
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 01 October 2021
Sec. Mood Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.713614
 Zijian Zhang1,2†
Zijian Zhang1,2† Yayun Chen1,2,3†
Yayun Chen1,2,3† Wei Wei1,2
Wei Wei1,2 Xiao Yang1,2
Xiao Yang1,2 Yajing Meng1,2
Yajing Meng1,2 Hua Yu1,2
Hua Yu1,2 Wanjun Guo1,2
Wanjun Guo1,2 Qiang Wang1,2
Qiang Wang1,2 Wei Deng1,2
Wei Deng1,2 Tao Li1,2
Tao Li1,2 Xiaohong Ma1,2*
Xiaohong Ma1,2*Background: Neurobiological mechanisms underlying the development of major depressive disorder (MDD) may differ depending on onset ages. Our aim was to determine whether regional homogeneity (ReHo) changes in early-onset depression (EOD) and late-onset depression (LOD) are different, which could also delineate EOD and LOD.
Methods: Ninety-one MDD patients and 115 healthy controls (HCs) were recruited, and resting-state functional magnetic resonance imaging data were collected. The ReHo comparison was conducted using analysis of variance.
Results: Compared with HCs, MDD patients showed decreased ReHo in the left precentral gyrus and the left middle cingulum area, and increased ReHo in the left middle orbital frontal gyrus and superior temporal gyrus. Compared with LOD patients, young HC separately, EOD patients had significantly increased ReHo in the right inferior frontal triangular gyrus and the left postcentral gyrus. However, compared with young HC, EOD patients showed decreased ReHo in the right superior frontal gyrus/supplementary motor area and the right medial frontal gyrus. ReHo in the right inferior frontal triangular gyrus was negatively correlated with the severity of cognitive disturbance in LOD patients (r = −0.47, p = 0.002), but not in EOD patients (r = 0.21, p = 0.178).
Conclusion: MDD patients with different onset ages may have different pathophysiological mechanisms; the EOD patients had more abnormal ReHo than LOD patients in the prefrontal lobe, especially the right inferior frontal triangular gyrus.
Major depressive disorder (MDD) is one of the most frequent psychiatric disorders with a lifetime prevalence of 15–30%, which is mainly characterized by depressed mood, decreased interest, lack of pleasure, slow thinking, worthlessness or guilt, reduced energy, impaired cognition, vegetative symptoms, and suicidal tendency (1–3). The current classification of MDD essentially relies on the presence of a defined number of clinical features and a time period over which these symptoms need to be present, as well as by identification of social function impairment (4, 5). MDD is a highly heterogeneous disorder, and there is a lack of consistent genetic and neurobiological biomarkers that can validate MDD as a distinct or even clear diagnostic entity (6, 7). Furthermore, heterogeneity of clinical presentations and individual differences make it difficult for us to deduce standardized and effective treatment strategies for MDD patients (8). Therefore, understanding the depressive subtypes is important to clarify the pathogenesis of MDD and further guide clinical treatment.
As mentioned above, clinical features of MDD patients during disease episode may vary largely. For instance, symptoms and characteristics of adults with early-onset depression (EOD) differ from those with late-onset depression (LOD) (9–11). EOD patients are associated with more suicide attempts and thoughts, sadness, irritability, diminished concentration, more psychiatric symptoms, agitation, more anxiety, neuroticism, atypical symptoms, higher prevalence of comorbid personality disorders, and higher prevalence of stressful life events preceding onset than LOD patients (10, 12–15). Several studies have indicated that EOD patients have poor outcomes, including unfavorable treatment response, longer duration of symptoms, greater illness severity, higher relapse rate (10, 11, 16, 17), and greater duration of illness, after adjusting for current age (12).
Furthermore, there seems to be a trend that MDD patients with earlier onset age may be more strongly affected by genetic factors (18–25). For example, a research from Sequenced Treatment Alternative to Relieving Depression (STAR * D) reported that MDD patients with positive family history for depression have a younger onset age than those without (26). In addition, the heritability of MDD is about 37% (27), and the heritability of EOD (<30 years) is higher than that of LOD (>30 years) (47 vs. 10%) (28). Tozzi et al. (19) reported that patients with onset age after 50 years old are not associated with a family history of MDD. A meta-review indicated that the different onset-age depression might be considered as different subtypes of depression (29). Taken together, the neurobiological mechanisms underlying the development of MDD in different onset age may vary. However, definitions of EOD and LOD in the literature have been inconsistent in their age cutoffs.
The median onset age for mood disorders is between 25 and 32 years (30). The cutoff age to differentiate EOD and LOD differs between studies and varies between 20 and 60 years old (9–11, 13, 15, 19, 31–34). A comparison of studies will result in inconsistent findings. Considering the role of different onset age in the pathology of MDD, it is important to define the age cutoff between EOD and LOD according to a biologically based criterium. Therefore, we chose a first-onset cutoff of 25 years, which is associated with family history of MDD (35, 36), and has been used as a cutoff in previous research on mood disorders (15, 34, 37–39) and genetic association studies (40). However, little is known about the neural mechanism underlying the difference between EOD and LOD subtypes.
For decades, neuroimaging has been widely used to explore the neuro- and pathomechanism of MDD. Regional homogeneity (ReHo) has been shown to be an effective index to measure spontaneous neural activity in resting-state functional magnetic resonance imaging (rs-fMRI) analysis (41). ReHo reflects the local neural activity coherence, which measures the homogeneity of the time course in a voxel and its nearest neighbors. Numerous studies have explored spontaneous brain activity in MDD using the ReHo method and provided some evidence of brain abnormalities, of which the abnormal ReHo in frontal cortex was most commonly been reported (42–46). Nevertheless, systematically collected neuroimaging data of medication-free EOD and LOD are still limited (39, 47–49). The regional neural activity difference between EOD and LOD remains unclear.
The aim of the current study was to explore the onset-age differences in the phenotypes of depression including clinical symptoms, brain abnormalities, and the relationship between these two aspects. Based on existing literatures, we hypothesized that (1) the MDD patients have decreased or increased regional neural activity homogeneity compared to HCs, especially in frontal lobe; (2) EOD patients may show more severe disturbance not only in clinical symptoms, but also in ReHo than LOD patients; and (3) specific brain alterations of EOD and LOD patients may be associated with different clinical symptoms.
This study recruited 206 right-handed participants including 91 patients with MDD and 115 gender- and age-matched healthy controls (HCs). Patients were recruited at the Mental Health Center of West China Hospital, Sichuan University. Diagnosis was based on the Structured Clinical Interview (SCID) for DSM-IV and no patients with MDD had current comorbid Axis I or II diagnosis (such as psychosis, substance abuse, and personality disorder). In addition, patients who had taken any antidepressants during the past 3 months before scanning were excluded. At the time of the study, 61 patients were drug-naive and 30 had been medication-free for at least 3 months. HCs were recruited by advertisements in local communities. HCs without a lifetime history of any DSM-IV psychiatric disorder were recruited and not taking any antipsychotics or antidepressants. Furthermore, HCs with a family history of psychiatric disorders were excluded. Written informed consent was given by all participants after the study procedure had been fully explained. Exclusion criteria of patients and HCs were as follows: pregnancy, neurological or internal systemic diseases, a history of acute physical illness, a history of head injury resulting in loss of consciousness, and major neurological disorders, cardiovascular disease, intellectual disability, alcohol or substance abuse, contraindications to MRI, and a previous history of electroconvulsive therapy within the last 6 months. All participants were Han Chinese between the ages of 18 and 53 years.
Data were collected on age, gender, education level, the number of episodes, age of onset, and illness duration of all participants from their medical record. Clinical symptoms were assessed using the 17-item Hamilton Rating Scale for Depression (HDRS), which provided a total score and could be divided into five factors (anxiety/somatization, weight, cognitive disturbance, retardation, and sleep disturbance) (50). All patients were experiencing a major depressive episode, with their HDRS total scores being at least 17 on the day of scanning. We divided all patients into two subgroups: the EOD group (18–25 years old) and the LOD group (26–53 years old). HCs were also divided into two subgroups (young HCs and old HCs) matching for each patient subgroup. The onset age is defined by the age at which depressive symptoms firstly presented according to patients' recalls. The symptoms meet the diagnostic criteria for major depressive episodes in the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV).
All magnetic resonance imaging (MRI) data were acquired on a Philips 3.0-T scanner (Achieva TX, Best, the Netherlands), equipped with an eight-channel phased-array head coil. Gradient-echo-planar imaging sequence was applied to collect the rs-fMRI data with the following parameters: time repetition (TR) = 2,000 ms, echo time (TE) = 30 ms, flip angle = 90°, 38 slices, in-plane matrix =64 × 64, field of view (FOV) = 240 × 240 mm2, and voxel size = 3.75 × 3.75 × 4 mm3. For each participant, the rs-fMRI scanning lasted for 480 s, and 240 volumes were obtained. During the scanning, participants were fitted with soft ear plugs and instructed to relax with their eyes closed, and stay awake without moving.
To spatially normalize and localize better, high-resolution T1 images were acquired by 3D magnetization-prepared rapid gradient-echo sequence as follows: TR = 8.37 ms, TE = 3.88 ms, flip angle = 7°, matrix = 256 × 256, FOV = 240 × 240 mm2, voxel size =1.00 × 1.00 × 1 mm3, and 188 slices.
These rs-fMRI images were analyzed using the Data Processing Assistant for Resting-State fMRI (DPARSF, http://rfmri.org/DPARSF) (51) and SPM8 (http://www.fil.ion.ucl.ac.uk/spm), which is implemented in Matlab 2016a (MathWorks, Natick, MA, USA). First, the first 10 volumes were discarded, and functional volumes were slice-time corrected and head motion corrected. All participants in this study had <2 mm displacement and 2° of rotation in any direction. Second, the EPI images were registered to the T1 image, and then spatial normalization was performed to a 3 × 3 × 3 mm3 Montreal Neurological Institute template in SPM8. Third, the linear systematic drift of resulting data was removed and filtered at 0.01–0.08Hz frequency spectrum at each voxel. Finally, mean signal of white matter (WM), cerebrospinal fluid (CSF), and Friston 24-parameter model of head motion were regressed out.
Individual ReHo maps were generated by calculating the Kendall's coefficient concordance of the time series of a given voxel with those of its nearest neighbors (26 voxels) in a voxel-wise manner (41). To reduce the effect of individual variations on the Kendall's coefficient of concordance value, ReHo maps were normalized by dividing the averaged Kendall's coefficient of concordance among each voxel of the whole brain. ReHo maps were smoothed with a 6-mm full-width half-maximum isotropic Gaussian kernel.
We compared the demographic and clinical data using the independent two-sample t-test, Chi-square test, or analysis of variance (ANOVA) between subgroups when appropriate, in Statistical package for Social Sciences (SPSS 22.0 for Windows). The significance threshold was set at p < 0.05.
Because the abnormal activation characteristic/pattern of brain often results from disease (depression), onset-age factor, and their interaction, a 2 × 2 full factorial model was used in SPM8, assessing the effects of onset age (young vs. old) and diagnosis (MDD vs. HCs) with age, gender, and education level as covariates; the interaction between onset age and diagnosis and the main effects of age-onset and diagnosis were tested. Multiple comparisons were corrected using the Monte Carlo simulation-based Alphasim program (1,000 times): the initial threshold on voxel level was set as p < 0.001, the cluster level was p < 0.05, and the corresponding cluster size was >16 (connection radius = 5 mm for correction).
Post-hoc analysis was conducted on extracted values of the clusters (Figure 1) that reached significance in the interaction analysis mentioned above. This analysis was completed in SPSS; Bonferroni-corrected pairwise comparisons were calculated to further investigate significant simple effects. To investigate the relationship between abnormal ReHo and clinical features, partial correlations were analyzed to explore the correlation between these abnormal values of ReHo and HDRS score or illness duration in each patient subgroup (age, gender, and years of education as covariates).
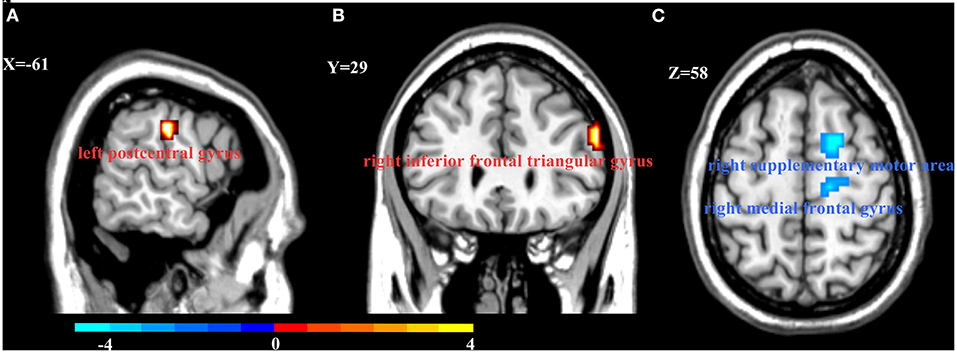
Figure 1. Interaction between onset age and depression on Regional homogeneity (ReHo). Compared with young HCs and LOD patients, EOD patients had increased ReHo in the left postcentral gyrus (A) and right inferior frontal triangular gyrus (B). However, EOD patients had significantly decreased ReHo in the right superior frontal gyrus/supplementary motor area and the right medial frontal gyrus (C) than young HCs. Color bar represent the t-value.
The study had 46 EOD patients, 45 LOD patients, 65 young HCs, and 50 old HCs. Patient subgroups did not differ significantly in gender, education years, number of episodes, HDRS scores, or illness course. Compared with each age-matched comparison group, no significant difference was observed in gender or age in the EOD and LOD groups except fewer education years in the old HC group. In addition, LOD patients have more severe sleep disturbance than EOD patients (Table 1).
There was a significant interaction between onset age and depression in the inferior frontal triangular gyrus, postcentral gyrus, superior frontal gyrus (supplementary motor area), and medial frontal gyrus. Further post-hoc analysis showed that compared with LOD patients and young HC, EOD patients had significantly increased ReHo in the right inferior frontal triangular gyrus and the left postcentral gyrus. However, compared with young HC, EOD patients showed decreased ReHo in the right superior frontal gyrus/supplementary motor area and the right medial frontal gyrus (Table 2; Figures 1, 2). The old HCs and LOD patients did not differ significantly in ReHo.
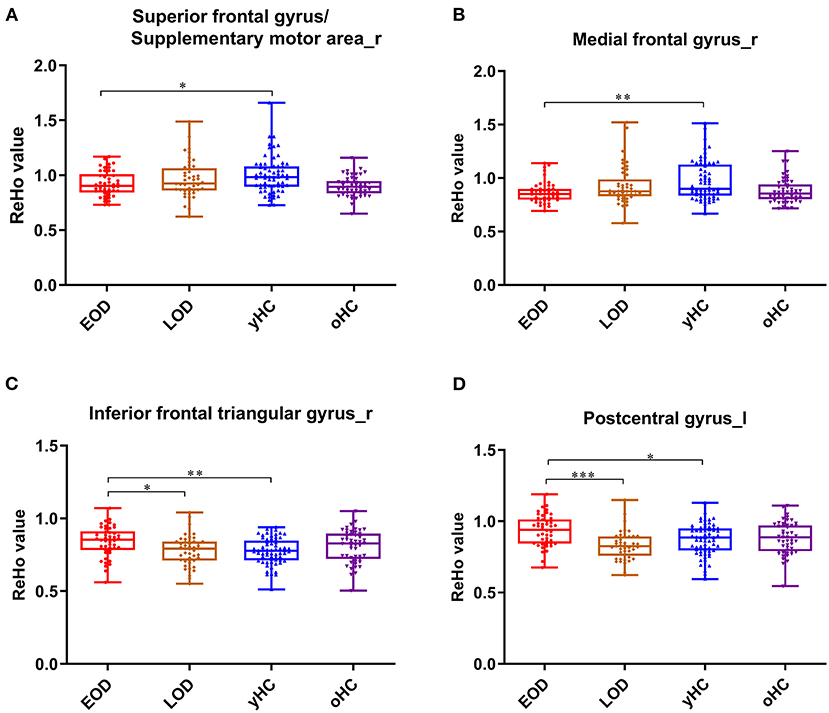
Figure 2. The box plots for the comparison between EOD, LOD patients, and young and old HCs. In the right superior frontal gyrus (supplementary motor area) (A), right medial frontal gyrus (B), right inferior frontal triangular gyrus (C), and lest postcentral gyrus (D). *p < 0.05, **p < 0.01, ***p < 0.001.
We also explored the main effects of onset age and depression separately (Table 2; Figure 3). We focused on the main effects of depression here; compared with HCs, MDD patients showed decreased ReHo in the left precentral gyrus and left middle cingulum area, and increased ReHo in the left middle orbital frontal gyrus and right superior temporal gyrus.
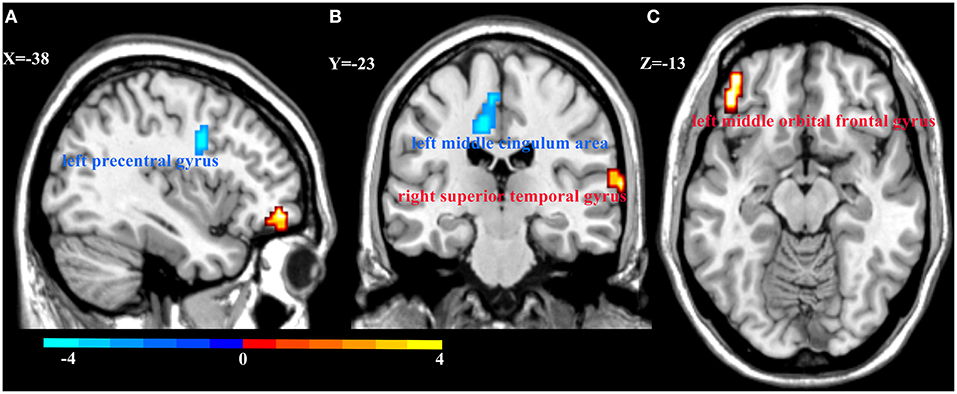
Figure 3. Regional homogeneity (ReHo) alterations in MDD patients and HCs. Compared with HCs, patients with MDD showed decreased ReHo in the left precentral gyrus (A) and left middle cingulum area (B), and increased ReHo in the right superior temporal gyrus (B) and left middle orbital frontal gyrus (C). Color bar represent the t-value.
With age, gender, and years of education as covariates, we found that ReHo in the right inferior frontal triangular gyrus was negatively correlated with the severity of cognitive disturbance in LOD patients (r = −0.47, p = 0.002). However, ReHo in the right inferior frontal triangular gyrus was positively correlated with the severity of cognitive disturbance in EOD patients, but not significant (r = 0.21, p = 0.178) (Figure 4). No other significant correlations were detected (Table 3).
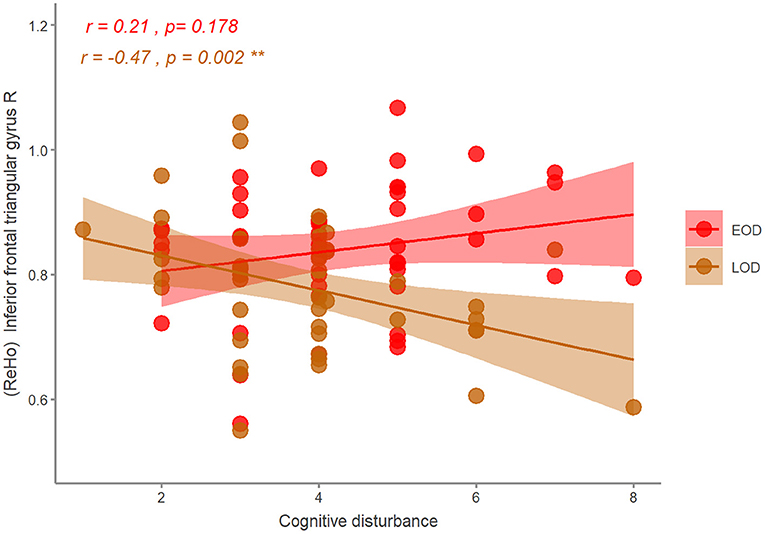
Figure 4. The correlation of the ReHo and HDRS symptom in EOD and LOD patients. EOD, early-onset depression; LOD, late-onset depression. **p < 0.01.
The present study is the first large-scale clinical study to examine the characteristics of medication-free MDD patients with different onset age by combining clinical presentation and neuroimaging phenotypes. Our study indicated that EOD patients were associated with greater disturbances in ReHo than LOD patients, and this disturbance was related to the symptoms of different onset-age patients. The present study provides novel insights into how development of depression is depicted by different onset age.
At the neuroimaging level, MDD patients showed decreased ReHo in the left precentral gyrus and the left middle cingulum area, and increased ReHo in the left middle orbital frontal gyrus and right superior temporal gyrus than HCs. These results may provide some evidence for the impairment of reward processing in MDD patients, consistent with previous studies. On the one hand, OFC is involved in non-reward and punishing events, and has increased functional connectivity with self-referential processes (medial prefrontal cortex, precuneus, and posterior cingulate cortex) causing loss, depression, and frustration (52–54). The increased ReHo of the right superior temporal gyrus lay in the auditory processing network, which may contribute to perceptual impairments in MDD patients (44, 45). On the other hand, cingulate gyrus dysfunction affects the release of dopamine and induces cognitive impairment, which mainly manifests with slow thinking and cognitive dysfunction in MDD patients. Bluhm et al. (55) reported that MDD patients had decreased connectivity between the PCC and the bilateral caudate, regions known to be involved in motivation and reward processing, which is characterized by lack of motivation and loss of pleasure and may be an early manifestation of MDD.
Further results showed that compared with LOD patients and young HCs, EOD patients had significantly increased ReHo in the right inferior frontal triangular gyrus and the left postcentral gyrus. The right inferior frontal triangular gyrus is an important component of the dorsolateral prefrontal cortex (DLPFC). The DLPFC dysfunction may be an early manifestation of MDD and that the right inferior frontal triangular gyrus plays an important role in the pathogenesis of the early stage of MDD; patients with mild DLPFC damage often appear to be uninterested in things, with slow thinking, poor memory, and lack of motivation (56–59). Recently, one study using machine learning methods reported the abnormal ReHo in the left postcentral gyrus and right fusiform gyrus with a high accuracy for discriminating MDD from HC (60). These results and our study provide further evidence for the important role of the prefrontal lobe in MDD, which may help distinguish the depression subtypes for accurate diagnosis and treatment in the future.
Our study found that compared with young HCs, EOD patients showed decreased ReHo in the right superior frontal gyrus/supplementary motor area and the right medial frontal gyrus. On the one hand, this trend of group difference was consistent with Guo et al. (61), who found that, using ALFF indicator, EOD patients had abnormal brain activation in the left superior/inferior temporal gyrus, left lingual gyrus, and right middle occipital gyrus compared to young HCs. On the other hand, these abnormal brain areas of EOD patients are mainly concentrated in the prefrontal gyrus (PFC) (39). The PFC is the executive control center for information processing, responsible for the processing of functional cognitive processes and related to the control of emotion and conflicting behaviors (62). Frontal hyperactivity has been viewed as an exaggerated or maladaptive compensatory process, serving to improve a persistent negative mood generated by abnormal chronic activity of limbic–subcortical structures. However, frontal hypo-metabolism may be associated with the failure to initiate or maintain a compensatory state (63), and then the depression became more serious. These studies suggest that the PFC may be the key area for the treatment of brain damage and disease in EOD patients. Moreover, our study found that compared with HCs, there were no significant changes in ReHo of LOD patients, which indicated that the degree of local function impairment is more serious in EOD patients than in LOD patients. The potential pathophysiological mechanisms of MDD patients at different onset age may be different.
Sleep disturbances are the major somatic symptoms in MDD patients (64), while LOD patients experience more severe sleep disturbances, especially early morning awakening. Sleep disturbance is a significant risk factor for the onset, exacerbation, and relapse of mood disorders (65). In addition to changes in sleep architecture, MDD patients show profoundly altered patterns of nocturnal hormone secretion, possibly through mechanisms that link regulation of sleep with neuroendocrine activity (66). Early relief of insomnia in MDD patients, in addition to alleviating other symptoms, may increase adherence to treatment and increase daytime performance and overall functioning, while complete relief of insomnia may improve prognosis (67). The difference on sleep disturbance between EOD and LOD patients may provide further evidence to distinguish these two subtypes of depression.
We found that ReHo in the right inferior frontal triangular gyrus was negatively correlated with the severity of cognitive disturbance in LOD patients. The inferior frontal triangular gyrus was located in the prefrontal lobe, close to the OFC and middle frontal gyrus, which was associated with cognitive control (68, 69). Orbitofrontal cortex plays an important role in decision-making, and decision-making impairment is associated with a susceptibility to suicidal symptoms (70), which is included in the cognitive disturbance. A previous study also reported the decreased ReHo in the right middle frontal gyrus was negatively correlated with depression symptoms in MDD patients (60). On the contrary with LOD patients, we found that the correlation between ReHo in the inferior frontal triangular gyrus and cognitive disturbance of EOD was positive (not significant). Although the ReHo in the right inferior frontal triangular gyrus of EOD was decreasing, it may not directly affect (or be affected by) cognition. A previous study has detected the older depressed patients with severer cognitive impairment (71). Some researchers found that the cortical thickness in the prefrontal cortex was correlated with cognitive impairment of LOD, but not EOD (72), similar to our results using ReHo indicator. This may indicate that the EOD and LOD subgroups have different cognitive impairment and relationship with ReHo in the prefrontal cortex. More studies are needed to clarify this in the future. The combination of clinical symptomology and brain imaging has again confirmed that the prefrontal lobe, especially the right inferior frontal triangular gyrus, is a key brain area to distinguish EOD patients from LOD patients.
However, our study still has some limitations. First, because the present study is a cross-sectional study, we could not exclude the possibility that some of our patients who manifested as having a depressive episode could be later diagnosed as having a bipolar disorder. Further follow-up study may be necessary to clarify the impact of prolonged depressive episodes on brain structure in the future. Second, as in other resting-state fMRI studies, inevitable physiological noises such as respiratory and cardiac fluctuations of the participants during scanning may influence the stability of resting-state fMRI signals. Although we have tried our best to correct such noises, their effects may have not been fully eliminated. In future studies, simultaneous cardiac recording may provide a rigorous correction. Last, there is no consensus on onset age to define EOD and LOD. However, the cutoff age in the present study has been used as a cutoff in previous research on mood disorders (15, 34, 37–39) and genetic association studies (40), and is associated with family history of MDD (35, 36).
We recruited a group of medication-free patients with major depressive disorder in our analysis to minimize confounding factors due to medication use, which could better elucidate ReHo changes that are directly related to the disease itself. MDD patients with different onset ages may have different pathophysiological mechanisms, and EOD patients had more abnormal ReHo than LOD patients in the prefrontal lobe, especially the right inferior frontal triangular gyrus.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of Sichuan University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZZ and YC wrote the main manuscript text. XM, WW, and TL conceived and designed the study. ZZ, YC, TL, WW, XY, YM, HY, WG, QW, and WD recruited the patients, administered assessment tools, and carried out data analysis. XM reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This research was partly funded by the National Natural Science Foundation of China (No. 82001432 and No.81671344), the China Postdoctoral Science Foundation (No. 2020TQ0213 and No. 2020M683319), and 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Prof. Dongtao Lin, Ph.D., of Sichuan University for his instruction in English usage, editorial support, and proofreading and all individuals who have participated in this study.
1. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the national comorbidity survey replication (NCS-R). JAMA. (2003) 289:3095–105. doi: 10.1001/jama.289.23.3095
2. Li CT, Lin CP, Chou KH, Chen IY, Hsieh JC, Wu CL, et al. Structural and cognitive deficits in remitting and non-remitting recurrent depression: a voxel-based morphometric study. Neuroimage. (2010) 50:347–56. doi: 10.1016/j.neuroimage.2009.11.021
3. Meng C, Brandl F, Tahmasian M, Shao J, Manoliu A, Scherr M, et al. Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain. (2014) 137:598–609. doi: 10.1093/brain/awt290
4. Paykel ES. Basic concepts of depression. Dial Clin Neurosci. (2008) 10:279–89. doi: 10.31887/DCNS.2008.10.3/espaykel
5. Baumeister H. A clinical significance criterion is essential for diagnosing subthreshold depression. Am J Psychiatry. (2010) 167:866; author reply 866–7. doi: 10.1176/appi.ajp.2010.10030398
6. Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. (2010) 167:1305–20. doi: 10.1176/appi.ajp.2009.10030434
7. Shyn SI, Hamilton SP. The genetics of major depression: moving beyond the monoamine hypothesis. Psychiatr Clin North Am. (2010) 33:125–40. doi: 10.1016/j.psc.2009.10.004
8. Baumeister H, Hutter N, Bengel J, Harter M. Quality of life in medically ill persons with comorbid mental disorders: a systematic review and meta-analysis. Psychother Psychosom. (2011) 80:275–86. doi: 10.1159/000323404
9. Charlton RA, Lamar M, Ajilore O, Kumar A. Preliminary analysis of age of illness onset effects on symptom profiles in major depressive disorder. Int J Geriatr Psychiatry. (2013) 28:1166–74. doi: 10.1002/gps.3939
10. Korten NC, Comijs HC, Lamers F, Penninx BW. Early and late onset depression in young and middle aged adults: differential symptomatology, characteristics and risk factors? J Affect Disord. (2012) 138:259–67. doi: 10.1016/j.jad.2012.01.042
11. Park SC, Hahn SW, Hwang TY, Kim JM, Jun TY, Lee MS, et al. Does age at onset of first major depressive episode indicate the subtype of major depressive disorder?: the clinical research center for depression study. Yonsei Med J. (2014) 55:1712–20. doi: 10.3349/ymj.2014.55.6.1712
12. Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, et al. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Res. (2004) 129:127–40. doi: 10.1016/j.psychres.2004.07.004
13. Bukh JD, Bock C, Vinberg M, Gether U, Kessing LV. Differences between early and late onset adult depression. Clin Pract Epidemiol Ment Health. (2011) 7:140–7. doi: 10.2174/1745017901107010140
14. Janssen J, Beekman AT, Comijs HC, Deeg DJ, Heeren TJ. Late-life depression: the differences between early- and late-onset illness in a community-based sample. Int J Geriatr Psychiatry. (2006) 21:86–93. doi: 10.1002/gps.1428
15. Parker G, Wilhelm K, Mitchell P, Austin MP, Roussos J, Gladstone G. The influence of anxiety as a risk to early onset major depression. J Affect Disord. (1999) 52:11–7. doi: 10.1016/S0165-0327(98)00084-6
16. Dekker MC, Ferdinand RF, van Lang ND, Bongers IL, van der Ende J, Verhulst FC. Developmental trajectories of depressive symptoms from early childhood to late adolescence: gender differences and adult outcome. J Child Psychol Psychiatry. (2007) 48:657–66. doi: 10.1111/j.1469-7610.2007.01742.x
17. Kemp AH, Gordon E, Rush AJ, Williams LM. Improving the prediction of treatment response in depression: integration of clinical, cognitive, psychophysiological, neuroimaging, genetic measures. CNS Spectr. (2008) 13:1066–86; quiz 1087–8. doi: 10.1017/S1092852900017120
18. Lavori PW, Warshaw M, Klerman G, Mueller TI, Leon A, Rice J, et al. Secular trends in lifetime onset of MDD stratified by selected sociodemographic risk factors. J Psychiatr Res. (1993) 27:95–109. doi: 10.1016/0022-3956(93)90054-6
19. Tozzi F, Prokopenko I, Perry JD, Kennedy JL, McCarthy AD, Holsboer F, et al. Family history of depression is associated with younger age of onset in patients with recurrent depression. Psychol Med. (2008) 38:641–9. doi: 10.1017/S0033291707002681
20. Weissman MM, Wickramaratne P, Nomura Y, Warner V, Verdeli H, Pilowsky DJ, et al. Families at high and low risk for depression: a 3-generation study. Arch Gen Psychiatry. (2005) 62:29–36. doi: 10.1001/archpsyc.62.1.29
21. Williamson DE, Birmaher B, Axelson DA, Ryan ND, Dahl RE. First episode of depression in children at low and high familial risk for depression. J Am Acad Child Adolesc Psychiatry. (2004) 43:291–7. doi: 10.1097/00004583-200403000-00010
22. Brodaty H, Peters K, Boyce P, Hickie I, Parker G, Mitchell P, et al. Age and depression. J Affect Disord. (1991) 23:137–49. doi: 10.1016/0165-0327(91)90026-O
23. Maier W, Lichtermann D, Minges J, Heun R, Hallmayer J, Klingler T. Unipolar depression in the aged: determinants of familial aggregation. J Affect Disord. (1991) 23:53–61. doi: 10.1016/0165-0327(91)90092-7
24. Weissman MM, Wickramaratne P, Merikangas KR, Leckman JF, Prusoff BA, Caruso KA, et al. Onset of major depression in early adulthood. Increased familial loading and specificity. Arch Gen Psychiatry. (1984) 41:1136–43. doi: 10.1001/archpsyc.1984.01790230022003
25. Williamson DE, Ryan ND, Birmaher B, Dahl RE, Kaufman J, Rao U, et al. A case-control family history study of depression in adolescents. J Am Acad Child Adolesc Psychiatry. (1995) 34:1596–607. doi: 10.1097/00004583-199512000-00010
26. Nierenberg AA, Trivedi MH, Fava M, Biggs MM, Shores-Wilson K, Wisniewski SR, et al. Family history of mood disorder and characteristics of major depressive disorder: a STAR*D (sequenced treatment alternatives to relieve depression) study. J Psychiatr Res. (2007) 41:214–21. doi: 10.1016/j.jpsychires.2006.02.005
27. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. (2000) 157:1552–62. doi: 10.1176/appi.ajp.157.10.1552
28. Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, et al. A registry-based twin study of depression in men. Arch Gen Psychiatry. (1998) 55:468–72. doi: 10.1001/archpsyc.55.5.468
29. Harald B, Gordon P. Meta-review of depressive subtyping models. J Affect Disord. (2012) 139:126–40. doi: 10.1016/j.jad.2011.07.015
30. Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry. (2005) 62:593–602. doi: 10.1001/archpsyc.62.6.593
31. Corruble E, Gorwood P, Falissard B. Association between age of onset and symptom profiles of late-life depression. Acta Psychiatr Scand. (2008) 118:389–94. doi: 10.1111/j.1600-0447.2008.01239.x
32. Gallagher D, Mhaolain AN, Greene E, Walsh C, Denihan A, Bruce I, et al. Late life depression: a comparison of risk factors and symptoms according to age of onset in community dwelling older adults. Int J Geriatr Psychiatry. (2010) 25:981–7. doi: 10.1002/gps.2438
33. Heun R, Kockler M, Papassotiropoulos A. Distinction of early- and late-onset depression in the elderly by their lifetime symptomatology. Int J Geriatr Psychiatry. (2000) 15:1138–42. doi: 10.1002/1099-1166(200012)15:12<1138::AID-GPS266>3.0.CO;2-7
34. Parker G, Wilhelm K, Asghari A. Early onset depression: the relevance of anxiety. Soc Psychiatry Psychiatr Epidemiol. (1997) 32:30–7. doi: 10.1007/BF00800665
35. Kendler KS, Fiske A, Gardner CO, Gatz M. Delineation of two genetic pathways to major depression. Biol Psychiatry. (2009) 65:808–11. doi: 10.1016/j.biopsych.2008.11.015
36. Kendler KS, Gatz M, Gardner CO, Pedersen NL. Age at onset and familial risk for major depression in a Swedish national twin sample. Psychol Med. (2005) 35:1573–9. doi: 10.1017/S0033291705005714
37. Bellivier F, Golmard JL, Rietschel M, Schulze TG, Malafosse A, Preisig M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. (2003) 160:999–1001. doi: 10.1176/appi.ajp.160.5.999
38. Parker G, Roy K, Hadzi-Pavlovic D, Mitchell P, Wilhelm K. Distinguishing early and late onset non-melancholic unipolar depression. J Affect Disord. (2003) 74:131–8. doi: 10.1016/S0165-0327(02)00002-2
39. Truong W, Minuzzi L, Soares CN, Frey BN, Evans AC, MacQueen GM, et al. Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Res. (2013) 214:204–11. doi: 10.1016/j.pscychresns.2013.09.003
40. Massat I, Souery D, Del-Favero J, Nothen M, Blackwood D, Muir W, et al. Association between COMT (Val158Met) functional polymorphism and early onset in patients with major depressive disorder in a European multicenter genetic association study. Mol Psychiatry. (2005) 10:598–605. doi: 10.1038/sj.mp.4001615
41. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. (2004) 22:394–400. doi: 10.1016/j.neuroimage.2003.12.030
42. Yao Z, Wang L, Lu Q, Liu H, Teng G. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. J Affect Disord. (2009) 115:430–8. doi: 10.1016/j.jad.2008.10.013
43. Guo WB, Liu F, Xue ZM, Yu Y, Ma CQ, Tan CL, et al. Abnormal neural activities in first-episode, treatment-naive, short-illness-duration, and treatment-response patients with major depressive disorder: a resting-state fMRI study. J Affect Disord. (2011) 135:326–31. doi: 10.1016/j.jad.2011.06.048
44. Guo WB, Sun XL, Liu L, Xu Q, Wu RR, Liu ZN, et al. Disrupted regional homogeneity in treatment-resistant depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:1297–302. doi: 10.1016/j.pnpbp.2011.02.006
45. Liu F, Hu M, Wang S, Guo W, Zhao J, Li J, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. (2012) 39:326–31. doi: 10.1016/j.pnpbp.2012.07.004
46. Wu QZ, Li DM, Kuang WH, Zhang TJ, Lui S, Huang XQ, et al. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum Brain Mapp. (2011) 32:1290–9. doi: 10.1002/hbm.21108
47. Chen JD, Liu F, Xun GL, Chen HF, Hu MR, Guo XF, et al. Early and late onset, first-episode, treatment-naive depression: same clinical symptoms, different regional neural activities. J Affect Disord. (2012) 143:56–63. doi: 10.1016/j.jad.2012.05.025
48. Shen Z, Jiang L, Yang S, Ye J, Dai N, Liu X, et al. Identify changes of brain regional homogeneity in early and later adult onset patients with first-episode depression using resting-state fMRI. PLoS ONE. (2017) 12:e0184712. doi: 10.1371/journal.pone.0184712
49. Shen Z, Cheng Y, Yang S, Dai N, Ye J, Liu X, et al. Changes of grey matter volume in first-episode drug-naive adult major depressive disorder patients with different age-onset. Neuroimage Clin. (2016) 12:492–498. doi: 10.1016/j.nicl.2016.08.016
50. Hamilton M. Development of a rating scale for primary depressive illness. Bri J Soc Clin Psychol. (1967) 6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x
51. Chao-Gan Y, Yu-Feng Z. Dparsf: a matlab toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. (2010) 4:13. doi: 10.3389/fnsys.2010.00013
52. Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. (2010) 35:192–216. doi: 10.1038/npp.2009.104
53. Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA. (2010) 107:11020–5. doi: 10.1073/pnas.1000446107
54. Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull. (2013) 39:358–65. doi: 10.1093/schbul/sbr151
55. Bluhm R, Williamson P, Lanius R, Theberge J, Densmore M, Bartha R, et al. Resting state default-mode network connectivity in early depression using a seed region-of-interest analysis: decreased connectivity with caudate nucleus. Psychiatry Clin Neurosci. (2009) 63:754–61. doi: 10.1111/j.1440-1819.2009.02030.x
56. Martin DM, McClintock SM, Forster JJ, Lo TY, Loo CK. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress Anxiety. (2017) 34:1029–39. doi: 10.1002/da.22658
57. Elliott R. Executive functions and their disorders. Br Med Bull. (2003) 65:49–59. doi: 10.1093/bmb/65.1.49
58. Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry. (2012) 169:693–703. doi: 10.1176/appi.ajp.2012.11071105
59. Li M, Xu H, Lu S. Neural basis of depression related to a dominant right hemisphere: a resting-state fMRI study. Behav Neurol. (2018) 2018:5024520. doi: 10.1155/2018/5024520
60. Yan M, He Y, Cui X, Liu F, Li H, Huang R, et al. Disrupted regional homogeneity in melancholic and non-melancholic major depressive disorder at rest. Front Psychiatry. (2021) 12:618805. doi: 10.3389/fpsyt.2021.618805
61. Guo WB, Liu F, Xun GL, Hu MR, Guo XF, Xiao CQ, et al. Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 40:153–9. doi: 10.1016/j.pnpbp.2012.08.014
62. Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. (2000) 126:890–909. doi: 10.1037/0033-2909.126.6.890
63. Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. (2003) 65:193–207. doi: 10.1093/bmb/65.1.193
64. Zhao D, Wu Z, Zhang H, Mellor D, Ding L, Wu H, et al. Somatic symptoms vary in major depressive disorder in China. Compr Psychiatry. (2018) 87:32–37. doi: 10.1016/j.comppsych.2018.08.013
65. Rumble ME, White KH, Benca RM. Sleep disturbances in mood disorders. Psychiatr Clin North Am. (2015) 38:743–59. doi: 10.1016/j.psc.2015.07.006
66. Holsboer-Trachsler E, Seifritz E. Sleep in depression and sleep deprivation: a brief conceptual review. World J Biol Psychiatry. (2000) 1:180–6. doi: 10.3109/15622970009150589
67. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry. (1999) 17(Suppl. 60):28–31; discussion 46–8.
68. Burks JD, Conner AK, Bonney PA, Glenn CA, Baker CM, Boettcher LB, et al. Anatomy and white matter connections of the orbitofrontal gyrus. J Neurosurg. (2018) 128:1865–72. doi: 10.3171/2017.3.JNS162070
69. Badre D. Frontal cortex and the hierarchical control of behavior. Trends Cogn Sci. (2018) 22:170–88. doi: 10.1016/j.tics.2017.11.005
70. Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. (2007) 12:360–6. doi: 10.1038/sj.mp.4001919
71. Richard-Devantoy S, Deguigne F, Annweiler C, Letourneau G, Beauchet O. Influence of gender and age on cognitive inhibition in late-onset depression: a case-control study. Int J Geriatr Psychiatry. (2013) 28:1125–30. doi: 10.1002/gps.3929
Keywords: resting-state functional magnetic resonance imaging, regional homogeneity, major depressive disorder, onset-age, hamilton rating scale for depression
Citation: Zhang Z, Chen Y, Wei W, Yang X, Meng Y, Yu H, Guo W, Wang Q, Deng W, Li T and Ma X (2021) Changes in Regional Homogeneity of Medication-Free Major Depressive Disorder Patients With Different Onset Ages. Front. Psychiatry 12:713614. doi: 10.3389/fpsyt.2021.713614
Received: 23 May 2021; Accepted: 30 August 2021;
Published: 01 October 2021.
Edited by:
Chien-Han Lai, National Yang-Ming University, TaiwanReviewed by:
Yonggui Yuan, Southeast University, ChinaCopyright © 2021 Zhang, Chen, Wei, Yang, Meng, Yu, Guo, Wang, Deng, Li and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Ma, bWF4aWFvaG9uZ0BzY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.