
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 07 February 2022
Sec. Mood Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.764656
This article is part of the Research Topic Insights in Mood and Anxiety Disorders: 2021 View all 18 articles
Background: Mental health problems after acute ischemic stroke (AIS) have caused wide public concerns, and the study on early identification of these disorders is still an open issue. This study aims to investigate the predictive effect of circulating neurofilament light (NfL) on long-term mental health status of AIS patients.
Methods: This study collected demographic information and mental health measurements from 304 AIS patients from May 1, 2016 to Dec 31, 2019. Baseline serum neurofilament light (NfL) was determined within 2 h since patient admission. Six months after AIS onset, the degree of symptoms of depression, anxiety, and insomnia was assessed by the Chinese versions of the 9-item Patient Health Questionnaire (PHQ-9), the 7-item Generalized Anxiety Disorder scale (GAD-7), the 7-item Insomnia Severity Index (ISI), respectively. Subjects were divided into the high NfL group and the low NfL group. Multivariate logistic regression analysis was performed to identify factors associated with these mental health problems.
Results: The high NfL group had significantly higher PHQ-9, GAD-7, and ISI scores than the low NfL group. The prediction of serum NfL for major depression generated a sensitivity of 70.27%, a specificity of 67.79% and an AUC of 0.694. The prediction of serum NfL for anxiety generated a sensitivity of 69.23%, a specificity of 64.02%, and an AUC of 0.683. The prediction of serum NfL for insomnia generated a sensitivity of 75.00%, a specificity of 66.43% and an AUC of 0.723. Higher serum NfL was a risk factor of post-AIS depression [ORs (95% CI): 4.427 (1.918, 10.217)], anxiety [ORs (95% CI): 3.063 (1.939, 6.692)], and insomnia [ORs (95% CI): 4.200 (1.526, 11.562)].
Conclusions: These findings imply that circulating NfL might be a potential biomarker of long-term mental health problems after AIS.
Acute ischemic stroke (AIS) is among the leading causes of death and disability worldwide (1). Except for neurological deficits, AIS patients also experience a variety of mental health problems, such as anxiety (2), depression (3), and insomnia (4) during the rehabilitation of the disease. Although these post-AIS consequences do not directly cause death or disability, they are closely related to the quality of life after stroke. Therefore, early identification of patients with risk of developing neuropsychological disorders is of significance for timely intervention to improve the mental health outcomes. Recent studies have identified a panel of blood-based biomarkers that is associated with stroke severity and prognosis (5). However, few convenient and effective biomarkers are available to evaluate the risk of neuropsychological problems after stroke.
Neurofilaments, including neurofilament light (NfL), neurofilament medium (NfM), and neurofilament heavy (NfH) are components of the neuronal cytoskeleton. Together with NfH and NfM, NfL represents one of the scaffolding proteins of the neuronal cytoskeleton and is released into the extracellular space following neuronal damage (6). NfL is increased in multiple neurological diseases, such as Alzheimer's disease (7), Parkinson's disease (8), and multiple sclerosis (9). It is also a well-validated prognostic biomarker of functional outcomes of AIS (10). But it is not clear yet whether NfL could predict mental health outcomes of this disease. This study aims to investigate the association between circulating NfL and mental health outcomes of AIS, including major depression, anxiety, and insomnia.
This study used the same cohort of patients in our previous study (11). Briefly, inpatients with AIS from the Department of Neurology, Sichuan Provincial People's Hospital and Ya'an People's Hospital during May 1, 2016 and Dec 31, 2019, were screened for eligibility for this study. Patients were excluded if they have one of the following conditions: (1) Have previously diagnosed depression, anxiety, and insomnia before AIS onset; (2) have other psychological disorders, such as schizophrenia and bipolar disorders et al. before AIS onset; (3) Cannot complete psychological tests due to hearing, language, or communicating disabilities; (4) Other severe neurological diseases which may affect circulating NfL levels, such as Parkinson's disease, Alzheimer's disease, and traumatic brain injury; (5) Refused to participate in this study. Written informed consent for participation was obtained from patients or their legal relatives. This study conformed with the principles of the Declaration of Helsinki and was approved by the investigational review board of the Sichuan Provincial People's Hospital.
The demographic information, including age, sex, education level, body mass index (BMI), smoking history, medical history, including oral anticoagulion or antiplatelet drug use, comorbidities such as hypertension, diabetes mellitus, hypercholesteremia, and arial fibrillation were collected from the medical records. AIS was diagnosed according to the World Health Organization (WHO) Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (WHO-MONICA) criteria and was verified by magnetic resonance imaging (MRI) performed within 24 h since symptom onset. The neurological deficits of patients were examined with the National Institutes of Health Stroke Scale (NIHSS) upon admission (12), performed by a certified stroke neurologist. AIS subtype was determined with the TOAST criteria.
We focused on symptoms of depression, anxiety, and insomnia for all participants 6 months after AIS onset, using Chinese versions of validated measurement tools. Accordingly, the 9-item Patient Health Questionnaire (PHQ-9; range, 0–27) (13), the 7-item Generalized Anxiety Disorder (GAD-7) scale (range, 0–21) (14), the 7-item Insomnia Severity Index (ISI; range, 0–28) (15), were used to assess the severity of symptoms of depression, anxiety, and insomnia, respectively. The total scores of these measurement tools were interpreted as follows: PHQ-9, normal (0–4), mild (5–9), moderate (10–14), and severe (15–27) depression; GAD-7, normal (0–4), mild (5–9), moderate (10–14), and severe (15–21) anxiety; ISI, normal (0–7), subthreshold (8–14), moderate (15–21), and severe (22–28) insomnia. These categories were based on cutoff values established in the literature (13–15). The cutoff value for detecting symptoms of major depression, anxiety, and insomnia distress were 10, 7, and 15, respectively. Participants with scores above the cutoff threshold were characterized as having major depression, anxiety and insomnia, respectively.
Blood was sampled within 2 h since admission, and serum was separated within 30 min after sampling and stored at −80°C until further analysis. Serum NfL was determined using the single-molecule (Simoa) array according to manufacturer's instructions (16). Each test was done in duplicates and the means of each test were used for statistical analysis. Monoclonal antibodies and purified bovine NFL were used as calibrators.
Continuous variables were tested for normality, and if they were normally distributed, an independent t-test was used, but if they were not normally distributed, a Mann-Whitney U-test was used. For categorical data, two-sample tests of proportions were used to compare proportions. Logistical regression models were utilized to investigate the association between serum NfL concentrations at baseline and mental health outcomes. We first fitted univariate models with a single candidate variable at one time. The potential risk factors as determined by a p-value < 0.2 were included in the final multivariate regression models. The receiver operating characteristic (ROC) curve analysis were utilized to test the predictive effects of baseline serum NfL on mental health outcomes at follow-up. Optimal sensitivity and specificity were determined via a non-parametric approach. The Youden index was calculated for the cutoff value to determine the cutoff value that maximized the discriminating power of the test. Statistical analyses were conducted using SPSS statistical package version 24 (IBM SPSS Statistics for Windows, Armonk, NY, USA) and a p-value < 0.05 was regarded as statistically significant.
We categorized the patients into two groups according to serum NfL concentrations, the high NfL group and the low NfL group. There was no significant difference in the mean age, median BMI, the frequency of family history of stroke, frequencies of antiplatelet drug use and anticoagulation drug use between the high NfL and low NfL group. Frequencies of hypertension, diabetes mellitus, hypercholesteremia and arial fibrillation between the high NfL and low NfL group were also not significantly different between groups. No significant difference was not observed in the distribution of infarction region and stroke etiology between the high and low NfL group. No significant difference was also not observed in the incidences of hemorrhagic transformation and recurrent AIS during follow-up between groups. The high NfL group had significantly higher incidences of PSD, PSA, and PSI than the low NfL group during follow-up (Table 1).
High NfL group had significantly higher PHQ-9, GAD-7, and ISI scores than low NfL group (Figure 1). Serum NfL concentrations were positively associated with PHQ-9, GAD-7, and ISI scores (Figure 2). Furthermore, serum NfL predicted major depression with a sensitivity of 70.27%, a specificity of 67.79% and an AUC of 0.694 (Figure 3A). Serum NfL predicted anxiety with a sensitivity of 69.23%, a specificity of 64.02% and an AUC of 0.683 (Figure 3B). Serum NfL predicted insomnia with a sensitivity of 75.00%, a specificity of 66.43% and an AUC of 0.723 (Figure 3C).
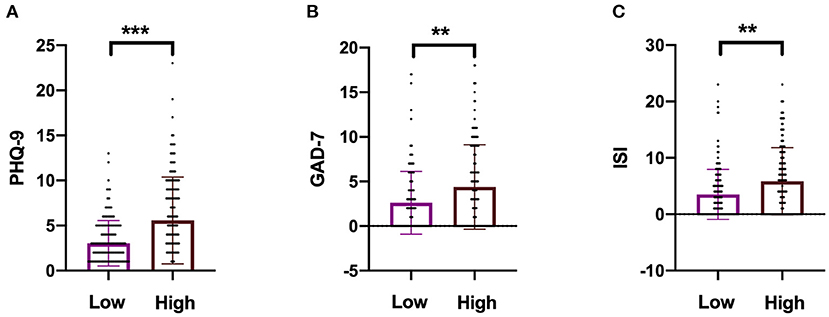
Figure 1. Comparison of the severity of depression, anxiety, and insomnia between high and low NfL group. (A) Comparison of PHQ-9 score between high and low NfL group. (B) Comparison of GAD-7 score between high and low NfL group. (C) Comparison of ISI score between high and low NfL group. PHQ-9, GAD-7, and ISI scales were used to determine the severity of depression, anxiety, and insomnia of subjects. Unimpaired t-test. **p < 0.01, ***p < 0.001.
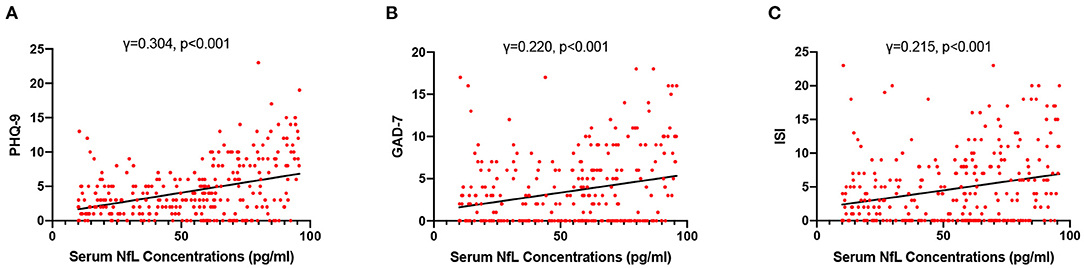
Figure 2. Association between serum NfL levels and post-AIS mental health outcomes. (A) Association between serum NfL concentrations and PHQ-9 score. (B) Association between serum NfL concentrations and GDA-7 score. (C) Association between serum NfL concentrations and ISS score. Spearman correlation analysis.
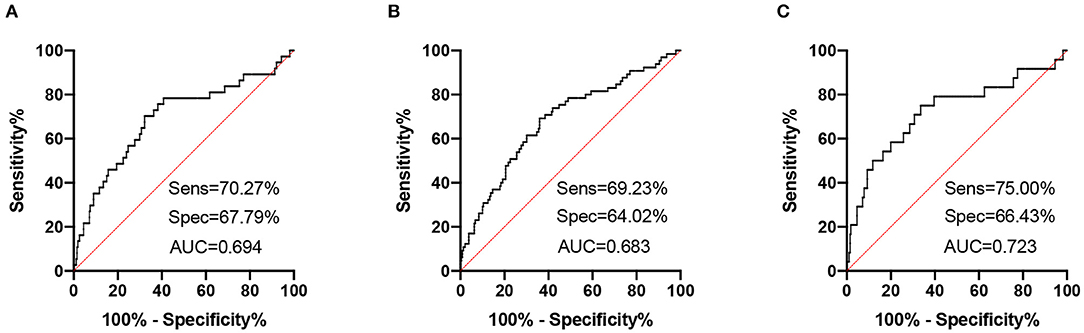
Figure 3. Receiver operating characteristic (ROC) curves of serum NfL for post-AIS mental health outcomes. (A) ROC curve of serum NfL and PHQ-9 score. (B) ROC curve of serum NfL and GAD-7 score. (C) ROC curve of serum NfL and ISI score.
We utilized three logistic regression models to investigate risk factors of major depression, anxiety and insomnia. In univariate analyses, family history of stroke [ORs (95% CI): 3.053 (1.021, 9.125)], DWI hyperintensity volume [ORs (95% CI): 1.037 (0.998, 1.077)], cerebral lobe infarction [ORs (95% CI): 1.984 (0.894, 4.402)], and high NfL level [ORs (95% CI): 4.244 (1.871, 9.625)] were found to be potential risk factors of major depression. Male sex was found to be a protective factor against major depression [ORs (95% CI): 0.526 (0.262, 1.053)]. However, only high NfL level remained to be a significant risk factor of major depression [ORs (95% CI): 4.427 (1.918, 10.217)] in the final multivariate model (Table 2).
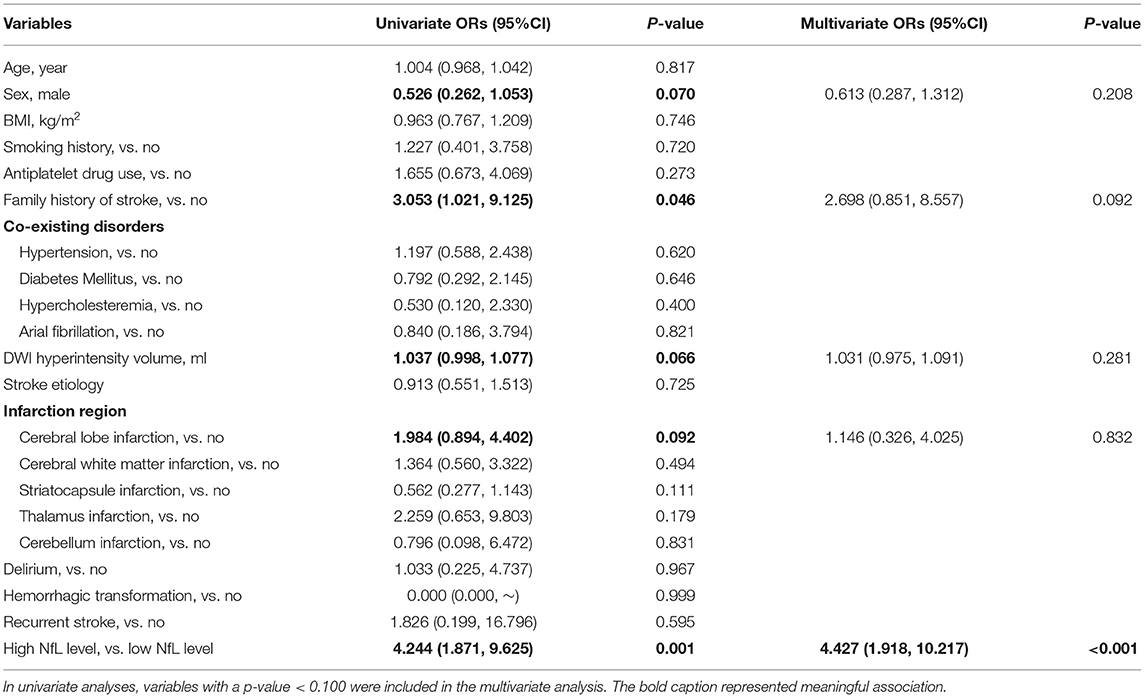
Table 2. A logistic regression model to evaluate the association between serum NfL and post-stroke major depression.
In univariate analyses, family history of stroke [ORs (95% CI): 2.502 (0.929, 6.736)], thalamus infarction [ORs (95% CI): 2.762 (0.847, 9.008)], and high NfL level [ORs (95% CI): 3.665 (1.993, 6.742)] were found to be potential risk factors of anxiety. Male sex was found to be a protective factor against anxiety [ORs (95% CI): 0.522 (0.300, 0.908)]. However, only high NfL level [ORs (95% CI): 3.063 (1.939, 6.692)] remained to be a significant risk factor of anxiety and male sex [ORs (95% CI): 0.514 (0.288, 0.917)] remained to be a protective factor against that in the final multivariate model (Supplementary Table 1).
In the analysis of risk factors of insomnia, only high NfL level [ORs (95% CI): 4.200 (1.526, 11.562)] was found to be a risk factor in both univariate and multivariate analyses. Collectively, these findings indicate that high serum NfL level might be a potential risk factor of major depression, anxiety, and insomnia (Supplementary Table 2).
In the present study, we investigated the associations between circulating NfL levels and mental health outcomes of AIS. We found that the incidences of mental health disorders after AIS, including anxiety, depression and insomnia, were significantly higher in the high NfL group in comparison with the low NfL group. ROC analyses found that serum NfL had a relatively high accuracy of predicting the occurrence of major depression, anxiety and insomnia. Furthermore, regression models identified high NfL level as a risk factor of these mental health disorders.
Post-stroke mental health disorders, including PSD (17), PSA (18), and PSI (19), are harmful to the quality of life in patients with AIS. Furthermore, these neuropsychological consequences of AIS have adverse effects on functional improvement of AIS. Therefore, early identification of patients at risk of developing these disorders is essential for timely intervention to achieve better prognosis. However, currently no reliable biomarker is available to predict the mental health outcomes of AIS.
Mounting evidence has demonstrated the association between circulating NfL levels and functional outcomes of AIS (10, 20). NfL is also suggested to be a reliable biomarker predicting post-stroke cognitive impairment (11, 21, 22). Mental health disorders, including depression, anxiety and insomnia, are commonly observed during the rehabilitation of AIS (23–25). The prevalence of PSD, PSA, and PSI was 12.17, 21.38, and 7.89%, respectively in this study, which is comparable to other reports in the Chinese population (2, 26). Therefore, in this study, we investigated the predictive value of circulating NfL for the 6-month neuropsychological outcomes of AIS.
We categorized the AIS patients into two subgroups according to serum NfL level, and it is interesting to see that high NfL group had substantially higher incidences of PSD, PSA, and PSI than low NfL group. Circulating NfL could predict post-AIS mental health disorders with a relatively high accuracy. In a recent study, it is demonstrated that elevated level of circulating NfL is associated with an increased risk of depression 3 months after stroke onset (27), which is consistent with our present findings. Although patients with anxiety (28) and insomnia (29) are found to have increased NfL levels, the association of circulating NfL with these disorders in AIS has not been reported yet.
The mechanisms underlying the associations between NfL and post-stroke mental health disorders could be multifactual and have not been thoroughly illustrated. NfL is also found to be increased in other psychiatric disorders, such as bipolar disorders (30), chronic insomnia disorder (29), and depression not due to stroke (31). These findings suggest that mental health disorders might contribute to neuronal damage, thus promoting the increase of NfL. In return, it is not clear why AIS patients with high circulating NfL levels are more prone to develop these mental illnesses. But we could speculate that mental health disorders are associated with the severity of neuroaxonal damage, as reflected by NfL levels. We propose that the disruption of the equilibrium of neurotransmitters induced by ischemic attack may induce neuroaxonal damage and promote might contribute to post-AIS mental health disorders (32).
This study has several limitations. First, this is a simple correlation analysis with a relatively small sample size, further large-scale investigations are needed to confirm the present findings. Second, only baseline NfL levels were determined, thus the association between the dynamic change of NfL and neuropsychological outcomes of AIS patients has not been illustrated. But in conclusion, this study found NfL as a potential biomarker of post-stroke mental health disorder, including depression, anxiety and insomnia. Furthermore, the median age of participants in this study is more than 60, thus it is unclear whether NfL could predict long-term mental health disorders in young AIS patients. Patients with increased NfL levels should be intensively monitored for delayed neuropsychological disorders.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Investigational Review Board of the Sichuan Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
J-HW designed the study and drafted the manuscript. D-ZW and F-QG collected the samples and patients' information. D-ZW, SY, and N-WY participated in the determination of NfL. D-ZW and N-WY conducted the statistical analysis. GL and WJ contributed to the revision of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all participants for their kindly participation in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.764656/full#supplementary-material
1. Gorelick PB. The global burden of stroke: persistent and disabling. Lancet Neurol. (2019) 18:417–8. doi: 10.1016/S1474-4422(19)30030-4
2. Li W, Xiao WM, Chen YK, Qu JF, Liu YL, Fang XW, et al. Anxiety in patients with acute ischemic stroke: risk factors and effects on functional status. Front Psychiatry. (2019) 10:257. doi: 10.3389/fpsyt.2019.00257
3. Mayman N, Stein LK, Erdman J, Kornspun A, Tuhrim S, Jette N, et al. Risk and predictors of depression following acute ischemic stroke in the elderly. Neurology. (2021) 96:e2184–91. doi: 10.1212/WNL.0000000000011828
4. Chen YK, Lu JY, Mok VC, Ungvari GS, Chu WC, Wong KS, et al. Clinical and radiologic correlates of insomnia symptoms in ischemic stroke patients. Int J Geriatr Psychiatry. (2011) 26:451–7. doi: 10.1002/gps.2547
5. Tiedt S, Brandmaier S, Kollmeier H, Duering M, Artati A, Adamski J, et al. Circulating metabolites differentiate acute ischemic stroke from stroke mimics. Ann Neurol. (2020) 88:736–46. doi: 10.1002/ana.25859
6. Yuan A, Rao MV, Veeranna V, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol. (2017) 9:a018309. doi: 10.1101/cshperspect.a018309
7. Quiroz YT, Zetterberg H, Reiman EM, Chen Y, Su Y, Fox-Fuller JT, et al. Plasma neurofilament light chain in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional and longitudinal cohort study. Lancet Neurol. (2020) 19:513–21. doi: 10.1016/S1474-4422(20)30137-X
8. Olsson B, Portelius E, Cullen NC, Sandelius A, Zetterberg H, Andreasson U, et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. J Am Med Assoc Neurol. (2019) 76:318–25. doi: 10.1001/jamaneurol.2018.3746
9. Kapoor R, Smith KE, Allegretta M, Arnold DL, Carroll W, Comabella M, et al. Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology. (2020) 95:436–44. doi: 10.1212/WNL.0000000000010346
10. Gendron TF, Badi MK, Heckman MG, Jansen-West KR, Vilanilam GK, Johnson PW, et al. Plasma neurofilament light predicts mortality in patients with stroke. Sci Transl Med. (2020) 12:aay1913. doi: 10.1126/scitranslmed.aay1913
11. Wang JH, Huang J, Guo FQ, Wang F, Yang S, Yu NW, et al. Circulating neurofilament light predicts cognitive decline in patients with post-stroke subjective cognitive impairment. Front Aging Neurosci. (2021) 13:665981. doi: 10.3389/fnagi.2021.665981
12. Goldstein LB, Samsa GP. Reliability of the national institutes of health stroke scale. Extension to non-neurologists in the context of a clinical trial. Stroke. (1997) 28:307–10. doi: 10.1161/01.STR.28.2.307
13. Zhang YL, Liang W, Chen ZM, Zhang HM, Zhang JH, Weng XQ, et al. Validity and reliability of patient health questionnaire-9 and patient health questionnaire-2 to screen for depression among college students in China. Asia Pac Psychiatry. (2013) 5:268–75. doi: 10.1111/appy.12103
14. He LC, Qian J, Cui H, Wu W. Reliability and validity of a generalized anxiety scale in general hospital outpatients. Shanghai Arch Psychiatry. (2010) 22:200–3. doi: 10.3969/j.issn.1002-0829.2010.04.002
15. Yu DS. Insomnia Severity Index: psychometric properties with Chinese community-dwelling older people. J Adv Nurs. (2010) 66:2350–9. doi: 10.1111/j.1365-2648.2010.05394.x
16. Li QF, Dong Y, Yang L, Xie JJ, Ma Y, Du YC, et al. Neurofilament light chain is a promising serum biomarker in spinocerebellar ataxia type 3. Mol Neurodegener. (2019) 14:39. doi: 10.1186/s13024-019-0338-0
17. Guo J, Wang J, Sun W, Liu X. The advances of post-stroke depression: 2021 update. J Neurol. (2021) 2021:4. doi: 10.1007/s00415-021-10597-4
18. Wang J, Zhao D, Lin M, Huang X, Shang X. Post-stroke anxiety analysis via machine learning methods. Front Aging Neurosci. (2021) 13:657937. doi: 10.3389/fnagi.2021.657937
19. Cai H, Wang XP, Yang GY. Sleep disorders in stroke: an update on management. Aging Dis. (2021) 12:570–85. doi: 10.14336/AD.2020.0707
20. Uphaus T, Bittner S, Groschel S, Steffen F, Muthuraman M, Wasser K, et al. NfL (neurofilament light chain) levels as a predictive marker for long-term outcome after ischemic stroke. Stroke. (2019) 50:3077–84. doi: 10.1161/STROKEAHA.119.026410
21. Peng Y, Li Q, Qin L, He Y, Luo X, Lan Y, et al. Combination of serum neurofilament light chain levels and MRI markers to predict cognitive function in ischemic stroke. Neurorehabil Neural Repair. (2021) 35:247–55. doi: 10.1177/1545968321989354
22. Wang Z, Wang R, Li Y, Li M, Zhang Y, Jiang L, et al. Plasma neurofilament light chain as a predictive biomarker for post-stroke cognitive impairment: a prospective cohort study. Front Aging Neurosci. (2021) 13:631738. doi: 10.3389/fnagi.2021.631738
23. Leppavuori A, Pohjasvaara T, Vataja R, Kaste M, Erkinjuntti T. Insomnia in ischemic stroke patients. Cerebrovasc Dis. (2002) 14:90–7. doi: 10.1159/000064737
24. Dollenberg A, Moeller S, Lucke C, Wang R, Lam AP, Philipsen A, et al. Prevalence and influencing factors of chronic post-traumatic stress disorder in patients with myocardial infarction, transient ischemic attack (TIA) and stroke - an exploratory, descriptive study. BMC Psychiatry. (2021) 21:295. doi: 10.1186/s12888-021-03303-1
25. Mayman NA, Tuhrim S, Jette N, Dhamoon MS, Stein LK. Sex differences in post-stroke depression in the elderly. J Stroke Cerebrovasc Dis. (2021) 30:105948. doi: 10.1016/j.jstrokecerebrovasdis.2021.105948
26. Liang Y, Chan YL, Deng M, Chen YK, Mok V, Wang F, et al. Enlarged perivascular spaces in the centrum semiovale are associated with poststroke depression: a 3-month prospective study. J Affect Disord. (2018) 228:166–72. doi: 10.1016/j.jad.2017.11.080
27. Zhao H, Mo M, Miao C, Li L, Yang H, Liu Y, et al. Association of serum biomarker neurofilament light concentration with post-stroke depression: a preliminary study. Gen Hosp Psychiatry. (2020) 64:17–25. doi: 10.1016/j.genhosppsych.2020.01.006
28. Tauil CB, Rocha-Lima AD, Ferrari BB, Silva FMD, Machado LA, Ramari C, et al. Depression and anxiety disorders in patients with multiple sclerosis: association with neurodegeneration and neurofilaments. Braz J Med Biol Res. (2021) 54:e10428. doi: 10.1590/1414-431x202010428
29. Ren CY, Liu PP, Li J, Li YQ, Zhang LJ, Chen GH, et al. Changes in telomere length and serum neurofilament light chain levels in female patients with chronic insomnia disorder. J Clin Sleep Med. (2021) 2021:9574. doi: 10.5664/jcsm.9574
30. Jakobsson J, Bjerke M, Ekman CJ, Sellgren C, Johansson AG, Zetterberg H, et al. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology. (2014) 39:2349–56. doi: 10.1038/npp.2014.81
31. Bavato F, Cathomas F, Klaus F, Gutter K, Barro C, Maceski A, et al. Altered neuroaxonal integrity in schizophrenia and major depressive disorder assessed with neurofilament light chain in serum. J Psychiatr Res. (2021) 140:141–8. doi: 10.1016/j.jpsychires.2021.05.072
Keywords: post-stroke, neurofilament light, depression, anxiety, insomnia
Citation: Wang D-Z, Guo F-Q, Guo L, Yang S, Yu N-W, Wang J and Wang J-H (2022) Serum Neurofilament Light Predicts 6-Month Mental Health Outcomes in a Cohort of Patients With Acute Ischemic Stroke. Front. Psychiatry 12:764656. doi: 10.3389/fpsyt.2021.764656
Received: 25 August 2021; Accepted: 10 December 2021;
Published: 07 February 2022.
Edited by:
Marco Grados, Johns Hopkins University, United StatesReviewed by:
Ye-Ran Wang, Third Military Medical University, ChinaCopyright © 2022 Wang, Guo, Guo, Yang, Yu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Hong Wang, amh3YW5nbmV1cm9sb2d5QHllYWgubmV0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.