- 1Department of Public Health and Management, Chongqing Three Gorges Medical College, Chongqing, China
- 2Key Laboratory of Cognition and Personality, Faculty of Psychology, Ministry of Education, Southwest University, Chongqing, China
- 3National Demonstration Center for Experimental Psychology Education, Southwest University, Chongqing, China
- 4College of Physical Education, Southwest University, Chongqing, China
- 5Mood Disorders Psychopharmacology Unit, Toronto, ON, Canada
- 6Yubei Center for Disease Control and Prevention, Chongqing, China
Alterations in the peripheral (e.g., serum, plasma, platelet) concentrations of arginine and its related catabolic products (i.e., ornithine, citrulline) in the urea and nitric oxide cycles have been reported to be associated with major depressive disorder (MDD). The meta-analysis herein aimed to explore the association between the concentration of peripheral arginine, its catabolic products and MDD, as well as to discuss the possible role of arginine catabolism in the onset and progression of MDD. PubMed, EMBASE, PsycINFO and Web of Science were searched from inception to June 2020. The protocol for the meta-analysis herein has been registered at the Open Science Framework [https://doi.org/10.17605/osf.io/7fn59]. In total, 745 (47.5%) subjects with MDD and 823 (52.5%) healthy controls (HCs) from 13 articles with 16 studies were included. Fifteen of the included studies assessed concentrations of peripheral arginine, eight assessed concentrations of ornithine, and six assessed concentrations of citrulline. Results indicated that: (1) the concentrations of arginine, ornithine, and citrulline were not significantly different between individuals with MDD and HCs when serum, plasma and platelet are analyzed together, (2) in the subgroups of serum samples, the concentrations of arginine were lower in individuals with MDD than HCs, and (3) concurrent administration of psychotropic medications may be a confounding variable affecting the concentrations of arginine, ornithine, and citrulline. Our findings herein do not support the hypothesis that arginine catabolism between individuals with MDD and HCs are significantly different. The medication status and sample types should be considered as a key future research avenue for assessing arginine catabolism in MDD.
Introduction
Major depressive disorder (MDD) is one of the most common mental disorders affecting more than 350 million people worldwide (1, 2). Furthermore, MDD is one of the leading causes of global burden of disease. Major depressive disorder affects approximately 16% of the world's population (3), continues to be a major cause of disability worldwide, and is the number one cause of suicide (4). Excessive inflammation and neurodegenerative changes have been reported in MDD, both of which have been associated with cognitive impairment (5). However, the underlying pathology of MDD remains poorly understood, in part due to the heterogeneity of genetic and environmental factors related to proposed mechanisms of action (6). Moreover, the pathogenesis of acute depression may be different from recurrent or chronic depression, which is characterized by long-term decline in social or occupational function and cognitive ability (7).
Intensified research efforts are devoted to predicting onset of MDD and treatment response through exploring possible biomarkers/biosignatures including but not limited to metabolic pathways (8–10). Extensive literature indicates that amino acids may be candidate biomarkers for a variety of diseases, including metabolic syndrome, cancer, and mental disorders (11–16). With increasing research focusing on the dysfunction of oxidative and nitrosative stress in MDD, arginine catabolism regulation has received increasing attention (17–19).
Arginine is a semi-essential amino acid and a substrate of important metabolic pathways in the physiological processes of the central nervous system and immune defense [e.g., urea and nitric oxide (NO) cycles] (20, 21). Arginine is transformed into NO and citrulline by endothelial nitric oxide synthase (eNOS), inducible nitric oxide synthase (iNOS) and neuronal nitric oxide synthase (22, 23). The alteration of arginine may lead to abnormalities of NO metabolism and the urea cycle metabolic pathway (24, 25).
The dysregulation of the L-arginine-NO metabolic pathway has been linked to the pathogenesis of severe depression (26, 27). For example, a study reported a positive correlation between increased plasma NO concentrations and suicide attempts in individuals with mild depression (28). A separate study reported that the inhibition of NOS induced antidepressant effects in rats (29). Hitherto, reducing or blocking the synthesis of NO (i.e., blocking NOS) in the brain may be protective against depression (i.e., antidepressant effects) (29, 63).
Available clinical evidence has demonstrated that the NO signaling pathway is associated with schizophrenia, anxiety disorders, and affective disorders (30). Notably, the NO system has previously been reported to be a potential target of antidepressant and anti-anxiety drugs in acute therapy and prevention (31).
Previous cross-sectional studies among patients with MDD and experimental studies based on animal models of depression have reported the altered arginine levels in blood related to the catabolite and NO imbalance in pathophysiology of MDD (18, 32). The dysfunction of NO signaling pathway has been suggested as a nexus between MDD and commonly encountered comorbidities via platelet activation, endothelial dysfunction (i.e., low circulating endothelial NO concentrations and impaired vasodilation), and elevated concentrations of proinflammatory circulating cytokines (33, 34).
Arginine, citrulline, and ornithine are key amino acids of the urea cycle (35). Arginine is the substrate for arginase, the enzyme that produces urea while converting arginine to ornithine (36). Previous studies have shown that arginase activity is elevated in people with depression (37). Moreover, L-arginine is reported to be a risk factor for the development of mild depression (38). It has been separately reported that patients with depression have lower circulating L-arginine concentrations (33). Arginine has also been reported to affect concentrations of aminobutyric acid and glutamate in the prefrontal cortex of the brain, which are important to cellular bioenergetics and oxidative stress (39). In addition, two clinical trials have shown that ketamine and esketamine, glutamatergic N-methyl-D aspartate receptor (NMDAR) antagonists with established antidepressant effects, contributed to the changes in arginine in the urea cycle (40, 41). Taken together, extensive literature has supported the notion that arginine may be implicated in mechanisms that are relevant to MDD.
Ornithine is a metabolite of arginine (42). Citrulline is derived not only from the production of NO but also from the action of the enzyme ornithine carbamoyltransferase (43). Previous findings regarding the function of arginine catabolism underlying MDD are inconsistent. L-Arginine competes with asymmetric dimethylarginine for NOS (44) and increases endothelial NO production and reverses the endothelial dysfunction associated with vascular risk factors (64). L-citrulline can be converted to L-arginine via the citrulline-NO cycles as well as the urea cycle. The possibility that decreased L-cirtulline contributes to decreased L-arginine concentrations in physically healthy patients with MDD cannot be excluded (45). It has been previously demonstrated that L-arginine may have the potential to treat MDD (46). Taken together, the foregoing studies provide the rational to assess the potential association between L-arginine concentrations and MDD.
To our knowledge, there has been no previous meta-analysis evaluating the association between arginine catabolism and MDD. Herein, the current meta-analysis aims to compare peripheral arginine and its related catabolic products (i.e., ornithine and citrulline) between patients with MDD and healthy controls (HCs) (i.e., healthy volunteers who do not have current or previous history of mental illness).
Methods
Literature Search
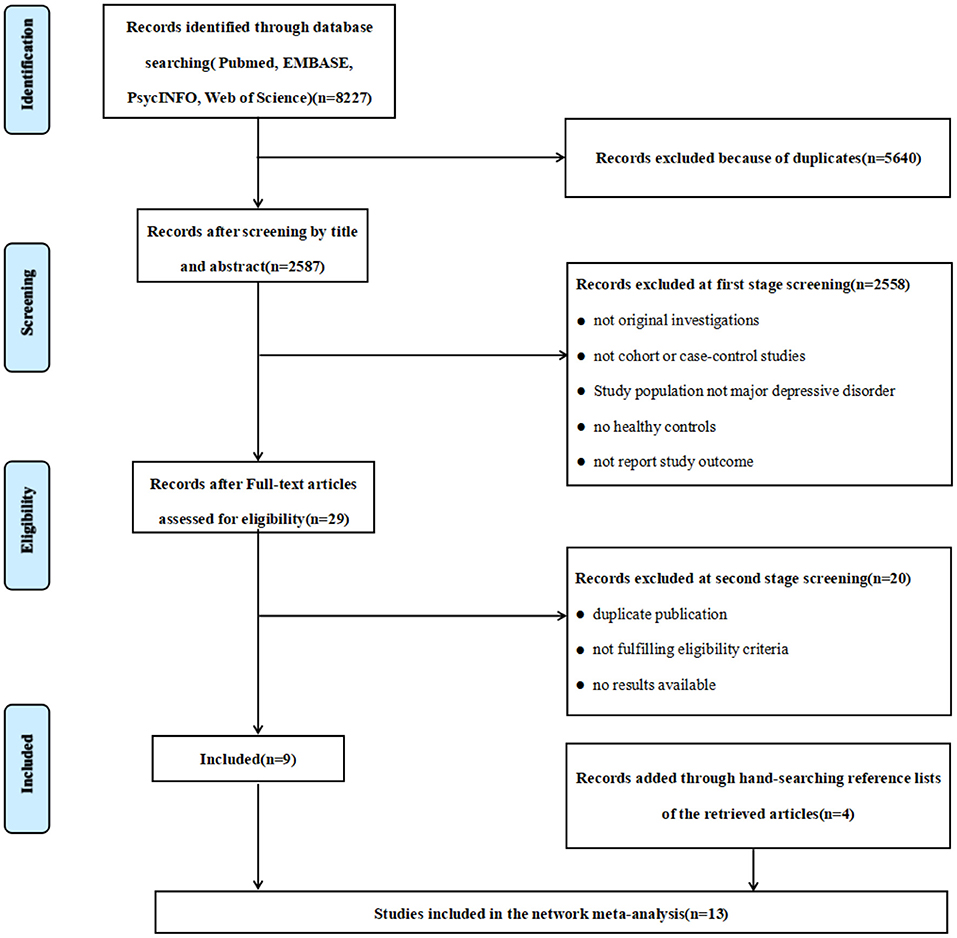
We performed this study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (47), and a systematic retrieval of literature from inception to June 2020. The literature search was conducted using the following online databases: PubMed, EMBASE, PsycINFO and Web of Science. The keywords of the search strategy were “major depressive disorder (MDD)”, “depression”, “mood disorder”, “arginine”, “ornithine”, “citrulline”, “L-arginine”, “amino acid”, “argininosuccinate”. The flow diagram outlining the study selection process is shown in Figure 1. The protocol of current meta-analysis has been registered at the Open Science Framework [https://doi.org/10.17605/osf.io/7fn59 (48)].
Selection Criteria
The inclusion of studies were based on the following criteria: (1) adults (≥ 18 years old) with Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnosed MDD (i.e., DSM-III-R, DSM-IV, DSM-IV-TR and DSM-V); (2) healthy volunteers who are not diagnosed with psychiatric illness and do not have a history of mental illness were used as the HC group; (3) measures of the concentrations of arginine, citrulline, or ornithine (one of which was sufficient) assessed in all subjects; and (4) the study type was either a case-control study or cohort study.
Studies were excluded based on the following criteria: (1) non-original research, articles or conference abstracts; (2) case reports, case studies, case series studies, clinical trials and other articles that did not meet the required research type; (3) non-human studies, the research objective did not include patients with MDD, or the case group contains other mental disorders (e.g., schizophrenia, bipolar disorder); (4) comparison of patients before and after treatment; people with other diseases as control group; (5) concentrations of arginine, citrulline, or ornithine were not available; (6) no full-text or studies were repetitive publications from the same datasets by the same or different authors.
Data Extraction and Quality Assessment
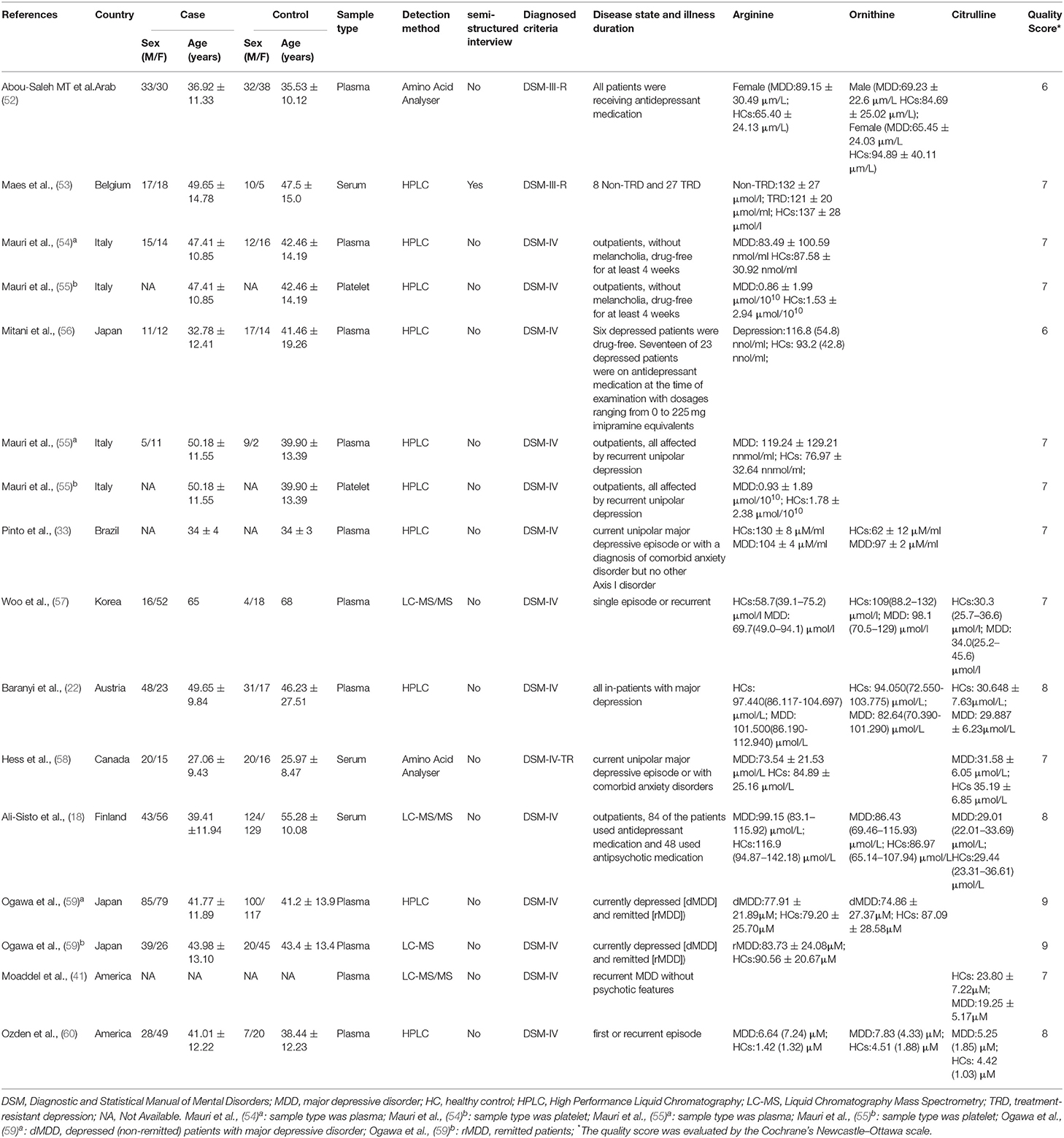
Two investigators (CB and LL) independently screened and reviewed articles, Supplementary Materials, and extracted relevant information. Several articles may not be captured by our search because their keywords are not exactly matched our search strategies. All reference lists of the retrieved articles were reviewed to identify potential studies for inclusion. The additional articles were manually retrieved from the official website of the journals from the reference lists. The study used standardized tables to extract information for each eligible article. The following information was extracted from each study: first author, publication year, study design, country, geographic location, age, sex (i.e., female, male), body mass index (BMI), type of blood sample specimen required for test (i.e., plasma, serum, platelet), sample detection method [i.e., High Performance Liquid Chromatography (HPLC), Liquid Chromatography Mass Spectrometry (LC-MS), or other], sample size, subjects' mean arginine or ornithine or citrulline concentrations, and standard deviation (SD).
Statistical Analysis
All data analysis was performed using Stata (version 15.0, Stata Corp LP, College Station, TX, USA). Forest plots was used to estimate the association between arginine, its related catabolic products, and MDD, which was evaluated by standardized mean difference (SMD) with a 95% confidence interval (CI). We assigned weights (%) based on the inverse of the variance. The greater weights represent the greater impact on the combined results. The heterogeneity of all studies was assessed by chi-square statistics and the I-Squared (I2) test. If P < 0.10 or I2 > 50%, we considered that the heterogeneity had statistical differences and a random effects model would be used. Otherwise, the fixed effect meta-analysis would be applied (9).
Subgroup analysis was performed to explore the potential impact of the inclusion characteristics of the studies on the pooled effect size. The effect sizes of arginine, ornithine and citrulline concentrations were calculated for each subgroup. The subgroups were created based on medication status (prescribed medication vs. medication-free), sample types (plasma, serum, or platelet), published year (before vs. after 2010), regional distribution (Asia, Europe, America, or Oceania) of arginine and detection method (Amino acid Analyzer, HPLC, LC-MS). To create subgroups based on living standards of past decade or earlier, studies were divided into two groups according to their publication date (i.e., before 2010 vs. after 2010) (49, 50). Meta-regression was used to investigate the source of heterogeneity, and the effects of both continuous and categorical factors on the study were assessed simultaneously. Sensitivity analysis was used to investigate whether any single study would have an effect on the heterogeneity of total measurements in each meta-analysis. The funnel plot with Begg's test and Egger's test were used to test publication bias.
The Newcastle-Ottawa Scale was used to detect the risk of bias in observational studies. According to the total scores of the Newcastle-Ottawa Scale, the observational studies were divided into three categories: extremely high risk of bias (0–3 points), high risk of bias (4–6 points) and low risk of bias (7–9 points) (51). The bias risk assessment of the included articles is shown in Supplementary Table 1.
Results
Basic Characteristics of Included Studies
A total of 8,227 articles were identified from the preliminary search. After screening titles and abstracts, excluding review articles and duplicated articles, and studies that did not meet the inclusion criteria, 29 articles were selected for full-text review. After evaluation, we identified 9 articles that met the inclusion criteria and were selected for analysis. Four articles were retrieved by manual search. The current meta-analysis includes 13 articles with 16 studies (18, 22, 33, 41, 52–60) (Figure 1). Of the thirteen articles included, ten articles reported the results of a single study each, and 3 articles each of which reported results from two studies.
The basic characteristics of included studies are illustrated in Table 1. The current meta-analysis included 745 (47.5%) individuals with MDD and 823 (52.5%) HCs. 15 of the studies assessed concentrations of arginine, 8 assessed ornithine concentrations, and 6 assessed citrulline concentrations. For the geographic location, 5 studies were conducted in Asia, 6 studies were conducted in Europe, 4 studies were conducted in the United States, and 1 study was conducted in Oceania. Most studies used the detection methods of HPLC (n = 10), followed by LC-MS (n = 4), and Amino Acid Analyses (n = 2). Only one study conducted semi-structured interviews, and the remaining studies conducted structured interviews for diagnosis. Participants in 9 studies had received antidepressant medications, and participants in 7 studies reported to be medication-free. The sample types for 11 studies was plasma, 3 studies were serum, and two were platelets. According to the results of the Newcastle-Ottawa Scale, there were 2 articles defined as “high bias risk” with scores of 6, while the others were all defined as “low bias risk” with scores higher than 7.
Homogeneity Analysis and Effect Estimation
Due to the high heterogeneity of arginine (I2 = 80.6%, P < 0.001), ornithine (I2 = 87.3%, P < 0.001) and citrulline (I2 = 72.1%, P = 0.003) reported in the included studies, random effect models were selected for the meta-analysis. No statistical differences in the concentrations of arginine (SMD = 0.02; 95%CI: −0.24, 0.29; P = 0.86), ornithine (SMD = −0.01; 95%CI: −0.38, 0.36; P = 0.96) and citrulline (SMD = −0.12; 95%CI: −0.43, 0.19; P = 0.46) were found between the subjects with MDD and HCs when serum, plasma and platelet are analyzed together. The forest plots are shown in Figure 2. Separate meta-analyses of arginine, ornithine and citrulline for serum, plasma and platelet are shown in Supplementary Figures 5–7.
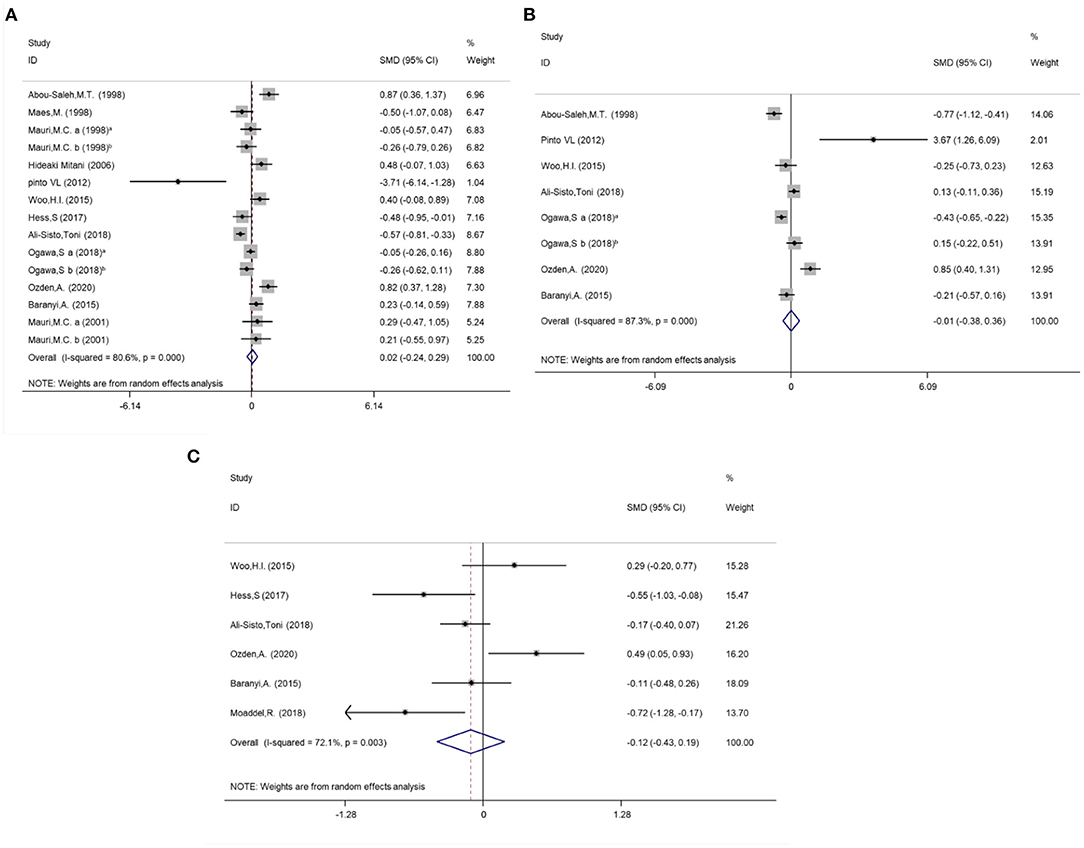
Figure 2. Meta-analysis for the difference of the Arginine, Ornithine and Citrulline concentrations between MDD individuals and controls by random effect analysis. (A) Arginine; (B) Ornithine. (C) Citrulline. Mauri et al. (54) a: sample type was plasma; Mauri et al., (54) b: sample type was platelet; Mauri et al., (55) a: sample type was plasma; Mauri et al., (55) b: sample type was platelet; Ogawa et al., (59) a: dMDD, depressed (non-remitted) individuals with major depressive disorder; Ogawa et al., (59) b: rMDD, remitted individuals.
Subgroup Analysis and Meta-Regression Results
Subgroup analysis were conducted to explore potential subgroup effects. For the subgroup analysis of arginine concentrations, the distribution trend of whether samples were prescribed medications (medication-free: SMD = −0.25; 95%CI: −0.83, 0.33; P = 0.39; I2 = 82.9%, P < 0.001; prescribed medications: SMD = 0.13; 95%CI: −0.17, 0.44; P = 0.39; I2 = 81.4%, P < 0.001) and different sample types (plasma: SMD = 0.23; 95%CI: −0.07, 0.53; P = 0.14; I2 = 76.1%, P < 0.001; serum: SMD = −0.54; P < 0.001; 95%CI: −0.74, −0.35; I2 = 0.0%, P = 0.93; platelet: SMD = −0.11; 95%CI: −0.54, 0.32; P = 0.62; I2 = 1.8%, P = 0.313) were also analyzed (Figure 3). The subgroup analysis results of medication status and sample types for ornithine and citrulline concentrations were shown in Supplementary Figure 1. We also conducted the subgroup analyses with year of publication, regional distribution, and detection methods (Supplementary Figure 2).
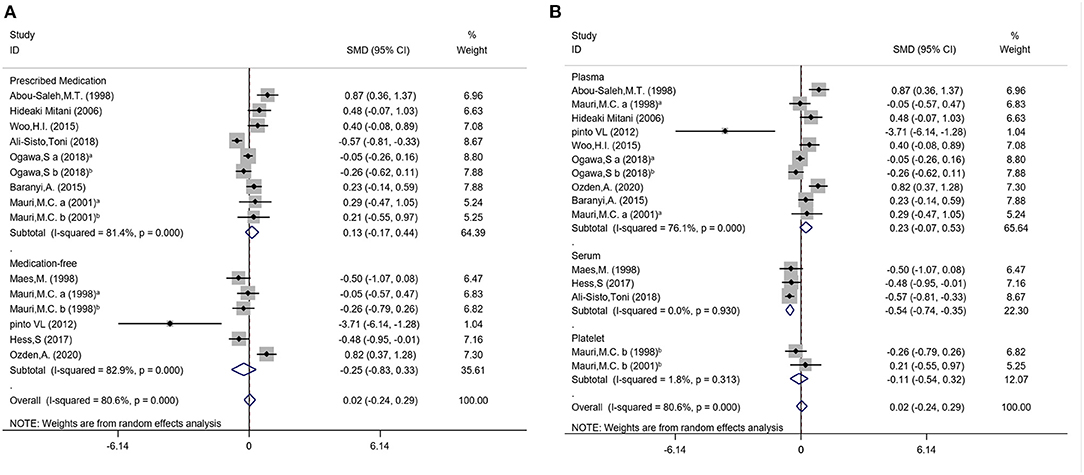
Figure 3. Subgroup differences of the arginine concentrations in subjects with MDD and controls. (A) Medication status of arginine; (B) Sample types of arginine.
Meta-regression was performed on five aspects (i.e., published year, medication use status, geographic location, sample types, and detection methods) to investigate the sources of heterogeneity. None of the variables above could explain the heterogeneity of meta-analysis (all P > 0.05).
Sensitivity Analysis and Publication Bias
The results of our sensitivity analysis indicated that there was no significant change by omitting a single study, indicating that the models were relatively robust (Supplementary Figure 3). The Egger's test and the Begg's test were used to evaluate the publication bias in the study (Supplementary Figure 4). The publication bias test was only performed for arginine studies since these were the only studies with a sample size greater than 10. The funnel plots of the included meta-analysis indicated that there was no publication bias of the included studies assessing the concentrations of arginine (Egger's intercept = 0.82; 95%CI = (−2.21, 3.85), P = 0.57 and Begg's test Z = 0.10, P = 0.92).
Discussion
The main findings of the meta-analysis herein are as follows: (1) the peripheral concentrations of arginine, ornithine, and citrulline were not significantly different between patients with MDD and HCs; (2) the peripheral concentrations of arginine were lower in individuals with MDD than the HCs in the subgroups of serum samples; and (3) concurrent medication may contribute to altered peripheral concentrations of arginine, ornithine, and citrulline.
Through subgroup analysis, we found that the status of medication and sample types may contribute to the reduced heterogeneity of the sample. Although no statistically significant results were observed in the subgroup analysis of the medication status, the various trends of prescribed medication and medication-free were observed in arginine concentrations between individuals with MDD and HCs (55). Arginine concentrations reported in individuals with MDD with prescribed medication were generally higher than that of the HC population, while the arginine concentrations in individuals with MDD who were not taking medication were lower in comparison to HCs (58). Ali-Sisto et al. did not report a significant difference of arginine concentrations between responders and non-responders of antidepressants at baseline (18).
The subgroup analysis of sample types indicated that the arginine and citrulline concentrations in the serum samples were significantly lower in individuals with MDD when compared with HCs. The samples from plasma and serum represent circulating concentrations of amino acids. A previous study also illustrated that serum L-arginine concentrations do reflect intracellular L-arginine (61). Pinto et al. reported that reduced plasma L-arginine concentrations were associated with reduced L-arginine flow in platelets in a small sample comparing individuals with MDD and HCs (33).
Both the medication status and fasting status were potential confounders for the amino acid concentrations in blood samples. Psychotropic medications may have direct/indirect effects on NO metabolite concentrations in individuals with MDD. For example, medication may alter the protein-binding of amino acids, possibly leading to dysregulation in renal clearance of these metabolites. Additionally, antidepressants may cause the inhibition of related enzymes (18).
Moreover, no previous research has indicated a significant difference in amino acid concentrations between serum and EDTA-K2 anticoagulated plasma samples in individuals with MDD. A recent methodological study reported that the concentrations of amino acids were higher when compared to heparin plasma, EDTA plasma, and fluoride plasma (62). From the current meta-analysis, we cannot determine the reasons behind the observed L-arginine differences in serum vs. plasma samples between cases and controls. The current findings provide instruction from a methodological perspective on the importance of evaluating serum vs. plasma highlighting the importance of evaluating these variables separately.
To our knowledge, this is the first meta-analysis to explore the associations between arginine and related catabolites and MDD. We cannot conclude that arginine catabolism or the bioavailability of arginine has the potential to decrease or increase in individuals with MDD. Herein, our findings provide a meaningful direction for researchers engaged in the study of the metabolic mechanism of MDD. Our current findings require replication, preferrably with a large, well-characterized sample, sufficient to conduct disparate covariate analysis including buy not limited to subgrouping on the basis of medication status.
Limitations
The findings in our study should be interpreted with caution due to the following methodological aspects affecting the outcomes of this review. Firstly, 16 studies from 13 articles were included in the current study, thus the sample sizes of overall and subgroup analysis were relatively small, and the results of subgroup analysis. Secondly, there was high heterogeneity in data comparison and data pooling. Through subgroup analysis and meta-regression analysis, the heterogeneity cannot be fully explained. Thirdly, studies that included populations categorized as “prescribed medication” may be limited in their results as some subjects may not have followed the medication regimen according to study protocol and/or included subjects that did not take any of the prescribed medication.
Conclusion
Taken together, using meta-analytic techniques we were unable to identify compelling evidence that arginine and/or its catabolic products exhibit significant alteration in adults with MDD vs. HCs. Subgroup analysis indicated that the concentrations of arginine were lower in individuals with MDD than HCs in the subgroups of serum samples. Our findings do not exclude the possibility of a type II error due to confounding factors.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MF, XG, and BC conceived and designed the study. BC and LLi collected the data. LLi performed the statistical analysis. MF, RM, PD, KT, ZR, and LLui contributed to the discussion. All authors revised the paper and approved the final version of this article.
Funding
This work was sponsored by the MOE (Ministry of Education in China) Project of Humanities and Social Sciences (21YJCZH004), Chongqing Municipal Education Commission Project of Science and Technology Innovation (KJCX2020003), and Chongqing Social Science Planning Project (2019PY57). The funding agents had no role in the design and conduct of the study; collection, management, analysis, interpretation of the data; preparation, review, or approval of the manuscript.
Conflict of Interest
RM has received research grant support from CIHR/GACD/Chinese National Natural Research Foundation; speaker/consultation fees from Lundbeck, Janssen, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie. RM is a CEO of Braxia Scientific Corp. KT has received personal fees from Braxia Scientific Corp. LLui is a contractor to Braxia Scientific Corp.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.686973/full#supplementary-material
References
1. Otte C, Gold S, Penninx B, Pariante C, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. (2016) 2:16065. doi: 10.1038/nrdp.2016.65
2. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990and#x2013;2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(17)32154-2
3. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. (2003) 289:3095–105. doi: 10.1001/jama.289.23.3095
4. Olin B, Jayewardene AK, Bunker M, Moreno F. Mortality and suicide risk in treatment-resistant depression: an observational study of the long-term impact of intervention. PLoS ONE. (2012) 7:e48002. doi: 10.1371/journal.pone.0048002
5. Krishnadas R, Cavanagh J. Depression: an inflammatory illness? J Neurol Neurosurg Psychiatry. (2012) 83:495–502. doi: 10.1136/jnnp-2011-301779
6. McIntosh A, Hall L, Zeng Y, Adams M, Gibson J, Wigmore E, et al. Genetic and environmental risk for chronic pain and the contribution of risk variants for major depressive disorder: a family-based mixed-model analysis. PLoS Med. (2016) 13:e1002090. doi: 10.1371/journal.pmed.1002090
7. Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. (2008) 358:55–68. doi: 10.1056/NEJMra073096
8. Block A, Schipf S, Van der Auwera S, Hannemann A, Nauck M, John U, et al. Sex- and age-specific associations between major depressive disorder and metabolic syndrome in two general population samples in Germany. Nord J Psychiatry. (2016) 70:611–20. doi: 10.1080/08039488.2016.1191535
9. Cao B, Chen Y, Brietzke E, Cha D, Shaukat A, Pan Z, et al. Leptin and adiponectin levels in major depressive disorder: a systematic review and meta-analysis. J Affect Disord. (2018) 238:101–10. doi: 10.1016/j.jad.2018.05.008
10. Nasca C, Bigio B, Lee F, Young S, Kautz M, Albright A, et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc Natl Acad Sci U S A. (2018) 115:8627–32. doi: 10.1073/pnas.1801609115
11. Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol. (1995) 5:71–75. doi: 10.1016/0924-977X(95)00033-L
12. Do K, Lauer C, Schreiber W, Zollinger M, Gutteck-Amsler U, Cuénod M, et al. gamma-Glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. J Neurochem. (1995) 65:2652–62. doi: 10.1046/j.1471-4159.1995.65062652.x
13. Fonteh AN, Harrington RJ, Tsai A, Liao P, Harrington MG. Free amino acid and dipeptide changes in the body fluids from Alzheimer's disease subjects. Amino Acids. (2007) 32:213–24. doi: 10.1007/s00726-006-0409-8
14. Cheng S, Rhee E, Larson M, Lewis G, McCabe E, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. (2012) 125:2222–31. doi: 10.1161/CIRCULATIONAHA.111.067827
15. Leichtle A, Nuoffer J, Ceglarek U, Kase J, Conrad T, Witzigmann H, et al. Serum amino acid profiles and their alterations in colorectal cancer. Metabolomics. (2012) 8:643–53. doi: 10.1007/s11306-011-0357-5
16. Xu H, Fang L, Hu Z, Chen Y, Chen J, Li F, et al. Potential clinical utility of plasma amino acid profiling in the detection of major depressive disorder. Psychiatry Res. (2012) 200:1054–7. doi: 10.1016/j.psychres.2012.05.027
17. Maurya P, Noto C, Rizzo L, Rios A, Nunes S, Barbosa D, et al. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Progress Neuro-Psychopharmacol Biol Psychiatr. (2016) 65:134–44. doi: 10.1016/j.pnpbp.2015.08.016
18. Ali-Sisto T, Tolmunen T, Viinamaki H, Mantyselka P, Valkonen-Korhonen M, Koivumaa-Honkanen H, et al. Global arginine bioavailability ratio is decreased in patients with major depressive disorder. J Affect Disord. (2018) 229:145–51. doi: 10.1016/j.jad.2017.12.030
19. Maes M, Landucci Bonifacio K, Morelli N, Vargas H, Barbosa D, Carvalho A, et al. Major differences in neurooxidative and neuronitrosative stress pathways between major depressive disorder and types I and II bipolar disorder. Mol Neurobiol. (2019) 56:141–56. doi: 10.1007/s12035-018-1051-7
20. Mariotti F, Petzke K, Bonnet D, Szezepanski I, Bos C, Huneau J, et al. Kinetics of the utilization of dietary arginine for nitric oxide and urea synthesis: insight into the arginine-nitric oxide metabolic system in humans. Am J Clin Nutr. (2013) 97:972–9. doi: 10.3945/ajcn.112.048025
21. Clark TC, Tinsley J, Sigholt T, Macqueen DJ, Martin SA. Arginine, ornithine and citrulline supplementation in rainbow trout: Free amino acid dynamics and gene expression responses to bacterial infection. Fish Shellfish Immunol. (2020) 98:374–90. doi: 10.1016/j.fsi.2020.01.026
22. Baranyi A, Amouzadeh-Ghadikolai O, Rothenhäusler H, Theokas S, Robier C, Baranyi M, et al. Nitric oxide-related biological pathways in patients with major depression. PLoS ONE. (2015) 10:e0143397. doi: 10.1371/journal.pone.0143397
23. Narvik J, Vanaveski T, Innos J, Philips M-A, Ottas A, Haring L, et al. Metabolic profile associated with distinct behavioral coping strategies of 129Sv and Bl6 mice in repeated motility test. Sci Rep. (2018) 8:1–11. doi: 10.1038/s41598-018-21752-9
24. Guelzim N, Mariotti F, Martin P, Lasserre F, Pineau T, Hermier DJA, et al. A role for PPARα in the regulation of arginine metabolism and nitric oxide synthesis. Amino Acids. (2011) 41:969–79. doi: 10.1007/s00726-010-0797-7
25. Zhang R, Zhang T, Ali A, Al Washih M, Pickard B, Watson DJC, et al. Metabolomic profiling of post-mortem brain reveals changes in amino acid and glucose metabolism in mental illness compared with controls. Comput Struct Biotechnol J. (2016) 14:106–16. doi: 10.1016/j.csbj.2016.02.003
26. Papageorgiou C, Grapsa E, Christodoulou NG, Zerefos N, Stamatelopoulos S, Christodoulou gnjp, et al. Association of serum nitric oxide levels with depressive symptoms: a study with end-stage renal failure patients. Psychother Psychosom. (2001) 70:216–20. doi: 10.1159/000056256
27. Suzuki E, Yagi G, Nakaki T, Kanba S, Asai MJJAD. Elevated plasma nitrate levels in depressive states. J Affect Disord. (2001) 63:221–4. doi: 10.1016/S0165-0327(00)00164-6
28. Kim YK, Paik JW, Lee SW, Yoon D, Han C, Lee BHJP, et al. Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Progress Neuro-Psychopharmacol Biol Psychiatr. (2006) 30:1091–6. doi: 10.1016/j.pnpbp.2006.04.008
29. Joca SRL, Guimarães FS. Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology. (2006) 185:298–305. doi: 10.1007/s00213-006-0326-2
30. Harvey BH and Experimental. Affective disorders and nitric oxide: a role in pathways to relapse and refractoriness? Human Psychopharmacol Clin Exper. (2015) 11:309–19. doi: 10.1002/(SICI)1099-1077(199607)11:4<309::AID-HUP775>3.0.CO;2-B
31. Wegener G, Volke V, Harvey BH. S.08.04 The nitric oxide pathway in anxiety and stress-related disorders. Eur Neuropsychopharmacol. (2007) 23:S2–3. doi: 10.1016/j.eurpsy.2008.01.010
32. Jesse CR, Bortolatto CF, Savegnago L, Rocha JB, Nogueira CW. Involvement of L-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effect of tramadol in the rat forced swimming test. Progress Neuro-Psychopharmacol Biol Psychiatr. (2008) 32:1838–43. doi: 10.1016/j.pnpbp.2008.08.010
33. Pinto VL, de Souza PF, Brunini TM, Oliveira MB, Moss MB, Siqueira MA, et al. Low plasma levels of L-arginine, impaired intraplatelet nitric oxide and platelet hyperaggregability: implications for cardiovascular disease in depressive patients. J Affect Disord. (2012) 140:187–92. doi: 10.1016/j.jad.2012.02.008
34. Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nature reviews. Drug Disc. (2015) 14:623–41. doi: 10.1038/nrd4623
35. Fromm HJ, Hargrove M. Essentials of Biochemistry. Springer Science & Business Media. (2012). doi: 10.1007/978-3-642-19624-9
36. Bersani FS, Wolkowitz OM, Lindqvist D, Yehuda R, Flory J, Bierer LM, et al. Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun. (2016) 52:153–60. doi: 10.1016/j.bbi.2015.10.015
37. Elgün S, Kumbasar H. Increased serum arginase activity in depressed patients. Progress Neuropsychopharmacol Biol Psychiatr. (2000) 24:227–32. doi: 10.1016/S0278-5846(99)00100-1
38. Mayoral-Mariles A, Cruz-Revilla C, Vega-Manriquez X, Aguirre-Hernandez R, Severiano-Perez P, Aburto-Arciniega E, et al. Plasma amino acid levels discriminate between control subjects and mildly depressed elderly women. Arch Med Res. (2012) 43:375–82. doi: 10.1016/j.arcmed.2012.07.006
39. Liu P, Jing Y, Zhang HJN. Age-related changes in arginine and its metabolites in memory-associated brain structures. Neuroscience. (2009) 164:611–28. doi: 10.1016/j.neuroscience.2009.08.029
40. Rotroff D, Corum D, Motsinger-Reif A, Fiehn O, Bottrel N, Drevets W, et al. Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: new mechanistic insights for rapid acting antidepressants. Transl Psychiatry. (2016) 6:e894. doi: 10.1038/tp.2016.145
41. Moaddel R, Shardell M, Khadeer M, Lovett J, Kadriu B, Ravichandran S, et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology (Berl). (2018) 235:3017–30. doi: 10.1007/s00213-018-4992-7
42. Kurata K, Shigemi K, Tomonaga S, Aoki M, Furuse MJN. L-Ornithine attenuates corticotropin-releasing factor-induced stress responses acting at GABA A receptors in neonatal chicks. Neuroscience. (2010) 172:226–31. doi: 10.1016/j.neuroscience.2010.10.076
43. Hoekstra R, Fekkes D, Pepplinkhuizen L, Loonen AJ, Tuinier S, Verhoeven WM. Nitric oxide and neopterin in bipolar affective disorder. Neuropsychobiology. (2006) 54:75–81. doi: 10.1159/000096042
44. Cooke RBSB-BASPTJCOTTBJ. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. (1998) 98:1842–7. doi: 10.1161/01.CIR.98.18.1842
45. Erez A, Nagamani SCS, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat Med. (2011) 17:1619–26. doi: 10.1038/nm.2544
46. Selley ML. Increased (E)-4-hydroxy-2-nonenal and asymmetric dimethylarginine concentrations and decreased nitric oxide concentrations in the plasma of patients with major depression. J Affect Disord. (2004) 80:249–56. doi: 10.1016/S0165-0327(03)00135-6
47. Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
48. Cao B, Deng R, Wang D, Li L, Ren Z, Xu L, et al. Association between arginine catabolism and major depressive disorder: a protocol for the systematic review and meta-analysis of metabolic pathway. Medicine. (2020) 99:e21068. doi: 10.1097/MD.0000000000021068
49. Shariati A, Dadashi M, Moghadam MT, van Belkum A, Yaslianifard S, Darban-Sarokhalil D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: a systematic review and meta-analysis. Sci Rep. (2020) 10:12689. doi: 10.1038/s41598-020-69058-z
50. Yi ZH, Luther Y, Xiong GH, Ni YL, Yun F, Chen J, et al. Association between diabetes mellitus and lung cancer: Meta-analysis. Eur J Clin Invest. (2020) 50:e13332. doi: 10.1111/eci.13332
51. Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
52. Abou-Saleh MT, Karim L, Krymsky M. The biology of depression in Arab culture. Nord J Psychiatry. (1998) 52:177–82. doi: 10.1080/08039489850139067
53. Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpe S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr Scand. (1998) 97:302–8. doi: 10.1111/j.1600-0447.1998.tb10004.x
54. Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. (1998) 37:124–9. doi: 10.1159/000026491
55. Mauri MC, Boscati L, Volonteri LS, Scalvini ME, Steinhilber CP, Laini V, et al. Predictive value of amino acids in the treatment of major depression with fluvoxamine. Neuropsychobiology. (2001) 44:134–8. doi: 10.1159/000054933
56. Mitani H, Shirayama Y, Yamada T, Maeda K, Ashby CRJr, Kawahara R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog Neuropsychopharmacol Biol Psychiatry. (2006) 30:1155–8. doi: 10.1016/j.pnpbp.2006.03.036
57. Woo HI, Chun MR, Yang JS, Lim SW, Kim MJ, Kim SW, et al. Plasma amino acid profiling in major depressive disorder treated with selective serotonin reuptake inhibitors. CNS Neurosci Ther. (2015) 21:417–24. doi: 10.1111/cns.12372
58. Hess S, Baker G, Gyenes G, Tsuyuki R, Newman S, Le Melledo JM. Decreased serum L-arginine and L-citrulline levels in major depression. Psychopharmacology. (2017) 234:3241–7. doi: 10.1007/s00213-017-4712-8
59. Ogawa S, Koga N, Hattori K, Matsuo J, Ota M, Hori H, et al. Plasma amino acid profile in major depressive disorder: Analyses in two independent case-control sample sets. J Psychiatr Res. (2018) 96:23–32. doi: 10.1016/j.jpsychires.2017.09.014
60. Ozden A, Angelos H, Feyza A, Elizabeth W, John P. Altered plasma levels of arginine metabolites in depression. J Psychiatr Res. (2020) 120:21–8. doi: 10.1016/j.jpsychires.2019.10.004
61. Loscalzo J. An experiment of nature: genetic L-arginine deficiency and NO insufficiency. J. Clin Invest. (2001) 108:663–4. doi: 10.1172/JCI13848
62. Sotelo-Orozco J, Chen SY, Hertz-Picciotto I, Slupsky CM. A comparison of serum and plasma blood collection tubes for the integration of epidemiological and metabolomics data. Front Mol Biosci. (2021) 8:682134. doi: 10.3389/fmolb.2021.682134
63. Dhir A, Kulkarni SK. Involvement of nitric oxide (NO) signaling pathway in the antidepressant action of bupropion, a dopamine reuptake inhibitor. Eur J Pharmacol. (2007) 568:177–85. doi: 10.1016/j.ejphar.2007.04.028
Keywords: arginine, depression, nitric oxide, metabolism, catabolism, bipolar disorder, cognition
Citation: Fan M, Gao X, Li L, Ren Z, Lui LMW, McIntyre RS, Teopiz KM, Deng P and Cao B (2021) The Association Between Concentrations of Arginine, Ornithine, Citrulline and Major Depressive Disorder: A Meta-Analysis. Front. Psychiatry 12:686973. doi: 10.3389/fpsyt.2021.686973
Received: 28 March 2021; Accepted: 19 October 2021;
Published: 18 November 2021.
Edited by:
Mario F. Juruena, King's College London, United KingdomReviewed by:
Jiajia Zhu, First Affiliated Hospital of Anhui Medical University, ChinaYan Li, Apellis Pharmaceuticals, United States
Copyright © 2021 Fan, Gao, Li, Ren, Lui, McIntyre, Teopiz, Deng and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Cao, YmluZ2Nhb0Bzd3UuZWR1LmNu; Peng Deng, ZHAwODg5NjVAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Mingyue Fan1†
Mingyue Fan1† Xiao Gao
Xiao Gao Li Li
Li Li Zhongyu Ren
Zhongyu Ren Leanna M. W. Lui
Leanna M. W. Lui Roger S. McIntyre
Roger S. McIntyre Bing Cao
Bing Cao