
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 05 August 2021
Sec. Public Mental Health
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.686514
This article is part of the Research Topic Individual Participant Data Meta-analysis: Approaches, Challenges and Considerations View all 6 articles
Women with primary dysmenorrhea are vulnerable to develop a depressive disorder, which is a common form of psycho-disturbance. However, clinical findings are inconsistent across studies, and the evidence has not been previously synthesized. This study aims to investigate whether primary dysmenorrhea is associated with a higher risk of depression via a cumulative analysis. Four electronic databases were systematically searched for the eligible studies. The combined effect was assessed by analyzing the relative risk (RR) and standard mean differences (SMD) with a 95% confidence interval (CI). This cumulative analysis was registered on the PROSPERO (ID: CRD42020169601). Of 972 publications, a total of 10 studies involving 4,691 participants were included. Pooled results from six included studies showed that primary dysmenorrhea was associated with a significant depressive disorder (RR = 1.72, 95%CI: 1.44 to 2.0, P < 0.001; heterogeneity: I2 = 0%, P = 0.544). In addition, synthesis results from two studies provided the BDI scores suggested that dysmenorrhea had significantly higher scores when compared to non-dysmenorrhea (SMD = 0.47, 95% CI: 0.31–0.62, P < 0.001; heterogeneity: I2 = 0%, P = 0.518). However, in the two studies providing the PROMIS T-Score, the pooled result showed that there was no significant difference between women with dysmenorrhea and those without dysmenorrhea (P = 0.466). The overall quality of the evidence in our study was judged to MODERATE. The present study has confirmed the positive relationship between primary dysmenorrhea and depression. Social supports and medical help from pain management physicians or psychologists are important interventions for women with dysmenorrhea-suffering depressive disorder.
Primary dysmenorrhea, a common gynecologic problem among women of reproductive age, is characterized by painful uterine cramps that occur before or during menstruation (1, 2). Women with dysmenorrhea are usually felt pain in the lower part of the abdomen without any pelvic diseases (i.e., endometriosis, pelvic inflammatory disease, uterine leiomyoma, or intrauterine contraceptive device) (3). Dysmenorrhea has even been considered a genuine chronic pain condition, occurring on a regular basis for most women. The primary cause of dysmenorrhea is thought to be correlated with prostaglandins, the hormones that induce muscle contractions and reduce blood flow and oxygen to the uterus (4). The prevalence of dysmenorrhea in young women varies from different countries or regions, ranging from 16 to 95% depending on the method of assessment (1, 5), which is severe in 2–29.8% of cases (6). Though dysmenorrhea is not life-threatening, it is one of the important factors that lower the quality of life, reduce social activities, and absent from school or work among young women. And surprisingly, dysmenorrhea has received slight scientific and clinical attention. Based on relevant studies investigating the different impacts on life in women with or without dysmenorrhea, women with dysmenorrhea are vulnerable to have higher levels of depression, anxiety, somatization, negative self-perception, and hostility (7). Besides, these women are also prone to reduce productivity, creativity, and job performance (8). Among these complications, depressive disorder is one of the most commonly reported issues in women with dysmenorrhea.
Mounting evidence has emerged suggesting that mood dysfunction was comorbid with dysmenorrhea condition (9). According to the previous studies, there is a positive association between primary dysmenorrhea and depressive disorder, and these two factors often cause a vicious circle of symptoms. It was suggested that depression might serve as a risk factor for dysmenorrhea (10). Tavallaee et al. (11) reported that women with higher depression tended to have more severe dysmenorrhea. Oppositely, some researchers showed that young women with dysmenorrhea have a higher risk of developing depression (12). A study reported by Liu et al. (13) indicated that subjects with primary dysmenorrhea were susceptible to depression when compared to those without dysmenorrhea (Self-rating distress scale: 34.36 ± 1.95 vs. 28.8 ± 1.58). In addition, Ambresin et al. (7) demonstrated that adolescent girls with severe dysmenorrhea had a 1.87-fold increased risk of depression as compared with those with no/mild/moderate dysmenorrhea. However, some studies failed to support such an association. In a large sample case-control study, László et al. (14) found that the prevalence of depressive symptoms in women with dysmenorrhea was comparable to the subjects without dysmenorrhea (6.46 vs. 5.0%, P = 0.08).
Though a positive association between dysmenorrhea and depression is speculated, the evidence is still controversial, and a directly calculated prevalence of depression is presently lacking. Therefore, the current systematic review and meta-analysis aim to summarize all the evidence focused on this topic and show a quantified result to better determine the level of risk in women with dysmenorrhea when compared to those healthy women without dysmenorrhea. If the synthesis results have confirmed this relationship, it is instructive and meaningful to help the clinicians being conscious of the hazardous effect of dysmenorrhea for developing depression and take some interventions to alleviate dysmenorrhea for the sufferers.
The protocol and report of this systematic review and meta-analysis were followed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The PRISMA checklist was illustrated in Supplementary Figure 1. Furthermore, we have registered this meta-analysis on the PROSPERO. For more detailed information, please visit the website of PROSPERO to access it (ID: CRD42020169601, http://www.crd.york.ac.uk/PROSPERO).
Four electronic databases, such as MEDLINE (PubMed), EMBASE (OVID), the Cochrane Library, and the PsychINFO were systemically retrieved by two authors up to January 07, 2021. The searching strategy applied for identifying the qualified studies in PubMed databases by using the MeSH and the terms was: ((((((((([“Depression”(Mesh)] OR Depressions) OR Depressive Symptoms) OR Depressive Symptom) OR Symptom, Depressive) OR Symptoms, Depressive) OR Emotional Depression) OR Depression, Emotional) OR Depressions, Emotional) OR Emotional Depressions) AND ((((((((([“Dysmenorrhea”(Mesh)] OR Dysmenorrheas) OR Pain, Menstrual) OR Menstrual Pain) OR Menstrual Pains) OR Pains, Menstrual) OR Menstruation, Painful) OR Menstruations, Painful) OR Painful Menstruation) OR Painful Menstruations).
Exactly, dysmenorrhea is a problem, rather than a disease, that periodically makes people uncomfortable due to menstrual cramps during the menstrual cycles. Assessments for dysmenorrhea or pain severity include but are not limited to specific questions, the Visual Analogue Scale (VAS), McGill Pain Questionnaire (MPQ), verbal multidimensional scoring system, Numeric Pain Rating Scale, the Wong-Baker FACES Pain Rating Scale, and scale of noncyclic pelvic pain. The definition of depression is qualified following the international classification of diseases (ICD) codes. Depression is an affective disorder manifested by either a dysphoric mood or loss of interest or pleasure in usual activities. Assessments for depression are according to Beck Depression Inventory (BDI), The NIH Patient Reported Outcomes Measurement Information System (PROMIS), the Edinburgh Depression Scale, the Depression Anxiety and Stress Scale (DASS-21), etc.
To be included in this meta-analysis, epidemiologic studies had to report the association between dysmenorrhea and depression. Only studies reporting with the English language and human participants were included. Those potential studies were reviewed and further assessed whether they met the predefined inclusion criteria. In line with the Patient, Intervention, Comparison, Outcome, and Study design (PICOS) standard, the question that guided the current meta-analysis was: Does dysmenorrhea increase the risk of depression/depressive disorder? The PICOS evidence for this meta-analysis has consisted of the following combinations: patients complained of dysmenorrhea or painful menstruation or menstrual Pain (P); a history of dysmenorrhea (I); compared with the healthy general population who did not suffer dysmenorrhea (C); diagnosed with depression or depressive disorder (O); no limitation on study designs (S). Besides, studies provided the odds ratios (OR), relative risk (RR), and standard mean differences (SMD) with 95% confidence intervals (CI) could be also included. In this study, we excluded the irrelevant studies according to the pre-specified exclusion criteria, including lack of information of the general populations who did not complain of dysmenorrhea; duplicated data or previous publications of the same clinical trials; review studies; case reports; letters; comments; meeting abstracts; and animal experiments. Based on the PICOS evidence and the inclusion criteria, the process of study selection was conducted by two authors independently. Any ambiguities or discrepancies were resolved by the corresponding author.
Two authors independently evaluated and extracted the necessary data from each included study by using a data collection form. Corresponding information includes the name of the first author; publication year; country/region of study origin; study design; mean age, total number, and cases of dysmenorrhea from the two groups; age at menarche; menstrual bleeding duration; RR with 95% CI) or SMD with 95% CI; assessment of dysmenorrhea or pain severity; and assessment of depression.
Study quality assessments were also conducted by two investigators independently. Interrater variability among manual scorers was evaluated from the agreement between the two scorers. Quality assessments of the eligible cross-sectional studies were performed using the cross-sectional study quality methodology checklist. This checklist consists of 11 items in which each criterion is assigned a score, and studies with scores of 0–3, 4–7, and 8–11 are rated too low quality, moderate quality, and high quality, respectively. The methodological quality of the case-control studies is evaluated by the Newcastle–Ottawa Scale. This scale contains nine domains and the conformity is assigned with one score. Eligible studies with scores of 0–3, 4–6, and 7–9 were rated as low quality, moderate quality, and high quality, respectively. Furthermore, we employed the grading of recommendations assessment, development, and evaluation (GRADE) approach to exert the absolute estimates of the risk of depression in patients with dysmenorrhea and meanwhile rank the overall quality of evidence.
Quantifying the association between dysmenorrhea and depression in women is the main objective of the current study. The dichotomous variables were calculated and presented with the RR and its 95% CI, while the continuous variables were calculated according to the SMD and its 95% CI. P < 0.05 were considered statistically significant. The heterogeneity test was performed using the I2 statistic and the Cochrane Q statistic (significance level at P < 0.10 with Q test; I2 > 50% was rated to substantial heterogeneity). In this meta-analysis, a random-effects model rather than a fixed-effects model was conducted due to a high likelihood of between-study variance for differences in the study design and the sample sizes. Sensitivity analyses were carried out to better detect the potential source of the heterogeneity. Publication bias analyses were conducted using the funnel plot as well as Begg's rank correlation test and Egger's regression asymmetry test. All statistical analyses were conducted via the STATA software (version 13.0, Stata Corp LP, Texas, USA).
The search flowchart for identifying the eligible studies was shown in Figure 1. The initial database search yielded 972 records, of which 376 from PubMed, 248 from Embase, 217 from the Cochrane Library, and 131 from the PsychINFO database. Finally, 10 relevant publications (14–23) contained data that met our predefined inclusion criteria. A total of 4,691 subjects and 2,130 dysmenorrhea cases were involved. Of the 10 included studies evaluated, 6 studies (14–19) provided the dichotomous variables (cases of depression) that could be calculated for the pooled RR via a meta-analysis, while 4 studies (20–23) provided the continuous variables (scores for depression), which were calculated for the pooled SMD. Among the 10 studies included in this meta-analysis, 5 studies (15, 17, 21–23) were cross-sectional designed and the remainder (14, 16, 18–20) were case-control designed. The trial publication years of the 10 selected studies ranged from 2006 to 2019. The mean age in women with dysmenorrhea and women without dysmenorrhea was 18–50 years and 18–43 years, respectively. One trial was conducted in Hungary (14), one in Gambia (15), one in Georgia (16), one in China (19), two in Iran (17, 21), two in Turkey (18–20), and two (22, 23) in the USA. The age at menarche of the participants ranged from 12.4 ± 1.1 years to 13.5 ± 1.2 years. Menstrual bleeding duration ranged from 4.5 ± 1.2 days to 6.4 ± 1.1 days. The characteristics of the 10 eligible studies are summarized in Table 1.
Across the 10 included studies, they were rated to a medium to high methodological quality. The proportion of high-quality studies was 20% (2/10). Among the five cross-sectional studies, four studies (15, 17, 22, 23) were assessed as moderate quality, and the remaining one study (21) was high quality (Supplementary Table 1). In the five case-control studies, four studies (14, 16, 18, 20) were judged to moderate quality, and only one study (19) was evaluated as high quality (Supplementary Table 2).
Table 2 showed the calculated results of the quality of evidence using the GRADE-pro. In the six included studies (14–19) reporting the cases of depression in both the study group and the control group, the rates of events of depression on average in women with dysmenorrhea were 332/1317 (25.2%), while the control subjects without dysmenorrhea were 226/1833 (12.3%); the absolute effect of dysmenorrhea on depression was 89 more per 1,000 (from 54 to 123 more); the overall quality of the evidence was MODERATE.
Based on the six included studies (14–19) providing the depression cases in women with dysmenorrhea and without dysmenorrhea, the pooled RR indicated that dysmenorrhea had a significantly higher risk of depression compared to non-dysmenorrhea (RR = 1.72, 95%CI: 1.44–2.0, P < 0.001; heterogeneity: I2 = 0%, P = 0.544; Figure 2). In line with this finding, synthesis results from two studies (20, 21) provided the BDI scores also suggested that women complained with dysmenorrhea had significantly higher values in BDI scores as compared with the control group without dysmenorrhea (SMD = 0.47, 95%CI: 0.31–0.62, P < 0.001; heterogeneity: I2 = 0%, P = 0.518; Figure 3A). However, when focused on the two eligible studies (22, 23) providing the PROMIS T-Score, the combined effect demonstrated that there was no significant difference between women with dysmenorrhea and those without dysmenorrhea (SMD = −0.84, 95%CI: −3.09–1.42, P = 0.466) and substantial heterogeneity had been identified (I2 = 98.9%, P < 0.001; Figure 3B).
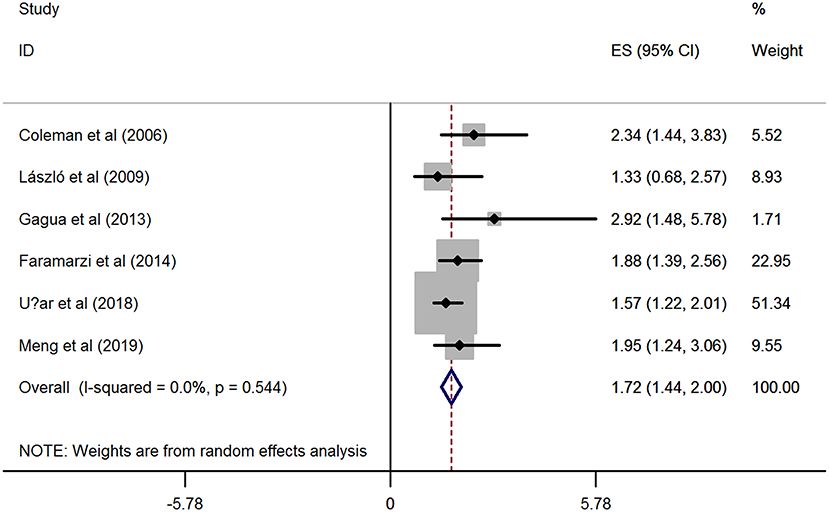
Figure 2. Forest plots of meta-analysis based on six eligible studies providing the cases of depression.
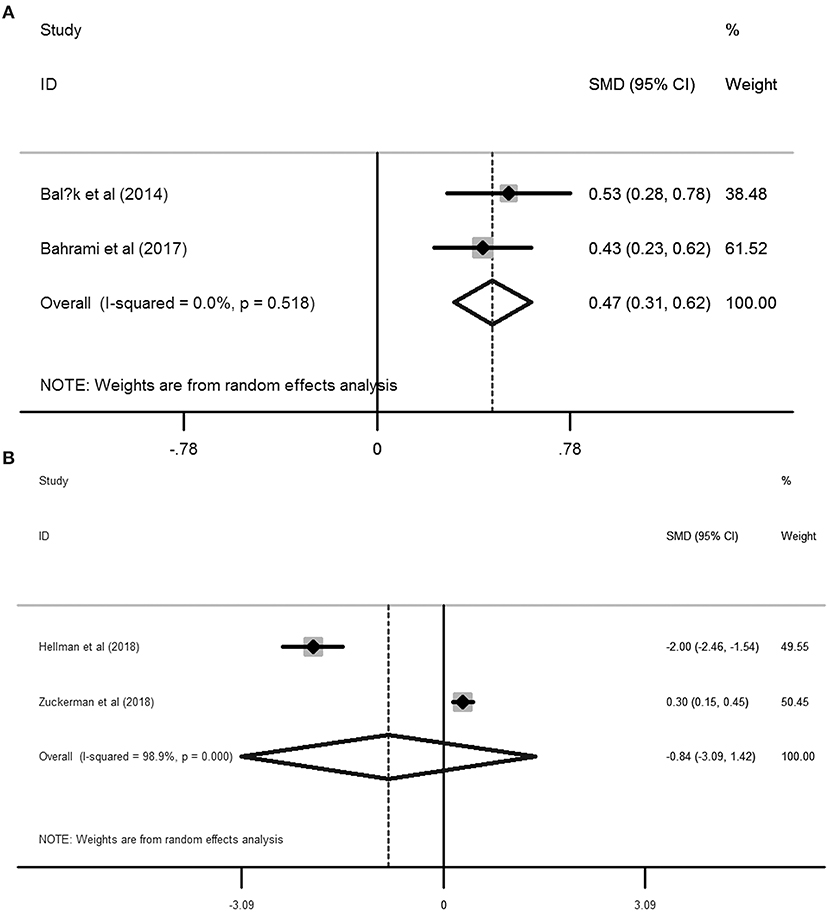
Figure 3. Forest plots of meta-analysis based on the two studies reporting the BDI scores (A) and the two studies reporting the PROMIS T-Score (B).
The above meta-analyses revealed that women with dysmenorrhea were more likely to encounter depressive disorder than those without dysmenorrhea. Nevertheless, pooled SMD from the two included studies (22, 23) reporting the PROMIS T-Score failed to present a positive association between dysmenorrhea and depression. Between the 2 studies, Zuckerman et al. (23) confirmed that dysmenorrhea was associated with a higher risk of depression than those without dysmenorrhea (PROMIS T-Score: 53.6 ± 9.2 vs. 50.9 ± 8.8), while in another study developed by Hellman et al. (22) indicated that dysmenorrhea was associated with a lower risk of depression (PROMIS T-Score: 53 ±1 vs. 55 ± 1). Therefore, substantial heterogeneity has been arisen due to these contradictory results.
To assess the influence of a single study on the overall RR, we have further conducted the sensitivity analyses based on the six included studies (14–19) providing the depression cases. As shown in Supplementary Figure 2 and Supplementary Table 3, sensitivity analyses revealed that there was no substantial change in the new overall pooled RR after omitting any of the six studies. The newly generated RR ranged from 1.67 (95% CI: 1.35 to 1.99, P < 0.001) to 1.88 (95% CI: 1.48 to 2.29, P <0.001). On the other hand, the heterogeneity also yielded similar results, all of the I2 were 0.0%. These results demonstrated that no single study dominated the overall synthetic RR on the association between dysmenorrhea and depression, in which the pooled effect estimate in this meta-analysis was robust.
The funnel plots, Begg's rank correlation test, and Egger's linear regression revealed that there was no significant publication bias among the included studies (Begg's, P > |z| = 0.133; Egger, P > |t| = 0.298, 95%CI: −1.76–4.44) (Figure 4).
Primary dysmenorrhea or menstrual pain, as characterized by painful menstrual cramps without any discernable macroscopic pelvic pathology, is the most prevalent gynecological disorder in those of child-bearing age. Despite having a high prevalence and worsening the quality of life, dysmenorrhea is disregarded in the pain community. In essence, dysmenorrhea could be classified as a genuine chronic pain condition and accompanied by headache, vomiting, nausea, and fatigue. Moreover, dysmenorrhea can affect the social life as well as the mental health of an individual. It is known that there is a close association between pain and depression (24). Since the first publication (25) reporting the relationship between dysmenorrhea and depressive symptoms, by Bloom et al. in 1978, there is mounting evidence indicates that dysmenorrhea patients are at high risk of depression (7, 10–14). However, we should also note that several studies (13, 14) failed to find a positive association between dysmenorrhea and depressive disorder.
The present meta-analysis revealed that women with primary dysmenorrhea conferred a 1.72-fold increased risk of depressive symptoms when compared to the healthy general population without dysmenorrhea (pooled RR = 1.72, 95%CI: 1.44–2.0, P < 0.001). Besides, we also found that women with dysmenorrhea had significantly higher values in BDI scores than non-dysmenorrhea individuals (SMD = 0.47, 95%CI: 0.31–0.62, P < 0.001). The average rates of depression events were 332/1,317 (25.2%) in women with dysmenorrhea and 226/1,833 (12.3%) in those healthy individuals without dysmenorrhea. The quality of the evidence of the present meta-analysis was regarded to MODERATE. In addition, some other features of the included studies also indicated our study was robust, including high methodological quality, no substantial heterogeneities, stable sensitivity analysis, and no significant publication bias of the eligible studies.
Though the present meta-analysis suggested that primary dysmenorrhea elevated the risk of depressive symptoms, the clear-cut etiology for such association has not yet been fully established (14, 15). Progressed researches indicate multifactorial mechanisms may contribute to the high prevalence of depressive disorder in women with primary dysmenorrhea, including recurrent and chronic menstrual pain (19), hormone-responsive disorders (20), increased pro-inflammatory cytokines (14, 16), and poor school or social performances (20, 21). These factors independently or collectively contribute to someone with primary dysmenorrhea are more liable to suffer from depression.
The association of depressive symptoms with dysmenorrhea could be explained as the adverse effect of chronic pain on women's mental health. Depressive disorders are known to exacerbate or co-occur with various forms of chronic pain (26). The prevalence of depression is high in subjects with chronic pain syndromes (24). Primary dysmenorrhea occurs on a regular menstrual cycle for most who menstruate, and this is considered to be a genuine chronic pain condition. Menstrual pain could be conceptualized as a stressor and exacerbate depressive symptoms (27). Depression commonly occurs in response to dysmenorrhea and can be anticipated in the next menstrual period (28).
The pathogenesis of primary dysmenorrhea is the excess secretion of uterine prostaglandins which are controlled by both progesterone and estrogen (29). Hormonal fluctuations during the menstrual cycle affect the regulation of emotions by their effects on the brain (30). Estrogen and progesterone have been implicated in depressive symptomatology in many women (31). It was suggested that variations in ovarian progesterone and estrogen level are associated with a high risk of depression (32). Both the high concentrations of estrogen and prostaglandin are probable mechanisms of dysmenorrhea (11). Estrogen leads to mood disorders via modulating the expression of genes that code for tryptophan hydroxylase and the serotonin transporter (33). Lokuge et al. (34) demonstrated that the variations of estrogen-related depression in women might be correlated to the regulation of the serotonin pathway, causing an alteration in serotonin neurotransmission. Progesterone, another key gonadal hormone for controlling the production of prostaglandin, causes dysfunctional mood regulation by regulating neurotransmitter synthesis, release, and transport (35). In addition to prostaglandins, estrogen, and progesterone, other chemicals such as vasopressin and phospholipids released during dysmenorrhea might also be associated with depressive disorder (36). Furthermore, aberrant circulating cortisol levels were associated with pain sensitivity in women with dysmenorrhea, which made them more liable to depression (37). For other physiological mechanisms, excessive and prolonged activation of the hypothalamic-pituitary-adrenal axis resulting in impaired follicular development might be one of the underlying pathogenesis between stress and dysmenorrhea (38). And there were numerous studies have confirmed the relationship between stress and depression (39). Therefore, the high prevalence of depressive symptoms in women with primary dysmenorrhea might be partially caused by high stress.
In addition to the effects of the fluctuating hormones, previous studies have revealed that depression was associated with the increased level of several pro-inflammatory cytokines (i.e., tumor necrosis factor α and interleukins) which was considered leading a deficit in serotonin and melatonin via the kynurenine pathway (40–42). Intriguingly, a case-control study (43) demonstrated that many pro-inflammatory cytokines were up-regulated in peripheral blood mononuclear cells in primary dysmenorrhoeic young women during the menstrual period.
Primary dysmenorrhea dramatically affects one's social life or school performance, which might also play a critical role in the association between primary dysmenorrhea and depression. Dysmenorrhea is one of the main causes of school and work absences among young women, with 14% to 52% reporting absenteeism (44). Those who complained about recurrent dysmenorrhea tended to have increased levels of serious stress in daily living and decreased productivity, creativity, and job performance, which were considered as the driving factors for depression (8, 45). The depressive disorder occurs in both sexes, but during puberty, girls are at three times higher risk than boys due to the great personal changes since that time (46). Ge et al. (47) also demonstrated that entry into adolescence is a period of elevated vulnerability to depression for girls.
Since a positive association between dysmenorrhea and depressive disorder, interventions to alleviate dysmenorrhea or depression may have a great impact on the quality of life for the sufferers. Several pharmacological interventions have been reported for the management of dysmenorrhea, including non-steroid anti-inflammatory drugs (NSAIDs), combined oral contraceptives, Danazol, and Leuprolide acetate (48, 49), etc. However, potential adverse events caused by these drugs should be noted. NSAIDs may cause gastrointestinal discomfort and hemorrhage. Hormone therapy may cause irregular uterine bleeding. In addition to the drugs used for dysmenorrhea, psychotropic medications for depression therapies may also play roles to wear off depression. However, long-term use of these drugs may cause drug dependency. As aforementioned, dysmenorrhea adversely affects mood and consequently affects the sufferer's attitude and relationships with family and peers, including poor concentration and an inability to participate in social activities. Therefore, more social supports may be a way to decrease the serious stress of the dysmenorrhea sufferers in daily living. Besides, it has been assumed that behavioral interventions, i.e., relaxation training, biofeedback, and mind-body awareness, may positively help to alleviate dysmenorrhea and thus decrease the risk of depression (50). However, these interventions need to be viewed with caution due to the data supporting these treatments are still limited. Therefore, more RCTs with large sample sizes are warranted to be done in this area.
To our knowledge, this is the first study for quantifying the association between primary dysmenorrhea and the risk of depression by conducting a meta-analysis. However, several inherent limitations were also identified in this study. On one hand, all the included studies were observational designed, either cross-sectional or case-control trials, which indicated that the direction of causality between dysmenorrhea and risk of depression was not so clear. On the other hand, though both the pooled RR for the six studies reporting the cases and the pooled SMD from the two included studies reporting BDI scores have confirmed the positive association between primary dysmenorrhea and depression, the synthetic SMD from the two included studies providing the PROMIS T-Score did not support women with dysmenorrhea have a significantly higher score than the healthy controls (SMD = −0.84, 95%CI: −3.09–1.42, P = 0.466, I2 = 98.9%, P < 0.001). This inconsistency might cause by the limited studies (two eligible studies) were included in this analysis. In line with the combined RR and the combined SMD derived from BDI scores, Zuckerman et al. (23) also observed a positive association between dysmenorrhea and higher risk of depression (SMD = 0.30, 95%CI: 0.15–0.45), while Hellman et al. (22) demonstrated that dysmenorrhea was correlated with a lower risk of depression (SMD = −2.00, 95%CI: −2.46 to −1.54; Figure 3B). This contradictory outcome contributes to a non-significant relationship between dysmenorrhea and depression (SMD = −0.84, 95%CI: −3.09–1.42, P = 0.466) as well as a substantial heterogeneity (I2 = 98.9%, P < 0.001; Figure 3B) when pooling these two studies. Unfortunately, the main topic of both Zuckerman et al. and Hellman et al.'s studies was not focused on the association between dysmenorrhea and depression, the authors just mentioned the PROMIS T-Score between the healthy controls and the dysmenorrhea sufferers and no further discussions related to dysmenorrhea and depression were performed in their studies. As a result, additional well-designed prospective cohorts with a large sample are still warranted to validate the evidence of the high risk of depressive disorder in women with primary dysmenorrhea. Last, we should also note that different validation tools were employed for assessing either primary dysmenorrhea or depression in the 10 included studies, which might affect the outcomes among different studies. In addition to different validation measurements, other characteristics of the included studies are also different, such as diverse study design, sample size, age of the participants, countries, age at menarche, menstrual bleeding duration, and comorbidities. Therefore, we should be cautious when interpreting our results in clinical practice because of these confounding factors.
In conclusion, the present systematic review and meta-analysis suggest that women with primary dysmenorrhea have a significant higher prevalence of depression than those without dysmenorrhea. Understanding such a potential relationship is important in increasing awareness of assessment for the depressive symptoms, improving the quality of life, and providing better-quality care and proper intervention for women with dysmenorrhea.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
SZ project development and data collection. WW data collection and conceptualization. RK methodology and investigation. XW and SZ original draft and methodology. All authors contributed to the article and approved the submitted version.
This work was supported by fund from the Health and Family Planning Commission of Hunan Province (No. B20180192), the Science and Technology Project of Hengyang (No. 2017KJ316), the Health and Family Planning Commission of Hunan Province (No. B20180174), and the High-level Hospital Construction Research Project of Maoming People's Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.686514/full#supplementary-material
Supplementary Figure 1. PRISMA checklist.
Supplementary Figure 2. Sensitivity analysis after each study was excluded by turns.
Supplementary Table 1. The methodological quality of the cross-sectional studies.
Supplementary Table 2. Newcastle-Ottawa Scale (NOS) assessment of the quality of the case-control studies.
Supplementary Table 3. Sensitivity analysis after each study was excluded by turns.
1. Tu F, Hellman K. Primary dysmenorrhea: diagnosis and therapy. Obstet Gynecol. (2021) 137:752. doi: 10.1097/AOG.0000000000004341
2. Bajalan Z, Moafi F, MoradiBaglooei M, Alimoradi Z. Mental health and primary dysmenorrhea: a systematic review. J Psychosom Obstet Gynaecol. (2019) 40:185–94. doi: 10.1080/0167482X.2018.1470619
3. Dias S, Pereira L, Oliveira AP, Santos R, Nunes L. Scientific and technological prospection on transdermal formulations and complementary therapies for the treatment of primary dysmenorrhea. Expert Opin Ther Pat. (2019) 29:115–26. doi: 10.1080/13543776.2019.1562547
4. Kitamura M, Takeda T, Koga S, Nagase S, Yaegashi N. Relationship between premenstrual symptoms and dysmenorrhea in Japanese high school students. Arch Womens Ment Health. (2012) 15:131–3. doi: 10.1007/s00737-012-0266-2
6. Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. (2014) 36:104–13. doi: 10.1093/epirev/mxt009
7. Kabukçu C, Kabukçu BB, Başay Ö. Primary dysmenorrhea in adolescents: association with attention deficit hyperactivity disorder and psychological symptoms. Taiwan J Obstet Gynecol. (2021) 60:311–7. doi: 10.1016/j.tjog.2021.01.033
8. Chawla A, Swindle R, Long S, Kennedy S, Sternfeld B. Premenstrual dysphoric disorder: is there an economic burden of illness? Med. Care. (2002) 40:1101–12. doi: 10.1097/00005650-200211000-00011
9. Pakpour AH, Kazemi F, Alimoradi Z, Griffiths MD. Depression, anxiety, stress, and dysmenorrhea: a protocol for a systematic review. Syst Rev. (2020) 9:65. doi: 10.1186/s13643-020-01319-4
10. Alonso C, Coe LC. Disruptions of social relationships accentuate the association between emotional distress and menstrual pain in young women. Health Psychol. (2001) 20:411–6. doi: 10.1037/0278-6133.20.6.411
11. Tavallaee M, Joffres MR, Corber SJ, Bayanzadeh M, Rad MM. The prevalence of menstrual pain and associated risk factors among Iranian women. J Obstet Gynaecol Res. (2011) 37:442–51. doi: 10.1111/j.1447-0756.2010.01362.x
12. Westling AM, Tu FF, Griffith JW, Hellman MK. The association of dysmenorrhea with noncyclic pelvic pain accounting for psychological factors. Am J Obstet Gynecol. (2013) 209:422.e1–422.e10. doi: 10.1016/j.ajog.2013.08.020
13. Liu J, Liu H, Mu J, Xu Q, Chen T, Dun W, et al. Altered white matter microarchitecture in the cingulum bundle in women with primary dysmenorrhea: a tract-based analysis study. Hum Brain Mapp. (2017) 38:4430–443. doi: 10.1002/hbm.23670
14. Laszlo KD, Kopp SM. Effort-reward imbalance and overcommitment at work are associated with painful menstruation: results from the Hungarostudy Epidemiological Panel 2006. J Occup Environ Med. (2009) 51:157–63. doi: 10.1097/JOM.0b013e318197ca89
15. Coleman R, Morison L, Paine K, Powell RA, Walraven G. Women's reproductive health and depression: a community survey in the Gambia, West Africa. Soc Psychiatry Psychiatr Epidemiol. (2006) 41:720–7. doi: 10.1007/s00127-006-0085-8
16. Gagua T, Tkeshelashvili B, Gagua D, McHedlishvili N. Assessment of anxiety and depression in adolescents with primary dysmenorrhea: a case-control study. J Pediatr Adolesc Gynecol. (2013) 26:350–4. doi: 10.1016/j.jpag.2013.06.018
17. Faramarzi M, Salmalian H. Association of psychologic and nonpsychologic factors with primary dysmenorrhea. Iran Red Crescent Med J. (2014) 16:e16307. doi: 10.5812/ircmj.16307
18. Ucar T, Timur TS, Aksoy DY, Nacar G. An analysis of dysmenorrhoea and depressive symptoms in university students: a case-control study. Int J Nurs Pract. (2018) 24:e12678. doi: 10.1111/ijn.12678
19. Meng L, Li J, Cheng Y, Wei T, Du Y, Peng S. Dysmenorrhea increased the risk of postpartum depression in Chinese Han parturients. Sci Rep. (2019) 9:16579. doi: 10.1038/s41598-019-53059-8
20. Balik G, Ustuner I, Kagitci M, Sahin KF. Is there a relationship between mood disorders and dysmenorrhea? J Pediatr Adolesc Gynecol. (2014) 27:371–4. doi: 10.1016/j.jpag.2014.01.108
21. Bahrami A, Sadeghnia H, Avan A, Mirmousavi SJ, Moslem A, Eslami S, et al. Neuropsychological function in relation to dysmenorrhea in adolescents. Eur J Obstet Gynecol Reprod Biol. (2017) 215:224–29. doi: 10.1016/j.ejogrb.2017.06.030
22. Hellman KM, Datta A, Steiner ND, Kane MJ, Garrison EF, Clauw DJ, et al. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol. (2018) 219:84.e1–84.e8. doi: 10.1016/j.ajog.2018.04.030
23. Zuckerman RM, Silton RL, Tu FF, Eng JS, Hellman MK. Somatic symptoms in women with dysmenorrhea and noncyclic pelvic pain. Arch Womens Ment Health. (2018) 21:533–41. doi: 10.1007/s00737-018-0823-4
24. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. (2003) 163:2433–45. doi: 10.1001/archinte.163.20.2433
25. Bloom LJ, Shelton JL, Michaels CA. Dysmenorrhea and personality. J Pers Assess. (1978) 42:272–6. doi: 10.1207/s15327752jpa4203_8
26. Rogers AH, Garey L, Bakhshaie J, Viana AG, Ditre JW, Zvolensky MJ. Anxiety, depression, and opioid misuse among adults with chronic pain: the role of anxiety sensitivity. Clin J Pain. (2020) 36:862–7. doi: 10.1097/AJP.0000000000000870
28. Ramspacher A, Neudert M, Koller A, Schlager S, Kofler B, Brunner MS. Influence of the regulatory peptide galanin on cytokine expression in human monocytes. Ann N Y Acad Sci. (2019) 1455:185–95. doi: 10.1111/nyas.14111
29. Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet. Gynecol. (2006) 108:428–41. doi: 10.1097/01.AOG.0000230214.26638.0c
30. Merz CJ, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, et al. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm Behav. (2012) 62:531–8. doi: 10.1016/j.yhbeh.2012.09.001
31. Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow RD. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. (1998) 338:209–16. doi: 10.1056/NEJM199801223380401
32. Studd J. Personal view: Hormones and depression in women. Climacteric. (2015) 18:3–5. doi: 10.3109/13697137.2014.918595
33. Maki PM, Dumas J. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med. (2009) 27:250–9. doi: 10.1055/s-0029-1216278
34. Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. Depression in women: windows of vulnerability and new insights into the link between estrogen and serotonin. J Clin Psychiatry. (2011) 72:e1563–9. doi: 10.4088/JCP.11com07089
35. Schiller CE, Meltzer-Brody S, Rubinow RD. The role of reproductive hormones in postpartum depression. CNS Spectr. (2015) 20:48–59. doi: 10.1017/S1092852914000480
36. Sahin N, Kasap B, Kirli U, Yeniceri N, Topal Y. Assessment of anxiety-depression levels and perceptions of quality of life in adolescents with dysmenorrhea. Reprod Health. (2018) 15:13. doi: 10.1186/s12978-018-0453-3
37. Vincent K, Warnaby C, Stagg CJ, Moore J, Kennedy S, Tracey I. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. (2011) 152:1966–75. doi: 10.1016/j.pain.2011.03.029
38. Wang L, Wang X, Wang W, Chen C, Ronnennberg AG, Guang W, et al. Stress and dysmenorrhoea: a population based prospective study. Occup Environ Med. (2004) 61:1021–6. doi: 10.1136/oem.2003.012302
39. LeMoult J, Humphreys KL, Tracy A, Hoffmeister JA, Ip E, Gotlib HI. Meta-analysis: exposure to early life stress and risk for depression in childhood and adolescence. J Am Acad Child Adolesc Psychiatry. (2019) 59:842–55. doi: 10.1016/j.jaac.2019.10.011
40. Catena-Dell'Osso M, Rotella F, Dell'Osso A, Fagiolini A, Marazziti D. Inflammation, serotonin and major depression. Curr Drug Targets. (2013) 14:571–7. doi: 10.2174/13894501113149990154
41. Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. (1999) 461:25–46. doi: 10.1007/978-0-585-37970-8_2
42. Galecki P, Talarowska M. Inflammatory theory of depression. Psychiatr Pol. (2018) 52:437–47. doi: 10.12740/PP/76863
43. Ma H, Hong M, Duan J, Liu P, Fan X, Shang E, et al. Altered cytokine gene expression in peripheral blood monocytes across the menstrual cycle in primary dysmenorrhea: a case-control study. PLoS ONE. (2013) 8:e55200. doi: 10.1371/journal.pone.0055200
44. Beal SJ, Dorn LD, Sucharew HJ, Sontag-Padilla L, Pabst S, Hillman J. Characterizing the longitudinal relations between depressive and menstrual symptoms in adolescent girls. Psychosom Med. (2014) 76:547–54. doi: 10.1097/PSY.0000000000000099
45. Dorn LD, Negriff S, Huang B, Pabst S, Hillman J, Braverman P, et al. Menstrual symptoms in adolescent girls: association with smoking, depressive symptoms, and anxiety. J Adolesc Health. (2009) 44:237–43. doi: 10.1016/j.jadohealth.2008.07.018
46. Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. (2003) 60:837–44. doi: 10.1001/archpsyc.60.8.837
47. Ge X, Natsuaki MN, Conger DR. Trajectories of depressive symptoms and stressful life events among male and female adolescents in divorced and nondivorced families. Dev Psychopathol. (2006) 18:253–73. doi: 10.1017/S0954579406060147
48. Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev. (2015) 2015:CD001751. doi: 10.1002/14651858.CD001751.pub3
Keywords: primary dysmenorrhea, depression, systematic review, cumulative analysis, risk
Citation: Zhao S, Wu W, Kang R and Wang X (2021) Significant Increase in Depression in Women With Primary Dysmenorrhea: A Systematic Review and Cumulative Analysis. Front. Psychiatry 12:686514. doi: 10.3389/fpsyt.2021.686514
Received: 27 March 2021; Accepted: 09 July 2021;
Published: 05 August 2021.
Edited by:
Anthony L. Vaccarino, Indoc Research, CanadaReviewed by:
Elizabeth Thomas, Monash Alfred Psychiatry Research Centre, AustraliaCopyright © 2021 Zhao, Wu, Kang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolan Wang, bGFubGFuNzQ4M0AxNjMuY29t orcid.org/0000-0002-5589-7244
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.