- 1Laboratory of Behavioral Neuroscience, Graduate Program in Health Sciences, University of Southern Santa Catarina, University of Southern Santa Catarina (UNISUL), Tubarão, Brazil
- 2Post Graduate Program in Pharmacology, Department of Pharmacology, Federal University of Santa Catarina, Federal University of Santa Catarina (UFSC), Florianópolis, Brazil
- 3Centro Brasileiro de Informações Sobre Drogas Psicotrópicas (CEBRID), Department of Preventive Medicine, Federal University of São Paulo, UNIFESP, São Paulo, Brazil
Although cannabis has been known for ages as an “alternative medicine” to provide relief from seizures, pain, anxiety, and inflammation, there had always been a limited scientific review to prove and establish its use in clinics. Early studies carried out by Carlini's group in Brazil suggested that cannabidiol (CBD), a non-psychotropic phytocannabinoid present in Cannabis sativa, has anticonvulsant properties in animal models and reduced seizure frequency in limited human trials. Over the past few years, the potential use of cannabis extract in refractory epilepsy, including childhood epilepsies such as Dravet's syndrome and Lennox-Gastaut Syndrome, has opened a new era of treating epileptic patients. Thus, a considerable number of pre-clinical and clinical studies have provided strong evidence that phytocannabinoids has anticonvulsant properties, as well as being promising in the treatment of different neuropsychiatric disorders, such as depression, anxiety, post-traumatic stress disorder (PTSD), addiction, neurodegenerative disorders and autism spectrum disorder (ASD). Given the advances of cannabinoids, especially CBD, in the treatment of epilepsy, would the same expectation regarding the treatment of other neuropsychiatric disorders be possible? The present review highlights some contributions from Brazilian researchers and other studies reported elsewhere on the history, pre-clinical and clinical data underlying the use of cannabinoids for the already widespread treatment of refractory epilepsies and the possibility of use in the treatment of some neuropsychiatric disorders.
Introduction
Cannabis sativa, a plant popularly known for giving rise to marijuana, has in its composition more than 140 compounds called phytocannabinoids. In addition to the phytocannabinoids present in the plant, endocannabinoids (eCB) are produced endogenously through physiological stimulation and cannabinoids of synthetic origin, all called cannabinoids. Both together and isolated, cannabinoids have a wide variety of effects on the nervous system, making these compounds promising psychopharmacological alternatives in treating many neuropsychiatric disorders (1–3). Among the possibilities for pharmacotherapeutic use, stand-depression, anxiety, post-traumatic stress disorder (PTSD), addiction, neurodegenerative disorders, autism spectrum disorder (ASD), and especially refractory epilepsy, among others (4–12).
Concerning the treatment of refractory epilepsies, the last few years have shown a significant increase in studies evaluating the risks and benefits of using cannabinoids in this context (13, 14). Epilepsy is a pathological condition that affects about 65 million people worldwide, and its principal characteristic is recurrent seizures, and its etiology can be varied, ranging from genetic syndromes to brain damage (15–18). It is also a condition that often does not respond to the pharmacotherapy used, and, in this sense, cannabinoids appear as a promising alternative. The two phytocannabinoids most researched for the treatment of epilepsies are delta-9-tetrahydrocannabinol (THC—main psychoactive compound) and especially cannabidiol (CBD—main non-psychoactive compound), which are useful in preventing seizures and reducing mortality, with low toxicity and high tolerability (11, 17–20). The path to the safe and effective use of cannabinoids in treating epilepsy seems to be unraveled by science; however, the next question: would the same expectation regarding the treatment of other neuropsychiatric disorders be possible? To shed light on this issue, this review, in addition to emphasizing the use of CBD in the treatment of epilepsy, examines the possibility of using this compound as an alternative to the treatment of some neuropsychiatric disorders. For more details about the botany, psychobiology, and the medical potential of cannabis, the readers can examine the various reviews available in the literature or direct toward an elegant review by Solymosi and Köfalvi (21).
“From an Alternative Medicine:” First Evidence and Carlini's Group Contribution
The use of Cannabis for the treatment of epilepsy has been going on for a long time, with evidence found in Sumerian tablets more than 3,800 years ago (14). The most recent reports started in the middle of the nineteenth century when the Irish surgeon William O'Shaughnessy announced the plant's therapeutic effects in the treatment of epilepsy, a fact that was soon reinforced by two other renowned English neurologists, J. R. Reynolds and W. Gowers (22, 23). Scientific publications from the 1940s, both in animal models (24) and in children with epilepsy (25), were the first reports of the therapeutic use of Cannabis for this condition. A significant step in the study of cannabinoids was taken by Mechoulam in the 1960s, when he isolated, clarified the structure, and synthesized THC and CBD, the most abundant and most studied phytocannabinoids in Cannabis to date, including for epilepsy (26–28).
In the sequence, and even before the discovery of the eCB system (which occurred only in the '90s), the Brazilian researcher Elisaldo Carlini started studies using CBD in animal models of epilepsy, suggesting the first scientific evidence about the therapeutic potential of CBD in treatment of this pathology (29, 30). Next, in partnership with the Mechoulam group, Carlini et al. conducted the first placebo-controlled study of CBD in patients with refractory epilepsy. At the time, the authors showed that two of the four epileptic patients treated with 200 mg of CBD daily showed an improvement in their epileptic status, without having any seizures within 3 months of treatment. The third patient had a partial improvement, while the fourth patient treated with CBD, and the other five patients in the placebo group showed no improvement. No toxic effects were observed, and this was the first study in humans from the “modern scientific era” to consider the possibility of CBD's therapeutic potential, isolated, in the treatment of refractory epilepsies (31).
Continuing the investigations, Carlini et al. published a series of studies that confirmed CBD's therapeutic potential in the treatment of seizures. In the early 1980s, a double-blind controlled trial was performed with CBD 200–300 mg/kg or placebo administered daily over more than 4 months in 15 patients with generalized epilepsy. Of the eight patients treated with CBD, four of them had practically no seizures throughout the experiment, and three had partial improvement, while the seven patients in the placebo group showed no improvement in the clinical picture of the seizures (32).
Subsequently, other studies by Carlini et al. have reinforced CBD's therapeutic potential, a non-psychoactive phytocannabinoid and, therefore, with fewer side effects than THC, in the treatment of epileptic conditions (33–35). Since then, different researchers have confirmed the pioneering studies of Carlini et al. since the 1970s, that is, that CBD can be a safe and effective therapeutic alternative for the treatment of epilepsy, a condition that affects millions of people across the world (11, 36–40). This contribution made an extensive article published recently (2020) in The NY Times about CBD, which considers Carlini as the “discoverer” of the use of this compound in epilepsy (41). A recent and simple search made based on scientific articles PubMed, using the descriptors “cannabidiol” and “epilepsy” lists ~470 publications addressing this topic. Although there is now much more evidence from studies in animals than in humans, in addition to few randomized controlled trials (28, 42), many clinical observations have suggested cannabinoids, not just CBD, as a new treatment for refractory epilepsies (for better visualization of the contribution of Carlini et al., see Figure 1).
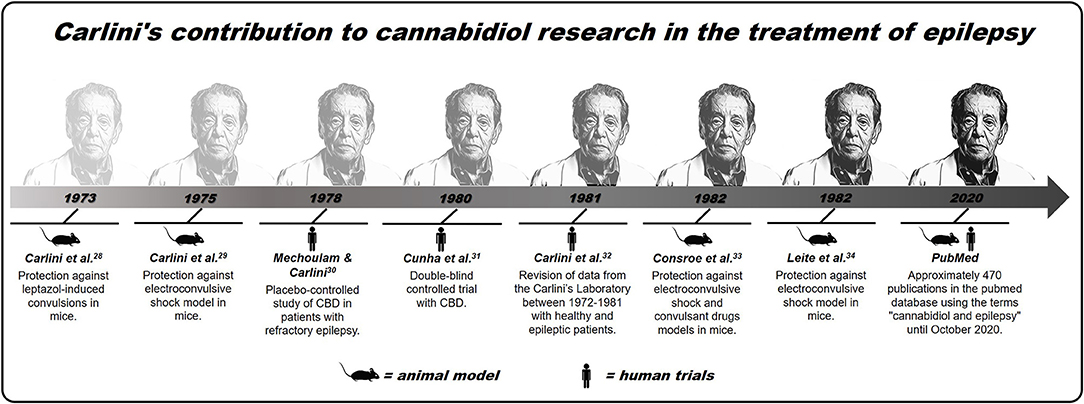
Figure 1. Brief history of Carlini's contribution to cannabidiol research in the treatment of epilepsy.
“To A New Treatment for Refractory Epilepsies:” Clinical Confirmation and The Role of Anecdotal Reports
The fact that medical cannabis is still illegal in several countries, coupled with the high financial cost for patients, when available, ends up favoring the use of artisanal extracts. In turn, these extracts do not always have strict quality control over the quantity and quality of the components present in the formulation. This situation makes it difficult to obtain reliable scientific data regarding the efficiency and safety of the drug use of cannabinoids. However, it is precisely anecdotal reports, mostly obtained through the use of cannabis herbal extract oil, that have provided a considerable amount of evidence for the use of cannabis and CBD in isolation as a treatment for epilepsies (43).
In a study of 74 children resistant to traditional treatment of epilepsy, treatment with CBD-rich herbal cannabis extract (20: 1 THC; ~10 mg/kg CBD per day) reduced the frequency of seizures by 89% of the studied population, with 43% of these children had a reduction that surpassed 50%. Only 6.7% of these children worsened in seizures, with treatment discontinue (44). In another prospective open-label study, using CBD-rich herbal cannabis extract (50: 1 THC; ~13 mg/kg CBD per day) to treat 20 children with Dravet's syndrome for 20 weeks, there was a reduction of more than 70% in seizures (45). Also, about the effectiveness of treatments using artisanal cannabis preparations, a retrospective study investigating the effects of CBD oil in 108 children with epilepsy is highlighted. In this study, 10% of children treated with CBD oil had no seizures, and 39% had a reduction more significant than 50% of those seizures, showing promising potential for this type of preparation. Less than 4% had sedation as an adverse effect (46). These studies, together, point to a direction and show that controlled clinical tests using different cannabinoid compounds, including standardized ones, are necessary, as well as they are promising in the treatment of epilepsy.
In this sense, some clinical studies have been done with standardized cannabinoid compounds, therefore, with better possibilities for the treatment's efficacy and safety. Among these, we highlight the open-label trial by Devinsky et al., which showed the effectiveness of Epidiolex in the control of refractory epilepsy in a study involving 162 patients. These patients received CBD 2 at 50 mg/kg per day, in stages, for 12 weeks. There was a reduction of ~36% in seizures compared to baseline. Mild adverse effects involving drowsiness, reduced appetite, diarrhea, fatigue, and seizures were reported in 79% of patients. Another 12% of patients had serious adverse effects (47). Besides, quality of life was measured in 48 of the 162 initial participants. There was an improvement in the scores obtained through the Quality of Life in Childhood Epilepsy (QOLCE) (48). In short, treatment with CBD proved to be relatively safe, reducing seizures and promoting an improvement in patients' quality of life (47, 48).
Facing the need for a double-blind/randomized/placebo-controlled trials, Devinsky et al. developed a study that reinforced the effectiveness of Epidiolex in the control of epilepsy in patients with Dravet's syndrome. The study included 120 subjects, including children and young adults, who were randomized to receive CBD (Epidiolex) at a dose of 20 mg/kg per day or placebo over 14 weeks. After treatment, 5% of participants who received CBD were free from seizures than the placebo group (0%). Also, 43% of patients treated with CBD had a 50% reduction in seizures, against 27% in the placebo group. It is also important to note that 93% of patients who received CBD treatment had an adverse effect, with the majority (89%) of these effects being considered mild or moderate (e.g., diarrhea, vomiting, drowsiness, etc.) (49). These results reinforce the evidence from the same group in previous studies (47, 48).
In another double-blind, placebo-controlled trial, two groups, one with 86 and 85 patients with Lennox-Gastaut syndrome, were treated, respectively, with CBD or placebo for 14 weeks. In this study, the group that received CBD, 20 mg/kg per day, had an average monthly reduction in epileptic seizures of around 43%, while the placebo group had an average of 21%. Mild and moderate adverse effects occurred in 86% of patients treated with CBD vs. 69% of patients in the placebo group, with diarrhea, drowsiness, decreased appetite, pyrexia, and vomiting being the most frequent. This study points to CBD as useful in treating seizures associated with Lennox-Gastaut syndrome and being relatively well-tolerated, as it has not caused severe adverse effects (50).
A point that has been discussed in the clinic and, therefore, deserves to be highlighted concerns the possible interactions of CBD with other anticonvulsant agents through the so-called polytherapy, so common in patients with refractory epilepsies. A better understanding of this adjunctive therapy is necessary so that there is greater clarification concerning the adverse effects, quality of life of the patient, as well as the effectiveness of the doses used. This understanding should involve, for example, genetic, pharmacodynamic, and pharmacokinetic issues that influence the effects of CBD in the presence of other drugs (and vice versa), thus providing greater security in choosing an appropriate pharmacotherapeutic strategy [for a more detailed review, see (51)]. One of these possibilities that have been documented is the interaction of CBD with benzodiazepine clobazam. Both are metabolized by the cytochrome P450 (CYP) pathway, and CBD could be potentiating the effects of clobazam by inhibiting this metabolism pathway. Likewise, clobazam could also be potentiating the antiepileptic effects of CBD by inhibiting its degradation pathway. This possibility raised a question about the effectiveness of CBD in the treatment of epilepsy, which was: would CBD have effects per se in the treatment of epilepsy, or would it need to be associated with clobazam? (52, 53) Although this answer is not yet clear, CBD in Europe has been approved only as an adjunctive treatment with clobazam. However, a study carried out with Lennox-Gastaut syndrome patients and Dravet syndrome who received CBD in the absence of clobazam (54), in addition to other studies (55, 56), strongly point to the fact that this phytocannabinoid exerts its therapeutic effects independently of its interaction with the mentioned benzodiazeinic. Therefore, the evidence suggests that the European Medicines Agency Public Assessment Report's prescription restriction is not supported. The lack of randomization for studies involving CBD interaction with clobazam may have contributed to some misconceptions (52).
In recent years, there have also been some systematic reviews of clinical trials, including meta-analysis, which have reinforced the effectiveness and safety of CBD or CBD-Rich Cannabis Extracts in the treatment of epilepsies (18, 40, 57). After a long time where anecdotal reports predominated, the evidence through well-conducted clinical studies indicates a safe use of cannabinoids, especially CBD, in the treatment of epilepsies. Further studies are needed to understand better the benefits to the possible risks of using cannabinoids in this situation. Studies are needed to better elucidate another prevalent issue among anecdotal reports, which concerns the interaction between CBD and THC influencing antiepileptic effects' effectiveness (20, 58). Would CBD alone be more effective, or would it need to interact with THC and other constituents of Cannabis sativa? Such an issue will be further discussed in the next section of this article.
The Possibility of The Entourage Effect: What Is Known About?
“One plus one is always more than two,” phrase of the song “O sal da terra” by Beto Guedes, a singer of Brazilian popular music. Obviously, he was not referring to cannabinoids but to the need to bring people together to build a better world. The same phrase can also make sense when speculating about the entourage effect observed in many studies that address the therapeutic application of cannabinoids.
The entourage effect is a term suggested referring to a situation where a group of endogenous compounds similar to eCB, when acting together, potentiate the effects mediated by cannabinoid receptors. This term was first mentioned by Bem-Shabat et al. (59), and soon expanded to also define the synergistic effects observed through the use of mixtures of plants in general, including concerning the different compounds present in cannabis. It is worth mentioning in this topic another pioneering aspect of Carlini and his group. Long before the advent of this terminology on cannabinoid effects, the Brazilian group published studies showing the relevance of the interaction between the different phytocannabinoids present in cannabis samples (60–62). Thus, evidence indicates that many of the therapeutic effects observed through the use of phytocannabinoids occur, in fact, much more from the complex and poorly understood interaction of all the compounds present in the plant (mainly THC and CBD) rather than the isolated action of a single compound (63, 64). In this case, can one plus one also be more than two?
Studies, mainly in animals but also in humans, have shown that the answer is yes. A recent meta-analysis study published by Pamplona et al. (40), which searched 199 articles (with 11 validated references) with a total of 670 patients, showed that CBD-rich extracts seem to present a better therapeutic profile than purified CBD. In this meta-analysis, 71% of patients treated with CBD-rich extracts reported some improvement in their epileptic condition, compared to 46% of patients treated with purified CBD. Patients treated with CBD-rich extracts also required lower doses (6 mg/kg/day) than patients who used only CBD (25.3 mg/kg/day), that is, an effect four times greater on the part of the unpurified extract. Still, in the same study, it was also possible to notice that patients treated with CBD-rich extracts had fewer side effects, both mild (33%) and severe (7%) when compared to those who received purified CBD (76% mild and 26% serious).
In addition to the main constituents of cannabis CBD and THC, another possibility that favors the entourage effect is the findings of the anticonvulsant potential of other phytocannabinoids, for example, delta-9-tetrahydrocannabivarin (THCV) and cannabidivarin (CBDV). These phytocannabinoids also proved useful anticonvulsants, with THCV having its effects via CB1 receptors, while CBDV did not (65–67). CBDV also had their anticonvulsant effects enhanced by CBD (66). Additional contributions to these specific topics through Carlini-trained researchers can be found in recent reviews (68–71). Considering the spectrum of possibilities of cannabis expanded to more than 200 terpenes present in the plant, the findings can be even more promising. Some terpenes are known to have pharmacological activities in the central nervous system, although they have not been tested in patients with epilepsy (63, 72). Although such interactions do seem to occur, more controlled clinical studies proving such a possibility are needed. However, regardless of whether they are better together or apart, one thing is sure, phytocannabinoids are proving to be increasingly influential in the treatment of epilepsies.
If Cannabidiol Works, How Does it Work?
To speculate about the neurobiological mechanisms involved in the antiepileptic activity of cannabinoids, including cannabidiol, it is necessary to understand a little more about the eCB system's physiology. This fact has been well-elucidated since the early 90s, when the discovery of this system occurred, which revolutionized the understanding of many neurophysiological responses. The eCB system consists of two receptors (CB1 and CB2), endogenous ligands (anandamide/AEA and 2-arachidonylglycerol/2-AG), and enzymes involved the synthesis and degradation of these ligands. It is a system with neuromodulatory functions, which regulate the presynaptic release of both excitatory and inhibitory neurotransmitters (73, 74). These neuromodulatory functions appear to play an essential role in controlling epilepsies, for example, through the activation of CB1 type cannabinoid receptors (75).
These receptors are located presynaptically, and their activation, either by endogenous ligands (e.g., AEA) or exogenous (e.g., THC), results in a transient hyperpolarization of the presynaptic membrane that, consequently, inhibits the release of excitatory neurotransmitters like glutamate (76). This fact agrees with the evidence that shows the downregulation of CB1 receptors in axial glutamatergic terminals extracted from the brain tissue of patients with epilepsy. On the other hand, the evidence also points to the upregulation of the same receptors at the GABAergic axonal terminals. In both possibilities, there is a loss of control over neuronal hyperexcitability, favoring epilepsy. The antiepileptic effects obtained from manipulating the eCB system or using exogenous phytocannabinoids may be related to the reestablishment of control over this hyperexcitability (19, 77–79).
In the case of phytocannabinoids, THC has a high affinity for the CB1 receptor and, through this receptor, can regulate neuronal excitability. This compound was the first phytocannabinoid to have its anticonvulsant properties evaluated, which must result from a reduction in the levels of excitatory neurotransmitters caused by its agonist action on CB1 receptors (19, 20). While CBD, the plant's non-psychoactive phytocannabinoid and possibly the most studied when it comes to antiepileptic properties, has a mechanism of action that has not yet been elucidated. This phytocannabinoid has a low affinity for CB1 receptors and may have its antiepileptic effect related to neuronal excitability's modulation through changes in the influx of Ca and Na ions, as well as actions in vanilloid receptors, adenosinergic and serotonergic systems (80–82). Another possibility to explain CBD's antiepileptic effects would be an eventual ability to inhibit both uptake/hydrolysis of the eCB and, thus, indirectly, to decrease neuronal excitability (83) by potentiating this system (84, 85). The fact is that this issue of the neurobiological mechanisms involved in the antiepileptic action of cannabinoids is not entirely defined and still requires a better understanding [for a complete review of the possible mechanisms of action of cannabinoids in epilepsy, see (80)]. As science grasps these mechanisms, this will result in more efficient pharmacotherapeutic approaches for the treatment of epilepsy and make it possible to expand the medicinal use of cannabinoids, including CBD, for other neuropsychiatric diseases.
Can The Success of Cannabidiol In The Treatment of Epilepsy Predict The Same Path for The Treatment of Neuropsychiatric Disorders?
It is not yet possible to say whether the use of CBD and other cannabinoids to treat different neuropsychiatric disorders will follow the same route observed for the treatment of epilepsy. However, significant steps have also been taken for these other possibilities. From depression to anxiety, including PTSD, addiction, neurodegenerative diseases, and ASD, these are disorders that, according to some studies, can use cannabinoids, especially CBD, as a pharmacotherapeutic alternative (4, 8, 86–88).
Depression
Regarding depression and anxiety, it is known that many cannabis users report its use for its relaxing effects; therefore, as a way to reduce the symptoms of these disorders (87, 89, 90). Additionally, several studies have pointed to the potentization of the eCB system or the use of exogenous ligands as promising possibilities in treating depression (5, 7, 91–97). Reinforcing this possibility, the blockade of this system, whether through the use of antagonists or genetic deletion, seems to lead to depressive and anxiety symptoms, which caused the withdrawal of the CB1 antagonist rimonabant, proposed for the treatment of obesity, from the market (98–100). According to this perspective, patients with major depression had reduced serum levels of eCBs, in addition to a lower density of CB1 receptors in the glial cells of the brain gray matter (101, 102). In this sense, a proposal to reestablish the eCB system's functions by inhibiting the degradation of its endogenous ligands can be explored as an antidepressant potential (103). Remembering that CBD, a phytocannabinoid with few side effects, may be acting in this way, potentiating the eCB system (84), even being reported in different studies as an effective antidepressant (6, 104, 105). Considering that traditional antidepressants (serotonin and/or noradrenaline reuptake inhibitors) have relatively low efficiency and still need weeks for their effects (106), it is suggested that the manipulation of the eCB system, which even has a response rate faster, can be an alternative for the treatment of depressive disorders [for a more detailed review, see (7, 91, 93, 96)].
Anxiety
About anxiety, CBD has also been shown to be a more exciting alternative, given the potentially anxiogenic effects of THC (107, 108). Several pre-clinical studies using different animal models (109–116), as well as some clinical studies (117–121), confirm the anxiolytic effects of CBD (122). In this research area, it is worth mentioning the vital participation of groups from the Faculty of Medicine of Rib. Preto—University São Paulo, BR, led by Zuardi and Guimaraes. In addition to the use of CBD, manipulation of the eCB system is an alternative in treating anxiety. This system is located in brain regions important for modulating responses related to fear and anxiety (123), with increased anandamide via inhibition of its degradation, promoting anxiolytic effects (109, 124–127). Considering the high abuse potential of benzodiazepines and the slow response of selective serotonin reuptake inhibitors (SSRIs), both CBD and potentiation of the eCB system are promising alternatives in pharmacotherapy of anxiety disorders [for a mor detailed review, see (87, 122, 127, 128)].
PTSD
Until recently considered as an anxiety disorder, post-traumatic stress disorder (PTSD), which from the DSM-5 was included in a new category called “trauma and stress-related disorders,” has also responded very well to research that involves cannabinoid treatment, especially CBD (8). The speculations started from the work of Marsicano et al. (129), showing the eCB system's role in the extinction of aversive memories. From then on, a series of pre-clinical studies started to indicate that the potentiation of the eCB system (130), the use of exogenous agonists for the CB1 receptor (131) or even the CBD (109, 132) could facilitate the extinction of aversive memories. In addition to facilitating the extinction process, different studies have shown the effect of cannabinoids impairing the processes of retrieval and consolidation of these memories, that is, more possibilities for intervention in the remembrance of traumatic events (133–135). In the face of so many reports of pre-clinical studies, it was not long before evidence also emerged from clinical studies (119, 136–140) and thus reinforced the potential of cannabinoids, including CBD, as a therapeutic alternative for the treatment of this disorder [for a more detailed review, see (8, 141–145)].
Addiction
In addition to PTSD, another neuropsychiatric condition where memories play a fundamental role, and that there is also evidence for the use of cannabinoids, is addiction/relapse to drugs of abuse. Although it seems to be a paradoxical variant, understanding the action of cannabinoids in the breakdown of hedonic or reinforcing memories can provide up-and-coming therapeutic alternatives. In this perspective, de Carvalho and Takahashi showed in a pioneering way the inhibitory effect of CBD in reactivation sessions in animals that previously had conditioned place preference induced by morphine or cocaine (88). This finding suggests CBD's disruptive effect on the reconsolidation of memories associated with drugs of abuse, thus reducing the risk of relapse (146). A similar result was reported by Luján et al. (147), showing that the CBD attenuated cocaine-induced conditioned place preference, in addition to reducing voluntary consumption by mice. Besides, this work showed that CBD increased the expression of CB1 receptors and neural cell proliferation in the hippocampus, reinforcing the ability of this cannabinoid to modulate both behavioral and molecular manifestations related to cocaine reinforcement (147). Cannabinoid receptors CB1 and CB2 even seem to perform opposite functions, and the antagonism of CB1 receptors has the same inhibitory effects seen in the activation of CB2 receptors concerning the modulation of cocaine-induced sensitization and conditioned place preference (CPP). These effects probably occur due to a block in neuronal activation of the hippocampus (148). Other studies have also shown the CBD's ability to also reduce alcohol consumption in animal models of an alcohol use disorder, in addition to reducing alcohol-related steatosis and fibrosis in the liver, and alcohol-related brain damage, preventing neuronal loss (149). From a clinical perspective, promising results showed that the voluntary use of cannabis caused a decrease in crack use and also promoted an improvement in the quality of life in individuals dependent on this substance (150–152). The evidence from CBD as a treatment for drug abuse disorders is still discreet but deserves a closer look [for a more detailed review, see (10, 149, 153)].
NDDs
One possibility that is increasingly attracting researchers' attention to the use of cannabinoids is related to the application of these compounds in neurodegenerative disorders (NDDs). These NDDs are strongly related to oxidative damage and a series of neuroinflammatory responses that ultimately lead to cell death (154). Among the NDDs, the most common are Parkinson's disease (PD) and Alzheimer's disease (AD), conditions in which the potentiation of the eCB system (155) or even the use of phytocannabinoids, especially CBD (156, 157), can play an auspicious role as neuroprotectants (4). This promising possibility on CBD is pointed out in vitro and in vivo studies (158–160), and even in clinical studies (161). Taking into account that the current classic treatments for NDDs do not stop and/or slow the progression of the disease, alternatives such as CBD or any other substances that target the eCB system can be good candidates as prototypes for the development of neuroprotective drugs [for a more detailed review, see (154–157, 161)].
ASD
Another neuropathology that appears to be associated with inflammatory processes and, therefore, can also be a target for cannabinoids is an autism spectrum disorder (ASD) (86, 162). This disorder is characterized by constant communication and social interaction deficits and restricted and repetitive behavior patterns, which still have unknown etiopathogenesis (163). One of the possibilities may be an imbalance in the eCB system, responsible for regulating some typically impaired functions in the ASD (164–168). This fact, associated with the anti-inflammatory activity of cannabinoids, has encouraged pre-clinical (169–171) and clinical (12, 172, 173) research to investigate the therapeutic potential of cannabinoids for the treatment of ASD. Among the possibilities, CBD seems to be the safest and most promising alternative (12, 172, 173), although other phytocannabinoids like CBDV also present themselves as candidates (171). The reestablishment of the balance of the eCB system and the anti-neuroinflammatory activity seems to support these compounds' activities as a treatment to ASD [for a more detailed review, see (86, 163, 164, 166, 174)].
There are still many other possibilities for neuropsychiatric disorders that can find cannabinoids as a possible therapeutic option. In this case, CBD, the main focus of this review, and other phytocannabinoids (e.g., THC, THCV, CBDV) appear to present quite promising pharmacotherapeutic alternatives for an increasingly broad number of neuropsychiatric disorders. However, for all these possibilities, including those mentioned here, further prospective, double-blind, placebo-controlled studies must be needed (for a summary, see Table 1). These studies are essential to ensure the effectiveness and safety of these compounds in each specific situation. In any case, this is a promising field of study where many pharmacotherapeutic alternatives may be revealed.
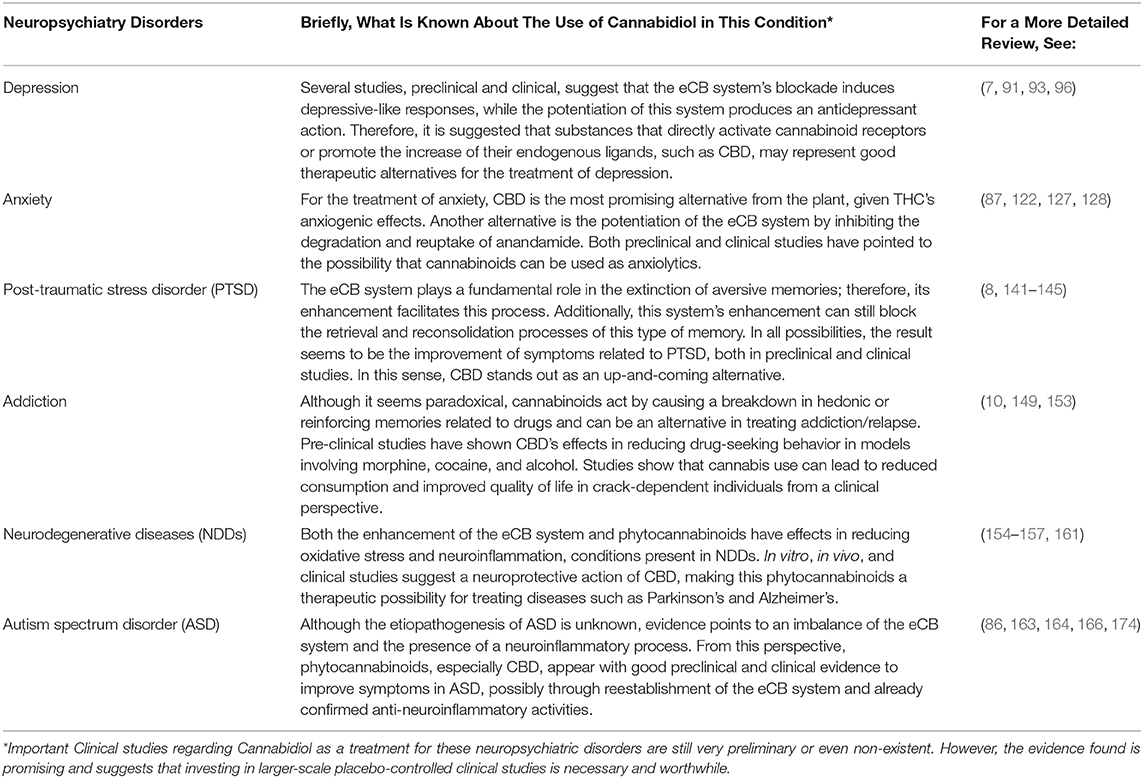
Table 1. CBD as a promising psychopharmacological alternative in the treatment of neuropsychiatric disorders.
Conclusion and Future Perspectives
In conclusion, we emphasize promising pre-clinical and clinical findings with cannabinoids, mostly from Brazilian-based researchers and other researchers worldwide. Specific studies have focused on the multifunctional phytocannabinoid, CBD, showing remarkable benefits, mainly for refractory epilepsy in children. These data contributed to the considered “prohibited substance” to enter the list of medicines for controlled use by the National Health Surveillance Agency (ANVISA), the regulatory agency responsible for the approval of new drugs in Brazil. Besides, one may anticipate other phytocannabinoid-based preparations and even new drugs acting at the endocannabinoid system as a promising therapeutic advance for other neuropsychiatric disorders, represented here by depression, anxiety-related disorders, PTSD, drug addiction and drug-induced relapse, neurodegenerative disorders, and ASD. If CBD (or other cannabinoids) with regard to neuropsychiatric disorders, will follow the same path observed for refractory epilepsies-from alternative medicine to a new treatment-, only advances in research can respond. While the definitive answers do not arrive, the fact is what we have so far allows us to glimpse a promising path.
Author Contributions
RB and RT: final form of the manuscript. All authors: conceived the review and prepared the first draft.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
First and foremost, the present review is dedicated to the memory of EC. We would thank EC who contributed to our knowledge and to his passionate support of the medicinal use of cannabis and its derivates. The authors wish to thank students Marina G. da Silva and Guilherme C. Darós at the Laboratory of Behavioral Neuroscience and all past students at the Laboratory of Psychopharmacology, UFSC, for their technical contributions.
Abbreviations
eCB, endocannabinoid; PTSD, post-traumatic stress disorder; ASD, autism spectrum disorder; THC, delta-9-tetrahydrocannabinol; CBD, cannabidiol; QOLCE, quality of life in childhood epilepsy; THCV, delta-9-tetrahydrocannabivarin; CBDV, cannabidivarin; CB1, cannabinoid receptor type 1; CB2, cannabinoid receptor type 2; AEA, anandamide; 2-AG, 2-arachidonylglycerol; GABA, gamma-aminobutyric Acid; SSRIs, selective serotonin reuptake inhibitors; DSM-5, Diagnostic and Statistical Manual; CPP, conditioned place preference; NDDs, neurodegenerative disorders; PD, Parkinson disease; AD, Alzheimer disease; ANVISA, National Health Surveillance Agency.
References
1. Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. (2016) 33:1357–92. doi: 10.1039/C6NP00074F
2. Howard P, Twycross R, Shuster J, Mihalyo M, Wilcock A. Cannabinoids. J Pain Symptom Manage. (2013) 46:142–9. doi: 10.1016/j.jpainsymman.2013.05.002
3. Mechoulam R, Hanuš LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. (2014) 15:757–64. doi: 10.1038/nrn3811
4. Campos AC, Fogaça MV, Sonego AB, Guimarães FS. Cannabidiol, Neuroprotection and Neuropsychiatric Disorders. Academic Press (2016). Available online at: https://www.sciencedirect.com/science/article/pii/S1043661816000396?via%3Dihub (accessed October 26, 2017).
5. Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. (2005) 115:3104–16. doi: 10.1172/JCI25509
6. Schier A, Ribeiro N, Coutinho D, Machado S, Arias-Carrion O, Crippa J, et al. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets. (2014) 13:953–60. doi: 10.2174/1871527313666140612114838
7. Poleszak E, Wośko S, Sławińska K, Szopa A, Wróbel A, Serefko A. Cannabinoids in depressive disorders. Life Sci. (2018) 213:18–24. doi: 10.1016/j.lfs.2018.09.058
8. Bitencourt RM, Takahashi RN. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: from bench research to confirmation in human trials. Front Neurosci. (2018) 12:502. doi: 10.3389/fnins.2018.00502
9. De Carvalho CR, Pamplona FA, Cruz JS, Takahashi RN. Endocannabinoids underlie reconsolidation of hedonic memories in Wistar rats. Psychopharmacology (Berl). (2014) 231:1417–25. doi: 10.1007/s00213-013-3331-2
10. Stern CAJ, de Carvalho CR, Bertoglio LJ, Takahashi RN. Effects of cannabinoid drugs on aversive or rewarding drug-associated memory extinction and reconsolidation. Neuroscience. (2018) 370:62–80. doi: 10.1016/j.neuroscience.2017.07.018
11. Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. (2014) 55:791–802. doi: 10.1111/epi.12631
12. Fleury-Teixeira P, Caixeta FV, da Silva LCR, Brasil-Neto JP, Malcher-Lopes R. Effects of cbd-enriched Cannabis sativa extract on autism spectrum disorder symptoms: an observational study of 18 participants undergoing compassionate use. Front Neurol. (2019) 10:1145. doi: 10.3389/fneur.2019.01145
13. Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, et al. Cannabis-based products for pediatric epilepsy: an updated systematic review. Seizure. (2020) 75:18–22. doi: 10.1016/j.seizure.2019.12.006
14. O'Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav. (2017) 70:341–8. doi: 10.1016/j.yebeh.2016.11.012
15. Fisher RS, Van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. (2005) 46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x
16. Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. (2007) 68:326–37. doi: 10.1212/01.wnl.0000252807.38124.a3
17. Lattanzi S, Cagnetti C, Matricardi S, Silvestrini M. Palliative non-resective surgery for drug-resistant epilepsy. Brain Dev. (2018) 40:512–3. doi: 10.1016/j.braindev.2017.12.012
18. Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, et al. Efficacy and safety of Cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. (2018) 78:1791–804. doi: 10.1007/s40265-018-0992-5
19. Huntsman RJ, Tang-Wai R, Shackelford AE. Cannabis for pediatric epilepsy. J Clin Neurophysiol. (2020) 37:2–8. doi: 10.1097/WNP.0000000000000641
20. Rosenberg EC, Tsien RW, Whalley BJ, Devinsky O. Cannabinoids and epilepsy. Neurotherapeutics. (2015) 12:747–68. doi: 10.1007/s13311-015-0375-5
21. Solymosi K, Kofalvi A. Cannabis: a treasure trove or pandora's box? Mini Rev Med Chem. (2016) 17:1223–91. doi: 10.2174/1389557516666161004162133
22. O'Shaughnessy WB. On the preparations of the Indian hemp, or Gunjah: cannabis indica their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. BMJ. (1843) s1-5:363–9. doi: 10.1136/bmj.s1-5.123.363
23. Reynolds JR. Epilepsy: its symptoms, treatment, and relation to other chronic convulsive diseases. Br Foreign Med Chir Rev. (1862) 30:309–12.
24. Loewe S, Goodman LS. Anticonvulsant action of marihuana-active substances. Fed Proc. (1947) 6:352.
25. Davis J, Proc HR-F. Antiepileptic Action of Marihuana Active Substances. (1949). calgarycmmc.com Available online at: https://www.calgarycmmc.com/Anti-epileptic-Action-of-Marijuana-Active-Substances-1947.pdf (accessed October 16, 2020).
26. Mechoulam R, Shvo Y. Hashish-I. The structure of cannabidiol. Tetrahedron. (1963) 19:2073–8. doi: 10.1016/0040-4020(63)85022-X
27. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. J Am Chem Soc. (1964) 86:1646–7. doi: 10.1021/ja01062a046
28. Hill AJ, Hill TDM, Whalley BJ. The development of cannabinoid based therapies for epilepsy. In: Murillo-Rodríguez, editor. Endocannabinoids: Molecular, Pharmacological, Behavioral and Clinical Features. Oak Park, IL: Bentham Science Publishers (2013). p. 164–204.
29. Carlini EA, Leite JR, Tannhauser M, Berardi AC. Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J Pharm Pharmacol. (1973) 25:664–5. doi: 10.1111/j.2042-7158.1973.tb10660.x
30. Carlini EA, Mechoulam R, Lander N. Anticonvulsant activity of four oxygenated cannabidiol derivatives. Res Commun Chem Pathol Pharmacol. (1975) 12:1–15.
31. Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Naturwissenschaften. (1978) 65:174–9. doi: 10.1007/BF00450585
32. Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. (1980) 21:175–85. doi: 10.1159/000137430
33. Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. (1981) 21:417S−27S. doi: 10.1002/j.1552-4604.1981.tb02622.x
34. Consroe P, Benedito MAC, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol. (1982) 83:293–8. doi: 10.1016/0014-2999(82)90264-3
35. Leite JR, Carlini EA, Lander N, Mechoulam R. Anticonvulsant effects of the (-) and (+)isomers of cannabidiol and their dimethylheptyl homologs. Pharmacology. (1982) 24:141–6. doi: 10.1159/000137588
36. Leo A, Russo E, Elia M. Cannabidiol and epilepsy: rationale and therapeutic potential. Pharmacol Res. (2016) 107:85–92. doi: 10.1016/j.phrs.2016.03.005
37. Samanta D. Cannabidiol: a review of clinical efficacy and safety in epilepsy. Pediatr Neurol. (2019) 96:24–9. doi: 10.1016/j.pediatrneurol.2019.03.014
38. Silvestro S, Mammana S, Cavalli E, Bramanti P, Mazzon E. Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules. (2019) 24:1459. doi: 10.3390/molecules24081459
39. Franco V, Perucca E. Pharmacological and therapeutic properties of cannabidiol for epilepsy. Drugs. (2019) 79:1435–54. doi: 10.1007/s40265-019-01171-4
40. Pamplona FA, Da Silva LR, Coan AC. Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: observational data meta-analysis. Front Neurol. (2018) 9:759. doi: 10.3389/fneur.2018.00759
41. A Hidden Origin Story of the CBD Craze. Available online at: https://www-nytimes-com.cdn.ampproject.org/c/s/www.nytimes.com/2020/05/23/sunday-review/coronavirus-cbd-oil.amp.html (accessed October 16, 2020).
42. Szaflarski JP, Martina Bebin E. Cannabis, cannabidiol, and epilepsy—From receptors to clinical response. Epilepsy Behav. (2014) 41:277–82. doi: 10.1016/j.yebeh.2014.08.135
43. Cilio MR, Thiele EA, Devinsky O. The case for assessing cannabidiol in epilepsy. Epilepsia. (2014) 55:787–90. doi: 10.1111/epi.12635
44. Tzadok M, Uliel-Siboni S, Linder I, Kramer U, Epstein O, Menascu S, et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: the current Israeli experience. Seizure. (2016) 35:41–4. doi: 10.1016/j.seizure.2016.01.004
45. McCoy B, Wang L, Zak M, Al-Mehmadi S, Kabir N, Alhadid K, et al. A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Ann Clin Transl Neurol. (2018) 5:1077–88. doi: 10.1002/acn3.621
46. Porcari GS, Fu C, Doll ED, Carter EG, Carson RP. Efficacy of artisanal preparations of cannabidiol for the treatment of epilepsy: practical experiences in a tertiary medical center. Epilepsy Behav. (2018) 80:240–6. doi: 10.1016/j.yebeh.2018.01.026
47. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. (2016) 15:270–8. doi: 10.1016/S1474-4422(15)00379-8
48. Rosenberg EC, Louik J, Conway E, Devinsky O, Friedman D. Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia. (2017) 58:e96–100. doi: 10.1111/epi.13815
49. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N Engl J Med. (2017) 376:2011–20. doi: 10.1056/NEJMoa1611618
50. Thiele EA, Marsh ED, French JA, Mazurkiewicz MB, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. (2018) 391:1085–96. doi: 10.1016/S0140-6736(18)30136-3
51. Raucci U, Pietrafusa N, Paolino MC, Di Nardo G, Villa MP, Pavone P, et al. Cannabidiol treatment for refractory epilepsies in pediatrics. Front Pharmacol. (2020) 11:1. doi: 10.3389/fphar.2020.586110
52. Lattanzi S, Trinka E, Striano P, Zaccara G, Del Giovane C, Nardone R, et al. Cannabidiol efficacy and clobazam status: a systematic review and meta-analysis. Epilepsia. (2020) 61:1090–8. doi: 10.1111/epi.16546
53. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug-drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. (2015) 56:1246–51. doi: 10.1111/epi.13060
54. Bialer M, Perucca E. Does cannabidiol have antiseizure activity independent of its interactions with clobazam? An appraisal of the evidence from randomized controlled trials. Epilepsia. (2020) 61:1082–9. doi: 10.1111/epi.16542
55. Savage TE, Sourbron J, Bruno PL, Skirvin LA, Wolper ES, Anagnos CJ, et al. Efficacy of cannabidiol in subjects with refractory epilepsy relative to concomitant use of clobazam. Epilepsy Res. (2020) 160:106263. doi: 10.1016/j.eplepsyres.2019.106263
56. Gaston TE, Bebin EM, Cutter GR, Ampah SB, Liu Y, Grayson LP, et al. Drug-drug interactions with cannabidiol (CBD) appear to have no effect on treatment response in an open-label expanded access program. Epilepsy Behav. (2019) 98:201–6. doi: 10.1016/j.yebeh.2019.07.008
57. Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, et al. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry. (2018) 89:741–53. doi: 10.1136/jnnp-2017-317168
58. Berkovic SF. Cannabinoids for epilepsy—real data, at last. N Engl J Med. (2017) 376:2075–6. doi: 10.1056/NEJMe1702205
59. Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee M-H, Vogel Z, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. (1998) 353:23–31. doi: 10.1016/S0014-2999(98)00392-6
60. Karniol IG, Carlini EA. The content of (-)Δ9-trans-tetrahydrocannabinol (Δ9-THC) does not explain all biological activity of some Brazilian marihuana samples. J Pharm Pharmacol. (1972) 24:833–5. doi: 10.1111/j.2042-7158.1972.tb08897.x
61. Karniol IG, Carlini EA. Pharmacological interaction between cannabidiol and δ9-tetrahydrocannabinol. Psychopharmacologia. (1973) 33:53–70. doi: 10.1007/BF00428793
62. Takahashi RN, Zuardi AW, Karniol IG. Composicao quimica e importancia dos diversos constituintes na atividade farmacologica de amostras de Cannabis sativa brasileiras. Brazilian J Med Biol Res. (1977) 10:379–85.
63. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. (2011) 163:1344–64. doi: 10.1111/j.1476-5381.2011.01238.x
64. Russo E, Guy GW. A tale of two cannabinoids : the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. (2006) 66:234–46. doi: 10.1016/j.mehy.2005.08.026
65. Hill AJ, Weston SE, Jones NA, Smith I, Bevan SA, Williamson EM, et al. 9-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia. (2010) 51:1522–32. doi: 10.1111/j.1528-1167.2010.02523.x
66. Hill TDM, Cascio MG, Romano B, Duncan M, Pertwee RG, Williams CM, et al. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol. (2013) 170:679–92. doi: 10.1111/bph.12321
67. Hill AJ, Mercier MS, Hill TDM, Glyn SE, Jones NA, Yamasaki Y, et al. Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol. (2012) 167:1629–42. doi: 10.1111/j.1476-5381.2012.02207.x
68. Vilela LR, Lima IV, Kunsch ÉB, Pinto HPP, de Miranda AS, Vieira ÉLM, de Oliveira ACP, et al. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. (2017) 75:29–35. doi: 10.1016/j.yebeh.2017.07.014
69. Asth L, Iglesias LP, De Oliveira AC, Moraes MFD, Moreira FA. Exploiting cannabinoid and vanilloid mechanisms for epilepsy treatment. Epilepsy Behav. (in press) 106832. doi: 10.1016/j.yebeh.2019.106832
70. dos Santos RG, Hallak JEC, Zuardi AW, de Souza Crippa AC, de Souza Crippa JA. Cannabidiol for the treatment of epilepsy: an overview of possible mechanisms of action and preclinical and human studies. In: Preedy VR, editor. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment. Amsterdam: Elsevier Inc. (2017). p. 795–801
71. Vilela LR, de Oliveira ACP, Moraes MF, Moreira FA, Takahashi RN. The endocannabinoid system as a target for new antiseizure drugs. In: Preedy VR, editor. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis, and Treatment. Amsterdam: Elsevier Inc. (2017). p. 605–15.
72. Russo EB, Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. In: Kendall D, Alexander SPH, editors. Advances in Pharmacology. Cambridge: Academic Press Inc. (2017). p. 67–134. (2019).
73. Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. (2009) 60:77–84. doi: 10.1016/j.phrs.2009.02.010
74. Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. (2012) 76:70–81. doi: 10.1016/j.neuron.2012.09.020
75. Sugaya Y, Kano M. Control of excessive neural circuit excitability and prevention of epileptic seizures by endocannabinoid signaling. Cell Mol Life Sci. (2018) 75:2793–811. doi: 10.1007/s00018-018-2834-8
76. Bitencourt RM, Alpár A, Cinquina V, Ferreira SG, Pinheiro BS, Lemos C, et al. Lack of presynaptic interaction between glucocorticoid and CB1 cannabinoid receptors in GABA- and glutamatergic terminals in the frontal cortex of laboratory rodents. Neurochem Int. (2014) 90:72–84. doi: 10.1016/j.neuint.2015.07.014
77. Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. (2015) 16:264–77. doi: 10.1038/nrn3937
78. Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. (2003) 302:84–8. doi: 10.1126/science.1088208
79. Lupica CR, Hu Y, Devinsky O, Hoffman AF. Cannabinoids as hippocampal network administrators. Neuropharmacology. (2017) 124:25–37. doi: 10.1016/j.neuropharm.2017.04.003
80. Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. (2016) 96:1593–659. doi: 10.1152/physrev.00002.2016
81. Jones NA, Glyn SE, Akiyama S, Hill TDM, Hill AJ, Weston SE, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure. (2012) 21:344–52. doi: 10.1016/j.seizure.2012.03.001
82. Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. (2017) 70:313–8. doi: 10.1016/j.yebeh.2016.11.016
83. Sylantyev S, Jensen TP, Ross RA, Rusakov DA. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci USA. (2013) 110:5193–8. doi: 10.1073/pnas.1211204110
84. Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. (2001) 134:845–52. doi: 10.1038/sj.bjp.0704327
85. Elmes MW, Kaczocha M, Berger WT, Leung KN, Ralph BP, Wang L, et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. (2015) 290:8711–21. doi: 10.1074/jbc.M114.618447
86. Poleg S, Golubchik P, Offen D, Weizman A. Cannabidiol as a suggested candidate for treatment of autism spectrum disorder. Prog Neuro-Psychopharmacology Biol Psychiatry. (2019) 89:90–6. doi: 10.1016/j.pnpbp.2018.08.030
87. Yin A qi, Wang F, Zhang X. Integrating endocannabinoid signaling in the regulation of anxiety and depression. Acta Pharmacol Sin. (2019) 40:336–41. doi: 10.1038/s41401-018-0051-5
88. de Carvalho CR, Takahashi RN. Cannabidiol disrupts the reconsolidation of contextual drug-associated memories in Wistar rats. Addict Biol. (2017) 22:742–51. doi: 10.1111/adb.12366
89. Volkow ND, Hampson AJ, Baler RD. Don't worry, be happy: endocannabinoids and cannabis at the intersection of stress and reward. Annu Rev Pharmacol Toxicol. (2017) 57:285–308. doi: 10.1146/annurev-pharmtox-010716-104615
90. Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: a longitudinal study. J Affect Disord. (2015) 172:211–8. doi: 10.1016/j.jad.2014.10.006
91. Bambico FR, Gobbi G. The cannabinoid CB1 receptor and the endocannabinoid anandamide: possible antidepressant targets. Expert Opin Ther Targets. (2008) 12:1347–66. doi: 10.1517/14728222.12.11.1347
92. Hillard C, Liu Q. Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr Pharm Des. (2014) 20:3795–811. doi: 10.2174/13816128113196660735
93. Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci. (2009) 30:484–93. doi: 10.1016/j.tips.2009.06.006
94. Griebel G, Stemmelin J, Lopez-Grancha M, Fauchey V, Slowinski F, Pichat P, et al. The selective reversible FAAH inhibitor, SSR411298, restores the development of maladaptive behaviors to acute and chronic stress in rodents. Sci Rep. (2018) 8:2416. doi: 10.1038/s41598-018-20895-z
95. Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther. (2013) 138:18–37. doi: 10.1016/j.pharmthera.2012.12.002
96. Micale V, Tabiova K, Kucerova J, Drago F. Role of the endocannabinoid system in depression: from preclinical to clinical evidence. In: Campolongo P, Fattore L, editors. Cannabinoid Modulation of Emotion, Memory, and Motivation. New York, NY: Springer (2015). p. 97–129.
97. Patel S, Hillard CJ. Role of endocannabinoid signaling in anxiety and depression. Curr Top Behav Neurosci. (2009) 1:347–71. doi: 10.1007/978-3-540-88955-7_14
98. Van Gaal L, Pi-Sunyer X, Després JP, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. (2008) 31(Suppl. 2):229–40. doi: 10.2337/dc08-s258
99. Aso E, Ozaita A, Serra MÀ, Maldonado R. Genes differentially expressed in CB1 knockout mice: involvement in the depressive-like phenotype. Eur Neuropsychopharmacol. (2011) 21:11–22. doi: 10.1016/j.euroneuro.2010.06.007
100. Valverde O, Torrens M. CB1 receptor-deficient mice as a model for depression. Neuroscience. (2012) 204:193–206. doi: 10.1016/j.neuroscience.2011.09.031
101. Hill MN, Miller GE, Ho WSV, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. (2008) 41:48–53. doi: 10.1055/s-2007-993211
102. Koethe D, Llenos IC, Dulay JR, Hoyer C, Torrey EF, Leweke FM, et al. Expression of CB1 cannabinoid receptor in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression. J Neural Transm. (2007) 114:1055–63. doi: 10.1007/s00702-007-0660-5
103. Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs. low CRH/NE states. Mol Psychiatry. (2002) 7:254–75. doi: 10.1038/sj.mp.4001032
104. Elsaid S, Kloiber S, Le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical and clinical findings. In: Rahman S, editor. Progress in Molecular Biology and Translational Science. Amsterdam: Elsevier B.V. (2019). p. 25–75.
105. Sales AJ, Fogaça MV, Sartim AG, Pereira VS, Wegener G, Guimarães FS, et al. Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol Neurobiol. (2019) 56:1070–81. doi: 10.1007/s12035-018-1143-4
106. Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. (2006) 163:28–40. doi: 10.1176/appi.ajp.163.1.28
107. Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by δ9-THC in normal subjects. Psychopharmacology (Berl). (1982) 76:245–50. doi: 10.1007/BF00432554
108. Martin-Santos R, Crippa JA, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des. (2012) 18:4966–79. doi: 10.2174/138161212802884780
109. Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. (2008) 18:849–59. doi: 10.1016/j.euroneuro.2008.07.001
110. Fogaça MV, Campos AC, Coelho LD, Duman RS, Guimarães FS. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: role of neurogenesis and dendritic remodeling. Neuropharmacology. (2018) 135:22–33. doi: 10.1016/j.neuropharm.2018.03.001
111. Shallcross J, Hámor P, Bechard AR, Romano M, Knackstedt L, Schwendt M. The divergent effects of cdppb and cannabidiol on fear extinction and anxiety in a predator scent stress model of ptsd in rats. Front Behav Neurosci. (2019) 13:91. doi: 10.3389/fnbeh.2019.00091
112. De Gregorio D, McLaughlin RJ, Posa L, Ochoa-Sanchez R, Enns J, Lopez-Canul M, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. (2019) 160:136–50. doi: 10.1097/j.pain.0000000000001386
113. Zieba J, Sinclair D, Sebree T, Bonn-Miller M, Gutterman D, Siegel S, et al. Cannabidiol (CBD) reduces anxiety-related behavior in mice via an FMRP-independent mechanism. Pharmacol Biochem Behav. (2019) 181:93–100. doi: 10.1016/j.pbb.2019.05.002
114. Granjeiro ÉM, Gomes FV, Guimarães FS, Corrêa FMA, Resstel LBM. Effects of intracisternal administration of cannabidiol on the cardiovascular and behavioral responses to acute restraint stress. Pharmacol Biochem Behav. (2011) 99:743–8. doi: 10.1016/j.pbb.2011.06.027
115. Fogaça MV, Reis FMCV, Campos AC, Guimarães FS. Effects of intra-prelimbic prefrontal cortex injection of cannabidiol on anxiety-like behavior: involvement of 5HT1A receptors and previous stressful experience. Eur Neuropsychopharmacol. (2014) 24:410–9. doi: 10.1016/j.euroneuro.2013.10.012
116. Guimarães FS, Aguiar JCD, Mechoulam R, Breuer A. Anxiolytic effect of cannabidiol derivatives in the elevated plus-maze. Gen Pharmacol. (1994) 25:161–4. doi: 10.1016/0306-3623(94)90027-2
117. Zuardi AW, Cosme RA, Graeff FG, Guimarães FS, Guimaraes FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. (1993) 7:82–8. doi: 10.1177/026988119300700112
118. Shannon S, Lewis N, Lee H, Hughes S. Cannabidiol in anxiety and sleep: a large case series. Perm J. (2019) 23:18–41. doi: 10.7812/TPP/18-041
119. Shannon S, Opila-Lehman J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: a case report. Perm J. (2016) 20:108–11. doi: 10.7812/TPP/16-005
120. Crippa JAS, Nogueira Derenusson G, Borduqui Ferrari T, Wichert-Ana L, Duran FLS, Martin-Santos R, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. (2011) 25:121–30. doi: 10.1177/0269881110379283
121. Bergamaschi MM, Queiroz RHC, Chagas MHN, De Oliveira DCG, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients. Neuropsychopharmacology. (2011) 36:1219–26. doi: 10.1038/npp.2011.6
122. Blessing EM, Steenkamp MM, Manzanares J, Marmar CR. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. (2015) 12:825–36. doi: 10.1007/s13311-015-0387-1
123. Demers CH, Drabant Conley E, Bogdan R, Hariri AR. Interactions between anandamide and corticotropin-releasing factor signaling modulate human amygdala function and risk for anxiety disorders: an imaging genetics strategy for modeling molecular interactions. Biol Psychiatry. (2016) 80:356–62. doi: 10.1016/j.biopsych.2015.12.021
124. Duan T, Gu N, Wang Y, Wang F, Zhu J, Fang Y, et al. Fatty acid amide hydrolase inhibitors produce rapid anti-anxiety responses through amygdala long-term depression in male rodents. J Psychiatry Neurosci. (2017) 42:230–41. doi: 10.1503/jpn.160116
125. Bluett RJ, Gamble-George JC, Hermanson DJ, Hartley ND, Marnett LJ, Patel S. Central anandamide deficiency predicts stress-induced anxiety: behavioral reversal through endocannabinoid augmentation. Transl Psychiatry. (2014) 4:e408. doi: 10.1038/tp.2014.53
126. Lomazzo E, Bindila L, Remmers F, Lerner R, Schwitter C, Hoheisel U, et al. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology. (2015) 40:488–501. doi: 10.1038/npp.2014.198
127. Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, et al. Chapter 5 the endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol. (2009) 85:57–72. doi: 10.1016/S0074-7742(09)85005-8
128. Papagianni EP, Stevenson CW. Cannabinoid regulation of fear and anxiety: an update. Curr Psychiatry Rep. (2019) 21:1–10. doi: 10.1007/s11920-019-1026-z
129. Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. (2002) 418:530–4. doi: 10.1038/nature00839
130. Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. (2005) 30:516–24. doi: 10.1038/sj.npp.1300655
131. Pandolfo P, Takahashi RN. The cannabinoid receptor agonist WIN 55, 212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl). (2006) 188:641–9. doi: 10.1007/s00213-006-0514-0
132. Do Monte FH, Souza RR, Bitencourt RM, Kroon JA, Takahashi RN. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav Brain Res. (2013) 250:23–7. doi: 10.1016/j.bbr.2013.04.045
133. Lemos JI, Resstel LB, Silveira Guimarães F. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res. (2010) 207:105–11. doi: 10.1016/j.bbr.2009.09.045
134. Stern CAJ, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: evidence from cannabidiol treatment. Neuropsychopharmacology. (2012) 37:2132–42. doi: 10.1038/npp.2012.63
135. Stern CAJ, Gazarini L, Vanvossen AC, Zuardi AW, Galve-Roperh I, Guimaraes FS, et al. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. (2015) 25:958–65. doi: 10.1016/j.euroneuro.2015.02.001
136. LaFrance EM, Glodosky NC, Bonn-Miller M, Cuttler C. Short and long-term effects of cannabis on symptoms of post-traumatic stress disorder. J Affect Disord. (2020) 274:298–304. doi: 10.1016/j.jad.2020.05.132
137. Elms L, Shannon S, Hughes S, Lewis N. Cannabidiol in the treatment of post-traumatic stress disorder: a case series. J Altern Complement Med. (2019) 25:392–7. doi: 10.1089/acm.2018.0437
138. Passie T, Emrich HM, Karst M, Brandt SD, Halpern JH. Mitigation of post-traumatic stress symptoms by Cannabis resin: a review of the clinical and neurobiological evidence. Drug Test Anal. (2012) 4:649–59. doi: 10.1002/dta.1377
139. Das RK, Kamboj SK, Ramadas M, Curran HV, Morgan CJA, Yogan K, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl). (2013) 226:781–92. doi: 10.1007/s00213-012-2955-y
140. Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico medical cannabis program. J Psychoactive Drugs. (2014) 46:73–7. doi: 10.1080/02791072.2013.873843
141. Lisboa SF, Vila-Verde C, Rosa J, Uliana DL, Stern CAJ, Bertoglio LJ, et al. Tempering aversive/traumatic memories with cannabinoids: a review of evidence from animal and human studies. Psychopharmacology (Berl). (2019) 236:201–26. doi: 10.1007/s00213-018-5127-x
142. Papini S, Sullivan GM, Hien DA, Shvil E, Neria Y. Toward a translational approach to targeting the endocannabinoid system in posttraumatic stress disorder: a critical review of preclinical research. Biol Psychol. (2015) 104:8–18. doi: 10.1016/j.biopsycho.2014.10.010
143. Sbarski B, Akirav I. Cannabinoids as therapeutics for PTSD. Pharmacol Ther. (2020) 211:107551. doi: 10.1016/j.pharmthera.2020.107551
144. Zer-Aviv TM, Segev A, Akirav I. Cannabinoids and post-traumatic stress disorder: clinical and preclinical evidence for treatment and prevention. Behav Pharmacol. (2016) 27:561–9. doi: 10.1097/FBP.0000000000000253
145. Hill MN, Campolongo P, Yehuda R, Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. (2018) 43:80–102. doi: 10.1038/npp.2017.162
146. Drug Memories: CBD & Addiction|Project CBD. Available online at: https://www.projectcbd.org/medicine/drug-memories-cbd-addiction (accessed November 16, 2020).
147. Luján MÁ, Castro-Zavala A, Alegre-Zurano L, Valverde O. Repeated cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology. (2018) 143:163–75. doi: 10.1016/j.neuropharm.2018.09.043
148. Lopes JB, Bastos JR, Costa RB, Aguiar DC, Moreira FA. The roles of cannabinoid CB1 and CB2 receptors in cocaine-induced behavioral sensitization and conditioned place preference in mice. Psychopharmacology (Berl). (2020) 237:385–94. doi: 10.1007/s00213-019-05370-5
149. De Ternay J, Naassila M, Nourredine M, Louvet A, Bailly F, Sescousse G, et al. Therapeutic prospects of cannabidiol for alcohol use disorder and alcohol-related damages on the liver and the brain. Front Pharmacol. (2019) 10:627. doi: 10.3389/fphar.2019.00627
150. Labigalini E, Rodrigues LR, DaSilveira DX. Therapeutic use of cannabis by crack addicts in Brazil. J Psychoactive Drugs. (1999) 31:451–5. doi: 10.1080/02791072.1999.10471776
151. Socías ME, Kerr T, Wood E, Dong H, Lake S, Hayashi K, et al. Intentional cannabis use to reduce crack cocaine use in a Canadian setting: a longitudinal analysis. Addict Behav. (2017) 72:138–43. doi: 10.1016/j.addbeh.2017.04.006
152. Goncalves JR, Nappo SA. Factors that lead to the use of crack cocaine in combination with marijuana in Brazil: a qualitative study health behavior, health promotion and society. BMC Public Health. (2015) 15:706. doi: 10.1186/s12889-015-2063-0
153. Lee JLC, Bertoglio LJ, Guimarães FS, Stevenson CW. Cannabidiol regulation of emotion and emotional memory processing: relevance for treating anxiety-related and substance abuse disorders. Br J Pharmacol. (2017) 174:3242–56. doi: 10.1111/bph.13724
154. Basavarajappa BS, Shivakumar M, Joshi V, Subbanna S. Endocannabinoid system in neurodegenerative disorders. J Neurochem. (2017) 142:624–48. doi: 10.1111/jnc.14098
155. Ren S-Y, Wang Z-Z, Zhang Y, Chen N-H. Potential application of endocannabinoid system agents in neuropsychiatric and neurodegenerative diseases-focusing on FAAH/MAGL inhibitors. Acta Pharmacol Sin. (2020) 41:1263–71. doi: 10.1038/s41401-020-0385-7
156. Cassano T, Villani R, Pace L, Carbone A, Bukke VN, Orkisz S, et al. From Cannabis sativa to cannabidiol: promising therapeutic candidate for the treatment of neurodegenerative diseases. Front Pharmacol. (2020) 11:124. doi: 10.3389/fphar.2020.00124
157. Watt G, Karl T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for alzheimer's disease. Front Pharmacol. (2017) 8:20. doi: 10.3389/fphar.2017.00020
158. Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: relevance to Parkinson's disease. Neurobiol Dis. (2005) 19:96–107. doi: 10.1016/j.nbd.2004.11.009
159. Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, et al. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE. (2011) 6:e28668. doi: 10.1371/journal.pone.0028668
160. Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in β-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-κB involvement. Neurosci Lett. (2006) 399:91–5. doi: 10.1016/j.neulet.2006.01.047
161. Crippa JAS, Hallak JEC, Zuardi AW, Guimarães FS, Tumas V, dos Santos RG. Is cannabidiol the ideal drug to treat non-motor Parkinson's disease symptoms? Eur Arch Psychiatry Clin Neurosci. (2019) 269:121–33. doi: 10.1007/s00406-019-00982-6
162. Siniscalco D, Schultz S, Brigida A, Antonucci N. Inflammation and neuro-immune dysregulations in autism spectrum disorders. Pharmaceuticals. (2018) 11:56. doi: 10.3390/ph11020056
163. Fusar-Poli L, Cavone V, Tinacci S, Concas I, Petralia A, Signorelli MS, et al. Cannabinoids for people with asd: a systematic review of published and ongoing studies. Brain Sci. (2020) 10:1–18. doi: 10.20944/preprints202007.0373.v1
164. Yeh ML, Levine ES. Perspectives on the role of endocannabinoids in autism spectrum disorders. OBM Neurobiol. (2017) 1:005. doi: 10.21926/obm.neurobiol.1702005
165. Aran A, Eylon M, Harel M, Polianski L, Nemirovski A, Tepper S, et al. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol Autism. (2019) 10:2. doi: 10.1186/s13229-019-0256-6
166. Pietropaolo S, Bellocchio L, Bouzón-Arnáiz I, Yee BK. The role of the endocannabinoid system in autism spectrum disorders: evidence from mouse studies. In: Ilieva M, Kwok-Wai Lau W, editors. Progress in Molecular Biology and Translational Science. Amsterdam: Elsevier B.V. (2020). p. 183–208. doi: 10.1016/bs.pmbts.2020.04.016
167. Karhson DS, Krasinska KM, Dallaire JA, Libove RA, Phillips JM, Chien AS, et al. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol Autism. (2018) 9:18. doi: 10.1186/s13229-018-0203-y
168. Kerr DM, Downey L, Conboy M, Finn DP, Roche M. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav Brain Res. (2013) 249:124–32. doi: 10.1016/j.bbr.2013.04.043
169. Kerr DM, Gilmartin A, Roche M. Pharmacological inhibition of fatty acid amide hydrolase attenuates social behavioural deficits in male rats prenatally exposed to valproic acid. Pharmacol Res. (2016) 113:228–35. doi: 10.1016/j.phrs.2016.08.033
170. Wu HF, Lu TY, Chu MC, Chen PS, Lee CW, Lin HC. Targeting the inhibition of fatty acid amide hydrolase ameliorate the endocannabinoid-mediated synaptic dysfunction in a valproic acid-induced rat model of Autism. Neuropharmacology. (2020) 162:107736. doi: 10.1016/j.neuropharm.2019.107736
171. Zamberletti E, Gabaglio M, Woolley-Roberts M, Bingham S, Rubino T, Parolaro D. Cannabidivarin treatment ameliorates autism-like behaviors and restores hippocampal endocannabinoid system and glia alterations induced by prenatal valproic acid exposure in rats. Front Cell Neurosci. (2019) 13:367. doi: 10.3389/fncel.2019.00367
172. Barchel D, Stolar O, De-Haan T, Ziv-Baran T, Saban N, Fuchs DO, et al. Oral cannabidiol use in children with autism spectrum disorder to treat related symptoms and Co-morbidities. Front Pharmacol. (2019) 9:1521. doi: 10.3389/fphar.2018.01521
173. Aran A, Cassuto H, Lubotzky A, Wattad N, Hazan E. Brief report: cannabidiol-rich cannabis in children with autism spectrum disorder and severe behavioral problems-a retrospective feasibility study. J Autism Dev Disord. (2019) 49:1284–8. doi: 10.1007/s10803-018-3808-2
Keywords: cannabis, phytocannabinoids, cannabidiol, epilepsy, neuropsychiatric disorders
Citation: Bitencourt RM, Takahashi RN and Carlini EA (2021) From an Alternative Medicine to a New Treatment for Refractory Epilepsies: Can Cannabidiol Follow the Same Path to Treat Neuropsychiatric Disorders? Front. Psychiatry 12:638032. doi: 10.3389/fpsyt.2021.638032
Received: 04 December 2020; Accepted: 12 January 2021;
Published: 11 February 2021.
Edited by:
Elaine Elisabetsky, Federal University of Rio Grande do Sul, BrazilReviewed by:
Pasquale Striano, University of Genoa, ItalyGary Stephens, University of Reading, United Kingdom
Copyright © 2021 Bitencourt, Takahashi and Carlini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rafael M. Bitencourt, cmFmYWVsLmJpdGVuY291cnQyQHVuaXN1bC5icg==
†In Memoriam
 Rafael M. Bitencourt
Rafael M. Bitencourt Reinaldo N. Takahashi2
Reinaldo N. Takahashi2