- 1Department of Medical Statistics and Epidemiology, School of Public Health, Sun Yat-sen University, Guangzhou, China
- 2Guangdong Provincial Key Laboratory of Food, Nutrition and Health, Sun Yat-sen University, Guangzhou, China
- 3Department of Prevention and Treatment of Common Diseases Among Students, Health Promotion Center for Primary and Secondary Schools, Guangzhou, China
Since the novel coronavirus disease 2019 (COVID-19) outbreak, adolescents' emerging mental health and behavior issues have been an international public health concern. This longitudinal study aimed to examine the situation of poor sleep quality, anxiety, and depressive symptoms among Chinese adolescents and to explore the associations between them before and during COVID-19. A total of 1,952 middle and high school students as eligible participants at baseline (pre-COVID-19, Wave 1; response rate: 98.79%), 1,831 eligible students were followed up at Wave 2 (October 2019 to December 2019, pre-COVID-19; retention rate: 93.80%), and 1,790 completed the follow-up at Wave 3 (during the COVID-19; retention rate: 97.80%). The mean age of the baseline students was 13.56 (SD: 1.46) years. The differences in anxiety and depressive symptoms between Wave 1, Wave 2, and Wave 3 were not statistically significant. The proportion of students with poor sleep quality increased over time, from Wave 1 (21.0%) to Wave 3 (26.0%, OR = 1.37, 95% CI = 1.17–1.60, P = 0.001) and from Wave 2 (21.9%) to Wave 3 (OR = 1.29, 95% CI = 1.11–1.51, P < 0.001). The cross-lagged generalized linear mixed models revealed that the concurrent and cross-lagged associations of poor sleep quality with anxiety symptoms across the three waves were significant (P < 0.05) and vice versa. Only a marginally significant positive cross-lagged association between poor sleep quality at Wave 2 and depressive symptoms at Wave 3 was found (standardized β estimate = 0.044, SE = 0.022, P = 0.045). Sleep quality was adversely affected during COVID-19, and the bidirectional associations of poor sleep quality with anxiety symptoms could not be neglected.
Introduction
Adolescence represents a developmental transition period between childhood and adulthood, characterized by marked changes in biological systems and physical maturation of the body and brain, rendering adolescents vulnerable to mental health and behavior problems, including anxiety symptoms, depressive symptoms, and poor sleep quality (1). Anxiety and depressive symptoms are among the most prevalent mental health problems presented in adolescents, resulting in considerable distress to the individual (e.g., impaired school functioning or developing into mental disorders), and then are the main contributors to the global burden of disease in this population (2). However, the causes of anxiety symptoms and depressive symptoms remained unclear. Previous evidence has suggested that sleep is an important predictor of adolescents' subsequent anxiety and depressive problems (3). Sleep is a core behavior of adolescents, consuming up to a third or more of each day, and contributes a vital role in adolescent brain development (4). With the increased incidence and prevalence of poor sleep quality reported in adolescents from different countries, adolescent sleep health is increasingly becoming a global public health concern. Numerous studies have found that poor sleep quality was associated with the elevated risks of anxiety symptoms or depressive symptoms (5, 6). Some evidence has also shown that anxiety symptoms or depressive symptoms can reversely predict poor sleep among adolescents (7), indicating that the associations of poor sleep quality with anxiety symptoms or depressive symptoms may be complex and bidirectional.
Since the outbreak of the novel coronavirus disease 2019 (COVID-19), the emerging mental health and behavior issues related to this global event have been an international public health concern (8). Previous cross-sectional and longitudinal studies in China during the initial stages of the COVID-19 pandemic demonstrated elevated levels of mental health problems among the general population (9, 10). The effects of the COVID-19 pandemic and the policies to contain it can be considered a stressor exposure, and their effects on mental health and behavioral problems are probably to be profound and long-lasting. However, to what extent such effects will last generalize to which population is unclear. During the early stages of the COVID-19 pandemic, most governments around the world have temporarily closed schools. As the outbreak of the COVID-19 during Chinese schools' winter vacation, all the school students were at home. It was notable that China's Ministry of Education issued school closure policies for the entire country between January 20 and February 8, and announced on January 27, 2020 that the 2020 spring semester for schools would be postponed to late April due to the COVID-19 outbreak, affecting a large number of students across primary and post-secondary grades in China (11). Some recent studies have discussed the influences of school closures during the COVID-19 pandemic on Chinese adolescents' anxiety or depressive symptoms and sleep behavior (12, 13). Nonetheless, most of the available evidence is cross-sectional or short-term and does not address the longer-term effects of COVID-19 and related policies on adolescents' mental health and behavior. Moreover, there is a scarce study on the longitudinal and reciprocal associations between poor sleep quality, anxiety symptoms, and depressive symptoms among Chinese adolescents before and during the COVID-19 pandemic. It is also notable that to minimize the negative impact of COVID-19 school closures on students, the government, National Health Commission, schools, families, and mental health professionals worked together to maintain students' routines and protect them from the impact of the pandemic (including guidelines, recommendations, or interventions during home confinement; online help services; a reduction in academic load) (14–16).
Therefore, longitudinal studies are needed to assess this gap in the literature. Cascade modeling, an analytic approach controlling within-time and across-time associations while estimating cross-lagged effects, is a strategy of examining the complex interplay in multiple factors across time (17). The purposes of this three-wave longitudinal study were to examine the situation of poor sleep quality, anxiety symptoms, and depressive symptoms among Chinese adolescents before and during COVID-19; to examine the concurrent and longitudinal associations among poor sleep quality, anxiety symptoms, and depressive symptoms among these adolescents at three-time points.
Materials and Methods
Study Design and Participants
Data were drawn from the Longitudinal Study of Adolescents' Mental and Behavioral Well-being Research in Guangzhou, China (Registration No. ChiCTR1900022032) (18), which used a multi-stage stratified cluster random sampling method to recruit 1,976 grade 7 and grade 10 students. Full details of the study sampling method are reported elsewhere (18). In brief, six middle schools and four high schools in Guangzhou were selected according to the representative and proportion of middle school and high school across districts, convenience for the data collection, and previous study collaboration. Two classes from the 7th grade in middle school and the 10th grade in high school were randomly selected among the chosen schools. All students presenting in the selected classes were invited to participate voluntarily. In this study, a total of 1,952 students as eligible participants at baseline (January to March 2019, pre-COVID-19, Wave 1; response rate: 98.79%). Among the recruited 1,952 students, 1,831 students were followed up at Wave 2 (October 2019 to December 2019, pre-COVID-19; retention rate: 93.80%), and 1,790 completed the follow-up at Wave 3 (October 2020 to December 2020, during the COVID-19; retention rate: 97.80%). After almost 3 months of lockdown between Wave 2 and Wave 3, all the surveyed middle and high schools in Guangzhou were reopened on May 11. In order to protect the privacy of participants and reduce information bias, self-reported questionnaires were blind to teachers during the three waves. After the study had been fully explained in detail, written informed consent was obtained from each participant and one of the student's legal guardians. This study was approved by the Sun Yat-sen University, School of Public Health Institutional Review Board (Ethics Number: L2017060).
Measures
Anxiety Symptoms
Anxiety symptoms were measured by the Chinese version of the Generalized Anxiety Disorder 7-Item Questionnaire (GAD-7) (19), which has been validated and widely used in Chinese adolescents with acceptable psychometric properties (20, 21). The Cronbach's alpha was 0.89, 0.91, and 0.91 with the study sample in Wave 1, Wave 2, and Wave3, respectively. The respondents were asked to rate the frequency of anxiety symptoms over the last 2 weeks, with the response options given on a four-point Likert scale (0 = not at all, 1 = several days, 2 = more than half the days, and 3 = nearly every day). The sum of scores ranges from 0 to 21, with higher scores indicating a higher level of anxiety symptoms severity, with the GAD-7 score ≥5 used to interpret as having anxiety symptoms (19).
Depressive Symptoms
Depressive symptoms were assessed by the Chinese version of the Center for Epidemiology Scale for Depression (CES-D), which has been validated and extensively utilized in Chinese adolescents (22, 23). The Cronbach's alpha was 0.80, 0.83, and 0.84 with the study sample in Wave 1, Wave 2, and Wave3, respectively. The CES-D comprises 20 items about the frequency of symptoms of depression over the past week, and the response options were rated in a four-point Likert scale (from 0 = rarely or none of the time to 3 = most or all of the time). The total CES-D score ranges from 0 to 60, with higher scores indicating more severe depressive symptomatology. A total CES-D score ≥16 was considered as having depressive symptoms (24).
Poor Sleep Quality
The level of sleep quality was assessed by the Chinese version of the Pittsburgh Sleep Quality Index (PSQI), which is commonly used to measure sleep quality and disturbance over 1 month. The Chinese version of PSQI has been validated and widely used among Chinese adolescents (25). The PSQI consists of 19 items constituting seven subscales: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, sleep medication use, and daytime dysfunction. The scores for each subscale are weighted equally and range from 0 to 3 points, and the sum of the scores for these seven subscales yields a global PSQI score, which ranges from 0 to 21, with higher scores indicating worse sleep quality. A global PSQI score >7 points indicates having poor sleep quality (25).
Other Variables
Demographic information including age, sex (1 = boy, 2 = girl), living arrangement (1 = living with both parents, 2 = living with a single parent, 3 = living with others), family relations (1 = good, 2 = average, 3 = poor), student-teacher relations (1 = good, 2 = average, 3 = poor), ever smoking (1 = yes, 2 = no), ever drinking (1 = yes, 2 = no) were also collected.
Statistical Analysis
All statistical analyses were conducted using Stata/MP (Stata Corp, LLC, College Station, TX) and Mplus version 8.3 (Muthén & Muthén, Los Angeles, CA, USA). First, descriptive analyses were used to describe sample characteristics across three waves, and the Chi-square tests for categorical variables and the one-way ANOVA analyses for continuous variables were performed. Second, considering this study utilized a complex multi-stage sampling design, students were grouped into classes, generalized linear mixed models (GLMMs) in which classes were treated as clusters were constructed to test the changes in anxiety symptoms, depressive symptoms, and poor sleep quality over the three waves for the whole sample (26). Third, the unadjusted and adjusted GLMMs were also adopted to examine the association of poor sleep quality (main effects) with anxiety symptoms or depressive symptoms over the three waves, including examination of the poor sleep quality by time interaction. Fourth, the cross-lagged autoregressive GLMMs were further used to examine the concurrent and cross-lagged associations of poor sleep quality with anxiety symptoms and depressive symptoms, and the unadjusted and adjusted cross-lagged autoregressive GLMMs were conducted in multiple steps. The comparative fit index (CFI), the standardized root mean square residual (SRMR), and the root mean square error of approximation (RMSEA) were used to measure model fit; CFI values >0.90 and RMSEA values <0.08 suggest acceptable model fit (27). Missing data were eliminated in the GLMMs. All statistical tests were two-sided, and a P < 0.05 was considered statistically significant.
Results
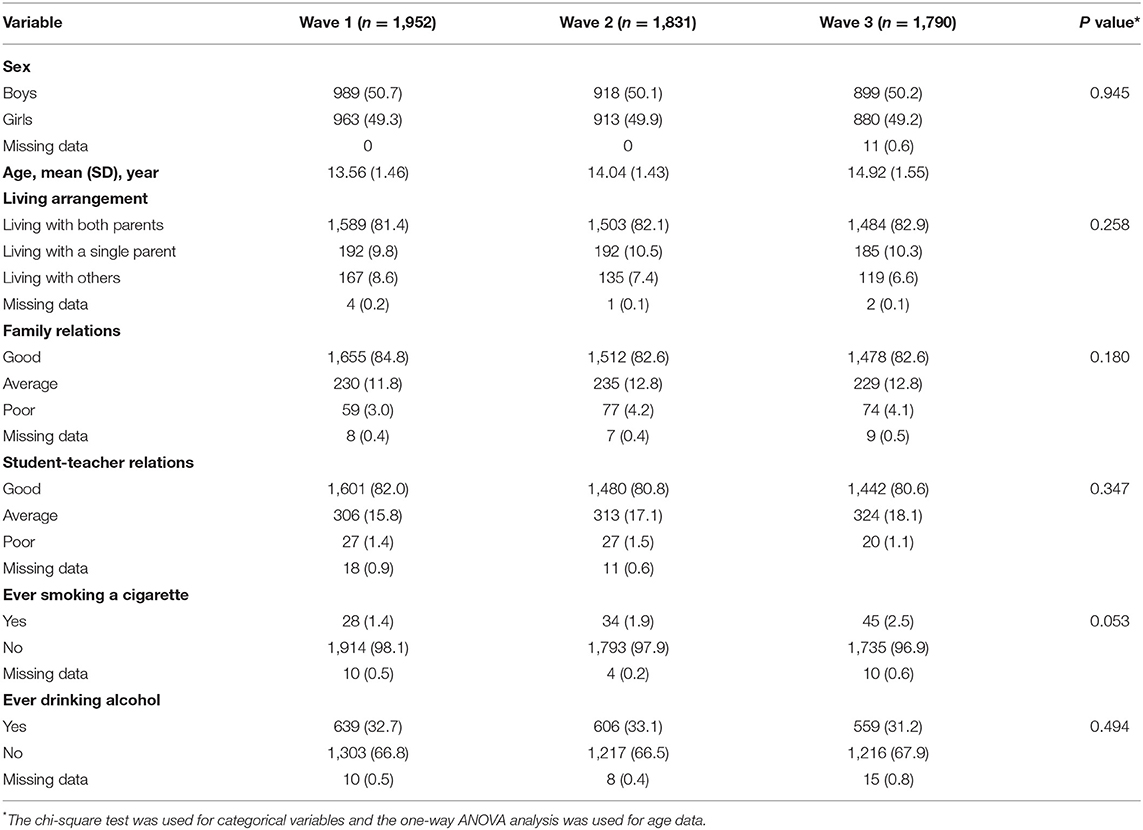
Table 1 shows sample demographic characteristics across waves. At wave 1, 50.7% of the participants were boys, and the mean (SD) age of the total students was 13.56 (SD: 1.46) years, with the age range from 13 to 18 years. The majority of students lived with both parents (81.4%), 3.0% reported poor family relations, and 1.4% reported their student-teacher relations as inferior. The proportion of students who reported ever smoking was 1.4% and reported ever drinking was 32.7%, respectively. There was no statistically significant difference in the demographic characteristics of the participants between the three waves (P > 0.05).
The mean GAD-7 score of anxiety symptoms at Wave 1, Wave 2, and Wave 3 was 3.75 (SD: 4.31), 3.60 (SD: 4.32), and 3.56 (SD: 4.22), respectively. Then mean CES-D score of depressive symptoms at Wave 1, Wave 2, and Wave 3 was 13.62 (SD: 10.07), 13.69 (SD: 10.53), and 13.44 (SD: 10.28), respectively. The differences in anxiety and depressive symptoms between Wave 1, Wave 2, and Wave 3 were not statistically significant. The mean PSQI score of sleep quality at Wave 1, Wave 2, and Wave 3 was 4.94 (SD: 2.44), 4.66 (SD: 2.84), and 5.08 (SD: 2.91), respectively; the difference between Wave 3 and Wave 2 was statistically significant (OR = 1.55, 95% CI 1.27–1.90, P < 0.001). Moreover, we also observed that compared with the sleep situation in Wave 1, there was a significant worsening of sleep duration (OR = 1.47, 95% CI = 1.35–1.60), sleep disturbance (OR = 2.11, 95% CI = 1.86–2.38), use of sleep medication (OR = 1.48, 95% CI = 1.10–1.99), daytime dysfunction (OR = 3.59, 95% CI = 3.22–4.00) in Wave 3, and a significant improvement in sleep latency (OR = 0.77, 95% CI = 0.71–0.84) in Wave 3. No significant differences were found between wave 1, wave 2, and wave 3 about subjective sleep quality and habitual sleep efficiency. The proportion of students with poor sleep quality increased over time, from Wave 1 (21.0%) to Wave 3 (26.0%, OR = 1.37, 95% CI = 1.17–1.60, P = 0.001) and from Wave 2 (21.9%) to Wave 3 (OR = 1.29, 95% CI = 1.11–1.51, P < 0.001), but not from Wave 1 to Wave 2 (OR = 1.05, 95% CI = 0.91–1.23, P = 0.471) (Table 2). Factors associated with anxiety symptoms, depressive symptoms, and poor sleep quality at Wave 1, Wave 2, and Wave 3 were presented in Supplementary Material.
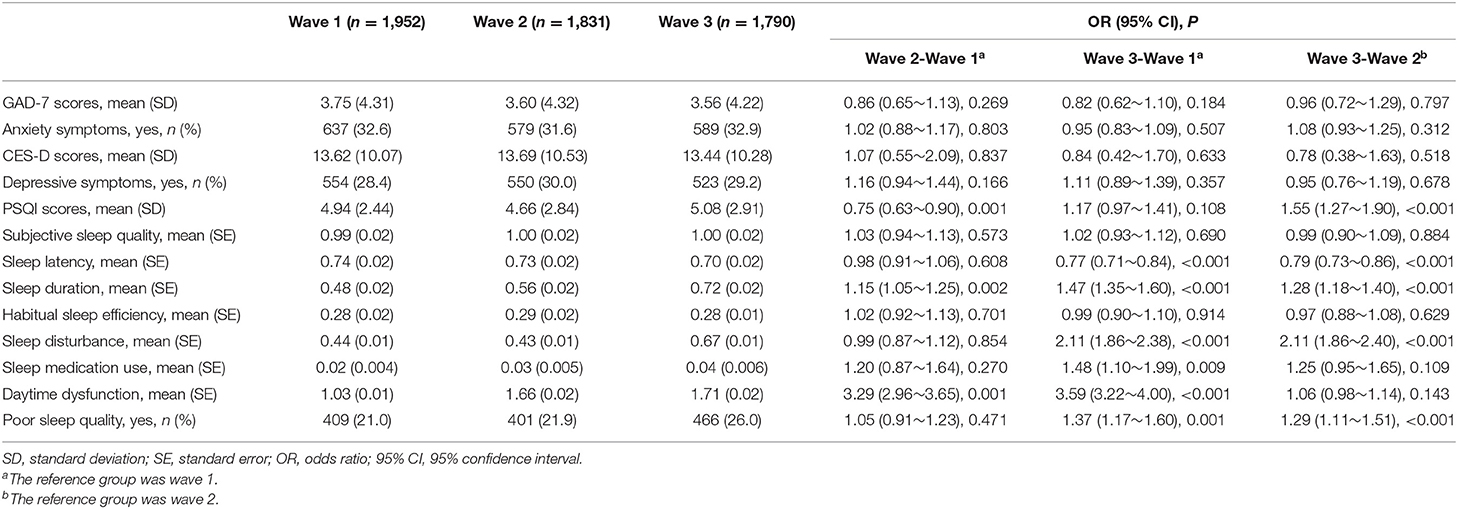
Table 2. Changes in anxiety symptoms, depressive symptoms, and poor sleep quality over the three waves with odds ratios (ORs) and 95% confidence interval.
As shown in Table 3, the unadjusted and adjusted GLMMs revealed the absence of a significant interactive effect of poor sleep quality by time on anxiety symptoms (P > 0.05) and depressive symptoms (P > 0.05) through the three-wave follow-up. Moreover, without adjusting for other variables, the total PSQI sleep quality scores were positively associated with anxiety symptoms and depressive symptoms (P < 0.05).
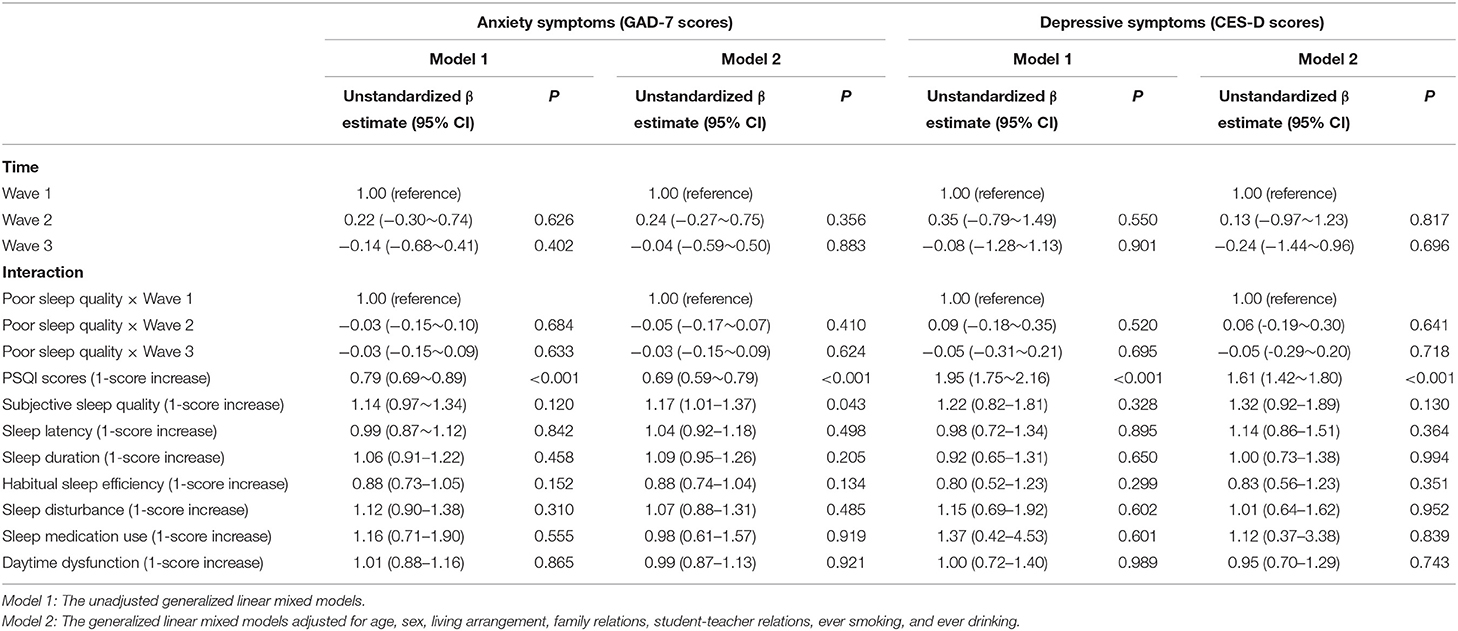
Table 3. Prospective associations of poor sleep quality with anxiety symptoms and depressive symptoms.
After adjusting for age, sex, living arrangement, family relations, student-teacher relations, ever smoking, and ever drinking, there was a significant main effect of the total PSQI scores with anxiety symptoms (unstandardized β estimate = 0.69, 95% CI = 0.59~0.79, P < 0.001) and depressive symptoms (unstandardized β estimate = 1.61, 95% CI = 1.42~1.80, P < 0.001) through the three waves. Regarding each PSQI component, the adjusted GLMMs found that subjective sleep quality was positively associated with anxiety symptoms (unstandardized β estimate = 1.17, 95% CI = 1.01~1.37, P < 0.05) through the three waves.
To further observe the associations of poor sleep quality with anxiety symptoms and depressive symptoms, we examined cross-lagged GLMMs. Figure 1 depicted the results of the path models for poor sleep quality and anxiety symptoms with acceptable model fits that included all cross-lagged pathways. After adjusting for age, sex, living arrangement, family relations, student-teacher relations, ever smoking, and ever drinking, the adjusted autoregressive associations of both variables from Wave 1 to Wave 3 were positive and significant, indicating that baseline levels of poor sleep quality (standardized β estimate: ranging from 0.225 to 0.348) and anxiety symptoms (standardized β estimate: ranging from 0.488 to 0.506) were predictive of the same variable at follow-up. Moreover, the concurrent associations of poor sleep quality and anxiety symptoms at Wave 1, Wave 2, and Wave 3 were significant (at Wave 1: standardized β estimate = 0.119, SE = 0.027, P < 0.001; at Wave 2: standardized β estimate = 0.279, SE = 0.025, P < 0.001; at Wave 3: standardized β estimate = 0.315, SE = 0.024, P < 0.001). The significant positive cross-lagged associations between poor sleep quality (at Wave 1 or Wave 2) and subsequent anxiety symptoms (at Wave 2: standardized β estimate = 0.061, SE = 0.024, P = 0.010; at Wave 3: standardized β estimate = 0.117, SE = 0.024, P < 0.001). Conversely, high level of anxiety symptoms (at Wave 1 or Wave 2) was also associated with increased levels of poor sleep quality later (at Wave 2: standardized β estimate = 0.256, SE = 0.025, P < 0.001, P < 0.001; at Wave 3: standardized β estimate = 0.054, SE = 0.027, P = 0.046).
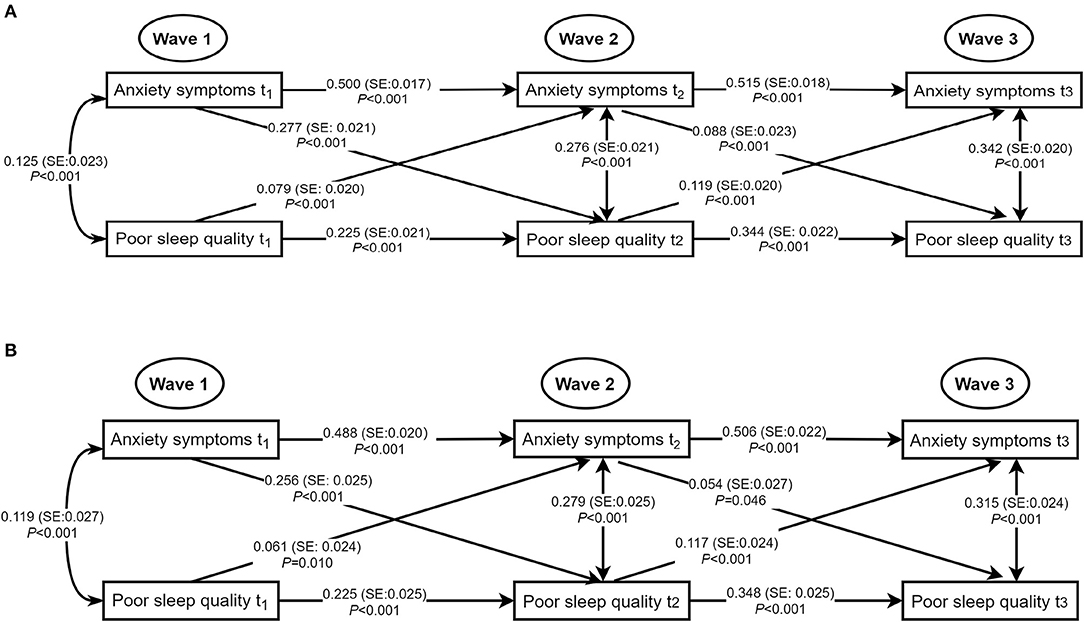
Figure 1. Concurrent and cross-lagged associations between poor sleep quality and anxiety symptoms. (A) Unadjusted models. (B) Models adjusting for age, sex, living arrangement, family relations, student-teacher relations, ever smoking, and ever drinking; for clarity of presentation, the standardized coefficients of the control variables were not displayed in the model but were included in the analyses. SE, standard error; the solid line meant the standardized coefficient was statistically significant.
Figure 2 showed significantly positive autoregressive associations of poor sleep quality and depressive symptoms with the same variable from Wave 1 to Wave 3 in the unadjusted and adjusted models with acceptable model fits. After adjusting for age, sex, living arrangement, family relations, student-teacher relations, ever smoking, and ever drinking, no concurrent associations of poor sleep quality with depressive symptoms were statistically significant at Wave 1, Wave 2, and Wave 3. A marginally significant positive cross-lagged association between poor sleep quality at Wave 2 and depressive symptoms at Wave 3 was observed (standardized β estimate = 0.044, SE = 0.022, P = 0.045).
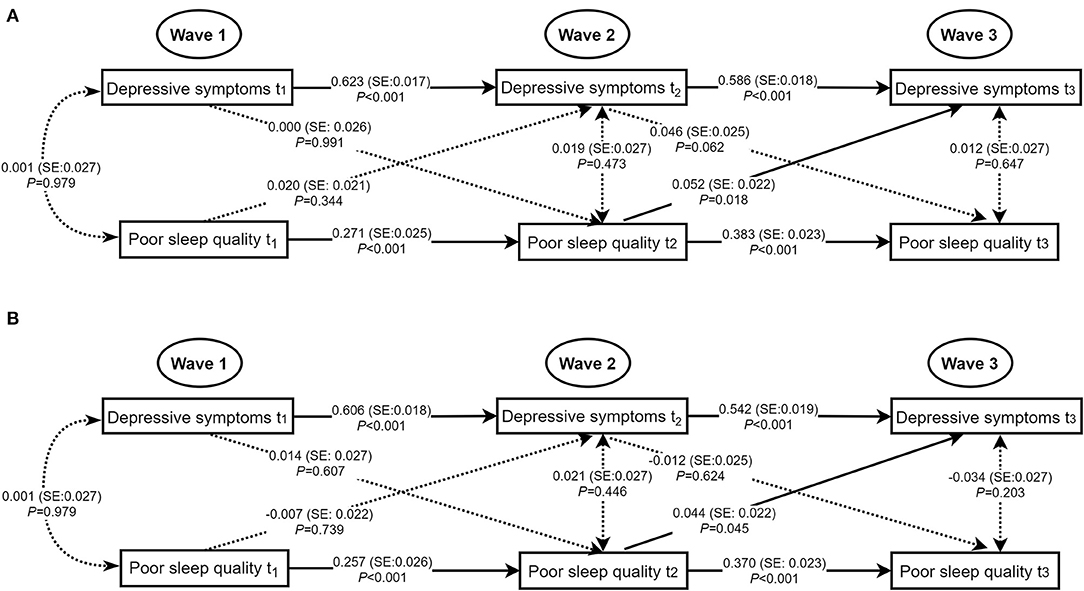
Figure 2. Concurrent and cross-lagged associations between poor sleep quality and depressive symptoms. (A) Unadjusted models. (B) Models adjusting for age, sex, living arrangement, family relations, student-teacher relations, ever smoking, and ever drinking; for clarity of presentation, the standardized coefficients of the control variables were not displayed in the model but were included in the analyses. SE, standard error; the solid line meant the standardized coefficient was statistically significant.
Discussion
This study first provides a detailed examination of anxiety and depressive symptoms and poor sleep quality of the Chinese adolescents at three-time points, including the period before and during COVID-19. Although the found rate of anxiety symptoms, depressive symptoms, or poor sleep quality at Wave 3 in this study was similar to results from previous cross-sectional studies conducted during the COVID-19 (10, 12), our three-wave longitudinal study found that except for poor sleep quality, the situation of anxiety and depressive symptoms among adolescents did not get worse at Wave 3, which is the time point that students have experienced almost 3 months lockdown and school closure due to the COVID-19 pandemic. However, a previous longitudinal study showed that depressive symptoms (not anxiety symptoms) among Chinese primary school students in Wave 2 (2 weeks after school reopening, mid-May 2020) increased from levels at Wave 1 (before the outbreak of COVID-19) (28). One explanation of the differences of change in depressive symptoms might be that our study population were middle and high school students (not primary school students). Another possible explanation was that the assessing point of Wave 3 in our study was almost five months after school reopening, mainly focusing on the long-term effects of school closing and opening during the COVID-19 pandemic. Besides, it also should be noted that the government, schools, families, and mental health professionals worked together to maintain students' routines and protect them from the impact of the pandemic (14–16), and superior to usual preventive strategies have also been highly recommended to be implemented (e.g., school mental health consultation or mental health education) after school reopening. Consistent with our findings, a longitudinal study of adults in the UK demonstrated that anxiety and depressive symptoms remained stable across waves during the first 6 weeks of the COVID-19 pandemic (29); a recent longitudinal study among high school students in São Paulo also showed that adolescents delayed bed and wake-up times and had increased the global PSQI score during the COVID-19 pandemic (30). Moreover, our study also observed that regarding each component of PSQI, sleep duration, sleep disturbance, use of sleep medication, and daytime dysfunction got worse at Wave 3 among these adolescents, while sleep latency got better at Wave 3. These findings were partially similar to a previous study among adults (31), and suggested that families, schools, and clinicians should be informed about the adverse effects of the COVID-19 pandemic in relation to adolescent sleep quality.
Although the direct long-term influence of the COVID-19 pandemic on anxiety or depressive symptoms was not observed in this study, this study observed significant main longitudinal effects of poor sleep quality on anxiety symptoms and depressive symptoms through the three waves in with and without adjusted GLMMs. Moreover, prior studies also demonstrated that individuals with poor sleep quality were at a higher risk of depressive symptoms and anxiety symptoms than those without (32). Poor sleep quality is nearly universal in individuals with mental health problems, and previous evidence has suggested individuals with depressive or anxiety symptoms would have sleep quality complaints (33). Therefore, this study also investigated the bidirectional associations of poor sleep quality with anxiety and depressive symptoms. This study revealed that after adjusting for age, sex, living arrangement, family relations, student-teacher relations, ever smoking, and ever drinking, poor sleep quality was positively associated with current as well as subsequent anxiety symptoms and vice versa. Only a marginally significant cross-lagged association between poor sleep quality at Wave 2 and depressive symptoms at Wave 3 was observed. Similarly, Kelly et al. showed that there were significant reciprocal associations of children's sleep/wake problems with anxiety and depressive symptoms using three waves data spanning 5 years (34). Robert et al. using data from a two-wave cohort study found that short sleep duration at baseline increased the risk of subsequent anxiety disorder twofold, but the reverse association was not significant (35). Shanahan et al. using a study of youth ages between 9 and 16, found that sleep problems predicted an increase in generalized anxiety disorders but only predicted depression when anxiety symptoms were also present, and vice versa (36). These results might be related to that several myriad mechanisms for the association between sleep problems and anxiety/depressive symptoms have been posited, including genes, hormones, regulatory systems (e.g., the hypothalamic-pituitary-adrenal axis), and cognitive process (37, 38). Sleep problems are sometimes a symptom of anxiety or depression, and sufficient sleep may serve an important role in developing adolescent brain regions (e.g., the prefrontal lobe), which is responsible for emotional regulation and cognitive function (39). Conversely, anxiety or depressive symptoms are disabling conditions among adolescents that result in emotional suffering and poor sleep quality (40). In this study, only poor sleep quality at Wave 2 predicting depressive symptoms at Wave 3 may reflect students' sleep quality was adversely affected by the COVID-19 pandemic. A possibility for the unobserved concurrent or cross-lagged associations between poor sleep quality and depressive symptoms may be that the autoregressive association between previous depressive symptoms and subsequent depressive symptoms is too strong to cover the effects of poor sleep quality.
As already mentioned, whereas symptoms of anxiety and depression remained stable across the three waves, sleep quality was adversely affected, and the bidirectional associations between poor sleep quality and anxiety or depressive symptoms could not be neglected. Thereby, the implications of this study highlight that prevention or intervention strategies targeted at cultivating healthy sleep habits and improving sleep quality are helpful to develop good mental health among adolescents, particularly during COVID-19.
The findings also highlight the importance of regularly assessing adolescents' mental health status, especially in adolescents with poor sleep quality. Although this study extends exiting literature on the associations between poor sleep quality, anxiety symptoms, and depressive symptoms among Chinese adolescents before and during COVID-19, several limitations should also be considered. First, the school-based longitudinal study only included school students as the study sample and did not include adolescents absent from schools. Second, indicators of poor sleep quality, anxiety symptoms, and depressive symptoms were based on self-report, then this study only comments on these symptoms of mental health and behaviors rather than psychiatric disorders. Third, given that most Chinese middle school students or high school students will transfer to other schools after graduation, this longitudinal study only invited junior grade one and senior grade one students to avoid loss of follow-up due to students going on for further study.
In conclusion, this three-wave longitudinal study found that anxiety and depressive symptoms among Chinese adolescents remained stable before and during COVID-19, while sleep quality among adolescents gets worse at the time point when students have experienced almost 3 months of lockdown and school closure due to the COVID-19 pandemic. Moreover, the concurrent and cross-lagged associations between poor sleep quality and anxiety symptoms across the three waves (before and during COVID-19) were statistically significant and vice versa. In contrast, only the cross-lagged effect of poor sleep quality at Wave 2 on depressive symptoms at Wave 3 was marginally significant. Considering the COVID-19 pandemic has been a global public health event, these study findings addressed that a proper surveillance system should be established in China to regularly assess adolescent mental health and behaviors. Schools and families should also be aware of the adverse effects of the COVID-19 pandemic, and positive education and intervention programs that cultivate adolescents' healthy sleep habits and positive emotion regulation are highly recommended to be developed to decrease risks of anxiety and depressive symptoms, especially among those with the current poor sleep quality.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Sun Yat-sen University, School of Public Health Institutional Review Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
LG and WW: full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, drafting of the manuscript, and statistical analysis. LG, WW, and CL: concept, design, administrative, technical, or material support. YG, XD, WL, and RW: acquisition, analysis, or interpretation of data. LG, WW, YG, XD, CL, RW, and WL: drafting of the manuscript. LG, WW, YG, and CL: supervision. All authors finally approve the submission of this paper.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81761128030 and 81903339) and Natural Science Foundation of Guangdong Province (Grant No. 2019A1515011091).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors like to thank all participating schools and students of the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.786640/full#supplementary-material
References
1. National Academies Of Sciences EAM, Division HAM, Education DOBA, Board On Children YAF, Applications COTN, Backes EP BRE. The Promise of Adolescence: Realizing Opportunity for All Youth. Washington, DC: National Academies Press (US). 2, Adolescent Development (2019). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK545476/
2. Erskine HE, Moffitt TE, Copeland WE, Costello EJ, Ferrari AJ, Patton G, et al. A heavy burden on young minds: the global burden of mental and substance use disorders in children and youth. Psychol Med. (2015) 45:1551–63. doi: 10.1017/S0033291714002888
3. Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev. (2014) 18:521–9. doi: 10.1016/j.smrv.2014.03.006
4. Tarokh L, Saletin JM, Carskadon MA. Sleep in adolescence: physiology, cognition and mental health. Neurosci Biobehav Rev. (2016) 70:182–8. doi: 10.1016/j.neubiorev.2016.08.008
5. Kuhlman KR, Chiang JJ, Bower JE, Irwin MR, Seeman TE, McCreath HE, et al. Sleep problems in adolescence are prospectively linked to later depressive symptoms via the cortisol awakening response. Dev Psychopathol. (2020) 32:997–1006. doi: 10.1017/S0954579419000762
6. Guo L, Deng J, He Y, Deng X, Huang J, Huang G, et al. Prevalence and correlates of sleep disturbance and depressive symptoms among Chinese adolescents: a cross-sectional survey study. BMJ Open. (2014) 4:e005517. doi: 10.1136/bmjopen-2014-005517
7. Alvaro PK, Roberts RM, Harris JK. The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med. (2014) 15:934–41. doi: 10.1016/j.sleep.2014.03.019
8. Galea S, Merchant RM, Lurie N. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention. JAMA Intern Med. (2020) 180:817–8. doi: 10.1001/jamainternmed.2020.1562
9. Wang C, Pan R, Wan X, Tan Y, Xu L, McIntyre RS, et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. (2020) 87:40–8. doi: 10.1016/j.bbi.2020.04.028
10. Chi X, Liang K, Chen ST, Huang Q, Huang L, Yu Q, et al. Mental health problems among Chinese adolescents during the COVID-19: the importance of nutrition and physical activity. Int J Clin Health Psychol. (2021) 21:100218. doi: 10.1016/j.ijchp.2020.100218
11. Cen X, Sun D, Rong M, Fekete G, Baker JS, Song Y, et al. The online education mode and reopening plans for Chinese schools during the COVID-19 pandemic: a mini review. Front Public Health. (2020) 8:566316. doi: 10.3389/fpubh.2020.566316
12. Zhou SJ, Wang LL, Yang R, Yang XJ, Zhang LG, Guo ZC, et al. Sleep problems among Chinese adolescents and young adults during the coronavirus-2019 pandemic. Sleep Med. (2020) 74:39–47. doi: 10.1016/j.sleep.2020.06.001
13. Zhang C, Ye M, Fu Y, Yang M, Luo F, Yuan J, et al. The psychological impact of the COVID-19 pandemic on teenagers in China. J Adolesc Health. (2020) 67:747–55. doi: 10.1016/j.jadohealth.2020.08.026
14. Wang G, Zhang Y, Zhao J, Zhang J, Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet. (2020) 395:945–7. doi: 10.1016/S0140-6736(20)30547-X
15. Liu S, Yang L, Zhang C, Xiang YT, Liu Z, Hu S, et al. Online mental health services in China during the COVID-19 outbreak. Lancet Psychiat. (2020) 7:e17–8. doi: 10.1016/S2215-0366(20)30077-8
16. National Health Commission of the People's Republic of China. Guideline for psychological crisis intervention during 2019-nCoV (2020). Available online at: http://www.nhc.gov.cn/jkj/s3577/202001/6adc08b966594253b2b791be5c3b9467.shtml
17. Masten AS, Cicchetti D. Developmental cascades. Dev Psychopathol. (2010) 22:491–5. doi: 10.1017/S0954579410000222
18. Wang W, Du X, Guo Y, Li W, Teopiz KM, Shi J, et al. The associations between sleep situations and mental health among Chinese adolescents: a longitudinal study. Sleep Med. (2021) 82:71–7. doi: 10.1016/j.sleep.2021.03.009
19. Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
20. Qi M, Zhou SJ, Guo ZC, Zhang LG, Min HJ, Li XM, et al. The effect of social support on mental health in Chinese adolescents during the outbreak of COVID-19. J Adolesc Health. (2020) 67:514–8. doi: 10.1016/j.jadohealth.2020.07.001
21. Sun J, Liang K, Chi X, Chen S. Psychometric properties of the generalized anxiety disorder scale-7 item (GAD-7) in a large sample of Chinese adolescents. Healthcare. (2021) 9:1709. doi: 10.3390/healthcare9121709
22. Cheng CP, Yen CF, Ko CH, Yen JY. Factor structure of the center for epidemiologic studies depression scale in Taiwanese adolescents. Compr Psychiatry. (2012) 53:299–307. doi: 10.1016/j.comppsych.2011.04.056
23. Lee SW, Stewart SM, Byrne BM, Wong JP, Ho SY, Lee PW, et al. Factor structure of the center for epidemiological studies depression scale in Hong Kong adolescents. J Pers Assess. (2008) 90:175–84. doi: 10.1080/00223890701845385
24. Radloff LS. The use of the center for epidemiologic studies depression scale in adolescents and young adults. J Youth Adolesc. (1991) 20:149–66. doi: 10.1007/BF01537606
25. Tsai PS, Wang SY, Wang MY, Su CT, Yang TT, Huang CJ, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
26. Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. (2004) 7:127–50. doi: 10.1177/1094428104263672
27. Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electron J Bus Res Methods. (2008) 1:53–60. doi: 10.21427/D7CF7R
28. Zhang L, Zhang D, Fang J, Wan Y, Tao F, Sun Y. Assessment of mental health of Chinese primary school students before and after school closing and opening during the COVID-19 pandemic. JAMA Netw Open. (2020) 3:e2021482. doi: 10.1001/jamanetworkopen.2020.21482
29. O'Connor RC, Wetherall K, Cleare S, McClelland H, Melson AJ, Niedzwiedz CL, et al. Mental health and well-being during the COVID-19 pandemic: longitudinal analyses of adults in the UK COVID-19 mental health & wellbeing study. Br J Psychiatry. (2020) 1–8. doi: 10.31234/osf.io/r8cdw
30. Genta FD, Rodrigues Neto GB, Velletri Sunfeld JP, Porto JF, Xavier AD, Moreno CRC, et al. COVID-19 pandemic impact on sleep habits, chronotype and health-related quality of life among high school students: a longitudinal study. J Clin Sleep Med. (2021) 17:1371–77. doi: 10.5664/jcsm.9196
31. Alfonsi V, Gorgoni M, Scarpelli S, Zivi P, Sdoia S, Mari E, et al. COVID-19 lockdown and poor sleep quality: not the whole story. J Sleep Res. (2021) 30:e13368. doi: 10.1111/jsr.13368
32. Leggett A, Assari S, Burgard S, Zivin K. The effect of sleep disturbance on the association between chronic medical conditions and depressive symptoms over time. Longit Life Course Stud. (2017) 8:138–51. doi: 10.14301/llcs.v8i2.433
33. Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. (2008) 10:473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen
34. Kelly RJ, El-Sheikh M. Reciprocal relations between children's sleep and their adjustment over time. Dev Psychol. (2014) 50:1137–47. doi: 10.1037/a0034501
35. Roberts RE, Duong HT. Is there an association between short sleep duration and adolescent anxiety disorders? Sleep Med. (2017) 30:82–7. doi: 10.1016/j.sleep.2016.02.007
36. Shanahan L, Copeland WE, Angold A, Bondy CL, Costello EJ. Sleep problems predict and are predicted by generalized anxiety/depression and oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. (2014) 53:550–8. doi: 10.1016/j.jaac.2013.12.029
37. Willis TA, Gregory AM. Anxiety disorders and sleep in children and adolescents. Sleep Med Clin. (2015) 10:125–31. doi: 10.1016/j.jsmc.2015.02.002
38. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. (2019) 23:2324–32. doi: 10.1111/jcmm.14170
39. Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annu Rev Clin Psychol. (2014) 10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716
Keywords: mental health, adolescent, longitudinal study, COVID-19, sleep problems
Citation: Wang W, Guo Y, Du X, Li W, Wu R, Guo L and Lu C (2022) Associations Between Poor Sleep Quality, Anxiety Symptoms, and Depressive Symptoms Among Chinese Adolescents Before and During COVID-19: A Longitudinal Study. Front. Psychiatry 12:786640. doi: 10.3389/fpsyt.2021.786640
Received: 30 September 2021; Accepted: 22 December 2021;
Published: 14 January 2022.
Edited by:
Miao Qu, Capital Medical University, ChinaReviewed by:
Luigi De Gennaro, Sapienza University of Rome, ItalyMei Hong Xiu, Peking University, China
Copyright © 2022 Wang, Guo, Du, Li, Wu, Guo and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Guo, Z3VvbGFuM0BtYWlsLnN5c3UuZWR1LmNu
 Wanxin Wang1,2
Wanxin Wang1,2 Lan Guo
Lan Guo Ciyong Lu
Ciyong Lu