background. J Affect Disord. (2016) 206:241–50. doi: 10.1016/j.jad.2016.07.029
92. Li J, Yoshikawa A, Meltzer HY. Replication of rs300774, a genetic biomarker near ACP1, associated with suicide attempts in patients with schizophrenia: relation to brain cholesterol biosynthesis. J Psychiatr Res. (2017) 94:54–61. doi: 10.1016/j.jpsychires.2017.06.005
93. Campos SB, Brasil Rocha PM, Neves FS, Miranda DM, Correa H. ACP1 gene polymorphism associated with suicide attempt type in bipolar disorder patients. Psychiatry Investig. (2017) 14:909–10. doi: 10.4306/pi.2017.14.6.909
94. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. (2003) 111:1805–12. doi: 10.1172/JCI18921
95. Suchankova P, Holm G, Träskman-Bendz L, Brundin L, Ekman A. The +1444C>T polymorphism in the CRP gene: a study on personality traits and suicidal behaviour. Psychiatr Genet. (2013) 23:70–6. doi: 10.1097/YPG.0b013e32835d71b6
96. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. (2017) 10:1178646917691938. doi: 10.1177/1178646917691938
97. Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. (1983) 6:101–97. doi: 10.1016/0098-2997(83)90005-5
98. Muneer A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: pathophysiologic and therapeutic considerations. Clin Psychopharmacol Neurosci. (2020) 18:507–26. doi: 10.9758/cpn.2020.18.4.507
99. Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. (2002) 1:609–20. doi: 10.1038/nrd870
100. Bryleva EY, Brundin L. Kynurenine pathway metabolites and suicidality. Neuropharmacology. (2017) 112:324–30. doi: 10.1016/j.neuropharm.2016.01.034
101. Schiefer J, Töpper R, Schmidt W, Block F, Heinrich PC, Noth J, et al. Expression of interleukin 6 in the rat striatum following stereotaxic injection of quinolinic acid. J Neuroimmunol. (1998) 89:168–76. doi: 10.1016/S0165-5728(98)00133-7
102. Ting KK, Brew BJ, Guillemin GJ. Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer’s disease. J Neuroinflammation. (2009) 6:36. doi: 10.1186/1742-2094-6-36
103. Varesio L, Clayton M, Blasi E, Ruffman R, Radzioch D. Picolinic acid, a catabolite of tryptophan, as the second signal in the activation of IFN-gamma-primed macrophages. J Immunol. (1990) 145:4265–71.
104. Baumgartner R, Forteza MJ, Ketelhuth DFJ. The interplay between cytokines and the kynurenine pathway in inflammation and atherosclerosis. Cytokine. (2019) 122:154148. doi: 10.1016/j.cyto.2017.09.004
105. Brundin L, Sellgren CM, Lim CK, Grit J, Pålsson E, Landén M, et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry. (2016) 6:e865. doi: 10.1038/tp.2016.133
106. Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. (2013) 38:743–52. doi: 10.1038/npp.2012.248
107. Lugo-Huitrón R, Ugalde Muñiz P, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev. (2013) 2013:104024. doi: 10.1155/2013/104024
108. Tam V, Patel N, Turcotte M, Bossé Y, Paré G, Meyre D. Benefits and limitations of genome-wide association studies. Nat Rev Genet. (2019) 20:8. doi: 10.1038/s41576-019-0127-1
109. Vlachiotis S, Andreakos E. Lambda interferons in immunity and autoimmunity. J Autoimmun. (2019) 104:102319. doi: 10.1016/j.jaut.2019.102319
110. Laje G, Allen AS, Akula N, Manji H, Rush AJ, McMahon FJ. Genome-wide association study of suicidal ideation emerging during citalopram treatment of depressed outpatients. Pharmacogenet Genomics. (2009) 19:666. doi: 10.1097/FPC.0b013e32832e4bcd
111. Puré E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. (2001) 7:213–21. doi: 10.1016/s1471-4914(01)01963-3
112. Galfalvy H, Zalsman G, Huang Y-Y, Murphy L, Rosoklija G, Dwork AJ, et al. A pilot genome wide association and gene expression array study of suicide with and without major depression. World J Biol Psychiatry. (2013) 14:574–82. doi: 10.3109/15622975.2011.597875
113. Galfalvy H, Haghighi F, Hodgkinson C, Goldman D, Oquendo MA, Burke A, et al. A genome-wide association study of suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. (2015) 168:557–63. doi: 10.1002/ajmg.b.32330
114. Zai CC, Gonçalves VF, Tiwari AK, Gagliano SA, Hosang G, de Luca V, et al. A genome-wide association study of suicide severity scores in bipolar disorder. J Psychiatr Res. (2015) 65:23–9. doi: 10.1016/j.jpsychires.2014.11.002
115. Arouca AB, Meirhaeghe A, Dallongeville J, Moreno LA, Lourenço GJ, Marcos A, et al. Interplay between the Mediterranean diet and C-reactive protein genetic polymorphisms towards inflammation in adolescents. Clin Nutr. (2020) 39:1919–26. doi: 10.1016/j.clnu.2019.08.016
116. Brull DJ, Serrano N, Zito F, Jones L, Montgomery HE, Rumley A, et al. Human CRP gene polymorphism influences CRP levels. Arterioscler Thromb Vasc Biol. (2003) 23:2063–9. doi: 10.1161/01.ATV.0000084640.21712.9C
117. Yang Y-H, Liu Y-Q, Zhang L, Li H, Li X-B, Ouyang Q, et al. Genetic polymorphisms of the TNF-α-308G/A are associated with metabolic syndrome in asthmatic patients from Hebei province, China. Int J Clin Exp Pathol. (2015) 8:13739–46.
118. Pan J, Hu J, Qi X, Xu L. Association study of a functional variant of TNF-α gene and serum TNF-α level with the susceptibility of congenital heart disease in a Chinese population. Postgrad Med J. (2019) 95:547–51. doi: 10.1136/postgradmedj-2019-136621
119. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc Natl Acad Sci USA. (1997) 94:3195–9. doi: 10.1073/pnas.94.7.3195
120. Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. (2000) 55:1023–7. doi: 10.1136/thorax.55.12.1023
121. Andia DC, de Oliveira NFP, Letra AM, Júnior FHN, Line SRP, de Souza AP. IL8 gene promoter polymorphism (rs4073) may contributes to chronic periodontitis. J Periodontol. (2011) 82:893–9. doi: 10.1902/jop.2010.100513
122. Chen J-Y, Liu J-H, Wu H-DI, Lin K-H, Chang K-C, Liou Y-M. Transforming growth factor-β1 T869C gene polymorphism is associated with acquired sick sinus syndrome via linking a higher serum protein level. PLoS One. (2016) 11:e0158676. doi: 10.1371/journal.pone.0158676
123. Suthanthiran M, Li B, Song JO, Ding R, Sharma VK, Schwartz JE, et al. Transforming growth factor-β1 hyperexpression in African-American hypertensives: a novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci USA. (2000) 97:3479–84.
124. Lv C, Wang Y, Wang J, Zhang H, Xu H, Zhang D. Association of interleukin-10 gene polymorphisms with ankylosing spondylitis. Clin Invest Med. (2011) 34:E370. doi: 10.25011/cim.v34i6.15898
125. Miteva LD, Stanilov NS, Deliysky TS, Stanilova SA. Significance of -1082A/G polymorphism of IL10 gene for progression of colorectal cancer and IL-10 expression. Tumour Biol. (2014) 35:12655–64. doi: 10.1007/s13277-014-2589-2
126. Wiebenga JXM, Heering HD, Eikelenboom M, van Hemert AM, van Oppen P, Penninx BWJH. Associations of three major physiological stress systems with suicidal ideation and suicide attempts in patients with a depressive and/or anxiety disorder. Brain Behav Immun. (2022) 102:195–205. doi: 10.1016/j.bbi.2022.02.021
127. Hong EP, Park JW. Sample size and statistical power calculation in genetic association studies. Genomics Inform. (2012) 10:117–22. doi: 10.5808/GI.2012.10.2.117
128. Valachis A, Mauri D, Neophytou C, Polyzos NP, Tsali L, Garras A, et al. Translational medicine and reliability of single-nucleotide polymorphism studies: can we believe in SNP reports or not? Int J Med Sci. (2011) 8:492–500. doi: 10.7150/ijms.8.492
129. Luciano M, Houlihan LM, Harris SE, Gow AJ, Hayward C, Starr JM, et al. Association of existing and new candidate genes for anxiety, depression and personality traits in older people. Behav Genet. (2010) 40:518–32. doi: 10.1007/s10519-009-9326-4
130. Cerri AP, Arosio B, Viazzoli C, Confalonieri R, Vergani C, Annoni G. The -308 (G/A) single nucleotide polymorphism in the TNF-alpha gene and the risk of major depression in the elderly. Int J Geriatr Psychiatry. (2010) 25:219–23. doi: 10.1002/gps.2323
131. Jun TY, Pae CU, Hoon-Han, Chae JH, Bahk WM, Kim KS, et al. Possible association between -G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population. Psychiatr Genet. (2003) 13:179–81. doi: 10.1097/00041444-200309000-00008
132. Yoon H-K, Kim Y-K. The T allele of the interferon-gamma +874A/T polymorphism is associated with bipolar disorder. Nord J Psychiatry. (2012) 66:14–8. doi: 10.3109/08039488.2011.593045
133. Oxenkrug G, Perianayagam M, Mikolich D, Requintina P, Shick L, Ruthazer R, et al. Interferon-gamma (+874) T/A genotypes and risk of IFN-alpha-induced depression. J Neural Transm (Vienna). (2011) 118:271–4. doi: 10.1007/s00702-010-0525-1
134. Giner L, Jaussent I, Olié E, Béziat S, Guillaume S, Baca-Garcia E, et al. Violent and serious suicide attempters: One step closer to suicide? J Clin Psychiatry. (2014) 75:e191–7. doi: 10.4088/JCP.13m08524
135. Jokinen J, Nordström AL, Nordström P. (2010). Cholesterol, CSF 5-HIAA, violence and intent in suicidal men. Psychiatry Res. 178:217–9. doi: 10.1016/j.psychres.2008.07.020
136. Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P, et al. Candidate endophenotypes for genetic studies of suicidal behavior. Biol Psychiatry. (2009) 65:556–63. doi: 10.1016/j.biopsych.2008.11.021
137. Turecki G. Dissecting the suicide phenotype: the role of impulsive–aggressive behaviours. J Psychiatry Neurosci. (2005) 30:398–408.
138. De Luca V, Tharmalingam S, Zai C, Potapova N, Strauss J, Vincent J, et al. Association of HPA axis genes with suicidal behaviour in schizophrenia. J Psychopharmacol. (2010) 24:677–82. doi: 10.1177/0269881108097817
139. Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch M-A. Interaction of FKBP5, A stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. (2010) 35:1674–83. doi: 10.1038/npp.2009.236
140. Roy A, Hodgkinson CA, DeLuca V, Goldman D, Enoch M-A. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J Psychiatr Res. (2012) 46:72–9. doi: 10.1016/j.jpsychires.2011.09.009
141. Kim J-M, Kang H-J, Kim S-Y, Kim S-W, Shin I-S, Kim H-R, et al. BDNF promoter methylation associated with suicidal ideation in patients with breast cancer. Int J Psychiatry Med. (2015) 49:75–94. doi: 10.1177/0091217415574439
Keywords: self-injurious behavior, suicide, polymorphism, SNP, cytokines, chemokines
Citation: Tamimou R, Lumbroso S, Mouzat K and Lopez-Castroman J (2022) Genetic variations related to inflammation in suicidal ideation and behavior: A systematic review. Front. Psychiatry 13:1003034. doi: 10.3389/fpsyt.2022.1003034
Received: 25 July 2022; Accepted: 29 September 2022;
Published: 17 October 2022.
Edited by:
Chao Xu, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Maria Skibińska, Poznan University of Medical Sciences, PolandCopyright © 2022 Tamimou, Lumbroso, Mouzat and Lopez-Castroman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jorge Lopez-Castroman, am9yZ2VjYXN0cm9tYW5AZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.