
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 23 December 2021
Sec. Public Mental Health
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.798453
This article is part of the Research Topic Global Excellence in Public Mental Health: Asia and Australasia View all 12 articles
Background: Face-to-face cognitive behavioral therapy (CBT) is one of the most widely used non-pharmacological treatment approaches for insomnia. The aim of this study is to assess the efficacy of face-to-face delivered CBT on health outcomes and to evaluate the effect of CBT components as subgroup variables to explain the efficacy of face-to-face delivered CBT on health outcomes in adults over 18 years old with insomnia.
Methods: Relevant randomized controlled trial studies published in the past 22 years were searched through the electronic databases. The Physiotherapy Evidence Database (PEDro) scale was used to assess the quality of the 31 included studies. The mean difference and standard deviation of outcome variables and subgroup variables were analyzed using random effect model, and the heterogeneity among the articles was assessed with the Q-test and I2. Egger regression analysis was used to assess publication bias.
Results: The meta-analysis showed a significant reduction in Insomnia Severity Index [standardized mean difference (SMD) = −2.56, 95% CI −3.81 to −1.30, p < 0.001], Pittsburgh Sleep Quality Index (SMD = −0.96, 95% CI −1.25 to −0.68, p < 0.001), sleep onset latency (SMD = −1.31, 95% CI −2.00 to −0.63, p < 0.001), wakening after sleep onset (SMD = −1.44, 95% CI −2.14 to −0.74, p < 0.001), number of awakenings (SMD = −1.18, 95% CI −2.10 to −0.26, p < 0.05), depression (SMD = −1.14, 95% CI −1.85 to −0.42, p < 0.01), and fatigue (SMD = −2.23, 95% CI −3.87 to −0.58, p < 0.01), and a significant increase in total sleep time (SMD = 0.63, 95% CI 0.28 to 0.98, p < 0.001), sleep efficiency (SMD = 1.61, 95% CI 0.92 to 2.29, p < 0.001), and physical health (SMD = 0.42, 95% CI 0.08 to 0.76, p < 0.05), in the CBT intervention group compared with the control group. There was no significant change in anxiety (SMD = −0.62, 95% CI −1.55 to 0.32, p > 0.05) and mental health (SMD = 1.09, 95% CI −0.59 to 2.77, p > 0.05) in CBT intervention group compared with control group. Group-delivered studies with larger number of intervention sessions and longer duration of single session provided a larger improvement in sleep quality.
Conclusion: Face-to-face delivered CBT is effective in increasing total sleep time, sleep efficiency, and physical health, and reducing Insomnia Severity Index scores, Pittsburgh Sleep Quality Index scores, sleep onset latency, wakening after sleep onset, number of awakenings, depression, anxiety, and fatigue in patients with insomnia. Face-to-face delivered CBT is more effective when delivered through a larger number of sessions with longer duration of each session, and when delivered in groups. Face-to-face CBT is recommended to provide treatment to patients with insomnia in clinical settings.
Systematic Review Registration: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200091, identifier: CRD4202020009.
Insomnia is one type of psychiatric disease that influences the quality, timing, and amount of sleep, resulting in fatigue and mental distress (1). Insomnia has a high prevalence across the world (2). It was reported that the global prevalence of insomnia was up to 30% (3). Mentally, insomnia can impair daytime concentration and generate anxious or depressed feels that may lead to or aggravate other psychiatric diseases such as depression and anxiety (4). Physically, a chronic condition of insomnia can increase the risks of a great amount of chronic diseases such as diabetes, cardiovascular diseases, and cancer (5).
Currently, insomnia is treated with pharmacological and non-pharmacological treatments. Cognitive behavioral therapy (CBT) is one of the widely studied non-pharmacological treatment option for insomnia, with marked long-term and short-term effects (6). It is currently the first-line therapy in managing all kinds of psychiatric diseases including depression, anxiety, and insomnia (7). The CBT approach perceives the psychiatric symptoms are underpinned by distorted cognition and related behaviors, and these symptoms could be reduced if the distorted cognition and behaviors are corrected (8). Based on the CBT principles, multiple CBT strategies were developed to change the thinking and behavioral patterns, and different strategies were involved in CBT treatments (9). For patients with insomnia, previous studies indicated that it was common for these patients to have dysfunctional understandings about sleep and worries about falling asleep (10). These thoughts may lead to behavioral changes such as spending more time in bed trying to fall asleep and irregular sleep times, which may make falling asleep more difficult, further providing reinforcement of the dysfunctional understandings and creating a vicious loop that exacerbates the existing insomnia symptoms (10). Applying CBT to these patients can break this loop from multiple directions. Cognitive behavioral therapy has been commonly delivered in a face-to face format, in which patients has face-to-face consultations with the therapists. The consultations can take place between one patient and a therapist individually (individual delivered) or one or more therapists and multiple patients (group delivered). The face-to-face delivery of CBT is commonly used to patients with insomnia because the components of CBT such as alliances building can be easily achieved in the face-to-face therapy mode. Previous studies have shown that CBT provided a similar efficacy in treating insomnia compared with pharmacological treatments (11). Furthermore, it has been found CBT treatment has fewer side effects than sleep medication intake (6).
Existing meta-analysis studies also found that CBT had a significant overall effect on sleep outcomes in patients with insomnia (12, 13), but these studies focused on sleep outcomes only, and subgroup analysis of CBT components was not included. In addition to sleep outcomes (Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, wakening after sleep onset, and number of awakenings), health outcomes were considered in this study, including psychiatric diseases (scores of depression and anxiety scales), fatigue (scores of fatigue scales), and quality-of-life related physical and mental health (scores of quality-of-life surveys). By assessing the effect of face-to-face CBT on these outcomes, our study will provide a comprehensive overview of the effect of face-to-face CBT on the health status of insomnia patients from a different perspective. We also conducted subgroup analysis to assess the effects of different CBT treatment designs and components on the sleep and psychiatric disease outcomes and the overall efficacy of face-to-face CBT to help develop a reasonable CBT treatment plan.
The procedure of this study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines (14). The protocol of this study was registered at the International Prospective Register of Systematic Reviews (PROSPERO Registration ID: CRD42020200091, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020200091).
The database search process was completed by two researchers (Xu and Sun) independently. Five electronic databases including PubMed, Scopus, EMBASE, Cochrane Library, and PsycINFO were searched for relevant studies by the two researchers. Gray literatures were acquired from ProQuest central: Dissertations and Thesis. The following Boolean approach for the keywords were used for the search: “insomnia” OR “sleep” AND “cognitive behavioral therapy” OR “cognitive behavior therapy” OR “CBT” AND “face-to-face delivered” OR “group delivered” OR “individual delivered” AND “randomized controlled trial” OR “randomized controlled trial” OR “RCT.” The search results from the defined five electronic databases were exported into the EndNote X9 software, and duplicates across databases were removed. Two researchers then examined the titles and abstracts of the articles and excluded articles that did not focus on insomnia or CBT. The full text of the rest of the articles were examined and selected based on the inclusion and exclusion criteria.
Full-text assessment of the articles was also completed by two researchers independently. In this study, PICO approach (15) was used to develop the inclusion and exclusion of articles. Only randomized controlled trial studies were included in this study. The studies must be published in English, and the publishing date should be from 1 January 1990 to 31 August 2021, with no restriction in sample size. Articles that were not peer reviewed or that scored 3 or less on the PEDro (16) scale were excluded from the study. If full text of the article was not available online, reasonable attempts were made, such as sending emails directly to the authors to request the full text of the articles.
P: The participants in the studies must be over 18 years in age and be self-reported or diagnosed with insomnia according to the International Classification of Sleep Disorders (ICSD), diagnostic and statistical manual of mental disorders fourth edition (DSM-IV) or fifth edition (DSM-V), or sleep questionnaires such as the Insomnia Severity Index (score > 7) (17), and Pittsburgh Sleep Quality Index (score > 4) (18). Participants should be randomly assigned to CBT intervention groups or control groups.
I: The face-to-face delivered CBT should be applied to the participants as interventions of insomnia. The CBT intervention must utilize at least two of the core components, including sleep restriction, stimulus control, cognitive therapy, and sleep hygiene. The number of other components in the intervention plan is not limited.
C: Participants in the control group in the studies received normal treatment, were placed in a waiting list (waiting list control, WLC), or received placebo treatments that did not provide significant effects on sleep.
O: The studies must report at least one of the primary outcomes required in this meta-analysis study. The primary outcomes include Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, wake after sleep onset, and number of awakenings.
P. The participants in the study were diagnosed with other severe psychiatric diseases including schizophrenia, severe depression, and severe bipolar disorders.
I. The intervention group received mindfulness-based intervention or remote-delivered CBT intervention.
C. No proper control groups presented in the study or patients in the control groups received any forms of face-to-face delivered CBT intervention.
O. The study did not provide complete data for the primary outcomes.
Studies passing the full-text assessment underwent a further quality assessment process conducted by two researchers independently using the Physiotherapy Evidence Database (PEDro) scale (16). The PEDro scale defines the quality of the articles by giving scores ranging from 0 to 10. The marking criteria include random allocation of participants, concealed allocation of participants, similar baseline characteristics between intervention and control groups, blinding to all participants, blinding to all therapists in the study, blinding to assessors who assessed the key outcomes of the study, enough outcome measures obtained, participants received intervention or control condition as study designed, presence of between-group comparison analysis, and presence of both point measures and measures of variability. For each of the criteria, 1 point would be given if the study completely met the criteria; otherwise, no point would be given. Articles scoring 3 or less were defined as poor quality articles (19). Scores 4–5 indicated moderate quality studies, scores 6–8 indicated good quality studies, and scores 9–10 indicated excellent quality studies (19).
The primary outcomes in this study were sleep outcomes, including severity of insomnia measured by the Insomnia Severity Index (17), sleep quality measured by the Pittsburgh Sleep Quality Index (18), total sleep time, sleep efficiency, sleep onset latency, wakening after sleep onset, and number of awakenings.
The secondary outcomes in this study included psychiatric disease outcomes, fatigue, and quality-of-life related physical and mental health. The psychiatric disease outcomes included scores of depression scales (20–24) and anxiety scales (25–27). The fatigue outcome was measured by the scores of various fatigue scales (28, 29). The quality-of-life related health outcomes were measured by the scores of the physical health section and mental health section in the short-form quality-of-life surveys (SF-12 and SF-36) (30).
The extraction of data was completed by two researchers independently. The third researcher was invited to confirm the data if disagreement occurred between the two researchers. If the article provided results for both post intervention and follow-up, the results in the longer intervention time for follow-up were extracted. Reasonable attempts, such as direct email requests to the authors, were also made to acquire the full datasets of the studies, if possible, when the published articles did not provide enough data.
Characteristics of the included studies and the PEDro scores are summarized in Table 1. The study characteristics include the location where the study was conducted (country), sample size, gender distribution (percentage of females), average age, type of disorder including any comorbid diseases, diagnosis criteria, drugs used in the study, intervention format, presence of manuals, number of intervention sessions, duration of a single session, total treatment time, type of therapist, percentage of participants that completed the treatment, type of control group, outcome variable names, and length of follow-up period.
The mean and standard deviation for the primary and secondary outcomes were extracted directly from the results sections, tables, and figures in the studies for both the CBT intervention group and the control group before and after the intervention. The mean difference was calculated by subtracting the mean before intervention from the mean after intervention, and the total pooled mean difference was also calculated. The standardized mean difference (SMD) was calculated by dividing the mean difference by the pooled standard deviation (31).
The designs and groupings of the subgroup variables were based on the recommended CBT design from the CBT for insomnia guidelines (32) written by Perlis and colleagues. Subgroup information was collected mainly based on the characteristics of intervention delivery and CBT components in each study. The characteristics of intervention were collected from each study and coded into subgroup manually. The subgroups include participant dropout rate (0 for <20% drop out rate and 1 for ≥20% drop out rate), number of treatment sessions (0 for <6 sessions and 1 for ≥6 sessions), duration of treatment sessions (0 for <1 h and 1 for ≥1 h), duration of treatment (0 for <6 weeks and 1 for ≥6 weeks), form of delivery (0 for group-delivered and 1 for individual-delivered), drugs used in the study (0 for no drugs, 1 for hypnotics, and 2 for other drugs including antidepressants and pain drugs), and co-morbid diseases (0 for no co-morbid diseases, 1 for psychiatric diseases, and 2 for chronic diseases). The CBT module component subgroups included treatment rationale, sleep hygiene, relapse prevention, relaxation training, and basic sleep information. The presence of each component was coded as 1, and the absence of certain module was coded as 0.
Because of expected heterogenicity, a random effects model, which provided a statistical parameter of the variations among the studies (33), was used to measure the mean difference and SMD of all outcome variables, and the results were presented as forest plots. A SMD between 0.2 and 0.5 indicated a small effect size, between 0.5 and 0.8 a medium effect size, and >0.8 a large effect size. In addition, the 95% confidence interval (CI) and p-value were determined for both mean difference and SMD. A p-value < 0.05 suggested that the result was statistically significant. The Q test and I2-values were used to assess the heterogenicity of the studies. The I2-value ranges from 0 to 100%. An I2-value larger than 50% indicates that the study has a high heterogenicity and a subgroup analysis is required to explore the possible causes. In addition, Egger regression analysis was used to assess the potential publication bias among the studies for each of the research outcomes, and the funnel plots were presented. A p-value >0.05 indicated that the publication bias for the research outcome was not significant. At the study level, sensitivity analysis was conducted by removing one study at a time vs. all the studies together to identify whether the overall publication bias results for the research outcome were related to any particular study or studies. The software used for data analysis was STATA 17.0.
A total of 2,854 studies were identified through database search and other sources. After removing the duplicates, 2,297 studies were screened by titles and abstracts. After the screening of titles and abstracts, 2,088 studies were excluded, and the remaining 209 studies underwent full-text assessment. Another 178 studies were excluded after full-text assessment. Among the 178 excluded studies, 19 were meta-analysis or systematic reviews. Thirty-three studies were not related to insomnia. Forty-two studies did not provide sufficient data for analysis. Twenty-seven studies did not have control groups. Twenty-eight studies provided PEDro scores lower than 3 and were considered as low-quality studies. Thirteen studies were study protocols for clinical trials. Sixteen studies involved participants aged under 18, which did not meet the inclusion criteria. The final number of eligible studies included for meta-analysis was 31. The detailed process of study screening for meta-analysis was presented in Figure 1.
A total number of 2,449 patients were included in the 31 studies (34–64), with 1,107 of which in the intervention group and 1,342 of which in the control group. A total of 12 studies (37, 39, 40, 46, 47, 51, 53, 54, 57, 58, 61, 63) received PEDro scores of 4–5, suggesting that they had moderate quality. The remaining 19 studies (34–36, 38, 41–45, 48–50, 52, 55, 56, 59, 60, 62, 64) received PEDro scores of 6–8, suggesting that they had good quality. The studies were carried out in nine different countries including USA (34, 37, 40, 43, 45, 47, 49–51, 53, 58, 59, 61–64), UK (41, 42), Canada (37, 39, 55), Australia (48, 54), China (38, 44), Japan (35), Sweden (36, 46, 52, 56), Norway (57), and Korea (60). The percentage of female taking part in the studies was 66.9%, and the average age of the participants was 54.6 years. All of the studies included patients with insomnia. Among of which, some of the studies included patients with different co-morbid diseases including alcohol dependence (34, 39), chronic pain (47, 50, 53, 58, 64), renal diseases (38, 44), hearing impairment (46), depression (37, 49, 52, 54), and posttraumatic stress disorder (61). The percentage of patients that reported co-morbid depression was 30.7% (34, 37–39, 46, 47, 49, 50, 52–55, 60–62), and the percentage of patients that reported co-morbid anxiety was 20.1% (34, 38, 46, 50, 54, 55, 60, 62). Thirteen studies (32, 34, 35, 40, 42, 43, 45–47, 49–51, 53, 55) used the ICSD as diagnosis criteria. Six studies (34, 35, 42, 49, 57, 61) diagnosed insomnia using the DSM-IV, while five studies (40, 41, 54, 62, 63) used the DSM-V. All of the included studies used either group-delivered or individual-delivered CBT in the intervention group, with 16 out of 31 studies (34–37, 39, 46, 47, 50, 52–55, 57, 58, 60, 61) using intervention manuals. Moreover, the total number of intervention sessions ranged from 1 to 36 and the time of a single session ranged from 15 to 120 min. The duration of the intervention ranged from 1 day (one single session) to 3 months. The CBT interventions were all delivered by trained professionals. In addition, an average of 88% of the patients completed the treatment across the 31 included studies. All of the included studies reported at least one of the sleep outcomes. The characteristics of studies included for meta-analysis were displayed in Table 1.
The results of meta-analysis were presented in Table 2. The forest plots were presented in Figures 2, 3.
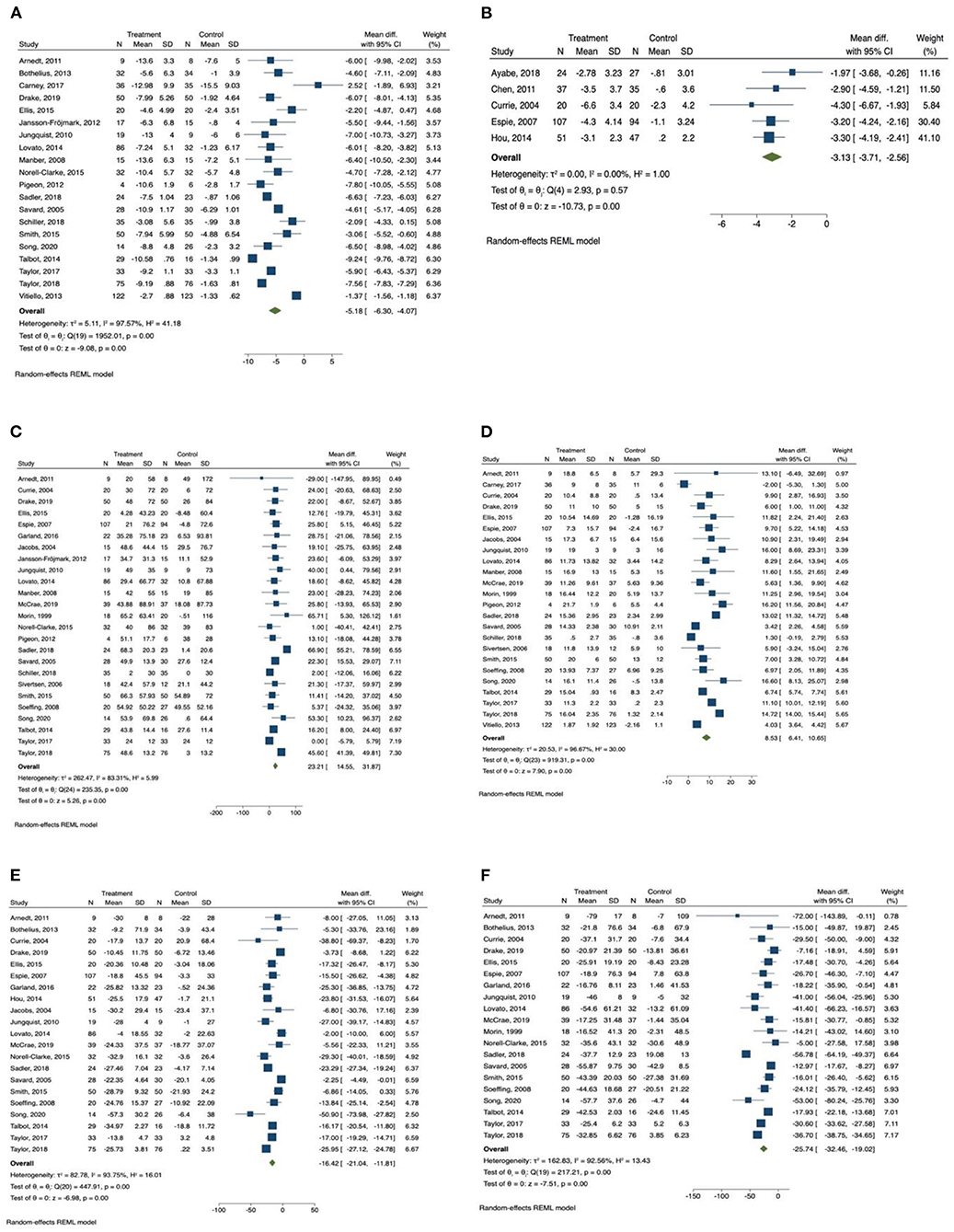
Figure 2. Forest plots of the effect of CBT for sleep outcomes. (A) Insomnia Severity Index, (B) Pittsburgh Sleep Quality Index, (C) total sleep time, (D) sleep efficiency, (E) sleep onset latency, (F) wake after sleep onset.
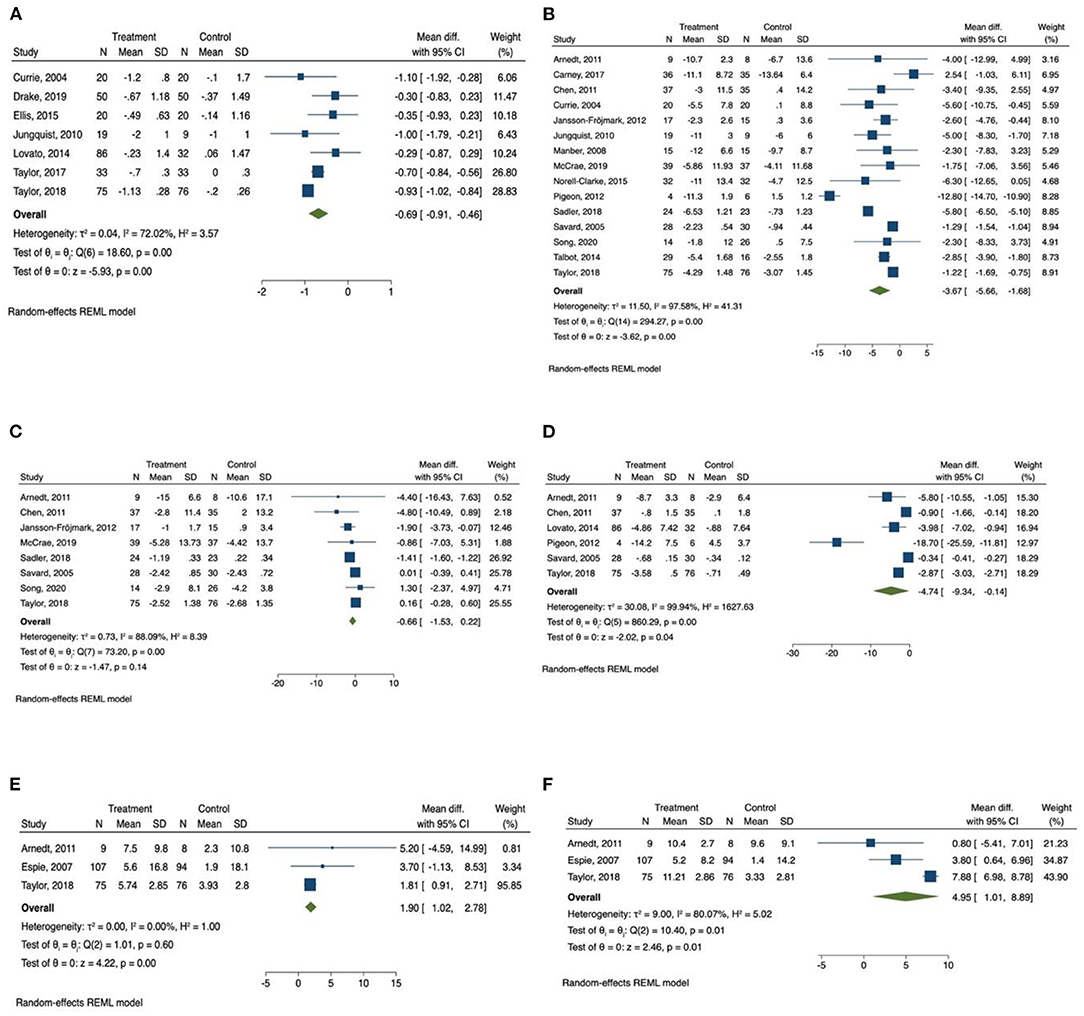
Figure 3. Forest plots of the effect of CBT for sleep and health outcomes. (A) Number of awakenings, (B) depression, (C) anxiety, (D) Fatigue, (E) physical health, (F) mental health.
Insomnia Severity Index was reported by 20 studies (34, 36, 37, 40, 41, 46–49, 52–56, 58, 60–64) as an outcome measurement. Patients in the CBT intervention group showed a significant reduction in insomnia severity as measured by Insomnia Severity Index (mean difference = −5.19, 95% CI −6.30 to −4.07, p < 0.001, I2 = 97.57) compared with the control group, with large effect size (SMD = −2.56, 95% CI −3.81 to −1.30, p < 0.001). Subgroup analysis was conducted for Insomnia Severity Index, and the results are presented in Table 3. We found that the subgroup variable that provided greatest effect in improving reducing insomnia severity was relapse prevention. Studies that included relapse prevention (mean difference = −6.765, 95% CI −8.117 to −5.413, p < 0.001, I2 = 84.05) reported a significantly greater improvement in insomnia severity scores than studies that did not include relapse prevention (mean difference = −6.042, 95% CI −7.106 to −4.978, p < 0.001, I2 = 90.56). Other effective subgroup variables included drop-out rate <20%, number of sessions ≥6, duration of one session <1 h, sleep hygiene, and relaxation training.
Pittsburgh Sleep Quality Index was reported in only five studies (35, 38, 39, 42, 44), and there was an overall significant improvement in sleep quality measured by Pittsburgh Sleep Quality Index in the intervention group (mean difference = −3.13, 95% CI −3.71 to −2.56, p < 0.001, I2 = 0.00) in comparison with the control group, with large effect size (SMD = −0.96, 95% CI −1.25 to −0.68, p < 0.001).
Among the sleep quality outcomes, the intervention group reported a significant increase in total sleep time (mean difference = 23.21, 95% CI 14.56 to 31.87, p < 0.001, I2 = 83.31) compared with the control group, with medium effect size (SMD = 0.63, 95% CI 0.28 to 0.98, p < 0.001). The results of subgroup analysis for total sleep time are displayed in Table 3. The duration of intervention sessions showed greatest effect on total sleep time. In studies in which the duration of one session was longer than 60 min (mean difference = 27.18, 95% CI 14.27 to 40.08, p < 0.001, I2 = 91.65), a greater effect of CBT was observed than in studies providing sessions shorter than 60 min (mean difference = 16.40, 95% CI 9.81 to 22.99, p < 0.001, I2 = 0.00). Other effective CBT characteristics included number of sessions ≥6, group delivered, treatment with drugs, sleep hygiene, relaxation training, and relapse prevention.
We also found that there was a significant increase in sleep efficiency (mean difference = 8.53, 95% CI 6.41 to 10.65, p < 0.001, I2 = 96.67) in the CBT intervention group compared with the control group, with large effect size (SMD = 1.61, 95% CI 0.92 to 2.29, p < 0.001). Subgroup analysis was conducted for sleep efficiency, and the results are presented in Table 4. Both relapse prevention and relaxation training showed a significant effect in improving sleep efficiency. Studies that included relapse prevention (mean difference = 10.834, 95% CI 7.572 to 14.097, p < 0.001, I2 = 87.34) had a greater effect on sleep efficiency than studies that did not include relapse prevention (mean difference = 7.476, 95% CI 4.920 to 10.031, p < 0.001, I2 = 97.01). Likewise, studies that included relaxation training reported a more significant increase in sleep efficiency (mean difference = 9.779, 95% CI 7.087 to 12.472, p < 0.001, I2 = 93.96) than studies that did not include relaxation training (mean difference = 6.787, 95% CI 3.737 to 9.837, p < 0.001, I2 = 95.83). Sleep efficiency was also significantly improved when drop-out rate was <20%, number of sessions were ≥6, length of intervention was ≤ 6 weeks, and sessions were group delivered.
In addition, sleep onset latency time was significantly improved in the CBT intervention group (mean difference = −16.43, 95% CI −21.04 to −11.81, p < 0.001, I2 = 93.75) in comparison with the control group, with large effect size (SMD = −1.31, 95% CI −2.00 to −0.63, p < 0.001). Subgroup analysis was also conducted for sleep onset latency, and the results are presented in Table 4. We found that the subgroup variable that provided greatest effect in improving sleep onset latency was comorbid disease. There was a significantly greater improvement in sleep onset latency when patients had comorbid psychiatric diseases (mean difference = −21.382, 95% CI −25.870 to −16.895, p < 0.001, I2 = 87.73) compared with patients who had comorbid chronic diseases (mean difference = −14.774, 95% CI −24.092 to −5.455, p < 0.01, I2 = 88.72) and patients who had no comorbid diseases (mean difference = −12.726, 95% CI −21.113 to −4.338, p < 0.01, I2 = 77.14). Other effective CBT characteristics and components included drop-out rate <20%, number of sessions <6, duration of one session ≥60 min, group delivered, relapse prevention, relaxation training, and basic sleep information.
Wake time after sleep onset was also significantly improved in the CBT intervention group (mean difference = −25.74, 95% CI −32.46 to −19.02, p < 0.001, I2 = 92.56) compared with the control group, with large effect size (SMD = −1.44, 95% CI −2.14 to −0.74, p < 0.001). The results of subgroup analysis for wakening after sleep onset are displayed in Table 5. The subgroup variables that provided greatest effect in improving wake time after sleep onset were duration of intervention session and relaxation training. The subgroup analysis indicated that wakening after sleep onset was more significantly improved in studies providing intervention sessions longer than 1 h (mean difference = −30.149, 95% CI −39.022 to −21.296, p < 0.001, I2 = 94.17) compared with studies providing sessions shorter than 1 h (mean difference = −17.375, 95% CI −20.768 to −13.987, p < 0.001, I2 = 0.00). A greater improvement in wakening after sleep onset was observed in studies that included relaxation training (mean difference = −30.034, 95% CI −40.118 to −19.950, p < 0.001, I2 = 94.89) compared with studies that did not include relaxation training (mean difference = −19.597, 95% CI −25.892 to −13.302, p < 0.001, I2 = 62.68). Other effective CBT characteristics and components included group delivered, comorbid psychiatric diseases, sleep hygiene, and relapse prevention.
The meta-analysis also showed that there was a significant improvement in number of awakenings (mean difference = −0.686, 95% CI −0.91 to −0.46, p < 0.001, I2 = 72.02) in the CBT intervention group compared with the control group, with large effect size (SMD = −1.18, 95% CI −2.10 to −0.26, p < 0.05).
The Q-test results were consistent with the I2 results in all analyses, indicating the same levels of significance of heterogenicity. The p-values between groups were all smaller than 0.001, suggesting that there was a significant difference between groups for each of the subgroup variables.
A total of 15 studies measured the effect of CBT on depression (31, 34–36, 43, 44, 46, 47, 49–52, 57–59) and the total number of participants in these studies was 753. We found that there was a significant improvement of depressive symptoms measured by depression scales (mean difference = −3.67, 95% CI −5.66 to −1.68, p < 0.001, I2 = 97.58) in CBT intervention groups compared with the control group, and there was a large effect size (SMD = −1.14, 95% CI −1.85 to −0.42, p < 0.01).
Subgroup analysis was also conducted on depression. The results for subgroup analysis on depression were presented in Table 5. The subgroup analysis showed that relapse prevention had greatest effect on depression. Studies that included relapse prevention reported a significantly greater improvement on depressive conditions (mean difference = −5.120, 95% CI −7.856 to −2.384, p < 0.001, I2 = 93.81) than studies that did not include relapse prevention (mean difference = −1.273, 95% CI −1.495 to −1.052, p < 0.001, I2 = 0.00). Other characteristics of the studies including drop-out rate <20%, number of sessions ≥6 sessions, duration of 1 session <1 h, length of intervention >6 weeks, individual delivered, using sleep drugs, and patients with co-morbid chronical diseases had significant effect on the reduction of depression. CBT intervention components including sleep hygiene, relaxation training, and basic sleep information had significant effect on the improvement of depression. The Q-test results were consistent with the I2 results, indicating the same levels of heterogenicity. The p between groups were all smaller than 0.001, suggesting that there was a significant difference between groups for each of the subgroup variables.
Anxiety was measured in eight studies (31, 35, 43, 47, 51, 52, 57, 59), and the total number of participants in these studies was 493. Compared with control groups, CBT intervention groups only reported a slight improvement in anxiety symptoms measured by anxiety scales (mean difference = −0.66, 95% CI −1.53 to 0.22, p > 0.05, I2 = 88.09), with moderate effect size (SMD = −0.62, 95% CI −1.55 to 0.32, p > 0.05). The Q-test result was consistent with the I2 result, indicating a high level of heterogenicity.
Fatigue was reported as outcome measurement in six studies (34, 38, 48, 53, 55, 62), and the total number of participants in these studies was 426. The meta-analysis found that there was a significant reduction of daytime fatigue measured by different fatigue scales (mean difference = −4.74, 95% CI −9.34 to −0.14, p < 0.05, I2 = 99.94) in CBT intervention groups compared with the control group with a large effect size (SMD = −2.23, 95% CI −3.87 to −0.58, p < 0.01). The Q-test result was consistent with the I2 result, indicating a high level of heterogenicity.
The scores of SF 12 and SF 36 quality of life survey were reported in three studies (34, 42, 62). The scores of physical health section and mental health section were collected from all of the three studies. Patients in the CBT intervention groups showed a significant improvement in physical health measured by SF 12 and SF 36 survey (1.90, 95% CI 1.02 to 2.78, p < 0.001, I2 = 0.00), with a medium effect size (SMD = 0.42, 95% CI 0.08 to 0.76, p < 0.05). Mental health measured by SF 12 and SF 36 survey was also improved in CBT intervention group (4.95, 95% CI 1.01 to 8.90, p < 0.05, I2 = 80.07) compared with control group, with a large but not statistically significant effect size (SMD = 1.09, 95% CI −0.59 to 2.77, p > 0.05). The Q-test results were consistent with the I2 results, indicating the same levels of heterogenicity.
The results of the Egger's regression test for all of the research outcomes were summarized in Table 2. The funnel plots of the outcome variables were provided in Supplementary Figures 1, 2. A symmetric distribution of mean difference in all outcomes was observed upon the visual inspection of the funnel plots, indicating that there was no significant publication bias. The results of Egger's test of all variables had no significant results including Insomnia Severity Index (t = 1.05, 95% CI −0.89 to 2.69, p > 0.05), Pittsburgh Sleep Quality Index (t = 0.20, 95% CI −3.99 to 4.52, p > 0.05), total sleep time (t = −0.18, 95% CI −1.19 to 1.00, p > 0.05), sleep efficiency (t = 1.51, 95% CI −0.36 to 2.27, p > 0.05), sleep onset latency (t = −0.87, 95% CI −2.02 to 0.84, p > 0.05), wakening after sleep onset (t = −0.42, 95% CI −1.76 to 1.18, p > 0.05), number of awakenings (t = 0.98, 95% CI −1.22 to 2.71, p > 0.05), depression (t = 0.04, 95% CI −1.82 to 1.89, p > 0.05), anxiety (t = −0.86, 95% CI −1.89 to 0.90, p > 0.05), physical health (t = 1.00, 95% CI −9.78 to 11.45, p > 0.05), and mental health (t = −3.18, 95% CI −14.87 to 8.91, p > 0.05). These results suggested that there is no significant publication bias for these outcomes. The study by Pigeon et al. (53) was excluded in the analysis of fatigue because it provided a high publication bias for this outcome. The Egger's t-value for fatigue after excluding this study was −1.77 (95% CI −5.01 to 1.43, p > 0.05), which suggested that there is no significant publication bias for fatigue in the rest of the studies. Removing any single study in other outcomes did not change the overall results. Therefore, all of the included studies contributed to the overall publication bias results in all outcomes except fatigue.
This study included 31 randomized controlled trial studies to assess the effect of face-to-face delivered CBT on different health outcomes in patients with insomnia. The results from this meta-analysis study showed that face-to-face delivered CBT had a significant positive effect in improving all of the sleep outcomes (Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, wakening after sleep onset, and number of awakenings), one of the psychiatric disease outcomes (depression), fatigue, and quality-of-life related physical and mental health. Face-to-face delivered CBT did not show significant effects on the other psychiatric disease outcome (anxiety).
The meta-analysis results were consistent with the findings from previous studies (12, 65), suggesting that face-to-face CBT had an overall significant effect in improving sleep quality and reducing insomnia symptoms. Previous studies (65, 66) mentioned that one of the major goals of CBT interventions on insomnia was to initially limit sleep opportunities at wrong time points in order to increase the pressure for sleep at right time points. Ultimately, the increased sleep drive would lead to an improvement of homeostatic regulation of sleep, and then right sleep opportunities at right time points. Variables such as sleep efficiency, sleep onset latency, and wake after sleep onset were related to homeostatic regulation of sleep, and these variables reported a relatively immediate improvement during the intervention, indicating an improvement of homeostatic regulation (65, 66). Other variables, including total sleep time and number of awakening, may show greater improvements over time as participants continue to practice the skills acquired during CBT intervention and gradually increase their opportunity for sleep (65, 66).
In randomized controlled trial studies, especially studies which were delivered in groups, frequent drop-out of participants may bring negative thoughts, such as untrust of the intervention and therapists, to other participants in the study. Participants would be less focused during the intervention sessions, and the overall effect of intervention would be reduced. Moreover, larger number of intervention sessions and longer duration of single sessions give the therapists a better chance to deliver the contents of the intervention in detail. Participants in these studies will get better understandings of the intervention contents and strategies and more likely to apply the strategies to their real life. We noticed that one of the included studies by Ellis (41) provided only one session of CBT intervention, but this study provided similar results on sleep outcomes compared with other studies which provided larger number of sessions. Considering this study was aimed to treat acute insomnia at early phase, further study was needed to confirm the effects of brief CBT on acute insomnia. In addition, longer intervention period could lead to the impatience of the participants and becoming unwilling to participate. Another important point is the form of delivery of the intervention. Patients in group-delivered CBT could discuss the contents of the interventions with peers, which promoted better understanding of the contents than patients who received CBT treatment alone. Also, the effect of face-to-face CBT was more significant when the patients had co-morbid psychiatric diseases or taking drugs such as hypnotics and antidepressants, which was consistent with the findings in previous study (67). A possible explanation for this result is that psychiatric diseases could be a main cause of insomnia. Although the studies included in this study all used CBT that was specially adapted for treating insomnia, some of the components such as cognitive therapy and relaxation training, still remained the functions of identifying and relieving anxious thoughts, which was effective in treating psychiatric disease symptoms. As a result, depressive or anxious symptoms were relieved due to CBT itself or other drug treatments. The relief of psychiatric disease symptoms led to a better mental health status, and therefore, insomnia was treated more effectively.
Sleep hygiene component was widely used as an individual treatment option in previous studies (68), which aimed to provide a better environment that is suitable for sleep. Although the effect of sleep hygiene alone on insomnia was very limited, a well-established bedroom environment is still advantageous when patients attempted to make behavioral changes (68). Consistent with the findings from previous study (69), relaxation training component was found to be effective. Relaxation training component taught the patients how to fall asleep more easily, establishing a positive mental condition toward insomnia, and patients with positive mental status are more determined to make behavioral changes and change their old sleep habits in order to improve sleep quality (69). As insomnia was a chronic condition, sustaining improvement is required after CBT intervention is finished. Therefore, relapse prevention is very important in CBT intervention program because the presence of relapse prevention component ensured the long-term effect of the intervention. In contrast, the rarely used components such as treatment rationale and basic sleep information did not seem to have great effects on sleep. These components only provided background information of disease and intervention to the patients, with limited effect on thoughts or behavioral changes. As the number of studies utilizing these components was limited, the effect of these components on sleep outcomes was uncertain.
It has been reported in prior study that face-to-face CBT has a significant effect in improving depressive symptoms (65), which is similar to our findings. A possible explanation for the result would be face-to-face CBT intervention established a regularized sleep-wake cycle, and previous study mentioned that patients with a regularized sleep-wake cycle was more likely to experience a reduction in daytime symptoms, including depression and pain (65).
In previous studies, traditional cognitive behavioral showed an overall significant effect on anxiety (70, 71), which was quite different from our findings. However, all of the studies included in this study used CBT for insomnia (CBT-I), a specially adapted form of CBT targeting insomnia only. Different to the traditional CBT interventions, the contents in the CBT-I intervention focused on sleep problems and topics on anxiety were not discussed. On the other hand, only five studies reported anxiety in this study. Due to the limited number of studies, the results of meta-analysis on anxiety may not be representative.
It is recognized that the most direct consequence of poor sleep quality caused by insomnia is fatigue during the day (72). Face-to-face CBT has already shown a significant effect in improving sleep quality. It is plausible that patients receiving CBT intervention have developed a more efficient rest in bed, which led to an apparent reduction in daytime fatigue.
The proposed mechanism for the improvement of physical and mental health is that face-to-face CBT intervention established a regularized sleep-wake cycle, which enabled the participants to get enough rest across the night. The adequate rest of the body would be helpful in reducing daytime symptoms such as depression, fatigue, and pain, leading to an overall increase in physical health. In this context, negative thoughts were rectified, and patients would have a more positive attitude toward life after the treatment. The improvement of both physical health and mental health contributed to the overall improvement of quality of life.
This study included a large number of high quality randomized controlled trial studies on the effectiveness of face-to-face delivered CBT in treating insomnia published in the last 22 years since the first RCT study (51) was published, and the results from subgroup analysis explained the contribution of specific subgroup variables to the overall effects. However, there are a number of limitations for the study. First, articles published in languages other than English were not included in this study. Second, although some of the research outcomes including Pittsburgh Sleep Quality Index, anxiety, fatigue, and scores of SF-12 and SF-36 health surveys were good indicators for sleep quality and health, they were reported in a very limited number of studies, making it hard to conclude if face-to-face CBT had a significant effect on those outcomes. Third, the effect of some CBT components was hard to measure because those components were presented in only one or two of the included studies, and we were not able to measure the heterogenicity and p-value between groups for those components. Therefore, further studies that include more trials with larger sample sizes, published in different languages, are needed to confirm the results of this study.
The using of face-to-face CBT when treating insomnia was highly recommended. When designing the CBT treatment plans, it is recommended that an appropriate design can include more than six sessions, duration of one session >1 h, length of intervention shorter than 6 months, and group delivered intervention. The retention of participants should be encouraged in the program, and the inclusion of the following components was recommended: sleep restriction, stimulus control, cognitive therapy sleep hygiene, relapse prevention, and relaxation training. Treatment rationale and basic sleep information was not recommended, but the inclusion of these components could still be considered if the circumstances permitted.
In conclusion, face-to-face delivered CBT is effective in improving the sleep-related outcomes, including Insomnia Severity Index, Pittsburgh Sleep Quality Index, total sleep time, sleep efficiency, sleep onset latency, and wake after sleep onset in patients with insomnia. It was also found that face-to-face delivered CBT can improve other health outcomes in insomnia patients, including depression, fatigue, physical health, and mental health. Face-to-face delivered CBT is more effective when delivered through a larger number of sessions with longer duration of each session, and when delivered in groups. Apart from the most traditional components such as sleep restriction, stimulus control, and cognitive therapy, other components including sleep hygiene, relapse prevention, and relaxation training also showed promising effects and can be considered to add into study designs and treatment plans. More studies with larger sample sizes are required in further studies to confirm the findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DX: data collection, data analysis, and writing of original draft. JS: conceptualization, methodology, data collection, and reviewing and editing of writing. EC and SB: critically reviewing and editing of writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.798453/full#supplementary-material
Supplementary Figure 1. Funnel plots for sleep outcomes. (A) Insomnia Severity Index, (B) Pittsburgh Sleep Quality Index, (C) total sleep time, (D) sleep efficiency, (E) sleep onset latency, (F) wake after sleep onset.
Supplementary Figure 2. Funnel plots for sleep and health outcomes. (A) Number of awakenings, (B) depression, (C) anxiety, (D) fatigue, (E) Physical health, (F) mental health.
1. American Psychiatric Association D, Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
2. Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. (2016) 5:780–4. doi: 10.4103/2249-4863.201153
3. Lee-Chiong TL. Sleep: A Comprehensive Handbook. Hoboken, NJ: John Wiley & Sons (2005). doi: 10.1002/0471751723
4. Institute of Medicine Committee on Sleep Medicine and Research. The National Academies Collection: Reports funded by National Institutes of Health. In: Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington, DC: National Academies Press. (2006).
5. Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. (2017) 9:151–61. doi: 10.2147/NSS.S134864
6. Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. (2012) 13:1–11. doi: 10.1186/1471-2296-13-40
7. Kay-Stacey M, Attarian H. Advances in the management of chronic insomnia. BMJ. (2016) 354:i2123. doi: 10.1136/bmj.i2123
8. Rothbaum BO, Meadows EA, Resick P, Foy DW. Cognitive-Behavioral Therapy. Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies. New York, NY: The Guilford Press (2000). p. 320–5.
10. Belanger L, Savard J, Morin CM. Clinical management of insomnia using cognitive therapy. Behav Sleep Med. (2006) 4:179–98. doi: 10.1207/s15402010bsm0403_4
11. Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. (2009) 13:205–14. doi: 10.1016/j.smrv.2008.06.001
12. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. (2015) 163:191–204. doi: 10.7326/M14-2841
13. van der Zweerde T, Bisdounis L, Kyle SD, Lancee J, van Straten A. Cognitive behavioral therapy for insomnia: a meta-analysis of long-term effects in controlled studies. Sleep Med Rev. (2019) 48:101208. doi: 10.1016/j.smrv.2019.08.002
14. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
15. Leonardo R, PICO. Model for clinical questions. Evid Based Med Pract. (2018) 3:2. doi: 10.4172/2471-9919.1000115
16. Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. (1998) 51:1235–41. doi: 10.1016/S0895-4356(98)00131-0
17. Morin CM. Insomnia: Psychological Assessment and Management. New York, NY: Guilford Press. (1993).
18. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
19. Cashin AG, McAuley JH. Clinimetrics: physiotherapy evidence database (PEDro) scale. J Physiother. (2020) 66:59. doi: 10.1016/j.jphys.2019.08.005
20. Beck AT, Steer RA, Brown GK. Beck Depression Inventory (BDI-II). New York, NY: Pearson. (1996). doi: 10.1037/t00742-000
21. Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R). In Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults. Mahwah, NJ: Lawrence Erlbaum Associates Publishers. (2004). p. 363–77. doi: 10.1037/t29280-000
22. Hamilton M. The Hamilton Rating Scale for Depression. Assessment of Depression: New York, NY: Springer (1986). p. 143–52. doi: 10.1007/978-3-642-70486-4_14
24. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
25. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. (1988) 56:893–7. doi: 10.1037/0022-006X.56.6.893
26. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
27. Bieling PJ, Antony MM, Swinson RP. The State–Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. (1998) 36:777–88. doi: 10.1016/S0005-7967(98)00023-0
28. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. (1989) 46:1121–3. doi: 10.1001/archneur.1989.00520460115022
29. Smets E, Garssen B. Bonke Bd, De Haes J. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. (1995) 39:315–25. doi: 10.1016/0022-3999(94)00125-O
30. Ware JE, Kosinski MA, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: Health Institute, New England Medical Center (1994).
31. Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press. (2014).
32. Perlis ML, Jungquist C, Smith M, Posner DA. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. New York, NY: Springer (2005). p. 1–182.
33. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. (2007) 28:105–14. doi: 10.1016/j.cct.2006.04.004
34. Arnedt JT, Conroy DA, Armitage R, Brower KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. (2011) 49:227–33. doi: 10.1016/j.brat.2011.02.003
35. Ayabe N, Okajima I, Nakajima S, Inoue Y, Watanabe N, Yamadera W, et al. Effectiveness of cognitive behavioral therapy for pharmacotherapy-resistant chronic insomnia: a multi-center randomized controlled trial in Japan. Sleep Med. (2018) 50:105–12. doi: 10.1016/j.sleep.2018.05.038
36. Bothelius K, Kyhle K, Espie CA, Broman JE. Manual-guided cognitive-behavioural therapy for insomnia delivered by ordinary primary care personnel in general medical practice: a randomized controlled effectiveness trial. J Sleep Res. (2013) 22:688–96. doi: 10.1111/jsr.12067
37. Carney CE, Edinger JD, Kuchibhatla M, Lachowski AM, Bogouslavsky O, Krystal AD, et al. Cognitive behavioral insomnia therapy for those with insomnia and depression: a randomized controlled clinical trial. Sleep. (2017). 40:zsx019. doi: 10.1093/sleep/zsx019
38. Chen HY, Cheng IC, Pan YJ, Chiu YL, Hsu SP, Pai MF, et al. Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int. (2011) 80:415–22. doi: 10.1038/ki.2011.151
39. Currie SR, Clark S, Hodgins DC, El-Guebaly N. Randomized controlled trial of brief cognitive–behavioural interventions for insomnia in recovering alcoholics. Addiction. (2004) 99:1121–32. doi: 10.1111/j.1360-0443.2004.00835.x
40. Drake CL, Kalmbach DA, Arnedt JT, Cheng P, Tonnu CV, Cuamatzi-Castelan A, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. (2019). 42:zsy217. doi: 10.1093/sleep/zsy217
41. Ellis JG, Cushing T, Germain A. Treating acute insomnia: a randomized controlled trial of a “single-shot” of cognitive behavioral therapy for insomnia. Sleep. (2015) 38:971–8. doi: 10.5665/sleep.4752
42. Espie CA, MacMahon KM, Kelly HL, Broomfield NM, Douglas NJ, Engleman HM, et al. Randomized clinical effectiveness trial of nurse-administered small-group cognitive behavior therapy for persistent insomnia in general practice. Sleep. (2007) 30:574–84. doi: 10.1093/sleep/30.5.574
43. Garland SN, Roscoe JA, Heckler CE, Barilla H, Gehrman P, Findley JC, et al. Effects of armodafinil and cognitive behavior therapy for insomnia on sleep continuity and daytime sleepiness in cancer survivors. Sleep Med. (2016) 20:18–24. doi: 10.1016/j.sleep.2015.12.010
44. Hou Y, Hu P, Liang Y, Mo Z. Effects of cognitive behavioral therapy on insomnia of maintenance hemodialysis patients. Cell Biochem Biophys. (2014) 69:531–7. doi: 10.1007/s12013-014-9828-4
45. Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: a randomized controlled trial and direct comparison. Arch Intern Med. (2004) 164:1888–96. doi: 10.1001/archinte.164.17.1888
46. Jansson-Fröjmark M, Linton SJ, Flink IK, Granberg S, Danermark B, Norell-Clarke A. Cognitive-behavioral therapy for insomnia co-morbid with hearing impairment: a randomized controlled trial. J Clin Psychol Med Settings. (2012) 19:224–34. doi: 10.1007/s10880-011-9275-y
47. Jungquist CR, O'Brien C, Matteson-Rusby S, Smith MT, Pigeon WR, Xia Y, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. (2010) 11:302–9. doi: 10.1016/j.sleep.2009.05.018
48. Lovato N, Lack L, Wright H, Kennaway DJ. Evaluation of a brief treatment program of cognitive behavior therapy for insomnia in older adults. Sleep. (2014) 37:117–26. doi: 10.5665/sleep.3320
49. Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. (2008) 31:489–95. doi: 10.1093/sleep/31.4.489
50. McCrae CS, Williams J, Roditi D, Anderson R, Mundt JM, Miller MB, et al. Cognitive behavioral treatments for insomnia and pain in adults with comorbid chronic insomnia and fibromyalgia: clinical outcomes from the SPIN randomized controlled trial. Sleep. (2019). 42:zsy234. doi: 10.1093/sleep/zsy234
51. Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. Jama. (1999) 281:991–9. doi: 10.1001/jama.281.11.991
52. Norell-Clarke A, Jansson-Fröjmark M, Tillfors M, Holländare F, Engström I. Group cognitive behavioural therapy for insomnia: effects on sleep and depressive symptomatology in a sample with comorbidity. Behav Res Ther. (2015) 74:80–93. doi: 10.1016/j.brat.2015.09.005
53. Pigeon WR, Moynihan J, Matteson-Rusby S, Jungquist CR, Xia Y, Tu X, et al. Comparative effectiveness of CBT interventions for co-morbid chronic pain and insomnia: a pilot study. Behav Res Ther. (2012) 50:685–9. doi: 10.1016/j.brat.2012.07.005
54. Sadler P, McLaren S, Klein B, Harvey J, Jenkins M. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. (2018). 41:zsy104. doi: 10.1093/sleep/zsy104
55. Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. (2005) 23:6083–96. doi: 10.1200/JCO.2005.09.548
56. Schiller H, Söderström M, Lekander M, Rajaleid K, Kecklund G, A. randomized controlled intervention of workplace-based group cognitive behavioral therapy for insomnia. Int Arch Occup Environ Health. (2018) 91:413–24. doi: 10.1007/s00420-018-1291-x
57. Sivertsen B, Omvik S, Pallesen S, Bjorvatn B, Havik OE, Kvale G, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. Jama. (2006) 295:2851–8. doi: 10.1001/jama.295.24.2851
58. Smith MT, Finan PH, Buenaver LF, Robinson M, Haque U, Quain A, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: a randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. (2015) 67:1221–33. doi: 10.1002/art.39048
59. Soeffing JP, Lichstein KL, Nau SD, McCrae CS, Wilson NM, Aguillard RN, et al. Psychological treatment of insomnia in hypnotic-dependant older adults. Sleep Med. (2008) 9:165–71. doi: 10.1016/j.sleep.2007.02.009
60. Song ML, Park KM, Motamedi GK, Cho YW. Cognitive behavioral therapy for insomnia in restless legs syndrome patients. Sleep Med. (2020) 74:227–34. doi: 10.1016/j.sleep.2020.07.011
61. Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, et al. Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep. (2014) 37:327–41. doi: 10.5665/sleep.3408
62. Taylor DJ, Peterson AL, Pruiksma KE, Hale WJ, Young-McCaughan S, Wilkerson A, et al. Impact of cognitive behavioral therapy for insomnia disorder on sleep and comorbid symptoms in military personnel: a randomized clinical trial. Sleep. (2018). 41:zsy069. doi: 10.1093/sleep/zsy069
63. Taylor DJ, Peterson AL, Pruiksma KE, Young-McCaughan S, Nicholson K, Mintz J. Internet and in-person cognitive behavioral therapy for insomnia in military personnel: a randomized clinical trial. Sleep. (2017). 40:zsx075. doi: 10.1093/sleep/zsx075
64. Vitiello MV, McCurry SM, Shortreed SM, Balderson BH, Baker LD, Keefe FJ, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: the lifestyles randomized controlled trial. J Am Geriatr Soc. (2013) 61:947–56. doi: 10.1111/jgs.12275
65. Koffel EA, Koffel JB, Gehrman PR, A. meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. (2015) 19:6–16. doi: 10.1016/j.smrv.2014.05.001
66. Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. (2002) 159:5–11. doi: 10.1176/appi.ajp.159.1.5
67. Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive Behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med. (2015) 175:1461–72. doi: 10.1001/jamainternmed.2015.3006
68. Chung KF, Lee CT, Yeung WF, Chan MS, Chung EW, Lin WL. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. (2018) 35:365–75. doi: 10.1093/fampra/cmx122
69. Garcia MC, Kozasa EH, Tufik S, Mello LEA, Hachul H. The effects of mindfulness and relaxation training for insomnia (MRTI) on postmenopausal women: a pilot study. Menopause. (2018) 25:992–1003. doi: 10.1097/GME.0000000000001118
70. Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. (2018) 35:502–14. doi: 10.1002/da.22728
71. Hall J, Kellett S, Berrios R, Bains MK, Scott S. Efficacy of cognitive behavioral therapy for generalized anxiety disorder in older adults: systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry. (2016) 24:1063–73. doi: 10.1016/j.jagp.2016.06.006
Keywords: cognitive behavioral therapy (CBT), face-to-face, insomnia, quality of sleep, health outcomes
Citation: Xu D, Cardell E, Broadley SA and Sun J (2021) Efficacy of Face-to-Face Delivered Cognitive Behavioral Therapy in Improving Health Status of Patients With Insomnia: A Meta-Analysis. Front. Psychiatry 12:798453. doi: 10.3389/fpsyt.2021.798453
Received: 20 October 2021; Accepted: 17 November 2021;
Published: 23 December 2021.
Edited by:
Liye Zou, Shenzhen University, ChinaCopyright © 2021 Xu, Cardell, Broadley and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Sun, ai5zdW5AZ3JpZmZpdGguZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.