- Division of Population and Behavioral Science, University of St. Andrews School of Medicine, St. Andrews, United Kingdom
Introduction: Impairments in the multifaceted neuropsychological construct of cognitive impulsivity are a main feature of chronic tobacco smokers. According to the literature, these cognitive impairments are relevant for the initiation and maintenance of the smoking behavior. However, the neuroanatomical correlates of cognitive impulsivity in chronic smokers remain under-investigated.
Methods: A sample of 28 chronic smokers (mean age = 28 years) not affected by polysubstance dependence and 24 matched non-smoker controls was recruited. Voxel Based Morphometry (VBM) was employed to assess Gray Matter (GM) volume differences between smokers and non-smokers. The relationships between GM volume and behavioral manifestations of impulsive choices (5 trial adjusting delay discounting task, ADT-5) and risky decision making (Cambridge Gambling Task, CGT) were also investigated.
Results: VBM results revealed GM volume reductions in cortical and striatal brain regions of chronic smokers compared to non-smokers. Additionally, smokers showed heightened impulsive choices (p < 0.01, Cohen's f = 0.50) and a riskier decision- making process (p < 0.01, Cohen's f = 0.40) compared to non-smokers. GM volume reductions in the left Anterior Cingulate Cortex (ACC) correlated with impaired impulsive and risky choices, while GM volume reductions in the left Ventrolateral Prefrontal Cortex (VLPFC) and Caudate correlated with heightened impulsive choices. Reduced GM volume in the left VLPFC correlated with younger age at smoking initiation (mean = 16 years).
Conclusion: Smokers displayed significant GM volume reductions and related cognitive impulsivity impairments compared to non-smoker individuals. Longitudinal studies would be required to assess whether these impairments underline neurocognitive endophenotypes or if they are a consequence of tobacco exposure on the adolescent brain.
Introduction
Tobacco smoking is the leading cause of preventable death worldwide (1). According to the World Health Organization (WHO) (1), ~7 million people die each year because of direct tobacco smoking, while 1.2 million individuals die because of second-hand smoke. Despite recent trends showing a decrease in tobacco smoking in several developed countries, the percentage of tobacco smokers worldwide remains dramatically high (1). In fact, it is estimated that there are ~1.3 billion current tobacco smokers, 80% of these living in low and middle-income countries (1).
Notably, impairments in the neuropsychological domain of “cognitive impulsivity” constitute one of the main features of substance users (2–5), including chronic tobacco smokers (6–8). Particularly, impairments in cognitive impulsivity are considered relevant for the initiation and chronicity of substance use (5). Cognitive impulsivity is characterized by the cognitive subdomains of choice impulsivity (delay discounting) and risky decision making (2, 4). Choice impulsivity refers to the tendency to opt for immediate pleasures/rewards (e.g., drug of abuse) over long- term gains (e.g., long-term health) (4), while “risky decision making” refers to a type of decisional process occurring when a an individual engages in decisions despite the risk of suffering known adverse consequences. Both cognitive impaired processes are strongly predictive of treatment outcomes for substance use and dependence, including tobacco smoking cessation treatments (8, 9). In fact, tobacco smokers displaying heightened impulsive choices (high delay discounting rates) are likely to relapse and to jeopardize cessation attempts (10, 11).
Advancements in neuroimaging techniques and lesion studies have helped to determine the neurobiological correlates of the multifaceted nature of cognitive impulsivity in healthy individuals by associating functional and structural disruptions in (a) the Ventrolateral PreFrontal Cortex (VLPFC), Dorsolateral PreFrontal Cortex (DLPFC), and lateral OrbitoFrontal Cortex (lOFC) to heightened impulsive choices, and (b) deficits in the Anterior Cingulate Cortex (ACC), Ventromedial PreFrontal Cortex (VMPFC), and medial/rostral OrbitoFrontal Cortex (mOFC) to impaired risky decision making (12). Additionally, disruptions in the ventral and dorsal striatum (caudate and putamen) have been related to both impaired impulsive and risky choices (12). According to the competing neurobehavioral decision systems theory of addiction (13) (CNDS), an imbalance between the reward system (comprising the striatum, midbrain, amygdala, and insula) and the executive decision system (comprising medial and lateral prefrontal brain regions) may explain the cognitive impulsivity impairments commonly manifested by substance users. Specifically, a hyperactive impulsive system and a hypoactive executive decision system may cause a reward bias and a lack of self-control toward the substance of abuse.
Notably, studies conducted on polysubstance users who were tobacco smokers in addition to being dependent to other substances such as cocaine (14), alcohol (15), and opioids (16), all revealed an association between Gray Matter (GM) volume reductions in fronto- cortical and striatal brain regions and behavioral manifestations of cognitive impulsivity. Despite studies conducted in the last decade revealing GM volume reductions in fronto-cortical and striatal brain regions of chronic tobacco smokers not affected by polysubstance dependence (17–19), the relationship between these GM volume reductions and impairments in cognitive impulsivity in chronic smokers remains under-investigated and limited to self-reported measures of impulsivity (20). To our knowledge, only one study conducted by Durazzo et al. (21) investigated the relationship between GM thickness and behavioral manifestations of cognitive impulsivity through a decision making task (Iowa gambling task, IGT) in 41 chronic smokers with a mean age of 46 years (21). Results of this study revealed an association between poorer decision making and reduced cortical thickness in the ACC and VMPFC of chronic smokers. However, no association between structural brain deficits in frontal brain regions and impulsive choices (delay discounting) was investigated, nor between reduced cortical thickness and decision making under risk outside a learning context (as assessed by the Cambridge Gambling Task, CGT) (22).
Therefore, the aim of the current study was to test the following hypotheses:
1) Chronic tobacco smokers not affected by polysubstance dependence, neurodegenerative conditions and/or psychiatric illnesses display GM volume reductions compared to non-smoker controls in a priori brain regions of interest such as VLPFC, lOFC, DLPFC, VMPFC, mOFC, ACC, insula, dorsal (caudate and putamen) and ventral striatum (globus pallidus, thalamus) in contrast to non-smokers healthy controls. A priori regions of interest were determined based on previous meta-analyses investigating GM volume reductions in chronic smokers compared to non-smokers, and on previous studies/reviews investigating the neuroanatomical (GM) correlates of cognitive impulsivity in substance users (studies are listed in Supplementary Tables 1a, 1b).
2) Chronic tobacco smokers display impairments in impulsive choices and risky decision-making compared to non-smokers as assessed by computerized measures of cognitive impulsivity such as the five- trial Adjusting Delay Discounting Task (ADT-5) (23) and the Cambridge Gambling Task (CGT) (22).
3) GM volume reductions in a priori regions of interest are correlated to heightened impulsive choices and impaired risky decision making in chronic smokers.
Materials and Methods
Recruitment
Community based chronic tobacco smokers and non-smokers were recruited through a convenient-sampling approach across the South Eastern regions of Scotland between October 2019 and March 2020. Different methods of recruitment were employed, these included: Internet advertisements posted on Gumtree and Craiglist websites, flyers distributed at local businesses (e.g., supermarkets, leisure centers), advertisements published on “The Courier” regional newspaper, and word of mouth. All participants provided written informed consent prior to the beginning of the study. They were rewarded a total of £100 for their full participation. The recruitment flow chart is depicted in Supplementary Figure 1.
Enrolled participants needed to attend two experimental sessions. These sessions were conducted on separate days within the same week (no more than 3 days apart): The first experimental session involved screening procedures and computerized measures of cognitive impulsivity (impulsive choices and risky decision making). This experimental session was conducted at the University of St Andrews School of Medicine in St Andrews. The second session involved a Magnetic Resonance Imaging (MRI) procedure at the Clinical Research Center (CRC), Ninewells Hospital, Dundee.
Inclusion and Exclusion Criteria
Participants were screened for eligibility (see Supplementary Table 2) through objective and subjective measurements. Specifically, an exhaled Carbon Monoxide (CO) test was utilized to measure CO levels in participants' breath, while a saliva drug testing kit was employed to determine presence (or not) for cotinine. The presence of cannabis, cocaine, morphine, methadone, amphetamine, methamphetamine, and benzodiazepines was assessed through a urine drug screening test.
Chronic tobacco smokers who reported a recreational use of cannabis (no more than twice per week) mixed with tobacco in the same “spliff” or “joint,” and did not present symptoms of acute intoxication (e.g., conjunctival injection, slurred speech, agitation) at the screening session, were not excluded from the study. In fact, the effect of recreational cannabis use on cortical brain structures is limited (24), whereas the effect of cannabis use on subcortical structures may be better explained by concomitant tobacco smoking (25). Meta-analytic findings also revealed a small effect of cannabis use on cognitive impulsivity with an effect size of 0.30 (26). The Mini International Neuropsychiatric Instrument (MINI) version 7.0.2 (27) was utilized to exclude the presence of DSM V psychiatric disorders (Axis I) for participants. A screening interview was utilized to exclude the presence of chronic conditions (e.g., HIV, diabetes), pregnancy, neurological disorders, and severe head injuries. Participants' patterns of tobacco use, smoking variables (pack years, cigarettes smoked per day, age at regular smoking onset), and weekly use of cannabis and alcohol were assessed through a paper and pencil questionnaire, while severity of nicotine dependence was assessed by using the Fagerström Test for Nicotine Dependence (FTND) (28). Participants' pre- morbid IQ was estimated through the Barona equations (29).
Instruments
Cognitive Measures
During this experimental session participants needed to complete two computerized measures of cognitive impulsivity: The 5 trial Adjusting Delay Discounting Task (ADT-5) (23) and the Cambridge Gambling Task (CGT) (22).
The ADT-5 measures choice impulsivity. Participants were presented with choices between two fixed hypothetical monetary rewards (£5 available immediately and £10 available at some time in the future) over five trials. The delay period was adjusted after each trial based on participants' previous choice. Outcome measures for this task consisted in Effective Delay 50% (ED50) values. ED50 represents “the delay that is effective in discounting the subjective value of the delayed reinforcer by 50%” 30. It consists in the inverse of the discounting rate k (ED50 = 1/k) (30).
The Cambridge Gambling Task (CGT) is a computerized measure of risky decision making outside a learning context (22) that is part of the Cambridge Neuropsychological Test Automated Battery (https://www.cambridgecognition.com/cantab/cognitive-tests/executive-function/cambridge-gambling-task-cgt/). During this task, a yellow token was hidden either in red or blue boxes over five stages. Participants needed to guess the location of the token by selecting a proportion of points to bet on their decision. Each participant started with 100 points. Participants lost or gained points depending on their choices. Specifically, participants gained points for each correct guess and lost points for each incorrect guess. The amount of points gained or lost consisted in the amount of points betted by the participants. Outcome domain measures for this task consisted in the multiple facets of the risky decision-making process commonly manifested by substance users (6, 16, 31). Specifically, outcome measures consisted in (a) the average number of points betted after choosing the most likely outcome (risk taking score); (b) the overall proportion of points betted during the task (overall proportion bet score) [both (a) and (b) outcome measures reflect a propensity toward risk]; (c) the proportion of all trials where the subject chose the majority box color, which reflects the rationality of the participants' decision making process (quality of decision making score); and (d) the ability to modify choices in light of information about the probability of different outcomes (risk adjustment score) (6, 16, 31).
Neuroimaging
Structural T1 weighted images were acquired through a Siemens 3T Prisma-FIT scanner (Siemens Healthineers, Erlangen, Germany). Specifically, an MP-RAGE (magnetization-prepared rapid acquisition gradient echo) sequence was utilized to acquire images with a voxel size 0.8 × 0.8 × 1.0 mm3 with whole brain coverage, repetition time (TR) = 1.9 s, echo time (TE) = 2.64 ms. Flip angle = 9°, FOV = 200 mm, matrix = 256 × 256, 176 slices, slice thickness 1 mm. Scans were reported by a consultant radiologist to rule out the presence of incidental findings.
Statistical Analysis
Data obtained from computerized measures of cognitive impulsivity (ADT-5, CGT) were analyzed through analyses of covariance (ANCOVAs) to test the null hypothesis of no differences between chronic tobacco smokers and non-smokers in relation to impulsive choices and risky decision-making outcome measures while controlling for relevant sociodemographic variables such as sex, age, premorbid IQ, educational level, social deprivation (SIMD), and other substances used (cannabis, alcohol). To be analyzed through ANCOVAs, data needed to meet the assumptions of normality, homoscedasticity, homogeneity of variances, and of homogeneity of regression slopes. Data failing assumption of normality and/or homogeneity of variances were Log10 transformed. The non-parametric Kruskall-Wallis H test was employed if data still violated assumptions of normality and/or of homogeneity of variances after transformation. Bonferroni-corrected pairwise comparisons with a significance level set at p < 0.05 were utilized to control for Type-1 error. SPSS v. 26 (SPSS Inc., USA) was utilized for this part of the analysis. Effect sizes (Choen's f) were computed through the software G*Power.
Neuroimaging data were analyzed by applying a whole-brain voxel-based morphometry (VBM) technique through SPM12 (https://www.fil.ion.ucl.ac.uk/spm/). T1 weighted images were segmented into gray and white matter probability maps, these were subsequently normalized to the Montreal Neurological Institute (MNI) template. Modulation was performed with Jacobian determinants to preserve the total amount of GM and WM in each probability map. Following segmentation and spatial normalization, images were smoothed with an 8 mm Gaussian kernel (32). Total intracranial volume was estimated by the Computational Anatomy Toolbox (http://www.neuro.uni-jena.de/cat/).
A two sample T-tests was utilized to assess for GM volume differences between chronic tobacco smokers and non-smokers across the whole brain, including a priori regions of interest (Supplementary Tables 1a, 1b). Whole-brain voxel-wise linear regression models were also computed to investigate the relationship between GM volume reductions and cognitive impulsivity outcome measures (ADT-5, CGT) in chronic tobacco smokers. Proof of concept analyses were conducted by computing whole-brain voxel-wise linear regression models investigating the relationship between GM volume reductions and tobacco exposure variables (cigarettes smoked per day, pack years, FTND, age at regular smoking onset).
Brain regions (including a priori regions of interest) were identified by converting MNI coordinates into Talairach coordinates (https://bioimagesuiteweb.github.io/webapp/mni2tal.html), and by inserting them into the Talairach Daemon Atlas (http://www.talairach.org/daemon.html) (16). A cluster forming significance threshold of p < 0.05 with a minimum of 100 contiguous voxels (k) per cluster was employed for two sample T-tests and for voxel-wise regression models testing the associations between GM volume and cognitive impulsivity measures. This threshold was obtained by applying Monte Carlo simulations (16, 33). For proof of concept analyses a more stringent threshold of p < 0.01 with a minimum of 100 contiguous voxels per cluster was employed. Covariates of no interest consisted in total intracranial volume (TIV), age, and biological sex for two sample T-tests and linear regression models. Results of whole-brain voxel-wise analyses are only reported in the text for a priori regions of interest.
Results
Sociodemographic and Smoking Characteristics
Chronic tobacco smokers and non-smokers were well-matched for age, Scottish index of multiple deprivation (SIMD) scores, and units of alcohol consumed per day at the time of the first experimental session as assessed by independent samples t-tests (p > 0.05). Chi-square test results did also reveal the absence of a statistically significant association between sex and smoking status, = 0.55, p = 0.46. However, chronic tobacco smokers displayed lower pre- morbid IQ and a lower educational level compared to non-smokers (p < 0.05). Sociodemographic and tobacco smoking characteristics of participants are displayed in Table 1. At the time of the second experimental session (MRI), chronic smoker participants were still matched for sex, age, SIMD, and units of alcohol consumed per day to non-smoker controls (Table 1). No significant changes in smoking characteristics were detected between the two sessions (paired samples t-test p > 0.05).
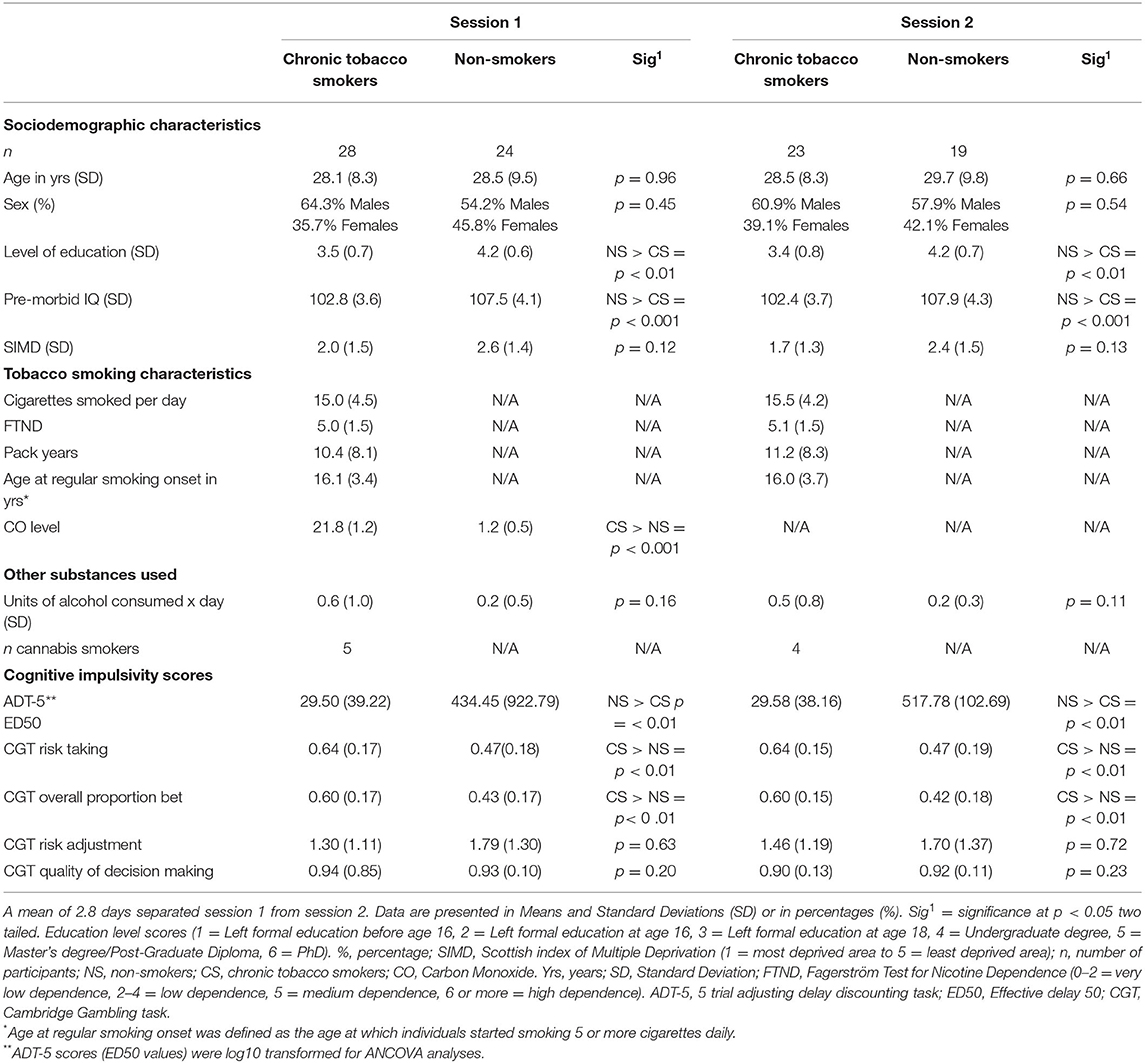
Table 1. Sociodemographic characteristics, smoking characteristics, and cognitive impulsivity scores of study participants.
Cognitive Tests
5-Trial Adjusting Delay Discounting Task
A statistically significant difference was detected between chronic tobacco smokers and non-smokers in relation to ED50 values [F(1,44) = 11.39, p < 0.01, partial η2 = 0.200,Cohen's f = 0.50] after adjusting for age, premorbid IQ, level of education, sex, units of alcohol consumed per day, occasional cannabis use, and SIMD covariates. Pairwise comparisons with Bonferroni adjustment revealed that non-smokers had significantly higher ED50 values (M = 1.89, SE = 0.21) compared to chronic smokers (M = 0.82, SE = 0.19) with a mean difference of 1.07 (95% CI, 0.43–1.71) p < 0.01. These results indicated that the delay at which £10 had lost 50% of its value (ED50) was longer for non-smokers compared to chronic smokers.
Cambridge Gambling Task
A statistically significant difference was also detected between chronic smokers and non-smokers in relation to “risk taking” scores [F(1,44) = 6.61, p < 0.01, partial η2 = 0.133, Cohen's f = 0.39] and “overall proportion bet” scores [F(1,44) = 7.28, p < 0.01, partial η2 = 0.153, Cohen's f = 0.42] after adjusting for relevant covariates. Pairwise comparisons with Bonferroni adjustment revealed that chronic smokers had significantly higher “risk taking” scores (M = 0.64, SE = 0.04) compared to non-smokers (M = 0.47, SE = 0.04) with a mean difference of 0.172 (95% CI, 0.03–0.30) p < 0.01. Similarly, chronic smokers had significantly higher “overall proportion bet” scores (M = 0.59, SE = 0.03) compared to non-smokers (M = 0.42, SE = 0.04) with a mean difference of 0.173 (95% CI, 0.04–0.30) p < 0.01. No statistically significant difference was found between chronic smokers and non-smokers in relation to “risk adjustment” scores [F(1,44) = 0.22, p = 0.63, partial η2 = 0.00]. Finally, no statistically significant difference was identified between chronic smokers and non-smokers in relation to “quality of decision making” scores as assessed by a Kruskall Wallis H test [H(1) = 1.63, p = 0.20].
post-hoc power calculations with a sample size of 52 participants and α = 0.05 revealed a power (1-β probability) of 0.94 for choice impulsivity (ADT-5) scores, a power of 0.84 for “overall proportion bet” scores (CGT), and a power of 0.78 for “risk taking” scores (CGT).
Considering the dropout of 10 participants (5 smokers and 5 non-smokers) prior to the MRI session, sensitivity analyses (ANCOVAs) were conducted to ascertain choice impulsivity (ADT-5) and risky decision making (CGT) differences between the remaining smokers (n = 23) and non-smokers (n = 19). Results still revealed a significant difference (p < 0.01) between smokers and non-smokers in relation to ED50 values, “risk taking,” and “overall proportion bet” scores with effect sizes (Cohen's f) of 0.57, 0.40, and 0.42 respectively.
Neuroimaging
Voxel based morphometry (VBM) results revealed GM volume reductions in chronic tobacco smokers compared to non-smokers in several cortical and striatal brain regions across the whole brain, predominantly in the left hemisphere. Structures presenting GM volume reductions, cluster sizes, Brodmann's areas (BA), and corresponding Montreal Neurological Institute (MNI) coordinates are reported in Supplementary Table 3. Regions of Interest displayed GM volume reductions in frontal cortices such as the bilateral ACC (14, 21, 29;−11, 26, 18; BA32), left DLPFC (−21, 41, 23; BA9), left VLPFC (−26, 35,−12; BA47), and left OFC (−5, 44, −27; BA11). Striatal gray matter reductions in chronic smokers compared to non-smokers were present in the left caudate (−11, 2, 12), and in the left putamen (−21, −9, 6).
Relationship Between Neuroimaging and Non-neuroimaging Measures
A positive relationship was detected between choice impulsivity scores (ED50 values) and GM volume in regions of interest. These included the left VLPFC (−50, 30, −18; BA47) (p = 0.002; T = 3.20; R2 = 0.251), left caudate (−18, −2, 30) (p = 0.002; T = 3.21; R2 = 0.336), right caudate (18, 0, 30) (p = 0.002; T = 3.37, R2 = 0.281), and left ACC (−17, 42, 5, BA32) (p < 0.0001; T = 4.57, R2 = 0.365) of chronic tobacco smokers. These associations are depicted in Figure 1. Furthermore, a negative relationship was detected between “risk taking” (p =0.001; T = 3.62; R2 = 0.409) and “overall proportion bet” (p = 0.002; T = 3.34; R2 = 0.371) scores of the CGT and GM volume in the left ACC (−9, 33, 21; BA 32) of chronic tobacco smokers (see Figure 2). Supplementary Tables 4, 5 depict the associations between risky decision making, impulsive choices and GM volume in brain regions of no interest (not related to the a priori hypotheses listed in the section Introduction).
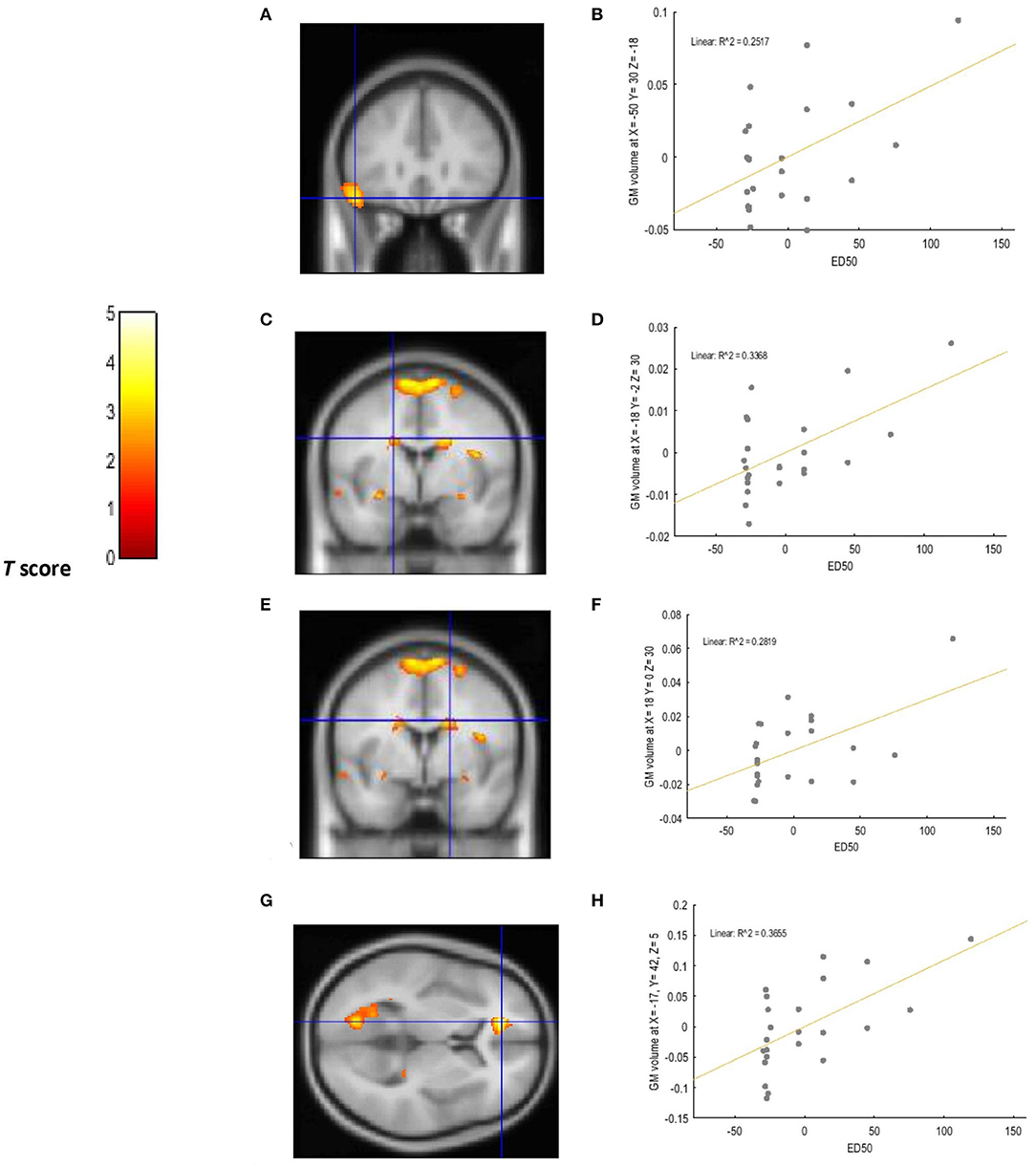
Figure 1. Voxel-wise regression results depicting positive relationships between ED50 values and GM volume in the left VLPFC, left ACC, and bilateral caudate while controlling for total intracranial volume (TIV), age, and biological sex. The cluster-forming threshold consisted in p < 0.05 with a minimum of 100 contiguous voxels per cluster. The figure shows the location of the left VLPFC (A), left caudate (C), and right caudate (E) voxel clusters in coronal slices. The location of the left ACC voxel cluster (G) is depicted in an axial slice. For visualization purposes, the scatterplot of adjusted response data (B) shows the significant (p < 0.005) reduction in GM volume as a function of ED50 values in the left VLPFC (cluster size: 1624 voxels). The scatterplot of adjusted response data (D) shows the significant (p < 0.005) reduction in GM volume as a function of ED50 values in the left caudate (cluster size: 469 voxels). The scatterplot of adjusted response data (F) shows the significant (p < 0.005) reduction in GM volume as a function of ED50 values in the right caudate (cluster size: 728 voxels). The scatterplot of adjusted response data (H) shows the significant (p < 0.005) reduction in GM volume as a function of ED50 values in the left ACC (cluster size: 732 voxels). Plotted values consist in residuals of mean GM volume (Y axis) and of mean ED50 values (X axis). R2: Coefficient of determination.
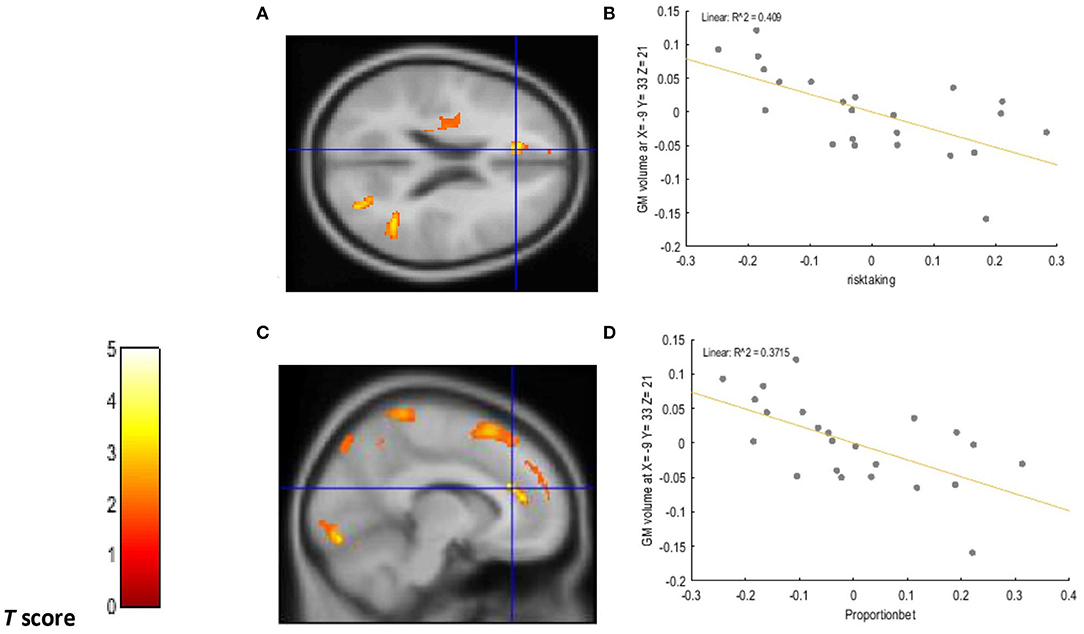
Figure 2. Voxel-wise regression results depicting negative relationships between risk taking scores, overall proportion bet scores, and GM volume in the left ACC while controlling for total intracranial volume (TIV), age, and biological sex. The cluster-forming threshold consisted in p < 0.05 with a minimum of 100 contiguous voxels per cluster. The figure shows the location of the left ACC voxel clusters in axial (A) and coronal (C) slices. For visualization purposes, the scatterplot of adjusted response data (B) shows the significant (p < 0.005) reduction in GM volume as a function of risk-taking scores in the left ACC (cluster size: 256 voxels). The scatterplot of adjusted response data (D) shows the significant (p < 0.005) reduction in GM volume as a function of overall proportion bet scores in the left ACC (cluster size: 227 voxels). Plotted values consist in residuals of mean GM volume (Y axis) and of mean risk taking and overall proportion bet scores (X axis). R2: Coefficient of determination.
Negative and positive relationships were also detected between GM volume in regions of interest and smoking variables. These are depicted in Table 2. Regression plots showing the directions of these associations are illustrated in Supplementary Figure 2. Supplementary Table 6 illustrates the associations between tobacco smoking variables and GM volume in brain regions of no interest.

Table 2. Voxel-wise regression results depicting significant associations between GM volume in regions of interest and smoking variables while controlling for TIV, age, and biological sex.
Discussion
Summary of Findings
a) Chronic tobacco smokers displayed GM volume reductions in various brain areas (predominantly in the left hemisphere of the brain), including striatal and cortical a priori regions of interest located in the prefrontal cortex, supporting hypothesis 1.
b) Chronic tobacco smokers displayed heightened impulsive choices and a riskier decision-making process in comparison to non-smoker controls as assessed by the ADT-5 and CGT tasks, supporting hypothesis 2.
c) GM volume reductions in cortical and striatal a priori regions of interest were correlated to impulsive choices (VLPFC, caudate, ACC) and risky decision making (ACC) in chronic tobacco smokers, supporting hypothesis 3.
Interpretation
These results are in line with recent findings demonstrating a strong association between chronic tobacco smoking, impulsive choices, and risky decision making (6, 7). That is, chronic tobacco smokers have a greater difficulty in delaying immediate gratification and display a riskier decision-making process in comparison to non- smokers. The current study adds up to the body of knowledge by revealing a cross- sectional relationship between impulsive choices, risky decision making, and GM volume reductions in chronic tobacco smokers not affected by psychiatric comorbidity, poly substance dependence (e.g., alcoholism), and/or chronic medical conditions (e.g., HIV). These deficits were detected in a relatively young sample of cigarettes smokers with a mean age of 28 years old. Thus, it is unlikely that participants were affected by neurocognitive impairments that are a consequence of the aging process (34).
Under a neuroanatomical point of view, the above findings are in line with previous studies revealing GM volume reductions in striatal and cortical structures located in the prefrontal cortex of chronic tobacco smokers (17–19). In accordance with these studies, the current research revealed a negative relationship between tobacco exposure variables and reduced GM volume in prefrontal and striatal brain regions. Specifically, longer pack years were associated to reduced GM volume in the left DLPFC, left ACC, left caudate, and left putamen. Severity of nicotine dependence (FTND score) was also associated with GM volume reductions in the left thalamus, an area strictly related to the development and maintenance of substance dependence for its dopaminergic projections (35). The current study provides support to the findings of Durazzo et al. (21) by revealing a correlation between GM volume reductions in the left ACC and risky decision making in chronic smokers. Furthermore, the current study revealed a positive association between GM volume reductions in the left ACC and the inability to delay gratification displayed by chronic smokers (ED50 values). Indeed, the ACC has been proposed to play a crucial role in the maintenance of addictive behaviors due to its connections with the limbic system and prefrontal cortex, therefore mediating maladaptive emotional and reward-based decisions (36). The association between GM volume reductions in the VLPFC and heightened impulsive choices displayed by chronic smokers extends previous work by endorsing disruptions in brain's executive system as a core feature of nicotine dependent individuals (36).
Notably, impairments in cognitive impulsivity for chronic smokers enrolled in the current study correlated to GM volume reductions localized predominantly in the left hemisphere of the brain. Despite GM deficits being reported frequently in left fronto-cortical and striatal brain regions in chronic tobacco smokers (19, 37, 38), no association between cognitive impairments and lateralization of structural brain deficits in chronic smokers was ever investigated. However, an fMRI study conducted by Clewett et al. (39) revealed greater functional coupling between the left fronto- parietal network and the left insular cortex in chronic tobacco smokers compared to non-smokers while performing a computerized Delay Discounting task (with hypothetical monetary rewards) (39). Greater functional connectivity between these areas was also associated to steeper discounting rates (39). Furthermore, a systematic review conducted by Gordon (40) showed greater peak activation in fronto-cortical brain areas located in the left hemisphere of tobacco deprived smokers while responding to cue-reactivity (craving) stimuli during fMRI investigations (40). Therefore, consistently with the CNDS model, the simultaneous hyperactivation of the left reward system to cigarettes cues, and the inability to delay gratification caused by GM volume reductions in the left VLPFC and left ACC, may induce cigarettes smokers to crave for, and to want immediately the drug of abuse (i.e. cigarettes) despite the known health risks associated to its usage. Indeed, impulsivity has been linked to tobacco craving and smoking relapses by the literature (41).
Even though the cross-sectional nature of the current research does not allow to directly imply causation, the above findings may be explained by neuroscience- based paradigms. According to the “tobacco Induced neurotoxicity theory of adolescent cognitive development” (TINACD) (42), exposure to tobacco during adolescence (a developmental period characterized by intense neurostructural and neurochemical maturation) may cause long-lasting deficits in frontal brain areas modulating cognitive functions mostly related to top- down inhibitory control and decision making (42). For this reason, adult smokers who initiated tobacco use during adolescence may have more difficulties quitting smoking, and relapse more frequently, in comparison to individuals who started smoking at a later developmental stage (42). This theory has been availed by animal models revealing unique, and long- lasting, cellular alterations and structural changes in fronto-cortical and striatal brain regions of rats exposed to nicotine during adolescence (43, 44). In support to the TINACD, the current study revealed a positive correlation between younger age at regular smoking onset (mean = 16 years) and GM volume reductions in the left VLPFC (BA 47), (Table 2). As described previously, GM volume reductions in the left VLPFC (BA 47) were also associated to heightened impulsive choices in the current sample (Figure 1).
An alternative interpretation to the above findings consists in the presence neurocognitive endophenotypes in substance dependent populations (45). In fact, studies have shown abnormal brain structures in fronto-cortical and striatal brain regions, and related cognitive impulsivity impairments, in substance dependent individuals and their drug- naïve biological siblings (46, 47). However, because of the lack of longitudinal studies, the causal relationship between chronic tobacco smoking and neurocognitive impairments remains unclear (48).
Clinical Relevance
Considering that impaired cognitive impulsivity may be determinant in fostering tobacco smoking during different stages of the drug addiction cycle (initiation, maintenance, relapse) (8, 11), results from this study may inform smoking cessation treatments, and neuroscience-based interventions for tobacco use and dependence. Specifically, the identification of GM volume reductions in brain areas related to different aspects of cognitive impulsivity (ACC to risky decision making, VLPFC, ACC, and caudate to impulsive choices), and the proposed left lateralization of these neurocognitive impairments, may aid the improvement of technology-based neuromodulation interventions such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (rTMS). In fact, such interventions have provided limited and contrasting results for the treatment of nicotine dependence (49).
Moreover, the inability to delay gratification, commonly featured by chronic smokers (7), should be considered a primary target for cognitive rehabilitation therapies (CRTs) (50). Studies have also proposed Episodic Future Thinking (ETF) interventions as effective means to decrease delay discounting rates, cigarette self- administration, and cigarette demand (51).
Strengths and Limitations
The current study should be considered in light of several limitations. First, the size of the sample enrolled in the current study was relatively small, therefore limiting the power of risky decision making (CGT) results. However, post-hoc power calculations for choice impulsivity (ADT-5) results revealed an achieved power of 0.94 with a very large effect size of 0.5. Additionally, participants were all recruited from the same deprived metropolitan area, thus generalizability may be limited to Scottish chronic smokers from a low socio-economic background (SES). Studies have proposed a relationship between low SES, brain development deficits, and cognitive impairments (52). Although, chronic tobacco smoking has been recently found to mediate these associations (53). Another limitation consists in the utilization of self- reported questionnaires to assess alcohol consumption patterns and smoking characteristics of participants (pack years, cigarettes smoked per day, age at regular smoking onset) as participants may have provided inaccurate information. Nonetheless, a rigorous screening procedure involving different objective measurements to assess smoking status (exhaled CO, salivary cotinine) and to exclude the presence of other substances in participants' system (urine analysis) was employed. The recruitment of a younger chronic smoker sample not affected by polysubstance dependence and characterized by minimal alcohol and cannabis use, with the utilization of stringent exclusion and inclusion criteria, may be considered strengths of the current study.
Conclusion
The current study revealed an association between left fronto-cortical and striatal GM volume reductions and impaired cognitive impulsivity in chronic tobacco smokers. GM volume reductions in the left VLPFC correlated to heightened impulsive choices and to younger age at regular smoking onset. Considering that the cross-sectional nature of the current research limits inferences of causal effects, longitudinal studies would be required to elucidate if these neurocognitive impairments are representations of pre-morbid endophenotypes or are caused by tobacco exposure on the developing adolescent brain.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by National Health Service (NHS) London Bromley Research Ethics Committee (REC) (REC Reference Number: 19/LO/1176), and by the University of St. Andrews Teaching and Research Ethics Committee (UTREC) (UTREC Approval Code: MD14516). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AC and AB were responsible for the study concept and design. AC contributed to the recruitment of participants, acquisition data, performed the analysis of data, and drafted the manuscript. AB supervised recruitment, screening procedures, assisted with data analysis, interpretation of findings, and provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
Funding
This study has been supported by a University of St. Andrews Endowment fund and by a self-funded PhD scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We wish to thank Professor Douglas Steele and Dr. Serenella Tolomeo for the theoretical advice and the support provided during the analysis of neuroimaging data. We wish to thank Dr Jennifer McFarlane and all radiographers at the Clinical Research Center (CRC) at Ninewells Hospital, Dundee for the support provided during the neuroimaging procedures. Finally, we wish to thank all participants for their involvement in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.708925/full#supplementary-material
References
1. World Health Organisation. Tobacco Key Facts. (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/tobacco (May 27, 2020).
2. Verdejo-Garcia A, Garcia-Fernandez G. Dom G. Cognition and addiction dialogues. Clin Neurosci. (2019) 21:281. doi: 10.31887/DCNS.2019.21.3/gdom
3. Lee RS, Hoppenbrouwers S. Franken IJ. A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychol Rev. (2019) 29:14–26. doi: 10.1007/s11065-019-09402-x
4. Bickel WK, Koffarnus MN, Moody L, Wilson AGJN. The behavioral-and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropsychopharmacology. (2014) 76:518–27. doi: 10.1016/j.neuropharm.2013.06.013
5. Yücel M, Oldenhof E, Ahmed SH, Belin D, Billieux J, Bowden-Jones H, et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. (2019) 114:1095–109. doi: 10.1111/add.14424
6. Chen S, Yang P, Chen T, Su H, Jiang H, Zhao MJP. Risky decision-making in individuals with substance use disorder: a meta-analysis and meta-regression review. Psychopharmacology. (2020) 237:1893–908. doi: 10.1007/s00213-020-05506-y
7. Conti AA, McLean L, Tolomeo S, Steele J, Baldacchino AB. Chronic tobacco smoking and neuropsychological impairments: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2019) 96:143–54. doi: 10.1016/j.neubiorev.2018.11.017
8. Bloom EL, Matsko SV, Cimino CRJAR. Theory. The relationship between cigarette smoking and impulsivity: a review of personality, behavioral, and neurobiological assessment. Addict Res Theory. (2014) 22:386–97. doi: 10.3109/16066359.2013.867432
9. Barlow P, McKee M, Reeves A, Galea G. Stuckler DJIjoe. Time-discounting and tobacco smoking: a systematic review and network analysis. Int J Epidemiol. (2017) 46:860–9. doi: 10.1093/ije/dyw233
10. González-Roz A, Secades-Villa R, Pericot-Valverde I, Weidberg S, Alonso-Pérez FJ. Effects of delay discounting and other predictors on smoking relapse. Span J Psychol. (2019) 22:E9. doi: 10.1017/sjp.2019.11
11. Sheffer CE, Christensen DR, Landes R, Carter LP, Jackson L, Bickel WK. Delay discounting rates: a strong prognostic indicator of smoking relapse. Addict Behav. (2014) 39:1682–9. doi: 10.1016/j.addbeh.2014.04.019
12. Dalley JW, Robbins TW. Fractionating impulsivity: neuropsychiatric implications. Nat Neurosci. (2017) 18:158. doi: 10.1038/nrn.2017.8
13. Bickel WK, Snider SE, Quisenberry AJ, Stein JS, Hanlon CA. Competing neurobehavioral decision systems theory of cocaine addiction: from mechanisms to therapeutic opportunities. Prog Brain Res. (2016) 223:269–93. doi: 10.1016/bs.pbr.2015.07.009
14. Meade CS, Bell RP, Towe SL, Hall SAJD, Dependence A. Cocaine-related alterations in fronto-parietal gray matter volume correlate with trait and behavioral impulsivity. Drug Alcohol Depend. (2020) 206:107757. doi: 10.1016/j.drugalcdep.2019.107757
15. Wang J, Fan Y, Dong Y, Ma M, Ma Y, Dong Y, et al. Alterations in brain structure and functional connectivity in alcohol dependent patients and possible association with impulsivity. PLoS ONE. (2016) 11:e0161956. doi: 10.1371/journal.pone.0161956
16. Tolomeo S, Gray S, Matthews K, Steele J, Baldacchino AB. Multifaceted impairments in impulsivity and brain structural abnormalities in opioid dependence and abstinence. Psychol Med. (2016) 46:2841–53. doi: 10.1017/S0033291716001513
17. Yang Z, Zhang Y, Cheng J, Zheng RJ. Meta-analysis of brain gray matter changes in chronic smokers. Eur J Radiol. (2020) 132:109300. doi: 10.1016/j.ejrad.2020.109300
18. Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA, et al. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct. (2016) 12:16. doi: 10.1186/s12993-016-0100-5
19. Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, et al. Lower subcortical gray matter volume in both younger smokers and established smokers relative to non- smokers. Addict Biol. (2016) 21:185–95. doi: 10.1111/adb.12171
20. Zsidó AN, Darnai G, Inhóf O, Perlaki G, Orsi G, Nagy SA, et al. Differentiation between young adult Internet addicts, smokers, and healthy controls by the interaction between impulsivity and temporal lobe thickness. J Behav Addict. (2019) 8:35–47. doi: 10.1556/2006.8.2019.03
21. Durazzo TC, Meyerhoff DJ, Yoder KK. Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimerdi.org/in. Drug Alcohol Depend. (2018) 192:277–84. doi: 10.1016/j.drugalcdep.2018.08.009
22. Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. (1999) 19:9029–38. doi: 10.1523/JNEUROSCI.19-20-09029.1999
23. Koffarnus MN, Bickel WK. A 5-trial adjusting delay discounting task: accurate discount rates in less than one minute. Exp Clin Psychopharmacol. (2014) 22:222–8. doi: 10.1037/a0035973
24. Orr JM, Paschall CJ, Banich MTJNC. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin. (2016) 12:47–56. doi: 10.1016/j.nicl.2016.06.006
25. Gillespie NA, Neale MC, Bates TC, Eyler LT, Fennema-Notestine C, Vassileva J, et al. Testing associations between cannabis use and subcortical volumes in two large population- based samples. Addiction. (2018) 113:1661–72. doi: 10.1111/add.14252
26. Figueiredo PR, Tolomeo S, Steele JD, Baldacchino A. Neurocognitive consequences of chronic cannabis use: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2020) 108:358–69. doi: 10.1016/j.neubiorev.2019.10.014
27. Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Amorim P, Janavs J, Weiller E, et al. The mini international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry. (1998) 59(Suppl. 20):22–33.
28. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. (1991) 86:1119–27.
29. Barona A, Reynolds CR, Chastain RJ, Psychology C. A demographically based index of premorbid intelligence for the WAIS—R. J Consult Clin Psychol. (1984) 52:885. doi: 10.1037/0022-006X.52.5.885
30. Yoon, Jin H, Higgins ST. Turning k on its head: comments on use of an ED50 in delay discounting research. Drug Alcoh Depend. (2008) 95:169–72. doi: 10.1016/j.drugalcdep.2007.12.011
31. Baldacchino A, Balfour DJ, Matthews K. Impulsivity and opioid drugs: differential effects of heroin, methadone and prescribed analgesic medication. Psychol Med. (2015) 45:1167–79. doi: 10.1017/S0033291714002189
32. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. (2005) 26:839–51. doi: 10.1016/j.neuroimage.2005.02.018
33. Slotnick SD, Moo LR, Segal JB, Hart J Jr. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. (2003) 17:75–82. doi: 10.1016/s0926-6410(03)00082-x
34. Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, et al. Age-associated cognitive decline. Br Med Bull. (2009) 92:135–52. doi: 10.1093/bmb/ldp033
35. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. (2016) 3:760–73. doi: 10.1016/S2215-0366(16)00104-8
36. Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. (2002) 159:1642–52. doi: 10.1176/appi.ajp.159.10.1642
37. Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein E. A factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. (2011) 54:42–8. doi: 10.1016/j.neuroimage.2010.08.008
38. Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. (2012) 17:977–80. doi: 10.1111/j.1369-1600.2010.00250.x
39. Clewett D, Luo S, Hsu E, Ainslie G, Mather M, Monterosso J. Increased functional coupling between the left fronto-parietal network and anterior insula predicts steeper delay discounting in smokers. Hum Brain Mapp. (2014) 35:3774–87. doi: 10.1002/hbm.22436
40. Gordon HW. Laterality of brain activation for risk factors of addiction. Curr Drug Abuse Rev. (2016) 9:1–18. doi: 10.2174/1874473709666151217121309
41. Potvin S, Tikàsz A, Dinh-Williams LL, Bourque J, Mendrek A. Cigarette cravings, impulsivity, and the brain. Front Psychiatry. (2015) 6:125. doi: 10.3389/fpsyt.2015.00125
42. DeBry SC, Tiffany ST. Tobacco-induced neurotoxicity of adolescent cognitive development (TINACD): a proposed model for the development of impulsivity in nicotine dependence. Nicotine Tob Res. (2008) 10:11–25. doi: 10.1080/14622200701767811
43. Yuan M, Cross SJ, Loughlin SE, Leslie FM. Nicotine and the adolescent brain. J Physiol. (2015) 593:3397–412. doi: 10.1113/JP270492
44. Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb Perspect Med. (2012) 2:a012120. doi: 10.1101/cshperspect.a012120
45. Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. (2012) 16:81–91. doi: 10.1016/j.tics.2011.11.009
46. Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. (2012) 335:601–4. doi: 10.1126/science.1214463
47. Long EC, Kaneva R, Vasilev G, Moeller FG, Vassileva J. Neurocognitive and psychiatric markers for addiction: common vs. specific endophenotypes for heroin and amphetamine dependence. Curr Top Med Chem. (2020) 20:585–97. doi: 10.2174/1568026620666200131124608
48. MacKillop J, Munafò MR. Commentary: delay discounting and smoking: robust correlation, but uncertain causation. Int J Epidemiol. (2017) 46:870–1. doi: 10.1093/ije/dyw303
49. Antonelli M, Fattore L, Sestito L, Di Giuda D, Diana M, Addolorato G. Transcranial magnetic stimulation: a review about its efficacy in the treatment of alcohol, tobacco and cocaine addiction. Addict Behav. (2021) 114:106760. doi: 10.1016/j.addbeh.2020.106760
50. Rezapour T, DeVito EE, Sofuoglu M, Ekhtiari H. Perspectives on neurocognitive rehabilitation as an adjunct treatment for addictive disorders: from cognitive improvement to relapse prevention. Prog Brain Res. (2016) 224:345–69. doi: 10.1016/bs.pbr.2015.07.022
51. Stein JS, Tegge AN, Turner JK, Bickel WK. Episodic future thinking reduces delay discounting and cigarette demand: an investigation of the good-subject effect. J Behav Med. (2018) 41:269–76. doi: 10.1007/s10865-017-9908-1
52. Brito NH, Noble KG. Socioeconomic status and structural brain development. Front Neurosci. (2014) 8:276. doi: 10.3389/fnins.2014.00276
Keywords: neuropsychology, nicotine, impulsivity, neuroimaging, tobacco, addictions, adolescents
Citation: Conti AA and Baldacchino AM (2021) Neuroanatomical Correlates of Impulsive Choices and Risky Decision Making in Young Chronic Tobacco Smokers: A Voxel-Based Morphometry Study. Front. Psychiatry 12:708925. doi: 10.3389/fpsyt.2021.708925
Received: 12 May 2021; Accepted: 13 August 2021;
Published: 30 August 2021.
Edited by:
Boris B. Quednow, University of Zurich, SwitzerlandReviewed by:
Liangsuo Ma, Virginia Commonwealth University, United StatesGabriela Gan, University of Heidelberg, Germany
Copyright © 2021 Conti and Baldacchino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aldo Alberto Conti, YWFjMjBAc3QtYW5kcmV3cy5hYy51aw==
 Aldo Alberto Conti
Aldo Alberto Conti Alexander Mario Baldacchino
Alexander Mario Baldacchino