
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 15 October 2021
Sec. Child and Adolescent Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.728952
This article is part of the Research TopicFragile X Spectrum DisordersView all 24 articles
FMR1 premutation is defined by 55–200 CGG repeats in the Fragile X Mental Retardation 1 (FMR1) gene. FMR1 premutation carriers are at risk of developing a neurodegenerative disease called fragile X-associated tremor/ataxia syndrome (FXTAS) and Fragile X-associated primary ovarian insufficiency (FXPOI) in adulthood. In the last years an increasingly board spectrum of clinical manifestations including psychiatric disorders have been described as occurring at a greater frequency among FMR1 premutation carriers. Herein, we reviewed the neuroimaging findings reported in relation with psychiatric symptomatology in adult FMR1 premutation carriers. A structured electronic literature search was conducted on FMR1 premutation and neuroimaging yielding a total of 3,229 articles examined. Of these, 7 articles were analyzed and are included in this review. The results showed that the main radiological findings among adult FMR1 premutation carriers presenting neuropsychiatric disorders were found on the amygdala and hippocampus, being the functional abnormalities more consistent and the volumetric changes more inconsistent among studies. From a molecular perspective, CGG repeat size, FMR1 mRNA and FMRP levels have been investigated in relation with the neuroimaging findings. Based on the published results, FMRP might play a key role in the pathophysiology of the psychiatric symptoms described among FMR1 premutation carriers. However, additional studies including further probes of brain function and a broader scope of psychiatric symptom measurement are required in order to obtain a comprehensive landscape of the neuropsychiatric phenotype associated with the FMR1 premutation.
Fragile X premutation carriers is defined by 55–200 CGG repeats in the Fragile X Mental Retardation 1 (FMR1) gene, whilst the full mutation is caused by >200 CGG repeats. Two major conditions associated with the FMR1 premutation have been well-established: the fragile X-associated Tremor/Ataxia Syndrome (FXTAS) and the fragile X-associated Primary Ovarian Insufficiency (FXPOI) [reviewed in (1)] FXTAS is a late onset neurodegenerative condition, and it is seen in around 40% of male premutation carriers and 16% of females (2). FXPOI is characterized by menopause before age 40 and it is seen in around 20% of women with the FMR1 premutation (3). Nevertheless, a broader clinical spectrum of symptoms, including psychiatric, sleep, and autoimmune conditions, has been described among FMR1 permutation carriers (4). Although the extent of all this group of conditions needs further delineation, in order to bring recognition to these problems, different names were proposed. Hagerman et al., proposed fragile X-associated Neuropsychiatric Disorders (FXAND) and the European Fragile X Network (EFXN) proposed to name them Fragile X-associated Neuropsychiatric Conditions (FXANC), and Fragile X Various Associated Conditions (FXVAC) to cover other physical conditions associated with the FMR1 premutation (51).
FMR1 premutation main neuropsychiatric disorders in adults include anxiety and depression and, to a lesser extent, obsessive compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), and substance abuse. Although most prior work has been performed examining different neuropsychiatric aspects in separate study samples, neuroimaging studies have depicted structural, functional, and connectivity changes within the brain associated to neuropsychiatric disorders. In regard to FMR1 premutation this relationship has been addressed, although with different approaches and with different population cohorts. Furthermore, a correlation on how neurostructural and neurofunctional effects might be associated with molecular aspects of the FMR1 premutation and psychiatric symptomatology has also been reported with varying results. This review focuses on the neuroimaging findings associated with neuropsychiatric disorders in adult FMR1 premutation carriers. Due to the wide clinical spectrum of neurological symptoms associated with the FMR1 premutation, including motor and cognitive impairment, executive and memory deficits, and psychiatric symptoms, we believe that a review focusing on the neuropathology, molecular underpinnings, and neuroimaging associated with neuropsychiatric findings could help to untangle the complex physiopathology associated with the FMR1 premutation.
A review of the literature was conducted. PubMed, Web of Science, Pschynfo and Cochrane Central Register of Controlled Trials were searched for eligible studies from 2000 to April 2021. Search terms are listed in Research Algorithm (Supplementary Table 1) where the complete search strategy is displayed. Inclusion criteria included FMR1 premutation carriers, both genders, from 18 to 99 years old and all ethnicities. As exclusion criteria, the search was only focused on pure psychiatric symptoms and therefore motor and cognitive impairments, executive function, or memory deficits were not considered.
Eligible qualitative studies were those that matched neuroimaging findings with psychiatric symptomatology in FMR1 premutation carriers. The type of studies selected were those of any design published in peer-reviewed academic journals with abstract available. Conference proceedings, theses, case reports and case series were excluded, and review articles, non-English articles, and studies on animal models were not considered (for a graphical summary of the selection procedure, see Figure 1).
After establishing and applying the inclusion criteria, two researchers (A. EM and L.RR) separately read the titles of the papers retrieved in the search and examined the structured abstract of the selected articles. Seven studies that described the relationship between neuroimaging and neuropsychiatric findings in adult FMR1 premutation were selected. All potential differences in interpretation between the reviewers were discussed to ensure that all the articles reviewed presented a satisfactory level of evidence.
This study is a review of previously published data and, as such, does not require ethics approval. The data were not used for any purpose other than those of the original study, and no new data were collected.
A total of 3,229 studies were identified from the initial search. After de-duplication and initial screening, 1,933 full-text articles were assessed for eligibility and 7 were included in this review (Figure 1, Table 1) (5–11).
Represented in these manuscripts were views from 413 study participants (244 FMR1 premutation carriers and 169 control individuals) from 2 countries (UK, USA). Participants included adult men and women ranging in age from 18 to over 79 years. FMR1 premutation carriers were recruited through screening of pedigrees of probands with FXS with a CGG repeat size ranging from 55 CGGs to 199 CGGs. All subjects who participated in Koldewyn et al. (6) also participated in the Hessl et al. (5) study and, therefore, were counted only once. Such relation was not mentioned in the rest of the studies. One study (11) included 3 FMR1 premutation mosaic participants and 1 intermediate allele participant within the premutation group and, thus, was not considered when evaluating the CGG range (Table 2).
All but one of the seven studies were performed in male population, whereas only one included both male and female permutation carriers. Four of them used functional magnetic resonance imaging (fMRI), five of them used volumetric measures and one used voxel-based morphometry. It is important to note that comorbid medical and psychiatric conditions, such as the mood of participants when performing tasks, were not considered, or at least not mentioned in any of the reviewed studies. Moreover, some of the participants enrolled in the studies were medicated with different drugs (Table 2), which might act as a potential confounding factor, and finally had an effect on the neuroimaging findings.
The synthesis of the 7 neuroimaging studies identified the amygdala and the hippocampus as the two major brain areas involved with FXAND. Table 3 summarizes results reported in the 7 studies.
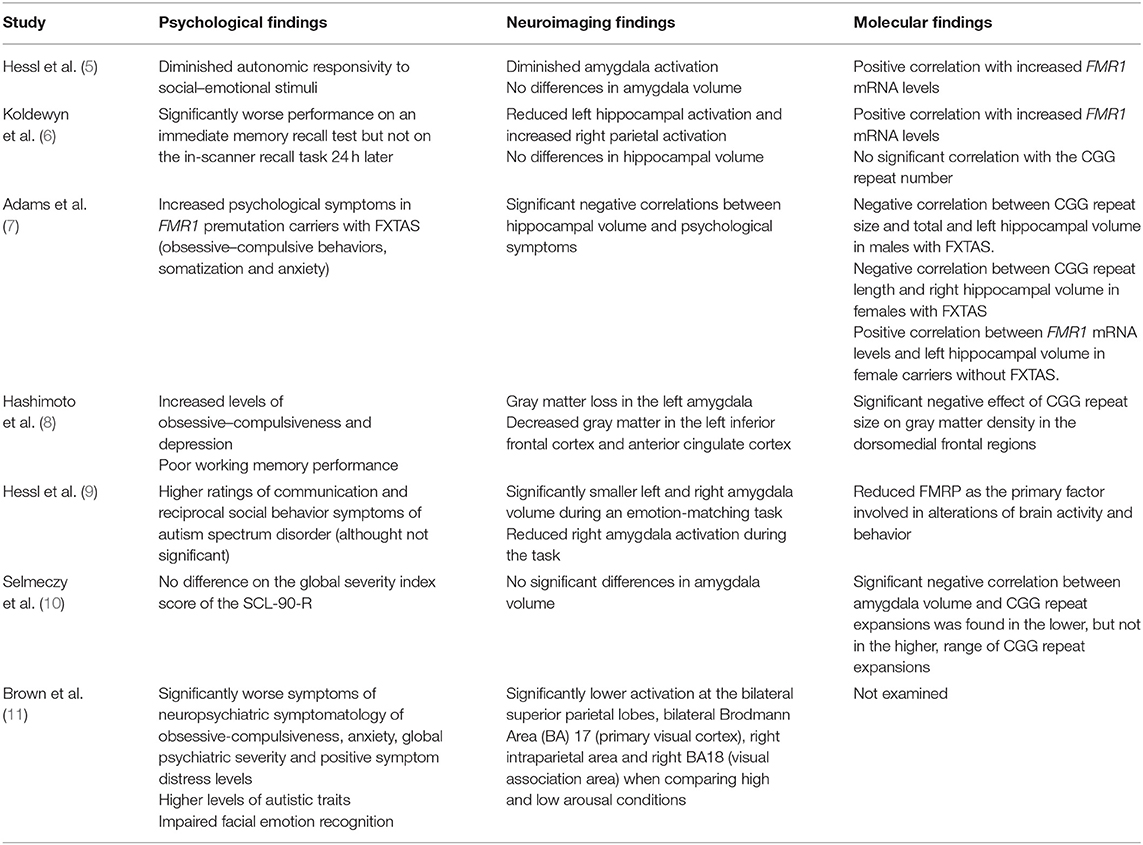
Table 3. Summary of the psychological, neuroimaging and molecular findings reported in FMR1 premutation carriers compared to control population in the papers reviewed.
The relationship between both the amygdala function and volume with psychological symptoms has been evaluated in several studies.
Regarding the amygdala function, male premutation carriers without FXTAS have been found to have decreased amygdala activation to emotional inputs which correlate with an increase of psychological symptoms. In 2007, Hessl et al. (5) described a negative correlation between psychological symptoms and amygdala activation compared to controls during an fMRI task consistent in passively viewing fearful vs. scrambled faces. Moreover, they also found that FMR1 premutation participants had decreased potentiation of the eye blink startle reflex to fearful faces, which is an indirect evidence of reduced amygdale activation, and diminished skin conductance response during a brief social stressor; evidencing reduced sympathetic activation. Similarly, Hessl et al. (9) described a negative correlation between higher ratings of autism spectrum symptoms and reduced left amygdala activation during an emotion processing fMRI task.
The reduced amygdala activation in FMR1 premutation carriers has been associated with abnormal elevation of FMR1 mRNA (5) and in particular, with reduced FMRP levels (9).
As for the amygdala volume and its correlation with psychological symptoms, results are controversial. While some studies did not find differences between groups in the amygdala volume and psychological symptoms (measured on the SCL-90-R) or cognitive ability (based on full scale IQ) (5, 10), others reported higher ratings of autism spectrum symptoms correlated with smaller bilateral amygdala volume (9). Additionally, Hashimoto et al. (8) demonstrated that increased levels of obsessive–compulsiveness and depression in male premutation carriers was associated with gray matter loss in the left amygdala evaluated by voxel-based morphometry. A plausible explanation for these discordant results might be the different neuroimaging methods used in each study. Whereas, Selmeczy et al. (10) used both 1.5 and 3.0 Tesla MRI, the study conducted by Hessel et al. (9) only used a 3.0 T structural imaging.
Interestingly, even though Selmeczy et al. (10) found no difference between groups in the amygdala volume, a significant negative correlation was found between amygdala volume and the lower range of CGG repeat expansion (CGG≥55 and <85), but not in the higher range of CGG repeat expansion (CGG≥85). This observation raises the intriguing possibility that different molecular mechanisms could be affecting the brain structure, and potentially the function, in FMR1 premutation carriers depending on the CGG repeat expansion size.
The relationship between psychological symptoms and hippocampal function and volume in FMR1 premutation carriers has also been examined.
Reduced hippocampal activation during an fMRI memory recall task has been associated to psychiatric symptomology in FMR1 premutation male carriers without FXTAS (6). Moreover, this reduction was found in association with parietal over-activation, which might be a feedback effect to compensate the decreased hippocampal involvement, and abnormal elevation of FMR1 mRNA.
A hippocampal volume effect has been associated with FMR1 premutation, albeit some inconsistent findings. While Jäkälä et al. (12) found reduced volumes; Loesch et al. (13) described increased volumes in this region in premutation carriers. On the other hand, Koldewyn et al. (6) did not find hippocampal volume effects associated with the premutation. The lack of consistent findings between these three studies may be due to specific cohort effects, especially if these cohorts included participants with and without FXTAS.
To our knowledge, only two papers explored the relationship between psychological measures and hippocampal size. While Koldewyn et al. (6) did not find hippocampal volume differences, Adams et al. (7) found a significant negative correlation between total hippocampal volume and anxiety in female carriers with and without FXTAS. This association seemed to be mainly driven by the right hippocampus since correlations were stronger. The association in male permutation carriers was weaker and was only significant for one of the psychological problems assessed (paranoid ideation). Furthermore, they found a negative correlation between CGG repeat size and total and left hippocampal volume in males with FXTAS and a similar correlation with CGG repeat length and right hippocampal volume in females with FXTAS (7).
The selected studies also showed other structural findings in FMR1 premutation carriers, even though there was no correlation with psychological symptoms. First, total brain volume has been found to be significantly decreased in older permutation carriers (13). Secondly, gray matter loss has also been examined in several studies: all of them evidencing reduced density in several brain regions in the permutation group. In this regard, Hashimoto et al. (8) reported significant gray matter loss in medial temporal lobe structures and cerebellar areas, such as the vermis lobule VII, and the cerebellar hemisphere lobule IX, as well as in multiple regions outside the cerebellum that have been related with several psychiatric conditions. Although significant loss was found when comparing the permutation group with controls, the correlation analysis failed to show significant association between all these areas and psychiatric problems in FMR1 premutation carriers. Finally, Brown et al. (11) found that permutation carriers exhibited significantly lower BOLD activation compared to controls at the bilateral superior parietal lobe, bilateral Brodmann Area (BA) 17 (V1) (primary visual cortex), right intraparietal area, and right BA18 (V2) (visual association area), when comparing high and low arousal conditions. However, no correlations were found between more psychiatric symptoms and higher levels of autistic traits observed in carriers and BOLD activation at the emotional processing fMRI task.
Overall, the studies herein reviewed provided valuable neuroimaging data of brain abnormalities in FMR1 premutation carriers related to neuropsychiatric disorders. However, the majority of them were conducted on small sample sizes of groups, which might have limited detection of true significances. Moreover, some of them included FMR1 premutation carriers with FXTAS which might also influence significant results. In addition, the neuroimaging methods used were different, which makes it difficult to compare results. It should also be noted that fMRI is a complex technique that can be influenced by many factors such as the paradigm design (the manner of stimulating the brain in order to obtain meaningful information), magnetic field strength, MRI acquisition parameter and subject collaboration. Furthermore, the parameters that have an influence in blood flow and oxygenation have an impact on fMRI signal and, overall, fMRI has a challenging data interpretation (14). Finally, there are several variables that have to be taken into account as potential confounding factors in all the studies reviewed. Aspects such as comorbid medical conditions, medication taken by the participants (psychotropic or psychoactive) or unmeasured (unobserved) factors, such as the mood or the stress of participants, could have influenced the results. The reviewed studies were aware of these aspects and tried to minimize their effect by matching FMR1 premutation and control groups, although this was not always possible.
FMR1 premutation carriers are at risk of developing an adult-onset neurodegenerative disorder named FXTAS (20). In addition, several studies reveal that young FMR1 premutation carriers are at increased risk for psychiatric conditions, memory problems and executive deficits (15–18). Indeed, brain function is also affected by FMR1 premutation status in relatively young premutation carriers without FXTAS who demonstrate no overt neurological symptoms (5, 6, 9, 11). Contrary to movement-related neurodegeneration, which increases over time, emotional symptoms seem to be consistent over the lifespan in FMR1 premutation carriers; suggesting a neurodevelopmental origin, different from the neurodegeneration seen in FXTAS (7, 11, 19).
FMR1 premutation carriers have elevated levels of FMR1 mRNA and can also have some degree of FMR1 protein (FMRP) deficiency, mainly at the high end of the FMR1 premutation range (20). FMRP is an RNA binding protein that regulates the translation of many gene products and has been implicated in dendritic maturation and in the formation of axons and myelin (21–23). In fact, FMRP activity is regulated in response to neuronal activity, and is an important mediator of synapse development, synaptic plasticity, learning and memory (reviewed in (52)). The mRNA targets of FMRP have received additional attention due to their enrichment for genes harboring risk to psychiatric disorders (24). Recently, Clifton et al. (52) has reported that a substantial proportion of FMRP targets have functions related to synaptic activity, anatomy or development. The association between synaptic plasticity and psychiatric disorders has been well-established with several genetic and functional studies describing the relevance of imbalanced of excitation and inhibition (25). FMRP levels have been reported to be reduced in FMR1 premutation brains of a mouse model (26), as well as in patients with psychiatric disorders such as autism, schizophrenia, bipolar disorder, and major depressive disorder (27). Whilst some studies had found a relationship between FMR1 mRNA levels and psychiatric symptomology or brain function in FMR1 premutation carriers (5, 6, 28), others have pointed out a stronger relationship with reduced FMRP levels (9). Taking into consideration the importance of FMRP in normal neurodevelopment and its association with psychiatric disorders, there is the possibility that moderate reductions in FMRP levels could play a role in the behavioral dysfunction seen in FMR1 premutation carriers.
Both structural and functional changes in the hippocampus and amygdala have been found to be altered in FMR1 premutation carriers and some studies have proved a relationship between such changes and psychiatric symptomatology (5–9). However, while functional changes have been consistently reported (5, 6, 9, 11), volumetric measures showed some inconsistent results, with some studies showing increase, decrease, or no significant differences between hippocampal and amygdala volumes or voxel density in FMR1 premutation carriers (6, 10, 12, 13, 29–31). The lack of consistent findings between studies may be due to a cohort effect either in the size, the inclusion of participants with and without FXTAS, gender of participants or differences in the CGG repeat size. Moreover, technical aspects such as the volumetric techniques used, the image quality or segmentation technique followed might also contribute to explain discrepancies. However, and in consonance, findings of amygdala and hippocampal volumes in mood disorders such as depression or anxiety in non FMR1 premutation carriers have also been conflicting, with some studies reporting positive, negative and no associations (32–46).
Although an association between the above described structural and functional changes and molecular aspects of the FMR1 pemutation carriers has been proven in some of the studies reported (Table 3), there is still need to better define them. What does seem certain, and evidence points to it, is that the limbic system is a brain structure particularly susceptible to RNA toxicity. During normal fetal development, the hippocampus is one of the areas in which FMR1 transcription is the highest (47) and in adult human brain, the hippocampus demonstrates one of the highest expression rates of FMR1 mRNA (48). Similarly, FMR1 mRNA levels are disproportionately increased in the amygdala of FMR1 premutation carriers (48, 49). Moreover, in post-mortem brain studies of male FMR1 premutation carriers with FXTAS, it has been shown that, compared to other brain areas, the hippocampi harbors high density of intranuclear inclusions (22, 23). Additionally, the knock-in mouse model of the FMR1 premutation showed a significantly reduced FMRP expression in several brain regions, including the hippocampus (50).
Future studies with larger and more homogeneous sample size are needed in order to increase statistical power and validate such findings. Furthermore, longitudinal studies will be needed to evaluate progression of the neuroimaging and clinical findings. In addition, looking for modifying factors, either predisposing or protective factors, able to modulate neuroimaging, and clinical symptomatology is a key point for the knowledge and understanding of the disease. It is also crucial to clarify the metabolic causes of brain toxicity and to identify early presymptomatic brain changes that precede, but which are ultimately associated with neuropsychiatry disorders. Finally, further investigations that include quantitative measurements of molecular changes will be of great interest in order to clarify the relative roles of increased FMR1 mRNA and FMRP protein changes in the Fragile X-associated phenotypes.
Overall this review would like to encourage all FMR1 research groups furthering investigating the neuropsychiatric involvement in FMR1 premutation with testing other brain systems, with additional probes of brain function and a broader scope of psychiatric symptom measurement. The combination of all these missing data would help to obtain a comprehensive landscape of the neuropsychiatric phenotype associated with the FMR1 premutation
The original contributions presented in the study are included in the article/Supplementary Files, further inquiries can be directed to the corresponding author/s.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was supported by the Instituto de Salud Carlos III (PI17/01067), co-financed by Fondo Europeo de Desarrollo Regional (FEDER) una manera de hacer Europa AGAUR from the Autonomous Catalan Government (2017SGR1134). The CIBER de Enfermedades Raras is an initiative of the Instituto de Salud Carlos III.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.728952/full#supplementary-material
1. Mila M, Alvarez-Mora MI, Madrigal I, Rodriguez-Revenga L. Fragile X syndrome: an overview and update of the FMR1 gene. Clin Genet. (2018) 93:197–205. doi: 10.1111/cge.13075
2. Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xunclà M, Badenas C, Kulisevsky J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Human Genet. (2009) 17:1359–62. doi: 10.1038/ejhg.2009.51
3. Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. (2000) 97:189–94. doi: 10.1002/1096-8628(200023)97:3<
4. Hagerman RJ, Protic D, Rajaratnam A, Salcedo-Arellano MJ, Aydin EY, Schneider A. Fragile X-Associated Neuropsychiatric Disorders (FXAND). Front Psychiatry. (2018) 9:564. doi: 10.3389/fpsyt.2018.00564
5. Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, et al. Amygdala dysfunction in men with the fragile X premutation. Brain. (2007) 130:404–16. doi: 10.1093/brain/awl338
6. Koldewyn K, Hessl D, Adams J, Tassone F, Hagerman PJ, Hagerman RJ, et al. Reduced hippocampal activation during recall is associated with elevated FMR1 mRNA and psychiatric symptoms in men with the Fragile X Premutation. Brain Imaging Behav. (2008) 2:105–16. doi: 10.1007/s11682-008-9020-9
7. Adams PE, Adams JS, Nguyen DV, Hessl D, Brunberg JA, Tassone F, et al. Psychological symptoms correlate with reduced hippocampal volume in fragile X premutation carriers. Am J Med Genet B Neuropsychiatr Genet. (2010) 53B:775–85. doi: 10.1002/ajmg.b.31046
8. Hashimoto R, Javan AK, Tassone F, Hagerman RJ, Rivera SM. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. (2011) 134:863–78. doi: 10.1093/brain/awq368
9. Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, et al. Decreased fragile X mental retardation protein expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biol Psychiatry. (2011) 70:859–65. doi: 10.1016/j.biopsych.2011.05.033
10. Selmeczy D, Koldewyn K, Wang JM, Lee A, Harvey D, Hessl DR, et al. Investigation of amygdala volume in men with the fragile X premutation. Brain Imaging Behav. (2011) 5:285–94. doi: 10.1007/s11682-011-9132-5
11. Brown SSG, Whalley HC, Kind PC, Stanfield AC. Decreased functional brain response to emotional arousal and increased psychiatric symptomology in FMR1 premutation carriers. Psychiatry Res Neuroimaging. (2019) 285:9–17. doi: 10.1016/j.pscychresns.2019.01.011
12. Jäkälä P, Hänninen T, Ryynänen M, Laakso M, Partanen K, Mannermaa A, et al. Fragile-X: neuropsychological test performance, CGG triplet repeat lengths, hippocampal volumes. J Clin Invest. (1997) 100:331–8. doi: 10.1172/JCI119538
13. Loesch DZ, Litewka L, Brotchie P, Huggins RM, Tassone F, Cook M. Magnetic resonance imaging study in older fragile X premutation male carriers. Ann Neurol. (2005) 58:326–30. doi: 10.1002/ana.20542
14. Specht K. Current challenges in translational and clinical fMRI and future directions. Front Psychiatry. (2020) 10:924. doi: 10.3389/fpsyt.2019.00924
15. Aziz M, Stathopulu E, Callias M, Taylor C, Turk J, Oostra B, et al. Clinical features of boys with fragile X premutations and intermediate alleles. Am J Med Genet B Neuropsychiatr Genet. (2003) 121B:119–27. doi: 10.1002/ajmg.b.20030
16. Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. (2006) 27:S137–44. doi: 10.1097/00004703-200604002-00012
17. Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, et al. No evidence for a difference in neuropsychological profile among carriers and noncarriers of the FMR1 premutation in adults under the age of 50. Am J Hum Genet. (2008) 83:692–702. doi: 10.1016/j.ajhg.2008.10.021
18. Chonchaiya W, Au J, Schneider A, Hessl D, Harris SW, Laird M, et al. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. (2012) 131:581–9. doi: 10.1007/s00439-011-1106-6
19. Brown SSG, Basu S, Whalley HC, Kind PC, Stanfield AC. Age-related functional brain changes in FMR1 premutation carriers. Neuroimage Clin. (2017) 17:761–7. doi: 10.1016/j.nicl.2017.12.016
20. Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. (2013) 12:786–98. doi: 10.1016/S1474-4422(13)70125-X
21. Inoue SB, Siomi MC, Siomi H. Molecular mechanisms of fragile X syndrome. J Med Invest. (2000) 47:101–7.
22. Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR, Jacquemont S, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. (2002) 125:1760–71. doi: 10.1093/brain/awf184
23. Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain. (2006) 129:243–55. doi: 10.1093/brain/awh683
24. Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. (2011) 146:247–61. doi: 10.1016/j.cell.2011.06.013
25. Howard DM, Adams MJ, Shirali M, Clarke TK, Marioni RE, Davies G, et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nature Commun. (2018) 9:1470. doi: 10.1038/s41467-018-03819-3
26. Qin M, Entezam A, Usdin K, Huang T, Liu ZH, Hoffman GE, et al. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiol Dis. (2011) 42:85–98. doi: 10.1016/j.nbd.2011.01.008
27. Fatemi SH, Folsom TD. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology. (2011) 60:1221–26. doi: 10.1016/j.neuropharm.2010.11.011
28. Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. (2005) 139B:115–21. doi: 10.1002/ajmg.b.30241
29. Moore CJ, Daly EM, Tassone F, Tysoe C, Schmitz N, Ng V, et al. The effect of pre-mutation of X chromosome CGG trinucleotide repeats on brain anatomy. Brain. (2004) 127:2672–81. doi: 10.1093/brain/awh256
30. Adams JS, Adams PE, Nguyen D, Brunberg JA, Tassone F, Zhang W, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS). Neurology. (2007) 69:851–9. doi: 10.1212/01.wnl.0000269781.10417.7b
31. Wang JY, Hessl DH, Hagerman RJ, Tassone F, Rivera SM. Age-dependent structural connectivity effects in fragile X premutation. Arch Neurol. (2012) 69:482–9. doi: 10.1001/archneurol.2011.2023
32. Hajek T, Kopecek M, Kozeny J, Gunde E, Alda M, Höschl C. Amygdala volumes in mood disorders–meta-analysis of magnetic resonance volumetry studies. J Affect Disord. (2009) 115:395–410. doi: 10.1016/j.jad.2008.10.007
33. Spampinato MV, Wood JN, De Simone V, Grafman J. Neural correlates of anxiety in healthy volunteers: a voxel-based morphometry study. J Neuropsychiatry Clin Neurosci. (2009) 21:199–205. doi: 10.1176/jnp.2009.21.2.199
34. Iidaka T, Matsumoto A, Ozaki N, Suzuki T, Iwata N, Yamamoto Y, et al. Volume of left amygdala subregion predicted temperamental trait of harm avoidance in female young subjects. A voxel-based morphometry study. Brain Res. (2006) 1125:85–93. doi: 10.1016/j.brainres.2006.09.015
35. Hu Y, Moore M, Bertels Z, Phan KL, Dolcos F, Dolcos S. Smaller amygdala volume and increased neuroticism predict anxiety symptoms in healthy subjects: a volumetric approach using manual tracing. Neuropsychologia. (2020) 145:106564. doi: 10.1016/j.neuropsychologia.2017.11.008
36. Barrós-Loscertales A, Meseguer V, Sanjuán A, Belloch V, Parcet MA, Torrubia R, et al. Behavioral inhibition system activity is associated with increased amygdala and hippocampal gray matter volume: a voxel-based morphometry study. Neuroimage. (2006) 33:1011–15. doi: 10.1016/j.neuroimage.2006.07.025
37. Baur V, Hänggi J, Jäncke L. Volumetric associations between uncinate fasciculus, amygdala, trait anxiety. BMC Neurosci. (2012) 13:4. doi: 10.1186/1471-2202-13-4
38. Potvin O, Catheline G, Bernard C, Meillon C, Bergua V, Allard M, et al. Gray matter characteristics associated with trait anxiety in older adults are moderated by depression. Int Psychogeriatr. (2015) 27:1813–24. doi: 10.1017/S1041610215000836
39. Blackmon K, Barr WB, Carlson C, Devinsky O, DuBois J, Pogash D, et al. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Res. (2011) 194:296–303. doi: 10.1016/j.pscychresns.2011.05.007
40. Taki Y, Kinomura S, Awata S, Inoue K, Sato K, Ito H, et al. Male elderly subthreshold depression patients have smaller volume of medial part of prefrontal cortex and precentral gyrus compared with age-matched normal subjects: a voxel-based morphometry. J Affect Disord. (2005) 88:313–20. doi: 10.1016/j.jad.2005.08.003
41. Spalletta G, Piras F, Caltagirone C, Fagioli S. Hippocampal multimodal structural changes and subclinical depression in healthy individuals. J Affect Disord. (2014) 152-154:105–12. doi: 10.1016/j.jad.2013.05.068
42. Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J Psychiatry Neurosci. (2009) 34:367–75.
43. O'Shea DM, Dotson VM, Woods AJ, Porges EC, Williamson JB, O'Shea A, et al. Depressive symptom dimensions and their association with hippocampal and entorhinal cortex volumes in community dwelling older adults. Front Aging Neurosci. (2018) 10:40. doi: 10.3389/fnagi.2018.00040
44. Osler M, Sørensen L, Rozing M, Calvo OP, Nielsen M, Rostrup E. Subclinical depressive symptoms during late midlife and structural brain alterations: a longitudinal study of Danish men born in 1953. Hum Brain Mapp. (2018) 39:1789–95. doi: 10.1002/hbm.23954
45. Goveas JS, Espeland MA, Hogan P, Dotson V, Tarima S, Coker LH, et al. Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women's Health Initiative MRI Study. J Affect Disord. (2011) 132:275–84. doi: 10.1016/j.jad.2011.01.020
46. Szymkowicz SM, Woods AJ, Dotson VM, Porges EC, Nissim NR, O'Shea A, et al. Associations between subclinical depressive symptoms and reduced brain volume in middle-aged to older adults. Aging Mental Health. (2019) 23:819–30. doi: 10.1080/13607863.2018.1432030
47. Abitbol M, Menini C, Delezoide AL, Rhyner T, Vekemans M, Mallet J. Nucleus basalis magnocellularis and hippocampus are the major sites of FMR-1 expression in the human fetal brain. Nature Genet. (1993) 4:147–53. doi: 10.1038/ng0693-147
48. Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. (2004) 41:e43. doi: 10.1136/jmg.2003.012518
49. Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS). RNA Biol. (2004) 1:103–5. doi: 10.4161/rna.1.2.1035
50. Entezam A, Biacsi R, Orrison B, Saha T, Hoffman GE, Grabczyk E, et al. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. (2007) 395:125–34. doi: 10.1016/j.gene.2007.02.026
51. Johnson K, Herring J, Richstein J. Fragile X Premutation Associated Conditions (FXPAC). Front Pediatr. (2020) 8:266. doi: 10.3389/fped.2020.00266
Keywords: FXAND, neuropathology, FMR1 premutation, neuroimaging, functional studies
Citation: Elias-Mas A, Alvarez-Mora MI, Caro-Benito C and Rodriguez-Revenga L (2021) Neuroimaging Insight Into Fragile X-Associated Neuropsychiatric Disorders: Literature Review. Front. Psychiatry 12:728952. doi: 10.3389/fpsyt.2021.728952
Received: 22 June 2021; Accepted: 17 September 2021;
Published: 15 October 2021.
Edited by:
Randi Jenssen Hagerman, MIND Institute, UC Davis, United StatesReviewed by:
Isabelle Esther Bauer, University of Texas Health Science Center at Houston, United StatesCopyright © 2021 Elias-Mas, Alvarez-Mora, Caro-Benito and Rodriguez-Revenga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laia Rodriguez-Revenga, bGJvZGlAY2xpbmljLmNhdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.