
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 11 November 2021
Sec. Sleep Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.754921
Background: Insomnia is a common clinical manifestation in patients with depression. Insomnia is not only a depression symptom but also an independent risk factor for recurrence. Cordyceps militaris (C. militaris) is thought to have the potential to treat insomnia. This study aimed to examine the efficacy and safety of duloxetine with C. militaris in improving sleep symptoms in patients with depression.
Methods: This study was a single-center, randomized, double-blind, placebo-controlled study that recruited outpatients admitted to Beijing Anding hospital from January 2018 to January 2019. Major depressive disorder (MDD) with insomnia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria and Mini-International Neuropsychiatric Interview (M.I.N.I.). Eligible subjects will be randomly assigned to two treatment groups in a 1:1 ratio, and receive treatment and follow-up of about 6 weeks of duloxetine plus Cordyceps militaris or placebo, respectively. The severity of depression and insomnia was evaluated at baseline and at 1, 2, 4, and 6 weeks using the 17-item Hamilton Depression Scale (HAMD-17) and Athens Insomnia Scale (AIS).
Results: A total of 59 subjects were included in the study (31 in the placebo group and 28 in the C. militaris group). 11 (18.6%) participants withdrew during the study period, 5 (17.9%) in the C. militaris group, and 6 (19.3%) in the placebo group. Depressive and sleep symptoms in all patients reduced over time. We found that the total scores of AIS and its subscales decreased more in the placebo group compared to the C. militaris group (p < 0.05). Secondary outcome revealed that there were no significant differences between the two groups in total HAMD-17 and its sleep factor scores (p > 0.05) at 1, 2, 4, and 6 weeks after treatment initiation. The incidences of adverse events were not significantly different between the two groups (all p > 0.05).
Conclusion: C. militaris at the current dose and duration did not improve sleep symptoms in patients with depression, but it is safe with rare side effects.
Major depressive disorder (MDD) is a common chronic psychiatric disorder with high morbidity, disability, and recurrence (1) that is projected to be one of the leading worldwide causes of disability by 2030 (2). Previous studies have shown that the balance between glutamate and γ-aminobutyric acid (GABA) is becoming increasingly relevant in the field of depression, thus α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor activation is recently considered as one of the most promising approaches for antidepressant therapies (3).
Accumulating evidence suggests that ~90% of patients with depression experience insomnia (4), which is considered not only a symptom of depression but also a significant predictor of depression (5). Although depressive symptoms improve after treatment (6–8). Insomnia often remains and predicts a shorter remission and increased risk of relapse (9). Clinically, depression is frequently accompanied by sleep disorders, which often seriously affect cognitive level, social function, and depression recovery.
Currently, there are two approaches to clinical treatment of sleep disorders, pharmacological and non-pharmacological. Pharmacological treatments of insomnia mainly include benzodiazepines, non-benzodiazepines, and sedating antidepressants (10). However, long-term use of these drugs has undesirable adverse effects, such as headache, forgetfulness, fatigue, dependence, changes in sleep structure, and hangover (11). In addition, antidepressant-induced insomnia can occur and delay recovery from MDD (12). Non-pharmacological therapy mainly consists of cognitive behavioral therapy (13). Its use is supported by evidence-based medicine and recommended by treatment guidelines. However, cognitive behavioral therapy resources are relatively scarce in China and it is underutilized. Other non-pharmacological therapies include diet therapy, aromatherapy, massage, homeopathy, and light therapy but clinical evidence supporting their use is lacking (14). Although clinical studies have shown that acupuncture is effective in treating insomnia (15), the studies were not rigorous and should be treated with caution (16). Biofeedback is another alternative insomnia treatment that has been used for many years (17). Recently, both drug therapy and non-drug therapy have some problems for depression with insomnia, including side effects and poor efficacy, resulting in unsatisfactory therapeutic effect. Therefore, novel and effective approaches to insomnia treatment in MDD with fewer adverse effects are needed.
Recently, sleep mechanism studies have found that the gradual accumulation of endogenous sleep-promoting factors during wakefulness form the basis of sleep (18). Further investigations have suggested that adenosine is a significant endogenous sleep-promoting substance (19–21). Systemic administration of adenosine analogs or inhibitors of its metabolism increase non-rapid eye movement (NREM) sleep in rats (22). Adenosine is a neuromodulator formed by hydrolysis of adenosine monophosphate (AMP) or S-adenosylhomocysteine (23). It promotes sleep by reacting with one of the four types of adenosine receptors, A1, A2A, A2B, and A3 (24). Melling et al. screened plant materials associated with adenosine receptor activity to identify supplements that improve sleep quality and found that Cordyceps militaris (C. militaris) contained cordycepin (3′-deoxyadenosine), an important bioactive constituent and naturally occurring adenosine analog that has been used in traditional medicine for treating insomnia for hundreds of years (25). Its long history of use alone provides good evidence for its safety. In 2009, the Ministry of Health of the People's Republic of China issued a notice to consider C. militaris as a new food resource. In addition, recent studies have shown that cordycepin promotes NREM sleep in rats (26). However, there were no other studies with C. militaris in humans yet. This study aimed to evaluate the efficacy of cordycepin in improving insomnia in patients with depression.
This double-blind, randomized, placebo-controlled clinical trial was conducted from January 1, 2018 to February 1, 2019. Adult patients diagnosed with depression were randomly allocated to receive either 6 weeks of C. militaris or placebo. The study was approved by the institutional review board of the Beijing Anding Hospital, Capital Medical University and registered with Clinical-Trials.gov (number ChiCTR-INR-17014074).
All participants provided written informed consent. Patients who met the following criteria were eligible: (1) age 18–65 years; (2) diagnosis of MDD with insomnia according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria and Mini-International Neuropsychiatric Interview (M.I.N.I.) (27); (3) moderate or greater severity of symptoms as indicated by a score of ≥ 17 on the Hamilton Depression Scale (HAMD-17) (28); (4) Athens Insomnia Scale (AIS) score >6 (29); and (5) no antipsychotic treatment for at least 3 months.
Exclusion criteria included the following: (1) serious comorbid cardiovascular, neurological, or other unstable medical condition; (2) suicidal ideation or attempts or aggressive behavior; (3) hepatic and renal function testing and/or electrocardiogram (ECG) beyond the normal reference range; (4) history of alcoholism or drug abuse within the previous 6 months; (5) allergy to duloxetine or C. sinensis; and (6) pregnancy or lactation.
Eligible subjects were randomly assigned to 1 of 2 groups in a 1:1 ratio: group A, duloxetine combined with C. militaris; group B, duloxetine combined with placebo. Random codes were produced in advance by an independent researcher not involved in the study via computerized number generation. Intervention allocation was stored in an envelope that was opened for statistical analysis after the data collection period was completed. All study personnel, including subjects, drug dispensers, outcome assessors, data collectors, and analysts, were blinded to allocation throughout the study period.
A fixed dosing schedule was employed throughout the study (duloxetine capsule, 60 mg/day; C. militaris or placebo tablet, 3 g/day). The drug dosage was selected according to the approved drug specification. C. militaris or matching placebo was administered using a double-blind format, while duloxetine was administered open-label. All participants received 1 duloxetine capsule daily after breakfast. C. militaris or placebo was administered as three tablets daily after dinner. The concomitant use of antipsychotics, other antidepressants, Chinese medicine affecting the central nervous system, or melatonin was prohibited from the washout period to the end of treatment. If necessary, medication for insomnia was only permitted after 2 weeks from the beginning of the trial and was only allowed for use <1 week.
Treatment outcome was evaluated at baseline and weeks 1, 2, 4, and 6. The AIS is a validated 8-item self-report questionnaire that assesses insomnia symptom. It has been shown to have appropriate diagnostic utility, including a set of items for assessing nocturnal sleep disturbance and daytime dysfunction. It is a useful instrument to screening insomnia, and the diagnostic accuracy of this scale (sensitivity and specificity) was high. A sum score is calculated (range: 0–24), with lower scores indicating fewer insomnia symptoms (29). The primary outcome was mean change in total AIS score and its subscales over the treatment period with day 1 as baseline. The HAMD-17 was used to assess the severity of depressive symptoms in the past week. The Chinese version of the HAMD-17 has been validated in Chinese populations and has good psychometric characteristics. The scale contains 17 variables and each item ranged from 0 to 2 or 0 to 4. A sum score is calculated (range: 0–52), with higher scores indicating more severe depressive symptoms (28). Secondary outcome was change in total HAMD-17 score and its sleep factor score.
Safety evaluation included physical examination and assessment of vital signs and adverse events at each follow-up visit. Adverse events were recorded, including date and time of onset, duration, severity, relationship to intervention, and action taken. Clinical laboratory tests and 12-lead ECGs were performed at screening and at the endpoint.
A total of 59 subjects were included in the study. Under the repeated measurement design, 80% power with a type I error of 0.05, the lowest effect size difference between the two groups was found (effect size = 0.3).
Efficacy analyses were performed on the intention-to-treat population who completed baseline and at least one evaluation after treatment. Linear mixed-effect models were used to compare differences in treatment outcome between the two groups. The model was established using time and group as categorical fixed factors and random intercepts. Baseline scores were included as covariates. An autoregressive covariance structure that produced the smallest Bayesian Information Criterion was applied. The safety analysis population included all randomized participants who took at least one dose of study medication. Categorical variables were analyzed using the Chi-square test. The Tukey test was used to detect differences in continuous variables between the two groups. Two-tailed p < 0.05 was considered significant. All analyses were conducted using SPSS software version 22.0.
A total of 61 participants were recruited and randomized, among which 2 subject who did not meet the inclusion criteria were excluded. So, there were 59 patients included in this study (31 in the placebo group and 28 in the C. militaris group). The study flowchart is shown in Figure 1. Patient characteristics were well-balanced between the two groups (Table 1). 11 (18.6%) participants withdrew during the study period, 5 (17.9%) in the C. militaris group, and 6 (19.3%) in the placebo group. Withdrawal reasons are provided in Figure 1.
Changes in overall AIS score and subscale scores are illustrated in Table 2 and Figure 2A. The linear mixed-effect model showed significant slope differences between the two groups for total AIS score (F = 6.22, p < 0.05), awakenings during the night (F = 16.02, p < 0.001), final awakening (F = 9.42, p < 0.05), total sleep duration (F = 6.87, p < 0.01), sleep quality (F = 9.70, p < 0.05), and functioning capacity during the day (F = 10.06, p < 0.01). Between-group comparisons showed a significantly greater reduction in AIS score in the placebo group compared to the C. militaris group (p < 0.05). The AIS score markedly decreased over the course of treatment in each group (p < 0.01).
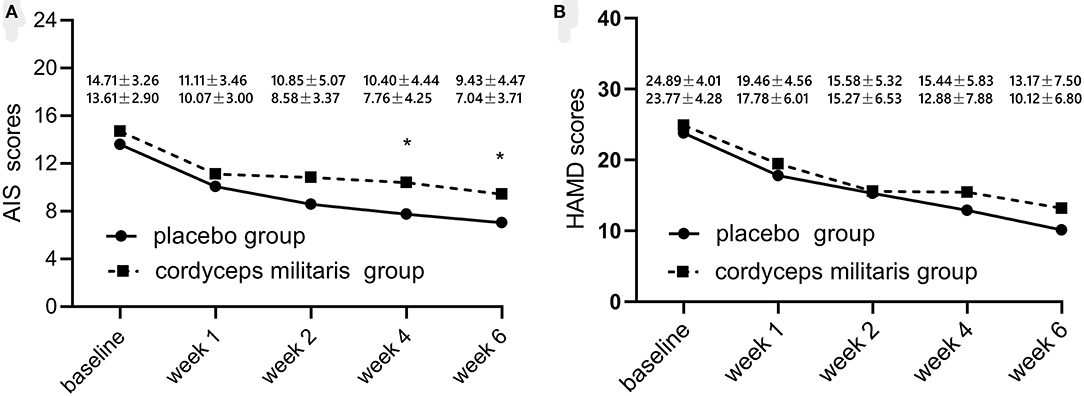
Figure 2. Changes in sleep quality (A) and HAMD score (B) with treatment from baseline to 6 weeks after treatment initiation. The placebo group is represented by the dotted line and the C. militaris group is represented by the solid line. *p < 0.05.
Table 3 shows the differences in HAMD and sleep factor scores between the two groups. The linear mixed-effect model showed that HAMD score did not significantly differ between the two groups from baseline to the end of treatment. The subcomponent scores also showed no significant differences between the two groups (F = 1.65, p > 0.05). Figure 2B shows that the HAMD score was not significantly different between the two groups at different time points. However, the HAMD score improved over time for each group (p < 0.01).
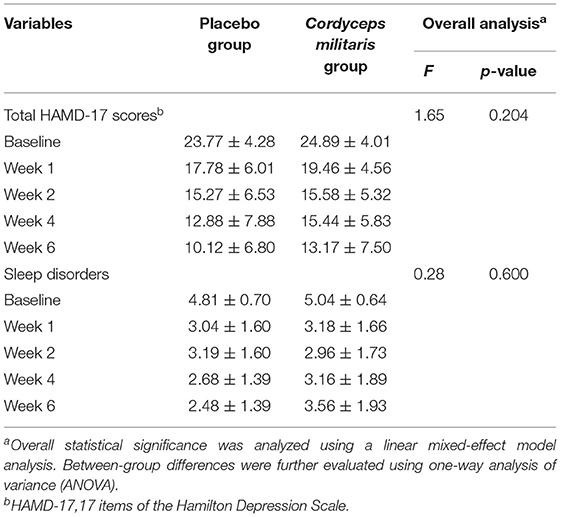
Table 3. Changes of HAMD sleep factors and total scores at baseline, week 1, week 2, week 4, and week 6.
The incidence of adverse events is shown in Supplementary Table 1. No severe adverse events were reported. The incidence of nausea, drowsiness, palpitation, dizziness, loss of appetite, vomiting, and excessive sweating was not significantly different between the two groups.
To the best of our knowledge, this preliminary study is the first double-blind, randomized, placebo-controlled clinical trial investigating the efficacy, tolerability, and safety of C. militaris as an additional treatment for insomnia symptoms in patients with MDD. C. militaris is an edible medicine product with confirmed anticancer, antibacterial, antiviral, immunomodulatory, neuroprotective, and antioxidant effects based on previous research (30, 31). Its main ingredient is an adenosine analog that has the potential to improve sleep through its physiological action on adenosine receptors; however, the mechanism remains controversial. C. militaris contained cordycepin (3′-deoxyadenosine). A previous study have proved that Cordycepin could produce a rapid and robust antidepressant Effect via enhancing pre-frontal AMPA receptor signaling pathway in male CD-1 mice (32). Although previous basic science studies of C. militaris have shown that it can extend the period of NREM sleep via association with adenosine receptors (26), no clinical trials have been conducted until now. Besides, there were no other studies with C. militaris in humans yet.
This study found that insomnia symptoms as measured by total AIS score were not improved by the addition of C. militaris to a 6-week course of duloxetine compared to placebo. However, the efficacy of duloxetine plus placebo was better than duloxetine plus C. militaris as measured by changes in total AIS and some subscale scores (awakenings during the night, final awakening, total sleep duration, sleep quality, and functioning capacity during the day). This interesting result may be due to the fact that this is a single-center exploratory study with limited sample size. Multi-center studies with large sample sizes in future might have different and more credible results.
In contrast to patient-reported insomnia symptoms, there was no significant difference in physician-assessed sleep improvement (HAMD-17 sleep factor score). This contrast suggests that reliance on subjective patient symptom assessment may be insufficient when evaluating insomnia and that objective sleep monitoring, such as sleep electroencephalography (EEG), is needed. In addition, the proportion of patients with first-episode depression in this study was relatively high. A previous meta-analysis found that the effectiveness of antidepressant single-drug treatment for first-episode depression is as high as 64.5% (31), and for most patients with first-episode depression, sleep difficulties improve along with reduction of depression. In this study, depressive symptoms significantly decreased in both groups after the first week of treatment, which we attributed to duloxetine. Furthermore, cordycepin has a short half-life in humans, unlike animals, and the dose and frequency of drug administration may also affect the results (33).
In addition, this study demonstrated no significant difference between the effects of 6 weeks of treatment with duloxetine plus C. militaris or duloxetine plus placebo on the secondary endpoints (i.e., change in total HAMD-17 and its sleep factor scores) or in the MDD treatment response. A significant improvement in HAMD-17 scores was found in both groups, consistent with previous findings (34).
The safety of C. militaris treatment for insomnia in patients with MDD is worth discussion. Minimal adverse events were reported for both C. militaris and placebo and treatment with duloxetine plus C. militaris did not increase the study withdrawal rate compared with duloxetine plus placebo. In addition, since 2009, when the ministry of health of China approved C. militaris as a new resource food (ministry of health announcement no. 3, 2009), it has been widely used and no adverse reactions have been reported, consistent with the results of this study (35).
The current study has several limitations, including small sample size, high number of participants with first-episode depression and mild sleep problems, reliance on patient reporting for measuring primary outcome, and a lack of objective sleep assessment.
Future multi-center studies should employ larger samples that include a wider representation of patients with MDD with sleep problems and utilize polysomnography to monitor sleep improvement.
C. militaris treatment at the current dose and duration is not recommended for the reduction of sleep symptoms in patients with MDD. Although C. militaris is safe and side effects are rare, its efficacy needs to be verified by future large-scale studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Beijing Anding Hospital, Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
GW designed the study. JJZ and XC wrote the protocol and analyzed the data. LX and JJZ commented on the protocol. JJZ and XC wrote the first draft of manuscript with input from LF. All authors commented on and approved the final manuscript.
The study was supported by the National Key R&D Program of China (2017YFC1311100), Beijing Brain Science Research Project (Z171100000117004), and the Beijing Municipal Science and Tech Commission (D171100007017001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the clinical staff at the National Clinical Research Center for Mental Disorders at Beijing Anding Hospital. Moreover, we thank the patients for this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.754921/full#supplementary-material
1. Hasler G. Pathophysiology of depression do we have any solid evidence of interest to clinicians? World Psychiatry. (2010) 9:155–61. doi: 10.1002/j.2051-5545.2010.tb00298.x
2. Organization WH. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization (2008).
3. Farley S, Apazoglou K, Witkin JM, Giros B, Tzavara ET. Antidepressant-like effects of an AMPA receptor potentiator under a chronic mild stress paradigm. Int J Neuropsychopharmacol. (2010) 13:1207–18. doi: 10.1017/S1461145709991076
4. Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. (2008) 10:473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen
5. Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. (2011) 135:10–9. doi: 10.1016/j.jad.2011.01.011
6. Cheng P, Luik AI, Fellman-Couture C, Peterson E, Joseph CLM, Tallent G, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. (2019) 49:491–500. doi: 10.1017/S0033291718001113
7. Cunningham JEA, Shapiro CM. Cognitive Behavioural Therapy for Insomnia (CBT-I) to treat depression: a systematic review. J Psychosom Res. (2018) 106:1–12. doi: 10.1016/j.jpsychores.2017.12.012
8. Freeman D, Sheaves B, Goodwin GM Yu LM, Nickless A, Harrison PJ, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. (2017) 4:749–58. doi: 10.1016/S2215-0366(17)30328-0
9. Reynolds CF III, Frank E, Houck PR, Mazumdar S, Dew MA, Cornes C, et al. Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am J Psychiatry. (1997) 154:958–62. doi: 10.1176/ajp.154.7.958
10. Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. (2017) 26:675–700. doi: 10.1111/jsr.12594
11. Wilt TJ, MacDonald R, Brasure M, Olson CM, Carlyle M, Fuchs E, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. (2016) 165:103–12. doi: 10.7326/M15-1781
12. Fabbri C, Marsano A, Balestri M, De Ronchi D, Serretti A. Clinical features and drug induced side effects in early versus late antidepressant responders. J Psychiatr Res. (2013) 47:1309–18. doi: 10.1016/j.jpsychires.2013.05.020
13. Ho FY, Chan CS, Lo WY, Leung JC. The effect of self-help cognitive behavioral therapy for insomnia on depressive symptoms: an updated meta-analysis of randomized controlled trials. J Affect Disord. (2020) 265:287–304. doi: 10.1016/j.jad.2020.01.062
14. Sarris J, Byrne GJ A. systematic review of insomnia and complementary medicine. Sleep Med Rev. (2011) 15:99–106. doi: 10.1016/j.smrv.2010.04.001
15. Wen X, Wu Q, Liu J, Xu Z, Fan L, Chen X, et al. Randomized single-blind multicenter trial comparing the effects of standard and augmented acupuncture protocols on sleep quality and depressive symptoms in patients with depression. Psychol Health Med. (2018) 23:375–90. doi: 10.1080/13548506.2017.1363399
16. Chen YF, Liu JH, Xu NG, Liang ZH, Xu ZH, Xu SJ, et al. Effects of acupuncture treatment on depression insomnia: a study protocol of a multicenter randomized controlled trial. Trials. (2013) 14:2. doi: 10.1186/1745-6215-14-2
17. Cortoos A, Verstraeten E, Cluydts R. Neurophysiological aspects of primary insomnia: implications for its treatment. Sleep Med Rev. (2006) 10:255–66. doi: 10.1016/j.smrv.2006.01.002
18. Lazarus M, Chen JF, Urade Y, Huang ZL. Role of the basal ganglia in the control of sleep and wakefulness. Curr Opin Neurobiol. (2013) 23:780–5. doi: 10.1016/j.conb.2013.02.001
19. Huang ZL, Zhang Z, Qu WM. Roles of adenosine and its receptors in sleep-wake regulation. Int Rev Neurobiol. (2014) 119C:349–71. doi: 10.1016/B978-0-12-801022-8.00014-3
20. Gvilia I, Suntsova N, Kostin A, Kalinchuk A, Mcginty D, Basheer R, et al. The role of adenosine in the maturation of sleep homeostasis in rats. J Neurophysiol. (2017) 117:327–35. doi: 10.1152/jn.00675.2016
21. Lazarus M, Chen JF, Huang ZL, Urade Y, Fredholm BB. Adenosine and Sleep. Handb Exp Pharmacol. (2019) 253:359–81. doi: 10.1007/164_2017_36
23. Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. (2007) 14:1315–523. doi: 10.1038/sj.cdd.4402132
24. Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. International Union of Basic and Clinical Pharmacology. LXXXI Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. (2011) 63:1–34. doi: 10.1124/pr.110.003285
25. Melling J, Belton FC, Kitching D, Stones WR. Production of pure cordycepin (3'-deoxyadenosine) from Cordyceps militaris. J Pharm Pharmacol. (1972) 24:125P.
26. Hu Z, Lee CI, Shah VK, Oh EH, Han JY, Bae JR, et al. Cordycepin increases nonrapid eye movement sleep via adenosine receptors in rats. Evid Based Complement Alternat Med. (2013) 2013:840134. doi: 10.1155/2013/840134
27. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59(Suppl. 20):22–33.
28. Hamilton. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
29. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. (2000) 48:555–60. doi: 10.1016/S0022-3999(00)00095-7
30. Wang F, Yin P, Lu Y, Zhou Z, Jiang C, Liu Y, et al. Cordycepin prevents oxidative stress-induced inhibition of osteogenesis. Oncotarget. (2015) 6:35496–508. doi: 10.18632/oncotarget.6072
31. Wang YT, Yang RM, Jiang XY. Meta-analysis of efficacy and safety of antidepressant monotherapy or combined olanzapine in the treatment of depression. Herald Med. (2011) 030:1295–7.
32. Li B, Hou Y, Zhu M, Bao H, Nie J, Zhang GY, et al. 3'-Deoxyadenosine (Cordycepin) produces a rapid and robust antidepressant effect via enhancing prefrontal AMPA receptor signaling pathway. Int J Neuropsychopharmacol. (2016) 19:pyv112. doi: 10.1093/ijnp/pyv112
33. Tsai YJ, Lin LC, Tsai TH. Pharmacokinetics of adenosine and cordycepin, a bioactive constituent of Cordyceps sinensis in rat. J Agric Food Chem. (2010) 58:4638. doi: 10.1021/jf100269g
34. Detke MJ, Lu Y, Goldstein DJ, Mcnamara RK, Demitrack MA. Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psychiatr Res. (2002) 36:383–90. doi: 10.1016/S0022-3956(02)00060-2
Keywords: depression, insomnia, Cordyceps militaris, duloxetine, efficacy, safety
Citation: Zhou J, Chen X, Xiao L, Zhou J, Feng L and Wang G (2021) Efficacy and Safety of Cordyceps militaris as an Adjuvant to Duloxetine in the Treatment of Insomnia in Patients With Depression: A 6-Week Double- Blind, Randomized, Placebo-Controlled Trial. Front. Psychiatry 12:754921. doi: 10.3389/fpsyt.2021.754921
Received: 07 August 2021; Accepted: 21 October 2021;
Published: 11 November 2021.
Edited by:
Jihui Zhang, The Chinese University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Sizhi Ai, The First Affiliated Hospital of Xinxiang Medical University, ChinaCopyright © 2021 Zhou, Chen, Xiao, Zhou, Feng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wang, d2FuZ2dhbmdfc3VhQHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.