
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 19 November 2021
Sec. Molecular Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.738358
This article is part of the Research Topic Insights in Molecular Psychiatry: 2021 View all 6 articles
 Hui Li1,2†
Hui Li1,2† Qingshuang Mu2†
Qingshuang Mu2† Yimin Kang3
Yimin Kang3 Xiaoyu Yang4
Xiaoyu Yang4 Ligang Shan5
Ligang Shan5 Meiling Wang3
Meiling Wang3 Cunbao Li3
Cunbao Li3 Yanlong Liu6,7*
Yanlong Liu6,7* Fan Wang8*
Fan Wang8*Objective: Cigarette smoking might accelerate cognitive impairment; however, this has never been investigated using human cerebrospinal fluid (CSF). We conducted this study to investigate the association between cigarette smoking and cognitive impairment through metal ions in CSF.
Methods: We obtained 5-ml CSF samples from routine lumbar puncture procedures in patients undergoing anterior cruciate ligament reconstruction before surgery in China. A total of 180 Chinese males were recruited (80 active smokers and 100 non-smokers). We measured specific cigarette-related neurotoxic metal ions in CSF, including iron, copper, zinc, lead, aluminum, and manganese. Sociodemographic data and history of smoking were obtained. The Montreal Cognitive Assessment (MoCA) was applied.
Results: Active smokers had fewer years of education (11.83 ± 3.13 vs. 13.17 ± 2.60, p = 0.01), and higher age (33.70 ± 10.20 vs. 29.76 ± 9.58, p = 0.01) and body mass index (25.84 ± 3.52 vs. 24.98 ± 4.06, p =0.03) than non-smokers. Compared to non-smokers, active smokers had significantly higher CSF levels of iron, zinc, lead, and aluminum and lower MoCA scores (all p < 0.05). Average daily numbers of cigarettes smoked negatively correlated with the MoCA scores (r = −0.244, p = 0.048). In young smokers, CSF manganese levels negatively correlated with MoCA scores (r = −0.373, p = 0.009).
Conclusions and Relevance: Cigarette smoking might be associated with male cognitive impairment, as shown by lower MoCA scores and higher levels of CSF iron, zinc, lead, and aluminum in active smokers. This might be early evidence of cigarette smoking accelerating male cognitive impairment.
Evidence suggests that cigarette smoking might accelerate brain aging (1). Metals found in cigarette smoke have been known to accumulate in tissues and fluids (2, 3), such as iron, copper, zinc aluminum, manganese and lead (4, 5). Metal accumulation in the nervous system could lead to heavy metal toxicity and accelerate cognitive impairment (6–8). Neurodegeneration, characterized by cognitive impairment, is the most common manifestation of heavy metal toxicity (9).
Increasing evidence suggests that dysregulation of iron, copper, and zinc homeostasis contributes to several neurodegenerative diseases (10, 11). Iron is involved in many fundamental biological processes in the brain, including oxygen transport, DNA synthesis, and mitochondrial respiration. Iron accumulation might be an essential factor contributing to neurodegenerative processes such as Alzheimer's disease (AD) (12). Copper is an active oxidation-reduction metal, as is iron, and both share toxicological consequences (13). Elevated copper levels may result in the generation of reactive oxygen species (ROS), DNA damage, and mitochondrial dysfunction (14). Disruption of the tightly regulated copper homeostasis in the brain can result in severe neurological malfunction and neurodegeneration. Studies suggested that the pathogenesis of neurodegenerative disorders such as AD involves an imbalanced copper homeostasis in the brain (15–17). Zinc's primary role is to stabilize the structure of several proteins, including signaling enzymes at all levels of cellular signal transduction and transcription factors. Excess zinc levels promote ROS production in the mitochondria, disrupting activities of metabolic enzymes, and activating apoptotic processes (18). Disruption of zinc homeostasis has been associated with AD (19).
Manganese is essential for human development and brain function. Excessive manganese levels are neurotoxic, as they disrupt mitochondrial function and induce oxidative stress (20). Chronic manganese exposure produces a cellular stress response that leads to neurodegenerative changes (21, 22). Mass spectrometry studies demonstrated that aluminum crosses the blood-brain barrier and accumulates in a semipermanent manner (23). Oral administration of aluminum to AD mice induced an increase in the amount of amyloid beta-protein and its deposition in plaques and aluminum to induce neurofibrillary degeneration and promote the appearance of tangle-like structures resembling AD patients (24). Also, aluminum exhibits an affinity for phosphates, thereby making DNA, RNA, and ATP perfect targets, affecting gene expression (6). A basic cellular and animal study elucidated the toxic actions of lead within the central nervous system (25). Lead has also been shown to induce latent changes in the aging brain and has been implicated in the pathogenesis of neurodegenerative diseases, particularly AD (26). Lead exposure in childhood could increase neurodegenerative disease risk in adulthood (25).
Components of cigarette smoke rapidly enter the brain in several ways and may cause the accumulation of these metal ions. Metal toxicity is associated with several neurodegenerative diseases, depending on levels of metal ions in the brain (27, 28). Nevertheless, the role of those metal ions in the association between cigarette smoking and cognitive impairment has never been reported. Therefore, this study was conducted to investigate the association of cognition and metal ions levels in CSF of cigarette smokers to further explore and support the effects of cigarette smoking on cognitive impairment.
Because there are few female subjects smokers in China, 180 Chinese males scheduled for anterior cruciate ligament reconstruction surgery were recruited from September 2014 to January 2016 [method as described in the literature (29)]. Of these, 80 were active smokers, and 100 were non-smokers. Sociodemographic data, including age, years of education, and body mass index (BMI), were collected. Clinical data, including a history of substance abuse and dependence, were obtained according to self-report and confirmed by the next of kin and family members. Exclusion criteria were as follows: (1) a family history of psychosis or neurological diseases, or CNS diseases determined by the Mini-International Neuropsychiatric Interview; and (2) systemic diseases based on the medical history and admitting diagnosis.
Participants who had never smoked and had no history of substance abuse or dependence were assigned to the non-smoker group. Active smokers were defined as those who consumed half a pack of cigarettes (half pack = 10 cigarettes) or more per day for more than 1 year. Smokers who smoked fewer than 10 cigarettes per day were excluded. No participants had a history of alcohol abuse or any psychiatric disorders, according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. All subjects were all independent without kinship. Active smokers were further grouped into younger smokers (n = 59, <40 y/o) and elder smokers (n = 21, ≥40 y/o), according to the literature (30). Based on World Health Organization criteria, active smokers were divided into moderate smokers (n = 46, >10 and <20 cigarettes per day for more than 1 year) and heavy smokers (n = 34, ≥ 20 cigarettes or more per day for more than 1 year. The maximum in this study was 40 cigarettes per day).
The present study was approved by the Institutional Review Board of Inner Mongolian Medical University and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects.
Smoking-related habit variables were obtained from active smokers, including age at smoking onset, years of cigarette smoking, average daily amount of cigarette smoking, and maximum daily amount of cigarette smoking. Cognition was assessed using the Montreal Cognitive Assessment (MoCA), a brief cognitive screening tool with high sensitivity and specificity for mild cognitive impairment (MCI), using a cutoff score of 26, with those scoring 25 or below being suspected of having MCI (31). The MoCA is a tool to differentiate healthy cognitive aging from MCI (32).
We recorded levels of high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine aminotransferase test (ALT), cholesterol (CHO), triglyceride (TG), gamma-glutamyl transferase (GGT) and aspartate aminotransferase (AST) which came from routine tests of the subjects to evaluate physical condition on admission. These peripheral metabolic marker levels were measured in the morning on the second hospital day after an overnight fasting period using a biochemistry analyzer (HITACH 7600, Hitachi Co., Tokyo, Japan).
Lumbar puncture is part of standard clinical procedure for patients undergoing anterior cruciate ligament reconstructive surgery in China. A licensed anesthetist performed a lumbar puncture in the morning before surgery, and a 5-ml CSF sample was obtained via intrathecal collection followed by immediately frozen at −80°C for storage. It takes <1 h to complete the entire anterior cruciate ligament reconstruction operation. The time from hospitalization to surgery was a maximum of 2 days.
Analyses were performed to measure CSF levels of iron, copper, zinc, lead, aluminum and manganese by atomic absorption spectrophotometry (33). Laboratory technicians were blinded to clinical data.
The normality of all variables was assessed using the Shapiro–Wilk test. Only the distribution of zinc, cooper, systolic pressure, and diastolic pressure were normally distributed (all p > 0.05). Consequently, the Mann-Whitney rank sum test was used (Table 1). The normality of the residuals from these models was assessed using the Shapiro–Wilk test. The homoscedasticity of residuals of the variances was verified using Levene's test; the residuals were all equally distributed (p > 0.05), except iron (p = 0.007). Therefore, analysis of covariance (ANCOVA) was used to compare differences in raw biomarkers between groups (Tables 2, 3). Multi-collinearity among covariates was estimated using tolerance and the variance inflation factor (VIF), using cutoffs thresholds for tolerance of < 0.1 and VIF >10 (34). Partial correlation analysis was performed to test the correlation between smoking habit variables and MoCA and between smoking habit variables and metal levels.
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Figures were created using GraphPad Prism version 8 (GraphPad Software Inc.). Since the variables under study might be heavily inter-dependent, especially the metals, and a possible effect worthy of further study did not wish to be missed in an exploratory context (35), therefore the Bonferroni correction was not conducted in the present study. All tests were two-sided, and the significance threshold was set at p < 0.05.
Compared to active smokers, non-smokers had significantly more years of education (13.17 ± 2.60 years vs. 11.83 ± 3.13 years, p = 0.01) and lower BMI (24.98 ± 4.06 vs. 25.84 ± 3.52 kg/m2, p = 0.03), and were younger (29.76 ± 9.58 years vs. 33.7 ± 10.20 years, p = 0.01). There were no differences between groups for other sociodemographic and clinical characteristics (Table 1). There were no significant correlations between BMI and metal levels and between age and metal levels in each group (all p > 0.05, Supplementary Material 1).
Using ANCOVA with age and education of years as covariates, the MoCA scores of non-smokers were significantly higher. Using ANCOVA with age and education as covariates, CSF levels of iron, zinc, lead, and aluminum were significantly higher in active smokers (all p < 0.05) (Table 2; Figure 1). No correlation was found between metal ions, age and MoCA scores in either group.
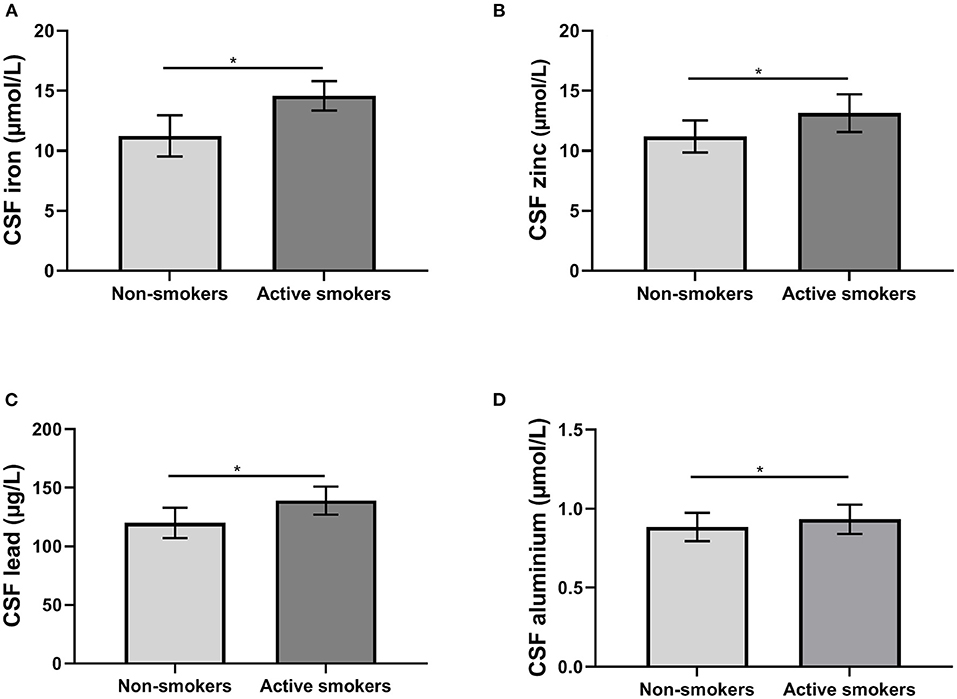
Figure 1. The differences of biomarkers in cerebral spinal fluid (CSF) between groups. (A) The differences of iron levels; (B) the differences of zinc levels; (C) the differences of lead levels; (D) the differences of aluminum levels. *p < 0.05.
Stepwise multiple regression analyses of six metals showed that no variable was removed from models (all tolerance >0.5 and VIF <3). Age was removed from models in active smokers (Table 3) since collinearity of age with age at smoking onset and years of cigarette smoking (both tolerance < 0.1 and VIF >30).
We calculated the correlation between smoking habit variables and MoCA, and smoking habit variables and the metals. Considering collinearity of age with age at smoking onset and years of cigarette smoking (both tolerance < 0.1 and VIF >30), average daily amount of cigarette smoking was negatively correlated with MoCA scores (r = −0.244, p = 0.048) with years of education and other smoking habits as covariates. With BMI, years of education, smoking habits, and other metals as covariates, there were no correlations between smoking habits and metal levels (all p > 0.05).
Active smokers were grouped into younger smokers (n = 59) and elder smokers (n = 21); 46 were moderate smokers, and 34 were heavy smokers. No differences were observed in CSF metal levels between younger and elder smokers or between moderate and heavy smokers (p > 0.05) with BMI, education, metals, and smoking habits as covariates. Compared to heavy smokers, there was a trend of higher MoCA scores in moderate smokers (p = 0.072) adjusted for age, years of education, metals, and smoking habits.
Figure 2 shows that CSF manganese levels negatively correlated with MoCA scores in young smokers (r = −0.373, p = 0.009) adjusted for years of education, BMI, other metals, and smoking habits.
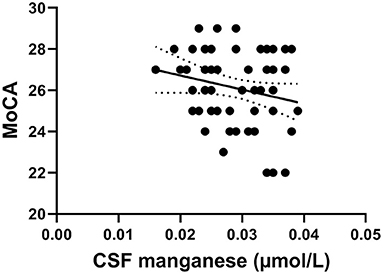
Figure 2. The negative correlation of manganese levels with MoCA scores in young smokers (r = −0.349, p = 0.012).
This is the first study to investigate the association of cigarette smoking and cognitive impairment through metal ions of CSF. We found that cigarette smoking might be associated with cognitive impairment, as shown by higher levels of iron, zinc, lead, and aluminum in CSF and lower MoCA scores in active smokers than non-smokers. The average daily amount of cigarette smoking was negative correlation with the MoCA scores.
Cigarette smoke might accelerate aging and cognitive impairment, including enhancing the risk of AD (36). Compared to non-smokers, middle-aged male smokers experienced a faster cognitive decline in global cognition and executive function (37). Heavy smoking is associated with cognitive impairment and cognitive decline in middle age (38). The differences in metal ion levels also might suggest an association between cigarette smoking and cognitive impairment.
Cigarette smoke can affect iron transporters (39); the principal ingredient (nicotine) blocks iron uptake by inhibiting iron release from transferrin and endocytosis (40). Brain iron is abnormally elevated early in several neurodegenerative disorders that impact memory, including AD (41). Studies reported a role for iron in neurodegenerative disorders, including increased iron levels in AD brains and iron involvement in the process of aging (42, 43). Moreover, iron is considered to accelerate cognitive impairment by inducing oxidative stress, ferroptotic cell death, or inflammatory responses (44). A recent cross-sectional study found a negative impact of chronic tobacco smoking on adult neuropsychological function, including alternating attention, working memory, short-term memory, long-term memory, processing accuracy, and executive function (45). In the present study, consistent with these previous studies, higher CSF iron levels and lower MoCA scores in active smokers, and average daily amount of cigarette smoking negative correlation with MoCA scores showed that cigarette smoking promoted the kind of change, suggesting cigarette smoking accelerating cognitive impairment.
Metallothionein (MT) is a group of metal-binding proteins in the blood-brain barrier. MT regulates the intracellular homeostasis of zinc. Because the copper-MT binding constant is much larger relative to zinc, MT exchanges zinc for copper when excess copper is present to defend against the more toxic copper (46). This phenomenon could explain the lack of difference in CSF copper levels between our two groups. A study showed that serum copper and zinc concentrations were significantly higher in smokers than in non-smokers; however, in rats, copper-zinc ratios in the liver, kidney, lung, and brain were significantly altered by nicotine treatment (47). This finding could partly explain higher CSF zinc levels in active smokers in our study. Zinc homeostasis is altered in aging, and there is deranged brain zinc homeostasis in AD. Although there are controversial views regarding zinc supplementation preventing AD pathology (48, 49), excessive zinc intake can lead to degeneration of cognitive function (50–52). This finding further suggests that higher CSF zinc levels as a feature of cigarette smoking accelerating cognitive impairment. Both iron and zinc have a higher binding affinity to Aβ and can promote its aggregation. Increased neuronal iron and zinc also bind to tau protein and facilitate the formation of neurofibrillary tangles to accelerate cognitive decline (6). Therefore, in conjunction with lower MoCA scores, levels of both metal ions were higher in CSF of active smokers, strongly suggesting the effects of cigarette smoking on cognitive impairment.
Manganese is an essential metal required for human development and brain function. Chronic overexposure to manganese may promote potent neurotoxic effects, including disrupting mitochondrial function and induction of oxidative stress (53). However, manganese not only competes with iron for the same binding protein transferrin, but also compete with other metal ions for divalent metal transporter DMT1 which non-selectively transports multiple divalent metals (54). These findings might explain no difference observed in CSF manganese levels between two groups in our study. Chronic manganese exposure can produce cognitive deficits in rats (55), children, and young men (56), which makes the negative correlation of manganese levels with MoCA scores in young smokers easy to understand.
There is increasing evidence supporting the notion of aluminum's involvement in hastening cognitive impairment, which is thought to increase the incidence of neurological diseases, including AD (57, 58). Epidemiological studies showed that occupational exposure to aluminum was associated with poor performance on cognitive tests (59). Chronic exposure of animals to aluminum is associated with evident deficits in learning and behavioral functions. Aluminum in tobacco can be inhaled via a number of ways, such as lung and oral epithelial tissues and accumulates in the brain over time (60). Aluminum is not essential for biological activities, and if accumulated in the brain, it induces amyloid β accumulation (61). It is toxic to the nervous system and induces irreversible cognitive impairment (58). In our study, higher CSF aluminum levels with lower MoCA scores were observed in active smokers than non-smokers, further suggesting that cigarette smoking accelerates cognitive impairment.
Cigarette smoking increases lead intake. Lead alters energy metabolism and blocks the release of calcium from mitochondria leading to the formation of ROS and apoptosis of the neuron and disrupts the formation of synapses (62). Lead causes significant adverse effects on the developing brain, including cognitive and learning disabilities (63). Any fraction of lead entering the brain cannot be neglected (64). Lead crosses the blood-brain barrier (65) and preferentially accumulates in the hippocampus and cerebral cortex in mice and humans (25) with consequent cognitive deficits (66, 67). Total brain volume, the volume of gray matter in the insula and cingulum, and white matter volume in the parietal lobes were reduced in a group of workers with chronic exposure to environmental lead (68). This finding explains the decline in cognitive function caused by lead toxicity. Therefore, the higher CSF lead levels and lower MoCA scores in active smokers than non-smokers in our study suggest that cigarette smoking accelerates cognitive decline in several ways.
Additionally, in the present study, the mediation analysis has been performed with smoking habits (X) as the independent variable and the sample size of active smokers (n = 80) was not enough to calculate the mediation effect efficiently, therefore, although there were no mediation effects of metal ions observed in our results, it is estimated that cigarette smoke contains thousands of chemical compounds and toxins that are deleterious to health (69), and there are the variety of toxic heavy metals in tobacco (69), many literatures cited here have shown that cigarette smoke increases the accumulation of metal ions in tissues and fluids, that their abnormal accumulation in the nervous system could lead to heavy metal toxicity and multiple neurodegenerative diseases including cognitive impairment, and that metal ions in tobacco could enter biological tissues and organs through cigarette smoke. Moreover, our subjects are all male Han people of northern China with similar living habits and environment. Therefore, it cannot be ruled out that cigarette smoke develops into a cognitive problem through abnormal deposition of metal ions. Taking together, higher levels of CSF iron, zinc, aluminum, and lead in smokers might be associated with cigarette smoking accelerating cognitive impairment.
There are some limitations to this study. First, CSF cannot directly reflect pathological changes in neurons; nevertheless, it represents biochemical changes in the brain. Second, subjects recruited in this study had anterior cruciate ligament injuries and were not an entirely healthy population, which might be seen as a confounder when interpreting the results. Finally, if there were more subjects and female subjects, our findings might be further supported.
Cigarette smoking might accelerate male cognitive impairment, as shown by lower MoCA scores and higher CSF iron, zinc, lead, and aluminum levels in active smokers. Higher levels of CSF iron, zinc, aluminum, and lead in smokers might be early evidence of cigarette smoking accelerating cognitive impairment. These broaden our understanding of cigarette smoke exposure associated with the development of neurodegenerative diseases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Inner Mongolian Medical University. The patients/participants provided their written informed consent to participate in this study.
FW and YL designed the study. FW, YL, CL, MW, and HL secured funding for the study. HL and QM led the drafting of the manuscript. HL led the statistical analyses. QM, XY, CL, MW, and LS collected the clinical data. All authors approved the final manuscript for submission.
This work was supported by the following grants: The Technology Support Project of Xinjiang (2017E0267), The 10th Inner Mongolia Autonomous Region Prairie excellence Project, Natural Science Foundation of Xinjiang Province (2018D01C228 and 2018D01C239), Outstanding Youth Science and Technology Talents of Xinjiang (2017Q007), Natural Science Foundation of China (81560229 and 81760252), Beijing Natural Science Foundation (7152074), Natural Science Foundation of Inner Mongolia Autonomous Region (2020MS08191), and the Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Professor Kuan-Pin Su and Doctor Hui-Chih Chang for valuable discussion and comments on the manuscript. They also thank all of the participants for their willingness to participate in the study and the time that they devoted to the study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.738358/full#supplementary-material
CSF, cerebral spinal fluid; AD, Alzheimer's disease; MCI, mild cognitive impairment; CNS, central nervous system; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ALT, alanine aminotransferase; CHO, cholesterol; TG, triglyceride; GGT, gamma-glutamyl transferase; AST, aspartate aminotransferase; BMI, body mass index; MoCA, Montreal Cognitive Assessment; ANCOVA, Analysis of covariance; SD, standard deviation; IQR, Interquartile Range.
1. Ho YS, Yang X, Yeung SC, Chiu K, Lau CF, Tsang AW, et al. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE. (2012) 7:e36752. doi: 10.1371/journal.pone.0036752
2. Ashraf MW. Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. Sci World J. (2012) 2012:729430. doi: 10.1100/2012/729430
3. Saffari A, Daher N, Ruprecht A, De Marco C, Pozzi P, Boffi R, et al. Particulate metals and organic compounds from electronic and tobacco-containing cigarettes: comparison of emission rates and secondhand exposure. Environ Sci Process Impacts. (2014) 16:2259–67. doi: 10.1039/C4EM00415A
4. Bernhard D, Rossmann A, Wick G. Metals in cigarette smoke. IUBMB Life. (2005) 57:805–9. doi: 10.1080/15216540500459667
5. Ghio AJ, Hilborn ED. Indices of iron homeostasis correlate with airway obstruction in an NHANES III cohort. Int J Chron Obstruct Pulmon Dis. (2017) 12:2075–84. doi: 10.2147/COPD.S138457
6. Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R. Metal toxicity links to Alzheimer's disease and neuroinflammation. J Mol Biol. (2019) 431:1843–68. doi: 10.1016/j.jmb.2019.01.018
7. Jia Q, Zhang Y, Liu S, Li Z, Zhou F, Shao L, et al. Analysis of search strategies for evaluating low-dose heavy metal mixture induced cognitive deficits in rats: an early sensitive toxicological approach. Ecotoxicol Environ Saf. (2020) 202:110900. doi: 10.1016/j.ecoenv.2020.110900
8. Pamphlett R, Mak R, Lee J, Buckland ME, Harding AJ, Kum Jew S, et al. Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus. PLoS ONE. (2020) 15:e0233300. doi: 10.1371/journal.pone.0233300
9. Patwa J, Flora SJS. Heavy metal-induced cerebral small vessel disease: insights into molecular mechanisms and possible reversal strategies. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21113862
10. Popescu BF, Nichol H. Mapping brain metals to evaluate therapies for neurodegenerative disease. CNS Neurosci Ther. (2011) 17:256–68. doi: 10.1111/j.1755-5949.2010.00149.x
11. Zheng W, Monnot AD. Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases. Pharmacol Ther. (2012) 133:177–88. doi: 10.1016/j.pharmthera.2011.10.006
12. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. (2014) 13:1045–60. doi: 10.1016/S1474-4422(14)70117-6
13. Coplan JD, Gorman JM. Treatment of anxiety disorder in patients with mood disorders. J Clin Psychiatry. (1990) 51(Suppl):9–13.
14. Angele-Martinez C, Nguyen KV, Ameer FS, Anker JN, Brumaghim JL. Reactive oxygen species generation by copper(II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology. (2017) 11:278–88. doi: 10.1080/17435390.2017.1293750
15. Bagheri S, Squitti R, Haertle T, Siotto M, Saboury AA. Role of copper in the onset of Alzheimer's disease compared to other metals. Front Aging Neurosci. (2017) 9:446. doi: 10.3389/fnagi.2017.00446
16. Bisaglia M, Bubacco L. Copper ions and Parkinson's disease: why is homeostasis so relevant? Biomolecules. (2020) 10:195. doi: 10.3390/biom10020195
17. Gromadzka G, Tarnacka B, Flaga A, Adamczyk A. Copper dyshomeostasis in neurodegenerative diseases-therapeutic implications. Int J Mol Sci. (2020) 21:9259. doi: 10.3390/ijms21239259
18. Wright RO, Baccarelli A. Metals and neurotoxicology. J Nutr. (2007) 137:2809–13. doi: 10.1093/jn/137.12.2809
19. Kawahara M, Tanaka KI, Kato-Negishi M. Zinc, Carnosine, and neurodegenerative diseases. Nutrients. (2018) 10:147. doi: 10.3390/nu10020147
20. Chtourou Y, Trabelsi K, Fetoui H, Mkannez G, Kallel H, Zeghal N. Manganese induces oxidative stress, redox state unbalance and disrupts membrane bound ATPases on murine neuroblastoma cells in vitro: protective role of silymarin. Neurochem Res. (2011) 36:1546–57. doi: 10.1007/s11064-011-0483-5
21. Langley MR, Ghaisas S, Ay M, Luo J, Palanisamy BN, Jin H, et al. Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson's disease: relevance to gene and environment interactions in metal neurotoxicity. Neurotoxicology. (2018) 64:240–55. doi: 10.1016/j.neuro.2017.06.002
22. Wang H, Zhang S, Yang F, Xin R, Wang S, Cui D, et al. The gut microbiota confers protection in the CNS against neurodegeneration induced by manganism. Biomed Pharmacother. (2020) 127:110150. doi: 10.1016/j.biopha.2020.110150
23. Yumoto S, Nagai H, Kobayashi K, Tamate A, Kakimi S, Matsuzaki H. 26Al incorporation into the brain of suckling rats through maternal milk. J Inorg Biochem. (2003) 97:155–60. doi: 10.1016/S0162-0134(03)00246-0
24. Mold MJ, O'Farrell A, Morris B, Exley C. Aluminum and neurofibrillary tangle co-localization in familial Alzheimer's disease and related neurological disorders. J Alzheimers Dis. (2020) 78:139–49. doi: 10.3233/JAD-200838
25. Reuben A. Childhood lead exposure and adult neurodegenerative disease. J Alzheimers Dis. (2018) 64:17–42. doi: 10.3233/JAD-180267
26. Kazi TG, Jalbani N, Arain MB, Jamali MK, Afridi HI, Sarfraz RA, et al. Toxic metals distribution in different components of Pakistani and imported cigarettes by electrothermal atomic absorption spectrometer. J Hazard Mater. (2009) 163:302–7. doi: 10.1016/j.jhazmat.2008.06.088
27. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. (1997) 388:482–8. doi: 10.1038/41343
28. Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. (2014) 7:60–72. doi: 10.2478/intox-2014-0009
29. Liu Y, Li H, Wang J, Xue Q, Yang X, Kang Y, et al. Association of cigarette smoking with cerebrospinal fluid biomarkers of neurodegeneration, neuroinflammation, and oxidation. JAMA Netw Open. (2020) 3:e2018777. doi: 10.1001/jamanetworkopen.2020.18777
30. Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. (2010) 7:3760–91. doi: 10.3390/ijerph7103760
31. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
32. Carson N, Leach L, Murphy KJ. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. (2018) 33:379–88. doi: 10.1002/gps.4756
33. Romaris EM, Cervantes II, Lopez JM, Marcen JF. Concentration of calcium and magnesium and trace elements (zinc, copper, iron and manganese) in cerebrospinal fluid: a try of a pathophysiological classification. J Trace Elem Med Biol. (2011) 25(Suppl. 1):S45–49. doi: 10.1016/j.jtemb.2010.10.009
34. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. (2019) 72:558–69. doi: 10.4097/kja.19087
35. Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. (2014) 34:502–8. doi: 10.1111/opo.12131
36. Durazzo TC, Mattsson N, Weiner MW, Alzheimer's Disease Neuroimaging Initiative. Smoking and increased Alzheimer's disease risk: a review of potential mechanisms. Alzheimers Dement. (2014) 10:S122–45. doi: 10.1016/j.jalz.2014.04.009
37. Sabia S, Elbaz A, Dugravot A, Head J, Shipley M, Hagger-Johnson G, et al. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch Gen Psychiatry. (2012) 69:627–35. doi: 10.1001/archgenpsychiatry.2011.2016
38. Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev. (2016) 9:76–9. doi: 10.2174/1874473709666160803101633
39. Bondy SC. Prolonged exposure to low levels of aluminum leads to changes associated with brain aging and neurodegeneration. Toxicology. (2014) 315:1–7. doi: 10.1016/j.tox.2013.10.008
40. Zhang WZ, Butler JJ, Cloonan SM. Smoking-induced iron dysregulation in the lung. Free Radic Biol Med. (2019) 133:238–47. doi: 10.1016/j.freeradbiomed.2018.07.024
41. Liu Y, Nguyen M, Robert A, Meunier B. Metal ions in Alzheimer's disease: a key role or not? Acc Chem Res. (2019) 52:2026–35. doi: 10.1021/acs.accounts.9b00248
42. Mesquita SD, Ferreira AC, Sousa JC, Santos NC, Correia-Neves M, Sousa N, et al. Modulation of iron metabolism in aging and in Alzheimer's disease: relevance of the choroid plexus. Front Cell Neurosci. (2012) 6:25. doi: 10.3389/fncel.2012.00025
43. Karama S, Ducharme S, Corley J, Chouinard-Decorte F, Starr JM, Wardlaw JM, et al. Cigarette smoking and thinning of the brain's cortex. Mol Psychiatry. (2015) 20:778–85. doi: 10.1038/mp.2014.187
44. Ayton S, Wang Y, Diouf I, Schneider JA, Brockman J, Morris MC, et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry. (2020) 25:2932–41. doi: 10.1038/s41380-019-0375-7
45. Nadar MS, Hasan AM, Alsaleh M. The negative impact of chronic tobacco smoking on adult neuropsychological function: a cross-sectional study. BMC Public Health. (2021) 21:1278. doi: 10.1186/s12889-021-11287-6
46. Ba LA, Doering M, Burkholz T, Jacob C. Metal trafficking: from maintaining the metal homeostasis to future drug design. Metallomics. (2009) 1:292–311. doi: 10.1039/b904533c
47. Dubick MA, Keen CL. Influence of nicotine on tissue trace element concentrations and tissue antioxidant defense. Biol Trace Elem Res. (1991) 31:97–109. doi: 10.1007/BF02990418
48. Linkous DH, Adlard PA, Wanschura PB, Conko KM, Flinn JM. The effects of enhanced zinc on spatial memory and plaque formation in transgenic mice. J Alzheimers Dis. (2009) 18:565–79. doi: 10.3233/JAD-2009-1162
49. Corona C, Masciopinto F, Silvestri E, Viscovo AD, Lattanzio R, Sorda RL, et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. (2010) 1:e91. doi: 10.1038/cddis.2010.73
50. Hedera P, Peltier A, Fink JK, Wilcock S, London Z, Brewer GJ. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin II. The denture cream is a primary source of excessive zinc. Neurotoxicology. (2009) 30:996–9. doi: 10.1016/j.neuro.2009.08.008
51. Nuttall JR, Oteiza PI. Zinc and the aging brain. Genes Nutr. (2014) 9:379. doi: 10.1007/s12263-013-0379-x
52. Takeda A, Tamano H. Cognitive decline due to excess synaptic Zn(2+) signaling in the hippocampus. Front Aging Neurosci. (2014) 6:26. doi: 10.3389/fnagi.2014.00026
53. Bjorklund G, Chartrand MS, Aaseth J. Manganese exposure and neurotoxic effects in children. Environ Res. (2017) 155:380–4. doi: 10.1016/j.envres.2017.03.003
54. Montalbetti N, Simonin A, Simonin C, Awale M, Reymond JL, Hediger MA. Discovery and characterization of a novel non-competitive inhibitor of the divalent metal transporter DMT1/SLC11A2. Biochem Pharmacol. (2015) 96:216–24. doi: 10.1016/j.bcp.2015.05.002
55. Liang G, Qin H, Zhang L, Ma S, Huang X, Lv Y, et al. Effects of chronic manganese exposure on the learning and memory of rats by observing the changes in the hippocampal cAMP signaling pathway. Food Chem Toxicol. (2015) 83:261–7. doi: 10.1016/j.fct.2015.07.005
56. Zoni S, Lucchini RG. Manganese exposure: cognitive, motor and behavioral effects on children: a review of recent findings. Curr Opin Pediatr. (2013) 25:255–60. doi: 10.1097/MOP.0b013e32835e906b
57. Maya S, Prakash T, Madhu KD, Goli D. Multifaceted effects of aluminium in neurodegenerative diseases: a review. Biomed Pharmacother. (2016) 83:746–54. doi: 10.1016/j.biopha.2016.07.035
58. Zhang J, Hao Y, Wang Y, Han Y, Zhang S, Niu Q. Relationship between the expression of TNFR1-RIP1/RIP3 in peripheral blood and cognitive function in occupational Al-exposed workers: a mediation effect study. Chemosphere. (2021) 278:130484. doi: 10.1016/j.chemosphere.2021.130484
59. Kumar V, Gill KD. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol. (2009) 83:965–78. doi: 10.1007/s00204-009-0455-6
60. Pappas RS, Watson CH, Valentin-Blasini L. Aluminum in tobacco products available in the United States. J Anal Toxicol. (2018) 42:637–41. doi: 10.1093/jat/bky034
61. Promyo K, Iqbal F, Chaidee N, Chetsawang B. Aluminum chloride-induced amyloid beta accumulation and endoplasmic reticulum stress in rat brain are averted by melatonin. Food Chem Toxicol. (2020) 146:111829. doi: 10.1016/j.fct.2020.111829
62. Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int. (2014) 2014:840547. doi: 10.1155/2014/840547
63. Liu X, Wei F, Cheng Y, Zhang Y, Jia G, Zhou J, et al. auditory training reverses lead (Pb)-toxicity-induced changes in sound-azimuth selectivity of cortical neurons. Cereb Cortex. (2019) 29:3294–304. doi: 10.1093/cercor/bhy199
64. Wallin C, Sholts SB, Osterlund N, Luo J, Jarvet J, Roos PM, et al. Alzheimer's disease and cigarette smoke components: effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-beta peptide aggregation. Sci Rep. (2017) 7:14423. doi: 10.1038/s41598-017-13759-5
65. Bakulski KM, Seo YA, Hickman RC, Brandt D, Vadari HS, Hu H, et al. Heavy metals exposure and alzheimer's disease and related dementias. J Alzheimers Dis. (2020) 76:1215–42. doi: 10.3233/JAD-200282
66. Schwartz BS, Chen S, Caffo B, Stewart WF, Bolla KI, Yousem D, et al. Relations of brain volumes with cognitive function in males 45 years and older with past lead exposure. Neuroimage. (2007) 37:633–41. doi: 10.1016/j.neuroimage.2007.05.035
67. Kordas K. Iron, lead, and children's behavior and cognition. Annu Rev Nutr. (2010) 30:123–48. doi: 10.1146/annurev.nutr.012809.104758
68. Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, et al. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. (2006) 66:1476–84. doi: 10.1212/01.wnl.0000216138.69777.15
69. Caruso RV, O'Connor RJ, Stephens WE, Cummings KM, Fong GT. Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: results from the International Tobacco Control (ITC) United States survey cohort. Int J Environ Res Public Health. (2013) 11:202–17. doi: 10.3390/ijerph110100202
Keywords: cigarette smoking, cognitive impairment, metal ion, cerebrospinal fluid, male
Citation: Li H, Mu Q, Kang Y, Yang X, Shan L, Wang M, Li C, Liu Y and Wang F (2021) Association of Cigarette Smoking With Male Cognitive Impairment and Metal Ions in Cerebrospinal Fluid. Front. Psychiatry 12:738358. doi: 10.3389/fpsyt.2021.738358
Received: 16 July 2021; Accepted: 28 October 2021;
Published: 19 November 2021.
Edited by:
Carlos M. Opazo, University of Melbourne, AustraliaReviewed by:
Amit Lotan, Hadassah-Hebrew University Medical Center, IsraelCopyright © 2021 Li, Mu, Kang, Yang, Shan, Wang, Li, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Wang, ZmFud2FuZ0Biam11LmVkdS5jbg==; ZmFud2FuZ0B4am11LmVkdS5jbg==; Yanlong Liu, YmVuamFtaW5seWxAd211LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.