
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 16 February 2021
Sec. Addictive Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.630247
This article is part of the Research TopicPsychological Aspects of Cannabis Use and Cannabis Use DisorderView all 14 articles
Background: Cannabis is known to have a broad range of effects on behavior, including experiencing a “high” and tranquility/relaxation. However, there are several adverse behavioral sequalae that can arise from cannabis use, depending on frequency of use, potency (e.g., THC content), age of onset, and cumulative exposure. This systematic review examined evidence for cannabis-related adverse behavioral sequalae in otherwise healthy human subjects.
Methods: Following PRISMA guidelines, we conducted a systematic review of cross-sectional and longitudinal studies from 1990 to 2020 that identified cannabis-related adverse behavioral outcomes in subjects without psychiatric and medical co-morbidities from PubMed and PsychInfo searches. Key search terms included “cannabis” OR “tetrahydrocannabinol” OR “cannabidiol” OR “marijuana” AND “anxiety” OR “depression” OR “psychosis” OR “schizophrenia” “OR “IQ” OR “memory” OR “attention” OR “impulsivity” OR “cognition” OR “education” OR “occupation”.
Results: Our search detected a total of 2,870 studies, from which we extracted 124 relevant studies from the literature on cannabis effects in the non-clinical population. Effects of cannabis on several behavioral sequelae including cognition, motivation, impulsivity, mood, anxiety, psychosis intelligence, and psychosocial functioning were identified. The preponderance of the evidence suggests that frequency of cannabis use, THC (but not CBD) content, age of onset, and cumulative cannabis exposure can all contribute to these adverse outcomes in individuals without a pre-existing medical condition or psychiatric disorder. The strongest evidence for the negative effects of cannabis are for psychosis and psychosocial functioning.
Conclusions: Although more research is needed to determine risk factors for development of adverse behavioral sequelae of cannabis use, these findings underline the importance of understanding vulnerability to the adverse effects of cannabis, which has implications for prevention and treatment of problematic cannabis use.
Following nicotine and alcohol, cannabis is the most commonly used psychoactive substance in the world, with a global prevalence of 5.1% in 2016 (1, 2). Cannabis is an illegal substance in most countries but is increasingly becoming a legal drug in various states in the USA, in Portugal and Uruguay, and, as of 2018, nationwide in Canada (3). The trend toward legalization of recreational cannabis use corresponds with heightened acceptance, reduced perception of risk, and an increase in cannabis use among adolescents and adults (4–6).
Cannabis contains over 100 distinct cannabinoids, several of which have demonstrated psychoactive properties (7). Two of the most widely researched cannabinoids are delta-9-tetrahydrocannabinol (THC), and cannabidiol (CBD), which directly modulate the endocannabinoid system in humans. The endocannabinoid system is comprised of at least two cannabinoid receptor types, CB1 and CB2, which are involved in various brain functions, including pain, motivation, memory, mood, and reward processing (8, 9).
THC is the principal psychoactive constituent of the cannabis plant and produces a wide range of transient and dose-dependent effects by acting as an agonist at CB1 receptors (10). In animal models, THC administration reduces anxiety at low doses but increases anxiety at higher doses (11). It also produces transient psychotomimetic effects, including perceptual distortions, paranoia, and euphoria (12). There is evidence that acute administration of THC interferes with numerous behavioral and cognitive processes, including emotional processing, episodic memory, attention, working memory, and reward processing [e.g., (13–15)].
In contrast to THC, CBD has a low affinity for CB1 and CB2 receptors, and its molecular mechanism of action remains poorly understood (7). CBD is thought to inhibit the hydrolysis and reuptake of endocannabinoids and modulate cannabinoid receptors (16, 17) CBD produces markedly different psychological effects in comparison to THC and does not adversely impact cognitive or motor performance during intoxication (18, 19). CBD is devoid of any psychomimetic effects, and both human and animal evidence suggests that CBD has anxiolytic properties (20, 21). Co-administration of CBD and THC may alter the pharmacological effect of THC, such that CBD enhances some of THC's desirable effects while attenuating some of its adverse effects (20, 22–24). For example, a recent systematic review investigating CBD's psychoactive properties, suggested that CBD may offset the psychosis-like effects of THC (25).
Recently, there have been concerns surrounding the increased levels of THC found in present day cannabis, combined with reduced levels of CBD (26). This high-potency cannabis is gaining popularity with recreational users despite a growing body of literature indicating that potent cannabis preparations are associated with adverse health outcomes, including increased risk of psychosis, hypomania, impulsivity, and cannabis use disorder (27–29). Furthermore, with substantial legalization, decreased perceptions of risk associated with cannabis may arise, which may further increase current cannabis use prevalence. For example, nationally representative data from adults across the United States indicated that the perceived risk of recreational cannabis use decreased from 51.3% in 2002 to 40.3% in 2012 (30), even though the THC potency of cannabis has increased from 8.9% in 2008 to 17.1% in 2017 (31). These changes in perception of risk emphasize the need for continued research on the behavioral effects of cannabis use, since there is significant variance concerning the potential harms and benefits of cannabis use.
This systematic review examines experimental, cohort, and cross-sectional studies to determine the effects of cannabis use on behavioral, cognitive, mental health and psychosocial adverse outcomes in non-clinical populations. We sought to address the following aims: (1) to determine the effects of cannabis use on the prevalence and severity of adverse outcomes in people without medical or psychiatric disorders and (2) to determine risk factors associated with the development of adverse outcomes in cannabis users.
Using PubMed and PsycINFO, original, peer-reviewed research articles were searched for based on the PRISMA guidelines by two of the authors (MS and RB) (See Figure 1) (32). Articles available online in the English language between 1990 through the end of October, 2020 were considered. Search terms (found in the title or abstract) utilized to obtain relevant articles were: “cannabis” OR “tetrahydrocannabinol” OR “cannabidiol” OR “marijuana” AND “anxiety” OR “depression” OR “psychosis” OR “schizophrenia” “OR “IQ” OR “memory” OR “attention” OR “impulsivity” OR “cognition” OR “motivation” OR “education” OR “occupation.” Titles and abstracts were screened for relevance by two of the authors (MS and RB), and articles passing this stage were downloaded and assessed for eligibility via full-text review by MS. All uncertainties were assessed and resolved by the senior author (TPG).
The inclusion criteria were: (1) Original studies with experimental, cross-sectional, or cohort designs; (2) studies in which the sample at baseline demonstrated no current medical condition or history of a psychiatric disorder with the exception of cannabis use disorder; (3) studies utilizing validated or objective measures to evaluate cannabis use (e.g., urine toxicology, scores from The Cannabis Use Disorders Identification Test [CUDIT], etc.) (4) studies utilizing validated or objective measures to evaluate the primary outcome (e.g., hospital records, scores from the Beck Depression Inventory, graduation GPA, etc.).
The exclusion criteria were: (1) reviews, meta-analyses, and case studies. Additionally, we employed the Newcastle Ottawa Scale (NOS) to evaluate the quality of eligible cross-sectional and cohort studies in the review (33). The NOS allows a maximum score of nine and evaluates studies on three broad domains; (1) the selection of the study groups; (2) the comparability of the groups; and (3) the verification of the outcome of interest. Studies receiving a score of 5 or lower on the NOS indicated a high risk of bias, and consequently were excluded from the review (See Supplementary Tables 2,3 for the quality ratings of the included and excluded studies, respectively).
We recorded the following variables from each study: author, publication year, study design, sample size, study population, follow-up time, outcome measures, level of cannabis use, matched variables, and relevant findings (see Supplementary Table 1 for further information). To determine whether there is a dose-dependent relationship between cannabis and the primary outcomes, we adapted a classification system utilized by Batalla et al. (34) which evaluated levels of cannabis use. We classified both cross-sectional and experimental designs. Cannabis dependent persons are those who meet the criteria for cannabis use disorder on the DSM-5 or DSM-IV at the time of the study. Chronic cannabis users are persons who do not meet the DSM-5 or DSM-IV criteria for cannabis use disorder but use cannabis 3+ times a week for at least 1 year. Recreational cannabis users are persons who use cannabis between one and four times a month, and controls were persons use had used cannabis <10 times in their lifetime.
To determine whether the accumulated evidence implicates a neutral or negative effect of cannabis for each domain, we calculated the proportion of studies evincing a negative effect of cannabis use against the total number of studies (See Table 2 for a summary of the level of evidence ratings) as follows:
1 = 0–19% corresponds with strong evidence of no effects of cannabis use
2 = 20–39% corresponds with moderate evidence of no effects of cannabis use
3 = 40–59% corresponds with mixed evidence of neutral or negative effects of cannabis use
4 = 60–79% corresponds with moderate evidence of negative effects of cannabis use
5 = 80–100% corresponds with strong evidence of negative effects of cannabis use.
We identified 2,870 hits of which 159 were considered potentially relevant, based on title and abstract inspection (See Figure 1 for methods overview). Detailed examination of the potentially relevant publications reduced the sample to 127 studies that were within the inclusion criteria set for this systematic review. However, after examining the quality of eligible studies via the NOS, three studies were excluded from the review. Overall, 124 studies were included in the review with publication dates ranging from 1995 to 2020. Studies were conducted in a broad range of countries, and comprised of 48 cohort designs, 26 placebo-controlled, counter-balanced, experimental designs, and 50 cross-sectional designs (see Table 1).
Four longitudinal studies evaluating the impact of cannabis use and memory were included (35, 42, 47, 63). Becker et al. (35) investigated differences among multiple cognitive domains within daily, adolescent, cannabis users and non-users at baseline and 2 years later. At follow-up, cannabis users demonstrated significant impairments in working memory and verbal learning relative to non-users. Moreover, an earlier age of cannabis use onset corresponded with more severe impairments at follow-up. Similarly, following a birth cohort of 1,037 individuals, Meier et al. (47) assessed neurocognitive functioning in participants at ages 13 and 38, while assessing cannabis use at ages 18, 21, 26, 32, and 38. Persistent cannabis use beginning in adolescence corresponded to impairments in working memory and verbal learning, in addition to other neurocognitive domains. However, a recent 14 year longitudinal study following adolescents annually, found no relationship between cannabis or alcohol use and performance in tasks evaluating verbal memory (63). Moreover, in a study comparing the effects of extended (28 day) of cannabis abstinence and reinstatement of cannabis use in people with schizophrenia vs. healthy controls with cannabis use disorder, there were no significant effects of 28 days of cannabis abstinence or reinstatement on verbal learning, verbal memory (% retention on HVLT-R task), and visuospatial memory in healthy controls, as compared to significant (>40%) improvements in verbal learning and memory with abstinence and impairment with cannabis use reinstatement in schizophrenia patients (62).
Twelve cross-sectional designs found no significant impairments in memory among cannabis users (24, 56–60, 64–69). Three of these studies investigated visuospatial working memory and independently employed fMRI to investigate potential differences in brain activity (67–69). Interestingly, although the three studies failed to suggest a behavioral deficit in cannabis users, the researchers all found unusual brain activity in cannabis users while performing the visuospatial memory task. Additionally, four other cross-sectional designs concluded no significant impairments among cannabis users in working memory (57–60). An fMRI study assessing working memory among a small sample of abstinent but frequent cannabis-using adolescents obtained no behavioral impairments on the task (60). However, during the task, cannabis users demonstrated increased brain activity in regions implicated in working memory, suggesting a reliance on neural compensatory strategies. These neurofunctional results were obtained in a similar study which found neural differences between cannabis users and non-users on a Sternberg-type working memory task, but no behavioral differences (57). In another study comparing adult, chronic cannabis users and controls on tests evaluating visuospatial memory, verbal memory, and executive functioning over a 28 day period, cannabis users demonstrated significant deficits in verbal memory during the first week of abstinence, but by Day 28, any significant differences between groups diminished (66). Moreover, no relationship between cannabis use frequency and performance at Day 28 emerged, presenting evidence for re-instatement of cognitive abilities with abstinence.
In contrast, 11 cross-sectional designs concluded that cannabis use is associated with memory impairments (24, 36, 39, 40, 43–46, 48, 49, 56). Morgan et al. (24) investigated whether the type of cannabinoid that users consumed led to differing impairments in memory. Splitting cannabis users into high THC/high CBD and high THC/low CBD groups, individuals who smoked high THC/high CBD cannabis did not demonstrate any deficits in immediate or delayed recall on an episodic memory task (24). However, high THC/low CBD users performed significantly worse on the task assessing episodic memory, leading to the authors' proposition that CBD may attenuate the harmful effects of THC on cognition. Additionally, three studies comparing 2–4 week cannabis abstinent users and non-users on measures of verbal learning and memory indicated that despite abstinence, users performed significantly worse than non-users (40, 44, 49). However, one of these studies found that among a 21 day cannabis abstinence intervention, slight improvements in verbal memory were obtained in cannabis users between Weeks 2 and 3, indicating partial recovery of neurocognitive functioning (40).
Similarly, the majority of experimental studies in which healthy volunteers received varying doses of THC suggest a negative impact of cannabis use on verbal learning, episodic memory, and working memory (13, 37, 41, 50, 51, 53). A recent study examined acute and delayed effects of THC intoxication on susceptibility to false memory among occasional cannabis users (50). Intoxicated participants generated significantly more spontaneous and suggestion-based false memories immediately after intoxication in comparison to placebo. The authors surmise that THC-intoxicated individuals may demonstrate a tendency toward more liberal responding to memory-related questions due to reductions in alertness and impaired abilities in forming learning associations. Additionally, the effects were most prominent at immediate in comparison to delayed recall. One experimental design administering vaporized THC (12%) or placebo to male, recreational, adolescent and adult cannabis users found that in comparison to adolescent users, adults exhibited greater impairments in a spatial working memory task following intoxication (41).
Three experimental designs found no relationship between acute cannabinoid intoxication and memory impairments (14, 61, 70). Bhattacharyya et al. (14) conducted an fMRI studying neurocognitive function during the Hopkins Verbal Learning Task after acute oral THC administration (10 mg) to healthy, occasional cannabis users. Although overall task performance was unaffected, THC attenuated brain activation in regions associated with episodic memory. These findings implicate that greater neural effort is required after THC administration to maintain normal levels of task performance. Additionally, Englund et al. (61) investigated whether a pre-treatment of 10 mg of oral Δ-9-tetrahydrocannabivarin (THCV) prior to 1 mg of THC intoxication would lead to cognitive and clinical effects among a non-clinical sample of men. The authors found that pre-treatment with THCV protected participants from memory impairments in a delayed memory recall task.
Overall, the literature suggests that acute THC intoxication produces acute impairments in verbal learning, episodic, and working memory. Moreover, the literature suggests a dose-dependent relationship between levels of cannabis use and long-term impairments within this cognitive domain.
Seven cross-sectional studies found a significant association between cannabis use and attention deficits (36, 40, 46, 64, 73, 74, 77). Fontes et al. (74) compared a sample of adult early-onset (before age 15), late-onset (after age 15), and non-users on a sustained attention task. Results indicated that early-onset users demonstrated significant impairments in comparison to controls and late-onset users. Similar findings were also obtained by Jacobsen et al. (73), where adolescent cannabis users performed significantly worse on a sustained attention task relative to controls and adolescent tobacco users. However, five cross-sectional designs indicated no differences in attention between cannabis users and non-users (44, 48, 57, 66, 78). Fridberg et al. (78) and Jager et al. (57) each investigated whether chronic cannabis users demonstrated impairments in selective attention, both in terms of behavioral performance and abnormal brain activity. Although both studies found that cannabis users performed equally fast and accurate as controls, cannabis users demonstrated abnormal brain activity as evaluated through fMRI and EEG, respectively.
Five experimental designs found that shortly after THC intoxication, adult participants without a medical or psychiatric disorder demonstrated significant deficits in attention (53, 70–72, 76). Desrosiers et al. (70) found that after acute, THC intoxication, recreational cannabis users performed significantly worse on a divided attention task in comparison to chronic users. The researchers surmised that the differences in performance are attributed to tolerance development in frequent smokers. Two major limitations of this study should be noted, however. First, performance while sober was not obtained in the study, so conclusions on overall attention impairments were limited. Additionally, occasional and frequent users' performance was not compared against a group of healthy controls to further evaluate the significance of their impairments. A more recent experimental design compared the effects of THC-dominant and THC/CBD equivalent cannabis ratios among a non-clinical sample on a task of divided attention (72). Unlike prior research (15, 24), the co-administration of CBD did not mitigate any adverse, cognitive effects of THC, and participants demonstrated significant attention deficits in both conditions. However, one experimental design administering either 0.015 or 0.03 mg/kg of THC to former, recreational cannabis users without a medical or psychiatric disorder found no impairments in sustained attention, as measured via the Rapid Visual Processing Task (79).
One longitudinal study following adolescents for 2 years found no relationship between cannabis use and speeded attention (35). However, users did demonstrate impairments in other cognitive domains, including working memory and planning. Rabin et al. (62) compared the effects of extended (28 day) cannabis abstinence and reinstatement of cannabis use in people with schizophrenia and healthy controls with cannabis use disorder and found no improvements in sustained attention among both groups of abstainers. Improvements were not obtained in multiple cognitive domains as well, including executive function and visuospatial working memory.
Overall, the literature suggests a relationship between chronic cannabis use and impairments in attention. However, more evidence is required to ascertain whether there is a dose-dependent effect of levels of cannabis use and deficits in this cognitive domain. Additionally, acute THC-intoxication appears to produce temporary impairments in attention.
Two cross-sectional studies and one experimental study found a relationship between cannabis use and impairments in processing speed (36, 64, 72). Thames et al. (36) evaluated whether cannabis use in the previous month impacted neurocognitive functioning in a non-clinical adult sample. Participants were classified as either recent users (last use within 4 weeks since study), remote cannabis users (last use >4 weeks since study), and non-users (no report of cannabis use). Recent users demonstrated impairments in information processing speed in addition to other cognitive domains (e.g., attention, working memory, and executive functioning) in comparison to past- and non-users (36). Interestingly, past users did not differ from non-users in any domain except for executive functioning, suggesting that cannabis abstinence can restore previous cognitive abilities. A rigorously controlled experimental design compared whether high THC/CBD vs. equivalent THC/CBD cannabis ratios would produce differential cognitive impairments among volunteers with no history of a psychiatric disorder (72). In an information processing speed task, 1:1 THC/CBD intoxication produced greater impairments in participants in comparison to the high THC/CBD condition, suggesting that CBD may not effectively prevent cognitive impairments associated with THC intoxication.
However, one recent 14 year longitudinal study following adolescents at baseline yearly, determined no relationship between cannabis or alcohol use and performance in tasks evaluating processing speed (63). Furthermore, two cross-sectional designs evaluating processing speed among older adults without a current medical condition or psychiatric disorder, no relationship between cannabis use and this cognitive domain emerged (56, 65). Burggren et al. (65) evaluated verbal memory, processing speed, and executive functioning among a sample of older adults who were former, chronic, cannabis users in addition to non-users and found that while former users performed worse than non-users on all cognitive domains, the differences were not significant. Similarly, Thayer et al. (56) obtained no behavioral differences in processing speed and other cognitive domains across a sample of older adults who were either current, chronic, cannabis users or had no history of cannabis use.
Overall, the evidence remains mixed on whether cannabis use alters processing speed, and whether there is a dose-dependent relationship between cannabis use and impairments in this neurocognitive ability.
Three longitudinal designs implicate a significant relationship between adolescent cannabis use and impairment in executive functioning (35, 47, 86). Castellanos et al. (86) concluded that frequent cannabis use among adolescent boys was associated with a significant decline in executive functioning by the end of high school. The relationship persisted even after controlling for high school graduation and other substance use. Additionally, a more recent longitudinal study obtained similar findings. Becker et al. (35) found that in comparison to adults without a history of cannabis use, chronic cannabis users who began use in adolescence, demonstrated significant reductions in planning and verbal learning at a 2 year follow-up. Additionally, reducing cannabis use did not ameliorate users' cognitive deficits.
Eight cross-sectional designs obtained a significant relationship between cannabis use and impairments in executive functioning (49, 74, 77, 80–83, 85). Four of these studies implicated that chronic cannabis users demonstrate significant impairments in decision-making in comparison to their non-using counterparts (77, 80, 81, 85). Two studies comparing early-onset (use began before age 16), late-onset (use began at or past age 16), and non-using controls in tasks evaluating executive functioning (e.g., Stroop task, Frontal Assessment Battery, and Wisconsin Card Sorting Test) suggested that only early-onset users performed poorly on these tests in comparison to both the late-onset users and healthy controls (74, 82).
However, seven cross-sectional studies found no deficits between cannabis users and non-users on tasks assessing executive functioning (48, 56, 65, 66, 87–89). In a study comparing numerous cognitive abilities between controls and older adults who were currently using cannabis, no differences between groups on any neuropsychological test emerged (56). Furthermore, employing structural MRI, the authors also obtained no significant differences between groups in white or gray matter density. However, one limitation of this study is that duration and levels of use was not specified, which may have impacted the findings. Moreover, employing fMRI, Hatchard et al. (88) obtained no performance differences between cannabis users and non-users on a Counting Stroop Task. However, in contrast to non-users, cannabis users displayed more intensive and extensive BOLD responses. The researchers surmise that the recruitment of additional brain regions among cannabis users may be a neural compensatory strategy to maintain their behavioral performance. Additionally, in one study comparing 25 day abstinent, adult, chronic cannabis users, cocaine users, and non-using controls on the Iowa Gambling Task, cocaine users demonstrated significant learning impairments in comparison to cannabis users and controls (89). Although abstinent-cannabis users did not significantly differ from controls on the task, their performance was consistently lower. This finding implicates that cannabis use may negatively impact executive functioning, but with abstinence, cognitive deficits are somewhat reversible. Finally, the researchers also obtained a dose-dependent effect of cannabis use on IGT performance, which implicates that this substance may affect cognition.
Overall, our review obtained a moderate level evidence concerning the effects of cannabis use and impairments in executive functioning. However, levels of cannabis use did not consistently correspond with greater impairments in this neurocognitive domain.
Only one longitudinal study investigated the role of cannabis use on the course on inhibitory control (63). Following adolescents between the ages of 12–15 annually, for 14 years, Infante et al. (63) found that greater cumulative cannabis use in adolescence was associated with deficits in inhibitory control in adulthood. The effects remained after controlling for relevant confounders including alcohol use.
Concerning cross-sectional designs, six studies also concluded that cannabis users exhibit greater impulsivity and poorer inhibitory control than non-users (44, 48, 64, 74, 91, 92). Lisdahl and Price (64) examined whether past-year cannabis use among adolescents and young adults corresponded with impairments in inhibitory control. After controlling for numerous confounders, cannabis use corresponded with poorer cognitive inhibition in a dose-dependent fashion. Additionally, there is some evidence that cannabis use corresponds with greater impulsivity in daily life. Ansell et al. (91) employed Ecological Momentary Assessment (EMA) to examine more immediate effects of cannabis on same day and subsequent day reports of impulsivity among a sample of chronic, cannabis-using adults. The authors found that independent of alcohol consumption, cannabis use was associated with same day increases in impulsivity and predicted next day increases in impulsivity.
However, three separate cross-sectional studies reported no impulsivity differences between cannabis users and non-users (38, 94, 95). In a study comparing inhibitory control among 28 day abstinent adolescent cannabis users and non-users, no significant differences between the groups' performance on a go/no-go task emerged (95). Similar findings were obtained by a more recent study, where regular, young-adult cannabis users and matched non-users performed similarly on an inhibitory task (94). However, when the authors compared early-onset (prior to age 16) and late-onset (ages 16 or later) cannabis users, individuals in the former group made greater errors of commission, but the results remained insignificant.
Although the literature is ambiguous on whether cannabis use produces long-term effects on impulsivity, there is evidence that acute administration of THC produces impairments in inhibitory control. Five experimental designs suggest a relationship between THC intoxication and increased impulsivity (41, 71, 76, 90, 93). Employing a double-blind, placebo-controlled design, Ramaekers et al. (71) compared the effects of 500 μg/kg of THC among occasional and chronic cannabis-using adults in the stop signal task (SST) 35 min, 3 h 30 min, 5 h 30 min, and 7 h 30 min post-THC administration. Both occasional and heavy cannabis users demonstrated impaired inhibitory control in the intoxicated condition, where worst performance arose 35 min after intoxication. To replicate this study's findings, Theunissen et al. (76) repeated the former study with a different group of occasional and chronic cannabis users and found that both users demonstrated significant impairments in the SST 45 min after THC administration.
Overall, whether chronic cannabis use reduces inhibitory control in a dose-dependent manner is less clear. However, the literature suggests that acute administration of THC leads to greater impulsivity and poorer inhibitory control.
Six longitudinal studies assessing the impact of cannabis use on IQ were included (42, 47, 86, 97–99). Two cohort studies concluded that there was a significant association between cannabis use beginning in adolescence and a decline in IQ (47, 86). Castellanos-Ryan et al. (86) concluded that frequent cannabis use among adolescent boys is associated with a significant decline in verbal IQ scores by the end of high school. Moreover, the authors found that poor short-term and working memory in pre-adolescence was associated with an earlier age of onset for cannabis use. Despite these findings, the relationship between cannabis use and verbal IQ was mediated by a reduction in high-school graduation rates. Meier et al. (47) conducted a large, national representative, birth-cohort design (N = 1,748) which explored the relationship between cannabis use from preadolescence into adulthood and potential changes in IQ and cognitive abilities. Individuals who received a diagnosis of cannabis use disorder before the age of 18 demonstrated an eight-point decline in IQ by the age of 38 in comparison to peers who never used cannabis or began use after the age of 18 (47). This relationship persisted after statistically adjusting for alcohol, tobacco and other drug use, schizophrenia, and educational level.
However, four cohort designs assessing cannabis use and IQ changes indicate that there is no direct relationship between these two variables (42, 97–99). In a birth-cohort twin study, adolescent cannabis use was associated with a decline in IQ and impaired executive functioning, but twins who used cannabis performed no worse than their co-twin without a history of cannabis use (42). The authors subsequently suggest that family background factors contribute to a spurious relationship between cannabis use and impaired executive functioning in the general population. An additional, cohort, twin design also indicated no relationship between cannabis use and IQ decline (99). Participants' IQ was measured prior to cannabis use between the ages of 9 to 12 years old, and again 8 years later. Cannabis use between this period corresponded with reduced scores in IQ at follow-up, however no clear relationship between frequency of use and IQ emerged. Consequently, the authors determined that the declines in IQ reflected the effects of familial or genetic factors that predated cannabis use onset. In a separate study investigating cognition and verbal IQ among 28 day abstinent early-onset (smoking before age 17) cannabis users, late-onset (smoking at or after age 17) cannabis users, and controls, only early-onset users performed poorly on measures evaluating verbal IQ (55). However, this relationship did not persist after controlling for relevant variables, including familial and childhood factors.
Currently, the literature remains mixed on whether chronic cannabis use impacts intelligence and IQ. Some studies suggest that there is a dose-dependent relationship between cannabis use and IQ scores, while other evidence suggests that there is no relationship between these variables.
Four cross-sectional studies included in the review implicate that cannabis users demonstrate motivation impairments in comparison to non-users (100–103). In a study examining the influence of reward on mood and performance on the spatial delayed response task in an adult sample of chronic cannabis users, tobacco smokers, and non-smoking controls, cannabis users rated their mood as significantly lower than smokers and controls during the reward conditions (101). The authors concluded that cannabis use may reduce reward processing at a behavioral level. Additionally, Lane et al. (103) found that on a monetary task assessing perseverative responding, adolescent cannabis users switched to the non-work, but less rewarding task significantly earlier than non-users.
However, two cross-sectional studies obtained no relationship between cannabis use and reductions in motivation (104, 105). In one study comparing adolescent cannabis users and non-users on a self-report battery examining high school students' motivation, no differences emerged (105). Additionally, Jager et al. (104) compared the performance of abstinent cannabis-using boys and non-using boys on the monetary incentive delay (MID) task, which assesses motivation and reward processing. Although no behavioral differences between groups emerged, a significant limitation of the study should be noted. Cannabis abstinence was not controlled for and varied from 1 to 16 weeks, which may have impacted the observed findings. Overall, the evidence suggests a dose-dependent relationship between cannabis use and impairments in motivation.
We identified eight longitudinal studies demonstrating that cannabis use has adverse effects on psychosocial functioning, including occupational and educational attainment (86, 106–112). Follow-up times ranged from 1 to 35 years. One study using a large, nationally representative sample found that among students from grade 9–12, individuals who used cannabis at baseline were less likely to attend class, complete their homework, and obtain or value high grades relative to their abstaining peers at year 2 and 3 (111). Furthermore, frequent cannabis use reduced the likelihood of planning to pursue either a graduate or professional degree post-graduation. A recent birth cohort study assessed four trajectories of cannabis use, including non-users, adolescent-limited, adult-onset, and chronic-adolescent users (107). Individuals who began cannabis use in adolescence and continued use throughout adulthood demonstrated the worst psychosocial functioning at age 35, while the non-user group reported the highest level of well-being. Moreover, heavy cannabis use in adolescence corresponded with an increased risk of adverse outcomes at ages 30–35, including a reduced likelihood to attain a postsecondary degree, a lower weekly income, a greater likelihood to rely on welfare, a greater likelihood of being unemployed, and a greater likelihood of being arrested. In contrast, one prospective, cohort design concluded that although there was a significant relationship between cannabis use at age 15 and educational performance at age 16, this relationship became non-significant after adjusting for relevant variables such as cigarette smoking, childhood conduct problems, and childhood depressive symptoms (98).
Overall, the evidence obtained in this review implicates a dose-dependent relationship between levels of cannabis use and poorer psychosocial outcomes.
We identified two experimental studies demonstrating a negative effect of THC in mood among a group of adults without a history of medical or psychiatric illness (15, 122). In one study, male participants received 10 mg of THC and reported their mood 1, 2, and 3 h post- THC administration. Relative to placebo, participants reported significantly elevated levels of dysphoria (15). However, two separate experimental studies administering THC to a non-clinical sample did not obtain similar findings (51, 61). Instead, both studies found that there was a dose-dependent relationship between THC consumption and pleasurable mood ratings.
Concerning longitudinal findings, we identified thirteen studies that suggested a relationship between cannabis use and a greater likelihood of developing major depression (107, 109, 113–121, 123, 131). However, six of these studies specifically outlined that only heavy or chronic (<4 times/week) adolescent cannabis use is a risk factor for depression in adulthood. Otten and Engels (123) investigated the relationship between cannabis use, depression, and the serotonin transporter gene (5-HTTLPR). The serotonin transporter gene is considered a significant candidate gene for its role in depression [for a review, see (154)]. Specifically, this gene encodes the serotonin transporter protein, which is responsible for the reuptake of serotonin from the synaptic cleft into the presynaptic neuron. The authors identified that cannabis use increases the risk for an increase in depressive symptoms over a 5 year period but only in users with the short allele of the 5-HTTLPR genotype. One study also investigated whether sex differences emerged when evaluating the effect of adolescent cannabis use and depressive symptomology in young adulthood (121). A state-wide secondary school sample of 1,601 students aged 14–15 were followed for 6 years. Daily use in female adolescents was associated with a 5-fold increase in the odds of reporting depression and anxiety after adjustment for concurrent use of other substances. Weekly or more frequent cannabis use in adolescents predicted an ~2-fold increase in risk for later depression and anxiety at follow-up, even after adjusting for potential confounders.
However, 10 separate cohort designs suggest that cannabis use is not associated with an increased risk of a future depression diagnosis (124–132, 155). Despina et al. (125) assessed 1,606 adolescents and obtained data on frequency of cannabis use and serious suicidal ideation at ages 15, 17, and 20 years. While cannabis use did not predict depressive symptomology or suicide ideation, depression predicted subsequent cannabis use, even after adjusting for possible confounders, including other substance use. Additionally, it is important to note that five of these longitudinal designs only considered adult cannabis use at baseline, which limited conclusions concerning the causal relationship between adolescent cannabis use and subsequent depressive symptomology (124, 126, 127, 131, 155).
Three cross-sectional designs also concluded that relative to non-users, cannabis users had more severe depressive symptomology (54, 100, 102). Employing positron emission tomography (PET), Bloomfield et al. (102) evaluated the relationship between dopaminergic function and subjective apathy in a sample of adult, chronic cannabis users. In comparison to normative data from adult without a history of cannabis use, cannabis users reported significantly greater levels of apathy and demonstrated reduced dopamine synthesis capacity.
Overall, the evidence obtained is mixed regarding the impact of cannabis use on depressive symptomology. Some studies suggest a dose-dependent relationship between levels of cannabis use and increased risk of depression, while other evidence found no relationship between these variables after controlling for relevant confounds.
Three prospective cohort designs implicate cannabis use as a significant risk factor for subclinical anxiety symptomology (116, 119, 133). One longitudinal study determined that among adolescent boys, increases in past-year cannabis use corresponded with increases in depressive and anxious symptomology the following year (119). Similarly, Hayatbakhsh et al. (116) found that after controlling for numerous confounding variables, cannabis use before the age of 15 correlated with greater anxious symptomology at age 21 among a national representative sample of adolescents (N = 3,239).
Four prospective designs suggest chronic cannabis use as a significant risk factor for the development of an anxiety disorder (107, 120, 121, 133). A birth-cohort study collecting information until participants reached 21 years old determined that frequent cannabis use in adolescence predicted a more than 2-fold increase in the diagnosis of an anxiety disorder by the final follow-up (133). This relationship persisted after controlling for tobacco, alcohol, and other substance use. Additionally, anxiety symptomology never predicted subsequent cannabis use. An additional birth-cohort longitudinal design investigated the impact of cannabis use and internalizing problems until age 35 (107). Adolescent-onset cannabis users and young-adulthood cannabis users were significantly more likely to be diagnosed with an anxiety disorder in comparison to adolescent-limited cannabis users, and non-users. The effect persisted even after controlling for childhood, other substance use, and familial factors. Additionally, one cohort study following a group of 14 year-olds for 15 years obtained no consistent relationship between adolescent cannabis use and a diagnosis of major depressive disorder at 29, but daily cannabis use in adolescence was a significant risk factor for development of generalized anxiety disorder at 29, even after adjusting for baseline confounders and other concurrent drug use (120). The researchers also found that overall, among participants, there was a reduction in cannabis use over young adulthood. However, among individuals who developed an anxiety disorder, the pattern of use was associated with either the maintenance or increasingly frequent use of cannabis throughout young adulthood. Despite these findings, six prospective studies obtained no relationship between cannabis use and an increased risk for a diagnosis of an anxiety disorder or an increase in subclinical anxiety symptomology (113, 115, 127, 129, 135, 155). One UK birth cohort study investigated the relationship between cannabis or cigarette use (at age 16) and diagnosis of depression or anxiety 2 years later (115). The authors determined that after adjusting for potential confounds and cigarette use, the relationship between cannabis use and anxiety symptomology diminished to a non-significant result. Similarly, in another population-based cohort design spanning 30 years, cannabis use in adolescence predicted depressive symptomology and suicidality at age 50, but not anxiety (113). Two other prospective cohort designs also determined that cannabis use in adulthood was not associated with anxiety symptomology, however these studies did not obtain or consider information regarding adolescent cannabis use (127, 135).
Two cross-sectional designs comparing non-clinical cannabis users to non-users suggested a relationship between cannabis use and greater subclinical anxiety symptomology (54, 100). One study classified cannabis users into those demonstrating presence of CBD in hair and those who did not, in addition to high- or low- THC levels. Users with high-THC levels in their hair and users with no CBD reported the most depressive and anxious symptomology, suggesting negative long-term effects of high-THC on mood (54). Wright et al. (100) investigated whether adult, non-clinical cannabis users differ from non-users in self-reports of anxiety, depression, and behavioral approach to rewards. In line with their hypothesis, users reported elevated depressive symptoms, and female users reported elevated anxiety symptoms than non-users.
Seven experimental studies in which healthy non-users received varying doses of THC produced greater anxious symptomology in comparison to placebo (13–15, 41, 52, 53, 90). McDonald et al. (90) administered either 7.5 or 15 mg of THC to a sample of men and women with no history of a psychiatric disorder prior to a neurocognitive battery and found a dose-dependent effect of THC on anxiety, anger, fatigue, and confusion. A more recent study provided recreational cannabis users either placebo (0 mg), 29, 49, and 69 mg of THC and obtained a dose-dependent effect of THC on increasing levels of anxiety (52). Additionally, the researchers found that subjective effects persisted up to 8 h post-intoxication.
Finally, two studies administering THC to volunteers without a history of a psychiatric disorder found no effects of THC on anxious symptomology while an additional two studies suggested an anxiolytic effect of CBD among a sample of healthy participants (61, 122, 134, 136). Zuardi et al. (136) and Linares et al. (134) both found that 300 mg of CBD reduced adult participants' self-report of speech-induced anxiety in comparison to placebo, 150 or 600 mg of CBD.
Overall, the literature implicates a dose-dependent relationship between greater levels of cannabis use and elevated anxious symptomology. However, the evidence suggests acute, anxiolytic effects of CBD, a constituent of cannabis sativa.
Thirteen longitudinal designs concluding a relationship between cannabis use and an increased risk for the development of a psychotic disorder were included in this review (124, 132, 138, 141–144, 147–152). One cohort study following Swedish male conscripts at ages 18–20 for 27 years obtained a dose-dependent relationship between cannabis use and a formal diagnosis of schizophrenia (143). However, the study was limited in that data regarding use of cannabis before conscription was unavailable. A more recent, nationally representative, birth cohort design (N = 6,534) also identified a dose-dependent, positive relationship between adolescent cannabis use and psychosis in adulthood (151). Cannabis use between the ages of 15–16 years was associated with a subsequent psychosis diagnosis by age 30, and this effect persisted after controlling for baseline prodromal symptoms, daily smoking, alcohol use, other substance use, and parental psychosis. A separate cohort design found that cannabis use at age 16 predicted psychotic symptoms at age 19 (152). However, psychotic symptoms at age 13 predicted cannabis use at, respectively, ages 16 and 19, providing support for a bidirectional causal association between the two variables. Only one cohort design did not obtain a significant relationship between cannabis use and an increased risk for psychosis after adjusting for relevant confounders, including tobacco use (153).
Two cross-sectional studies comparing cannabis users and non-users without a psychiatric disorder concluded that users report greater psychotic symptoms than non-users (54, 137). Morgan et al. (54) classified cannabis users via hair samples into those who use both THC and CBD, and those who only use THC. Cannabis users who only demonstrated use of THC reported significantly more psychotic symptomology than THC and CBD users and non-users, implicating a relationship between THC and psychotic symptomology. Similarly, a more recent cross-sectional design attained a dose-dependent relationship between cannabis use frequency and severity of psychotic symptoms including mania, paranoia, and presence of auditory hallucinations (137). The effects persisted even after adjusting for relevant confounds, such as sex, age, and other substance use (137).
One pilot, within-subjects, placebo-controlled, double-blind design obtained no effect of acute THC administration on psychotic, anxious, or depressive symptomology following a 5 day pre-treatment of THCV (61). An earlier study led by the same team of researchers also found that pre-administration of CBD reduced participants' self-reports of psychotic symptomology on the Positive and Negative Affect Syndrome Scale (PANSS) (140). However, relative to placebo, this difference did not reach significance, suggesting that CBD may not fully attenuate the psychotic symptoms produced by THC.
Overall, the literature presents strong evidence that acute THC intoxication increases reports of psychotic symptomology. Moreover, the literature suggests a dose-dependent relationship between levels of cannabis use and increased risk for the development of a psychotic disorder.
Concerning cognitive impairments, there is moderate evidence that chronic cannabis use and acute THC intoxication may negatively impact verbal, working, and episodic memory, executive functioning, and divided and sustained attention in users. However, cannabis does not appear to affect all cognitive domains, as impairments in visuospatial memory, processing speed, inhibitory control, and IQ were less consistent with mixed results.
Our review suggests a dose-dependent relationship between chronic cannabis use and verbal, episodic, and working memory impairments, but primarily among individuals who began use in adolescence. These findings coincide with preclinical animal models which have found that repeated exposure to THC during adolescence, but not adulthood, negatively impacts multiple cognitive domains, including memory, throughout the lifespan (156, 157). We also found modest evidence for improvements in cognition with cannabis abstinence, as some studies comparing non-users and past users demonstrated similar levels of performance among laboratory assessments (36, 65, 66). Given the brain's plasticity, restoration of neurocognitive abilities may be expected. A recent meta-analysis investigating residual cognitive impairments in cannabis users found no significant deficits among individuals who had abstained for at least 25 days (158). However, additional well-controlled prospective designs monitoring cognition from current use through cessation of use and over extended periods of abstinence are needed.
Among studies investigating the effects of acute THC administration, the evidence implicates greater impairment in cognition among recreational cannabis users and non-users in comparison to chronic cannabis users [e.g., (48, 61, 70, 79)]. In fact, a recent review evaluating the development of tolerance among cannabis users obtained comparable findings suggesting that cognition was most impaired upon acute THC intoxication (159), suggesting minimal tolerance.
Despite these findings, mixed evidence for numerous cognitive domains in this review arose, which may be due to variability in the control variables employed, cognitive tests utilized, operationalization of cognitive domains, participants' cannabis use histories, and cannabis exposure heterogeneity. Irrespective of these limitations, the effects obtained in this systematic review suggest that impairment of numerous cognitive domains can persist well-beyond the period of acute intoxication and consequently adversely impact everyday functioning in cannabis users.
Although the research concerning the effects of cannabis on motivation among non-clinical populations is limited, the evidence suggests a moderate negative relationship between cannabis use and motivation. These findings align with the “amotivational syndrome,” a term first coined by Smith [(160), p. 43] which purports that individuals who use cannabis are characterized by “a loss of desire to work or compete”, in addition to reduced emotional reactivity and interest in attaining goals (161).
While cannabis use may adversely impact motivation, the results need to be interpreted with caution due to certain methodological limitations. All of the included studies were cross-sectional, which significantly limits our understanding concerning the directionality between these two variables. Moreover, the conception and operationalization of motivation greatly differed across studies. While some of the included studies employed self-report measures such as the Apathy Evaluation Scale (AES) to measure apathy, other studies utilized performance-based tasks and operationalized motivation as perseverance in working for a monetary reward. Despite these methodological limitations, the evidence concerning cannabis use and reduced motivation aligns with studies suggesting that chronic cannabis users demonstrate reduced occupational and educational attainment compared to non-users [e.g., (107, 111, 112)]. Nevertheless, the field can significantly benefit from controlled, large-scale prospective designs evaluating motivation throughout the life trajectory while considering the impacts of the frequency of cannabis use, age of onset for use, and the influence of other substance use on motivational outcomes.
With a global trend toward legalization and decriminalization of cannabis for medical and/or recreational uses, the potential psychosocial harms accompanying cannabis use remain a major public health concern. Use of cannabis by adolescents is widespread and our findings suggest that chronic cannabis use throughout this developmental period is strongly associated with reduced educational and occupational attainment among this population. Nevertheless, the mechanisms linking cannabis and educational and economic risks are unclear. However, early age of onset, frequent or heavy use, and predictors of early use (e.g., childhood conduct or depressive symptoms), may undermine educational and occupational attainment. Consequently, delaying use onset and reducing the frequency of cannabis use patterns among adolescents may be beneficial in minimizing disruptions to educational goals and economic success.
While much of the evidence focuses on post-secondary or high school educational attainment, only one study included in the review followed a birth cohort until middle adulthood (107). The authors found that persistent cannabis use throughout adulthood corresponded with adverse mental health, substance use, and psychosocial outcomes, even after controlling for relevant confounding factors. Moreover, the authors denoted individual and childhood factors predicting persistent cannabis use in adulthood, including novelty-seeking, parental substance use, deviant peer affiliation, and conduct disorder diagnosis in adolescence. While additional, well-controlled studies are required to substantiate these findings, regardless of causality, our review indicates that individuals who utilize heavy amounts of cannabis for an extended period may experience adverse consequences to their social and economic well-being throughout the lifespan.
The review obtained mixed evidence concerning the relationship between cannabis use and depressive symptomology. Numerous studies implicated a dose-dependent relationship between cannabis use beginning in adolescence and increases in depressive symptomology. However, this effect was inconsistent across studies, which may be attributed to methodological differences within the literature. For example, differences surrounding the follow-up period and assessments of cannabis use frequency and levels may have precluded other authors from distinguishing a relationship between cannabis use and depressive symptomology. Moreover, the reported association between cannabis use and depression may have been influenced by variation among the controlled variables across the studies reviewed. A modest proportion of the cohort studies obtaining a significant relationship between adolescent cannabis use and increased depressive symptomology in adulthood did not account for additional substance use, such as nicotine and alcohol [e.g., (117, 118)]. This is of significance because alcohol and tobacco use are prevalent among cannabis users, and these substances may independently elevate individual susceptibility to depression if used throughout adolescence (162–164).
Concerning the effects of cannabis use on anxious symptomology, while individuals frequently report cannabis as an effective agent to relieve anxiety, [e.g., (165)] the evidence suggests a moderate dose-dependent relationship between cannabis use and heightened anxious symptomology. One potential exception surrounding these findings is in the case of cannabidiol (CBD). Among two randomized, double-blind, placebo-controlled, experimental designs, relative to placebo, CBD reduced anxiety on a social stress test among a sample of non-clinical participants (134, 136). While these findings implicate an anxiolytic effect of CBD, future research is necessary to evaluate whether these effects persist long-term.
Despite these findings within the anxiety literature, there are some noteworthy methodological considerations. Similar to the findings surrounding cannabis and depression, controlled factors varied across studies. Several cohort designs did not obtain significant relationships between cannabis use and anxiety disorders after controlling for other substance use and childhood psychosocial variables [e.g., (117, 127, 135)], while studies that did not control for one or more of these variables did obtain significant findings [e.g., ((63, 129)]. Secondly, follow-up times of cohort designs significantly varied, ranging from 1 to 35 years, with attention directed toward adolescents and young adults. Consequently, the effects of cannabis use on anxious symptomology during middle and late adulthood are poorly understood.
Although the review suggests cannabis as heightening anxious symptomology and possibly depressive symptomology, the mechanism underlying this relationship has not been clearly established. A neurobiological explanation has been put forward suggesting that THC may perturb endocannabinoid system CB1 receptor signaling, which has been linked to psychopathology and dysregulation of emotional experiences (166). Animal models have also demonstrated that administering THC during adolescence elevates symptoms reflecting anhedonia and anxiety in adulthood and is paralleled by neurotransmitter changes, including a diminution in serotonin, which is a neurotransmitter linked to depression, and increases in norepinephrine, which is a neurotransmitter linked with anxiety (167, 168). Interestingly, a recent preliminary study of 28 days of cannabis abstinence in people with major depression and cannabis use disorder suggests clinically relevant improvements in depression, anxiety and motivation (169).
A second explanation for the relationship between cannabis and elevated anxious and depressive symptomology utilizes a psychosocial lens (170). Cannabis use is associated with numerous adverse psychosocial outcomes, including unemployment, increased affiliation with deviant peers, and poorer educational outcomes (106, 112), which are all factors that may increase risk of developing an anxious or depressive disorder.
We found a strong relationship between chronic cannabis use and an increased risk for psychosis. This relationship persisted independent of alcohol [e.g., (143, 153)] and tobacco [e.g., (124, 138, 152)] use. In comparison to non-users, cannabis users have an earlier age of onset of psychotic disorders (143, 147, 151). Moreover, the association between cannabis use and psychotic symptomology is elevated with heavier, more frequent, and earlier use (27, 132, 138, 143). These findings coincide with a large meta-analysis which analyzed over 20,000 subjects, and found that the onset of psychosis is 2.7 years earlier in cannabis users than in non-users (171).
Although the evidence supports a relationship between cannabis use and psychotic symptoms, these findings may be confounded by tobacco use, as a significant proportion of cannabis users also smoke cigarettes. Moreover, many longitudinal studies observing a relationship between cannabis use and increased psychotic symptomology or a diagnosis of a psychotic disorder, had not recorded tobacco use [e.g., (138, 144, 148)]. A recent meta-analysis found that daily tobacco use correlates with an increased risk of psychosis, in addition to an earlier onset of a psychotic disorder (172). However, other evidence suggests that acute nicotine or tobacco use does not exacerbate the positive and negative symptoms of psychosis in schizophrenia [e.g., (173, 174)], and that abstinence does not alter schizophrenia psychosis (175).
Despite the potential confounding role of tobacco use in longitudinal designs, direct evidence obtained within experimental studies demonstrate a clear temporal association between THC-intoxication and increased psychotic symptomology, including positive, negative, and cognitive symptoms [e.g., (24, 53, 146)]. Further, reports of psychosis within randomized, placebo-controlled, experimental studies of THC administration are commonly made, and among some individuals, psychosis persists beyond the acute intoxication phase (15, 176, 177). Therefore, the primary symptom clusters present in schizophrenia are also frequently present in varying degrees during THC-intoxication.
One of the major strengths of this review includes its behavioral focus: we addressed important questions concerning the clinical, cognitive, and psychosocial outcomes associated with cannabis use. Our findings provide evidence that there are numerous risks associated with cannabis use, which is likely of significant interest to public health officials, educators, policymakers, researchers, healthcare practitioners, and the general public. Additionally, extending our review to a broad array of outcomes allowed us to detect major gaps in the current literature. A final strength of this review is that we employed a methodologically rigorous and comprehensive approach in collecting our evidence by following PRISMA guidelines (32).
Although we performed a comprehensive, systematic review, there are important limitations to note. As previously discussed, many of the studies across the investigated domains did not control for important confounding factors such as alcohol, tobacco and other substance use, or familial and other psychosocial variables [e.g., (58, 63, 74, 129)]. Accordingly, the possibility that the negative impacts of cannabis use upon the outcomes explored are attributed to confounding factors cannot be dismissed. An additional limitation of the present systematic review is the heterogeneity of the included studies insofar as study methodology, outcome measures assessed, and duration of follow-up. Participants differed on various socio-demographic characteristics and cannabis use parameters, including age of onset, lifetime use, abstinence periods for former users, and frequency of use. Moreover, the potency of cannabis used, and relative concentrations of THC and CBD are also important to consider and were infrequently discussed in the included studies. These methodological differences have likely contributed to the mixed findings, and should be addressed in future research.
Despite these limitations, we did identify a trend where frequent or heavy cannabis use in adolescence was typically a significant risk factor for numerous adverse outcomes in adulthood, including worsened educational attainment, reduced IQ, and the development of an anxiety disorder, major depressive disorder and psychosis (See Table 2 for a summary of the quality of the evidence).
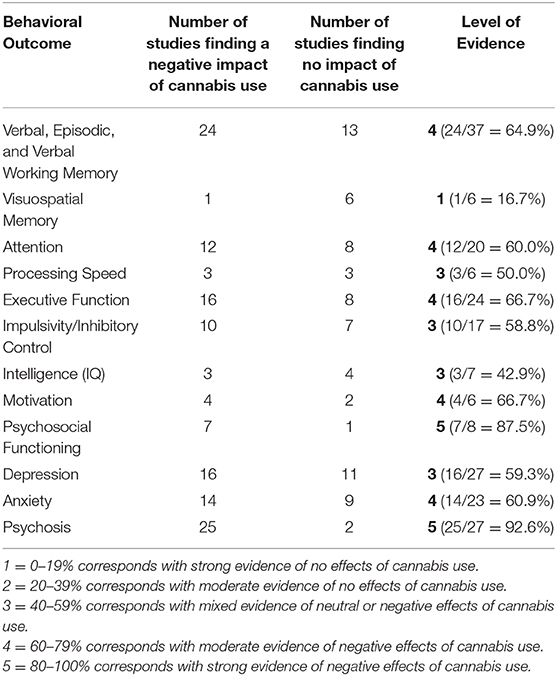
Table 2. The strength of evidence concerning cannabis and cannabinoids in behavioral outcomes among persons without a medical condition or history of a psychiatric disorder.
Our findings suggest that the adolescent brain is especially vulnerable to the effects of cannabinoids (especially THC) in comparison to the adult brain. Prior research has demonstrated that exposure to endogenous cannabinoids modifies the endocannabinoid system, which is a major player in shaping neurodevelopmental processes, including modulating neuroplasticity and regulating synaptic connections (178–180). It is possible that cannabis use during adolescence disrupts these neurodevelopmental processes. Consequently, this may produce enduring changes in brain structure and function that underlie many of the adverse cognitive and clinical outcomes associated with adolescent cannabis use.
The use of cannabis is extensive, ranging from occasional use to daily use and CUD. Although the desired effects sought by recreational cannabis users include relaxation, euphoria, and decreased anxiety, our review obtained evidence of adverse, acute and chronic sequelae of cannabis use, including impairments in cognition, increased risk for psychosis, depression, and anxiety, and poorer psychosocial functioning.
Although the evidence obtained in this systematic review implicates numerous adverse consequences of cannabis use beginning in adolescence, cannabis use among this age group is increasing (5, 181, 182). Moreover, with Canada's recent legalization of recreational cannabis and as more US states (now 10 states plus the District of Columbia) and nations consider legalizing medical or recreational cannabis use, the perceived risk of cannabis has also been trending downward cite (6, 183). Consequently, further investigation of the effects of these policies on usage patterns and related outcomes are a major public health concern.
Future research investigating the impact of cannabinoids during aging processes in middle and late adulthood is strongly needed, as little attention has been paid to this demographic. This is of concern because cannabis use is also increasing among this population (184), yet the effects of this substance in late adulthood are unknown. These studies will be crucial in depicting a more complete picture concerning the replicability and robustness of observed effects.
Finally, our work highlights a clear need for well-controlled longitudinal cohort and experimental vs. cross-sectional designs that utilize standardized measures of cognition, intelligence, motivation, psychiatric symptoms, and psychosocial functioning. Future studies should also be of adequate duration to assess cannabis and constituent effects, and have adequate follow-up periods to evaluate cannabis abstinence effects on these outcome measures. This would permit more accurate measurement of cannabis-related effect sizes on these outcomes. Such refinements in future methodologies would allow rigorous meta-analyses of cannabis effects on these various behavioral sequelae which may ultimately inform clinical and political decision-making.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
MS and TG conceived and designed the presented idea. MS and RB were involved in collecting data and designing the tables. MS wrote the manuscript with support from TG. TG encouraged MS to investigate specific topics for the review and supervised the findings of this work. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.630247/full#supplementary-material
1. Abuse S, Administration MHS. Key Substance Use and Mental Health Indicators in the United States: Results From the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17-5044, NSDUH Series H-52). Substance Abuse and Mental Health Services Administration (2017).
2. United Nations Office on Drugs and Crime.World Drug Report. Executive Summary. Conclusions and Policy Implications (2018).
3. MacKay R, Phillips K, Tiedemann M. Bill C-45: An Act Respecting Cannabis and to Amend the Controlled Drugs and Substances Act, the Criminal Code and other Acts. Library of Parliament (2018).
4. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. Lancet Psychiatry. (2016) 3:954–64. doi: 10.1016/S2215-0366(16)30208-5
5. Estoup AC, Moise-Campbell C, Varma M, Stewart DG. The impact of marijuana legalization on adolescent use, consequences, and perceived risk. Substance Use Misuse. (2016) 51:1881–7. doi: 10.1080/10826084.2016.1200623
6. Sarvet AL, Wall MM, Keyes KM, Cerdá M, Schulenberg JE, O'Malley PM, et al. Recent rapid decrease in adolescents' perception that marijuana is harmful, but no concurrent increase in use. Drug Alcohol Depend. (2018) 186:68–74. doi: 10.1016/j.drugalcdep.2017.12.041
8. Piomelli D, Giuffrida A, Calignano A, de Fonseca FRG. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. (2000) 21:218–24. doi: 10.1016/S0165-6147(00)01482-6
10. Lambert DM, Fowler CJ. The endocannabinoid system: drug targets, lead compounds, and potential therapeutic applications. J Med Chem. (2005) 48:5059–87. doi: 10.1021/jm058183t
11. Rubino T, Sala M, Vigano D, Braida D, Castiglioni C, Limonta V, et al. Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Δ 9-tetrahydrocannabinol in rats. Neuropsychopharmacology. (2007) 32:2036–45. doi: 10.1038/sj.npp.1301330
12. Morgan CJ, Freeman TP, Hindocha C, Schafer G, Gardner C, Curran HV. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry. (2018) 8:1–10. doi: 10.1038/s41398-018-0191-x
13. D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, et al. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. (2005) 57:594–608. doi: 10.1016/j.biopsych.2004.12.006
14. Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Δ9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. (2009) 66:442–51. doi: 10.1001/archgenpsychiatry.2009.17
15. Martin-Santos R, a Crippa J, Batalla A, Bhattacharyya S, Atakan Z, Borgwardt S, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Design. (2012) 18:4966–79. doi: 10.2174/138161212802884780
16. Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. (2015) 12:692–8. doi: 10.1007/s13311-015-0374-6
17. Bih CI, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. (2015) 12:699–730. doi: 10.1007/s13311-015-0377-3
18. Consroe P, Carlini EA, Zwicker AP, Lacerda LA. Interaction of cannabidiol and alcohol in humans. Psychopharmacology. (1979) 66:45–50. doi: 10.1007/BF00431988
19. Belgrave B, Bird K, Chesher G, Jackson D, Lubble K, Starmer G, et al. The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacology. (1979) 64:243–6. doi: 10.1007/BF00496070
20. Zuardi AW, Hallak JEC, Crippa JAS. Interaction between cannabidiol (CBD) andΔ 9-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology. (2012) 219:247–9. doi: 10.1007/s00213-011-2495-x
21. Schubart C, Sommer I, Fusar-Poli P, De Witte L, Kahn R, Boks M. Cannabidiol as a potential treatment for psychosis. Eur Neuropsychopharmacol. (2014) 24:51–64. doi: 10.1016/j.euroneuro.2013.11.002
22. Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. (2010) 35:764–74. doi: 10.1038/npp.2009.184
23. Morgan CJ, Curran HV. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br J Psychiatry. (2008) 192:306–7. doi: 10.1192/bjp.bp.107.046649
24. Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br J Psychiatry. (2010) 197:285–90. doi: 10.1192/bjp.bp.110.077503
25. Iseger TA, Bossong MG. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr Res. (2015) 162:153–61. doi: 10.1016/j.schres.2015.01.033
26. Cascini F, Aiello C, Di Tanna G. Increasing delta-9-tetrahydrocannabinol (Δ-9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr Drug Abuse Rev. (2012) 5:32–40. doi: 10.2174/1874473711205010032
27. Di Forti M, Morgan C, Dazzan P, Pariante C, Mondelli V, Marques TR, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. (2009) 195:488–91. doi: 10.1192/bjp.bp.109.064220
28. Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. (2014) 44:797–810. doi: 10.1017/S0033291713001438
29. Lowe DJ, Sasiadek JD, Coles AS, George TP. Cannabis and mental illness: a review. Eur Arch Psychiatry Clin Neurosci. (2019) 269:107–20. doi: 10.1007/s00406-018-0970-7
30. Pacek LR, Mauro PM, Martins SS. Perceived risk of regular cannabis use in the United States from 2002 to 2012: differences by sex, age, and race/ethnicity. Drug Alcohol Depend. (2015) 149:232–44. doi: 10.1016/j.drugalcdep.2015.02.009
31. Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci. (2019) 269:5–15. doi: 10.1007/s00406-019-00983-5
32. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
33. Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa, ON: Ottawa Hospital Research Institute (2011).
34. Batalla A, Bhattacharyya S, Yuecel M, Fusar-Poli P, Crippa JA, Nogue S, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS ONE. (2013) 8:e55821. doi: 10.1371/journal.pone.0055821
35. Becker MP, Collins PF, Schultz A, Urošević S, Schmaling B, Luciana M. Longitudinal changes in cognition in young adult cannabis users. J Clin Exp Neuropsychol. (2018) 40:529–43. doi: 10.1080/13803395.2017.1385729
36. Thames AD, Arbid N, Sayegh P. Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addict Behav. (2014) 39:994–9. doi: 10.1016/j.addbeh.2014.01.019
37. Curran VH, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Δ 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. (2002) 164:61–70. doi: 10.1007/s00213-002-1169-0
38. Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behaviour and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. (2012) 34:962–76. doi: 10.1080/13803395.2012.703642
39. Mercuri K, Terrett G, Henry JD, Curran HV, Elliott M, Rendell PG. Episodic foresight deficits in regular, but not recreational, cannabis users. J Psychopharmacol. (2018) 32:876–82. doi: 10.1177/0269881118776672
40. Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. (2007) 13:807. doi: 10.1017/S1355617707071032
41. Mokrysz C, Freeman TP, Korkki S, Griffiths K, Curran HV. Are adolescents more vulnerable to the harmful effects of cannabis than adults? A placebo-controlled study in human males. Transl Psychiatry. (2016) 6:e961. doi: 10.1038/tp.2016.225
42. Meier MH, Caspi A, Danese A, Fisher HL, Houts R, Arseneault L, et al. Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction. (2018) 113:257–65. doi: 10.1111/add.13946
43. Carey SE, Nestor L, Jones J, Garavan H, Hester R. Impaired learning from errors in cannabis users: dorsal anterior cingulate cortex and hippocampus hypoactivity. Drug Alcohol Depend. (2015) 155:175–82. doi: 10.1016/j.drugalcdep.2015.07.671
44. Lovell M, Bruno R, Johnston J, Matthews A, McGregor I, Allsop D, et al. Cognitive, physical, and mental health outcomes between long-term cannabis and tobacco users. Addict Behav. (2018) 79:178–88. doi: 10.1016/j.addbeh.2017.12.009
45. Blest-Hopley G, O'Neill A, Wilson R, Giampietro V, Bhattacharyya S. Disrupted parahippocampal and midbrain function underlie slower verbal learning in adolescent-onset regular cannabis use. Psychopharmacology. (2019) 1–17. doi: 10.1007/s00213-019-05407-9
46. Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology. (2011) 216:131–44. doi: 10.1007/s00213-011-2203-x
47. Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. (2012) 109:E2657–64. doi: 10.1073/pnas.1206820109
48. Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, et al. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology. (2013) 226:307–19. doi: 10.1007/s00213-012-2908-5
49. Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. (2002) 59:1337–43. doi: 10.1212/01.WNL.0000031422.66442.49
50. Kloft L, Otgaar H, Blokland A, Monds LA, Toennes SW, Loftus EF, et al. Cannabis increases susceptibility to false memory. Proc Natl Acad Sci USA. (2020) 117:4585–9. doi: 10.1073/pnas.1920162117
51. Matheson J, Sproule B, Di Ciano P, Fares A, Le Foll B, Mann RE, et al. Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology. (2020) 237:305–16. doi: 10.1007/s00213-019-05369-y
52. Hunault CC, Böcker KB, Stellato R, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology. (2014) 231:4723–33. doi: 10.1007/s00213-014-3630-2
53. Morrison P, Zois V, McKeown D, Lee T, Holt D, Powell J, et al. The acute effects of synthetic intravenous [Delta] 9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. (2009) 39:1607. doi: 10.1017/S0033291709005522
54. Morgan C, Gardener C, Schafer G, Swan S, Demarchi C, Freeman T, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med. (2012) 42:391. doi: 10.1017/S0033291711001322
55. Pope Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. (2003) 69:303–10. doi: 10.1016/S0376-8716(02)00334-4
56. Thayer RE, YorkWilliams SL, Hutchison KE, Bryan AD. Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Res Neuroimaging. (2019) 285:58–63. doi: 10.1016/j.pscychresns.2019.02.001
57. Jager G, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology. (2006) 185:358–68. doi: 10.1007/s00213-005-0298-7
58. Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog Neuro Psychopharmacol Biol Psychiatry. (2010) 34:837–45. doi: 10.1016/j.pnpbp.2010.03.032
59. Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: a prospective fMRI study. Hum Brain Mapp. (2014) 35:2470–82. doi: 10.1002/hbm.22342
60. Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. (2010) 49:561–72. e3. doi: 10.1016/j.jaac.2010.02.001
61. Englund A, Atakan Z, Kralj A, Tunstall N, Murray R, Morrison P. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: a placebo-controlled, double-blind, crossover pilot trial. J Psychopharmacol. (2016) 30:140–51. doi: 10.1177/0269881115615104
62. Rabin RA, Barr MS, Goodman MS, Herman Y, Zakzanis KK, Kish SJ, et al. Effects of extended cannabis abstinence on cognitive outcomes in cannabis dependent patients with schizophrenia vs non-psychiatric controls. Neuropsychopharmacology. (2017) 42:2259–71. doi: 10.1038/npp.2017.85
63. Infante MA, Nguyen-Louie TT, Worley M, Courtney KE, Coronado C, Jacobus J. Neuropsychological trajectories associated with adolescent alcohol and cannabis use: a prospective 14-year study. J Int Neuropsychol Soc. (2020) 26:480–91. doi: 10.1017/S1355617719001395
64. Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc. (2012) 18:678–88. doi: 10.1017/S1355617712000276
65. Burggren AC, Siddarth P, Mahmood Z, London ED, Harrison TM, Merrill DA, et al. Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis Cannabinoid Res. (2018) 3:242–51. doi: 10.1089/can.2018.0035
66. Pope Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol. (2002) 42:41–7S. doi: 10.1002/j.1552-4604.2002.tb06002.x
67. Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology. (2004) 176:239–47. doi: 10.1007/s00213-004-1885-8
68. Smith AM, Longo CA, Fried PA, Hogan MJ, Cameron I. Effects of marijuana on visuospatial working memory: an fMRI study in young adults. Psychopharmacology. (2010) 210:429–38. doi: 10.1007/s00213-010-1841-8
69. Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol Addict Behav. (2007) 21:478. doi: 10.1037/0893-164X.21.4.478
70. Desrosiers NA, Ramaekers JG, Chauchard E, Gorelick DA, Huestis MA. Smoked cannabis' psychomotor and neurocognitive effects in occasional and frequent smokers. J Anal Toxicol. (2015) 39:251–61. doi: 10.1093/jat/bkv012
71. Ramaekers JG, Kauert G, Theunissen E, Toennes SW, Moeller M. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. (2009) 23:266–77. doi: 10.1177/0269881108092393
72. Arkell TR, Lintzeris N, Kevin RC, Ramaekers JG, Vandrey R, Irwin C, et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology. (2019) 236:2713–24. doi: 10.1007/s00213-019-05246-8
73. Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci. (2004) 1021:384–90. doi: 10.1196/annals.1308.053
74. Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry. (2011) 198:442–7. doi: 10.1192/bjp.bp.110.077479
75. Solowij N, Michie PT, Fox AM. Differential impairments of selective attention due to frequency and duration of cannabis use. Biol Psychiatry. (1995) 37:731–9. doi: 10.1016/0006-3223(94)00178-6
76. Theunissen EL, Kauert GF, Toennes SW, Moeller MR, Sambeth A, Blanchard MM, et al. Neurophysiological functioning of occasional and heavy cannabis users during THC intoxication. Psychopharmacology. (2012) 220:341–50. doi: 10.1007/s00213-011-2479-x
77. Shannon EE, Mathias CW, Dougherty DM, Liguori A. Cognitive impairments in adolescent cannabis users are related to THC levels. Addict Disord Their Treat. (2010) 9:158–63. doi: 10.1097/ADT.0b013e3181c8c667
78. Fridberg DJ, Skosnik PD, Hetrick WP, O'Donnell BF. Neural correlates of performance monitoring in chronic cannabis users and cannabis-naive controls. J Psychopharmacol. (2013) 27:515–25. doi: 10.1177/0269881113477745
79. Boggs DL, Cortes-Briones JA, Surti T, Luddy C, Ranganathan M, Cahill JD, et al. The dose-dependent psychomotor effects of intravenous delta-9-tetrahydrocannabinol (Δ9-THC) in humans. J Psychopharmacol. (2018) 32:1308–18. doi: 10.1177/0269881118799953
80. Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. (2005) 26:480–92. doi: 10.1016/j.neuroimage.2005.02.012
81. Casey JL, Cservenka A. Effects of frequent marijuana use on risky decision-making in young adult college students. Addict Behav Rep. (2020) 11:100253. doi: 10.1016/j.abrep.2020.100253
82. Sagar KA, Dahlgren MK, Gönenç A, Racine MT, Dreman MW, Gruber SA. The impact of initiation: early onset marijuana smokers demonstrate altered Stroop performance and brain activation. Dev Cogn Neurosci. (2015) 16:84–92. doi: 10.1016/j.dcn.2015.03.003
83. Scott JC, Wolf DH, Calkins ME, Bach EC, Weidner J, Ruparel K, et al. Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia Neurodevelopmental Cohort. Psychol Addict Behav. (2017) 31:423. doi: 10.1037/adb0000268
84. Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. (2006) 31:2296–303. doi: 10.1038/sj.npp.1301068
85. Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res Neuroimaging. (2011) 191:51–9. doi: 10.1016/j.pscychresns.2010.10.002
86. Castellanos-Ryan N, Pingault J-B, Parent S, Vitaro F, Tremblay RE, Seguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol. (2017) 29:1253. doi: 10.1017/S0954579416001280
87. Vaidya JG, Block RI, O'leary DS, Ponto LB, Ghoneim MM, Bechara A. Effects of chronic marijuana use on brain activity during monetary decision-making. Neuropsychopharmacology. (2012) 37:618–29. doi: 10.1038/npp.2011.227
88. Hatchard T, Fried P, Hogan M, Cameron I, Smith A. Marijuana use impacts cognitive interference: an fMRI investigation in young adults performing the counting Stroop task. J Addict Res Ther. (2014) 5:197–203. doi: 10.4172/2155-6105.1000197
89. Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet J-L, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug Alcohol Depend. (2007) 90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004
90. McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioural measures of impulsivity in humans. Neuropsychopharmacology. (2003) 28:1356–65. doi: 10.1038/sj.npp.1300176
91. Ansell EB, Laws HB, Roche MJ, Sinha R. Effects of marijuana use on impulsivity and hostility in daily life. Drug Alcohol Depend. (2015) 148:136–42. doi: 10.1016/j.drugalcdep.2014.12.029
92. Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. (2014) 231:1455–65. doi: 10.1007/s00213-013-3326-z
93. Atakan Z, Bhattacharyya S, Allen P, Martin-Santos R, Crippa J, Borgwardt S, et al. Cannabis affects people differently: inter-subject variation in the psychotogenic effects of Δ9-tetrahydrocannabinol: a functional magnetic resonance imaging study with healthy volunteers. Psychol Med. (2013) 43:1255–67. doi: 10.1017/S0033291712001924
94. Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WD. Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett. (2012) 511:89–94. doi: 10.1016/j.neulet.2012.01.039
95. Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. (2007) 194:173–83. doi: 10.1007/s00213-007-0823-y
96. Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Δ-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. (2008) 64:966–73. doi: 10.1016/j.biopsych.2008.05.011
97. Boccio CM, Beaver KM. Examining the influence of adolescent marijuana use on adult intelligence: further evidence in the causation versus spuriousness debate. Drug Alcohol Depend. (2017) 177:199–206. doi: 10.1016/j.drugalcdep.2017.04.007
98. Mokrysz C, Landy R, Gage SH, Munafo MR, Roiser JP, Curran HV. Are IQ and educational outcomes in teenagers related to their cannabis use? A prospective cohort study. J Psychopharmacol. (2016) 30:159–68. doi: 10.1177/0269881115622241
99. Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, et al. Impact of adolescent marijuana use on intelligence: results from two longitudinal twin studies. Proc Natl Acad Sci USA. (2016) 113:E500–8. doi: 10.1073/pnas.1516648113
100. Wright NE, Scerpella D, Lisdahl KM. Marijuana use is associated with behavioural approach and depressive symptoms in adolescents and emerging adults. PloS ONE. (2016) 11:e0166005. doi: 10.1371/journal.pone.0166005
101. Martin-Soelch C, Kobel M, Stoecklin M, Michael T, Weber S, Krebs B, et al. Reduced response to reward in smokers and cannabis users. Neuropsychobiology. (2009) 60:94–103. doi: 10.1159/000239685
102. Bloomfield MA, Morgan CJ, Kapur S, Curran HV, Howes OD. The link between dopamine function and apathy in cannabis users: an [18 F]-DOPA PET imaging study. Psychopharmacology. (2014) 231:2251–9. doi: 10.1007/s00213-014-3523-4
103. Lane SD, Cherek DR, Pietras CJ, Steinberg JL. Performance of heavy marijuana-smoking adolescents on a laboratory measure of motivation. Addict Behav. (2005) 30:815–28. doi: 10.1016/j.addbeh.2004.08.026
104. Jager G, Block RI, Luijten M, Ramsey NF. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: a cross-sectional multicenter fMRI study. J Psychoact Drugs. (2013) 45:156–67. doi: 10.1080/02791072.2013.785837
105. Pacheco-Colón I, Coxe S, Musser ED, Duperrouzel JC, Ross JM, Gonzalez R. Is cannabis use associated with various indices of motivation among adolescents? Subst Use Misuse. (2018) 53:1158–69. doi: 10.1080/10826084.2017.1400566
106. Homel J, Thompson K, Leadbeater B. Trajectories of marijuana use in youth ages 15–25: implications for postsecondary education experiences. J Stud Alcohol Drugs. (2014) 75:674–83. doi: 10.15288/jsad.2014.75.674
107. Boden JM, Dhakal B, Foulds JA, Horwood LJ. Life-course trajectories of cannabis use: a latent class analysis of a New Zealand birth cohort. Addiction. (2020) 115:279–90. doi: 10.1111/add.14814
108. Stiby AI, Hickman M, Munafò MR, Heron J, Yip VL, Macleod J. Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction. (2015) 110:658–68. doi: 10.1111/add.12827
109. Epstein M, Hill KG, Nevell AM, Guttmannova K, Bailey JA, Abbott RD, et al. Trajectories of marijuana use from adolescence into adulthood: environmental and individual correlates. Dev Psychol. (2015) 51:1650. doi: 10.1037/dev0000054
110. Meier MH, Hill ML, Small PJ, Luthar SS. Associations of adolescent cannabis use with academic performance and mental health: a longitudinal study of upper middle class youth. Drug Alcohol Depend. (2015) 156:207–12. doi: 10.1016/j.drugalcdep.2015.09.010
111. Patte KA, Qian W, Leatherdale ST. Marijuana and alcohol use as predictors of academic achievement: a longitudinal analysis among youth in the COMPASS study. J Sch Health. (2017) 87:310–8. doi: 10.1111/josh.12498
112. Thompson K, Leadbeater B, Ames M, Merrin GJ. Associations between marijuana use trajectories and educational and occupational success in young adulthood. Prev Sci. (2019) 20:257–69. doi: 10.1007/s11121-018-0904-7
113. Hengartner MP, Angst J, Ajdacic-Gross V, Rössler W. Cannabis use during adolescence and the occurrence of depression, suicidality and anxiety disorder across adulthood: findings from a longitudinal cohort study over 30 years. J Affect Disord. (2020) 272:98–103. doi: 10.1016/j.jad.2020.03.126
114. Schoeler T, Theobald D, Pingault J-B, Farrington D, Coid J, Bhattacharyya S. Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. Psychol Med. (2018) 48:2169–76. doi: 10.1017/S0033291717003658
115. Gage SH, Hickman M, Heron J, Munafò MR, Lewis G, Macleod J, et al. Associations of cannabis and cigarette use with depression and anxiety at age 18: findings from the Avon Longitudinal Study of Parents and Children. PloS ONE. (2015) 10:e0122896. doi: 10.1371/journal.pone.0122896
116. Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and anxiety and depression in young adults: a large prospective study. J Am Acad Child Adolesc Psychiatry. (2007) 46:408–17. doi: 10.1097/chi.0b013e31802dc54d
117. Van Laar M, Van Dorsselaer S, Monshouwer K, De Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. (2007) 102:1251–60. doi: 10.1111/j.1360-0443.2007.01875.x
118. Assari S, Mistry R, Caldwell CH, Zimmerman MA. Marijuana use and depressive symptoms; gender differences in African American adolescents. Front Psychol. (2018) 9:2135. doi: 10.3389/fpsyg.2018.02135
119. Meier MH, Beardslee J, Pardini D. Associations between recent and cumulative cannabis use and internalizing problems in boys from adolescence to young adulthood. J Abnormal Child Psychol. (2020) 48:771–82. doi: 10.1007/s10802-020-00641-8
120. Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. (2013) 108:124–33. doi: 10.1111/j.1360-0443.2012.04015.x
121. Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. (2002) 325:1195–8. doi: 10.1136/bmj.325.7374.1195
122. Childs E, Lutz JA, de Wit H. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug Alcohol Depend. (2017) 177:136–44. doi: 10.1016/j.drugalcdep.2017.03.030
123. Otten R, Engels RC. Testing bidirectional effects between cannabis use and depressive symptoms: moderation by the serotonin transporter gene. Addict Biol. (2013) 18:826–35. doi: 10.1111/j.1369-1600.2011.00380.x
124. Manrique-Garcia E, Zammit S, Dalman C, Hemmingsson T, Allebeck P. Cannabis use and depression: a longitudinal study of a national cohort of Swedish conscripts. BMC Psychiatry. (2012) 12:112. doi: 10.1186/1471-244X-12-112
125. Despina B, Massimiliano O, Natalie C-R, Johanne R, Tina M, Michel B, et al. Cannabis use, depression and suicidal ideation in adolescence: direction of associations in a population based cohort. J Affect Disord. (2020) 274:1076–83. doi: 10.1016/j.jad.2020.05.136
126. Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: a longitudinal study. J Affect Disord. (2015) 172:211–8. doi: 10.1016/j.jad.2014.10.006
127. Danielsson A-K, Lundin A, Agardh E, Allebeck P, Forsell Y. Cannabis use, depression and anxiety: a 3-year prospective population-based study. J Affect Disord. (2016) 193:103–8. doi: 10.1016/j.jad.2015.12.045
128. Griffith-Lendering M, Huijbregts SC, Mooijaart A, Vollebergh W, Swaab H. Cannabis use and development of externalizing and internalizing behaviour problems in early adolescence: a TRAILS study. Drug Alcohol Depend. (2011) 116:11–7. doi: 10.1016/j.drugalcdep.2010.11.024
129. Grunberg VA, Cordova KA, Bidwell L, Ito TA. Can marijuana make it better? Prospective effects of marijuana and temperament on risk for anxiety and depression. Psychol Addict Behav. (2015) 29:590. doi: 10.1037/adb0000109
130. Baggio S, N'Goran AA, Deline S, Studer J, Dupuis M, Henchoz Y, et al. Patterns of cannabis use and prospective associations with health issues among young males. Addiction. (2014) 109:937–45. doi: 10.1111/add.12490
131. Harder VS, Morral AR, Arkes J. Marijuana use and depression among adults: testing for causal associations. Addiction. (2006) 101:1463–72. doi: 10.1111/j.1360-0443.2006.01545.x
132. Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. (2002) 325:1212–3. doi: 10.1136/bmj.325.7374.1212
133. Duperrouzel J, Hawes SW, Lopez-Quintero C, Pacheco-Colón I, Comer J, Gonzalez R. The association between adolescent cannabis use and anxiety: a parallel process analysis. Addict Behav. (2018) 78:107–13. doi: 10.1016/j.addbeh.2017.11.005
134. Linares IM, Zuardi AW, Pereira LC, Queiroz RH, Mechoulam R, Guimaraes FS, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Brazil J Psychiatry. (2019) 41:9–14. doi: 10.1590/1516-4446-2017-0015
135. Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and anxiety disorders: results from a population-based representative sample. Eur Neuropsychopharmacol. (2016) 26:493–505. doi: 10.1016/j.euroneuro.2015.12.037
136. Zuardi AW, Rodrigues NP, Silva AL, Bernardo SA, Hallak JE, Guimarães FS, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. (2017) 8:259. doi: 10.3389/fphar.2017.00259
137. Ruiz-Veguilla M, Barrigón ML, Hernández L, Rubio JL, Gurpegui M, Sarramea F, et al. Dose–response effect between cannabis use and psychosis liability in a non-clinical population: evidence from a snowball sample. J Psychiatr Res. (2013) 47:1036–43. doi: 10.1016/j.jpsychires.2013.03.003
138. Van Os J, Bak M, Hanssen M, Bijl R, De Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. (2002) 156:319–27. doi: 10.1093/aje/kwf043
139. Mokrysz C, Shaban ND, Freeman TP, Lawn W, Pope RA, Hindocha C, et al. Acute effects of cannabis on speech illusions and psychotic-like symptoms: two studies testing the moderating effects of cannabidiol and adolescence. Psychol Med. (2020) 1–9. doi: 10.1017/S0033291720001038
140. Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. (2013) 27:19–27. doi: 10.1177/0269881112460109
141. Fergusson DM, Horwood LJ, Ridder EM. Tests of causal linkages between cannabis use and psychotic symptoms. Addiction. (2005) 100:354–66. doi: 10.1111/j.1360-0443.2005.01001.x
142. Rössler W, Hengartner MP, Angst J, Ajdacic-Gross V. Linking substance use with symptoms of subclinical psychosis in a community cohort over 30 years. Addiction. (2012) 107:1174–84. doi: 10.1111/j.1360-0443.2011.03760.x
143. Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ. (2002) 325:1199. doi: 10.1136/bmj.325.7374.1199
144. Ferdinand RF, Sondeijker F, Van Der Ende J, Selten JP, Huizink A, Verhulst FC. Cannabis use predicts future psychotic symptoms, and vice versa. Addiction. (2005) 100:612–8. doi: 10.1111/j.1360-0443.2005.01070.x
145. Kaufmann R, Kraft B, Frey R, Winkler D, Weiszenbichler S, Bäcker C, et al. Acute psychotropic effects of oral cannabis extract with a defined content of Δ9-tetrahydrocannabinol (THC) in healthy volunteers. Pharmacopsychiatry. (2010) 43:24–32. doi: 10.1055/s-0029-1237397
146. Morrison P, Stone J. Synthetic delta-9-tetrahydrocannabinol elicits schizophrenia-like negative symptoms which are distinct from sedation. Hum Psychopharmacol Clin Exp. (2011) 26:77–80. doi: 10.1002/hup.1166
147. Bechtold J, Hipwell A, Lewis DA, Loeber R, Pardini D. Concurrent and sustained cumulative effects of adolescent marijuana use on subclinical psychotic symptoms. Am J Psychiatry. (2016) 173:781–9. doi: 10.1176/appi.ajp.2016.15070878
148. Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. (2005) 57:1117–27. doi: 10.1016/j.biopsych.2005.01.026
149. Kuepper R, van Os J, Lieb R, Wittchen H-U, Höfler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. (2011) 342:d738. doi: 10.1136/bmj.d738
150. Miettunen J, Törmänen S, Murray GK, Jones PB, Mäki P, Ebeling H, et al. Association of cannabis use with prodromal symptoms of psychosis in adolescence. Br J Psychiatry. (2008) 192:470–1. doi: 10.1192/bjp.bp.107.045740
151. Mustonen A, Niemelä S, Nordström T, Murray GK, Mäki P, Jääskeläinen E, et al. Adolescent cannabis use, baseline prodromal symptoms and the risk of psychosis. Br J Psychiatry. (2018) 212:227–33. doi: 10.1192/bjp.2017.52
152. Griffith-Lendering M, Wigman J, Prince van Leeuwen A, Huijbregts S, Huizink AC, Ormel J, et al. Cannabis use and vulnerability for psychosis in early adolescence–a TRAILS study. Addiction. (2013) 108:733–40. doi: 10.1111/add.12050
153. Gage S, Hickman M, Heron J, Munafò M, Lewis G, Macleod J, et al. Associations of cannabis and cigarette use with psychotic experiences at age 18: findings from the Avon Longitudinal Study of Parents and Children. Psychol Med. (2014) 44:3435–44. doi: 10.1017/S0033291714000531
154. Levinson DF. The genetics of depression: a review. Biol Psychiatry. (2006) 60:84–92. doi: 10.1016/j.biopsych.2005.08.024
155. Blanco C, Hasin DS, Wall MM, Flórez-Salamanca L, Hoertel N, Wang S, et al. Cannabis use and risk of psychiatric disorders: prospective evidence from a US national longitudinal study. JAMA Psychiatry. (2016) 73:388–95. doi: 10.1001/jamapsychiatry.2015.3229
156. Panlilio LV, Ferré S, Yasar S, Thorndike EB, Schindler CW, Goldberg SR. Combined effects of THC and caffeine on working memory in rats. Br J Pharmacol. (2012) 165:2529–38. doi: 10.1111/j.1476-5381.2011.01554.x
157. Irimia C, Polis IY, Stouffer D, Parsons LH. Persistent effects of chronic Δ9-THC exposure on motor impulsivity in rats. Psychopharmacology. (2015) 232:3033–43. doi: 10.1007/s00213-015-3942-x
158. Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol. (2012) 20:420. doi: 10.1037/a0029117
159. Colizzi M, Bhattacharyya S. Cannabis use and the development of tolerance: a systematic review of human evidence. Neurosci Biobehav Rev. (2018) 93:1–25. doi: 10.1016/j.neubiorev.2018.07.014
160. Smith DE. Acute and chronic toxicity of marijuana. J Psychedelic Drugs. (1968) 2:37–48. doi: 10.1080/02791072.1968.10524399
161. McGlothlin WH, West LJ. The marihuana problem: an overview. Am J Psychiatry. (1968) 125:370–8. doi: 10.1176/ajp.125.3.370
162. Chaiton MO, Cohen JE, O'Loughlin J, Rehm J. A systematic review of longitudinal studies on the association between depression and smoking in adolescents. BMC Public Health. (2009) 9:356. doi: 10.1186/1471-2458-9-356
163. Marmorstein NR. Longitudinal associations between alcohol problems and depressive symptoms: early adolescence through early adulthood. Alcoholism Clin Exp Res. (2009) 33:49–59. doi: 10.1111/j.1530-0277.2008.00810.x
164. Fluharty M, Taylor AE, Grabski M, Munafò MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tobacco Res. (2016) 19:3–13. doi: 10.1093/ntr/ntw140
165. Kosiba JD, Maisto SA, Ditre JW. Patient-reported use of medical cannabis for pain, anxiety, and depression symptoms: Systematic review and meta-analysis. Soc Sci Med. (2019) 233:181–92. doi: 10.1016/j.socscimed.2019.06.005
166. Vinod KY, Hungund BL. Role of the endocannabinoid system in depression and suicide. Trends Pharmacol Sci. (2006) 27:539–45. doi: 10.1016/j.tips.2006.08.006
167. Stopponi S, Soverchia L, Ubaldi M, Cippitelli A, Serpelloni G, Ciccocioppo R. Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur Neuropsychopharmacol. (2014) 24:1037–45. doi: 10.1016/j.euroneuro.2013.12.012
168. Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry. (2016) 79:578–85. doi: 10.1016/j.biopsych.2015.07.024
169. Lucatch AM, Klober S, Rizvi S, Meyer JM, George T.P. Effects of cannabis abstinence on clinical e outcomes in people with cannabis use disorder and major depression. Can J Addict. (2020) 3:33–41. doi: 10.1097/CXA.0000000000000090
170. Degenhardt L, Hall W, Lynskey M. Exploring the association between cannabis use and depression. Addiction. (2003) 98:1493–504. doi: 10.1046/j.1360-0443.2003.00437.x
171. Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. (2011) 68:555–61. doi: 10.1001/archgenpsychiatry.2011.5
172. Gurillo P, Jauhar S, Murray RM, MacCabe JH. Does tobacco use cause psychosis? Systematic review and meta-analysis. Lancet Psychiatry. (2015) 2:718–25. doi: 10.1016/S2215-0366(15)00152-2
173. Sacco K, Termine A, Seyal A, Dudas MM, Jatlow PI, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. (2005) 62:649–59. doi: 10.1001/archpsyc.62.6.649
174. Jubelt LE, Barr RS, Goff DC, Weiss AP, Evins A.E. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology. (2008) 199:89–98. doi: 10.1007/s00213-008-1133-8
175. George TP, Vessicchio J.C., Termine A, Sahady DM, Head CA, Wexler BE, Kosten T.R. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacol. (2002) 26:75–85. doi: 10.1016/S0893-133X(01)00296-2
176. Favrat B, Ménétrey A, Augsburger M, Rothuizen LE, Appenzeller M, Buclin T, et al. Two cases of “cannabis acute psychosis” following the administration of oral cannabis. BMC Psychiatry. (2005) 5:1–6. doi: 10.1186/1471-244X-5-17
177. Wilkinson ST, Radhakrishnan R, D'Souza DC. Impact of cannabis use on the development of psychotic disorders. Curr Addict Rep. (2014) 1:115–28. doi: 10.1007/s40429-014-0018-7
178. Galve-Roperh I, Palazuelos J, Aguado T, Guzmán M. The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. (2009) 259:371–82. doi: 10.1007/s00406-009-0028-y
179. Harkany T, Keimpema E, Barabás K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Mol Cell Endocrinol. (2008) 286:S84–90. doi: 10.1016/j.mce.2008.02.011
180. Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji S-P, Bai G, et al. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic-and antidepressant-like effects. J Clin Investig. (2005) 115:3104–16. doi: 10.1172/JCI25509
181. Johnston LD, Miech RA, O'Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future National Survey Results on Drug Use, 1975-2018: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research (2019).
182. Weinberger AH, Zhu J, Lee J, Anastasiou E, Copeland J, Goodwin RD. Cannabis use among youth in the United States, 2004–2016: faster rate of increase among youth with depression. Drug Alcohol Depend. (2020) 209:107894. doi: 10.1016/j.drugalcdep.2020.107894
183. Carliner H, Brown QL, Sarvet AL, Hasin DS. Cannabis use, attitudes, and legal status in the US: a review. Prev Med. (2017) 104:13–23. doi: 10.1016/j.ypmed.2017.07.008
Keywords: cannabis, healthy subjects, cognition, mood, anxiety, psychosis, motivation, intelligence
Citation: Sorkhou M, Bedder RH and George TP (2021) The Behavioral Sequelae of Cannabis Use in Healthy People: A Systematic Review. Front. Psychiatry 12:630247. doi: 10.3389/fpsyt.2021.630247
Received: 17 November 2020; Accepted: 25 January 2021;
Published: 16 February 2021.
Edited by:
Daniel Feingold, Ariel University, IsraelReviewed by:
Michael Patrick Schaub, University of Zurich, SwitzerlandCopyright © 2021 Sorkhou, Bedder and George. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tony P. George, dG9ueS5nZW9yZ2VAY2FtaC5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.