- 1Laboratory for Traumatic Stress Studies and Center for Genetics and BioMedical Informatics Research, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
- 2Department of Psychology, University of Chinese Academy of Sciences, Beijing, China
- 3People's Hospital of Deyang City, Deyang, China
The adenylate cyclase activating polypeptide 1 (pituitary) receptor (ADCYAP1R1) gene is associated with the hypothalamic-pituitary-adrenal (HPA) axis, which controls stress responses. The single-nucleotide polymorphism of ADCYAP1R1, rs2267735, has been investigated in many studies to test its association with posttraumatic stress disorder (PTSD), but the results have not been consistent. It is worth systematically exploring the role of rs2267735 in PTSD development. In this study, we analyzed rs2267735 in 1,132 trauma-exposed Chinese individuals (772 females and 360 males). We utilized the PTSD checklist for DSM-5 (PCL-5) to measure the PTSD symptoms. Then, we analyzed the main, G × E (rs2267735 × trauma exposure), and G × G (with other HPA axis gene polymorphisms) effects of rs2267735 on PTSD severity (total symptoms). There were no significant main or G × E effects (P > 0.05). The G × G ADCYAP1R1-FKBP5 interaction (rs2267735 × rs1360780) was associated with PTSD severity (beta = −1.31 and P = 0.049) based on all subjects, and the G × G ADCYAP1R1-CRHR1 interaction (rs2267735 × rs242924) was correlated with PTSD severity in men (beta = −4.72 and P = 0.023). Our study indicated that the ADCYAP1R1 polymorphism rs2267735 may affect PTSD development through diverse gene-gene interactions.
Introduction
ADCYAP1R1, an HPA Axis Gene, Is Associated With Stress Response
The pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptor (PAC1) are widely distributed in hypothalamic and limbic structures (1–6). This peptide and receptor help regulate responses to anxiety-provoking stimuli or stress (3, 7–10). The ADCYAP1R1 gene, which encodes the receptor PAC1, is associated with the neuroendocrine system hypothalamic–pituitary–adrenal (HPA) axis that is involved in controlling mammalian stress responses (11, 12).
There are many single-nucleotide polymorphisms (SNPs) in ADCYAP1R1. One of them, rs2267735, was reported to play a role in anxiety symptoms, fear-related cognitions, and stress-related responses (13–15). For example, rs2267735 could regulate the normal stress response by affecting the bind of estrogen receptors alpha and estrogen response element (16).
PTSD Is a Stress-Related Mental Disorder Related to ADCYAP1R1 and Other HPA Axis Genes
Posttraumatic stress disorder (PTSD) is a trauma- and stressor-related disorder (17) that can occur after exposure to traumatic events (18). Many studies have shown that PTSD is associated with ADCYAP1R1 and ADCYAP1R1–environment interactions [e.g., (19–22)]. In a Chinese population, a significant association between ADCYAP1R1 and the PTSD emotional numbing cluster was found, a finding that extended previous results (23).
Moreover, PTSD is correlated with other genes that are associated with the HPA axis or regulation of its activity. The FKBP5 gene regulates the HPA axis by encoding FK506 binding protein 5 (FKBP5). FKBP5 can bind to glucocorticoid receptors (24). The FKBP5 gene was detected to have a significant correlation with PTSD in a meta-analysis study (25, 26) and could reflect the risk of co-morbidity of PTSD and depression in mild trauma exposure (27). FKBP5-environment interactions predicted the risk of PTSD in adults (28–30). The CRHR1 gene and the CRHR2 gene regulate the HPA axis together by binding their encoding products, corticotropin-releasing hormone (CRH) receptors CRF1 and CRF2, to CRH. The CRHR1 gene has been suggested to be associated with PTSD (31–36). The CRHR2 gene has been shown to significantly affect PTSD (32, 37, 38) as well as through a gene–environment interaction (39).
Associations Between ADCYAP1R1 and PTSD Are Worth Further Exploration, and Genetic Studies Should Consider Main G × E and G × G Effects
Numerous neurogenetic studies of the ADCYAP1R1 gene have been performed. Some of them gave out a significant main effect on PTSD. For example, Ressler and her colleagues have found that ADCYAP1R1 could contribute to the diagnosis of PTSD and the severity of PTSD symptoms in females who suffered a major trauma (21). Later, Lind et al. (20) used a meta-analysis to show that the C allele at rs2267735 of ADCYAP1R1 significantly increased the risk of PTSD in women. A review supported as well that the epigenetic regulation of ADCYAP1R1 might predict PTSD risk (40). However, not all studies performed the same, and they even led to no effects. In order to replicate the finding of Ressler et al. (21), two independent samples were newly selected, and their results failed to repeat such (41). In addition, a recent investigation did not show that ADCYAP1R1 was strongly correlated with PTSD (42). The possible reasons for why these study results have not been consistent may be due to the types of trauma exposure (43, 44) and the sample characteristics (23, 41). However, the underlying factors have been most likely not singular. Therefore, we need more studies to enrich the evidence of the main or gene-environment effects of ADCYAP1R1 on PTSD.
Furthermore, the four genes ADCYAP1R1, FKBP5, CRHR1, and CRHR2 are all associated with PTSD and have a close relationship with each other by regulating the HPA axis together. Since genes functionally interact with each other, a phenomenon that is usually called epistasis (i.e., gene-gene interaction) in genetics (45), the gene-gene interactions between ADCYAP1R1 and other HPA axis genes need to be investigated. However, to our knowledge, a neurogenetic study that investigates the gene–gene interactions between ADCYAP1R1 and other HPA axis genes in PTSD has not been conducted. Thus, the correlation between the interactions of ADCYAP1R1 with other HPA axis genes (including FKBP5, CRHR1, andCRHR2) and PTSD is worth further exploring.
Brief Study Design
To further explore the role of ADCYAP1R1 in PTSD development and the physiological underpinnings of the stress response, this study examined the main, G × E (rs267735 × trauma exposure), and G × G (with other HPA axis gene polymorphisms) effects of rs2267735 on PTSD severity in a cohort of 2008 Chinese Wenchuan earthquake survivors.
Materials and Methods
Participants
In the present study, we selected 1,132 survivors of the Wenchuan earthquake in Hanwang Town, Sichuan Province, China. These participants have been described previously (38, 46), and we provide the detailed information for the cohort here again for convenience.
The participants were adults (older than 18 years old) without any mental illness or intellectual disability history. Regarding self-reported gender results, 68.19% of the participants were women, and the remaining participants were men. Moreover, most participants reported that their ethnicities were Han (99.65%), and the remaining were reported to be Qiang. Furthermore, the marital status of 13.07% of the participants was unmarried. In addition, 32.50% of the participants had an educational level involving high school or above.
The measurement occurred in a large rebuilt community of Hanwang Town from November 6–8 in 2013. The investigators were all trained clinical psychologists, psychotherapists, and psychology graduate students. Our study was approved by the institutional review board of the Institute of Psychology, Chinese Academy of Sciences, and was completely in compliance with national legislation and the Declaration of Helsinki. All the participants signed informed consent forms.
Measures
Earthquake-related trauma exposure was assessed by a self-reported questionnaire (46). The participants needed to answer 10 yes (1) or no (0) questions about whether they experienced (a) being trapped under a rubble, (b) being injured, (c) being disabled due to injuries, (d) participating in rescue efforts, (e) witnessing the death of someone, (f) being exposed to mutilated bodies, (g) the traumatic death of a family member, (h) the traumatic injury of a family member, (i) the traumatic death of a friend or neighbor, and (j) losing livelihood due to a disaster. The level of trauma exposure was defined as the sum score of the 10 items.
PTSD symptoms were assessed by a self-reported questionnaire, the PTSD Checklist of DSM-5 [PCL-5; (47, 48)]. The 20 items included on the PCL-5 used a five-point Likert scale (from 0 = never to 4 = most of the time). The Chinese version has shown good validity and reliability (49). The participants needed to complete the PCL-5 Chinese version according to their PTSD symptom occurrence frequency and severity in the past month of the earthquake. The final score indicating PTSD symptom severity was the sum of all the item scores.
Depressive symptoms were measured by a self-report questionnaire, the Center for Epidemiological Studies-Depression (CES-D) scale (50). The 20 items included on the CES-D used a four-point Likert scale (from 0 = rare or none of the time/ <1 day to 3 = most or all of the time/5–7 days). The Chinese version has been validated and widely used in Chinese populations (51). The participants were required to answer the Chinese version of the questionnaire on the basis of their personal experiences in the past week. The final score indicating depressive symptoms was reflected by the sum of all the item scores.
SNP Selection and Genotyping
Four HPA axis genes with nine relevant SNPs were selected for study: ADCYAP1R1 (rs2267735), FKBP5 (rs9296158, rs3800373, rs1360780, and rs9470080), CRHR1 (rs4458044 and rs242924), and CRHR2 (rs8192496 and rs2267715). These SNPs were genotyped in a previous study (38); thus, we directly used their data.
We used PLINK (52) to perform the Hardy–Weinberg equilibrium (HWE) test (53). In addition, we also calculated the SNP call rate and minor allele frequency (MAF).
Statistical Analyses
PLINK 1.07 and R 3.4.4 (https://www.r-project.org/) were used to conduct all the statistical analyses in our study. A pairwise comparison of demographic variables and the SNP rs2267735 was tested by analysis of variance (ANOVA) in R. A linkage disequilibrium (LD) analysis has been done previously (38), and we directly referenced those results.
The associations between rs2267735 (coded as minor allele counts per subject) and PTSD severity were detected by a linear regression model and adjusted for age, sex (1: women and 0: men), education level (1: high school or above and 0: other), marital status (1: single and 0: married), the environmental factor trauma exposure, and depressive symptoms. The main, gene–environment interaction (G × E), and gene–gene interaction (G × G) effects were considered. When studying the G × E effects, we further added a linear model analysis with all gene–covariate and environment–covariate interactions (54). The all-interactions model was used to control for potential confounders and compared to the single-interaction model. A subset analysis for women and men was performed to detect potential sex differences. All P-values of linear regressions in our study are two-sided.
For adjustment of possible bias and correction for multiple comparisons, the SNP-related variables were subjected to a permutation test with 100,000 cycles. The results with significant P and permutation P-values are reported. Moreover, we calculated the effect size (semipartial correlation) as well.
Results
Overall Results
The summary of trauma exposure, PTSD severity (total symptom) scores, and depressive symptoms (CES-D scale total score) are shown in Table 1. The mean value of depressive symptoms in all samples is 37.00, and the standard error of the mean was 8.63. According to the DSM-5, we inferred probable PTSD in our participants. As our likely diagnoses showed, the probable PTSD prevalence was 13.78% (14.89% for women and 11.38% for men). All of the above-mentioned data were exactly the same as those of our previous study (38) since they were based on the same cohort, and we provided the data here for convenience.
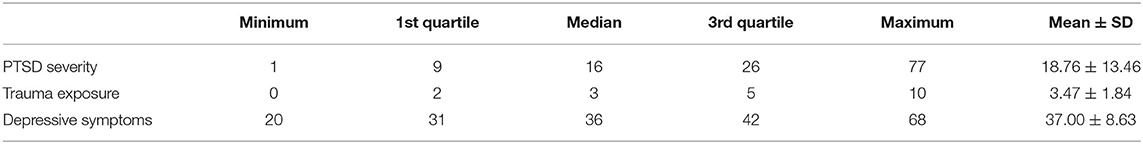
Table 1. Summary of posttraumatic stress disorder (PTSD) severity (total symptoms), trauma exposure, and depressive symptom scores.
The HWE test indicated that the genotype frequencies of rs2267735 agreed with the Hardy–Weinberger equilibrium (P = 0.812). The call rate was 100%, and the MAF (for allele G) was 0.485. The pairwise comparisons of the demographic variables and SNP rs2267735 are shown in Supplementary Table 1. There was no significant result between the SNP and depression.
Based on previous LD analysis results (38), during the following analyses, we used rs2267735 × rs1360780 to index the G × G effect of ADCYAP1R1–FKBP5 and rs2267735 × rs242924 to index the G × G effect of ADCYAP1R1–CRHR1.
Main Effects and G × E Effects of ADCYAP1R1 on PTSD Severity
As shown, the minor allele G of rs2267735 of ADCYAP1R1 was not significantly associated with PTSD severity. The G × E ADCYAP1R1–environment (rs2267735 × trauma exposure) effect on PTSD severity was also not significant. The trauma exposure was measured by a 10-item scale in the present study [see Supplementary Tables 2, 3 (single-interaction model) and Supplementary Table 4 (all-interaction model) for details].
G × G Effects of ADCYAP1R1 Genes on PTSD Severity
The G × G ADCYAP1R1–FKBP5 (rs2267735 × rs1360780) effect on PTSD severity was significant in all samples (P = 0.049). The G × G ADCYAP1R1–CRHR1 (rs2267735 × rs242924) effect on PTSD severity was significant in men (P = 0.023; see Tables 2, 3 for details). Figure 1 shows the G × G (epistasis) effects represented by the distributions of PTSD severity across genotype combinations. We also provide all the other results of the G × G analysis (Supplementary Table 5).
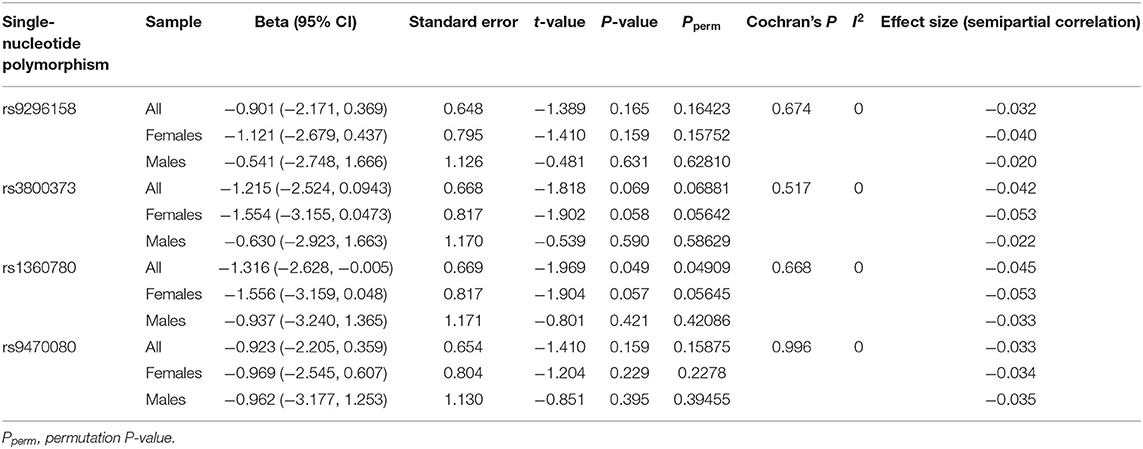
Table 2. G × G effects of ADCYAP1R1–FKBP5 (rs2267735 × rs9296158, rs3800373, rs1360780, and rs9470080) on posttraumatic stress disorder severity.
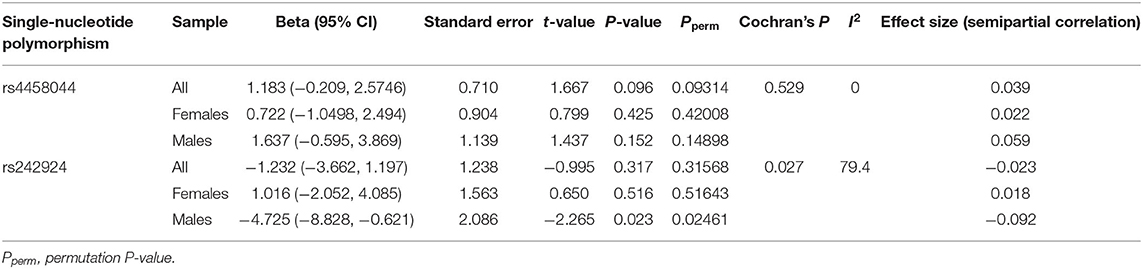
Table 3. G × G effects of ADCYAP1R1–CRHR1 (rs2267735 × rs4458044 and rs242924) on posttraumatic stress disorder severity.
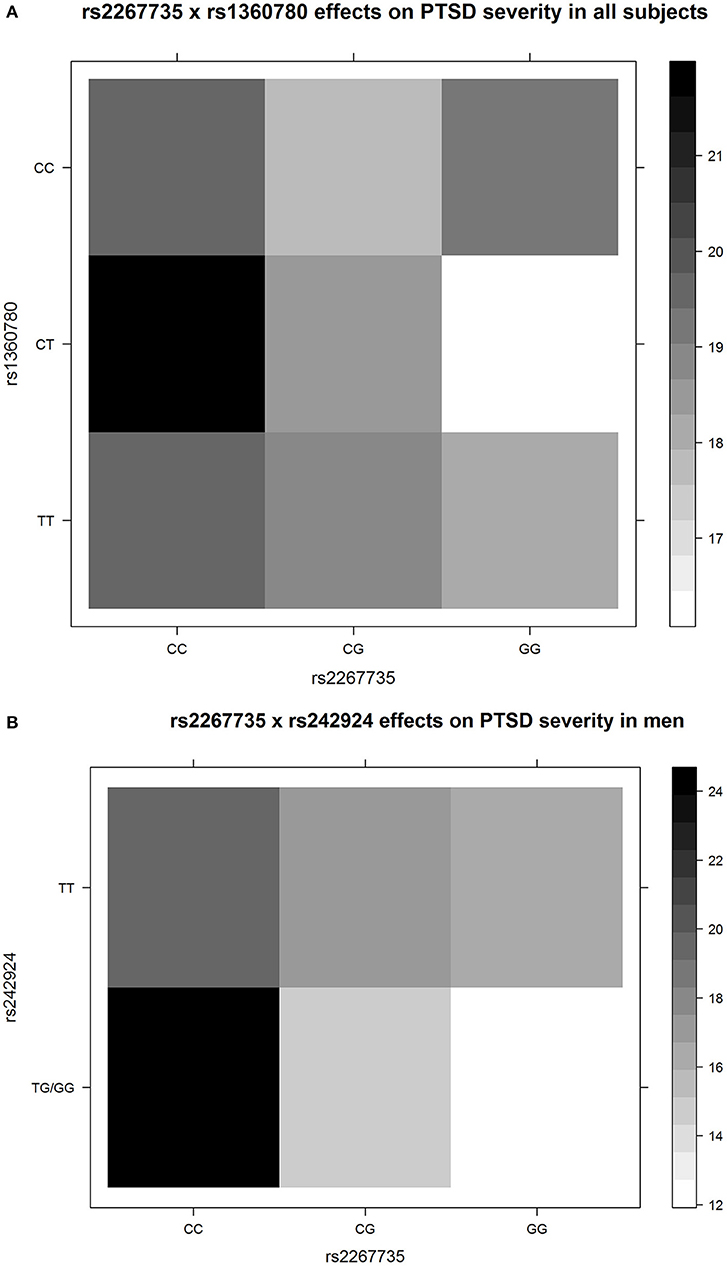
Figure 1. The G × G (epistasis) effects represented by the distributions of posttraumatic stress disorder (PTSD) severity across genotype combinations. The colors of each unit were filled according to the means of PTSD severity of the subjects with the corresponding specific genotype combinations. The upper panel (A) shows rs2267735 × rs1360780 for all subjects, and the lower panel (B) shows rs2267735 × rs242924 for men. Only three men had the rs242924 genotype GG; thus, we merged GG and TG. The figures directly show epistasis. For example, in (A), for the subjects with rs1360780 genotype CC, the minor allele G of rs2267735 is not correlated to PTSD severity; for the subjects with rs1360780 genotypes CT and TT, the minor allele G of rs2267735 corresponds to decreased PTSD severity, with different effect sizes. The figures were drawn by R3.4.4.
Discussion
Summary of Results
In this study, two gene–gene interactions (G × G) were identified. An ADCYAP1R1–FKBP5 effect was associated with PTSD severity in all subjects, and an ADCYAP1R1–CRHR1 effect was found in men.
An FKBP5-environment interaction and ADCYAP1R1 have been found to be associated with PTSD (19–22, 28–30), but to our knowledge, few studies have shown a G × G effect of FKBP5 and ADCYAP1R1. Therefore, this is the first study showing that the ADCYAP1R1 polymorphism rs2267735 could affect PTSD development through a novel ADCYAP1R1–FKBP5 interaction. Moreover, this finding was observed under the conditions of earthquake trauma type and in a Chinese ethnic group.
In addition, CRHR1 has been reported in prospective studies to moderate childhood maltreatment effects on PTSD symptoms (28, 30, 31). The gene–gene–environment (CRHR1 × 5–HTTLPR × childhood maltreatment) interaction could predict adult depressive symptoms among black people of lower socioeconomic status (55). Here the identified G × G effect between ADCYAP1R1 and CRHR1 on PTSD in men was interesting and further revealed the influence of gene–gene interactions on PTSD.
An Indication of the Results of G × G Effects From a Physiological Perspective
Earlier findings of PTSD candidate gene studies have shown that rs2267735 in ADCYAP1R1 could increase amygdala reactivity and reduce functional connectivity between the amygdala and hippocampus (21, 56). In addition, four SNPs in FKBP5 (rs9296158, rs3800373, rs1360780, and rs9470080), which are related to regulating the stress response system, were shown to increase amygdala reactivity to threat stimuli and the severity of PTSD symptoms in adulthood (28, 57). Thus, the ADCYAP1R1–FKBP5 interaction may influence PTSD development by affecting amygdala reactivity together.
Both ADCYAP1R1 and CRHR1 are involved in stress response by modulating CRF function and the release of cortisol through the adrenal cortex (58). Therefore, the ADCYAP1R1–CRHR1 interaction suggests that their gene expression may influence PTSD by regulating CRF together.
One G × G Was Sex Nonspecific, and the Other One Was Related to Sex
The genotypes of rs2267735 were differentially distributed in females and males (Supplementary Table 2). To explore sex differences in the two G × G (ADCYAP1R1–FKBP5 and ADCYAP1R1–CRHR1) interactions, we used Cochran's Q statistic test by PLINK. To investigate heterogeneity between results from women and men (i.e., the sex–G × G interactions), we also calculated the heterogeneity index I2 (range, 0–100). The ADCYAP1R1-FKBP5 interactions were not different between women and men (Cochran's Q statistic P < 0.668 and I2 = 0), which indicated that the effects were sex non-specific. The ADCYAP1R1-CRHR1 interactions were different between sexes (Cochran's Q statistic P < 0.027 and I2 = 79.4), which showed that sex might moderate the effects. Many studies have shown that sex could regulate the relationship between ADCYAP1R1 and PTSD. Women with the C allele at rs2267735 of ADCYAP1R1 had a higher PTSD risk than those with the G allele (20, 21). Moreover, a genome-wide association study indicated that CRHR1 is a genetic factor related to PTSD reexperiencing symptom (33). Our findings suggested that ADCYAP1R1 might also affect PTSD in men in a specific manner by interacting with CRHR1.
The Limitations of This Study
Our study had some limitations. One was the scales used in the current study. The PTSD was measured by a self-reported scale, and a clinical assessment could be used in the future. The trauma exposure was measured by a 10-item scale. Although it has shown great reliability and validity (38, 46, 59–61), other questionnaires with more items could be tried to be applied further. Depression was regarded as a covariate, so it was mentioned less in the study. The second was that the sample size was relatively small, and the number of women was approximately two times that of men. Large sample sizes and sex-balanced samples should be considered. The single ethnic group and trauma exposure type also need to be broadened. The third was about the definition of the G × E effect. Because this was a candidate gene study, so the “G” was only considered as specific genotype instead of genetics, and the “E” was only concentrated on earthquake, which was the most related environment factor to the PTSD symptom measured in the present study. Other possible related factors, such as rearing environment, social support, socioeconomic status, parenting styles, and education, could be taken into consideration in the future. The fourth was the sex difference of ADCYAP1R1–CRHR1 interactions which might be due to the sex hormone level which was different between men and women. This point could be detected through physiological experiments and indications.
Conclusions
We have provided additional genetic findings regarding the HPA axis and its involvement in PTSD and indicated that the ADCYAP1R1 polymorphism rs2267735 may affect PTSD development through diverse gene–gene interactions. Our analysis provided new insight into PTSD genetics.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the institutional review board of the Institute of Psychology, Chinese Academy of Sciences. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LW and KZ conceived and designed the overall study. LW collected the samples. KZ, JZ, YZ, and GZ performed the statistical analysis. CC maintained genotyping. PL and SL contributed to collecting samples. KZ, JZ, and LW wrote the manuscript. GL and RF helped revise the manuscript. All the authors read and approved the final version of the manuscript.
Funding
This study was partially supported by the National Natural Science Foundation of China (nos. 31471004, 31470070, and 31971020), the Key Project of the National Social Science Foundation of China (no. 20ZDA079), the Key Project of Research Base of Humanities and Social Sciences of Ministry of Education (no. 16JJD190006), and the Key Research Program of the Chinese Academy of Sciences (no. ZDRW-XH-2019-4).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We really thank the two reviewers for their helpful and constructive comments on our work. We also would like to acknowledge the participants of this study for their active cooperation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.665599/full#supplementary-material
References
1. Cho, J-H, Zushida K, Shumyatsky GP, Carlezon WA Jr, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci. (2012) 32:14165–77. doi: 10.1523/JNEUROSCI.1402-12.2012
2. Hashimoto H, Shintani N, Tanida M, Hayata A, Hashimoto R, Baba A. PACAP is implicated in the stress axes. Curr Pharm Des. (2011) 17:985–9. doi: 10.2174/138161211795589382
3. Johnson GC, Parsons R, May V, Hammack SE. The role of pituitary adenylate cyclase-activating polypeptide (PACAP) signaling in the hippocampal dentate gyrus. Front Cell Neurosci. (2020) 14:111. doi: 10.3389/fncel.2020.00111
4. Meloni EG, Kaye KT, Venkataraman A, Carlezon WA. PACAP increases ARC/ARG 3.1 expression within the extended amygdala after fear conditioning in rats. Neurobiol Learn Mem. (2019) 157:24–34. doi: 10.1016/j.nlm.2018.11.011
5. Missig G, Mei LD, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, et al. Parabrachial pituitary adenylate cyclaseactivating polypeptide activation of amygdala endosomal extracellular signal-regulated kinase signaling regulates the emotional component of pain. Biol Psychiatry. (2017) 81:671–82. doi: 10.1016/j.biopsych.2016.08.025
6. Varodayan FP, Minnig MA, Steinman MQ, Oleata CS, Riley MW, Sabino V, et al. PACAP regulation of central amygdala GABAergic synapses is altered by restraint stress. Neuropharmacology. (2020) 168:107752. doi: 10.1016/j.neuropharm.2019.107752
7. Kirry AJ, Herbst MR, Poirier SE, Maskeri MM, Rothwell AC, Twining RC, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) signaling in the prefrontal cortex modulates cued fear learning, but not spatial working memory, in female rats. Neuropharmacology. (2018) 133:145–54. doi: 10.1016/j.neuropharm.2018.01.010
8. Legradi G, Hannibal J, Lechan RM. Pituitary adenylate cyclase-activating polypeptide-nerve terminals densely innervate corticotropin-releasing hormone-neurons in the hypothalamic paraventricular nucleus of the rat. Neurosci Lett. (1998) 246:145–8. doi: 10.1016/S0304-3940(98)00255-9
9. Ross RA, Hoeppner SS, Hellberg SN, O'Day EB, Rosencrans PL, Ressler KJ, et al. Circulating PACAP peptide and PAC1R genotype as possible transdiagnostic biomarkers for anxiety disorders in women: a preliminary study. Neuropsychopharmacology. (2020) 45:1125–33. doi: 10.1038/s41386-020-0604-4
10. Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. (2003) 463:199–216. doi: 10.1016/S0014-2999(03)01282-2
11. Gonzalez P, Martinez KG. The role of stress and fear in the development of mental disorders. Psychiatr Clin N Am. (2014) 37:535. doi: 10.1016/j.psc.2014.08.010
12. Oyola MG, Handa RJ. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress Int J Biol Stress. (2017) 20:476–94. doi: 10.1080/10253890.2017.1369523
13. Jovanovic T, Stenson AF, Thompson N, Clifford A, Compton A, Minton S, et al. Impact of ADCYAP1R1 genotype on longitudinal fear conditioning in children: interaction with trauma and sex. Neuropsychopharmacology. (2020) 45:1603–8. doi: 10.1038/s41386-020-0748-2
14. Stroth N, Holighaus Y, Ait-Ali D, Eiden LE. PACAP: a master regulator of neuroendocrine stress circuits and the cellular stress response. Ann N Y Acad Sci. (2011) 1220:49–59. doi: 10.1111/j.1749-6632.2011.05904.x
15. Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. (2009) 61:283–357. doi: 10.1124/pr.109.001370
16. Mercer KB, Dias B, Shafer D, Maddox SA, Mulle JG, Hu P, et al. Functional evaluation of a PTSD-associated genetic variant: estradiol regulation and ADCYAP1R1. Transl Psychiatry. (2016) 6:e978. doi: 10.1038/tp.2016.241
17. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edn. Arlington, VA: American Psychiatric Publishing (2013). doi: 10.1176/appi.books.9780890425596
18. Bisson JI, Cosgrove S, Lewis C, Roberts NP. Post-traumatic stress disorder. BMJ. (2015) 351:h6161. doi: 10.1136/bmj.h6161
19. Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, et al. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet Part B Neuropsychiatr Genet. (2013) 162B:262–72. doi: 10.1002/ajmg.b.32145
20. Lind MJ, Marraccini ME, Sheerin CM, Bountress K, Bacanu SA, Amstadter AB, et al. Association of posttraumatic stress disorder with rs2267735 in the ADCYAP1R1 gene: a meta-analysis. J Traum Stress. (2017) 30:389–98. doi: 10.1002/jts.22211
21. Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. (2011) 470:492–7. doi: 10.1038/nature09856
22. Sharma S, Ressler KJ. Genomic updates in understanding PTSD. Prog Neuro-Psychopharmacol Biol Psychiatry. (2019) 90:197–203. doi: 10.1016/j.pnpbp.2018.11.010
23. Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD's emotional numbing symptoms in Chinese earthquake survivors. J Affect Disord. (2013) 150:156–9. doi: 10.1016/j.jad.2013.01.010
24. Guidotti G, Calabrese F, Anacker C, Racagni G, Pariante CM, Riva MA. Glucocorticoid receptor and FKBP5 expression is altered following exposure to chronic stress: modulation by antidepressant treatment. Neuropsychopharmacology. (2013) 38:616–27. doi: 10.1038/npp.2012.225
25. Sheerin CM, Lind MJ, Bountress KE, Marraccini ME, Amstadter AB, Bacanu A, et al. Meta-analysis of associations between hypothalamic-pituitary-adrenal axis genes and risk of posttraumatic stress disorder. J Traum Stress. (2020) 33:688–98. doi: 10.1002/jts.22484
26. uiHu Y, Chu X, Urosevich TG, Hoffman SN, Kirchner HL, Adams RE, et al. Predictors of current DSM-5 PTSD diagnosis and symptom severity among deployed veterans: significance of predisposition, stress exposure, and genetics. Neuropsychiatr Dis Treat. (2020) 16:43–54. doi: 10.2147/NDT.S228802
27. Li G, Wang L, Zhang K, Cao C, Cao X, Fang R, et al. FKBP5 genotype linked to combined PTSD-depression symptom in Chinese earthquake survivors. Can J Psychiatry. (2019) 64:863–71. doi: 10.1177/0706743719870505
28. Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. J Am Med Assoc. (2008) 299:1291–305. doi: 10.1001/jama.299.11.1291
29. Wang Q, Shelton RC, Dwivedi Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: a systematic review and meta-analysis. J Affect Disord. (2018) 225:422–8. doi: 10.1016/j.jad.2017.08.066
30. Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. (2010) 35:1684–92. doi: 10.1038/npp.2010.37
31. Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, et al. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Mark. (2011) 30:89–99. doi: 10.1155/2011/928497
32. Carvalho CM, Coimbra BM, Ota VK, Mello MF, Belangero SI. Single-nucleotide polymorphisms in genes related to the hypothalamic-pituitary-adrenal axis as risk factors for posttraumatic stress disorder. Am J Med Genet Part B Neuropsychiatr Genet. (2017) 174:671–82. doi: 10.1002/ajmg.b.32564
33. Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, et al. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci. (2019) 22:1394–401. doi: 10.1038/s41593-019-0447-7
34. Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. (2009) 26:984–92. doi: 10.1002/da.20605
35. von Wolff G, Avrabos C, Stepan J, Wurst W, Deussing JM, Holsboer F, et al. Voltage-sensitive dye imaging demonstrates an enhancing effect of corticotropin-releasing hormone on neuronal activity propagation through the hippocampal formation. J Psychiatr Res. (2011) 45:256–61. doi: 10.1016/j.jpsychires.2010.06.007
36. White S, Acierno R, Ruggiero KJ, Koenen KC, Kilpatrick DG, Galea S, et al. Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord. (2013) 27:678–83. doi: 10.1016/j.janxdis.2013.08.003
37. Toth M, Flandreau EI, Deslauriers J, Geyer MA, Mansuy IM, Pich EM, et al. Overexpression of forebrain CRH during early life increases trauma susceptibility in adulthood. Neuropsychopharmacology. (2016) 41:1681–90. doi: 10.1038/npp.2015.338
38. Zhang K, Wang L, Li G, Cao C, Fang R, Liu P, et al. Correlation between hypothalamic-pituitary-adrenal axis gene polymorphisms and posttraumatic stress disorder symptoms. Horm Behav. (2020) 117:104604. doi: 10.1016/j.yhbeh.2019.104604
39. Wolf EJ, Mitchell KS, Logue MW, Baldwin CT, Reardon AF, Humphries DE, et al. Corticotropin releasing hormone receptor 2 (CRHR-2) gene is associated with decreased risk and severity of posttraumatic stress disorder in Women. Depress Anxiety. (2013) 30:1161–9. doi: 10.1002/da.22176
40. Ramikie TS, Ressler KJ. Mechanisms of sex differences in fear and posttraumatic stress disorder. Biol Psychiatry. (2018) 83:876–85. doi: 10.1016/j.biopsych.2017.11.016
41. Chang SC, Xie P, Anton RF, De Vivo I, Farrer LA, Kranzler HR, et al. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Mol Psychiatry. (2012) 17:239–41. doi: 10.1038/mp.2011.118
42. Young KA, Morissette SB, Jamroz R, Meyer EC, Stanford MS, Wan L, et al. 5-HTTLPR and DISC1 risk genotypes for elevated PTSD symptoms in US military veterans. World Psychiatry. (2017) 16:109–10. doi: 10.1002/wps.20359
43. Green BL, Krupnick JL, Stockton P, Goodman L, Corcoran C, Petty R. Psychological outcomes associated with traumatic loss in a sample of young women. Am Behav Sci. (2001) 44:817–37. doi: 10.1177/00027640121956511
44. Kelley LP, Weathers FW, McDevitt-Murphy ME, Eakin DE, Flood AM. A comparison of PTSD symptom patterns in three types of civilian trauma. J Traum Stress. (2009) 22:227–35. doi: 10.1002/jts.20406
45. Wolf JB, Brodie ED, Wade MJ. Epistasis and the Evolutionary Process. New York, NY: Oxford University Press (2000).
46. Liu P, Wang L, Cao C, Wang R, Zhang J, Zhang B, et al. The underlying dimensions of DSM-5 posttraumatic stress disorder symptoms in an epidemiological sample of Chinese earthquake survivors. J Anxiety Disord. (2014) 28:345–51. doi: 10.1016/j.janxdis.2014.03.008
47. Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Traum Stress. (2015) 28:489–98. doi: 10.1002/jts.22059
48. Wortmann JH, Jordan AH, Weathers FW, Resick PA, Dondanville KA, Hall-Clark B, et al. Psychometric analysis of the PTSD checklist-5 (PCL-5) among treatment-seeking military service members. Psychol Assess. (2016) 28:1392–403. doi: 10.1037/pas0000260
49. Wang L, Cao X, Cao C, Fang R, Yang H, Elhai JD. Factor structure of DSM-5 PTSD symptoms in trauma-exposed adolescents: examining stability across time. J Anxiety Disord. (2017) 52:88–94. doi: 10.1016/j.janxdis.2017.07.001
50. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. (1977) 1:385–401. doi: 10.1177/014662167700100306
51. Cao X, Wang L, Cao C, Zhang J, Liu P, Zhang B, et al. Patterns of DSM-5 posttraumatic stress disorder and depression symptoms in an epidemiological sample of Chinese earthquake survivors: a latent profile analysis. J Affect Disord. (2015) 186:58–65. doi: 10.1016/j.jad.2015.06.058
52. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. (2007) 81:559–75. doi: 10.1086/519795
53. Wigginton JE, Cutler DJ, Abecasis GR. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. (2005) 76:887–93. doi: 10.1086/429864
54. Keller MC. Gene × environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry. (2014) 75:18–24. doi: 10.1016/j.biopsych.2013.09.006
55. Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, Gillespie CF, et al. Polymorphisms in CRHR1 and the serotonin transporter loci: gene x gene x environment interactions on depressive symptoms. Am J Med Genet Part B Neuropsychiatr Genet. (2010) 153B:812–24. doi: 10.1002/ajmg.b.31052
56. Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, et al. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress Anxiety. (2013) 30:251–8. doi: 10.1002/da.22037
57. Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. (2013) 16:33–41. doi: 10.1038/nn.3275
58. Seligowski AV, Hill SB, King CD, Wingo AP, Ressler KJ. Chapter 10 - Understanding resilience: biological approaches in at-risk populations. In: Chen A, editor. Stress Resilience. Academic Press (2020). p. 133–48. doi: 10.1016/B978-0-12-813983-7.00010-0
59. Cao C, Wang L, Cao X, Dong C, Liu P, Luo S, et al. Support for the association between RORA gene polymorphisms and the DSM-5 posttraumatic stress disorder symptoms in male earthquake survivors in China. Asian J Psychiatry. (2017) 25:138–41. doi: 10.1016/j.ajp.2016.10.028
60. Zhang K, Wang L, Cao C, Li G, Fang R, Liu P, et al. A DRD2/ANNK1-COMT interaction, consisting of functional variants, confers risk of post-traumatic stress disorder in traumatized Chinese. Front Psychiatry. (2018) 9:170. doi: 10.3389/fpsyt.2018.00170
Keywords: ADCYAP1R1, posttraumatic stress disorder, HPA axis, SNP, gene-gene interaction
Citation: Wang L, Zhang J, Li G, Cao C, Fang R, Liu P, Luo S, Zhao G, Zhang Y and Zhang K (2021) The ADCYAP1R1 Gene Is Correlated With Posttraumatic Stress Disorder Symptoms Through Diverse Epistases in a Traumatized Chinese Population. Front. Psychiatry 12:665599. doi: 10.3389/fpsyt.2021.665599
Received: 25 February 2021; Accepted: 22 April 2021;
Published: 07 June 2021.
Edited by:
Divya Mehta, Queensland University of Technology, AustraliaReviewed by:
Fengchun Wu, Guangzhou Medical University, ChinaSeth Davin Norrholm, Wayne State University, United States
Copyright © 2021 Wang, Zhang, Li, Cao, Fang, Liu, Luo, Zhao, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunlin Zhang, emhhbmdrbEBwc3ljaC5hYy5jbg==
 Li Wang
Li Wang Jingyi Zhang1,2
Jingyi Zhang1,2 Guangyi Zhao
Guangyi Zhao Yingqian Zhang
Yingqian Zhang Kunlin Zhang
Kunlin Zhang