
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 06 January 2022
Sec. Forensic Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.788240
This article is part of the Research Topic Insights in Forensic Psychiatry: 2021 View all 6 articles
 Nathan J. Kolla1,2,3,4,5*
Nathan J. Kolla1,2,3,4,5* Areti Smaragdi6
Areti Smaragdi6 George Gainham7
George Gainham7 Karolina H. Karas8
Karolina H. Karas8 Colin Hawco1,3
Colin Hawco1,3 Justin Haas9
Justin Haas9 Tracey A. Skilling1,3
Tracey A. Skilling1,3 Margaret Walsh10
Margaret Walsh10 Leena Augimeri10
Leena Augimeri10Background: Stop, Now And Plan (SNAP) is a cognitive behavioral-based psychosocial intervention that has a strong evidence base for treating youth with high aggression and externalizing behaviors, many of whom have disruptive behavior disorders. In a pre-post design, we tested whether SNAP could improve externalizing behaviors, assessed by the parent-rated Child Behavior Checklist (CBCL) and also improve behavioral measures of impulsivity in children with high aggression and impulsivity. We then investigated whether any improvement in externalizing behavior or impulsivity was associated with gray matter volume (GMV) changes assessed using structural magnetic resonance imaging (sMRI). We also recruited typically developing youth who were assessed twice without undergoing the SNAP intervention.
Methods: Ten children who were participating in SNAP treatment completed the entire study protocol. CBCL measures, behavioral measures of impulsivity, and sMRI scanning was conducted pre-SNAP and then 13 weeks later post-SNAP. Twelve healthy controls also completed the study; they were rated on the CBCL, performed the same behavioral measure of impulsivity, and underwent sMRI twice, separated by 13 weeks. They did not receive the SNAP intervention.
Result: At baseline, SNAP participants had higher CBCL scores and performed worse on the impulsivity task compared with the healthy controls. At the second visit, SNAP participants still had higher scores on the CBCL compared with normally-developing controls, but their performance on the impulsivity task had improved to the point where their results were indistinguishable from the healthy controls. Structural magnetic resonance imaging in the SNAP participants further revealed that improvements in impulsivity were associated with GMV changes in the frontotemporal region.
Conclusion: These results suggest that SNAP led to improvement in behavioral measures of impulsivity in a cohort of boys with high externalizing behavior. Improvement in impulsivity was also associated with increased GMV changes. The mechanism behind these brain changes is unknown but could relate to cognitive behavioral therapy and contingency management interventions, important components of SNAP, that target frontotemporal brain regions. Clinically, this study offers new evidence for the potential targeting of brain regions by non-invasive modalities, such as repetitive transcranial magnetic stimulation, to improve externalizing behavior and impulsivity.
Disruptive behavior disorders (DBDs) are characterized by symptoms of conduct-disordered behaviors and oppositionality that are among the most prevalent classes of problems affecting young children (1–4). Data suggest that one in 11 preschoolers meet diagnostic criteria for a DBD (1, 2, 5). Among the negative consequences of DBDs include risk for later life psychopathology, family dysfunction, and criminality (6–8).
Stop, Now and Plan (SNAP) is a cognitive behavioral-based psychosocial intervention for youth with high aggression and externalizing behaviors. SNAP has a rich evidence base for helping children improve their self-control and externalizing behaviors. For example, one investigation compared SNAP participants to wait-list controls (318 boys and girls) and reported significant improvements on the Child Behavior Checklist (CBCL) (9) and Social Skills Improvement Scale (10) among the SNAP youth (11). Wait-list controls were children who were on waitlists for SNAP treatment and who were not yet receiving SNAP. Other program evaluation studies compared 252 boys who were randomized to SNAP or standard community services as usual. At follow-up, CBCL scores on the aggression, conduct problems, and externalizing behavior were significantly decreased in the SNAP condition relative to treatment as usual. In fact, boys who displayed the most diverse behavioral difficulties evidenced the most improvement (12). SNAP model programs also show evidence for reducing offending (12), reducing conduct problems in boys with high levels of conduct problems (13), and decreasing financial costs of chronic delinquency to society (14). For example, a cost benefit analysis found that every $1 spent on SNAP yielded an estimated savings between $2.05 and $3.75 (14).
As mentioned, enhanced self-control is a key theoretical mechanism for change in the SNAP intervention (12). Self-control has been described as a distinctive individual concept that is reliably associated with individuals' capacity to override immediately rewarding behaviors and engage in continued, effortful, goal-oriented behavior (15–17). Impulsivity may represent an important contributory factor to self-control (18). In impulsive choice, self-control relates to the ability to delay gratification and select the larger, delayed reward. Self-control can also be expressed by choosing the smaller, certain reward versus the temptation of gambling and selecting the larger, uncertain reward (19). Interestingly, longitudinal analysis revealed that physiological self-regulation or control moderated the effect of trait impulsivity on externalizing behaviors (20) in a non-clinical sample of adolescents, while another study of a community sample concluded that the effects of stress on self-control were mediated by impulsivity (18).
To the best of our knowledge, only one study has investigated biomarkers of change among children participating in an evidence-based, multimodal treatment for high externalizing behavior. This investigation examined neurobiological changes associated with positive outcomes related to self-regulation in SNAP. In this study, neural markers of self-regulation using electroencephalography in SNAP participants aged 8–12 years old were measured (21). Compared with a non-clinical, age- and gender-matched control group, SNAP participants were found to have larger N2 magnitudes and smaller frontal P3 magnitudes. Interestingly, SNAP children who improved with treatment exhibited a significant decrease in the magnitude of the N2 versus children who did not improve. Among the children who improved, source analysis during the time of the N2 evidenced a reduction in activation of the ventral and dorsomedial prefrontal cortex, in addition to the anterior medial temporal lobe. Reductions in activation of these brain regions are consistent with improvement in self-control (22, 23).
Emboldened by these results, we originally planned a task-based functional magnetic resonance imaging (fMRI) experiment of inhibitory control in SNAP youth, while also collecting structural imaging data. We intended to study the relationship between pre-post fMRI data and any changes in clinical and behavioral measures of the SNAP participants. Unfortunately, participant motion in the MRI scanner was too severe to use the majority of the functional data. As a result, we analyzed the well-preserved structural imaging data to investigate whether other biomarkers could be plausibly linked to anticipated improvements in clinical and behavioral measures among the SNAP participants. Accordingly, we investigated whether SNAP treatment would lead to improvement in clinical and behavioral measures and whether any improvement would be associated with brain gray matter volume (GMV) changes using structural magnetic resonance imaging (sMRI).
This study was approved by the Centre for Addiction and Mental Health (CAMH) Research Ethics Board in Toronto, Ontario, Canada. All participants provided informed written consent prior to enrollment. All parents of the children participants signed informed consents for the study, while all children who participated in the investigation signed age-appropriate informed assent forms. All methods were performed in accordance with the relevant guidelines and regulations.
This study was registered as a Clinical Trial: ClinicalTrials.gov ID: NCT0299807. The protocol ID is 0702016.
SNAP is an evidence-based psychosocial intervention that is aimed at 6–11-year-olds who present with externalizing behaviors, many of whom have DBDs (11). The 13-week core cognitive-behavioral groups (SNAP Boys and SNAP Girls) component teaches physiological awareness of emotional responding that may promote aggressive behaviors, and it also helps to improve self-control and impart problem-solving skills to make more adaptive choices. Central to the program is working with affected children, their families, peers, and schools during critical developmental stages. Another core SNAP component includes a concurrent 13-week SNAP Caregiver Group that focuses on effective parent management strategies and also helping caregivers learn the SNAP strategy to support their child. Supplementary program components include individual child counseling and mentoring, family counseling, school advocacy, teacher consultation, and SNAP youth leadership. The focus of each SNAP component is to generalize SNAP strategy skills across a multitude of settings. The SNAP model adopts a holistic treatment approach emphasizing the importance of how self-control and self-regulation fit in the broader context of risk and prosocial factors.
As discussed above, SNAP was offered to children, which is based on a cognitive behavioral therapy (CBT) model and parent management training. The two core SNAP components, SNAP Boys/SNAP Girls Group and the SNAP Caregiver Group are delivered for 90 min concurrently each week over 13-weeks at community agencies. CBT promotes efficient regulation of emotion and impulses through strategies such as cognitive restructuring, problem-solving, role-playing, social and token reinforcements, and generalization activities (24). Parent management training imparts positive parenting practices, including skill encouragement, problem-solving, and monitoring in addition to substitution of coercive or lax discipline strategies with mild sanctions addressing misbehavior (25, 26).
Thirteen SNAP participants were recruited and 12 sex-matched healthy control participants were recruited for the study. SNAP participants were recruited from the group of children who were commencing SNAP treatment at a community agency. The study was explained to SNAP children and their parents prior to the beginning of treatment for different SNAP treatment groups held during the lifespan of the study. Interested individuals were then encouraged to contact the Research Analyst leading the study. Healthy controls were recruited from the community in response to social media posts. All participants across both groups were male and right-handed. The only inclusion criteria for the SNAP children were that they were male and that they were participating in SNAP. Three SNAP participants were excluded from analysis as two individuals withdrew from the study (not SNAP treatment) before their post-SNAP second visit and another participant was unable to attend the post-SNAP second visit due to the COVID-19 pandemic. Thus, we had complete clinical, behavioral, and sMRI data for 10 SNAP participants and 12 healthy control participants.
Demographic and clinical variables of interest included the following: age, ethnicity, handedness, past traumatic brain injury, and socioeconomic status (SES). SES was measured using the Nam-Powers-Boyd scale (27). Full-scale IQ was measured using the Wechsler Abbreviated Scale of Intelligence [WASI-II; (28)], which is a reliable and well-validated tool for the assessment of cognitive ability in individuals age six to 89 years. Independent samples t-tests were used to compare continuous variables, and chi-square tests were employed to compare categorical variables. Data were analyzed using SPSS 25 (IBM Corp., 2017). Significant values were defined by p-values < 0.05.
Psychiatric diagnoses were generated using the Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5 criteria [K-SADS-PL5; (29)], which is a semi-structured interview tool. All SNAP participants met criteria for a DBD, save one participant who was only diagnosed with attention deficit hyperactivity disorder (ADHD). Testing positive for any psychiatric disorder was an exclusion criterion for the healthy group participants. Diagnoses for the SNAP participants are presented in Table 1.
Because of the focus on the CBCL in prior SNAP studies, we designated parent-rated CBCL scores as a measure of psychosocial functioning that was obtained pre- and post-SNAP visits for the SNAP participants and pre- and post-visits, separated by 13 weeks, for the healthy controls for consistency/replication purposes. The CBCL is an extensively validated measure administered to a child's caregiver to assess various domains of psychopathology in youth. The measure consists of 112 items that the respondent can endorse as “not true,” “sometimes true,” or “very true.” The CBCL provides broadband (e.g., Internalizing and Externalizing) and narrowband (e.g., Anxious/Depressed, Withdrawn/Depressed, Aggressive, and Rule-Breaking) scales, with higher scores signaling the presence of impaired functioning on the characteristic of interest. Parents were directed to answer the CBCL questionnaire based on their child's presentation at the beginning of SNAP treatment and at the post-SNAP assessment period.
We also explored a laboratory measure of impulsivity as an outcome measure. The measure used was the computerized Two Choice Impulsivity Paradigm [TCIP; (30)]. The TCIP is a discrete-choice task used to assess preference for a smaller, immediate reward or a larger, delayed reward. Participants were instructed to earn as many points as possible, such that a proportional amount of points was rewarded according to the amount of time the participant chose to wait to make the selection. For example, if participants chose to wait for 5 s (e.g., the immediate choice), they received five points. Alternatively, participants were awarded 10 points for choosing to wait 10 s (e.g., the delayed choice). Participants who chose to wait less time earned less points and were deemed more impulsive compared with participants who chose to wait longer and earn more points. Thirty trials of the TCIP was employed as the threshold. Variables of interest for the TCIP included the number of immediate choices, the proportion of immediate choices, and the highest maximum consecutive number of immediate choices. We operationalized higher values for these variables as indicative of greater impulsivity.
In order to elucidate group × interaction effects for CBCL and impulsivity measures, we utilized a generalized estimating equation. All models were performed with an exchangeable working correlation matrix. Due to distributions being skewed or otherwise having a non-normal distribution, gamma or negative binomial distribution models were used, both with a log link function, depending if data were continuous or count variables, respectively. However, when the data had a symmetrical appearing distribution, a normal model with identity link was used. There were two levels for each variable: SNAP participant and healthy control and time 1 and time 2 (initiation of SNAP and post-SNAP for the SNAP participants and time 1 and time 2 separated by 13 weeks for the healthy controls). A p-value < 0.05 indicated significance.
All participants underwent two MRI scanning sessions. For the SNAP participants, they were scanned at the start of SNAP treatment (time 1) and post-SNAP (time 2). For the healthy controls, they were scanned 13 weeks apart. All MRI scans were conducted using a 3T GE MR750 scanner (MR750, GE Healthcare, Milwaukee, USA) located at CAMH, Toronto, ON. Structural images were captured with a sagittal three-dimensional magnetization, which prepared three-dimensional, inversion recovery prepped, fast spoiled gradient echo (3D IR FSPGR) sequences (TR = 6.7 ms; TE = 3.0 ms; TI = 650 ms; frequency FoV = 230 × 230 mm; slice thickness = 0.9 mm; flip angle = 8°; matrix = 256 × 256; voxel size = 0.9 × 0.9 × 0.9 mm3; acquisition time = 4:41 min). T1 images were visually inspected for abnormalities. Pre-processing and voxel-based morphometry (VBM) analyses were completed in Statistical Parametric Mapping 12 [SPM12; (31)] running with MATLAB R2016a (MATLAB, 2016). Structural images were automatically segmented into three tissue classes: gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) (32). Subsequent inter-subject alignment and spatial normalization was performed using DARTEL (33–35) with a Gaussian kernel of 10 mm (FWHM).
GM volume differences in the SNAP group between time 1 and time 2 were analyzed with a multiple regression factorial design in the VBM option of SPM12. Because the current study aimed to investigate the SNAP program effect on CBCL measures and/or impulsivity measures, regression analyses were performed for those variables where SNAP participants were significantly improved at time 2 vs. time 1, relative to healthy participants. Change in brain volumes following SNAP treatment were calculated by subtracting the VBM GM volumes at time 1 from time 2, using fslmaths [https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Fslutils; (36)]. For each variable, a change score was calculated by subtracting the impulsivity baseline score from the post-treatment score. Age at second scan and total intracranial volume (TIV) were included as covariates in the analyses. TIV was calculated by summating participant-level GM, WM, and CSF volumes and averaging across both MRI scans. Tissue volume calculations were performed using the “Tissue Volume” utility in SPM12, which requires segmentation files outputted from the segmentation step during pre-processing. Voxel extent threshold was set at p < 0.001 and clusters were considered significant at p < 0.05 FDR, corrected for cluster size. Further information and support for the post-processing analysis adopted by this investigation can be found in the following reference: (37) and websites: (https://www.jiscmail.ac.uk/cgi-bin/wa-jisc.exe?A2=ind1705&L=SPM&P=R26026; https://www.jiscmail.ac.uk/cgi-bin/wa-jisc.exe?A2=ind1310&L=SPM&P=R1986).
Regarding demographic variables, the SNAP participants were older than the healthy participants (10.6 ± 1.3 vs. 8.9 ± 1.8 years). Therefore, age was used as a covariate for all subsequent analyses. There was no significant difference between groups for any of the other demographic variables. Demographic data are presented in Table 2.
The generalizing estimating equations analysis yielded non-significant results for all CBCL variables. However, significant group × time effects were detected for all three measures of impulsivity. Results revealed that the TCIP number of immediate choices, proportion of immediate choices, and maximum number of consecutive immediate choices were all significantly lower in the SNAP group at time 2. Results similarly revealed that healthy controls had no significant change in their impulsivity scores from time 1 to time 2 (see Table 3, Figures 1–3).
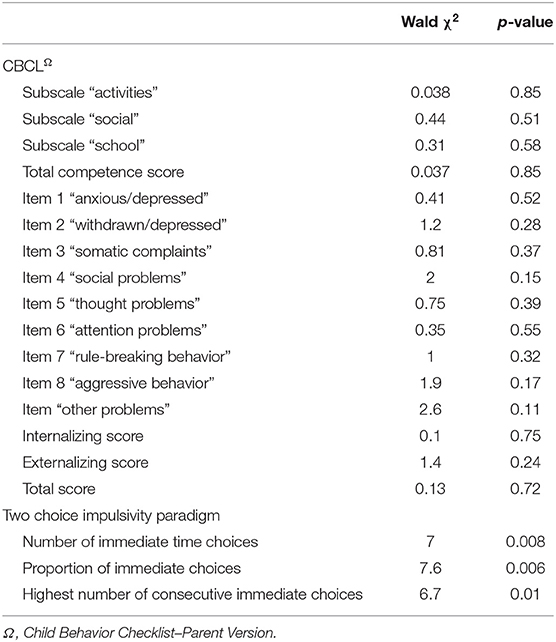
Table 3. Group × time interaction effects for clinical variables measured with a generalized estimating equations model.
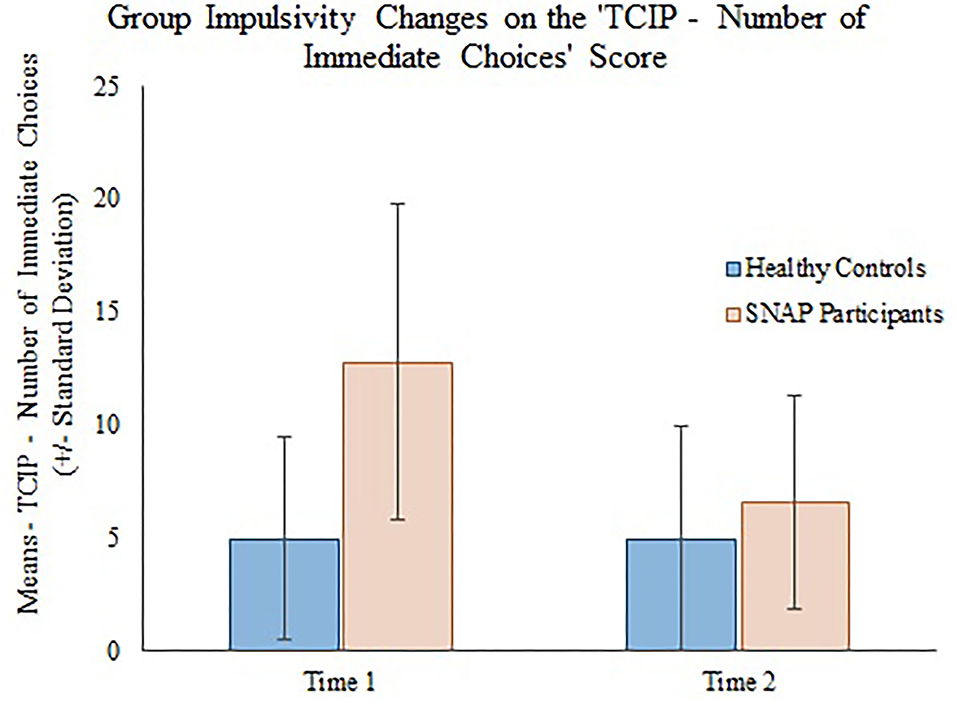
Figure 1. Group impulsivity changes on the Two Choice Impulsivity Paradigm–Number of Immediate Choices score.

Figure 2. Group impulsivity changes on the Two Choice Impulsivity Paradigm–Proportion of Immediate Choices score.
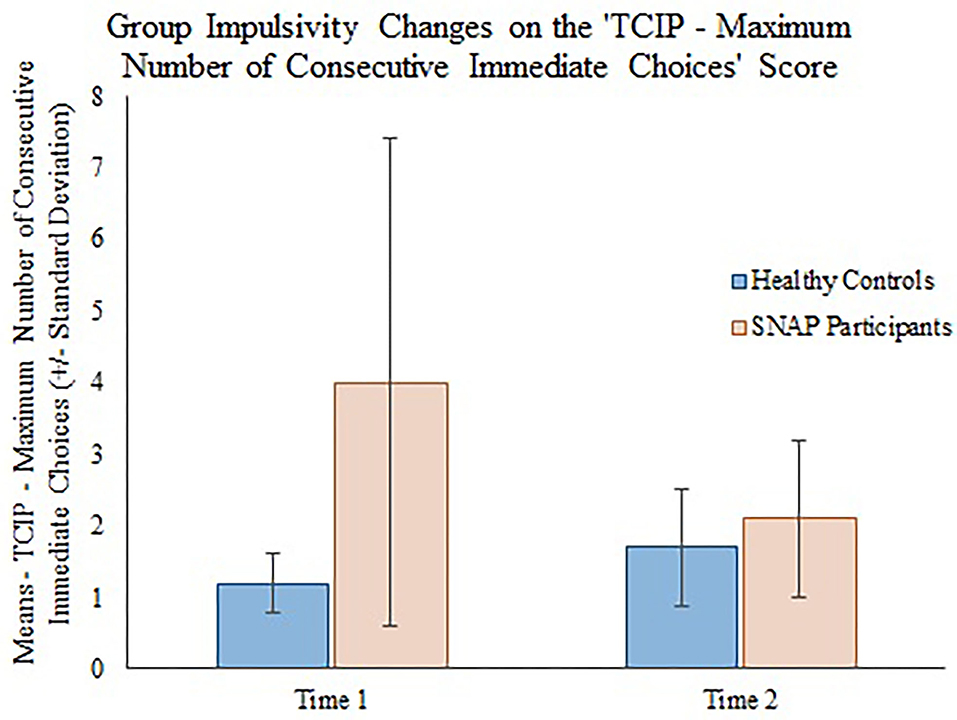
Figure 3. Group impulsivity changes on the Two Choice Impulsivity Paradigm–Maximum Number of Consecutive Immediate Choices score.
Brain imaging analysis demonstrated significant correlations between change in brain volume following SNAP treatment and the change score (e.g., improvement) for the impulsivity measure maximum number of consecutive immediate choices. Specifically, positive associations were detected in largely frontotemporal regions in the following clusters (p < 0.05, FDR-corrected at peak level): left middle temporal gyrus, right inferior temporal gyrus, right postcentral gyrus, right superior frontal gyrus, right temporal pole, left postcentral gyrus, and the left lateral occipital cortex. These results suggest that reduced impulsivity was associated with increases in the volumes of the aforementioned brain regions at time 2 in the SNAP sample. Negative correlations were detected in the following clusters (p < 0.05, FDR-corrected at peak level): left temporal pole, right lateral occipital cortex, and right intracalcarine cortex. These results suggest that improvements in impulsivity were associated with decreases in the volumes of the aforementioned brain regions at time 2 in the SNAP sample. The two other measures of impulsivity (e.g., number and proportion of immediate choices) were not associated with any GMV changes (see Figure 4 and Table 4).
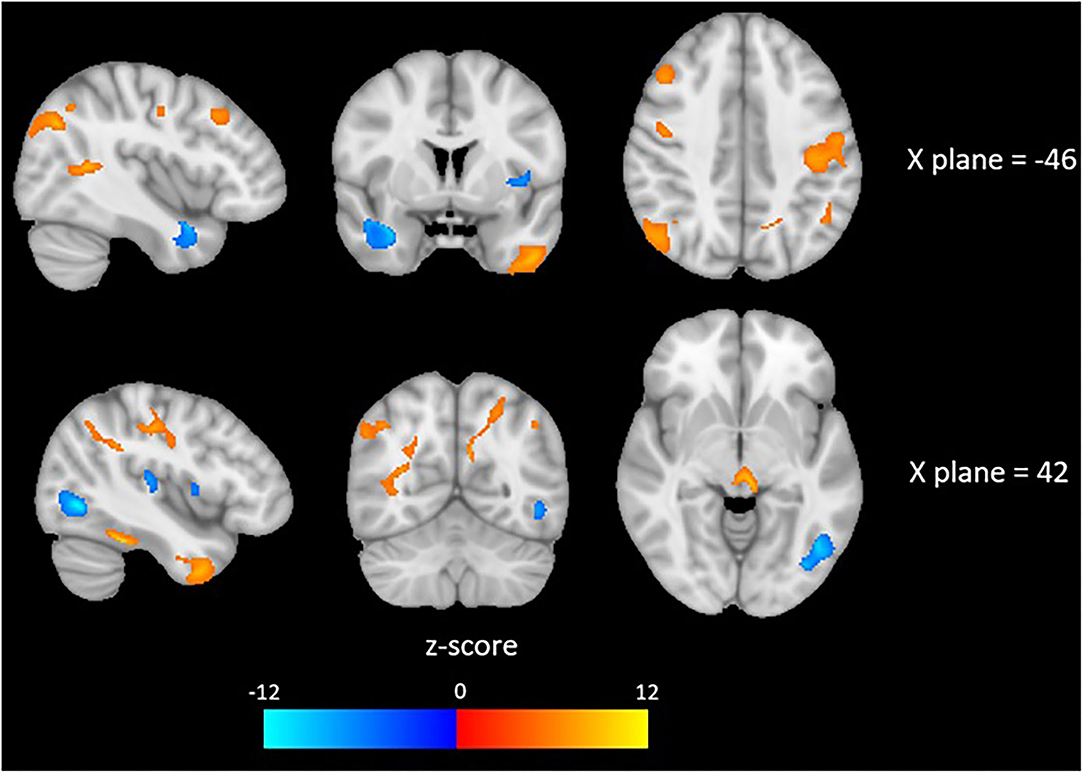
Figure 4. Red colors indicate a positive association and blue colors indicate a negative association between depicted brain areas and impulsivity, as measured by the Two Choice Impulsivity Paradigm–Maximum Number of Consecutive Immediate Choices score.
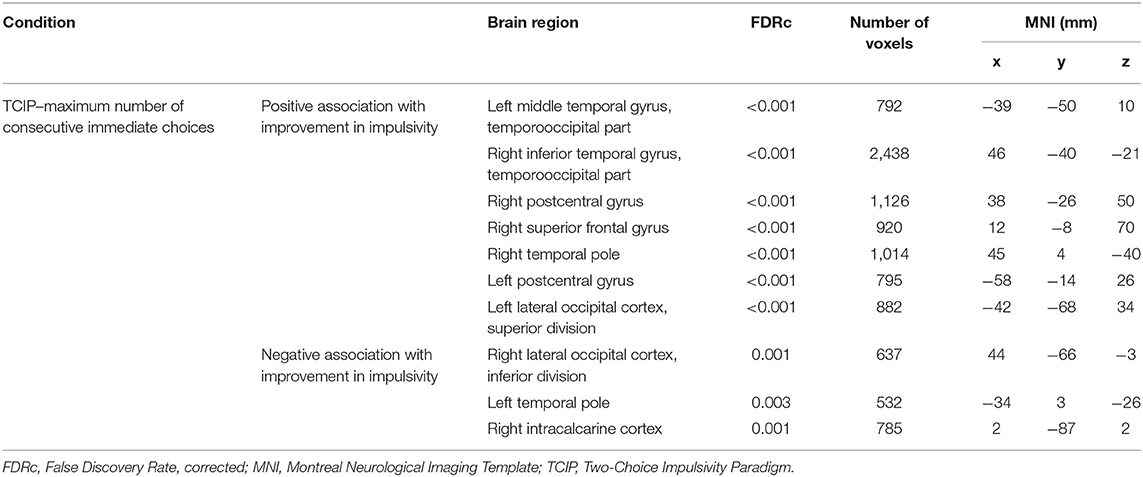
Table 4. Positive and negative associations between gray matter volume and positive impulsivity change score.
This exploratory clinical trial aimed to test whether SNAP treatment would improve parent-rated CBCL scores and behavioral measures of a delay discounting impulsivity task. We then investigated whether any clinical improvement in these variables would be associated with GMV changes assessed using sMRI. We found that SNAP was associated with improvement on all behavioral measures of impulsivity but was not associated with change in CBCL scores. We also determined that improvement in one behavioral measure of impulsivity was associated with GMV changes in the SNAP sample post-treatment. From the outset, we emphasize that all results should be interpreted with caution given the relatively small sample size and exploratory nature of the analyses.
Our main finding was that all behavioral measures of a delay discounting task were significantly improved in the SNAP participants post-treatment. Prior to the SNAP treatment, the SNAP cohort performed worse on the task compared with the healthy controls, as evidenced by the selection of greater number and proportion of immediate choices and selection of a greater maximum number of consecutive immediate choices. However, after SNAP treatment, the SNAP participants' results had improved to the level where there was no distinguishable difference in performance between the SNAP participants and healthy controls at time 2. Because SNAP participants received interventions designed to improve self-control between time 1 and 2, whereas the healthy group did not receive an intervention, we attribute the improvement in the SNAP participants to the effects of SNAP treatment. Several components of the SNAP program could have contributed to positive change. Some, but not all investigations (38), demonstrate that CBT can reduce impulsivity and improve self-control in clinical populations (39). For example, a 12-week course of CBT was shown to reduce impulsivity, ascertained by improvement on Barratt Impulsiveness Scale 11 (40) scale scores, in a sample of depressed outpatients at the end of treatment and at follow-up 30 days later (41). In outpatient substance abusers (42), 12 weeks of group CBT treatment was associated with reductions in UPPS Impulsive Behavior Scale (43) scores. Contingency management (CM), which aims to influence decision-making processes by shifting preference for delayed versus immediate rewards (44), has also been shown to reduce delay discounting among substance users (45, 46). Given that SNAP is predicated on a CBT model that includes social and token reinforcement (11, 24), we tentatively suggest that CBT and CM interventions may have contributed to reductions in delay discounting among SNAP participants post-treatment.
It is also possible that the parent management training component of SNAP contributed to the improvement in impulsivity seen in the SNAP youth. There is evidence that parent skills training interventions can reduce impulsivity in youth with externalizing disorders. For example, one pre-post study of a structured parent skill training intervention found that parent-rated impulsivity of children with ADHD significantly improved after completion of the intervention (47). Therefore, modifications in the family environment may have also been responsible for the reduced impulsivity observed among the SNAP participants.
Contrary to other studies investigating the impact of SNAP on externalizing behaviors, we found that CBCL scores of SNAP participants were not significantly changed post-intervention compared with healthy controls. One possibility to explain this discrepancy is that we did not assess participants for changes in CBCL scores beyond time 2. Previous research has demonstrated that improvement on primary measures of antisocial behavior may be greatest at 15 months from baseline assessment (12). Thus, it is possible that had we assessed the SNAP participants beyond 13 weeks, we would have seen improvement on externalizing behaviors. Given that the CBCL is validated for answering questions about children's presentation during the past six months, it may not have been an ideal measure for assessing short term change. Anecdotally, it is often the case that CBCL scores do not decrease or, conversely, actually increase immediately following SNAP treatment, presumably because parent training during SNAP treatment makes parents more cognizant of their children's issues and behaviors and more likely to rate their children higher on the CBCL. However, it is also possible that our study was under-powered to detect changes in CBCL scores. Future investigations assessing neurobiological mechanisms of clinical improvement in SNAP participants should consider recruiting larger sample sizes.
We found that improvement on the delay discounting impulsivity task was associated with GMV changes in the SNAP participants. Most of the alterations involved increases in GMV in frontotemporal regions at time 2 that correlated with less delay discounting. The brain regions implicated in the current investigation are consistent with those identified in the SNAP EEG study that predicted positive change, namely the prefrontal cortex. Studies examining the neural networks underlying impulsivity consistently link it with network-level alterations in the prefrontal cortex and temporal cortex (48–51). For example, in healthy adults, lower trait impulsivity was correlated with greater cortical thickness in left middle frontal, orbital frontal, and superior frontal regions (52), whereas in a clinical population with high impulsivity, lower trait impulsivity was associated with greater left and right temporal cortex thickness (53). Delay-discounting tasks appear, in particular, to recruit frontotemporal and limbic neural systems (54, 55). According to one model of delay discounting, functioning of the lateral temporal lobe is germane to cognitive processes, such as theory of mind, and reflection on one's own mental state (56–59), while the medial temporal lobe is pertinent to simulation of potential future experiences and creation of mental images (59, 60). Thus, the cognitive functions of these regions can be understood in the context of mental tasks required of delay discounting, including imagining the future and contemplating one's own preferences (61). What is the mechanism behind changes in GMV volume following SNAP treatment? We can only speculate on this point, but one possibility is that CBT interventions promoted brain changes in the SNAP participants. For example, pre- and post-intervention studies in patients with chronic pain and chronic fatigue syndrome found that CBT treatment was associated with increases in prefrontal GMV (62, 63). Several reports have documented the up-regulation of neuroplasticity in rodents and mammals following enrichment of the environment (64–66). Accordingly, we cautiously propose that CBT interventions may have led to increased neurogenesis in frontotemporal regions among the SNAP cohort. This information is important clinically, because it pinpoints brain regions that may be amenable to biological treatment interventions, such as repetitive transcranial magnetic stimulation, that could help reduce impulsivity and externalizing behaviors.
Another potential explanation for the increased GMV following SNAP psychosocial treatment is that SNAP may have upregulated levels of brain derived neurotrophic factor (BDNF). BDNF is the best studied member of the growth factor neurotrophic family and is the most prevalent growth factor in the central nervous system (CNS) (67). BDNF plays a critical role in development and plasticity of the brain and is implicated in the pathology of many psychiatric disorders (68). Several studies have now reported that CBT and other behavioral therapies can increase peripheral levels of BDNF (69, 70). For example, one recent study found that CBT for chronic stress increased serum levels of BDNF, in addition to improving sleep, depressive symptoms, and emotional exhaustion (70). Some evidence suggests that serum levels of BDNF correlate with markers of neuronal integrity in the CNS (71). Therefore, we cautiously propose that increased GMV following SNAP treatment may have been associated with altered levels of BDNF that could promote neural plasticity. Future biochemical studies of BDNF and/or magnetic resonance spectroscopy studies of interventions for youth with high externalizing behaviors would be needed to test this hypothesis.
Several limitations of the current study must be noted. First, the sample size of the experimental group was relatively small. However, we were able to recruit a comparable control group. As previously noted, our smaller sample size may have rendered some analyses under-powered to detect significant change for some measures. In general, we found it challenging to recruit a sample of youth with high externalizing behavior who were willing to present twice for assessment of clinical variables and MRI scanning who were at the same time undergoing SNAP treatment. To promote future neurobiologically-informed research of aggression and externalizing behavior in youths, robust treatment programs, such as SNAP, may consider embedding scanning and other biological measures into their treatment protocol. Second, we did not consider any potential effects of psychotropic medication on the results obtained. Two SNAP participants were taking psychotropic medication throughout the duration of SNAP (one participant was taking a psychostimulant and another participant was taking a psychostimulant and an antidepressant). Given the small number of SNAP participants, it was not feasible to conduct subgroup analyses involving the medicated participants. Third, as we conducted group-level analyses, it was not possible to determine whether improvement on the impulsivity task was linked to GMV changes at the individual level.
In conclusion, these preliminary results demonstrated that SNAP treatment was associated with improvement on a delay discounting impulsivity task and GMV changes. Overall, there is very little research investigating biomarkers of change in children undergoing evidence-based treatment for externalizing and conduct-disordered behavior. This exploratory study adds to the literature base by using MRI to detect GMV changes following SNAP treatment. Although we caution the interpretation of our results given the smaller sample size, we believe that this work is still valuable as it provides a foundation for neurobiologically- informed research underlying improvement in impulsivity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study involving human participants was reviewed and approved by the Centre for Addiction and Mental Health Research Ethics Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
NJK conceived and designed the study, was responsible for writing the first draft, and approved the final draft for submission. AS designed and conceived the study, was responsible for data collection, and approved the final draft for submission. GG was responsible for data collection and statistical analysis and approved the final draft for submission. KAK and JH was responsible for data collection and approved the final draft for submission. CH was responsible for statistical and MRI analysis and approved the final draft for submission. TAS, MW, and LA conceived and designed the study and approved the final draft for submission. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This research was funded by an Ontario Mental Health Foundation New Investigator Fellowship and a Slaight Family Centre For Youth in Transition Research Award, both awarded to NJK.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Egger HL, Angold A. Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. J Child Psychol Psychiatry. (2006) 47:313–37. doi: 10.1111/j.1469-7610.2006.01618.x
2. Keenan K, Wakschlag LS, Danis B, Hill C, Humphries M, Duax J. Further evidence of the reliability and validity of DSM-IV ODD and CD in preschool children. J Am Acad Child Adolesc Psychiatry. (2007) 46:457–68. doi: 10.1097/CHI.0b013e31803062d3
3. Birmaher B, Ehmann M, Axelson DA, Goldstein BI, Monk K, Kalas C. Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL) for the assessment of preschool children–a preliminary psychometric study. J Psychiatr Res. (2009) 43:680–6. doi: 10.1016/j.jpsychires.2008.10.003
4. Baillargeon RH, Sward GD, Keenan K, Cao G. Opposition-defiance in the second year of life: a population-based cohort study. Infancy. (2011) 16:418–34. doi: 10.1111/j.1532-7078.2010.00043.x
5. Egger HL, Erkanli A, Keeler G, Potts E, Walter BK, Angold A. Test-retest reliability of the preschool age psychiatric assessment (PAPA). J Am Acad Child Adolesc Psychiatry. (2006) 45:538–49. doi: 10.1097/01.chi.0000205705.71194.b8
6. Kim-Cohen J, Moffitt TE, Taylor A, Pawlby SJ, Caspi A. Maternal depression and children's antisocial behavior: nature and nurture effects. Arch Gen Psychiatry. (2005) 62:173–81. doi: 10.1001/archpsyc.62.2.173
7. Copeland WE, Miller-Johnson S, Keeler G, Angold A, Costello EJ. Childhood psychiatric disorders and young adult crime: a prospective, population-based study. Am J Psychiatry. (2007) 164:1668–75. doi: 10.1176/appi.ajp.2007.06122026
8. Burke JD, Waldman I, Lahey BB. Predictive validity of childhood oppositional defiant disorder and conduct disorder: implications for the DSM-V. J Abnorm Psychol. (2010) 119:739–51. doi: 10.1037/a0019708
9. Achenbach TM, Ruffle TM. The child behavior checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev. (2000) 21:265–71. doi: 10.1542/pir.21-8-265
10. Gresham FM, Elliott SN. Social skills improvement system rating scales manual. Minneapolis, MN: NCS Pearson (2008).
11. Augimeri LK, Walsh M, Donato A, Blackman A, Piquero AR. SNAP (stop now and plan): helping children improve their self-control and externalizing behavior problems. J Crim Justice. (2018) 56:7. doi: 10.1016/j.jcrimjus.2017.08.010
12. Burke JD, Loeber R. The effectiveness of the stop now and plan (SNAP) program for boys at risk for violence and delinquency. Prev Sci. (2015) 16:242–53. doi: 10.1007/s11121-014-0490-2
13. Smaragdi A, Blackman A, Donato A, Walsh M, Augimeri L. Sex differences in the classification of conduct problems: implications for treatment. J Dev Life-Course Criminol. (2020) 6:16. doi: 10.1007/s40865-020-00149-1
14. Farrington DP, Koegl CJ. Monetary benefits and costs of the stop now and plan program for boys aged 6-11, based on the prevention of later offending. J Quant Criminol. (2015) 31:24. doi: 10.1007/s10940-014-9240-7
15. Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. (2004) 72:271–324. doi: 10.1111/j.0022-3506.2004.00263.x
16. de Ridder DTD, Lensvelt-Mulders G, Finkenauer C, Marjin Stok F, Baumeister RF. Taking stock of self-control: a meta-analysis of how trait self-control relates to a wide range of behaviors. Pers Soc Psychol Rev. (2012) 16:76–99. doi: 10.1177/1088868311418749
17. Allom V, Mullan B, Hagger M. Does inhibitory control training improve health behaviour? a meta-analysis. Health Psychol Rev. (2016) 10:168–86. doi: 10.1080/17437199.2015.1051078
18. Hamilton KR, Sinha R, Potenza MN. Self-reported impulsivity, but not behavioral approach or inhibition, mediates the relationship between stress and self-control. Addict Behav. (2014) 39:1557–64. doi: 10.1016/j.addbeh.2014.01.003
19. Smith T, Panfil K, Bailey C, Kirkpatrick K. Cognitive and behavioral training interventions to promote self-control. J Exp Psychol Anim Learn Cogn. (2019) 45:259–79. doi: 10.1037/xan0000208
20. Kuhn MA, Ahles JJ, Aldrich JT, Wielgus MD, Mezulis AH. Physiological self-regulation buffers the relationship between impulsivity and externalizing behaviors among nonclinical adolescents. J Youth Adolesc. (2018) 47:829–41. doi: 10.1007/s10964-017-0689-1
21. Woltering S, Granic I, Lamm C, Lewis MD. Neural changes associated with treatment outcome in children with externalizing problems. Biol Psychiatry. (2011) 70:873–9. doi: 10.1016/j.biopsych.2011.05.029
22. Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. (2004) 55:11–29. doi: 10.1016/S0278-2626(03)00277-X
23. Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. (2007) 10:1116–24. doi: 10.1038/nn1944
24. Barkley RA. Commentary on the multimodal treatment study of children with ADHD. J Abnorm Child Psychol. (2000) 28:595–9. doi: 10.1023/A:1005139300209
25. Forehand R, Lautenschlager GJ, Faust J, Graziano WG. Parent perceptions and parent-child interactions in clinic-referred children: a preliminary investigation of the effects of maternal depressive moods. Behav Res Ther. (1986) 24:73–5. doi: 10.1016/0005-7967(86)90152-X
26. Martinez CRJr, Forgatch MS. Preventing problems with boys' noncompliance: effects of a parent training intervention for divorcing mothers. J Consult Clin Psychol. (2001) 69:416–28. doi: 10.1037/0022-006X.69.3.416
27. Boyd M. A socioeconomic scale for Canada; measuring occupational status from the census. Can Rev Sociol. (2008) 45:40. doi: 10.1111/j.1755-618X.2008.00003.x
28. Wechsler D. Wechsler Abbreviated Scale of Intelligence-Second Edition (WASI-II). San Antonio, TX: NCS Pearson (2011).
29. Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N. The schedule for affective disorders and schizophrenia for school-age children–present and lifetime version for DSM5 (K-SADS-PL-DSM5). Western Psychiatric Institute and Clinic: Advanced Center for Intervention and Services Research (ACISR) for Early Onset Mood and Anxiety Disorders, Yale University: Child and Adolescent Research and Education (CARE) Program (2016).
30. Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav Res Methods. (2005) 37:82–90. doi: 10.3758/BF03206401
31. Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical parametric mapping: the analysis of functional brain images. London, UK: Elsevier (2011).
32. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. (2005) 26:839–51. doi: 10.1016/j.neuroimage.2005.02.018
33. Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Curr Med Imaging Rev. (2005) 8. doi: 10.2174/1573405054038726
34. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. (2007) 38:95–113. doi: 10.1016/j.neuroimage.2007.07.007
35. Ashburner J. Computational anatomy with the SPM software. Magn Reson Imaging. (2009) 27:1163–74. doi: 10.1016/j.mri.2009.01.006
36. Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T. Bayesian analysis of neuroimaging data in FSL. Neuroimage. (2009) 45:S173–86. doi: 10.1016/j.neuroimage.2008.10.055
37. Zhu X, Suarez-Jimenez B, Zilcha-Mano S, Lazarov A, Arnon S, Lowell AL. Neural changes following equine-assisted therapy for posttraumatic stress disorder: A longitudinal multimodal imaging study. Hum Brain Mapp. (2021) 42:1930–9. doi: 10.1002/hbm.25360
38. Scholten H, Scheres A, de Water E, Graf E, Granic UI, Luijten M. Behavioral trainings and manipulations to reduce delay discounting: A systematic review. Psychon Bull Rev. (2019) 26:1803–49. doi: 10.3758/s13423-019-01629-2
39. Leeman RF, Bogart D, Fucito LM, Boettiger CA. “Killing two birds with one stone”: alcohol use reduction interventions with potential efficacy in enhancing self-control. Curr Addict Rep. (2014) 1:41–52. doi: 10.1007/s40429-013-0008-1
40. Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. (1995) 51:768–74.
41. Sedighrad G, Asgari P, Heidari A, Seraj Khorami N. Effectiveness of cognitive-behavioral therapy on reducing impulsive behaviors, alexithymia, and despair in depressed patients at counseling centers in Ahvaz. Int J Health Stud. (2021) 7:5.
42. Hatami-Nasari F, Azadfallah P, Tork M, Eydi-Baygi M, Gharaee-Ardakani S. Examining the effectiveness of group cognitive-behavioral therapy based on schema on impulsivity of substance abusers. Ann Mil Health Sci Res. (2015) 13:5.
43. Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif. (2001) 30:21. doi: 10.1016/S0191-8869(00)00064-7
44. Stanger C, Budney AJ, Bickel WK. A developmental perspective on neuroeconomic mechanisms of contingency management. Psychol Addict Behav. (2013) 27:13. doi: 10.1037/a0028748
45. Yi R, Johnson MW, Giordano LA, Landes RD, Badger GJ, Bickel WK. The effects of reduced cigarette smoking on discounting future rewards: an initial evaluation. Psychol Rec. (2008) 58:12. doi: 10.1007/BF03395609
46. Yoon J, Higgins H, Bradstreet ST, Badger MP, Thomas GJCS. Changes in the relative reinforcing effects of cigarette smoking as a function of initial abstinence. Psychopharmacology. (2009) 205:14. doi: 10.1007/s00213-009-1541-4
47. Shah R, Sharma A, Grover S, Sachdeva D, Chakrabarti S, Avasthi A. Development and effectiveness of parent skills training intervention for Indian families having children with attention-deficit/hyperactivity disorder (ADHD). Asian J Psychiatr. (2021) 64:102762. doi: 10.1016/j.ajp.2021.102762
48. Snowden JS, Bathgate D, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. (2001) 70:323–32. doi: 10.1136/jnnp.70.3.323
49. Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. (2007) 34:905–23. doi: 10.1016/j.neuroimage.2006.09.046
50. Goswami R, Dufort P, Tartaglia MC, Green RE, Crawley A, Tator CH. Frontotemporal correlates of impulsivity and machine learning in retired professional athletes with a history of multiple concussions. Brain Struct Funct. (2016) 221:1911–25. doi: 10.1007/s00429-015-1012-0
51. McDonald V, Hauner KK, Chau A, Krueger F, Grafman J. Networks underlying trait impulsivity: Evidence from voxel-based lesion-symptom mapping. Hum Brain Mapp. (2017) 38:656–65. doi: 10.1002/hbm.23406
52. Schilling C, Kuhn S, Romanowski A, Schubert F, Kathmann N, Gallinat J. Cortical thickness correlates with impulsiveness in healthy adults. Neuroimage. (2012) 59:824–30. doi: 10.1016/j.neuroimage.2011.07.058
53. Zsido AN, Darnai G, Inhof O, Perlaki G, Orsi G, Nagy SA. Differentiation between young adult internet addicts, smokers, and healthy controls by the interaction between impulsivity and temporal lobe thickness. J Behav Addict. (2019) 8:35–47. doi: 10.1556/2006.8.2019.03
54. McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. (2004) 306:503–7. doi: 10.1126/science.1100907
55. Wang Q, Chen C, Cai Y, Li S, Zhao X, Zheng L. Dissociated neural substrates underlying impulsive choice and impulsive action. Neuroimage. (2016) 134:540–9. doi: 10.1016/j.neuroimage.2016.04.010
56. Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. (2007) 58:259–89. doi: 10.1146/annurev.psych.58.110405.085654
57. Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. (2009) 19:2767–96. doi: 10.1093/cercor/bhp055
58. Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. (2012) 24:1742–52. doi: 10.1162/jocn_a_00233
59. Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. (2014) 1316:29–52. doi: 10.1111/nyas.12360
60. Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. (2007) 11:280–9. doi: 10.1016/j.tics.2007.05.005
61. Owens MM, Gray JC, Amlung MT, Oshri A, Sweet LH, MacKillop J. Neuroanatomical foundations of delayed reward discounting decision making. Neuroimage. (2017) 161:261–70. doi: 10.1016/j.neuroimage.2017.08.045
62. de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer PJW, et al. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. (2008) 131(Pt 8):2172–80. doi: 10.1093/brain/awn140
63. Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain. (2013) 14:1573–84. doi: 10.1016/j.jpain.2013.07.020
64. Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav Brain Res. (1996) 78:57–65. doi: 10.1016/0166-4328(95)00216-2
65. Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. (2000) 1:67–73. doi: 10.1038/35036235
66. van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. (2000) 1:191–8. doi: 10.1038/35044558
67. Piotrkowicz M, Janoska-Jazdzik M, Koweszko T, Szulc A. The influence of psychotherapy on peripheral brain-derived neurotrophic factor concentration levels and gene methylation status: a systematic review. J Clin Med. (2021) 10. doi: 10.3390/jcm10194424
68. Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. (2012) 64:238–58. doi: 10.1124/pr.111.005108
69. Yamada H, Yoshimura C, Nakajima T, Nagata T. Recovery of low plasma BDNF over the course of treatment among patients with bulimia nervosa. Psychiatry Res. (2012) 198:448–51. doi: 10.1016/j.psychres.2011.12.015
70. Orosz A, Federspiel A, Eckert A, Seeher C, Dierks T, Tschitsaz A. Exploring the effectiveness of a specialized therapy programme for burnout using subjective report and biomarkers of stress. Clin Psychol Psychother. (2021) 28:852–61. doi: 10.1002/cpp.2539
Keywords: externalizing behavior, aggression, impulsivity, cognitive behavioral therapy, structural magnetic resonance imaging
Citation: Kolla NJ, Smaragdi A, Gainham G, Karas KH, Hawco C, Haas J, Skilling TA, Walsh M and Augimeri L (2022) Psychosocial Intervention for Youth With High Externalizing Behaviors and Aggression Is Associated With Improvement in Impulsivity and Brain Gray Matter Volume Changes. Front. Psychiatry 12:788240. doi: 10.3389/fpsyt.2021.788240
Received: 01 October 2021; Accepted: 24 November 2021;
Published: 06 January 2022.
Edited by:
Thomas Nilsson, University of Gothenburg, SwedenReviewed by:
Pietro Muratori, Fondazione Stella Maris (IRCCS), ItalyCopyright © 2022 Kolla, Smaragdi, Gainham, Karas, Hawco, Haas, Skilling, Walsh and Augimeri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nathan J. Kolla, bmF0aGFuLmtvbGxhQGNhbWguY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.