
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 07 May 2021
Sec. Mood Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.659121
This article is part of the Research Topic Neuroimaging Biomarkers in Mood and Anxiety Disorders View all 8 articles
Functional neuroimaging studies have implicated alterations in frontostriatal and frontoparietal circuits in obsessive-compulsive disorder (OCD) during various tasks. To date, however, brain activation for visuospatial function in conjunction with symptoms in OCD has not been comprehensively evaluated. To elucidate the relationship between neural activity, cognitive function, and obsessive-compulsive symptoms, we investigated regional brain activation during the performance of a visuospatial task in patients with OCD using functional magnetic resonance imaging (fMRI). Seventeen medication-free patients with OCD and 21 age-, sex-, and IQ-matched healthy controls participated in this study. Functional magnetic resonance imaging data were obtained while the subjects performed a mental rotation (MR) task. Brain activation during the task was compared between the two groups using a two-sample t-test. Voxel-wise whole-brain multiple regression analyses were also performed to examine the relationship between obsessive-compulsive symptom severity and neural activity during the task. The two groups did not differ in MR task performance. Both groups also showed similar task-related activation patterns in frontoparietal regions with no significant differences. Activation in the right dorsolateral prefrontal cortex in patients with OCD during the MR task was positively associated with their total Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) scores. This study identified the specific brain areas associated with the interaction between symptom severity and visuospatial cognitive function during an MR task in medication-free patients with OCD. These findings may serve as potential neuromodulation targets for OCD treatment.
Obsessive-compulsive disorder (OCD) is a disabling neuropsychiatric condition characterized by recurrent, unwanted thoughts (obsession) and/or repetitive behaviors (compulsion), with a prevalence of 2–3% (1). Accumulated evidence from neuropsychological and neuroimaging studies in OCD has consistently demonstrated impairments in executive cognitive functions, such as inhibitory control and planning (2–4), and in the related neurocircuitry in the frontal-subcortical areas, called the cortico-striato-thalamo-cortical (CSTC) circuits (5–7). Recent studies also suggested the involvement of frontoparietal regions associated with working memory, particularly non-verbal memory, and visuospatial processing in OCD, although the involvement was rather inconsistent across studies (7–9). In addition, some evidence indicates that dysconnectivity of the frontoparietal network might have an important role in underpinning OCD symptomatology (10, 11).
Mental rotation (MR) is the ability to rotate mental representations for two- and three-dimensional objects in the mind (12), and an MR task is one of the well-known tasks for measuring visuospatial function (13, 14). Many studies of neuropsychological functions in OCD have documented deficits in visuospatial abilities (15), but the results of the MR task have not been consistent. Several studies have reported that patients with OCD are impaired significantly in MR ability compared with healthy controls (HCs) (16, 17). However, Moritz et al. (18) found no group difference in MR task performance when subjects were asked not only to count the sides of three-dimensional figures that were displayed but also sides that were not visible. Their subsequent study (9) also showed that OCD patients had no significant impairment in MR task performance compared to anxiety patients and HCs. It seems possible that these conflicting results are due to variations in study design, task condition, and task difficulty. Therefore, it would be helpful to implement an MR paradigm that has been well-validated and widely used (19).
The brain regions that are activated during an MR task are the superior parietal, frontal, and inferior temporal cortices (20). The posterior parietal/occipital cortex integrates visual and somatosensory information and is involved in implementing maps of space that code the locations of the targets of intended actions (21). Hence, these brain regions are suggested to be the main regions mediating MR performance (22). Performance on the MR task also requires the involvement of working memory, which recruits prefrontal areas such as the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate gyrus (23). Neuroimaging studies employing MR tasks have provided a valuable means to elucidate the behavior of frontoparietal circuits underlying spatial working memory processes during MR in those with mental disorders. For example, Silk et al. (24) investigated alterations in brain activation in adolescents with attention-deficit hyperactivity disorder (ADHD) during an MR task and found that the ADHD group showed less activation in the “action-attentional” system (including Brodmann areas (BAs) 46, 39, and 40: the DLPFC and inferior parietal areas) and the superior parietal and middle frontal regions, suggesting a model of dysfunction of frontoparietal networks in ADHD. To date, however, no studies have investigated the functional neural correlates of MR in patients with OCD.
Therefore, the present study aimed to investigate the functioning of frontoparietal networks related to visuospatial abilities in medication-free patients with OCD. We first compared the performance of the MR task in OCD patients with HCs. Then, we used functional MRI (fMRI) to examine whether brain activation patterns during the MR task differed between OCD patients and HCs. In addition, the association of neural activation during the MR task with patients' symptoms was investigated to reveal the relationship between symptoms, cognitive function, and brain activity in OCD patients. Also, we hypothesized that a certain region belonging to frontoparietal circuits of OCD patients would be associated with obsessive-compulsive symptoms.
We recruited 26 medication-free patients with OCD (9 drug-naïve patients and 17 patients who were drug-free for at least 4 weeks) from the OCD clinic at Seoul National University Hospital. All patients underwent the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and fulfilled the criteria for OCD. Twenty-six HCs matched for sex, age, and IQ were also recruited from the community. For all participants, the exclusion criteria were a lifetime history of psychosis, substance abuse/dependence, Tourette's disorder, or other tic-related conditions as well as a history of seizure disorder, head injury, or intellectual disability. Patients were assessed with the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (25), the Hamilton Rating Scale for Depression (HAM-D) (26), and the Hamilton Anxiety Rating Scale (HAM-A) (27) to measure the severity of their obsessive-compulsive symptomatology, depression, and anxiety, respectively. From each participant, we collected T1-weighted high-resolution anatomical MRI, resting-state fMRI, diffusion tensor imaging, and fMRI scans during the MR task and the Tower of London (ToL) task. In this study, we focused on neural mechanisms associated with visuospatial ability using MR task-based fMRI data. Resting-state fMRI and ToL task-based fMRI data from this dataset were previously reported elsewhere (28, 29).
After pre-processing the fMRI data, 3 patients and 2 HCs were excluded due to excessive head motion [>2.5 mm of translation or 2.5° of rotation and >0.30 mm for mean framewise displacement (FD)] (30). One HC was also excluded due to missing data, and 8 subjects (OCD = 6, HCs = 2) dropped out after inclusion. Therefore, the final sample consisted of 17 patients with OCD (7 drug-naïve and 10 unmedicated for at least 4 weeks) and 21 matched HCs. Eight patients were free of Axis I or Axis II psychiatric comorbid disorders, and 9 had the following axis I psychiatric comorbidities: dysthymic disorder (n = 3) and depressive disorder not otherwise specified (n = 6). This study was approved by the Institutional Review Board of Seoul National University Hospital, and all participants signed an informed consent form prior to their participation. The demographic and clinical information for both groups is provided in Table 1.
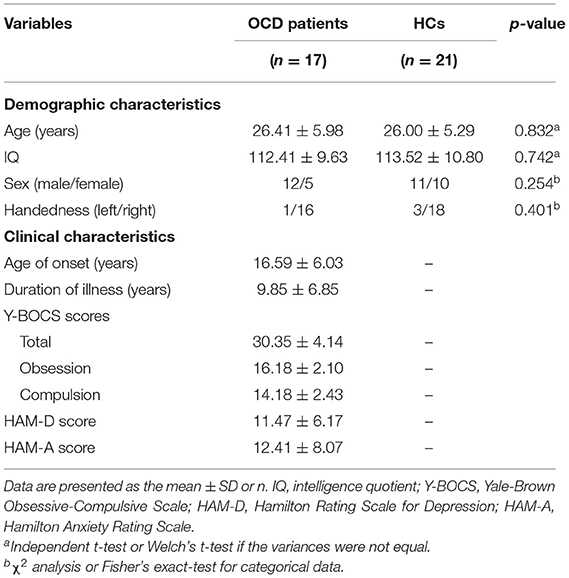
Table 1. Demographic and clinical characteristics of patients with obsessive-compulsive disorder (OCD) and healthy controls (HCs).
Participants performed a block-designed MR task with the following two conditions: (i) the MR condition and (ii) the zero rotation (ZR) condition as a control condition (Figure 1). For both the MR and ZR conditions, stimuli were three letters and three numbers (F, G, R, 2, 4, and 5). Because these alphanumeric stimuli are highly familiar that are usually displayed in an upright position, rotated characters are especially suitable for automatically triggering MR (31). The task consisted of four MR blocks and two ZR blocks. Stimuli in each block were presented pseudorandomly. Each block included 9 trials and was divided into resting fixation blocks of 20 s. The task was self-paced, with instructions to complete the task as quickly as possible without sacrificing speed for accuracy. In each trial, participants saw four rotated and/or mirrored characters (three identical mirrored characters and a different mirrored one) on the screen. Participants had to determine which of the alphanumeric characters was different from the others in each trial. For the MR condition, stimuli were rotated by four different angles (40, 80, 120, and 160 degrees), and these rotated characters were mirrored. For the ZR condition, only the characters generated by mirroring the image along the horizontal plane of the picture were used without any rotation (0 degrees). Before scanning, the participant practiced the task to learn the rules.
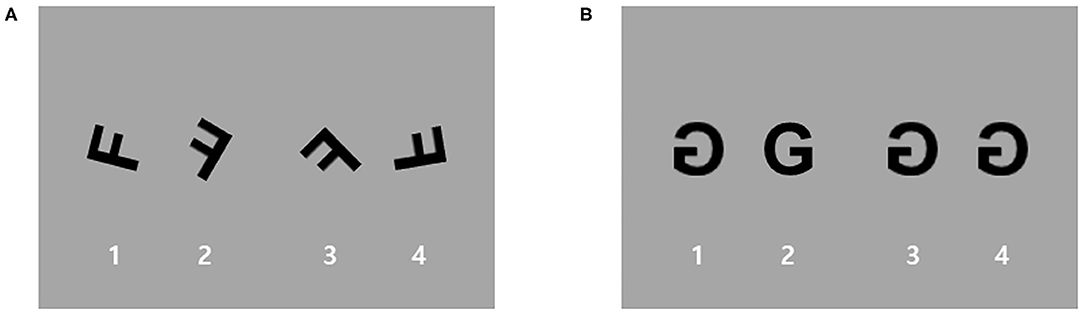
Figure 1. An example of stimuli used for the mental rotation (MR) task. Participants performed a block-designed MR task with two conditions: (A) an MR condition and (B) a zero rotation (ZR) condition as a control condition. For the MR condition, stimuli were rotated by four different angles (40, 80, 120, and 160 degrees), and these rotated characters were mirrored. For the ZR condition, only the characters generated by mirroring the image along the horizontal plane of the picture without any rotation (0 degrees) were used.
Image data were acquired with a 3T scanner (Siemens Magnetom Trio, Erlangen, Germany) using a T2*-weighted gradient echo-planar imaging (EPI) sequence during the task. The parameters were echo time (TE) = 30 ms, repetition time (TR) = 2 s, flip angle (FA) = 90°, voxel size = 3.4 × 3.4 × 4.0 mm3, interleaved axial slices = 27, and number of volumes = ~360 per subject. High-resolution T1-weighted magnetization-prepared rapid-gradient echo anatomical images were obtained (TE = 1.89 ms, TR = 1.670 ms, FA = 9°, voxel size = 1.0 × 0.98 × 0.98 mm3, and sagittal slices = 208). To minimize possible motion artifacts, head cushions were used, and the participants were asked to move as little as possible during the acquisition.
Comparisons between OCD patients and HCs for demographic characteristics and behavioral performance during the MR task were made with independent t-tests or Mann-Whitney U-tests. A chi-square analysis or Fisher's exact-test was used for categorical data.
Image pre-processing and statistical analyses were performed using SPM12 software (http://www.fil.ion.ucl.ac.uk/spm). After discarding the first 4 volumes, data were corrected for both slice timing acquisition and head motion and spatially normalized to the Montreal Neurological Institute (MNI) reference brain. Then, data were smoothed with a 6-mm full-width at half-maximum (FWHM) Gaussian kernel.
For the first-level analysis, two regressors were generated by modeling the neural response to each of the MR and ZR conditions as a box car with an onset cycle equal to the length of each block, convolved with a hemodynamic response function. We computed the contrasts for the MR condition vs. the resting fixation condition and for the MR condition and ZR (control) condition for each subject. Next, these first-level contrast images were entered into second-level random effect analysis. One-sample t-tests were used for the patients and control groups separately to identify regions of significant activation during the MR task compared with the ZR and fixation condition. Two-sample t-tests were used to identify areas showing significant differences in activation in patients with OCD compared with HCs.
Voxel-wise whole-brain multiple regression analyses were also performed to examine whether there was an association between patient symptom severity (total Y-BOCS score) and neural activation during the MR task. Patients' HAM-D scores were included in the model as a covariate to control for their depressive symptoms. All statistical results were set at a cluster-level threshold of p < 0.05, family-wise error (FWE) corrected for multiple comparisons, and a voxel-level threshold of p < 0.001, uncorrected.
Table 2 shows behavioral performance, including response times, accuracy, and efficiency (defined by the accuracy divided by the response time), for each group during the MR task and the statistical results. No significant between-group difference was observed for any of the performance variables. There was a trend, however, for OCD patients to show slower responses and increased error rates than HCs during the task.
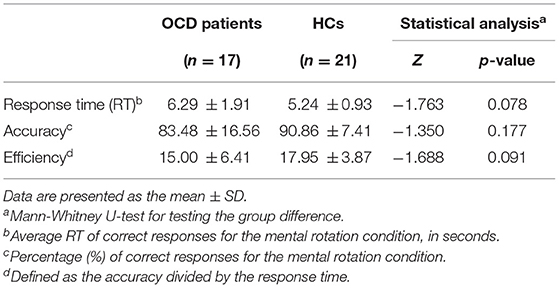
Table 2. Behavioral performance during the mental rotation task in patients with obsessive-compulsive disorder (OCD) and healthy controls (HCs).
Figure 2 shows the main effect of the MR condition vs. fixation (or ZR) condition for each group. In a comparison of the MR condition and the fixation condition, both groups exhibited bilateral activation in the parietal lobe, predominantly in the superior and inferior areas, extending into the occipital lobe. Activation was also found in a considerable portion of the bilateral frontal areas, including the superior frontal gyrus, middle frontal gyrus, and superior medial frontal gyrus, and subcortical areas, including the striatum and thalamus. A comparison between the MR condition and the ZR condition revealed an overall similar activation pattern to that observed in the comparison between the MR condition and the fixation condition. Both groups showed bilateral activation in a considerable portion of the parietal and frontal areas. However, few activations in subcortical regions were observed. On the other hand, between-group analysis did not show any significant differences in brain activation during the MR task between patients with OCD and HCs.
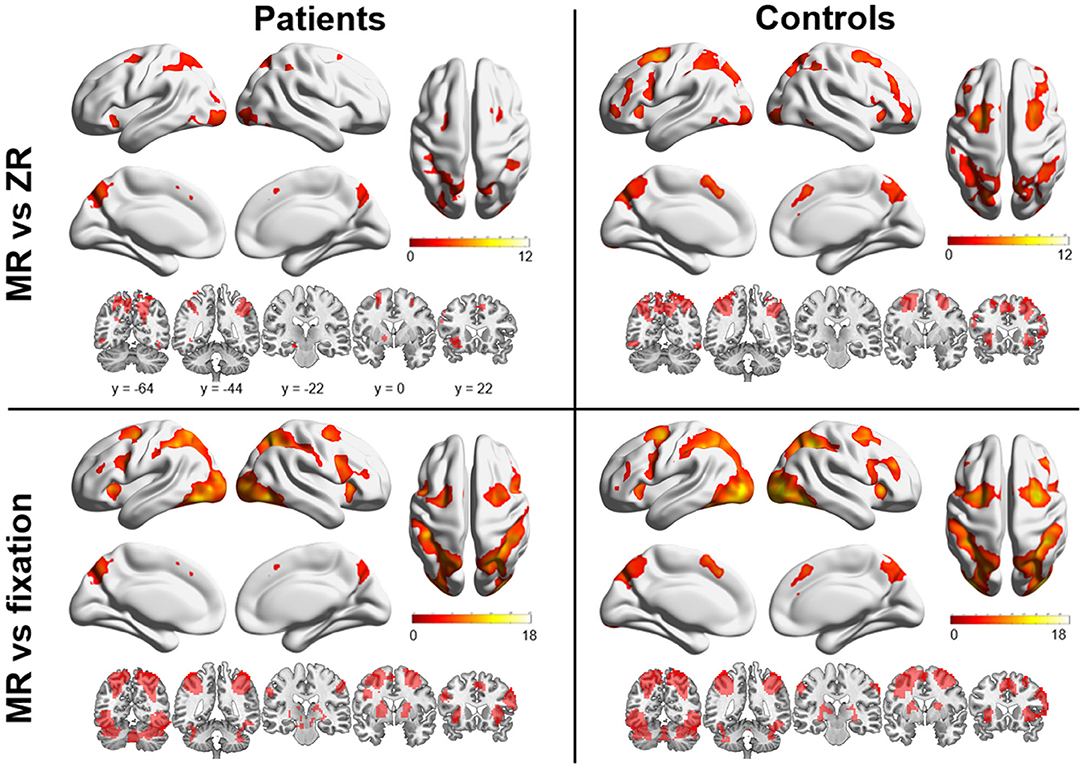
Figure 2. Brain regions are shown that were significantly activated in patients with OCD and healthy controls (HCs) during the mental rotation (MR) condition vs. the zero rotation (ZR) condition and in the MR condition vs. the fixation condition. Markedly increased activation was observed in the frontal and parietal areas in both groups.
The multiple regression analysis results are presented in Figure 3. The voxel-wise whole-brain multiple regression analysis revealed a significant positive association between the contrast images of the MR condition vs. the ZR condition and clinical symptom severity in OCD patients, showing that the more severe the obsessive-compulsive symptoms were, the greater the activation was in the right DLPFC (x, y, z = 51, 33, 33; t-/z-values = 6.39/4.30; cluster size = 75 voxels) of patients.
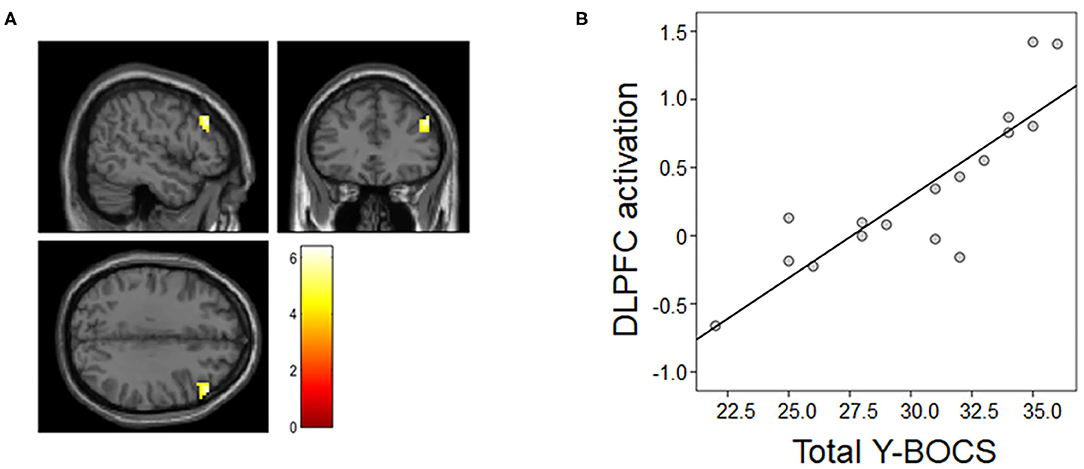
Figure 3. Association between neural activation during the MR task and symptom severity. (A) Patients' total Y-BOCS scores were associated with right dorsolateral prefrontal cortex (DLPFC) activation in the mental rotation (MR) condition vs. the zero rotation (ZR) condition. (B) For illustration purposes, we extracted the magnitude of DLPFC activation showing the above association and then assessed Pearson's correlation between the magnitude of activation and the total Y-BOCS score.
To the best of our knowledge, the present study was the first to evaluate the neural responses of patients with OCD while they were performing an MR task. Particularly, all patients were medication-free at the time of the MRI scan. We found that frontoparietal regions showed significant activation during the MR task in both OCD patients and HCs, but there were no significant group differences in brain activation. A conspicuous finding from our data was that higher clinical symptom severity in patients with OCD was significantly associated with greater activation in the right DLPFC. Therefore, these findings suggest that the frontoparietal network is implicated in visuospatial cognition, and the DLPFC in this network is an important element in the pathophysiology of OCD.
The brain circuits activated during the MR task were almost the same in patients and HCs and included the bilateral parietal, occipital, and frontal regions. These regions have been reported consistently in previous neuroimaging studies of MR (20). This finding further supports the idea that MR is a form of spatial working memory task that activates frontoparietal networks (23). Specifically, activation of the superior parietal cortex and adjacent regions appears to reflect visuospatial processing components of MR (23, 32, 33), and the activation of motor regions in the pre-central cortex implies the use of motor stimulation to solve MR problems (20, 34). The additional activation of prefrontal areas may reflect the role of working memory in MR (22, 35).
However, we were unable to identify any regions showing significantly increased or decreased activation in OCD patients compared with HCs during the MR task. This result may be explained by the fact that the patients demonstrated a level of performance on the MR task that was similar to that of the control subjects. Likewise, Nakao et al. (36) found that the patients with OCD and normal controls did not differ in their performance on neuropsychological tests and showed similar brain activation on fMRI during the Stroop test. Given the evidence of impaired visuospatial ability in patients with OCD (37), it is somewhat surprising that there were no significant group differences in MR task performance. Several factors could be attributed to this finding. Because of their comparably high intelligence, OCD patients may have compensated for suboptimal behavioral performance (38, 39). A number of studies have reported sex differences in the MR task, with men performing better than women (40–42). Our study included a slightly larger proportion of males in the patient group relative to control subjects (although there were no significant differences in demographic characteristics), possibly resulting in an overestimation of the performance scores on average in the OCD group. Furthermore, the low difficulty of the MR task that we used in the present study may have masked subtle behavioral or neurocognitive differences between the two groups. We used simple two-dimensional alphanumeric stimuli with relatively low difficulty rather than a two-dimensional representation of tri-dimensional figures (17). Indeed, it has been observed in neuropsychological studies that OCD patients show normal cognitive performance on low-demand cognitive tasks but show cognitive deficits at tasks with higher cognitive demands (43, 44). Although the task consisting of rotated alphanumeric stimuli properly activates the frontoparietal areas subserving MR (22, 31), further studies using more adequate task conditions that can generate cognitive load sufficiently are suggested.
The most important clinically relevant finding was that neural activation in the right DLPFC during the MR task was positively associated with obsessive-compulsive symptom severity. Previous studies suggested that there was a relationship between the orbitofrontal-striatal circuit and the generation or severity of obsessive-compulsive symptoms (45, 46). In addition to dysfunction of this circuitry, there has been considerable recent evidence that the frontoparietal networks, including the DLPFC and parietal regions, are also affected in OCD (5, 7). Indeed, de Vries et al. (47) found that OCD patients showed task-related hyperactivation in the left dorsal frontal areas and left precuneus associated with better task performance. Moon and Jeong (48) reported that a BOLD signal change in the DLPFC of patients with OCD during face-recognition tasks was negatively correlated with their Y-BOCS scores. In prior resting-state fMRI studies, altered connectivity regarding the DLPFC has been found in OCD patients relative to HCs, suggesting that the DLPFC might be central to OCD pathophysiology (11, 49). A more recent rs-fMRI study using bivariate Granger causality analysis demonstrated the abnormal causal interactions of DLPFC-related circuits in patients with OCD (50). Our finding that greater activation in the DLPFC during the MR task was associated with severe obsessive-compulsive symptoms may be related to patients' efforts to resist their symptoms during cognitive tasks. This idea is supported by data that the DLPFC is activated when patients with OCD inhibit their obsessive processes (5) or compulsive behaviors (51). Furthermore, it has been reported that the DLPFC is directly connected with the frontostriatal circuit of OCD patients (52) and is important in the integration of emotion and cognition (53). These roles and abnormalities of the DLPFC have made it an attractive target for neuromodulation. Indeed, a recent meta-analysis of randomized controlled trials demonstrated that repetitive transcranial magnetic stimulation (rTMS) targeting the DLPFC yielded greater improvements in obsessive-compulsive symptoms than sham rTMS (54). Taken together, our finding of the association between visuospatial task-related activation in the DLPFC and obsessive-compulsive symptom severity seems to be consistent with these earlier findings. This finding also provides new evidence of the involvement of abnormalities in the frontoparietal circuits in the pathophysiology of OCD.
Several limitations to this study need to be acknowledged. First, the sample size in the final analysis was relatively small. Some participants were excluded due to the quality of the functional imaging data, and the decreased number of subjects might have affected the statistical power of the results. Therefore, future studies with larger sample sizes are needed to confirm the findings of the present study. Second, nine of the 17 patients with OCD had comorbid axis I disorders such as dysthymic disorder or depressive disorder not otherwise specified. Given that depressive symptoms influence task performance and decreased performance is related to imaging results (18), it is important to bear clinical characteristics in mind when interpreting these results. Third, no information was collected about what participants subjectively experienced (e.g., whether they felt distressed, irritated, or compulsive) during the scan. These emotions may have affected their cognitive task performance and/or neural activity (55). We should also consider the learning effect on the MR task because all subjects practiced the task prior to the fMRI scan. Last, our patient sample consisted largely of males, and sex has been suggested to contribute to the biological heterogeneity of OCD (56); thus, this high proportion of males in our sample limits the generalization of our findings.
In conclusion, this fMRI study provides the first evidence that neural activation in unmedicated patients with OCD when performing an MR task is not different from that in HCs but is associated with obsessive-compulsive symptom severity. These results improve the present understanding of the neural mechanisms of MR associated with symptoms in OCD and thus provide the potential to identify neuromodulation targets for managing patients' symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Seoul National University Hospital. The patients/participants provided their written informed consent to participate in this study.
WJ, GS, and JK designed the study. WJ, TK, and GS contributed to the acquisition of MRI data. JK collected the clinical data, interpreted the data, and gave critical comments on the manuscript. SO and WJ performed data analysis and wrote the first manuscript. All authors critically reviewed the manuscript content and approved the final version for publication.
This work was supported by the Basic Science Research Program and the Basic Research Laboratory Program through the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT, and Future Planning (Grant Nos. 2018R1A4A1025891 and 2019R1A2B5B03100844).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank Dr. Da-Jung Shin and Mrs. Soo-Bin Lee for their help with data acquisition and all subjects who participated in this study.
1. Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol Psychiatry. (2010) 15:53–63. doi: 10.1038/mp.2008.94
2. Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. (2005) 29:399–419. doi: 10.1016/j.neubiorev.2004.11.006
3. Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. (2014) 44:1121–30. doi: 10.1017/S0033291713001803
4. Snyder HR, Kaiser RH, Warren SL, Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: a meta-analysis. Clin Psychol Sci. (2015) 3:301–30. doi: 10.1177/2167702614534210
5. Kwon JS, Jang JH, Choi JS, Kang DH. Neuroimaging in obsessive-compulsive disorder. Expert Rev Neurother. (2009) 9:255–69. doi: 10.1586/14737175.9.2.255
6. Norman LJ, Taylor SF, Liu Y, Radua J, Chye Y, De Wit SJ, et al. Error processing and inhibitory control in obsessive-compulsive disorder: a meta-analysis using statistical parametric maps. Biol Psychiatry. (2019) 85:713–25. doi: 10.1016/j.biopsych.2018.11.010
7. Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. (2019) 5:52. doi: 10.1038/s41572-019-0102-3
8. Jung WH, Gu BM, Kang DH, Park JY, Yoo SY, Choi CH, et al. BOLD response during visual perception of biological motion in obsessive-compulsive disorder: an fMRI study using the dynamic point-light animation paradigm. Eur Arch Psychiatry Clin Neurosci. (2009) 259:46–54. doi: 10.1007/s00406-008-0833-8
9. Moritz S, Kloss M, Jacobsen D, Kellner M, Andresen B, Fricke S, et al. Extent, profile and specificity of visuospatial impairment in obsessive-compulsive disorder (OCD). J Clin Exp Neuropsychol. (2005) 27:795–814. doi: 10.1080/13803390490918480
10. Goncalves OF, Soares JM, Carvalho S, Leite J, Ganho-Avila A, Fernandes-Goncalves A. Patterns of default mode network deactivation in obsessive compulsive disorder. Sci Rep. (2017) 7:44468. doi: 10.1038/srep44468
11. Gursel DA, Avram M, Sorg C, Brandl F, Koch K. Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev. (2018) 87:151–60. doi: 10.1016/j.neubiorev.2018.01.016
12. Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. (1971) 171:701–3. doi: 10.1126/science.171.3972.701
13. Caissie AF, Vigneau F, Bors DA. What does the mental rotation test measure? An analysis of item difficulty and item characteristics. Open Psychol J. (2009) 2:94–102. doi: 10.2174/1874350100902010094
14. Carpenter PA, Just MA, Keller TA, Eddy W, Thulborn K. Graded functional activation in the visuospatial system with the amount of task demand. J Cogn Neurosci. (1999) 11:9–24. doi: 10.1162/089892999563210
15. Tukel R, Gurvit H, Ertekin BA, Oflaz S, Ertekin E, Baran B, et al. Neuropsychological function in obsessive-compulsive disorder. Compr Psychiatry. (2012) 53:167–75. doi: 10.1016/j.comppsych.2011.03.007
16. Purcell R, Maruff P, Kyrios M, Pantelis C. Neuropsychological deficits in obsessive-compulsive disorder: a comparison with unipolar depression, panic disorder, and normal controls. Arch Gen Psychiatry. (1998) 55:415–23. doi: 10.1001/archpsyc.55.5.415
17. Savage CR, Baer L, Keuthen NJ, Brown HD, Rauch SL, Jenike MA. Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biol Psychiatry. (1999) 45:905–16. doi: 10.1016/s0006-3223(98)00278-9
18. Moritz S, Kloss M, Jahn H, Schick M, Hand I. Impact of comorbid depressive symptoms on nonverbal memory and visuospatial performance in obsessive-compulsive disorder. Cogn Neuropsychiatry. (2003) 8:261–72. doi: 10.1080/135468000344000020
19. Liesefeld HR, Zimmer HD. The advantage of mentally rotating clockwise. Brain Cogn. (2011) 75:101–10. doi: 10.1016/j.bandc.2010.10.012
20. Zacks JM. Neuroimaging studies of mental rotation: a meta-analysis and review. J Cogn Neurosci. (2008) 20:1–19. doi: 10.1162/jocn.2008.20013
21. Podzebenko K, Egan GF, Watson JD. Widespread dorsal stream activation during a parametric mental rotation task, revealed with functional magnetic resonance imaging. Neuroimage. (2002) 15:547–58. doi: 10.1006/nimg.2001.0999
22. Gogos A, Gavrilescu M, Davison S, Searle K, Adams J, Rossell SL, et al. Greater superior than inferior parietal lobule activation with increasing rotation angle during mental rotation: an fMRI study. Neuropsychologia. (2010) 48:529–35. doi: 10.1016/j.neuropsychologia.2009.10.013
23. Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, et al. Changes in cortical activity during mental rotation. A mapping study using functional MRI. Brain. (1996) 119:89–100. doi: 10.1093/brain/119.1.89
24. Silk T, Vance A, Rinehart N, Egan G, O'Boyle M, Bradshaw JL, et al. Fronto-parietal activation in attention-deficit hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry. (2005) 187:282–3. doi: 10.1192/bjp.187.3.282
25. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The yale-brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry. (1989) 46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007
26. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
27. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
28. Kim M, Jung WH, Shim G, Kwon JS. The effects of selective serotonin reuptake inhibitors on brain functional networks during goal-directed planning in obsessive-compulsive disorder. Sci Rep. (2020) 10:20619. doi: 10.1038/s41598-020-77814-4
29. Shin DJ, Jung WH, He Y, Wang J, Shim G, Byun MS, et al. The effects of pharmacological treatment on functional brain connectome in obsessive-compulsive disorder. Biol Psychiatry. (2014) 75:606–14. doi: 10.1016/j.biopsych.2013.09.002
30. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. (2012) 59:2142–54. doi: 10.1016/j.neuroimage.2011.10.018
31. Weiss MM, Wolbers T, Peller M, Witt K, Marshall L, Buchel C, et al. Rotated alphanumeric characters do not automatically activate frontoparietal areas subserving mental rotation. Neuroimage. (2009) 44:1063–73. doi: 10.1016/j.neuroimage.2008.09.042
32. Jordan K, Heinze HJ, Lutz K, Kanowski M, Jancke L. Cortical activations during the mental rotation of different visual objects. Neuroimage. (2001) 13:143–52. doi: 10.1006/nimg.2000.0677
33. Koshino H, Carpenter PA, Keller TA, Just MA. Interactions between the dorsal and the ventral pathways in mental rotation: an fMRI study. Cogn Affect Behav Neurosci. (2005) 5:54–66. doi: 10.3758/cabn.5.1.54
34. Michelon P, Vettel JM, Zacks JM. Lateral somatotopic organization during imagined and prepared movements. J Neurophysiol. (2006) 95:811–22. doi: 10.1152/jn.00488.2005
35. Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O'Boyle MW, et al. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. Am J Psychiatry. (2006) 163:1440–3. doi: 10.1176/ajp.2006.163.8.1440
36. Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res. (2005) 139:101–14. doi: 10.1016/j.pscychresns.2004.12.004
37. Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clin Psychol Rev. (2013) 33:1163–71. doi: 10.1016/j.cpr.2013.09.004
38. Heath CJ, Horst NK, Picciotto MR. Oral nicotine consumption does not affect maternal care or early development in mice but results in modest hyperactivity in adolescence. Physiol Behav. (2010) 101:764–9. doi: 10.1016/j.physbeh.2010.08.021
39. Norman LJ, Carlisi CO, Christakou A, Murphy CM, Chantiluke K, Giampietro V, et al. Frontostriatal dysfunction during decision making in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. (2018) 3:694–703. doi: 10.1016/j.bpsc.2018.03.009
40. Parsons TD, Larson P, Kratz K, Thiebaux M, Bluestein B, Buckwalter JG, et al. Sex differences in mental rotation and spatial rotation in a virtual environment. Neuropsychologia. (2004) 42:555–62. doi: 10.1016/j.neuropsychologia.2003.08.014
41. Semrud-Clikeman M, Fine JG, Bledsoe J, Zhu DC. Gender differences in brain activation on a mental rotation task. Int J Neurosci. (2012) 122:590–7. doi: 10.3109/00207454.2012.693999
42. Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. (1995) 117:250–70. doi: 10.1037/0033-2909.117.2.250
43. Purcell R, Maruff P, Kyrios M, Pantelis C. Cognitive deficits in obsessive-compulsive disorder on tests of frontal-striatal function. Biol Psychiatry. (1998) 43:348–57. doi: 10.1016/s0006-3223(97)00201-1
44. van der Wee NJ, Ramsey NF, Jansma JM, Denys DA, van Megen HJ, Westenberg HM, et al. Spatial working memory deficits in obsessive compulsive disorder are associated with excessive engagement of the medial frontal cortex. Neuroimage. (2003) 20:2271–80. doi: 10.1016/j.neuroimage.2003.05.001
45. Maia TV, Cooney RE, Peterson BS. The neural bases of obsessive-compulsive disorder in children and adults. Dev Psychopathol. (2008) 20:1251–83. doi: 10.1017/S0954579408000606
46. Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive-compulsive disorder. Semin Clin Neuropsychiatry. (2001) 6:82–101. doi: 10.1053/scnp.2001.21833
47. de Vries FE, de Wit SJ, Cath DC, van der Werf YD, van der Borden V, van Rossum TB, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. (2014) 76:878–87. doi: 10.1016/j.biopsych.2013.11.021
48. Moon CM, Jeong GW. Associations of neurofunctional, morphometric and metabolic abnormalities with clinical symptom severity and recognition deficit in obsessive-compulsive disorder. J Affect Disord. (2018) 227:603–12. doi: 10.1016/j.jad.2017.11.059
49. Vaghi MM, Vertes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry. (2017) 81:708–17. doi: 10.1016/j.biopsych.2016.08.009
50. Li H, Hu X, Gao Y, Cao L, Zhang L, Bu X, et al. Neural primacy of the dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder. Neuroimage Clin. (2020) 28:102432. doi: 10.1016/j.nicl.2020.102432
51. Nakao T, Okada K, Kanba S. Neurobiological model of obsessive-compulsive disorder: evidence from recent neuropsychological and neuroimaging findings. Psychiatry Clin Neurosci. (2014) 68:587–605. doi: 10.1111/pcn.12195
52. Melloni M, Urbistondo C, Sedeno L, Gelormini C, Kichic R, Ibanez A. The extended fronto-striatal model of obsessive compulsive disorder: convergence from event-related potentials, neuropsychology and neuroimaging. Front Hum Neurosci. (2012) 6:259. doi: 10.3389/fnhum.2012.00259
53. Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci USA. (2002) 99:4115–20. doi: 10.1073/pnas.062381899
54. Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets used in repetitive transcranial magnetic stimulation (rTMS) for the treatment of Obsessive-Compulsive Disorder (OCD). Psychiatr Q. (2018) 89:645–65. doi: 10.1007/s11126-018-9566-7
55. Osaka M, Yaoi K, Minamoto T, Osaka N. When do negative and positive emotions modulate working memory performance? Sci Rep. (2013) 3:1375. doi: 10.1038/srep01375
Keywords: obsessive-compulsive disorder, functional MRI, mental rotation task, visuospatial function, dorsolateral prefrontal cortex
Citation: Oh S, Jung WH, Kim T, Shim G and Kwon JS (2021) Brain Activation of Patients With Obsessive-Compulsive Disorder During a Mental Rotation Task: A Functional MRI Study. Front. Psychiatry 12:659121. doi: 10.3389/fpsyt.2021.659121
Received: 27 January 2021; Accepted: 06 April 2021;
Published: 07 May 2021.
Edited by:
Chien-Han Lai, National Yang-Ming University, TaiwanReviewed by:
Tomohiro Nakao, Kyushu University, JapanCopyright © 2021 Oh, Jung, Kim, Shim and Kwon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Soo Kwon, a3dvbmpzQHNudS5hYy5rcg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.