
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 20 April 2021
Sec. Mood Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.651196
This article is part of the Research TopicObsessive-Compulsive Disorder (OCD) Across the Lifespan: Current Diagnostic Challenges and the Search for Personalized Treatment View all 26 articles
 Srinivas Balachander1,2*
Srinivas Balachander1,2* Sandra Meier3
Sandra Meier3 Manuel Matthiesen3,4,5,6
Manuel Matthiesen3,4,5,6 Furkhan Ali1,2
Furkhan Ali1,2 Anand Jose Kannampuzha1,2
Anand Jose Kannampuzha1,2 Mahashweta Bhattacharya1,2
Mahashweta Bhattacharya1,2 Ravi Kumar Nadella2
Ravi Kumar Nadella2 Vanteemar S. Sreeraj2
Vanteemar S. Sreeraj2 Dhruva Ithal2
Dhruva Ithal2 Bharath Holla2
Bharath Holla2 Janardhanan C. Narayanaswamy1,2
Janardhanan C. Narayanaswamy1,2 Shyam Sundar Arumugham1
Shyam Sundar Arumugham1 Sanjeev Jain2
Sanjeev Jain2 YC Janardhan Reddy1,2
YC Janardhan Reddy1,2 Biju Viswanath2
Biju Viswanath2Background: Obsessive-compulsive disorder (OCD) is a heterogeneous illness, and emerging evidence suggests that different symptom dimensions may have distinct underlying neurobiological mechanisms. We aimed to look for familial patterns in the occurrence of these symptom dimensions in a sample of families with at least two individuals affected with OCD.
Methods: Data from 153 families (total number of individuals diagnosed with DSM-5 OCD = 330) recruited as part of the Accelerator Program for Discovery in Brain Disorders using Stem Cells (ADBS) was used for the current analysis. Multidimensional Item Response Theory (IRT) was used to extract dimensional scores from the Yale-Brown Obsessive-Compulsive Scale (YBOCS) checklist data. Using linear mixed-effects regression models, intra-class correlation coefficients (ICC), for each symptom dimension, and within each relationship type were estimated.
Results: IRT yielded a four-factor solution with Factor 1 (Sexual/Religious/Aggressive), Factor 2 (Doubts/Checking), Factor 3 (Symmetry/Arranging), and Factor 4 (Contamination/Washing). All except for Factor 1 were found to have significant ICCs, highest for Factor 3 (0.41) followed by Factor 4 (0.29) and then Factor 2 (0.27). Sex-concordant dyads were found to have higher ICC values than discordant ones, for all the symptom dimensions. No major differences in the ICC values between parent-offspring and sib-pairs were seen.
Conclusions: Our findings indicate that there is a high concordance of OCD symptom dimensions within multiplex families. Symptom dimensions of OCD might thus have significant heritability. In view of this, future genetic and neurobiological studies in OCD should include symptom dimensions as a key parameter in their analyses.
Obsessive-compulsive disorder (OCD) is a complex neuropsychiatric illness, with a prevalence of 2–3% in the general population (1). Controlled family studies have identified an elevated risk of OCD in first-degree relatives of around 23% (2, 3), with odds ratios ranging from 11 to 32. Twin studies have also found heritability estimates of OCD to be around 30–60% (4), with higher heritability in pediatric OCD samples. Gene discovery efforts for OCD, especially those using genome-wide approaches have, however, yielded few consistent markers (5). Inability to replicate findings, in genetic and neurobiological research, is commonly attributed to the heterogeneity in the phenotypic presentation of OCD (6). To tackle this heterogeneity, several approaches have been employed to subtype the illness. These include using the age at onset (7–9), degree of insight (10–12), comorbidity profile [e.g. tic disorder (13, 14), depression/anxiety (15–17)], and familiality (18–20). One important approach in this direction has been that of OCD symptom dimensions.
Several factor analytic studies on OCD symptomatology have confirmed the existence of 5 factors (or dimensions, used interchangeably), which are contamination/washing, doubts/checking, symmetry/arranging, unacceptable/taboo thoughts (aggressive, sexual, religious) and hoarding (21, 22). Certain symptom dimensions are found to have specific clinical correlates, for e.g. symmetry/arranging is associated with earlier age at onset & family history (19, 23), greater comorbid depression & anxiety in those with forbidden thoughts (17, 24). Owing to major differences in neurobiology (25), treatment response (26) and other clinical features of patients with hoarding, it is now considered a separate diagnosis (27). Research on how the other symptom dimensions may differ from each other with respect to familial aggregation, genetics, or neurobiology, is still in its early stages (28).
Several studies have examined the familiality of broadly-defined OCD & clinical correlates of the familial form of OCD, but only a few of them have examined the familiality of individual symptom dimensions. Table 1 summarizes these studies. The largest of these studies (31) done in clinical populations analyzed the sample from the Obsessive-Compulsive Collaborative Genetics Study (OCGS), found significant co-occurrence between siblings, of contamination and hoarding dimensions. They also found that gender could play a role in the degree of sharing between the sibling pairs (35). Also reported similar findings with respect to contamination and hoarding dimensions (35). However, the ascertainment of information regarding OC symptoms in relatives was done only through administering a family history screen to the probands. A few other studies have found high concordance particularly for contamination symptoms (30, 33). Two twin studies have shown conflicting results regarding the commonality, i.e. shared vs. specific heritability of symptom dimensions. The smaller of the two studies (33) found commonality between all dimensions with specific heritability for contamination. However, the study done in the TwinsUK sample (34), in a much larger sample found that the best-fit model was one that included common and unique genetic/environmental factors for the symptom dimensions, and hoarding was found to have the lowest loading on the common factor.
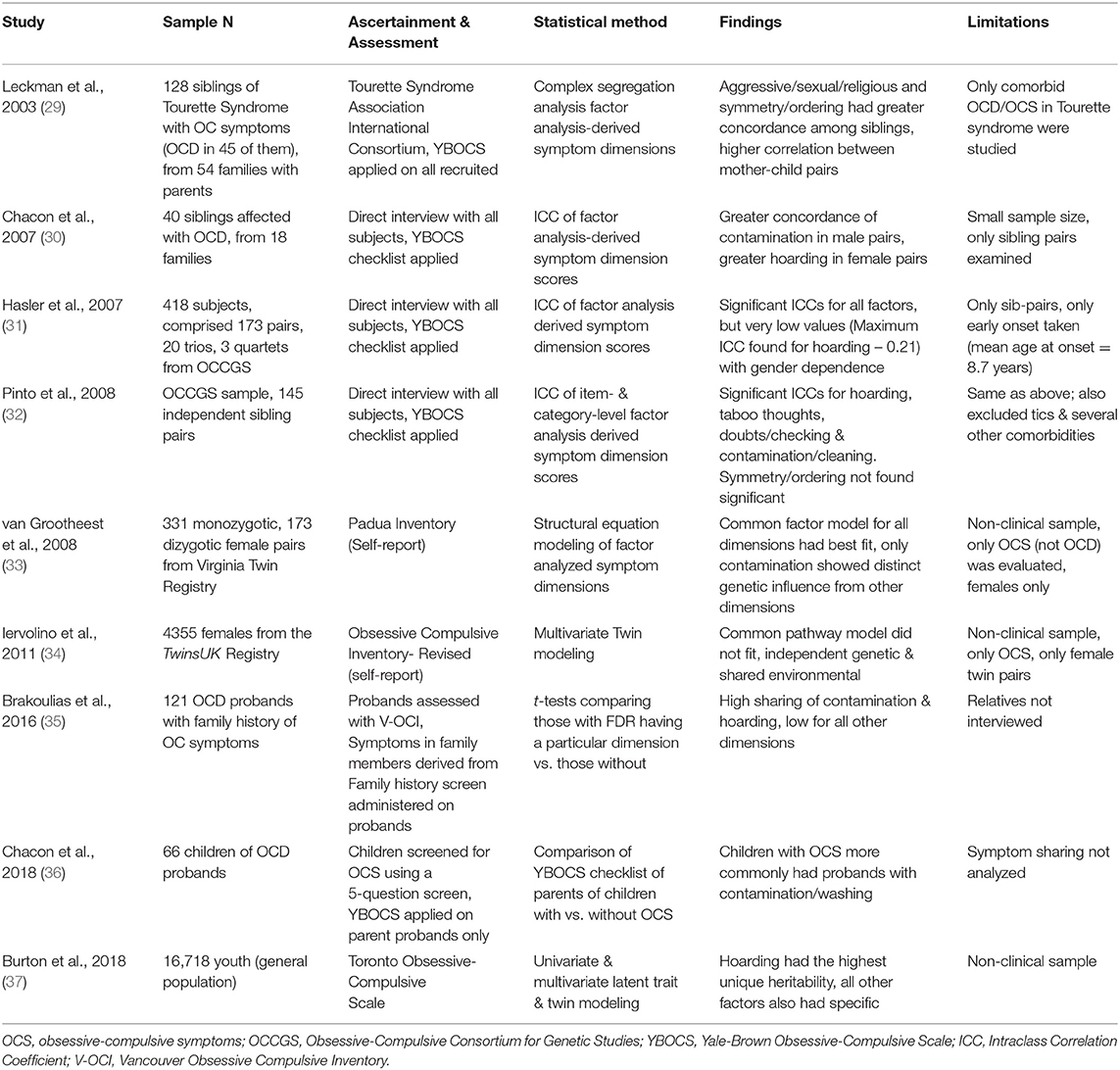
Table 1. Studies that have examined familial sharing of symptom dimensions in Obsessive Compulsive Disorder (OCD).
Overall, the studies have shown heterogeneous findings, which might result from the varying methodology. For example, some of the studies have focused primarily on a particular phenotype, such as comorbid Tourette syndrome, early-onset symptoms, female subjects etc., which may limit the generalizability of the results. Some studies have been conducted on non-clinical analog populations. Other methodological issues include varying methods of clinical assessment and type of relationships with probands studied (some studies have focused on sibling/twin pairs alone).
Hence, from the available research, it is still difficult to conclude whether the individual symptom dimensions in OCD are heritable, or at least have a familial concordance. This is important to study, especially in clinical populations, as familiality is one of the criteria originally proposed by Robins & Guze (38), to establish the validity of a construct. Additionally, there are no studies on the effect of specific relationships, like sex-concordance and parent-of-origin (i.e. imprinting) in the transmission of the OC symptom dimensions.
The aim of this current study was to examine the familial patterns in the co-aggregation of these specific symptom dimensions in a sample of families with multiple first-degree relatives affected with OCD. We hypothesized that all symptom dimensions would show familial concordance and that the degree of concordance may differ based on gender and type of relationship between the affected individuals.
We screened all individuals seeking treatment for OCD at the speciality OCD Clinic of the National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore between July 2016 and December 2019 for the presence of OCD in their first-degree relatives. Individuals were asked about a family history of OCD for the purpose of recruitment into the Accelerator Program for Discovery in Brain Disorders using Stem Cells (ADBS) (39). The study is approved by the Institute Ethics Committee and all participants gave written informed consent to participate in the study.
Out of a total of 1,354 subjects with OCD, 330 (24%) individuals, belonging to 153 families were found to have familial OCD (that is having a first-degree relative, either a parent or a sibling, with OCD). A diagnosis of OCD was ascertained first by interviewing at least three family members, for a family history of OCD and then confirmed later by directly interviewing the affected family members by asking questions from the OCD section of the MINI International Neuropsychiatric Interview (MINI) 7.0.0 (40).
All subjects underwent a detailed clinical assessment using the Mini International Neuropsychiatric Interview (MINI) 7.0.0 (40) and the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) symptom checklist and the severity measure (41, 42). The diagnosis of OCD was confirmed by two clinicians, at least one being a consultant psychiatrist specialized in the diagnosis of OCD. All raters underwent training with inter-rater reliability exercises for the Y-BOCS every 3 months using interview transcripts, which yielded high reliability indices for the total score (Cronbach's alpha = 0.83–0.89), and for all the main symptom categories in the checklist (Cohen's kappa = 0.90–0.96).
Sample size estimation & post-hoc power analysis was carried out (43) using the package ICC Sample Size (44). With the given sample size of 153 families, the minimum ICC value which can be reliably detected with a statistical power of 0.8 is 0.20. As we intend to also look at pairs of specific relationship types within the sample, we extrapolated this power analysis for various sample sizes and ICC estimates, as shown in Supplementary Figure 1. The ICC value increases to 0.24 at N = 100 and to 0.34 at N = 50.
The Item response theory (IRT) has gained popularity as a method to identify latent traits or dimensions within categorical/binary data. It is known to have several advantages over approaches based on classical test theory, such as factor analysis. IRT involves the estimation of certain parameters that helps in understanding the relationship between each item in the scale and the latent trait/dimension(s) that we aim to measure. One of the most commonly used IRT methods is the 2-parameter logistic (2-PL) model, wherein each scale item is gauged based on a “discrimination” parameter and a “difficulty” parameter. The discrimination parameter indicates the degree of specificity of that item that latent trait, and the difficulty parameter indicates the likelihood (or ‘ability’) of a subject endorsing the item. These are represented graphically as item response characteristics curves, with difficulty indicated in the x-axis and discrimination in the y-axis, respectively. Hence, the identification of latent traits/dimensions and their scores, are considered to have greater accuracy with IRT than with the other methods (45).
Using the irt.fa function in the “psych” package in R (46), multidimensional item response theory analysis (MIRT) with the 2-parameter logistic (2-PL) model was carried out, with the main categories of the Y-BOCS checklist items. From the “Miscellaneous” categories of the obsession and compulsion checklists, only those items which were present in more than 10% of the sample were included. As part of the MIRT, exploratory factor analysis was done using the “generalized least squares” method, from a tetrachoric correlation matrix of the Y-BOCS symptom checklist items. An orthogonal rotation using the “varimax” method was employed. The resultant loadings from the factor analysis are transformed to item discrimination parameters. The “tau” parameter from the tetrachoric correlations, combined with the item factor loading are then used to estimate item difficulties. As the number of factors to be extracted can be pre-specified, we ran the same analysis starting from 2-factor up to a 6-factor model. We compared the fit indices (Bayesian Information Criteria, Comparative Fit Index & Root Mean Square of Approximation) of each of these models. The final model was chosen after considering both the fit indices as well as concordance with the existing literature on symptom dimensions from the factor-analytic studies on OCD (47). Using the parameter estimates for discrimination and difficulty IRT-based scores were derived for each individual subject, to take up for the familiality analysis.
Using the “lme4” (48) and “performance” (49) packages in R, we used a linear mixed-effects model to compute the intra-class correlation coefficient (ICC) values for each symptom dimension. The ICC has been used in several other studies (31) to measure the level of sharing of phenotypic traits between family members, and was originally developed for this purpose (50).
Sex and age at onset were included as fixed-effect covariates, in order to regress out their influences on phenotypic expression. Several previous studies have indicated that symptom dimensions vary based on sex (51–53) and age at onset of illness (9, 54). The “Family ID” was included as a random-effects variable, and the ICC was calculated as the ratio of the residual variance between families (or pairs) to the total variance between all subjects (55). We report “adjusted-ICC” values in the output, due to the non-Gaussian distribution of the residuals (56). Similar analyses were carried out separately to estimate ICCs for each type of relationship (e.g. parent-offspring, sibling-sibling, sex-concordant and sex-discordant). Sex was not added as a covariate while analyzing the gender-concordant pairs, but the age at onset was included in all of them. In order to estimate standard errors and 95% confidence intervals for the ICCs, a bootstrapping procedure, run for 10,000 iterations, was employed for each of the mixed-effect models.
Figure 1 shows four representative pedigrees from our sample, along with the principal symptom dimension of the affected individuals in the family. The sample consisted of 132 families with two affected members, 19 families with three affected members, one family with three affected members and one family with five affected members. There were no families with concordant twins (monozygotic or dizygotic) in the sample.
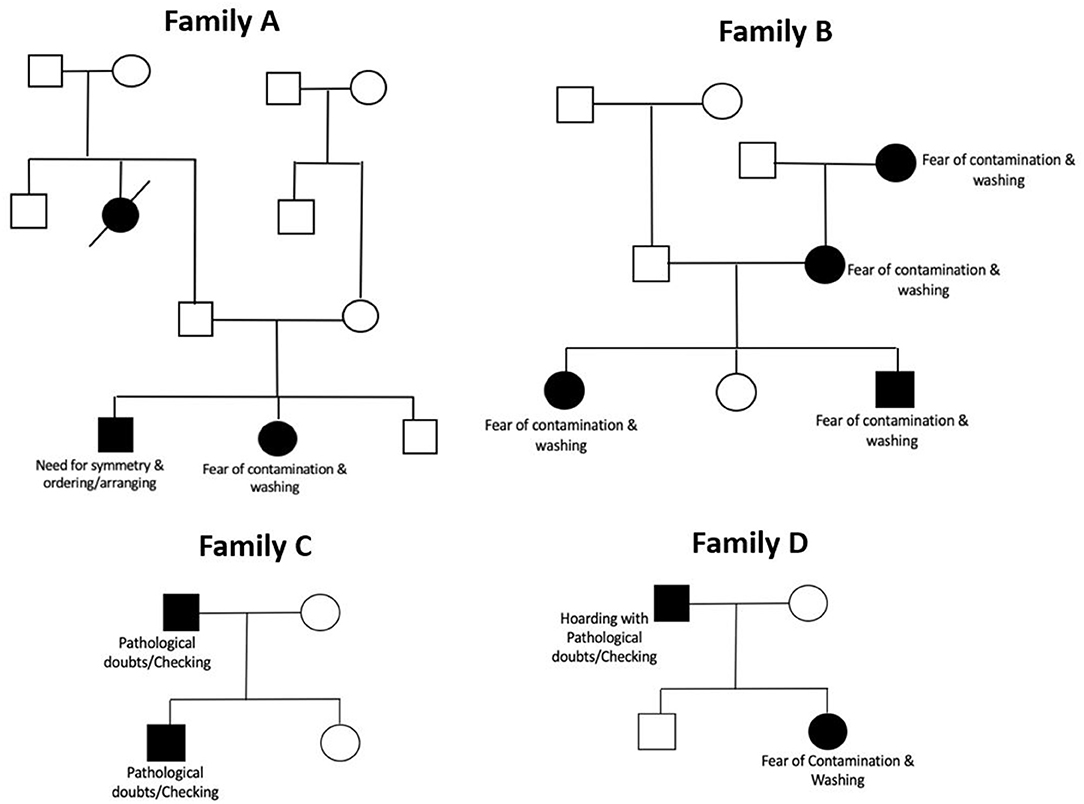
Figure 1. Representative pedigrees from the sample showing principal symptoms in affected member. The pedigrees have been illustrated using standardized pedigree nomenclature (57). Boxes represent male sex, and circles represent female sex. Black shading within a box/circle indicates the disease affectation status (OCD), while an unshaded box/circle indicates that the individual is unaffected. Diagonal line through a square/circle indicates deceased status.
Table 2 shows the clinical and sociodemographic details of the total sample. Juvenile-onset OCD (age at onset before 18 years) was seen in 112 (34%) of the sample. Supplementary Figures 1, 2 also show the differences in the age at onset of OCD by generation, and be sex. Additionally, a majority of the sample (84%) had at least one lifetime comorbidity, as assessed using the MINI.
The results of the item-response theory analysis are shown in Figure 2. As shown in the figure, the four factors were as follows: Factor 1 included mental compulsions along with sexual, religious and aggressive obsessions, Factor 2 included pathological doubts with checking, repeating and counting compulsions, Factor 3 included need for symmetry obsessions along with ordering/arranging compulsions, Factor 4 was fear of contamination with cleaning/washing compulsions. The YBOCS checklist item of “somatic” obsessions did not appear to have significant loading with any of the factors. This model was found to have the following fit indices: the cumulative variance explained by the factor analysis step was 68%, the comparative fit index was 0.95, and the root mean square error of approximation was 0.076 (90% CI 0.067–0.085), all of which indicate an acceptable level fit for the model.
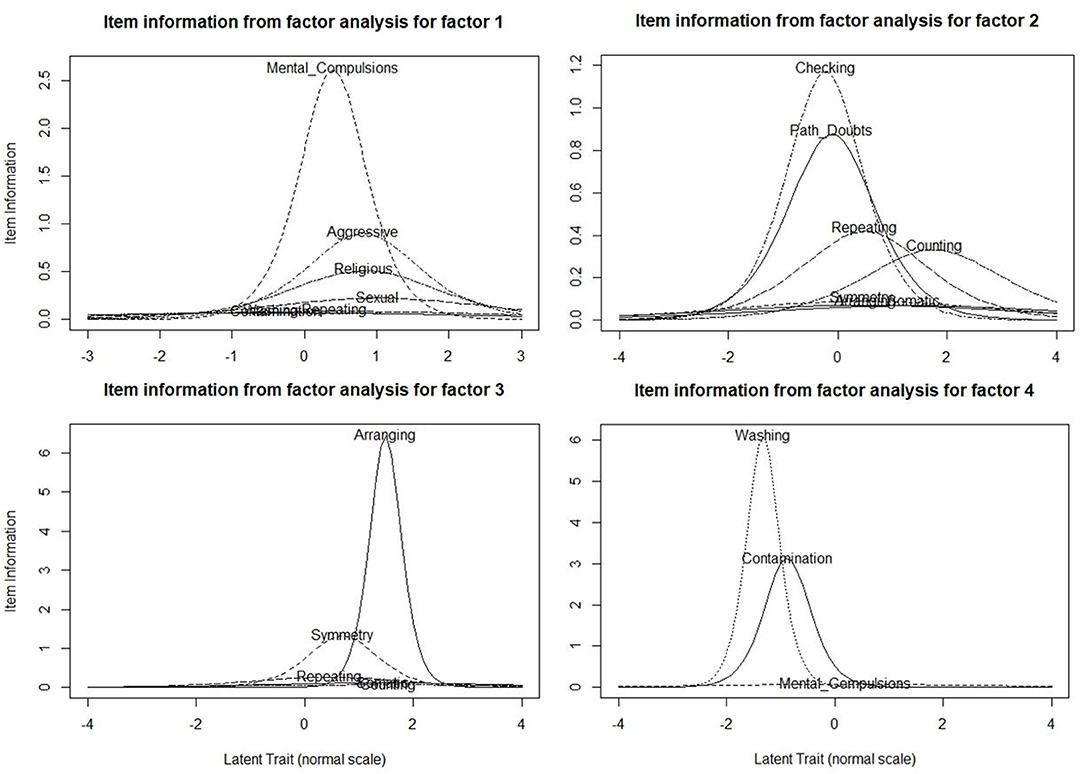
Figure 2. Item Information Curve (IIC) Plots of the Multi-dimensional Item Response Theory analysis done with the Yale-Brown Obsessive-Compulsive Checklist Items (N = 330). These plots represent the item information curves (IICs) for the items that are loaded within each factor. The x-axes represent the “difficulty” parameter (lesser “difficulty” means greater likelihood of the subject endorsing this item), and the y-axes represent the “discrimination” parameter. IICs with high peaks and relatively narrow spread indicate high discrimination, or high specificity of the item for that particular factor.
Figure 3 shows the results of the ICC values derived from the mixed-effect modeling, for the overall sample and for each specific relationship type (see Supplementary Table 1 for the actual ICC values). Only Factor 2 (Doubts/Checking), Factor 3 (Symmetry/Arranging) and Factor 4 (Contamination/Washing) were found to have significant ICC values when all members within families were included, regardless of the gender or type of relationship. The highest ICC was seen for symmetry/ordering (0.41), followed by contamination/washing (0.29) and then in pathological doubts/checking (0.27) dimension. The ICC values in the sex-concordant pairs were higher than those in the sex-discordant pairs for every factor. The ICC values and their 95% confidence intervals do not appear to deviate markedly from each other when parent-offspring and sibling pairs were looked into specifically. Significant ICC values were found in every relationship type for symmetry/ordering and contamination/washing dimension. ICC values were not found to be significant in any of the specific relationship types for Factor 1 (“Forbidden thoughts”), and in the gender-discordant pairs for doubts/checking dimension. We also conducted post-hoc analyses on a subset of families having multiple members (≥2) having OCD with comorbid depression (either Major Depressive Disorder or Dysthymia). We found similar results even in this subset; significant ICC values were found for all factors except Factor 1 (“forbidden thoughts”) (Supplementary Table 2).
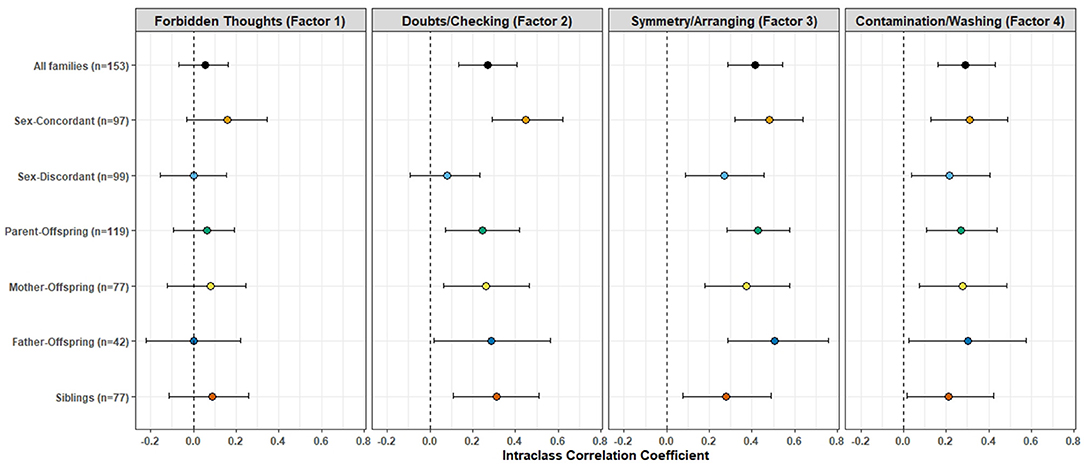
Figure 3. Intra-class Correlation Coefficients (ICC) of the factor analysis-derived symptom dimension scores between first-degree relative pairs. The colored dots represent the ICC value, the error bars represent their 95% confidence intervals, for each symptom dimension and across different relationship types. See Supplementary Table 1 for the source data.
To our knowledge, this is the first study to report familial aggregation of symptom dimensions among first-degree relatives affected with OCD in a large sample of multiplex OCD families. The study also analyzed how the sharing of symptom dimensions might be influenced by the type of relationships between the affected members, and gender.
We originally hypothesized that all symptom dimensions would show strong familial concordance. The main finding of our study showed that only three of the symptom dimensions, which include “symmetry/arranging,” “contamination/washing” and “doubts/checking” had significant familial concordance. The “forbidden thoughts” dimension, which includes aggressive, sexual and religious obsessions along with mental compulsions, did not show significant concordance. Also, higher degrees of concordance for all symptom dimensions was found when the affected members within a family were of the same sex, in contrast to when they were of the opposite sex. There were no major differences between parent-offspring pairs (both mother-offspring as well as father-offspring) and sibling pairs.
Similar findings of high familial concordance for the contamination/washing dimensions have been demonstrated in two previous studies (31, 35). A previous twin study that analyzed the sample from the Virginia Twin Cohort (33) found contamination to have a distinct genetic heritability, all other dimensions were better explained by a latent common factor model. In contrast, a subsequent study from the TwinsUK registry with a much larger sample size found only the hoarding dimension to have distinct genetic influences (34). However, as these studies were done in non-clinical populations, it is not clear how these self-reported “OC-like” behaviors may differ from symptoms in OCD. The heritability of the contamination/washing dimension hence needs to be examined further.
Studies that have compared familial and sporadic OCD have indicated the high occurrence of symmetry/arranging dimension in familial OCD samples (18, 23, 58). This was also shown when comparing early-onset OCD to adult-onset and tic-related OCD to non-tic related OCD, showing higher rates of symmetry/arranging (14). A recent candidate gene study from our center, evaluating a polymorphism in the DRD4 gene found a specific association with the symmetry/arranging dimension (59). All of these indicate that there may be a higher genetic contribution associated with this factor.
The “forbidden thoughts” factor was found to have the least degree of familial concordance, even after accounting for comorbid depression. The low familiality of this dimension, especially between siblings, is in contrast with the findings of the OCD Collaborative Genetics Study (OCGS) (31), which reported the highest concordance for this factor among sibling pairs. Their sample consisted of early-onset OCD with predominantly females (70%), and were Caucasians. It may hence be important to examine this separately in early and late onset cohorts, and further across different ethnicities as well.
Previous factor analysis studies in OCD have shown discrepant findings with respect to aggressive/harm & checking- related symptoms. While some studies have shown checking compulsions to load with aggressive obsessions (60, 61), many others (32, 62, 63) including several from our center (12, 64, 65) found doubts & checking to load separately from aggressive obsessions (which loads with forbidden/taboo thoughts). In the current study “doubts” were coded separately from aggressive obsessions, which could have resulted in a factor structure different from the OCGS study. This could have thus influenced the findings with respect to the familiality of the “forbidden thoughts” dimension.
There are several strengths to this study. First, the study is unique in that the sample included multiplex OCD families of OCD which is different from the previous studies that have looked at only sibling pairs or one study which looked only at parent-offspring pairs. This helped in examining the patterns between specific relationship types in the sample. All participants were evaluated by interviewing them directly, and the assessments were carried out by trained raters with high inter-rater reliability. This is a key advantage over several of the studies, which used self-report tools or assessed only one of the subjects within the family (see Table 1 for details).
The sample was ascertained from a tertiary-care speciality OCD clinic, and information was collected about all first and second-degree relatives in the families. Despite this, there was an uneven sex distribution in the parental generation. Although the overall sex ratio was even (nearly 1:1, as shown in Table 2), in the parent-child pairs, the ratio of number of female: male parents was 77:42 (as depicted in Figure 3). One might also speculate that there may be a “cohort effect.” That is, the males in the older generation who had the phenotype of familial OCD with an earlier age at onset of symptoms, greater comorbidities and possibly poorer overall outcome, may have had lesser fecundity and hence were poorly represented in our sample. Females, on the other hand, have a later onset of OCD, and are also commonly known to have onset of OCD after the first child-birth (66). This phenomenon, of a “cohort effect” has been reported previously in longitudinal cohort studies of schizophrenia (67).
Despite the relatively large overall sample size (330 subjects from 153 families), the power analysis indicated that the minimum ICC that could be reliably estimated was 0.2 with the total sample, and this increased gradually for smaller sample size. Hence, the results of the sub-analyses done for the specific relationship pairs need to be interpreted with caution.
Another limitation of our study was the use of a checklist for assessing OCD symptoms, which is categorical/dichotomous measure, hence the factor scores that were derived for each subject may not indicate a true “severity” of that particular dimension for the subject. This could have been overcome by the use of the dimensional YBOCS (D-YBOCS), which gives a separate severity score ranging from 0 to 15 across each symptom dimension (68). However, the D-YBOCS is used only as a cross-sectional measure and its reliability in measuring the lifetime severity of these symptom dimensions is uncertain. Unaffected FDRs were not included in the analysis as very few of them reported symptoms that could be tapped by the YBOCS checklist. Possibly, the additional use of either the D-YBOCS or a self-reported measure like the Obsessive-Compulsive Inventory – Revised (OCI-R) (69) or the Padua Inventory (70) may have been more sensitive to pick up sub-threshold OC symptoms and symptoms with forbidden/taboo content.
In addition to the above limitation, it is difficult to draw inferences about genetic mechanisms such as imprinting/silencing due to confounding environmental/psychosocial influence. One might still argue that these could be behaviors that are “learned” or “taught” between family members. Family accommodation is one such factor that can play a significant role in the sharing of symptoms. Accommodation refers to responses of the patient's family (typically parents, spouse or even children) to his/her obsessive-compulsive symptoms, and includes behaviors such as directly participating in compulsions, or helping to avoid triggers of obsessions or distress (71). Investigating if such accommodative behaviors may have preceded the onset of OCD in the affected FDRs in multiplex OCD families would help understand this further. However, large-scale studies of OC symptoms in non-clinical twin samples have found that the sharing of symptoms between twin pairs is more likely to be due to genetic than environmental factors, with heritability estimates of around 60–100% (34, 37).
We conclude that the symptom dimensions, particularly checking, washing & arranging have a robust familial basis. Efforts are being made to validate symptom dimensions by identifying each of their unique clinical and neurobiological correlates. High familiality of these specific symptom dimensions further emphasizes the need for such an approach, in order to deconstruct the complex phenotype of OCD. Stratifying patients into such homogeneous sub-groups based on symptom dimensions may substantially improve statistical power and facilitate discovery of reproducible genetic and imaging signatures of the illness. Further research into the clinical utility of these symptom dimensions, such as response to specific treatments is also warranted and likely to have an important role in developing “personalized” treatment options for OCD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institute Ethics Committee, National Institute of Mental Health&Neuro Sciences (NIMHANS), Bangalore. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
SB, FA, AK, MB, and DI were involved in the data collection for this study. SB, VS, RK, and BH were involved in the statistical analysis. SM and MM critically reviewed the manuscript and provided inputs to improve the analysis & interpretation of the study results. SB, SJ, BV, YR, JN, and SA contributed to study planning, conceptualization, and manuscript preparation & review. All authors contributed to the article and approved the submitted version.
This study was funded by the Accelerator Program for Discovery in Brain disorders using Stem cells (ADBS) of the Department of Biotechnology, Government of India (BT/PR17316/MED/31/326/2015).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.651196/full#supplementary-material
1. Fontenelle LF, Mendlowicz MV, Versiani M. The descriptive epidemiology of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2006) 30:327–37. doi: 10.1016/j.pnpbp.2005.11.001
2. Nestadt G, Samuels J, Riddle M, Bienvenu OJ, Liang KY, LaBuda M, et al. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. (2000) 57:358–63. doi: 10.1001/archpsyc.57.4.358
3. do Rosario-Campos MC, Leckman JF, Curi M, Quatrano S, Katsovitch L, Miguel EC, et al. A family study of early-onset obsessive-compulsive disorder. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. (2005) 136B:92–7. doi: 10.1002/ajmg.b.30149
4. van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive-compulsive disorder: a review. Twin Res Hum Genet Off J Int Soc Twin Stud. (2005) 8:450–8. doi: 10.1375/twin.8.5.450
5. Arnold PD, Askland KD, Barlassina C, Bellodi L, Bienvenu OJ, Black D, et al. International obsessive compulsive disorder foundation genetics collaborative (IOCDF-GC) and OCD collaborative genetics association studies (OCGAS). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. (2018) 23:1181–8. doi: 10.1038/mp.2017.154
6. Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, et al. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol Psychiatry. (2005) 10:258–75. doi: 10.1038/sj.mp.4001617
7. Jaisoorya TS, Janardhan Reddy YC, Srinath S. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder?–Findings from an Indian study. Eur Child Adolesc Psychiatry. (2003) 12:290–7. doi: 10.1007/s00787-003-0342-2
8. Hemmings SMJ, Kinnear CJ, Lochner C, Niehaus DJH, Knowles JA, Moolman-Smook JC, et al. Early- versus late-onset obsessive-compulsive disorder: investigating genetic and clinical correlates. Psychiatry Res. (2004) 128:175–82. doi: 10.1016/j.psychres.2004.05.007
9. Narayanaswamy JC, Viswanath B, Veshnal Cherian A, Bada Math S, Kandavel T, Janardhan Reddy YC. Impact of age of onset of illness on clinical phenotype in OCD. Psychiatry Res. (2012) 200:554–9. doi: 10.1016/j.psychres.2012.03.037
10. Eisen JL, Phillips KA, Coles ME, Rasmussen SA. Insight in obsessive compulsive disorder and body dysmorphic disorder. Compr Psychiatry. (2004) 45:10–15. doi: 10.1016/j.comppsych.2003.09.010
11. Ravi Kishore V, Samar R, Janardhan Reddy YC, Chandrasekhar CR, Thennarasu K. Clinical characteristics and treatment response in poor and good insight obsessive-compulsive disorder. Eur Psychiatry J Assoc Eur Psychiatr. (2004) 19:202–8. doi: 10.1016/j.eurpsy.2003.12.005
12. Cherian AV, Narayanaswamy JC, Srinivasaraju R, Viswanath B, Math SB, Kandavel T, et al. Does insight have specific correlation with symptom dimensions in OCD? J Affect Disord. (2012) 138:352–9. doi: 10.1016/j.jad.2012.01.017
13. Leckman JF, Grice DE, Barr LC, de Vries AL, Martin C, Cohen DJ, et al. Tic-related vs. non-tic-related obsessive compulsive disorder. Anxiety. (1994) 1:208–15.
14. Jaisoorya TS, Reddy YCJ, Srinath S, Thennarasu K. Obsessive-compulsive disorder with and without tic disorder: a comparative study from India. CNS Spectr. (2008) 13:705–11. doi: 10.1017/S1092852900013791
15. Overbeek T, Schruers K, Vermetten E, Griez E. Comorbidity of obsessive-compulsive disorder and depression: prevalence, symptom severity, and treatment effect. J Clin Psychiatry. (2002) 63:1106–12. doi: 10.4088/JCP.v63n1204
16. Hasler G, LaSalle-Ricci VH, Ronquillo JG, Crawley SA, Cochran LW, Kazuba D, et al. Obsessive-compulsive disorder symptom dimensions show specific relationships to psychiatric comorbidity. Psychiatry Res. (2005) 135:121–32. doi: 10.1016/j.psychres.2005.03.003
17. Viswanath B, Narayanaswamy JC, Rajkumar RP, Cherian AV, Kandavel T, Math SB, et al. Impact of depressive and anxiety disorder comorbidity on the clinical expression of obsessive-compulsive disorder. Compr Psychiatry. (2012) 53:775–82. doi: 10.1016/j.comppsych.2011.10.008
18. Hanna GL, Fischer DJ, Chadha KR, Himle JA, Van Etten M. Familial and sporadic subtypes of early-onset obsessive-compulsive disorder. Biol Psychiatry. (2005) 57:895–900. doi: 10.1016/j.biopsych.2004.12.022
19. Viswanath B, Narayanaswamy JC, Cherian AV, Reddy YCJ, Math SB. Is familial obsessive-compulsive disorder different from sporadic obsessive-compulsive disorder? A comparison of clinical characteristics, comorbidity and treatment response. Psychopathology. (2011) 44:83–9. doi: 10.1159/000317776
20. Arumugham SS, Cherian AV, Baruah U, Viswanath B, Narayanaswamy JC, Math SB, et al. Comparison of clinical characteristics of familial and sporadic obsessive-compulsive disorder. Compr Psychiatry. (2014) 55:1520–5. doi: 10.1016/j.comppsych.2014.07.006
21. Bloch MH, Landeros-Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry. (2008) 165:1532–42. doi: 10.1176/appi.ajp.2008.08020320
22. Brakoulias V. Diagnostic subtyping of obsessive-compulsive disorder: have we got it all wrong? Aust N Z J Psychiatry. (2013) 47:23–5. doi: 10.1177/0004867412455851
23. Cullen B, Brown CH, Riddle MA, Grados M, Bienvenu OJ, Hoehn-Saric R, et al. Factor analysis of the yale-brown obsessive compulsive scale in a family study of obsessive-compulsive disorder. Depress Anxiety. (2007) 24:130–8. doi: 10.1002/da.20204
24. Prabhu L, Cherian AV, Viswanath B, Kandavel T, Bada Math S, Janardhan Reddy YC. Symptom dimensions in OCD and their association with clinical characteristics and comorbid disorders. J Obsessive-Compuls Relat Disord. (2013) 2:14–21. doi: 10.1016/j.jocrd.2012.10.002
25. An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, et al. To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Mol Psychiatry. (2009) 14:318–31. doi: 10.1038/sj.mp.4002129
26. Bloch MH, Bartley CA, Zipperer L, Jakubovski E, Landeros-Weisenberger A, Pittenger C, et al. Meta-analysis: hoarding symptoms associated with poor treatment outcome in obsessive-compulsive disorder. Mol Psychiatry. (2014) 19:1025–30. doi: 10.1038/mp.2014.50
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. St. Arlington, TX; VA (2013).
28. Thorsen AL, Kvale G, Hansen B, van den Heuvel OA. Symptom dimensions in obsessive-compulsive disorder as predictors of neurobiology and treatment response. Curr Treat Options Psychiatry. (2018) 5:182–94. doi: 10.1007/s40501-018-0142-4
29. Leckman JF, Pauls DL, Zhang H, Rosario-Campos MC, Katsovich L, Kidd KK, et al. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. (2003) 116B:60–8. doi: 10.1002/ajmg.b.10001
30. Chacon P, Rosario-Campos MC, Pauls DL, Hounie AG, Curi M, Akkerman F, et al. Obsessive-compulsive symptoms in sibling pairs concordant for obsessive-compulsive disorder. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. (2007) 144B:551–5. doi: 10.1002/ajmg.b.30457
31. Hasler G, Pinto A, Greenberg BD, Samuels J, Fyer AJ, Pauls D, et al. Familiality of factor analysis-derived YBOCS dimensions in OCD-affected sibling pairs from the OCD collaborative genetics study. Biol Psychiatry. (2007) 61:617–25. doi: 10.1016/j.biopsych.2006.05.040
32. Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, Samuels JF, Murphy DL, et al. Further development of YBOCS dimensions in the OCD collaborative genetics study: symptoms vs. categories. Psychiatry Res. (2008) 160:83–93. doi: 10.1016/j.psychres.2007.07.010
33. van Grootheest DS, Boomsma DI, Hettema JM, Kendler KS. Heritability of obsessive-compulsive symptom dimensions. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. (2008) 147B:473–8. doi: 10.1002/ajmg.b.30622
34. Iervolino AC, Rijsdijk FV, Cherkas L, Fullana MA, Mataix-Cols D. A multivariate twin study of obsessive-compulsive symptom dimensions. Arch Gen Psychiatry. (2011) 68:637–44. doi: 10.1001/archgenpsychiatry.2011.54
35. Brakoulias V, Starcevic V, Martin A, Berle D, Milicevic D, Viswasam K. The familiality of specific symptoms of obsessive-compulsive disorder. Psychiatry Res. (2016) 239:315–9. doi: 10.1016/j.psychres.2016.03.047
36. Chacon P, Bernardes E, Faggian L, Batistuzzo M, Moriyama T, Miguel EC, et al. Obsessive-compulsive symptoms in children with first degree relatives diagnosed with obsessive-compulsive disorder. Rev Bras Psiquiatr São Paulo Braz 1999. (2018) 40:388–93. doi: 10.1590/1516-4446-2017-2321
37. Burton CL, Park LS, Corfield EC, Forget-Dubois N, Dupuis A, Sinopoli VM, et al. Heritability of obsessive-compulsive trait dimensions in youth from the general population. Transl Psychiatry. (2018) 8:191. doi: 10.1038/s41398-018-0249-9
38. Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. (1970) 126:983–7. doi: 10.1176/ajp.126.7.983
39. Viswanath B, Rao NP, Narayanaswamy JC, Sivakumar PT, Kandasamy A, Kesavan M, et al. Discovery biology of neuropsychiatric syndromes (DBNS): a center for integrating clinical medicine and basic science. BMC Psychiatry. (2018) 18:106. doi: 10.1186/s12888-018-1674-2
40. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-International neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59(Suppl. 20):22–33; quiz 34–57.
41. Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The yale-brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry. (1989) 46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007
42. Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The yale-brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry. (1989) 46:1012–6. doi: 10.1001/archpsyc.1989.01810110054008
43. Zou GY. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat Med. (2012) 31:3972–81. doi: 10.1002/sim.5466
44. Rathbone A, Shaw S, Kumbhare D. ICC Sample Size: Calculation of Sample Size and Power for ICC. (2015). Available online at: https://cran.r-project.org/package=ICC.Sample.Size (accessed August 1, 2020).
45. Streiner DL. Measure for measure: new developments in measurement and item response theory. Can J Psychiatry Rev Can Psychiatr. (2010) 55:180–6. doi: 10.1177/070674371005500310
46. Revelle W. Psych: Procedures for Psychological, Psychometric, and Personality Research. Evanston: Northwestern University (2020).
47. Cameron DH, Streiner DL, Summerfeldt LJ, Rowa K, McKinnon MC, McCabe RE. A comparison of cluster and factor analytic techniques for identifying symptom-based dimensions of obsessive-compulsive disorder. Psychiatry Res. (2019) 278:86–96. doi: 10.1016/j.psychres.2019.05.040
48. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. (2015) 67:1–48. doi: 10.18637/jss.v067.i01
49. Lüdecke D, Makowski D, Waggoner P, Patil I. Performance: assessment of regression models performance. CRAN. (2020). doi: 10.5281/zenodo.3952174
50. Fisher RA. Statistical Methods for Research Workers. In: Kotz S, Johnson NL, editors. Breakthroughs in Statistics. New York, NY: Springer New York (1992). p. 66–70.
51. Torresan RC, Ramos-Cerqueira AT de A, de Mathis MA, Diniz JB, Ferrão YA, Miguel EC, et al. Sex differences in the phenotypic expression of obsessive-compulsive disorder: an exploratory study from Brazil. Compr Psychiatry. (2009) 50:63–9. doi: 10.1016/j.comppsych.2008.05.005
52. Raines AM, Oglesby ME, Allan NP, Mathes BM, Sutton CA, Schmidt NB. Examining the role of sex differences in obsessive-compulsive symptom dimensions. Psychiatry Res. (2018) 259:265–9. doi: 10.1016/j.psychres.2017.10.038
53. Tripathi A, Avasthi A, Grover S, Sharma E, Lakdawala BM, Thirunavukarasu M, et al. Gender differences in obsessive-compulsive disorder: findings from a multicentric study from India. Asian J Psychiatry. (2018) 37:3–9. doi: 10.1016/j.ajp.2018.07.022
54. Kichuk SA, Torres AR, Fontenelle LF, Rosário MC, Shavitt RG, Miguel EC, et al. Symptom dimensions are associated with age of onset and clinical course of obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 44:233–9. doi: 10.1016/j.pnpbp.2013.02.003
55. Nakagawa S, Johnson PCD, Schielzeth H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface. (2017) 14:20170213. doi: 10.1098/rsif.2017.0213
56. Johnson PC. Extension of Nakagawa & Schielzeth's R2GLMM to random slopes models. Methods Ecol Evol. (2014) 5:944–6. doi: 10.1111/2041-210X.12225
57. Bennett RL, French KS, Resta RG, Doyle DL. Standardized human pedigree nomenclature: update and assessment of the recommendations of the national society of genetic counselors. J Genet Couns. (2008) 17:424–33. doi: 10.1007/s10897-008-9169-9
58. Alsobrook JP II, Leckman JF, Goodman WK, Rasmussen SA, Pauls DL. Segregation analysis of obsessive-compulsive disorder using symptom-based factor scores. Am J Med Genet. (1999) 88:669–75. doi: 10.1002/(SICI)1096-8628(19991215)88:6&<669::AID-AJMG17&>3.0.CO;2-N
59. Taj MJRJ, Viswanath B, Purushottam M, Kandavel T, Janardhan Reddy YC, Jain S. DRD4 gene and obsessive compulsive disorder: do symptom dimensions have specific genetic correlates? Prog Neuropsychopharmacol Biol Psychiatry. (2013) 41:18–23. doi: 10.1016/j.pnpbp.2012.10.023
60. Matsunaga H, Maebayashi K, Hayashida K, Okino K, Matsui T, Iketani T, et al. Symptom structure in Japanese patients with obsessive-compulsive disorder. Am J Psychiatry. (2008) 165:251–3. doi: 10.1176/appi.ajp.2007.07020340
61. Mataix-Cols D, Rauch SL, Manzo PA, Jenike MA, Baer L. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry. (1999) 156:1409–16.
62. Pinto A, Eisen JL, Mancebo MC, Greenberg BD, Stout RL, Rasmussen SA. Taboo thoughts and doubt/checking: a refinement of the factor structure for obsessive-compulsive disorder symptoms. Psychiatry Res. (2007) 151:255–8. doi: 10.1016/j.psychres.2006.09.005
63. Katerberg H, Delucchi KL, Stewart SE, Lochner C, Denys DAJP, Stack DE, et al. Symptom dimensions in OCD: item-level factor analysis and heritability estimates. Behav Genet. (2010) 40:505–17. doi: 10.1007/s10519-010-9339-z
64. Balachander S, Bajaj A, Hazari N, Kumar A, Anand N, Manjula M, et al. Long-term outcomes of intensive inpatient care for severe, resistant obsessive-compulsive disorder: résultats à long terme de soins intensifs à des patients hospitalisés pour un trouble obsessionnel-compulsif grave et résistant. Can J Psychiatry Rev Can Psychiatr. (2020) 65:779–89. doi: 10.1177/0706743720927830
65. Kashyap H, Kumar JK, Kandavel T, Reddy YCJ. Relationships between neuropsychological variables and factor-analysed symptom dimensions in obsessive compulsive disorder. Psychiatry Res. (2017) 249:58–64. doi: 10.1016/j.psychres.2016.12.044
66. Forray A, Focseneanu M, Pittman B, McDougle CJ, Epperson CN. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. (2010) 71:1061–1068. doi: 10.4088/JCP.09m05381blu
67. Di Maggio C, Martinez M, Ménard JF, Petit M, Thibaut F. Evidence of a cohort effect for age at onset of schizophrenia. Am J Psychiatry. (2001) 158:489–92. doi: 10.1176/appi.ajp.158.3.489
68. Rosario-Campos MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, et al. The dimensional yale-brown obsessive-compulsive scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol Psychiatry. (2006) 11:495–504. doi: 10.1038/sj.mp.4001798
69. Wootton BM, Diefenbach GJ, Bragdon LB, Steketee G, Frost RO, Tolin DF. A contemporary psychometric evaluation of the obsessive compulsive inventory-Revised (OCI-R). Psychol Assess. (2015) 27:874–82. doi: 10.1037/pas0000075
70. Burns GL, Keortge SG, Formea GM, Sternberger LG. Revision of the padua inventory of obsessive compulsive disorder symptoms: distinctions between worry, obsessions, and compulsions. Behav Res Ther. (1996) 34:163–73. doi: 10.1016/0005-7967(95)00035-6
Keywords: OCD, obsessive-compulsive, familial, heritability, symptomatology, symptom dimensions, dimensions
Citation: Balachander S, Meier S, Matthiesen M, Ali F, Kannampuzha AJ, Bhattacharya M, Kumar Nadella R, Sreeraj VS, Ithal D, Holla B, Narayanaswamy JC, Arumugham SS, Jain S, Reddy YCJ and Viswanath B (2021) Are There Familial Patterns of Symptom Dimensions in Obsessive-Compulsive Disorder? Front. Psychiatry 12:651196. doi: 10.3389/fpsyt.2021.651196
Received: 08 January 2021; Accepted: 11 March 2021;
Published: 20 April 2021.
Edited by:
Yuan-Pang Wang, University of São Paulo, BrazilReviewed by:
Beatrice Benatti, Luigi Sacco Hospital, ItalyCopyright © 2021 Balachander, Meier, Matthiesen, Ali, Kannampuzha, Bhattacharya, Kumar Nadella, Sreeraj, Ithal, Holla, Narayanaswamy, Arumugham, Jain, Reddy and Viswanath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Srinivas Balachander, c3Jpbml2YXNiYWxhY2hhbmRlckBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.