
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 11 January 2022
Sec. Addictive Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.797578
This article is part of the Research TopicPolysubstance Abuse and Cognitive DysfunctionView all 6 articles
 Zoe Bourgault1,2†
Zoe Bourgault1,2† Dafna Sara Rubin-Kahana3,4†
Dafna Sara Rubin-Kahana3,4† Ahmed Nabeel Hassan1,2,5,6
Ahmed Nabeel Hassan1,2,5,6 Marcos Sanches5,7
Marcos Sanches5,7 Bernard Le Foll1,2,3,5,8,9*
Bernard Le Foll1,2,3,5,8,9*Polysubstance use is a growing public health concern that has been associated with poor clinical outcomes. Compared to single-drug users, this population suffers greater deficits in cognitive function, which hinder treatment success and recovery. Despite its high prevalence and poor prognosis, epidemiological research on polysubstance use and accompanying cognitive profile is lacking. We investigated associations between numbers of past-year co-occurring substance use disorders (SUDs) and self-reported cognitive function using data from the National Epidemiologic Survey for Alcohol and Related Conditions III (NESARC-III). Regression analyses revealed a significant negative association between cognitive scores and numbers of past-year SUDs, which was moderated by sex. After adjusting for confounding variables, greater numbers of SUDs were associated with declining self-reported cognitive function, and this relationship was stronger among females. Our findings expand on current literature on cognitive impairments among polysubstance users and provide a novel, nuanced description of this relationship among the general population. We highlight the need for targeted and individualized treatment approaches in order to improve outcomes in this population.
The majority of individuals affected by substance use disorders (SUDs) report the habitual use of multiple drugs (1). In clinical settings, it is common for SUD patients to have a history of polysubstance use or to meet diagnostic criteria for multiple SUDs at a time (1–3). Polysubstance users represent up to 91% of treatment-seeking drug-users, who consume an average of 3.5 concurrent substances (4). These individuals tend to be males, young adults, and from lower socioeconomic status (5). Compared to single-drug users, polysubstance users are younger at the onset of drug use, and suffer a more persistent disorder course, complicated by higher rates of comorbid psychiatric diagnoses and medical problems (2, 3, 6). Overall, both epidemiological and prospective studies have established polysubstance use as a growing public health concern associated with poorer outcomes and higher rates of mortality (1, 2, 7, 8).
SUDs are commonly associated with deficits in cognitive function. A large body of neuropsychological research has described significant impairments in executive function, attention, and decision-making that are thought to contribute to SUD onset and relapse (9, 10). Cognitive dysfunction has been associated with predictors of treatment failure such as reduced attendance, shorter periods of drug abstinence, and higher rates of dropout (11–13). There is substantial evidence indicating that polysubstance use results in more severe neurocognitive deficits than single-drug use (14–16). This has been demonstrated as poorer performance in tasks assessing processes such as executive function, processing speed, working memory, and visuospatial ability (14, 17, 18). For instance, when compared to patients with alcohol use disorder, polysubstance users show lower performance on multiple measures of learning and memory, and score higher on measures of impulsivity (16). Neuroimaging studies have associated these deficits with structural and chemical abnormalities that differ from those seen in both single-drug users and controls (18–20). Whether impairments are a consequence of the repeated use of multiple drugs, or alternatively predispose certain individuals to developing multiple SUDs is unclear.
In addition to greater cognitive deficit, polysubstance use has been associated with significantly reduced cognitive recovery during drug abstinence. Improvements in cognitive performance have been correlated to duration of abstinence in single drug-users, whereas impaired performance persists over time in those who used multiple substances (14). Even following 1 year of abstinence, polysubstance users still showed lower cognitive functions compared to controls (21). The severe and enduring neuropsychological consequences associated with the abuse of multiple drugs may thus explain the poorer outcomes in this population.
A number of studies have described neurocognitive deficits that occur with polysubstance use. However, research distinguishing between polysubstance use of different numbers of drugs is lacking. One study found evidence of an association between declining performance on a verbal memory task and number of DSM-IV diagnoses of substance dependence in women (22). Neuroimaging research has also found structural abnormalities in polysubstance users that increase in severity with greater numbers of substances used. The prefrontal localization of these abnormalities may reflect the cognitive deficits seen in this population (23, 24). In the present study, our primary aim was to determine whether a relationship exists between the number of past-year co-occurring SUDs and self-reported cognitive function. We also sought to investigate whether the strength of this relationship is moderated by sex, due to the significant sex-differences in the clinical presentation of SUDs. Compared to males, females suffering from SUDs are more likely to meet diagnostic criteria for comorbid psychiatric disorders, report higher levels of distress, and are more likely to have a history of trauma or abuse (25). Females have also been found to progress more quickly from initial drug exposure to dependence (26). Studies on adolescent substance use have found that females appear to be more severely affected by cognitive deficits compared to males (27, 28). Current literature has not adequately addressed sex differences in the clinical presentation of polysubstance use. Importantly, potential sex-specific neurocognitive outcomes may have significant treatment implications and require further study.
We addressed these questions using data from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III). As the majority of polysubstance users do not seek treatment (6), this large national sample provides a more adequate representation than would a clinical sample. We hypothesized that self-reported cognition would worsen with increasing numbers of past-year SUDs. Regarding sex differences, we hypothesized that the relationship between self-reported cognitive function and number of past-year SUDs would be stronger among females. To the best of our knowledge, this is the first study assessing the association between cognitive function and numbers of SUDs in a national survey. We are also the first to investigate the effect of sex on this relationship.
The NESARC-III is a cross-sectional, nationally representative survey conducted in the United States in 2012-2013. The participants were 36,309 civilian adults in households and selected group quarters (29). Our study included 35,916 respondents after excluding 393 respondents (1.1% of the sample) who had missing responses for any of the Executive Function Index (EFI) items.
The survey respondents were selected through a multi-stage probability sampling procedure, and data were weighted to represent the US population based on the 2012 American Community Survey (“Income and Poverty in the United States: 2016”). Face-to-face interviews took place in respondents' homes. The household response rate was 72%, the person-level response rate was 84%, and the overall response rate was 60.1%. NESARC-III methodology is described further elsewhere (29). The National Institutes of Health institutional review boards approved the survey.
The EFI is a self-reported cognitive functioning questionnaire designed for use in community surveys (30). NESARC-III included 12 items from the EFI scales. Nine out of the twelve items fitted a two-factor model, representing two subscales: executive functions (four items) and attention (five items) and a total score, with acceptable reliability and validity (31). Respondents rate each self-statement item in the questionnaire as not at all, a little, somewhat, a lot, or very much. Responses were coded 0-4, with higher values indicating better functioning.
The diagnostic interview used in NESARC-III was the Alcohol Use Disorder and Associated Disabilities Interview Schedule-5 (AUDADIS-5). It is a structured, computer-assisted diagnostic interview designed for lay interviewers (32). It covers the frequency and amount of drug and alcohol use, DSM-5 substance use disorders and psychiatric disorders.
NESARC-III participants were asked to self-report past year use, including alcohol, tobacco, cannabis, opioids, stimulants, sedatives, club drugs, hallucinogens and others. Participants who reported recreational substance use were asked about the criteria for substance use disorder (SUD).
Consistent with DSM-5, the diagnosis of SUD required at least 2/11 criteria. Recreational substance use that did not meet diagnostic criteria was not included in SUD groups. For test-retest reliability and procedural validity, please see Hasin et al. (32, 33). Although nicotine was found to enhance cognition (34, 35), there is evidence supporting lower cognitive function among smokers compared to non-smokers (35, 36). We also checked our data, and people with tobacco use disorder (TUD) reported lower cognitive function compares to people without SUD. Thus, we decided to include TUD in this study.
The frequency and amount of drug and alcohol use will not be analyzed in this manuscript.
Sociodemographic covariates included sex (male, female), race/ethnicity (non-Hispanic Whites, non-Hispanic Blacks, Native, Asian, Hispanic), age (18–90), marital status (married/living with someone as if married, widowed/divorced/separated, never married), education (some high school or less, high school graduate, some college or higher) and past-year household income ($0-$9,999, $10,000-$39,999, $40,000-$109,999, $110,000 or greater).
Statistical analysis was performed in “R” version 4.1.0 using the “survey” package (37) to implement NESARC-III's complex survey design (Taylor series linearization).
For Table 1, all descriptive results have accounted for survey weights and survey design.
The regression models presented in Tables 2, 4 were estimated using Ordinary Least Squares (OLS) with controls for sociodemographic characteristics (sex, race, marital status, age, education and income). Linearity diagnostic were checked through visual inspection of scatterplots. Estimation was done using the “svyglm” function (with a “gaussian” distribution and an “identity” link function). To test whether sex has a moderation effect on the association between Number of SUDs and cognitive functions, we added an interaction between sex and number of SUDs to the model, while still controlling for the sociodemographic factors. Regression coefficients were transformed into standardized effect sizes equivalent to Cohen's d related to one additional SUD by dividing it by the standard deviation of the model residuals.
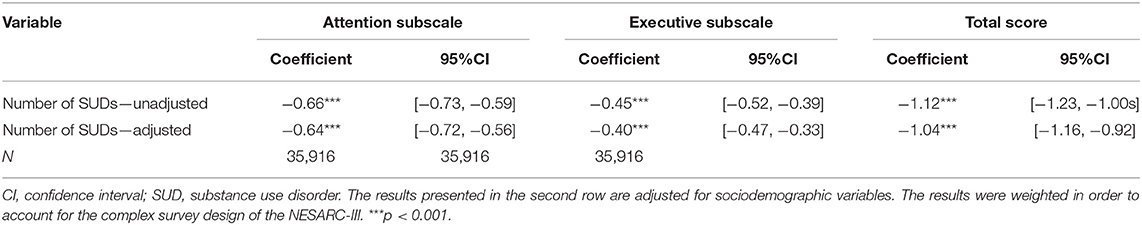
Table 2. Linear regression estimating the association between the number of substance use disorders and Executive Function Index scores.
All results were considered statistically significant if the p-value was below 0.05.
Participants with polysubstance SUDs tended to be skewed toward young males, with lower education and household income. Tobacco, alcohol and cannabis were the most prevalent use disorders. Demographic characteristics of the sample are presented in Table 1, weighted means and population shares (percentage) were calculated for continuous and categorical characteristics, respectively. Numbers in Table 1 are reported for five separate groups by the number of Substance Use Disorders (SUDs), from no SUD to four SUDs and above.
Figure 1 presents the estimated marginal means of EFI scores and respective standard error (SE) (means and SE adjusted for all above mentioned sociodemographic controls except sex) for males/females/all participants by the number of SUDs. It is apparent that mean scores decline with additional SUDs. In addition, self-reported cognition seems to be higher for males vs. females.
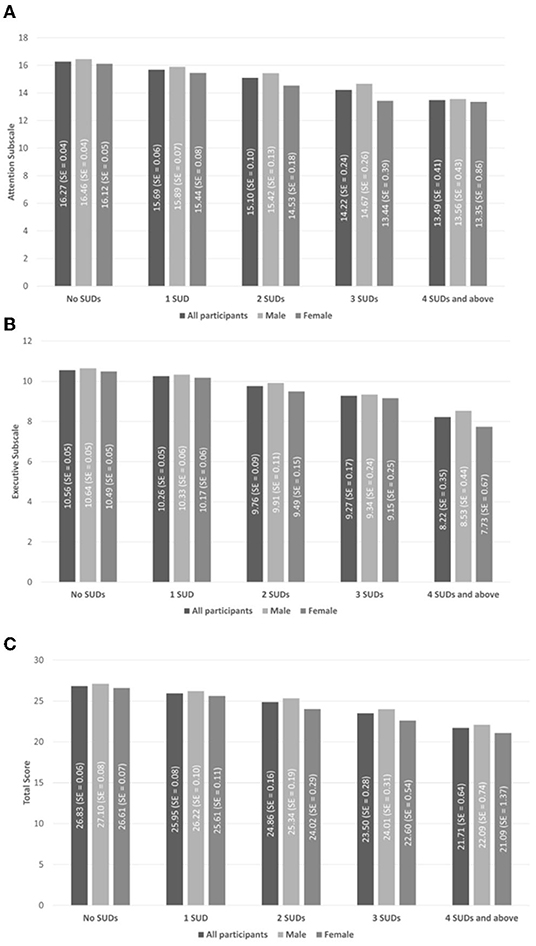
Figure 1. Estimated marginal means of executive function index (EFI) scores and respective standard error (SE) (means and SE adjusted for all above mentioned sociodemographic controls except sex) for males, females, and all participants by number of substance use disorders (SUDs). (A) The attention subscale (maximal score = 20). (B) The executive function subscale (maximal score = 16). (C) The total score (maximal score = 46). Values presented here were adjusted using NESARC-III Survey weights but not for covariates.
For our main hypothesis, using three OLS regressions (one for each EFI scale), we test the statistical significance of the association between the number of SUDs and EFI score. A negative and significant coefficient for the “Number of SUDs” measure, mean there is a negative association between number of SUDs and EFI scores. Or in other words, an additional SUD is associated with a decrease in self-reported cognition. Table 2 presents both adjusted and unadjusted results.
The number of SUDs is significantly associated with worse self-reported cognition in all subscales and in the total score, in both the adjusted and unadjusted models. In the adjusted models, on average, an additional SUD is associated with a decrease of 0.64 [95% CI = −0.72 to −0.56], 0.40 [95% CI = −0.47 to −0.33] and 1.04 [95% CI = −1.16 to −0.92] points in the attention, executive function and the total scores, respectively.
Table 3 presents the prevalence of specific SUD, by sex and by number of SUDs. For our second hypothesis (presented in Table 4), we tested the same association while interacting sex with Number of SUDs. The “Number of SUDs: Females” measure is the association between number of SUDs and EFI score for females only and the same goes for “Number of SUDs: Males.” Table 4 also includes the p-value of testing whether the difference between the male and female association is statistically significant.
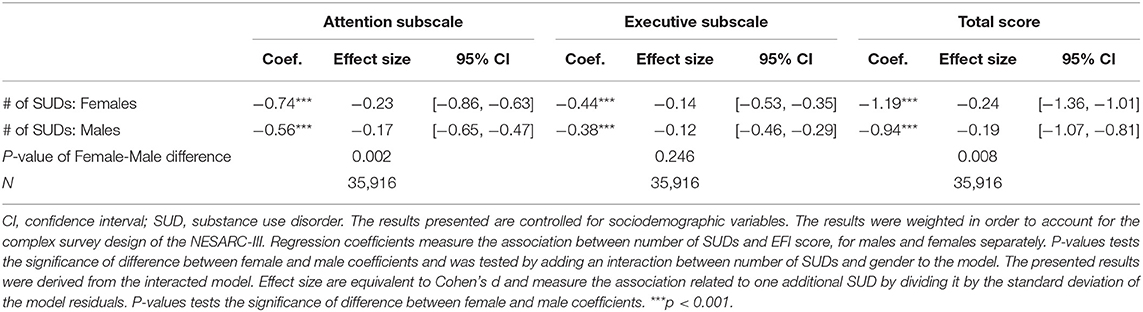
Table 4. Linear regression estimating the association between the number of substance use disorders and executive Function Index scores by sex.
As seen in Table 4, the association between the number of SUDs and self-reported cognition is found to be significant for both females and males. In addition, the interaction between sex and number of SUDs was significant for both the Attention subscale and Total score.
Attention subscale was found to be significantly more associated to number of SUDs for females than for males (p-value = 0.002). Among females, an extra SUD condition is related to a decrease of 0.74 [95% CI = −0.86 to −0.63, Cohen's d = −0.23] in the attention subscale, while among males the decrease was found to be smaller at 0.56 [95% CI = −0.65 to −0.47, Cohen's d = −0.17].
In contrast, the executive subscale was found not to be significantly more associated to number of SUDs for females than for males (p-value = 0.246). Among females, an extra SUD condition is related to a decrease of 0.44 [95% CI = −0.53 to −0.35, Cohen's d = −0.14] in the executive subscale, while among males the association was found to be −0.38 [95% CI = −0.46 to −0.29, Cohen's d = −0.12].
Total EFI scale was found to be significantly more associated to number of SUDs for females than for males (p-value = 0.008). Among females, an extra SUD condition is related to a decrease of 1.19 [95% CI = −1.36 to −1.01, Cohen's d = −0.24] in the total scale, while among males the decrease was found to be smaller at 0.94 [95% CI = −1.07 to −0.81, Cohen's d = −0.19].
The present study provides novel insights on the relationship between polysubstance use and cognitive function. Using data obtained from a large sample of U.S. adults, we sought to determine whether self-reported cognition varied across groups of individuals with differing numbers of SUDs. After controlling for relevant socio-demographic variables, our linear regression models indicate a significant negative association between cognition scores and increasing numbers of SUDs. In both males and females, having more SUD diagnoses was associated with worsening scores in both EFI subscales and total EFI ratings. Our sex-based analysis revealed that the decline in cognitive function observed with the presence of additional SUDs was significantly greater in females.
Previous studies have associated polysubstance use with greater deficits in cognition compared to single drug use (14, 15). It has been proposed that sequential or simultaneous use of multiple drugs may have additive neurotoxic effects, resulting in greater impairment in polysubstance users compared to single drug users (20, 38). In line with these findings, our results could indicate cumulative deleterious effects of additional substances, explaining the decline in EFI scores with increasing numbers of SUD. Alternatively, pre-existing deficits in cognitive function may predispose certain individuals to developing SUDs by more impulsive use of substances (39, 40). Our results may therefore be indicative of premorbid cognitive ability, with higher numbers of SUDs resulting from poorer baseline cognition. Due to our cross-sectional methodology, temporal relationships and consequently the direction of causality between these factors cannot be established. Similar findings of increasing severity with greater numbers of substances have been found for other clinical outcomes including mental distress, psychiatric disorders, and medical issues (3, 41). Longitudinal assessments of cognitive markers in different groups of polysubstance users are required in order to draw further conclusions from our findings.
Our analyses showed a moderating effect of sex on the relationship between polysubstance use and cognition. Higher numbers of SUDs were associated with a significantly greater decline in scores on the attention subscale and total EFI ratings reported by females. Studies investigating sex-differences in polysubstance use are limited; however, current evidence indicates that females may experience more severe cognitive deficits in the context of single-substance use compared to males (28). Neuroimaging studies investigating adolescent alcohol use have shown greater alterations in neural activation patterns as well as smaller prefrontal volumes in females (42, 43). Adolescent females also appear to be more vulnerable to deficits in working memory and executive function associated with cannabis use (27, 28). The faster rate of maturation of prefrontal networks among females may explain their increased susceptibility to cognitive deficits during adolescent onset of substance use (28). In addition, sex differences in the prevalence of psychiatric disorders among substance users have been previously reported and may have contributed to our results (44). Concurrent diagnoses of major depression and posttraumatic stress disorder are more common among females with SUDs compared to males (26, 45). Notably, these diagnoses involve pervasive impairments in cognitive functioning (46, 47), and have been associated with polysubstance use (3). The possibility that differences in psychiatric comorbidities contributed to the sex-specific findings in our sample warrants further investigation.
The results from this study should be considered in light of certain limitations. Our ability to establish temporal relationships between variables and infer causality from our data was limited by the cross-sectional study design. Past-year diagnoses of SUDs were associated with self-assessments of current cognitive function, therefore our results do not account for the timing of SUD onset relative to changes in cognition over time. The cognitive outcomes included in the survey were limited to the Executive Function Index, therefore our conclusions cannot be generalized to other cognitive domains such as learning and memory. Additionally, the clinical outcomes assessed by the NESARC survey were obtained through self-reports and are subject to recall error. The NESARC also surveyed adults aged 18 and over, which limits our ability to generalize our findings to adolescent populations who report high rates of polysubstance use (15). It is also important to note that our definition of substance use was restricted to cases meeting DSM-5 criteria for SUDs and did not include subthreshold drug use. We also did not take frequency or amount of consumption into account, nor did we distinguish between simultaneous and sequential drug use. Whether our findings would vary with other definitions of drug exposure (e.g., misuse, abuse) or according to different patterns of use is unknown. We also did not investigate the contributions of different substances or their combinations, which could have differential effects on the severity of outcomes. For instance, the cognitive deficits associated with chronic alcohol use may be more frequent and severe than those observed in cocaine or cannabis users (48, 49). The effects of heavy alcohol consumption were not addressed in our analysis and should be accounted for in future research. There are additional clinical and lifestyle factors to consider that may have contributed to our findings. In addition to depression and PTSD, co-occurring personality disorders as well as history of childhood trauma are associated with cognitive dysfunction (50, 51). The frequent malnutrition and medical problems seen in this population have also been associated with impairments in cognition (52). Finally, the power of our analyses may have been limited by the small sample size of certain groups when considering individuals with many SUDs. Despite these limitations, we observed a strong dose-dependent relationship between past-year numbers of SUDs and cognition. Our current understanding of SUDs implies that substance use must significantly interfere with an individual's life and functioning (53). Regardless of which drugs are used, higher numbers of SUDs reflect increasing levels of distress and harm, which impair an individual's ability to function. It is likely that the cumulative impact of increasing numbers of SUD diagnoses underlies our findings, rather than the substances themselves.
The current study has important implications in the understanding of SUD patients who often present with extensive polysubstance use and cognitive impairments. Due to its severe and persistent presentation, the treatment of polysubstance use remains a significant challenge in clinical practice. Despite reporting higher levels of psychological distress (41), individuals affected by multiple SUDs are less likely to seek treatment than those suffering from only one (5). Even in clinical settings, these patients are at a heightened risk of treatment dropout, which may result from the severity of their cognitive profile (2). We have provided novel evidence of a sex-specific relationship between cognitive deficits and multiple SUDs. We suggest that these variables be carefully considered when assessing patients and establishing individualized treatment plans. The present study as well as future research will allow the development and implementation of more effective therapeutic approaches, leading to improved outcomes in this understudied population.
Publicly available datasets were analyzed in this study. This data can be found at: https://www.niaaa.nih.gov/research/nesarc-iii.
The studies involving human participants were reviewed and approved by Centre for Addiction and Mental Health Research Ethics Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
DR-K, AH, MS, and BL contributed to conception and design of the study. DR-K performed the statistical analysis. ZB and DR-K wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
DR-K was supported by the O'Brien Scholars Program within the Child and Youth Mental Health Collaborative at CAMH and The Hospital for Sick Children, Toronto, Canada. BL is supported by CAMH, a clinician-scientist award from the department of Family and Community Medicine of the University of Toronto and a Chair in Addiction Psychiatry from the department of Psychiatry of University of Toronto.
BL has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator-initiated projects. BL has participated in a session of a National Advisory Board Meeting (Emerging Trends BUP-XR) for Indivior Canada and has been consultant for Shinogi. He has some in-kind donation of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. He has obtained industry funding from Canopy (through research grants handled by CAMH or University of Toronto), Bioprojet, ACS and Alkermes. He has received in kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Crummy EA, O'Neal TJ, Baskin BM, Ferguson SM. One is not enough: understanding and modeling polysubstance use. Front Neurosci. (2020) 14:569. doi: 10.3389/fnins.2020.00569
2. Preti E, Prunas A, Ravera F, Madeddu F. Polydrug abuse and personality disorders in a sample of substance-abusing inpatients. Mental Health Subst Use. (2011) 4:256–66. doi: 10.1080/17523281.2011.577751
3. Bhalla IP, Stefanovics EA, Rosenheck RA. Clinical epidemiology of single versus multiple substance use disorders: polysubstance use disorder. Med Care. (2017) 55(Suppl 92):S24-32. doi: 10.1097/MLR.0000000000000731
4. Onyeka IN, Uosukainen H, Korhonen MJ, Beynon C, Bell JS, Ronkainen K, et al. Sociodemographic characteristics and drug abuse patterns of treatment-seeking illicit drug abusers in Finland, 1997–2008: the Huuti Study. J Addict Dis. (2012) 31:350–62. doi: 10.1080/10550887.2012.735563
5. McCabe SE, West BT, Jutkiewicz EM, Boyd CJ. Multiple DSM-5 substance use disorders: a national study of US adults. Hum Psychopharmacol. (2017) 32:e2625. doi: 10.1002/hup.2625
6. McCabe SE, West BT. The 3-year course of multiple substance use disorders in the United States: a national longitudinal study. J Clin Psychiatry. (2017) 78:e537–44. doi: 10.4088/JCP.16m10657
7. Day C. Benzodiazepines in combination with opioid pain relievers or alcohol: greater risk of more serious ED visit outcomes. In: The CBHSQ Report. Rockville, MD: Substance Abuse and Mental Health Services Administration (US) (2013). p. 1–9.
8. McClure FL, Niles JK, Kaufman HW, Gudin J. Concurrent use of opioids and benzodiazepines: evaluation of prescription drug monitoring by a United States Laboratory. J Addict Med. (2017) 11:420–6. doi: 10.1097/ADM.0000000000000354
9. Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Substance Abuse Treat. (2014) 47:58–72. doi: 10.1016/j.jsat.2014.01.008
10. Ramey T, Regier PS. Cognitive impairment in substance use disorders. CNS Spectr. (2019) 24:102–13. doi: 10.1017/S1092852918001426
11. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. (2006) 81:313–22. doi: 10.1016/j.drugalcdep.2005.08.003
12. Copersino ML, Schretlen DJ, Fitzmaurice GM, Lukas SE, Faberman J, Sokoloff J, et al. Effects of cognitive impairment on substance abuse treatment attendance: predictive validation of a brief cognitive screening measure. Am J Drug Alcohol Abuse. (2012) 38:246–50. doi: 10.3109/00952990.2012.670866
13. Sømhovd M, Hagen E, Bergly T, Arnevik EA. The Montreal cognitive assessment as a predictor of dropout from residential substance use disorder treatment. Heliyon. (2019) 5:e01282. doi: 10.1016/j.heliyon.2019.e01282
14. Selby MJ, Azrin RL. Neuropsychological functioning in drug abusers. Drug Alcohol Depend. (1998) 50:39–45. doi: 10.1016/S0376-8716(98)00002-7
15. Connor JP, Gullo MJ, White A, Kelly AB. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opin Psychiatry. (2014) 27:269-75. doi: 10.1097/YCO.0000000000000069
16. Schmidt TP, Pennington DL, Cardoos SL, Durazzo TC, Meyerhoff DJ. Neurocognition and inhibitory control in polysubstance use disorders: comparison with alcohol use disorders and changes with abstinence. J Clin Exp Neuropsychol. (2017) 39:22–34. doi: 10.1080/13803395.2016.1196165
17. Fernández-Serrano MJ, Pérez-García M, Schmidt Río-Valle J, Verdejo-García A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. (2010) 24:1317–32. doi: 10.1177/0269881109349841
18. Abé C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ. Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Depend. (2013) 130:30–7. doi: 10.1016/j.drugalcdep.2012.10.004
19. Pennington DL, Durazzo TC, Schmidt TP, Abé C, Mon A, Meyerhoff DJ. Alcohol use disorder with and without stimulant use: brain morphometry and its associations with cigarette smoking, cognition, inhibitory control. PLoS ONE. (2015) 10:e0122505. doi: 10.1371/journal.pone.0122505
20. Sung YH, Carey PD, Stein DJ, Ferrett HL, Spottiswoode BS, Renshaw PF, et al. Decreased frontal N-acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behav Brain Res. (2013) 246:154–61. doi: 10.1016/j.bbr.2013.02.028
21. Hagen E, Erga AH, Hagen KP, Nesvåg SM, McKay JR, Lundervold AJ, et al. One-year sobriety improves satisfaction with life, executive functions and psychological distress among patients with polysubstance use disorder. J Subst Abuse Treat. (2017) 76:81–7. doi: 10.1016/j.jsat.2017.01.016
22. Medina KL, Shear PK, Schafer J, Armstrong TG, Dyer P. Cognitive functioning and length of abstinence in polysubstance dependent men. Arch Clin Neuropsychol. (2004) 19:245–58. doi: 10.1016/S0887-6177(03)00043-X
23. Kaag AM, van Wingen GA, Caan MWA, Homberg JR, van den Brink W, Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict Biol. (2017) 22:1048–56. doi: 10.1111/adb.12375
24. Kaag AM, Schulte MHJ, Jansen JM, van Wingen G, Homberg J, van den Brink W, et al. The relation between gray matter volume and the use of alcohol, tobacco, cocaine and cannabis in male polysubstance users. Drug Alcohol Depend. (2018) 187:186–94. doi: 10.1016/j.drugalcdep.2018.03.010
25. Rodriguez AS, Robinson LD, Kelly PJ, Hudson S. Polysubstance use classes and health outcomes among women attending specialist substance use treatment services. Drug Alcohol Rev. (2021). doi: 10.1111/dar.13375. [Epub ahead of print].
26. McHugh RK, Votaw VR, Sugarman DE, Greenfield SF. Sex and gender differences in substance use disorders. Clin Psychol Rev. (2018) 66:12–23. doi: 10.1016/j.cpr.2017.10.012
27. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. (2009) 14:457–68. doi: 10.1111/j.1369-1600.2009.00166.x
28. Noorbakhsh S, Afzali MH, Boers E, Conrod PJ. Cognitive function impairments linked to alcohol and cannabis use during adolescence: a study of gender differences. Front Hum Neurosci. (2020) 14:95. doi: 10.3389/fnhum.2020.00095
29. Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. (2015) 72:757–66. doi: 10.1001/jamapsychiatry.2015.0584
30. Spinella M. Self-rated executive function: development of the executive function index. Int J Neurosci. (2005) 115:649–67. doi: 10.1080/00207450590524304
31. Aharonovich E, Shmulewitz D, Wall MM, Grant BF, Hasin DS. Self-reported cognitive scales in a US National Survey: reliability, validity, and preliminary evidence for associations with alcohol and drug use. Addiction. (2017) 112:2132–43. doi: 10.1111/add.13911
32. Hasin DS, Grant B. NESARC findings on increased prevalence of marijuana use disorders-consistent with other sources of information. JAMA Psychiatry. (2016) 73:532. doi: 10.1001/jamapsychiatry.2015.3158
33. Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012-2013: findings from the national epidemiologic survey on alcohol and related conditions-III. Am J Psychiatry. (2016) 173:588–99. doi: 10.1176/appi.ajp.2015.15070907
34. Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. (2001) 49:258–67. doi: 10.1016/S0006-3223(00)01094-5
35. Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev. (2007) 17:259–73. doi: 10.1007/s11065-007-9035-9
36. Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. (2005) 57:56–66. doi: 10.1016/j.biopsych.2004.10.022
37. Lumley T. Analysis of complex survey samples. J Stat Softw. (2004) 9:1–19. doi: 10.18637/jss.v009.i08
38. Medina KL, Shear PK, Schafer J. Memory functioning in polysubstance dependent women. Drug Alcohol Depend. (2006) 84:248–55. doi: 10.1016/j.drugalcdep.2006.02.009
39. Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. (2006) 45:468–75. doi: 10.1097/01.chi.0000199028.76452.a9
40. Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. (2012) 169:926–36. doi: 10.1176/appi.ajp.2012.11091421
41. Burdzovic Andreas J, Lauritzen G, Nordfjærn T. Co-occurrence between mental distress and poly-drug use: a ten year prospective study of patients from substance abuse treatment. Addict Behav. (2015) 48:71–8. doi: 10.1016/j.addbeh.2015.05.001
42. Caldwell LC, Schweinsburg AD, Nagel BJ, Bartlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (Blood Oxygen Level Dependent) response to spatial working memory. Alcohol Alcohol. (2005) 40:194–200. doi: 10.1093/alcalc/agh134
43. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. (2008) 32:386–94. doi: 10.1111/j.1530-0277.2007.00602.x
44. Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. (2010) 33:339–55. doi: 10.1016/j.psc.2010.01.004
45. Torchalla I, Strehlau V, Li K, Aube Linden I, Noel F, and Krausz M. Posttraumatic stress disorder and substance use disorder comorbidity in homeless adults: Prevalence, correlates, and sex differences. Psychol Addict Behav. (2014) 28:443–52. doi: 10.1037/a0033674.
46. Sumner JA, Hagan K, Grodstein F, Roberts AL, Harel B, Koenen KC. Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety. (2017) 34:356–66. doi: 10.1002/da.22600
47. Perini G, Cotta Ramusino M, Sinforiani E, Bernini S, Petrachi R, Costa A. Cognitive impairment in depression: recent advances and novel treatments. Neuropsychiatr Dis Treat. (2019) 15:1249–58. doi: 10.2147/NDT.S199746
48. Robinson JE, Heaton RK, O'Malley SS. Neuropsychological functioning in cocaine abusers with and without alcohol dependence. J Int Neuropsychol Soc. (1999) 5:10–9. doi: 10.1017/S1355617799511028
49. Bruijnen C, Dijkstra BAG, Walvoort SJW, Markus W, VanDerNagel JEL, Kessels RPC, et al. Prevalence of cognitive impairment in patients with substance use disorder. Drug Alcohol Rev. (2019) 38:435–42. doi: 10.1111/dar.12922
50. Monarch ES, Saykin AJ, Flashman LA. Neuropsychological impairment in borderline personality disorder. Psychiatr Clin North Am. (2004) 27:67–82, viii–ix. doi: 10.1016/S0193-953X(03)00109-6
51. Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res. (2012) 46:500–6. doi: 10.1016/j.jpsychires.2012.01.005
52. Ritz L, Coulbault L, Lannuzel C, Boudehent C, Segobin S, Eustache F, et al. Clinical and biological risk factors for neuropsychological impairment in alcohol use disorder. PLoS ONE. (2016) 11:e0159616. doi: 10.1371/journal.pone.0159616
Keywords: polysubstance use, substance use disorder, cognition, sex differences, addiction
Citation: Bourgault Z, Rubin-Kahana DS, Hassan AN, Sanches M and Le Foll B (2022) Multiple Substance Use Disorders and Self-Reported Cognitive Function in U.S. Adults: Associations and Sex-Differences in a Nationally Representative Sample. Front. Psychiatry 12:797578. doi: 10.3389/fpsyt.2021.797578
Received: 18 October 2021; Accepted: 13 December 2021;
Published: 11 January 2022.
Edited by:
Saulo Gantes Tractenberg, Pontifical Catholic University of Rio Grande do Sul, BrazilReviewed by:
Felix Henrique Paim Kessler, Federal University of Rio Grande do Sul, BrazilCopyright © 2022 Bourgault, Rubin-Kahana, Hassan, Sanches and Le Foll. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernard Le Foll, YmVybmFyZC5sZWZvbGxAY2FtaC5jYQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.