
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 25 February 2021
Sec. Sleep Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.630162
This article is part of the Research Topic The Complex Interaction between Biological, Metabolic and Neurologic Dysregulation in Obstructive Sleep Apnea View all 6 articles
 Frangiskos Frangopoulos1
Frangiskos Frangopoulos1 Savvas Zannetos2
Savvas Zannetos2 Ivi Nicolaou1
Ivi Nicolaou1 Nicholas-Tiberio Economou3
Nicholas-Tiberio Economou3 Tonia Adamide1
Tonia Adamide1 Andreas Georgiou1
Andreas Georgiou1 Pantelis T. Nikolaidis4
Pantelis T. Nikolaidis4 Thomas Rosemann5
Thomas Rosemann5 Beat Knechtle5
Beat Knechtle5 Georgia Trakada3*
Georgia Trakada3*Introduction: Little information exists in the general population whether clinical presentation phenotypes of obstructive sleep apnea (OSA) differ in terms of sleep quality and comorbidities.
Aim: The purpose of our study was to assess possible differences between symptomatic and asymptomatic OSA patients concerning syndrome's severity, patients' sleep quality, and comorbidities.
Subjects and methods: First, in a nationwide, stratified, epidemiological survey, 4,118 Cypriot adult participants were interviewed about sleep habits and complaints. In the second stage of the survey, 264 randomly selected adults underwent a type III sleep study for possible OSA. Additionally, they completed the Greek version of Pittsburgh Sleep Quality Index (Gr-PSQI), Epworth Sleepiness Scale (ESS), Athens Insomnia Scale (AIS), and Hospital Anxiety and Depression Scale (HADS).
Results: From 264 enrolled participants, 155 individuals (40 females and 115 males) were first diagnosed with OSA. Among these 155 patients, 34% had ESS ≥ 10 and 49% AIS ≥ 6. One or both symptoms present categorized the individual as symptomatic (60%) and neither major symptom as asymptomatic (40%). There were no significant statistical differences (SSDs) between the two groups (symptomatic–asymptomatic) with regard to anthropometrics [age or gender; neck, abdomen, and hip circumferences; and body mass index (BMI)]. The two groups had no differences in OSA severity—as expressed by apnea–hypopnea index (AHI), oxygen desaturation index (ODI), and mean oxyhemoglobin saturation (SaO2)—and in cardiometabolic comorbidities. Symptomatic patients expressed anxiety and depression more often than asymptomatics (p < 0.001) and had poorer subjective sleep quality (Gr-PSQI, p < 0.001). According to PSQI questionnaire, there were no SSDs regarding hours in bed and the use of sleep medications, but there were significant differences in the subjective perception of sleep quality (p < 0.001), sleep efficiency (p < 0.001), duration of sleep (p = 0.001), sleep latency (p = 0.007), daytime dysfunction (p < 0.001), and finally sleep disturbances (p < 0.001).
Conclusion: According to our data, OSA patients reporting insomnia-like symptoms and/or sleepiness do not represent a more severe phenotype, by the classic definition of OSA, but their subjective sleep quality is compromised, causing a vicious cycle of anxiety or depression.
Obstructive sleep apnea (OSA) is defined as a disorder of sleep presenting repetitive (either complete or partial) closure of the upper airway. These apneas and hypopneas lead to oxygen desaturation, activation of the autonomous nervous system, and micro arousals. OSA is related with increased morbidity and mortality, and literature indicates an association among OSA, hypertension, cardiovascular disease (CVD), and insulin resistance (1). OSA is a very common disorder with a great additive impact on public health. Epidemiologic data in Northern Europe estimate the prevalence of moderate and severe OSA to 23.4% in women and 49.7% in men (2). A recent epidemiologic study in the general population of Cyprus approximates the intermediate-to-high risk for OSA prevalence to be 50% in males and 18% in females (3). The increase of reported OSA prevalence over time is attributed to the obesity epidemic, the advanced polysomnographic recording techniques, and the revision of the diagnostic criteria (4). In a South American population-based epidemiologic study, 32.8% of the participants had OSA and 16.9% had an apnea–hypopnea index (AHI) ≥ 15. In the same study, Epworth Sleepiness Scale (ESS) > 9 and/or frequencies higher than once a week of the eighth question of Pittsburgh Sleep Quality Index (PSQI) classified the participants in the 55% of the population experiencing sleepiness (5). A study in Spain recorded hypersomnolence in 18% of the subjects and was not related with OSA (6). Interestingly, a longitudinal study of the Wisconsin Sleep Cohort estimated a 3-fold greater mortality risk for participants with severe sleep-disordered breathing (SDB), independently of sleepiness (7). In a large cluster analysis, depression was the lowest (5.2%) in the group with young overweight, minimally symptomatic without comorbidities group and greater (26.4%) in the middle-aged symptomatic multimorbid OSA group (8). Even less evidences exist in the literature concerning insomnia and OSA, as initially it was not considered a symptom related to OSA. What could be the clinical importance of daytime symptoms is yet to be answered.
Several screening questionnaires have been validated to identify patients with OSA, based mainly on symptoms and demographics data. Patients often complain about sleepiness and/or insomnia-like symptoms. There is considerable variability in symptom perception and expression, biological severity, and consequences of the syndrome and sleep quality among patients, even though OSA diagnosis is usually defined by AHI.
Recent studies have suggested that this heterogeneity of OSA could be due to different phenotypes in terms of symptoms and have aimed to identify clinical subtypes of OSA, taking into account demographics, severity of disease, symptoms, and comorbidities (9–12). A pioneer study identified three groups: (a) disturbed sleep (insomnia and restless sleep), (b) minimally symptomatic, and (c) excessive sleepiness during daytime (9). Consequent studies identified similar or more specific-oriented groups. For example, a multicenter study described five clinical phenotypes: (a) disturbed sleep, (b) minimally symptomatic, (c) upper airway symptoms with sleepiness, (d) upper airway symptoms dominant, and (e) sleepiness dominant (with few other symptoms) (10). A prospective longitudinal study of adult patients with OSA (AHI of ≥5/h) examined four clinical presentation phenotypes considering daytime symptoms described as excessive daytime sleepiness (EDS) and nocturnal sleep problems other than OSA (insomnia): (1) EDS, (2) EDS/insomnia, (3) non-EDS/non-insomnia, (4) and insomnia phenotype (11). In another attempt to investigate treatment outcomes on different clinical phenotypes, a study identified five distinct clusters with marked clinical differences (12). Sleepiness, insomnia, and lack of symptoms are the common components of all taxonomy efforts.
Multiple subcategorizations, though interesting, minimally improve our diagnostic abilities, treatment options, progression of the syndrome, and outcome prediction. Our attempt was to investigate potential differences between two main groups: those expressing major symptoms (sleepiness and/or insomnia) and those with minimal symptoms. The latent is a silent group, difficult to identify during screening and with potentially the same harmful consequences of OSA. We designed this study in order to assess possible differences between symptomatic and asymptomatic patients, concerning the severity of the syndrome, the prevalence of comorbidities, the subjective sleep quality, and common mental disturbances, namely, anxiety and depression.
In a large-scale epidemiologic study conducted in the adult general population, 4,118 adult participants were interviewed in order to estimate OSA prevalence in Cyprus [Cyprus Sleep Apnea Epidemiological Study (CySAES)] (3). The initial sample consisted of adult individuals residing in Cyprus. Inclusion criteria were (1) age ≥ 18 years, (2) Cypriot citizens, and (3) consent to participate in the study. The sample was categorized based on the last demographic report (2016) by district, rural, or urban area; gender; and age (13). The questionnaire was administered using computer-assisted telephone interviewing (CATI) method (14). First, all eligible participants were interviewed by phone and answered a modified STOP-Bang questionnaire in order to estimate OSA risk. A secondary cross-sectional nationwide survey was piloted to examine the validity of the estimated screening results. From the initial representative sample, 344 adults were randomly selected to participate in the second stage procedure by undertaking a type III sleep study. A type III sleep testing device monitors a minimum of four channels that include one or more channels of respiratory effort, airflow, oxygen saturation, and heart rate/electrocardiogram. No strict inclusion or exclusion criteria were applied in this second phase of the study, in order to guarantee no bias in the selection, minimize the necessary sample size, and achieve reliable results. Participants were excluded from the analyses only if they had a previous known history of sleep apnea and/or were under treatment with continuous positive airway pressure (CPAP) or other therapies. The flowchart of the study is summarized in Figure 1.
A total of 264 adults (76.74%, age: 21–83 years) finally underwent a type III sleep study assessment for possible OSA. Standards from the American Academy of Sleep Medicine (AASM) manual were used to score respiratory events (15). The AHI was calculated as the mean number of apneas and hypopneas per hour of sleep study. AHI ≥ 5 was considered diagnostic for sleep apnea regardless of symptoms.
Additionally, all subjects provided a self-reported medical history about previously diagnosed comorbidities (hypertension, arrhythmias, heart failure, ischemic heart disease, previous stroke, and diabetes mellitus) and answered the Greek version of PSQI (Gr-PSQI), ESS, Athens Insomnia Scale (AIS), and Hospital Anxiety and Depression Scale (HADS).
PSQI questionnaire contains 19 self-rated questions and 5 questions rated by the bed partner or roommate. Only self-rated questions are included in the scoring. The 19 self-rated questions are combined to form 7 component scores; each one has a range of 0–3 points and represents 7 clinically derived domains of sleep difficulties. In all cases, 0 indicates no difficulty, while 3 indicates severe difficulty. The seven component scores are then summed to yield total surrogate PSQI score (global PSQI) with a range of 0–21 points, with 21 indicating severe difficulties in all areas (16). Self-administered score of >5 has a diagnostic sensitivity of 89.6% and specificity of 86.5% to distinguish “poor” sleepers from “good” sleepers, even though it is not related to objective sleep measures [actigraphy and polysomnography (PSG)] in a community sample.
ESS measures the general level of sleepiness by asking people to rate their usual chances of dozing off or falling asleep in eight different situations or activities of their daily lives. Although a score > 10 is considered affirmative for excessive, self-rated, daytime sleepiness, the correlation between ESS and mean sleep latency or measures of sleep apnea severity is low (17).
AIS is designed to assess the nature, severity, and impact of insomnia and monitor treatment response in adults. A cutoff score of ≥6 is used to establish the diagnosis of insomnia (18). The eradication of primary and secondary definitions of insomnia allows the clinicians to diagnose insomnia regardless of the causes of the disorder.
Finally, HADS is a reliable instrument for detecting and separating the states of depression and anxiety, by excluding somatic symptoms (19). Scores of >10 are indicative of psychological morbidity, whereas scores between 8 and 10 are considered borderline.
Known psychiatric pathologies were ruled out at the time of inclusion, by using a self-reported medical history table describing the major psychiatric and neurologic pathologies and medication that could affect the scores of the symptom questionnaires.
We divided newly diagnosed sleep apneic patients in symptomatics (Group A) and asymptomatics (Group B) in order to investigate possible differences between the two groups concerning syndrome's severity, patients' sleep quality, and comorbidities. One or both symptoms (sleepiness and/or insomnia-like) present categorized the individual as symptomatic (93 individuals, 60%) and neither major symptom as asymptomatic (62 individuals, 40%).
The study protocol was approved by the Institutional Review Board of both the General Hospital in Nicosia, Cyprus, and the “Alexandra” University Hospital in Greece, and the Cyprus Bioethics Committee (EEBK/EP/2016/35). All subjects gave consent to participate in the study after appropriate information was given.
Statistical analysis included summarization of the data in tables and charts, and it was performed by using a statistical analysis software platform (IBM SPSS Statistics v.25 program). Specifically, χ2 test was performed when comparing nominal variables, and t-tests were performed when comparing continuous variables. Continuous variables are summarized with means and standard deviations and compared using t-tests. Categorical variables are summarized using frequencies and percentages and compared among groups using chi-squared. Descriptive statistics procedures for complex survey data (chi-square) were used to examine demographic and health characteristics for all participants. Two-sided hypothesis testing was performed in order to reject or not the null hypothesis. All results reported are based on two-sided tests. Tests were adjusted for all pairwise comparisons within a row of each innermost sub-table using the Bonferroni method of correction. A p < 0.05 was regarded as statistically significant.
From 4,118 eligible responders, stratified to represent the Cypriot population, a cohort of 344 individuals—randomly selected by SPSS—were enrolled; and 264 subjects (77%) underwent type III sleep test. According to the diagnostic criteria for breathing disturbances, 155 had AHI ≥ 5. From these 155 (40 female and 115 male) individuals, 34% had ESS > 10 and 49% AIS ≥ 6. The characteristics of the sleep disturbance (SDB) group are summarized in Table 1. One or both symptoms present categorized the individual as symptomatic (Group A, 93 individuals, 60%) and neither major symptom as asymptomatic (Group B, 62 individuals, 40%).
Males were 72.9% of symptomatic and 74.35 of asymptomatic OSA patients. Age was 55.78 ± 12.5 in symptomatics and 59.29 ± 12.77 in asymptomatics. To ensure the internal validity of our research, the two groups were tested for confounding factors, namely, gender, age, and body mass index (BMI). There were no significant statistical differences (SSDs) between the two groups (symptomatic–asymptomatic) concerning anthropometrics (neck, abdomen, and hip circumferences; BMI; age; or gender; Table 2). The two groups had also no SSD in OSA severity as concluded by AHI, oxygen desaturation index (ODI), mean oxygen saturation (SaO2), and comorbidities. Sleep test data are summarized in Table 3.
Symptomatics had statistically significant poorer sleep quality than asymptomatics (GR-PSQI, 8.41 ± 4.23, vs. 4.88 ± 2.43, p = 0.000), with no SSD regarding hours in bed (7.14 ± 1.05 vs. 7.24 ± 1.21) and the use of sleep medications (0.45 ± 1.06 vs. 0.18 ± 0.65, comp6). SSDs between OSA patients with and without symptoms were observed in the subjective perception of sleep quality (1.61 ± 0.93 vs. 0.81 ± 0.61, p = 0.000, comp1), sleep latency (1.28 ± 1.05 vs. 0.85 ± 0.87, p = 0.007, comp2), sleep duration (1.62 ± 0.94 vs. 1.18 ± 0.75, p = 0.001, comp3), sleep efficiency (1.07 ± 1.25 vs. 0.43 ± 0.80, p = 0.000, comp4), sleep disturbance (1.6 ± 0.68 vs. 1.07 ± 0.47, p = 0.000, comp5), and daytime dysfunction (0.79 ± 0.73 vs. 0.38 ± 0.52, p = 0.000, comp7).
The global PSQI score for symptomatics was mainly determined by sleep disturbance (22.42%), duration of sleep (20.63%), and subjective sleep quality (19.53%). For asymptomatics, the decisive factors were primarily duration of sleep (27.36%) and sleep disturbance (24.62%) (Figure 2). There was SSD only in the defining contribution of duration of sleep (p = 0.013) in the configuration of global PSQI between the two groups (6.20 ± 1.15 for symptomatics vs. 6.66 ± 1.19 for asymptomatics, p = 0.049), as no SSD was observed in sleep onset and offset and time in bed.
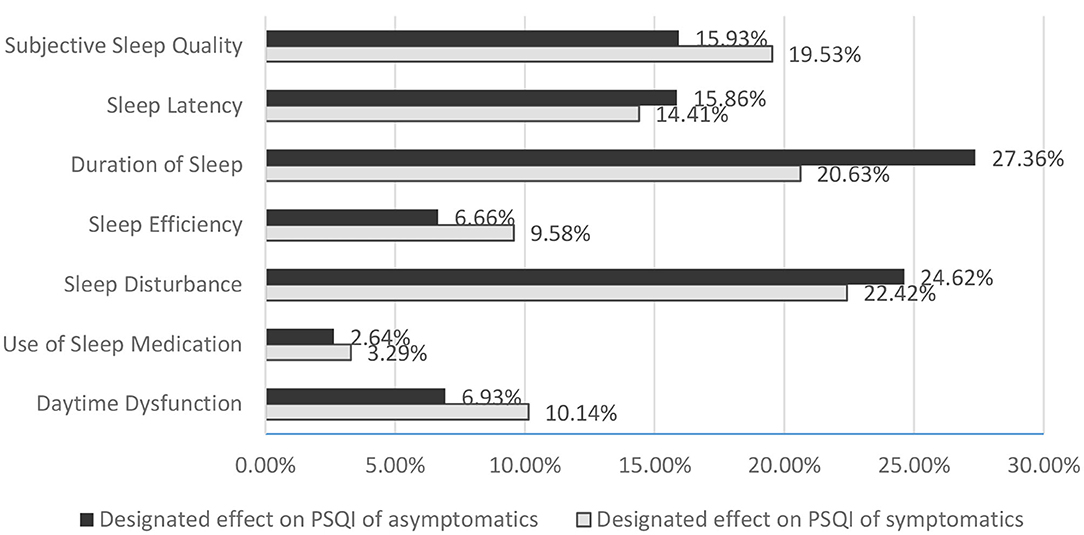
Figure 2. Designated effect of categories on PSQI in OSA patients with and without symptoms. PSQI, Pittsburgh Sleep Quality Index; OSA, obstructive sleep apnea.
Finally, when OSA patients with insomnia-like symptoms were compared with the rest individuals with AHI ≥ 5, all components of PSQI were SSDs, including the use of sleep medication. On the contrary, when the sleepy group was compared with the rest individuals with AHI ≥ 5, there was no SSD for hours in bed, sleep latency, and use of medication (Table 4).
The prevalence and severity of anxiety, depression, and fatigue were higher in patients with symptoms.
Anxiety was present in 18.1% of symptomatic OSA patients compared with 2.9% of asymptomatics (p = 0.003), whereas depression was likely in 12% of symptomatic patients compared with 2.9% of asymptomatics (p = 0.037). Symptomatics were more anxious (6.55 ± 4.37 vs. 3.65 ± 3.01, p = 0.000) and more depressed (6.08 ± 3.81 vs. 3.26 ± 3.28, p = 0.000) than asymptomatics, according to the HADS score. No SSD of previous mental illnesses existed between the two groups (11.1% of symptomatics and 8.7% of asymptomatics). Moreover, 63.4% of symptomatics complained about fatigue vs. 30.3% of asymptomatics (p = 0.000), with a total score of 4.65 ± 1.61 vs. 3.16 ± 1.6 (p = 0.000).
We further included the possible confounder PSQI total score as a variable in our regression models; in this way, we controlled for the impact of the confounding variable. The estimated measure of association before and after adjusting for confounding was examined. The coefficient for symptomatics dropped more than 10%, when total PSQI was introduced into the model, meaning that PSQI was a confounding variable that affected anxiety (p < 0.001, dependent variable) in a causal relationship, as well as the symptoms (p = 0.027, independent variable). It was also a confounding variable related with the depression (p < 0.001) and symptomatic groups (p = 0.011).
To further support our findings, comparisons were conducted between a baseline–non-OSA population sample. Sleep quality was estimated in a control sample of 109 non-SDB subjects with normal AHI (<5/h). The control sample was not divided into symptomatics and asymptomatics, as the AHI was the controlled coefficient. The control sample was summoned after the analysis of the sleep studies (Figure 1). When compared with the subjects with abnormal AHI (≥5/h), there was no SSD regarding HADS, AIS, PSQI, and all its components. The PSQI score was similar between the two groups: it was 6.33 ± 3.39 in the control group vs. 6.75 ± 3.99 in the SDB group. This reinforces the outcomes of the study, suggesting the importance of symptoms in subjective sleep quality. ESS score was significantly higher in AHI ≥ 5 group (6.26 ± 4.16 in the control group vs. 8.31 ± 5.52, p = 0.001). To further strengthen the clinical relevance of the results of the study, a separate analysis between subjects with AHI <15 and AHI ≥ 15 was performed. There was no significant difference for AIS, ESS, PSQI, depression, and anxiety. An extra proof that AHI severity is almost irrelevant to the investigated parameters.
Although the AHI or REI metrics are subject to criticism nowadays, they remain the way we assess patients (20); therefore, a further classification according to the standard severity of OSA was conducted. Interestingly, only ESS was significantly higher between severe OSA and control group (6.26 ± 4.16 in the control group vs. 9.47 ± 5.32, p = 0.007), while insomnia, anxiety, and depression were almost evenly distributed between groups. PSQI was also insignificantly different, pointing out the importance of symptoms and not AHI in the quality of sleep. Therefore, the usual markers of OSA severity do not address efficiently the sleep quality and psychiatric consequences of the syndrome if the questionnaires centered on patient-reported symptoms are not applied. Nevertheless, the small numbers of the four groups did not allow us to extract solid results between classically determined severity groups.
The analysis was repeated by using a cutoff of 15/h, which is the current way to establish the need for treatment. There was a significant difference only for sleep efficiency (0.74 ± 1.01 in the control vs. 0.84 ± 1.23, p = 0.005, comp4). Interestingly, anxiety and depression had no significant difference between the two groups.
The addition of a control group in the comparison did not allow us to establish that the symptoms reported, namely, insomnia and sleepiness, are solely due to the associated OSA, as these daytime symptoms are expressions of multiple different conditions and only sleepiness in severe OSA was statistically different to the control group. Nevertheless, such an establishment was not the objective of the study. The comparison with the control group fortified the importance of symptoms in sleep quality and psychiatric disturbances, as they differ not because of AHI but of the symptoms per se. In this regard, the analysis of classic OSA severity markers did not help in establishing more firm OSA phenotypes, as the sample was limited.
Further verification analysis was performed; results are depicted in Table 5. We compared the patients from the SDB group according to their response to AIS, ESS, and both. For patients with or without insomnia, there were strong significant differences for PSQI (p < 0.000), anxiety (p < 0.000), and depression (p < 0.000); similar differences were recorded for patients with or without both symptoms (PSQI, p < 0.000; anxiety, p < 0.000; and depression, p < 0.000). For patients with sleepiness compared with patients without sleepiness, there were also SSDs for PSQI (p < 0.005), anxiety (p < 0.012), and depression (p < 0.009). A similar sensitivity analysis for four categories of patients, with solely sleepiness or only insomnia, both symptoms, or no symptoms, was conducted. There were significant differences for PSQI between the no symptoms group and the only insomnia group (p < 0.000) and the no symptoms group and the both symptoms group (p < 0.000), but not with the only somnolence group. In an analogous pattern, there were significant differences for anxiety between the no symptoms group and the only insomnia group (p < 0.000) and the no symptoms group and the both symptoms group (p < 0.000), but not with the only somnolence group. Similarly, for depression, there were SSDs of the no symptoms group, respectively, with the only insomnia group (p < 0.000) and both symptoms group (p < 0.000), but not the only somnolence group. The results verify the impact of major sleep symptoms to the psychiatric disturbances, primarily insomnia, but somnolence cannot be acquitted due to the small sample groups.
The concept of apnea index was first introduced by Guilleminault as a metric for sleep apnea syndromes (21). Hypopneas were officially embraced in the index in a consensus report by the AASM (22). Subsequently, a persistent argument raised concerning the cutoff level of desaturation for scoring hypopneas and the tallying of arousals following respiratory events in the severity index (respiratory disturbance index) (23). The index multiplies when lower cutoff points for hypopneas are introduced and arousals are added in the calculation. AHI is criticized for correlating weakly with endotype (underlying etiology), phenotype (symptoms and adverse outcomes), and response to treatment (23, 24). Rephrasing an elegant editorial by Levy et al. (25) AHI reflects only a metric in OSA with limited impact and meaning on a complex entity. That is why novel composite scores taking into account subjective complaints, comorbidities, and AHI (26) or integrated scores for multiple constituents of disease severity (27) gain ground in describing OSA severity. The widely accepted severity cutoffs 5, 15, and 30 per hour were used for further analysis even though they are considered invalid for clinical decision making, as they correlate poorly with symptoms, comorbidities, and outcomes (28).
It is nowadays broadly recognized that the sole calculation of the AHI is not sufficient to correctly classify our apneic patients. Furthermore, excessive daytime hypersomnolence is not associated with AHI, while insomnia is the dominant symptom of OSA. Multiple phenotypes with different clinical and demographic characteristics have been reported, as the syndrome is more complex by definition. The attempt is to enhance categorization of OSA patients, link each category to a favorable treatment option, and ultimately to accomplish precision-based medicine for OSA patients (24).
Our study intended to address some of the most important clinical questions and challenges regarding (1) whether clinical phenotyping of OSA by means of symptom expression, subjectively measured with ESS and AIS, is related to disease severity as measured by AHI or other proposed indexes and (2) whether EDS and/or insomnia-like symptoms in OSA have adverse effect on PSQI and subsequently or bilaterally neuropsychiatric disorders (NPDs). According to our data in a general population of Cyprus, OSA patients reporting insomnia-like symptoms and/or sleepiness do not represent a more severe phenotype, by the classic definition of OSA with AHI, but their subjective sleep quality is compromised, causing a vicious cycle of anxiety or depression.
The current state of knowledge indicates that the diagnosis and treatment of OSA, focusing on the number of respiratory events during sleep, are an oversimplified taxonomy (26). The complex pathophysiology, the variety of clinical presentation (e.g., daytime sleepiness, insomnia-like and mood disturbances, or minimal symptoms), and the relevant comorbidities (recognized to be highly associated with OSA, e.g., arterial hypertension) comprise a heterogeneous syndrome.
Recent concepts on differing clinical phenotypes provide opportunities for a better understanding of the syndrome. Clustering of symptoms and comorbidities allows discrimination between clinical subgroups with different characteristics. There were several attempts to identify clinical subtypes of OSA (8, 9, 11, 12, 29, 30); however, the generalizability of available data is limited due to methodological differences. Nevertheless, three generally accepted subgroups are patients with paucity of symptoms, patients with EDS, and patients with complaints of insomnia-like sleep disturbance. The conventional description of a typical OSA patient has focused on symptoms of increased daytime sleepiness; however, insomnia patients represent the dominant phenotype in clinical practice. The frequency of reported insomnia symptoms in different OSA cohorts varies between 39 and 55% (31). Not < 56% in a cohort were labeled as an EDS-insomnia or insomnia phenotype (11).
Taking into account the frequent coexistence of symptoms (EDS-insomnia), we recognized the existence of two clusters of patients: one with relatively low symptom burden and another with predominant insomnia-like sleep disturbance symptoms and/or daytime sleepiness, among newly diagnosed patients with AHI ≥ 5, in a general population-based study. These differences in expressing or not nocturnal or diurnal symptoms may be an add on risk factor for sleep apnea severity, comorbidities, mental symptoms, and, last but not the least, sleep quality. Moreover, it is important to know whether phenotyping the patients for symptoms that are characteristic but not exclusively attributed to OSA has any clinical importance. Finally, understanding of the silent asymptomatic cluster of OSA patients is important in order to re-establish our screening tools and referral patterns. Simplification to the two most common specific OSA presentation—asymptomatic vs. symptomatic—groups is essentially sufficient to assess severity profiles and collateral consequences.
Symptoms in OSA (EDS, insomnia, depression, fatigue, etc.) are considered to be influenced by sex, age, and the presence of other comorbidities (32). In our study, there were no statistical differences between the two groups (symptomatic–asymptomatic) in terms of gender, age, or comorbidities. Actually, there were different outcomes in the literature concerning EDS and insomnia-like symptoms. Although sleepiness was linked to cardiovascular morbidity and mortality outcomes (33), it was not associated with an increase of prevalence in CVD in a large cohort (34). In another population-based, cross-sectional study, insomnia prevalence did not differ between subjects with and without OSA, but moderate-to-severe OSA subjects reported less insomnia symptoms than subjects without OSA (35). It is possible that other coexisting sleep disorders, definition, and assessment of the symptoms and the studied population also affect the results (9, 36).
PSG is the reference assessment tool for the diagnosis of OSA (20). Cumulative data demonstrated a weak relationship between daytime excessive sleepiness and the conventional measures of OSA severity (e.g., the AHI), and that was also confirmed in our results. The two groups—symptomatic vs. asymptomatic—had no differences in terms of AHI, ODI, and mean SaO2 magnitude. Patients with a high AHI may score low on symptom scales and vice versa (37). This diversity may be attributed to differences in individual susceptibility to the systemic effects of OSA. The clinical definition of OSA based on the combination of AHI and daytime symptoms is compromised by the high prevalence of elevated AHI in the general population and by the poor correlation of EDS with AHI (38). Type III studies do not include sleep staging and are expected to give a lower AHI compared with the calculation based on PSG where periods of wakefulness during the sleep study are excluded in the calculation of AHI (39). Underestimation of AHI did not affect our results, as the dependent factor was symptoms and both groups were subject to the same bias.
However, our symptomatic group reported poorer sleep quality on PSQI and complained more often about symptoms of depression, anxiety, and fatigue than did the asymptomatic group. The global PSQI score for symptomatics was mainly determined by sleep disturbance, duration of sleep, and subjective sleep quality, whereas for asymptomatics, the decisive factors were primarily duration of sleep and sleep disturbance.
Duration of sleep was less for symptomatics than for asymptomatics and the best predictor of subjective sleep quality, but there was no SSD on time in bed. Wake after sleep onset (WASO) time is not reflected on PSQI calculation, but in our opinion, it is an important sleep quality factor. In a sleep quality study in renal transplant patients, the patients with PSQI > 5 were consider as poor sleepers and showed a higher total medical comorbidity score, poorer mental health, and more severe anxiety but no difference in depressive symptoms when compared with the good sleepers group (40). In another sleep quality study, subjective sleep quality was strongly negative correlated with depression score, physical symptoms, and trait anxiety (41), similar to our results. Subjective sleep quality's association with sleep onset latency was stronger than with sleep duration. In a community-dwelling adults study, with mean PSQI score of 6.3, PSQI and ESS were related poorly with each other. Participants grouped by either cluster analysis of PSQI and ESS scores differed from each other on psychological/stress symptoms, but not on polysomnographic indices. Higher PSQI scores were associated with greater psychological distress and larger sleep disturbance on sleep diaries. Finally, the PSQI was more closely related to psychological symptom ratings and sleep diary measures than the ESS (42).
Poor sleep quality has a major long-term impact on mental and physical health. Our study identified a cause-and-effect relationship between PSQI and symptoms of anxiety and depression. PSQI had a causal relation to the symptoms, especially insomnia; and symptoms' expression correlated with poor sleep quality and separately with anxiety and depression. There is growing evidence for an increased frequency of OSA in a variety of NPDs, including stroke, neurodegenerative/muscular disorders, major depression, and post-traumatic stress disorder (43, 44). Several studies suggest that OSA not only may be frequent but also represents an independent risk factor for the subsequent development of NPDs, such as depression (45).
AHI was not related to the risk for hospitalization for depression in a study by Kendzerska et al. (46), and a causal link between OSA and severe depression was not supported. Nevertheless, higher depressive symptoms were reported in OSA patients (47), almost doubled in prevalence for the OSA group compared with the no-OSA group in a population-based study (48). About 17% of OSA patients had a major depressive disorder in a large community sample (49) and up to 40% in clinical samples (47, 50). Moreover, a large general population study found an association between EDS, rather than OSA per se, and depression (51) and introduced symptoms in the equation. Association between OSA and depressive symptoms is questioned, but findings indicate that depression is a consequence of OSA (52, 53), and major sleep-related symptoms may be the mediators in this relation. Correction for confounders for depression like obesity, young age, female sex, and hypnotic medication use was not applied, as there were no SSDs between groups for these characteristics.
AHI proved once more a convenient metric but with limited clinical implications. The results of our study suggest that patients with depression should be routinely questioned for symptoms of insomnia and/or sleepiness, as further assessment and treatment for sleep-disordered breathing may mitigate depressive symptoms. The effects of OSA treatment in depression and anxiety especially in the symptomatic phenotype have to be assessed.
The major strength of our research is the validity of our findings, which is documented by the large representative population-based sample size and by the high response rate. This allows us to generalize the results, enabling extrapolation of findings to the original population. Moreover, we included all adult age groups, from 18+ to 80+ years old. Another major advantage of our study is the community-based, randomly selected sample that is optimal for epidemiological studies. As subjects were recruited from the community and not from clinical sleep canters, there is no referral bias, causing a spurious association of OSA with risk of comorbidities. The study identified a sleep lab-naive sample; and as OSA patients were excluded from the study, a better assessment of the natural history of untreated OSA is possible. A strength of our study is also the application of common questionnaires and simple phenotyping methodology across a population-based sample. Our study did not include all possible symptoms and comorbidities nor complicated phenotyping but only focused on major sleep symptoms that may occur in patients with OSA. This simplification in two groups minimizes the need for a larger sample to support SSD. Moreover, most of the previous studies included patients with moderate-to-severe OSA, and the clusters found may not be generalizable to patients with milder OSA. An important feature of our study is that all individuals with AHI ≥ 5 were introduced in the study. Reflecting the established demographic risk factors for OSA, the cohort was generally middle-aged, moderately obese, and predominantly male.
Limitations must also be acknowledged. A self-reported questionnaire concerning the history of comorbid diseases was assembled, but no medical assessment was provided in those with a negative history in order to identify non-diagnosed comorbidities. Another limitation is the absence of a psychiatric revision of the participants according to their self-reported mental questionnaires. Any differences with previous reports may be attributed to cultural or regional differences in symptom reporting (54, 55) or referral strategies and access to care, together with existing known variation in OSA etiology across ethnic groups (56–60).
As Young stated, there is more to be done in the quantification of the adverse health consequences of OSA in order to define the overall social burden (61). Experts recognizing the poor correlation between AHI and daytime symptoms, as well the multivariate expression of the syndrome, advised a revision of the diagnostic criteria and severity thresholds for OSA, taking into consideration the different clinical and pathophysiological phenotypes and relevant comorbidities (26). To conclude, identification of two distinct groups according to the expressed daytime symptoms, symptomatics and asymptomatics, requires future surveys concerning diagnostic screening, consequences, and effective treatments.
According to our study, OSA patients reporting insomnia and or sleepiness do not represent a more severe phenotype as to the classic taxonomy of syndrome's gravity categorized by the number of apneas and hypopneas per hour of sleep. Neither do symptomatics report a greater number of comorbidities. Nevertheless, symptomatics express poor sleep quality and mood disturbances significantly different from asymptomatics. The explanation given is that their sleep quality is compromised, causing a vicious cycle of anxiety or depression.
The results of the study indicate that the severity of the sleep respiratory pathology represented by AHI is inadequate. Even with the addition of oxygenation indices for the cardiovascular manifestations of the syndrome as we demonstrated in a previous study (62) and the daytime symptoms as we proposed in this study, one is uncertain to suggest a novel classification, as there are more elements missing. Nevertheless, objective assessment using symptom questionnaires is in our opinion essential and should be compulsory, as they illuminate the sleep quality aspect and predisposition for psychic imbalance.
This study contributes to the understanding of the impact of EDS and insomnia in OSA. According to our results, we ought to reconsider our screening techniques, customized to the patient's complaints, probably with the utility of sleep quality questionnaires and screening tools for NPDs. Moreover, it will be desirable to validate clinical assessment methods that correctly classify a new OSA patient. This understanding could enhance personalized treatment approaches in OSA patients. Finally, a new conceptual framework to evaluate disease severity of OSA may be developed. The diagnostic workup should incorporate this multifactorial approach and define severity, not only considering AHI but also including EDS, NPDs (e.g., cognitive impairment and depression), related sleep disturbances (e.g., insomnia), consequences, and prognoses.
A confirmation of the current findings in longitudinal studies would be needed to more precisely evaluate the value of defining clinical presentation phenotype.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the study protocol was approved by the Institutional Review Board of both the General Hospital in Nicosia, Cyprus and the Alexandra University Hospital, in Greece and the Cyprus Bioethics Committee (EEBK/EP/2016/35). All subjects gave consent to participate in the study after appropriate information was given. The patients/participants provided their written informed consent to participate in this study.
IN and TA contributed in the acquisition of the data. N-TE conceived and designed the study. SZ performed the data analysis. FF wrote the paper. GT, PN, TR, and BK participated in the interpretation of the data and revised them critically for content. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Allan I. Pack advances in sleep-disordered breathing. Am J Respir Crit Care Med. (2006) 173:7–15. doi: 10.1164/rccm.200509-1478OE
2. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the hypnolaus study. Lancet Respir Med. (2015) 3:310–8. doi: 10.1016/S2213-2600(15)00043-0
3. Frangopoulos F, Nicolaou I, Zannetos S, Economou NT, Adamide T, Georgiou A, et al. Estimating obstructive sleep apnea in Cyprus: a randomised, stratified epidemiological study using STOP-Bang sleep apnea questionnaire. Sleep Med. (2019) 61:37–43. doi: 10.1016/j.sleep.2019.04.013
4. Senaratna CV, Perret JL, Lodge C, Lowe A, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. (2016) 34:70–81. doi: 10.1016/j.smrv.2016.07.002
5. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LRA. Obstructive sleep apnea syndrome in the saò paulo epidemiologic sleep study. Sleep Med. (2010) 11:441–6. doi: 10.1016/j.sleep.2009.10.005
6. Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea–hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 Yr. Am J Respir Crit Care Med. (2001) 163:685–9. doi: 10.1164/ajrccm.163.3.2005065
7. Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the wisconsin sleep cohort. Sleep. (2008) 31:1071–8.
8. Bailly S, Destors M, Grillet Y, Richard P, Stach B, Vivodtzev I, et al. Obstructive sleep apnea: a cluster analysis at time of diagnosis. PLoS ONE. (2016) 11:e0157318. doi: 10.1371/journal.pone.0157318
9. Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Arnardottir ES, Pack AI, et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. (2014) 44:1600–7. doi: 10.1183/09031936.00032314
10. Keenan BT, Kim J, Singh B, Bittencourt L, Chen NH, Cistulli PA, et al. Recognizable clinical subtypes of obstructive sleep apnea across international sleep centers: a cluster analysis. Sleep J. (2018) 41:zsx214. doi: 10.1093/sleep/zsx214
11. Saaresranta T, Hedner J, Bonsignore MR, Riha RL, McNicholas WT, Penzel T, et al. Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS ONE. (2016). 11:e0163439. doi: 10.1371/journal.pone.0163439
12. Gagnadoux F, Le Vaillant M, Paris A, Pigeanne T, Leclair-Visonneau L, Bizieux-Thaminy A, et al. Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest. (2016). 149:288–90. doi: 10.1016/j.chest.2015.09.032
13. Tyrer S, Heyman B. Sampling in epidemiological research: issues, hazards and pitfalls. BJPsych Bull. (2016) 40:57–60. doi: 10.1192/pb.bp.114.050203
14. Kempf AM, Remington PL. New challenges for telephone survey research in the twenty-first century. Annu Rev Public Health. (2007) 28:113–26. doi: 10.1146/annurev.publhealth.28.021406.144059
15. Iber C. American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine. (2007).
16. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburg sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1988) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
17. Johns MW. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
18. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. The diagnostic validity of the athens insomnia scale. J Psychosom Res. (2002) 55:263–7. doi: 10.1016/S0022-3999(02)00604-9
19. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
20. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an american academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:479–504. doi: 10.5664/jcsm.6506
21. Guilleminault C, Van Den Hoed J, Mitler M. Clinical overview of the sleep apnea syndromes. In: Guilleminault C, Dement W, editors. Sleep Apnea Syndromes. New York, NY: Alan R Liss (1978). p. 1–12.
22. The Report of an American Academy of Sleep Medicine TaskForce. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. (1999) 22:667–89. doi: 10.1093/sleep/22.5.667
23. Pevernagie DA, Gnidovec-Strazisar B, Grote L, Heinzer R, McNicholas WT, Penzel T, et al. On the rise and fall of the apnea–hypopnea index: a historical review and critical appraisal. J Sleep Res. (2020) 29:e13066. doi: 10.1111/jsr.13066
24. Edwards BA, Redline S, Sands SA, Owens RL. More than the sum of the respiratory events: personalized medicine approaches for obstructive sleep apnea. Am J Respir Crit Care Med. (2019) 200:691–703. doi: 10.1164/rccm.201901-0014TR
25. Lévy P, Tamisier R, Pépin JL. Assessment of sleep-disordered-breathing: quest for a metric or search for meaning? J Sleep Res. (2020) 29:e1314. doi: 10.1111/jsr.13143
26. Randerath W, Bassetti CL, Bonsignore MR, Farre R, Ferini-Strambi L, Grote L, et al. Challenges and perspectives in obstructive sleep apnoea report by an ad hoc working group of the sleep disordered breathing group of the european respiratory society and the european sleep research society. Eur Respir J. (2018) 52:1702616. doi: 10.1183/13993003.02616-2017
27. Dieltjens M, Verbraecken JA, Hedner J, Vanderveken OM, Steiropoulos P, Kvamme JA. Use of the clinical global impression scale in sleep apnea patients –results from the ESADA database. Sleep Med. (2019) 59:56–65. doi: 10.1016/j.sleep.2018.10.028
28. Hudgel DW. Sleep apnea severity classification-revisited. Sleep. (2016) 39:1165–6. doi: 10.5665/sleep.5776
29. Vavougios GD, George DG, Pastaka C, Zarogiannis SG, Gourgoulianis KI. Phenotypes of comorbidity in OSAS patients: combining categorical principal component analysis with cluster analysis. J Sleep Res. (2016) 25:31–8. doi: 10.1111/jsr.12344
30. Lacedonia D, Carpagnano GE, Sabato R, Lo Storto MM, Palmiotti GA, Capozzi V, et al. Characterization of obstructive sleep apnea-hypopnea syndrome (OSA) population by means of cluster analysis. J Sleep Res. (2016) 25:724–30. doi: 10.1111/jsr.12429
31. Luyster FS, Buysse DJ, Strollo PJ Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. (2010). 6:196–204. doi: 10.5664/jcsm.27772
32. Levy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. (2015) 1:15015. doi: 10.1038/nrdp.2015.15
33. Ren R, Li Y, Zhang J, Zhang J, Zhou J, Sun Y, et al. Obstructive sleep apnea with objective daytime sleepiness is associated with hypertension. Hypertension. (2016) 68:1264–70. doi: 10.1161/HYPERTENSIONAHA.115.06941
34. Oglivie RP, Lakshminarayan K, Iber C, Patel SR, Lutsey PL. Joint effects of OSA and self-reported sleepiness on incident CHD and stroke. Sleep Med. (2018) 44:32–7. doi: 10.1016/j.sleep.2018.01.004
35. Uhlig BL, Hagen K, Stjern EM, Gravdahl GB, Sand T. The relationship between obstructive sleep apnea and insomnia: a population-based cross sectional polysombographic study. Sleep Med. (2019) 54:126–33. doi: 10.1016/j.sleep.2018.10.026
36. Adams RJ, Appleton SL, Vakulin A, Lang C, Martin SA, Taylor AW, et al. Association of daytime sleepiness with obstructive sleep apnoea and comorbidities varies by sleepiness definition in a population cohort of men. Respirology. (2016) 21:1314–21. doi: 10.1111/resp.12829
37. Deegan PC, McNicholas WT. Predictive value of clinical features for the obstructive sleep apnoea syndrome. Eur Respir J. (1996) 9:117–24. doi: 10.1183/09031936.96.09010117
38. Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. (2016) 47:194–202. doi: 10.1183/13993003.01148-2015
39. Escourrou P, Grote L, Penzel T, McNicholas WT, Verbraecken J, Tkacova R, et al. The diagnostic method has a strong influence on classification of obstructive sleep apnea. J Sleep Res. (2015) 24:730–8. doi: 10.1111/jsr.12318
40. Kachuee H, Ameli J, Taheri S, Assari S, Riahipour F, Khedmat H, et al. Sleep Quality and its correlates in renal transplant patients. Transpant Proc. (2007) 39:1095–7. doi: 10.1016/j.transproceed.2007.04.001
41. Augner C. Association of subjective sleep quality with depression score, anxiety. Physical symptoms and sleep onset latency in students. Cenr Eur J Public Health. (2011) 19:115–7. doi: 10.21101/cejph.a3647
42. Buysse DJ, Hall ML, Strollo PJ, Kamarck TW, Owens J, Lee L, et al. Relationships between the pittsburgh sleep quality index (PSQI), epworth sleepiness scale (ESS), and clinical/polysomnographic measures in a community sample. J Clin Sleep Med. (2008) 4:563–71. doi: 10.5664/jcsm.27351
43. Gupta M, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. (2015) 11:165–75. doi: 10.5664/jcsm.4466
44. Knechtle B, Economou NT, Nikolaidis PT, Velentza L, Kallianos A, Steiropoulos P, et al. Clinical characteristics of obstructive sleep apnea in psychiatric disease. J Clin Med. (2019) 8:534. doi: 10.3390/jcm8040534
45. Haba-Rubio J, Marti-Soler H, Tobback N, Andries D, Marques-Vidal P, Waeber G, et al. Sleep characteristics and cognitive impairment in the general population: the hypnolaus study. Neurology. (2017) 88:463–9. doi: 10.1212/WNL.0000000000003557
46. Kendzerska T, Gershon AS, Hawker GA, Tomlinson GA, Leung RS. Obstructive sleep apnoea is not a risk factor for incident hospitalised depression: a historical cohort study. Eur Respir J. (2017) 49:1601361. doi: 10.1183/13993003.01361-2016
47. Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Sleep Med Rev. (2009) 13:437–44. doi: 10.1016/j.smrv.2009.04.001
48. Chen YH, Keller JK, Kang JH, Hsieh HJ, Lin HC. Obstructive sleep apnea and the subsequent risk of depressive disorder: a population-based follow-up study. J Clin Sleep Med. (2013) 9:417–23. doi: 10.5664/jcsm.2652
49. Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry. (2003) 64:1195–00. doi: 10.4088/JCP.v64n1009
50. Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler EO, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. (2008) 4:19–25. doi: 10.5664/jcsm.27074
51. LaGrotte C, Fernandez-Mendoza J, Calhoun SL, Liao D, Bixler EO, Vgontzas AN. The relative association of obstructive sleep apnea, obesity and excessive daytime sleepiness with incident depression: a longitudinal, population-based study. Int J Obes. (2016) 40:1397–404. doi: 10.1038/ijo.2016.87
52. Bixler EO, Gaines J, Vgontzas AV. Obstructive sleep apnoea and depression: is there an association? Eur Respir J. (2017) 49:1700858. doi: 10.1183/13993003.00858-2017
53. Kales A, Caldwell AB, Cadieux RJ, Soldatos CR, Kales JD. Severe obstructive sleep apnea–II: associated psychopathology and psychosocial consequences. J Chronic Dis. (1985) 38:427–34. doi: 10.1016/0021-9681(85)90138-9
54. Im EO, Ko Y, Chee W. Symptom clusters among multiethnic groups of cancer patients with pain. Palliat Support Care. (2013) 11:295–305. doi: 10.1017/S1478951512000314
55. Bauer AM, Chen CN, Alegría M. Prevalence of physical symptoms and their association with race/ethnicity and acculturation in the United States. Gen Hosp Psychiatry. (2012) 34:323–31. doi: 10.1016/j.genhosppsych.2012.02.007
56. Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spryet K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. (1997) 155:186–92. doi: 10.1164/ajrccm.155.1.9001310
57. Ong KC, Clerk AA. Comparison of the severity of sleep-disordered breathing in Asian and Caucasian patients seen at a sleep disorders center. Respir Med. (1998) 92:843–8. doi: 10.1016/S0954-6111(98)90386-9
58. Li KK, Kushida C, Powell NB, Riley RW, Guilleminaultet C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. (2000) 110(10 Pt 1):1689–93. doi: 10.1097/00005537-200010000-00022
59. Lee RW, Vasudavan S, Hui DS, Prvan T, Petocz P, Darendeliler A, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. (2010) 33:1075–80. doi: 10.1093/sleep/33.8.1075
60. O'Connor GT, Lind BK, Lee ET, Nieto FJ, Redline S, Samet JM, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the sleep heart health study. Sleep. (2003). 26:74–9.
61. Young T. Rationale, design and findings from the Wisconsin sleep cohort study: toward understanding the total societal burden of sleep disordered breathing. Sleep Med Clin. (2009) 4:37–46. doi: 10.1016/j.jsmc.2008.11.003
Keywords: obstructive sleep apnoea, sleep quality, anxiety, depression, cardiometabolic comorbidities
Citation: Frangopoulos F, Zannetos S, Nicolaou I, Economou N-T, Adamide T, Georgiou A, Nikolaidis PT, Rosemann T, Knechtle B and Trakada G (2021) The Complex Interaction Between the Major Sleep Symptoms, the Severity of Obstructive Sleep Apnea, and Sleep Quality. Front. Psychiatry 12:630162. doi: 10.3389/fpsyt.2021.630162
Received: 17 November 2020; Accepted: 29 January 2021;
Published: 25 February 2021.
Edited by:
Ingo Fietze, Charité—Universitätsmedizin Berlin, GermanyReviewed by:
Maria Rosaria Bonsignore, University of Palermo, ItalyCopyright © 2021 Frangopoulos, Zannetos, Nicolaou, Economou, Adamide, Georgiou, Nikolaidis, Rosemann, Knechtle and Trakada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgia Trakada, Z3RyYWthZGFAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.