- 1Sociology of Medicine and Psychobiology, Department of Physical Activity and Health, University of Potsdam, Potsdam, Germany
- 2Faculty of Health Sciences Brandenburg, University of Potsdam, the Brandenburg Medical School Theodor Fontane and the Brandenburg University of Technology Cottbus, Senftenberg, Germany
- 3Department of Sports Medicine and Exercise Physiology, Goethe University Frankfurt, Frankfurt am Main, Germany
- 4Statistical Consulting Unit StaBLab, Ludwig-Maximilians-Universität München, Munich, Germany
- 5University Hospital Carl Gustav Carus at Technical University Dresden, Dresden, Germany
- 6Department of Preventive and Sports Medicine, Institute of Occupational, Social and Environmental Medicine, Goethe University Frankfurt, Frankfurt am Main, Germany
- 7Orthopädiezentrum Theresie, Munich, Germany
- 8Pain Management, Centre of Orthopaedics and Trauma Surgery, Conservative Orthopaedics and Pain Management, Heidelberg University Hospital, Heidelberg, Germany
- 9Centre of Sports Medicine, University Outpatient Clinic, University of Potsdam, Potsdam, Germany
The effects of exercise interventions on unspecific chronic low back pain (CLBP) have been investigated in many studies, but the results are inconclusive regarding exercise types, efficiency, and sustainability. This may be because the influence of psychosocial factors on exercise induced adaptation regarding CLBP is neglected. Therefore, this study assessed psychosocial characteristics, which moderate and mediate the effects of sensorimotor exercise on LBP. A single-blind 3-arm multicenter randomized controlled trial was conducted for 12-weeks. Three exercise groups, sensorimotor exercise (SMT), sensorimotor and behavioral training (SMT-BT), and regular routines (CG) were randomly assigned to 662 volunteers. Primary outcomes (pain intensity and disability) and psychosocial characteristics were assessed at baseline (M1) and follow-up (3/6/12/24 weeks, M2-M5). Multiple regression models were used to analyze whether psychosocial characteristics are moderators of the relationship between exercise and pain, meaning that psychosocial factors and exercise interact. Causal mediation analysis were conducted to analyze, whether psychosocial characteristics mediate the exercise effect on pain. A total of 453 participants with intermittent pain (mean age = 39.5 ± 12.2 years, f = 62%) completed the training. It was shown, that depressive symptomatology (at M4, M5), vital exhaustion (at M4), and perceived social support (at M5) are significant moderators of the relationship between exercise and the reduction of pain intensity. Further depressive mood (at M4), social-satisfaction (at M4), and anxiety (at M5 SMT) significantly moderate the exercise effect on pain disability. The amount of moderation was of clinical relevance. In contrast, there were no psychosocial variables which mediated exercise effects on pain. In conclusion it was shown, that psychosocial variables can be moderators in the relationship between sensorimotor exercise induced adaptation on CLBP which may explain conflicting results in the past regarding the merit of exercise interventions in CLBP. Results suggest further an early identification of psychosocial risk factors by diagnostic tools, which may essential support the planning of personalized exercise therapy.
Level of Evidence: Level I.
Clinical Trial Registration: DRKS00004977, LOE: I, MiSpEx: grant-number: 080102A/11-14. https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00004977.
Perspective
The effect of sensorimotor exercise on LBP can be moderated by psychosocial factors. Therefore, diagnostic tools that can be applied as a prescreening tool to design a personalized exercise intervention protocol and to optimize the effect of the exercise on LBP are important.
Introduction
Unspecific chronic low back pain (CLBP, abbreviations in Appendix A) is a worldwide health problem that has not been satisfactorily mitigated with current knowledge and treatment approaches (1). One single solution is not effective; rather, a collective interdisciplinary approach has been recommended by international clinic guidelines (2). The large number of physical and psychological LBP-therapies and their relatively small effects (3–5) have led to the call for individualization of therapies (6) based on diagnostic tools to determine which therapy would be most appropriate for the person (7–9). Unfortunately, solely the personalization of therapies does not solve this problem completely (10). Diagnostic tools may not be precise enough to detect and delineate different areas of risk, (11) or the dose-response relationship between treatment and adaption needs to be investigated more (12).
Exercise can be an important treatment for CLBP (13). However, the effects of exercise on self-reported pain severity and psychological improvements, which only range from small to moderate, are inconsistent (14) and often of weak long-term effects (15). An explanation for this inconsistency may be the impact of psychosocial factors and the reciprocal relationship between exercise and pain. There is evidence that specific psychosocial factors, also called “yellow flags” (16), influence the course of pain. These include distress (17–19), fear avoidance, catastrophizing, and social isolation (20, 21). Some of them have a dosage-related U-shaped relationship with pain (22), a possible explanation for the variation in intervention success of exercise (12, 23). For example, moderate exercise can reduce stress and therefore, it impacts pain (mediation). On the other hand, stress influences the effects of exercise on pain (moderation). Although it is to assume that psychosocial variables account, to some extent, for the relationship between exercise and pain, the direction of these pathways is still unknown (see Figure 1). Theoretically, there are different explanations. For example, stress-related neural pattern alterations influence peripheral nerve function (24), pain transmission (25, 26), and myelination due to metabolic changes (24), all contributing to increased peripheral sensitization (27). Further, psychological stress and depressed mood influence molecular and functional recalibrations among mitochondria and mitochondrial energetics (28), contributing to fatigue and tissue alterations [e.g., nerve growth factor (NGF), innervation density, and bone metabolism (29)] that all tackle the effects of exercising. Therefore, stress-related alterations may influence the sensorimotor system and the adaption to sensorimotor exercise stimuli (30).
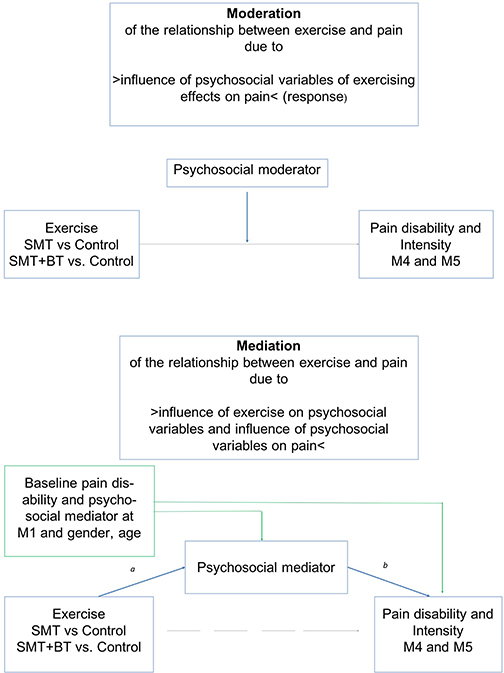
Figure 1. Demonstration of moderation (objective 1) and mediation (objective 2) of psychosocial risk factors on the relationship of exercise and pain. All analyses are controlled for age and sex (mechanisms of interest: blue lines; direct effects: gray lines; possible confounder: green lines).
This raises the question: to what extent do psychosocial factors alter the response of sensorimotor exercise on pain reduction, the dose-response, and how these psychosocial factors interact with sensorimotor exercise. So far, little is known about the moderation or mediation effect of psychosocial factors on the response to sensorimotor exercise; to the best of our knowledge, there is only one systematic review that stated that psychosocial factors may be moderators of general pain treatments (31), while there are more studies which suggest that fear, catastrophizing, and depression are possible mediators of pain and disability in the context of physical activity treatments (32–34). Further, there is a lack of research that includes various psychosocial factors and investigates the question of moderation and mediation simultaneously. The aim of this randomized controlled trial was to analyze whether there is (1) a moderation of exercise effects on LBP due to psychosocial factors and (2) a mediation of exercise effects on LBP due to psychosocial factors (Figure 1). Study questions were addressed in a three armed intervention design, in which beside a regular therapy group two sensorimotor exercise groups exist. The multimodal module with three additional units [details see (35)] addressed consequently above described psychoneuroendocrinological and molecular pathways: (a) A cognitive distraction module (working memory task) during exercising should target the processing of pain stimuli and their emotional classification within the pain-brain-network; (b) A body scan module should harmonize neuronal activity and neurotransmitter concentration and thus reducing peripheral pain sensitization and transmission; (c) A psychoeducation module inform about pain processing, uncomfortable pain behavior (fear avoidance), and mechanisms of becoming chronic pain.
Materials and Methods
Participants
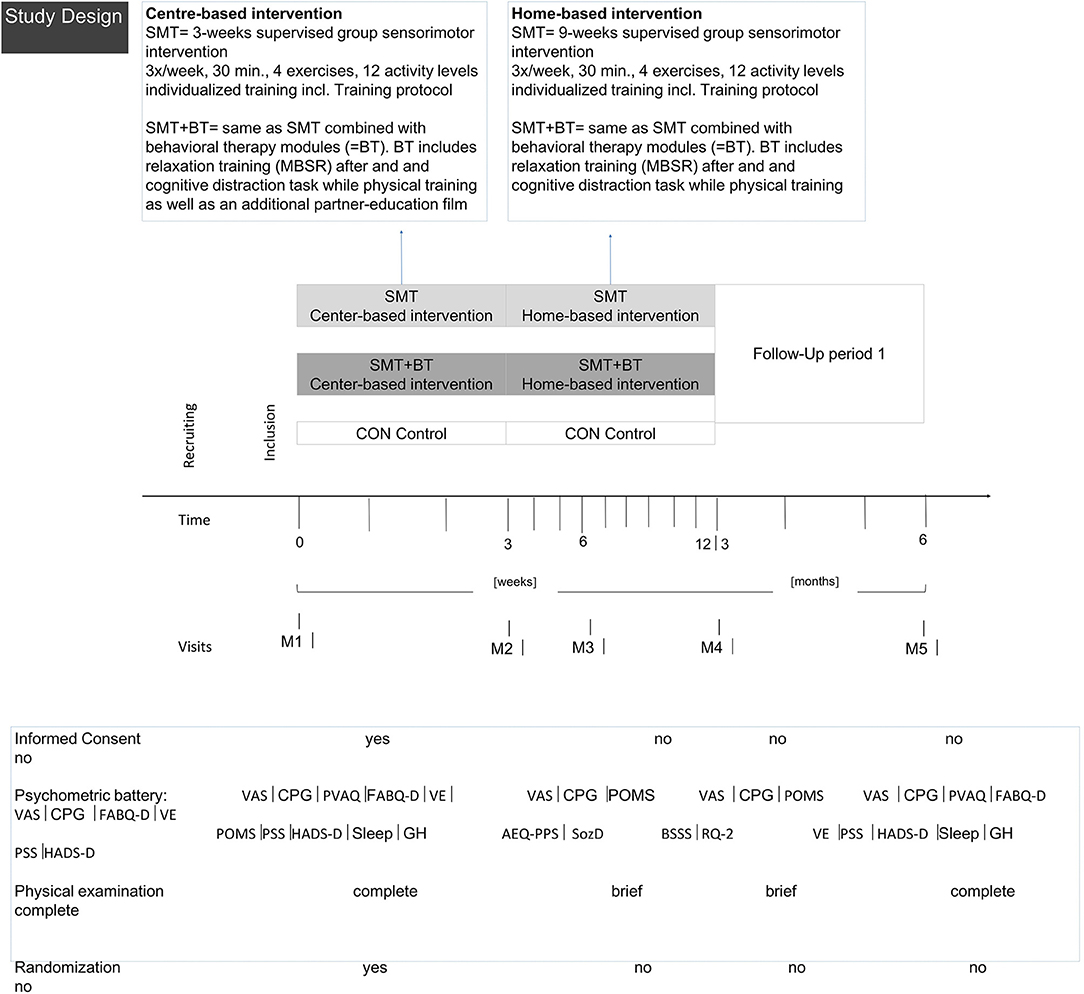
Participants were recruited from advertising and while consulting orthopedic outpatient clinics of university sites across Germany for intermittent low back pain for the first time. Study sites are located at different German federal states, offer primary care for general population and for athletes (Center of German Sports Medicine). All five study sites are members of the National Research Network for Medicine in Spine Exercise (MiSpEx, www.mispex.de) and followed the same study routines. Inclusion criteria were as follows: age 18-65 years, non-acute (low) back pain (>20 on a 100-point Visual-Analog Pain Scale). Persons who suffered from acute infections (<7 days), acute back pain (>7 days prior to study), or illnesses/syndromes that contraindicate physical activity (e.g., red flags such as inflammatory, traumatic, or systematic processes), were pregnant, were not able to stand upright independently, and were unable to get up from a lying position or unable to fill out a questionnaire independently were excluded. In total, 744 persons were screened and 662 were included after receiving written and oral information and having signed the informed consent form (enrolment Figure 2). Sample size calculation was based on a feasibility study [α ≤ 0.05; 1-β = 0.999, drop out 30%, power analysis by G*Power, (36) effect size f = 0.25, sample size: n = 600].
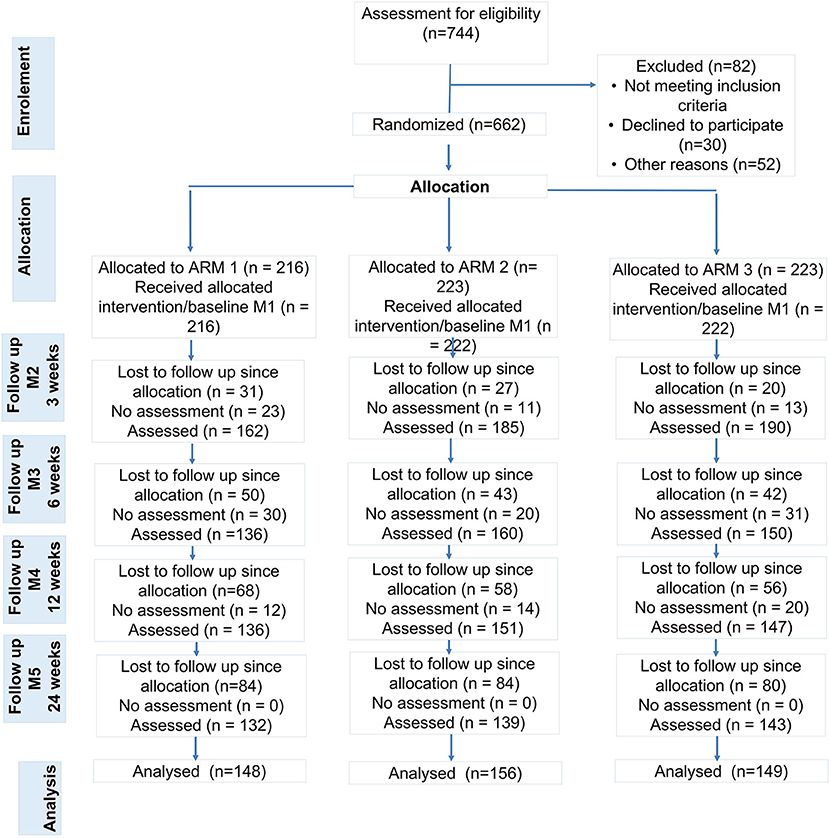
Figure 2. Study Flow (ARM 1: control group, ARM2: unimodal sensorimotor treatment, SMT; ARM 3: multidisciplinary sensorimotor exercise and behavioral treatment, SMT-BT). People with no pain (pain class 0, n = 41), athletes (n = 11) and people with missing cases at M1, M4 and M5 were excluded from analyses.
Design and Procedure
The multicenter study was a single-blind 3-arm randomized controlled trial of a 12-week intervention [Clinical-Trial-Register 05/16/2013, No-DRKS00004977, 06/2013-12/2014, for further information and findings see (37–39)]. Test-retest studies at all study sites guaranteed the validation of measures, methods and intervention routines before the RCT started.
Participants were randomly allocated (nblock= 18, basis 1:1; www.randomization.com) to a control group (CG, usual care), a unimodal intervention group (SMT, sensorimotor treatment), or a multidisciplinary group (SMT-BT, sensorimotor treatment with behavioral modules) as SMT is one of the most effective exercises for decreasing pain intensity (40–43). An additional intervention description is provided in Appendix B. Exercise diaries secured compliance control.
After baseline (M1), measurements were taken after 3 (M2), 6 (M3), 12 (M4), and 24 weeks (M5). Assessments consisted of questionnaires, physical examination, and functional measurements (Figure 3). Study personnel were blinded; participants were instructed not to communicate their group allocation to other participants or the staff. The study was conducted according to the Declaration of Helsinki (ethics approval 01/25/2012, ethics review board University of Potsdam No. 36/2011) and complied with the Consolidated Standards of Reporting Trials (CONSORT).
Instruments
Primary Outcome Criteria
Pain intensity and disability in the past 3 months were assessed by the subscales “characteristic pain intensity” and “subjective pain disability” of the Chronic Pain Grade questionnaire (CPG) (44).
Moderators and Mediators
Psychosocial risk factors were captured by different questionnaires and reported separately in three domains (pain experience, stress, and social context).
Pain Experience
Anxiety and depression during the previous week were assessed using the Hospital Anxiety and Depression Scale (HADS, two subscales, range: 0 [no anxiety/depression] up to 21 [severe anxiety/depression]; clinical cut off: anxiety >11, depression > 9 points) (45).
Awareness and preoccupation with pain were assessed using the Pain Vigilance and Awareness Questionnaire (PVAQ-D) (46). Higher scores on a single scale (range 0-80) indicate higher levels of awareness and attention to pain.
Fear-avoidance-beliefs were measured using the Fear-Avoidance-Belief-Questionnaire (FABQ-D) (47). Higher scores (range 0-30) indicate stronger fear-avoidance-beliefs (only “Pain caused by physical activity” subscale is presented in this paper).
Stress
Subjective stress was measured using the Perceived Stress Scale (PSS) (48). The final score ranges from 0 (no perceived stress) to 40 (high perceived stress), within the last 3 months.
Vital exhaustion (VE), a state of extreme physical fatigue, irritability, and hopelessness “in previous time” was measured with the Maastricht Vital Exhaustion Questionnaire (VE) (49). The final score ranges from 0 to 18 (0-3 = no exhaustion, 4-10 = mild to moderate exhaustion, 11-14 = considerable exhaustion, and <14 points=severe vital exhaustion).
Social Environment
Demographics (sex, age, education, employment, and household composition) and lifestyle factors (physical activity, medication, alcohol, and nicotine consumption) were assessed using standardized questions. Social support in the previous week was assessed using the Berliner Social Support Scales (BSSS) (50).
Statistical Analysis
As study objectives are not an evaluation of treatment main effects (e.g., intention to treat analysis), only complete cases were analyzed. Participants with no pain (defined as Korff pain class 0) and with more than 10 h of physical activity/week (athletes) were excluded from the analysis because of a higher level of structural physiological adaptation at baseline, the different psychosocial life-context, and pain tolerance in comparison to untrained people (51). Missing data imputation were carried out in accordance with questionnaire manuals and American Psychology Association guidelines (52). Missing cases (e.g., missing questionnaire at measurement point) were not respected (complete case analysis).
Moderation Analysis
Moderation analysis was performed in R (53), whereby regression models for each psychosocial factor on the dependent outcome, pain intensity and disability, included an interaction between the groups (CG, SMT, SMT-BT) and psychosocial factors (linear interaction models from M1 to M4 and to M5, conservative calculation). Results were presented as the difference between the expected value of the outcome variable of an intervention-group participant and of a control-group participant, given all other parameters held constant. The difference in the effects on pain outcome between the intervention and control groups depended on the value of the moderating factor. Therefore, we present this difference once with the value given by the 20% quantile and a second time by the 80% quantile of each factor (as we are especially interested in people with higher psychosocial risk). For each model, an F-test was performed to test whether the regression coefficients for the interaction significantly improved model fit to the data. All models were controlled for baseline pain, age, sex, physical activity (logarithmic sports variable), and study center.
Mediation Analyses
Mediation analysis was conducted with the mediation R package (53, 54) under the counterfactual framework (55) which is based on the Imai and colleagues approach to extend Structural Equation Modeling (SEM) by using causal inference methods (56). From the two general analytical approaches for mediation [statistical/traditional (57) and causal], the second one comprehend interactions and assumptions testing's by means of sensitivity analysis (58).
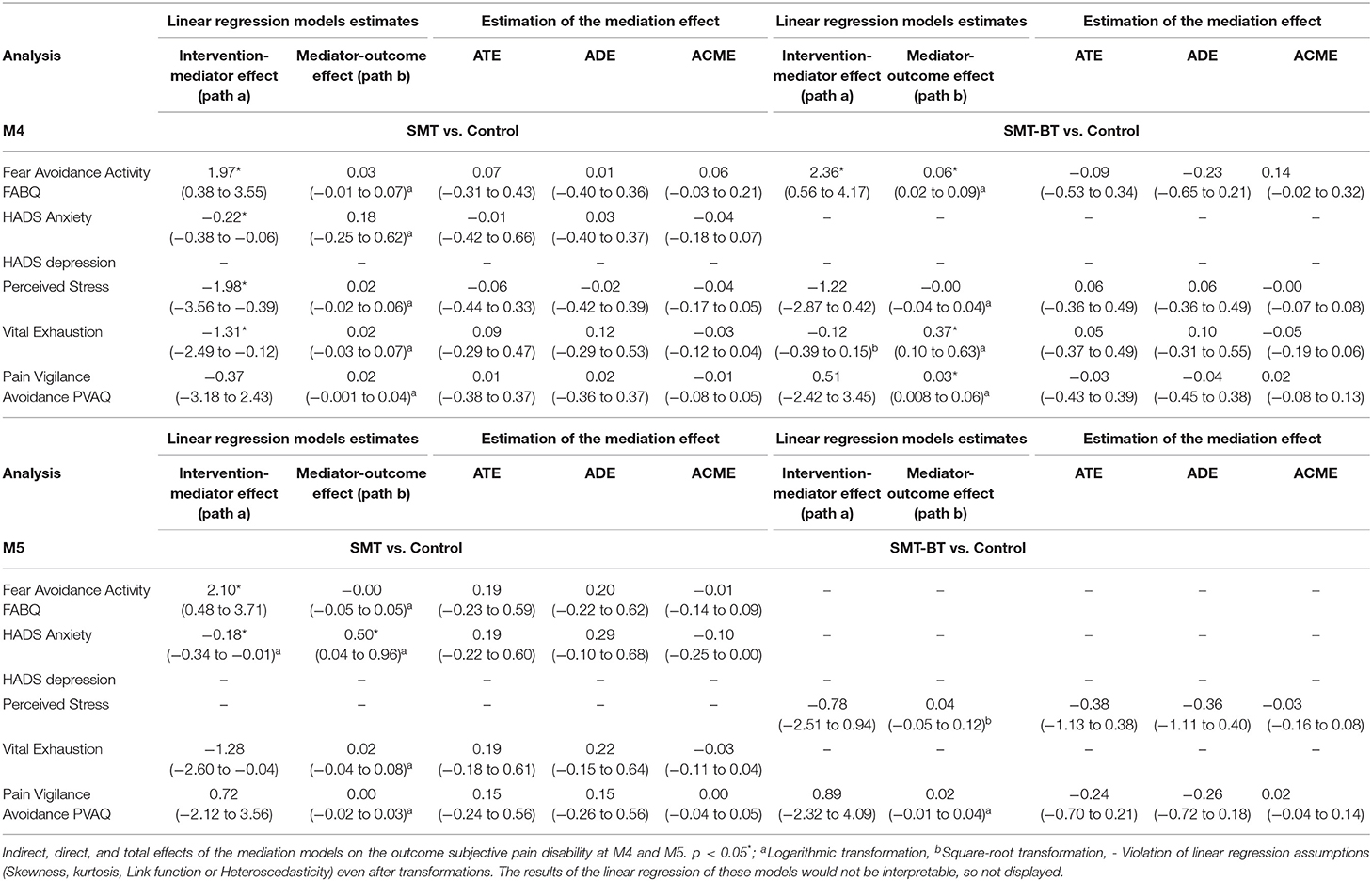
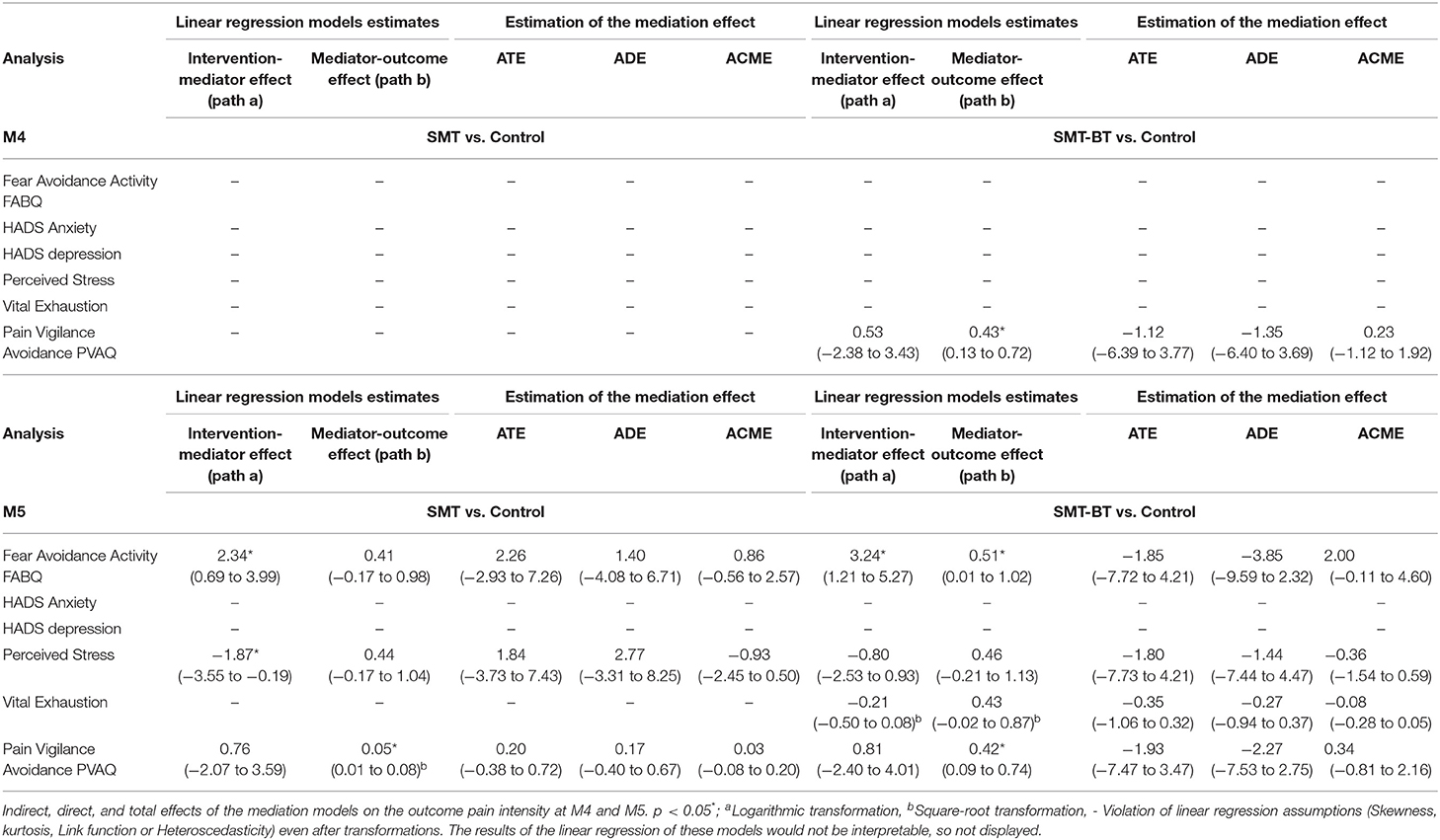
Our causal mediation analysis explored the linear relationship between the intervention (SMT and SMT-BT), a psychosocial factor and the main outcomes (subjective pain disability and pain intensity at M4 and M5). We analyzed the psychosocial factors from the tree yellow flag domains (pain experience, stress, and social environment) assessed at M4 as mediators.
Estimation of the mediation effect followed two steps. First, two statistical models were fitted separately; (54) to begin with “the mediator model” comprised the mediator (psychosocial factor, e.g., HADS Anxiety) as the dependent variable from the intervention (e.g., SMT). Following, “the outcome model” with pain (subjective pain disability and pain intensity) as a function of the mediator and intervention. In both models, we controlled for baseline measures of pain and mediators (at M1), gender and age. We only used linear regression models, with proper transformations (e.g., logarithmic, square root) when needed to comply with statistical assumptions (e.g., skewness/heteroscedasticity) for these first step.
Second, the mediate function incorporated both models to compute the total (ATE), direct (ADE) and indirect effect (ACME). The ACME is the portion of the total effect of the intervention on the outcome that is conducted though the mediator. While ADE is the remaining effect of the intervention owed to other causal mechanisms (59). In these second step, we applied the non-parametric bootstrap for variance estimation with 1,000 random samples (59).
Further, a test for a potential interaction between intervention and mediator in the “outcome model” was performed. If the interaction test (test.Tmint from mediation package R) showed a positive result (p-value <0.05), the treatment–mediator interaction was included.
Only if a mediation effect is detected a sensitivity analysis is planned. The presented approach relies on the “sequential ignorability assumption,” defined as: accounting the observed confounders, the treatment assignment is presumed to be ignorable, namely, independent of potential outcomes and potential mediators (54). When the sequential ignorability assumption is violated a sensitivity analysis is outlined in order to determine the robustness of the ACME estimate (mediation effect) (54, 60).
Results
Descriptive
Six hundred and sixty volunteers participated, of which 453 (age M [SD] = 39.5 [12.2] years, f = 62%, higher education 45%, pain medication intake 10%, living in partnership 55%) were finally available for the statistical analysis (complete case for M1, M4, and M5, minimum pain CPG-class and exclusion of 11 athletes). The sample examined had moderate pain (for disability: M [SD] = 20.86 [22.75], and intensity M [SD] = 35.10 [18.98]), average BMI M [SD] = 24.30 [3.66], and less comorbidity with a low psychosocial risk at baseline. Only a small portion of the participants showed an elevated psychosocial risk profile, although mostly in the low to moderate range (values for each group see Table 1, Figure 4). Significant differences at baseline was shown for DISS only between SMT+BT and CG (p = 0.01) and for BMI between SMT+BT and the other groups. The mean training frequency in the center-based phase was M (SD) = 2.64 (0.61) times per week and during the home-based phase M (SD) = 2.40 (0.87) times per week.
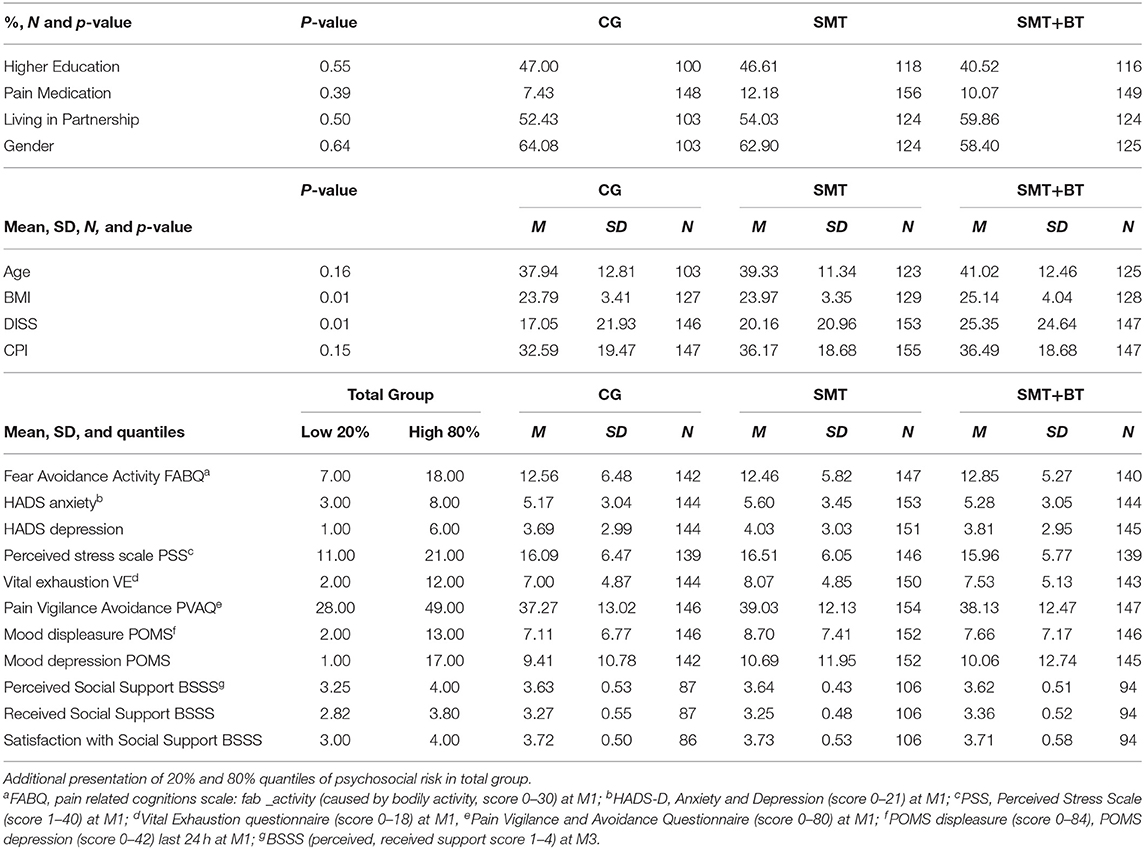
Table 1. Baseline sample and descriptive characteristics (M, SD) of psychosocial factors (separated for each scale/group).
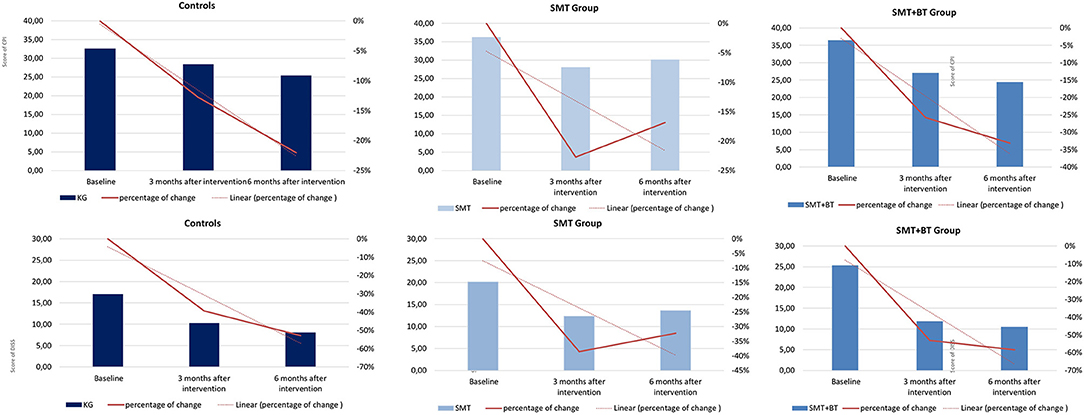
Figure 4. Graphical demonstration of the improvement of pain in percentage from baseline (M1) up to 12 weeks (M4) and 24 weeks (M5) in all groups (pain intensity [above] and pain disability [below]).
Role of Psychosocial Moderators on the Intervention Effect on Pain
Pain Experience
There was no significant moderation of the intervention effects by fear avoidance regarding physical activity (kinesiophobia), by pain vigilance-avoidance and by anxiety after the intervention (M4). In contrast, depressive mood (POMS) and depressive symptomatology (HADS) at baseline were systematic moderators of the intervention effects on CPI. For the multidisciplinary group (SMT-BT), for example, the expected CPI value in M4 was 8.62 points lower than the expected CPI in the control group for patients with a HADS depression value of 6.00. Furthermore, depressive symptoms (HADS) significantly moderated pain disability (Table 2). By subtracting from the value of SMT-BT the value of SMT for a given quantile one is able to derive the expected difference in CPI/DISS between SMT-BT and SMT patients. For example the expected CPI value in M4 for a patient in SMT-BT with a HADS depression value of 6.00 (80% quantile) is −8.62 - (-4.21) = −4.41 points i.e. 4.41 points lower than for a patient in SMT. Remark: A negative difference implies a lower, a positive difference a higher value for SMT-BT compared to SMT.
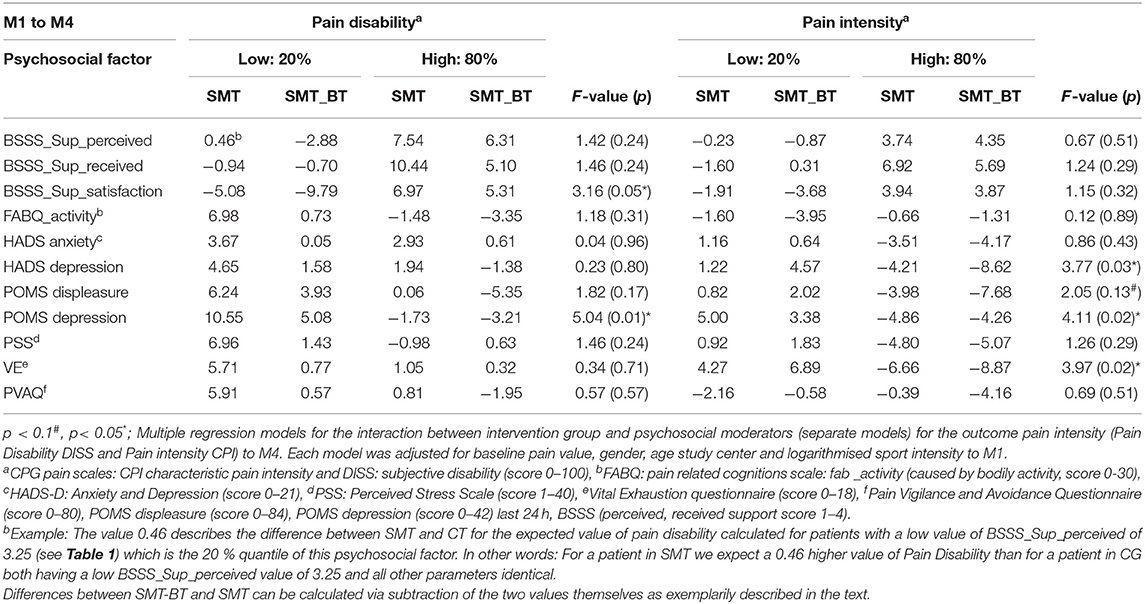
Table 2. Interactions-metrics of intervention effects for subjective pain disability and pain intensity 12 weeks (M4) after the intervention (20%, 80% quantiles).
Regarding the sustainability of the programs (M5), it was shown that depressive symptomatology is a significant moderator of the intervention effects (8.81 CPG points in pain intensity in the multidisciplinary group); no improvement was observed in the SMT group. The opposite was true for the significant moderation due to anxiety: participants who were anxious at the beginning of the intervention and improved in the meantime, deteriorated again, especially in the SMT-BT group (Table 3).
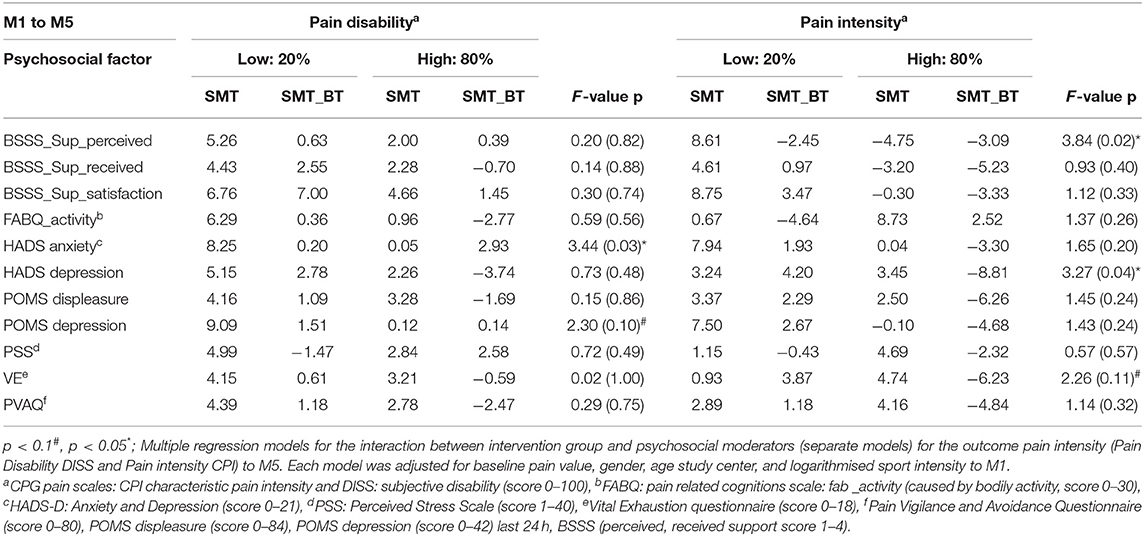
Table 3. Interactions-metrics of intervention effects for subjective pain disability and pain intensity 24 weeks (M5) after the intervention (20%, 80% quantiles).
Stress
A significant moderation of the intervention effects on CPI after the intervention (M4) was observed by vital exhaustion. This means that highly exhausted participants, each with a VE value of 12 at baseline, would improve their CPI intervention effect scores in comparison to a control group participant around 8.87 CGP points in the SMT-BT group and around 6.66 points in the SMT group. No significant moderation was observed for the Perceived Stress Scale (Table 2 and Figure 5). At follow-up, there was no sustainable effect due to domain stress, although vital exhaustion showed a trend toward improvement of pain intensity in the multidisciplinary group (Table 3).
Social Environment
Satisfaction with social support was a moderator of the intervention effects on pain disability. Social support in the center-based treated control group significantly moderated the intervention effects on pain disability in comparison to the home-based intervention groups (Table 2). Regarding the sustainability of the intervention effects, the opposite was observed: perceived social support was a significant moderator of the improvement of intervention effects on pain intensity for the home-based intervention groups (Table 3).
Role of Psychosocial Mediators on the Intervention Effect on Pain
None of the models showed a significant average total effect (overall intervention effect) and no psychosocial factor played a significant role as a mediator for the relationship between intervention (SMT or SMT-BT) and subjective pain disability (at M4 or M5, Table 4) as well as CPI (at M4 or M5, Table 5).
Discussion
This paper addressed the question whether psychosocial risk factors are moderators or mediators of the effects of sensorimotor exercise intervention on LBP.
Moderation
Role of Psychosocial Moderators
Results show a moderation of the intervention effects on pain syndromes in both exercise interventions compared to the controls in the short-term and further for the multidisciplinary group in the long-term. The magnitude of depressive symptomatology, vital exhaustion, and social support is a moderator of the relationship between exercise efficacy and pain. Results suggest that people with high depressive symptomatology should be better treated with multidisciplinary interventions, for which moderation amplitude reaches a clinically relevant reduction in pain intensity and disability (61–63). Further of clinical relevance was the moderation of sustainability effects of the multidisciplinary therapy due to a depressive symptomatology. While the moderation of exercise effects through vital exhaustion for the short- and long-term efficacy were comparable, the sustainability for anxiety decreased, but without clinical relevance. In summary, both of the identified moderators “depression” and “vital exhaustion” may limit long-term adaptions of the sensorimotor system to exercise stimuli regarding pain.
The reported pain syndromes of the participants may be best sub-summarized in the ICD-11 category nociplastic/algopathic/nocipathic pain, (64–66) in which pain syndromes can be explained by a central sensitization including altered nociception. Further, the two identified moderators that may influence exercise efficacy in terms of pain reduction can be sub-summarized to a “symptom triad” often described in patients with non-specific pain, which is, a co-occurrence of depression, fatigue, and pain. Different research groups try to explain this phenomenon and essentially points to an overload or “catabolic state” of the body (18, 67, 68).
However, several explanations are given for this. As introduced, a stress-induced catabolic state may alter myelination (24) innervation density (29), peripheral nerve function (24), pain transmission (25, 26), contributing to increased peripheral sensitization (27) and possibly altering sensorimotor system response. Also, the changes in neurotransmitter concentration and inflammatory response (e.g., neuropeptides, norepinephrine) (69) may be a profound explanation for the presented results (19, 70), in which vital exhaustion and depressive symptoms mainly moderated exercised induced pain intensity outcomes. Exercise stimuli may be differently processed depending on the exercise dose/intensity.
Moderation Within Specific Treatments
Furthermore, it is known that people with an altered nociceptive function respond better to therapies targeting central rather than peripheral pain mechanisms (64); the presented results confirm this by showing a higher benefit from the multidisciplinary intervention for people suffering on depressive symptoms. As the multimodal intervention triggers an activation of executive functions while exercising, the central processing of pain and emotional classification will be altered (71). Effects of an unimodal sensorimotor exercising such a regulation of neural circuits influencing mitochondrial energetics, can be moderated by fatigue as it is seen in the results as well. This gives notice about the catabolic state of the body limiting the adaptive capacity to neuromuscular exercise stimuli. In both cases, the implication for clinicians might be, to tailor exercise therapy to individual patient needs as far as possible.
Moderators Within Clinical Setting
As observed by many therapists, the ambulatory setting of regular routines leads to a specific comfortable effect, measured by a significant moderation of the treatment effects by social satisfaction in the control group during the intervention. Interestingly, after the end of the intervention, perceived support in exercising groups moderated the sustainability within their home-based social environment. This decisive factor, to give the patient the feeling of being “perceived” and “accepted,” may be implemented quite inexpensively in therapeutic and preventive everyday life.
Mediation
No mediation of fear avoidance, anxiety, stress, vital exhaustion, or pain vigilance on exercise effects was observed. Pain-killing medication and lower body mass index, potential positive treatment effect modifiers in other studies (72), had no influence (pain-killing medication). Although results are in line with a recently published trial (73), some studies suggesting that effects on pain can be mediated by depression, fear avoidance or pain catastrophizing (32, 33, 74) and further that these mediation effects can also play a role within a physical activity context (33, 34). A closer look to these studies give notice, that mostly pain disability is chosen as potential outcome and that exercise therapy is not specified. Studies examine mediation processes of physical activity in general, which can be influenced by age, gender, weight and social setting and are rarely controlled in the analysis. Further the methodological quality of most of these studies were low (32). Only one study showing a pain catastrophizing mediation effect after exercising referring to concrete exercise routines (aerobic and endurance training) (34). Although most studies about mediation refer to general exercise treatments they are very valuable in demonstrating the need for physical activity in everyday life regarding the preservation of function and prevention of pain.
Who Comes First: Moderation or Mediation
Regarding the literature it is obviously that psychosocial factors significantly influence the effectiveness of pain management interventions. But till now, it is still unclear whether this is based on moderation or mediation. The presented results suggest that the expression of pain intensity due to different interventions is moderated by psychosocial factors, which was elaborated here very specifically for sensorimotor training and strict controlled (baseline pain, age, gender physical activity in general) and which can be well-justified by the addressed underlying physiological processes of the treatment forms. Moreover, a stronger moderation can be seen predominantly short-term and lasts mainly over the active period-i.e. during continuous training execution. This also fits well with existing literature, which suggests that pain intensity alter more rapidly by interventions than disability or function. The moderation of exercise effects on pain intensity and disability decrease over time in presented data, although we further still do not see a mediation. Mediation processes may play role in their long-term influence on physical activity and psychosomatic symptoms such as depression in general, but they are strongly conflicted by lifestyle factors and methodologically difficult to extract, which was not study objective here.
Limitations
First, for mediation no non-linear regression techniques to model the intervention-mediator and mediator-outcome effects were explored. Further, no use of latent growth modeling which allows inclusion of multiple time points (at least 3 measurements over different time points), providing a more accurate estimate of change both within and between participants (75). However, all mediation analysis were controlled for baseline measures of pain and respective mediators. Second, sample size calculation was considered for testing treatment main effects (e.g., intention to treat analysis), which was not study objective here. As the analysis of pathways would be strongly afflicted by imputation of missing cases (e.g., missing measurement point) we preferred a complete case analysis and controlled further for age, gender, baseline pain, and physical activity in general which might be a strength of this study. However, this reduction of the sample size may limit the statistical power (e.g., exclusion of athletes, inclusion of four study-centers). Third, participant could not be blinded, although study personal was (single blinding). Fourth, due to the high number of statistical tests in moderation and mediation analyses multiple testing problems substantially increase the probability of incorrectly rejecting null hypotheses. Finally, effect sizes of the exercise intervention on pain were low (max. d = 0.2, although this is typical for exercise intervention studies) (72).
Conclusion
The results regarding a possible moderation of the response to a sensorimotor-exercise-intervention on LBP are of high relevance and raise hope for an explanation of the long-lasting heterogeneity of exercise effects on CLBP. They provide insight into a possible interaction between pain and exercise, and its dose-response relationship. Further research in this area is needed for the development of diagnostic tools (76, 77) and personalized therapy modules with reinforcing effects for self-management care (35). This would lead to a great benefit for future therapy and prevention concepts.
Data Availability Statement
The datasets for this study can be found in the MiSpEx Network, the doi is requested at University of Potsdam.
Ethics Statement
All clinical investigations have been conducted according to the principles expressed in the Declaration of Helsinki. Final ethical approval has been provided 01/25/2012 by the major institutional ethics review board of the University of Potsdam, Germany (number 36/2011). The study was registered as a clinical trial 05/16/2013 in the German Clinical Trial Register with the identification number: DRKS00004977. Study conduction between 06/2013-12/2014. All adverse events were reported in the manuscript.
Author Contributions
P-MW: writing original draft. DN: editing. P-MW and FM: conceptualization and data curation. HB, MS, CS, WB, and FM: investigation. DD and P-MW: methodology and formal analysis. All authors: resources. FM: funding, prinicipal investigator (PI), and project administration.
Funding
The present study was funded by the German Federal Institute of Sport Science on behalf of the Federal Ministry of the Interior of Germany as the major funder. It is realized within MiSpEx – the National Research Network for Medicine in Spine Exercise (grant-number: 080102A/11-14). The funder does not influence data collection, analysis, and interpretation or writing of the manuscript. The authors declare that they have no competing interests. We further acknowledge the support of the Deutsche Forschungsgemeinschaft the Open Access Publishing Fund of the University of Potsdam.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Our gratitude goes to Laura Puerto Valencia and Helmut Küchenhoff for statistical support, to Sanne Houtenbos for proof reading and to Juliane Müller, Steffen Müller, Josephine Stoll, Philipp Flößel, Martin Handel, Thore Haag, Ann-Christin Pfeifer, Simone Gantz, Lutz Vogt, Jessica de Witt Huberts, Anja Weiffen, and Anne-Kathrin Puschmann for the invaluable support in the study. We prospectively thank both the technical and medical staff at study sites for their investment during study conduction.
References
1. Henschke N, Kamper SJ, Maher CG. The epidemiology and economic consequences of pain. Mayo Clin Proc. (2015) 90:139-47. doi: 10.1016/j.mayocp.2014.09.010
2. Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. (2018) 2018:2368-83. doi: 10.1016/S0140-6736(18)30489-6
3. Hayden JA, van Tulder MW, Malmivaara A, Koes BW. Exercise therapy for treatment of non-specific low back pain. Cochrane Database Syst Rev. (2005) 1:CD000335. doi: 10.1002/14651858.CD000335.pub2
4. Henschke N, Ostelo RW, van Tulder MW, Vlaeyen JW, Morley S, Assendelft WJ, et al. Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev. (2010) 7:1-98. doi: 10.1002/14651858.CD002014.pub3
5. Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. (2014) 2014:1-11. doi: 10.1002/14651858.CD000963.pub3
6. O'Keeffe M, O'Sullivan P, Purtill H, Bargary N, O'Sullivan K. Cognitive functional therapy compared with a group-based exercise and education intervention for chronic low back pain: a multicentre randomised controlled trial (RCT). Br J Sports Med. (2019) 2019:782-9. doi: 10.1136/bjsports-2019-100780
7. Lentz TA, Beneciuk JM, Bialosky JE, Zeppieri G Jr, Dai Y, Wu SS, et al. Development of a yellow flag assessment tool for orthopaedic physical therapists: results from the optimal screening for prediction of referral and outcome (OSPRO) cohort. J Orthop Sports Phys Ther. (2016) 46:327-43. doi: 10.2519/jospt.2016.6487
8. Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. (2008) 59:632-41. doi: 10.1002/art.23563
9. Traeger A, Henschke N, Hübscher M, Williams CM, Kamper SJ, Maher CG, et al. Estimating the risk of chronic pain: development and validation of a prognostic model (PICKUP) for patients with acute low back pain. PLoS Med. (2016) 13:e1002019. doi: 10.1371/journal.pmed.1002019
10. Hill JC, Whitehurst DGT, Lewis M, Bryan S, Dunn KM, Foster NE, et al. Comparison of stratified primary care managment for low back pain with current best practice (STarTBack): a randomised controlled trial. Lancet. (2011) 378:1560–71. doi: 10.1016/S0140-6736(11)60937-9
11. Karran EL, Traeger AC, McAuley JH, Hillier SL, Yau YH, Moseley GL. The value of prognostic screening for patients with low back pain in secondary care. J Pain. (2017) 18:673–86. doi: 10.1016/j.jpain.2016.12.020
12. Wippert P-M, Wiebking C. Stress and alterations in the pain matrix: a biopsychosocial perspective on back pain and its prevention and treatment. Int J Environ Res Public Health. (2018) 15:1–11. doi: 10.3390/ijerph15040785
13. Oliveira CB, Franco MR, Maher CG, Tiedemann A, Silva FG, Damato TM, et al. The efficacy of a multimodal physical activity intervention with supervised exercises, health coaching and an activity monitor on physical activity levels of patients with chronic, nonspecific low back pain (Physical Activity for Back Pain (PAyBACK) trial): study protocol for a randomised controlled trial. Trials. (2018) 19:1–10. doi: 10.1186/s13063-017-2436-z
14. Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst- Rev. (2017) 2017:1–66. doi: 10.1002/14651858.CD011279.pub2
15. Saragiotto BT, Maher CG, Yamato TP, Costa LO, Menezes Costa LC, Ostelo RW, et al. Motor control exercise for chronic non-specific low back pain. Chocrane Database Syst Rev. (2016) 2016:1–129. doi: 10.1002/14651858.CD012004
16. Nicholas MK, Linton SJ, Watson PJ, Main CJ. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther. (2011) 96:1–17. doi: 10.2522/ptj.20100224
17. Ellingson LD, Koltyn KF, Kim JS, Cook DB. Does exercise induce hypoalgesia through conditioned pain modulation? Psychophysiology. (2014) 51:267–76. doi: 10.1111/psyp.12168
18. McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. (2010) 59:9–15. doi: 10.1016/j.metabol.2010.07.012
19. Van Houdenhove B, Luyten P. Beyond dualism: the role of life stress in chronic pain. Pain. (2005) 113:238–47. doi: 10.1016/j.pain.2004.10.010
20. Alhowimel A, AlOtaibi M, Radford K, Coulson N. Psychosocial factors associated with change in pain and disability outcomes in chronic low back pain patients treated by physiotherapist: A systematic review. SAGE Open Med. (2018) 6:1–8. doi: 10.1177/2050312118757387
21. Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. (2016) 17:T70–92. doi: 10.1016/j.jpain.2016.01.001
22. Heneweer H, Vanhees L, Picavet HS. Physical activity and low back pain: a U-shaped relation? Pain. (2009) 143:21–5. doi: 10.1016/j.pain.2008.12.033
23. Cook DB, Stegner AJ, Ellingson LD. Exercise alters pain sensitivity in Gulf War veterans with chronic musculoskeletal pain. J Pain. (2010) 11:764–72. doi: 10.1016/j.jpain.2009.11.010
24. Verheijen M, Chrast R, Burrola P, Lemke G. Local regulation of fat metabolism in peripheral nerves. Genes Dev. (2003) 17:2450–64. doi: 10.1101/gad.1116203
25. Amsalem M, Poilbout C, Ferracci G, Delmas P, Padilla F. Membrane cholesterol depletion as a trigger of Nav1.9 channel-mediated inflammatory pain. EMBO J. (2018) 37:e97349 doi: 10.15252/embj.201797349
26. Huang K, Ji Y, Huang J, Chen Q. Calculating myelin sheath thickness with watershed algorithm for biological analysis. J Teknol. (2016) 78:21–7. doi: 10.11113/jt.v78.8633
27. Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Penninx BW, et al. Reduced hypothalamic-pituitary-adrenal axis activity in chronic multi-site musculoskeletal pain: partly masked by depressive and anxiety disorders. BMC Musculoskelet Disord. (2014) 15:227. doi: 10.1186/1471-2474-15-227
28. Picard M, McManus MJ, Gray JD, Nasca C, Moffat C, Kopinski PK, et al. Mitochondrial functions moudlate neuroendocrine, metabolic, inflammatory and transcriptional responses to acute psychological stress. Proc Natl Acad Sci USA. (2015) 112:E6614–23. doi: 10.1073/pnas.1515733112
29. Wippert P-M, Block A, Mansuy IM, Peters EMJ, Rose M, Rapp MA, et al. Alterations in bone homeostasis and microstructure related to depression and allostatic load. Psychother Psychosom. (2019) 2019:383–5. doi: 10.1159/000503640
30. Picard M, Russel TH, Burelle Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol. (2012) 302:C629–41. doi: 10.1152/ajpcell.00368.2011
31. Gurung T, Ellard DR, Mistry D, Patel S, Underwood M. Identifying potential moderators for response to treatment in low back pain: a systematic review. Physiotherapy. (2015) 101:243–51. doi: 10.1016/j.physio.2015.01.006
32. Lee H, Hübscher M, Moseley GL, Kamper SJ, Traeger AC, Mansell G, et al. How does pain lead to disability? A systematic review and meta-analysis of mediation studies in people with back and neck pain. Pain. (2015) 156:988–97. doi: 10.1097/j.pain.0000000000000146
33. Marshall PMW, Schabrun S, Know MF. Physical activity and the mediating effect of fear, depression, anxiety, and catastrophizing on pain related disability in people with chronic low back pain. PLoS ONE. (2017) 7:e180788. doi: 10.1371/journal.pone.0180788
34. Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. (2006) 7:261–71. doi: 10.1016/j.jpain.2005.10.011
35. Wippert P.-M, Drießlein D, Beck H, Schneider C, Puschmann A-K, Banzer W, et al. (2020). The feasibility and effectiveness of a new practical multidisciplinary treatment for low-back pain: a randomized controlled trial. J Clin Med. 9:115. doi: 10.3390/jcm9010115
36. Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
37. Wippert PM, de Witt Huberts J, Klipker K, Gantz S, Schiltenwolf M, Mayer F. Beschreibung und empirische Fundierung des verhaltenstherapeutischen Moduls der MiSpEx-Intervention. Der Schmerz. (2015) 29:658–63. doi: 10.1007/s00482-015-0044-y
38. Mayer F, Arampatzis A, Banzer W, Beck H, Brüggemann GP, Hasenbring M, et al. Medicine in Spine Exercise [MiSpEx] – A national research network to evaluate back pain in high-performance sports as well as the general population. Z Sportmedizin. (2018) 69:229–35. doi: 10.5960/dzsm.2018.340
39. Hönning A, Stengel D CG. Statistical strategies to address main research questions of the MiSpEx network and meta-analytical approaches. German J Sports Med. (2018) 69:236–9. doi: 10.5960/dzsm.2018.339
40. Searle A, Spink M, Ho A, Chuter V. Exercise interventions for the treatment of chronic low back pain: a systematic review and meta-analysis of randomised controlled trials. Clin Rehabil. (2015) 29:1155–67. doi: 10.1177/0269215515570379
41. Arampatzis A, Schroll A, Moreno Catalá M, Laube G, Schüler S, Dreinhofer K. A random-perturbation therapy in chronic non-specific low-back pain patients: a randomised controlled trial. Eur J Appl Physiol. (2017) 117:2547–60. doi: 10.1007/s00421-017-3742-6
42. Mueller J, Stoll J, Mueller S, Mayer F. Dose-response relationship of core-specific sensorimotor interventions in healthy, well-trained participants: study protocol for a (MiSpEx) randomized controlled trial. Trials. (2018) 19:1–8. doi: 10.1186/s13063-018-2799-9
43. Owen PJ, Miller CT, Mundell NL, Verswijveren SJJM, Tagliaferri SD, Brisby H, et al. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br J Sports Med. (2019) 2019:1–12. doi: 10.1136/bjsports-2019-100886
44. Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. (1992) 50:133–49. doi: 10.1016/0304-3959(92)90154-4
45. Herrmann-Lingen C, Buss U, Snaith RP. Hospital Anxiety and Depression Scale - Deutsche Version (HADS-D), ed Manual. Bern: Hans Huber (1995).
46. Kunz M, Capito ES, Horn-Hofmann C, Baum C, Scheel J, Karmann AJ, et al. Psychometric properties of the German version of the Pain Vigilance and Awareness Questionnaire (PVAQ) in pain-free samples and samples with acute and chronic pain. Int J Behav Med. (2017) 24:260–71. doi: 10.1007/s12529-016-9585-4
47. Pfingsten M, Leibing E, Franz C, Bansemer D, Busch O, Hildebrandt J. [Fear-avoidance-beliefs in patients with backpain]. Schmerz. (1997) 11:387–95. doi: 10.1007/s004820050114
48. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. (1983) 24:385–96. doi: 10.2307/2136404
49. Appels A, Hoppener P, Mulder A. A questionnaire to assess premonitory symptoms of myocardial infarction. Int J Cardiol. (1987) 17:15–24. doi: 10.1016/0167-5273(87)90029-5
51. Tesarz J, Schuster AK, Hartmann M, Gerhardt A, Eich W. Pain perception in athletes compared to normally active controls: a systematic review with meta-analysis. Pain. (2012) 153:1253–62. doi: 10.1016/j.pain.2012.03.005
52. APS. Publication Manual of the American Psychological Association, Seventh Edition. Washington, DC: Association AP (2020).
53. R-Core-Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2019).
54. Tingley D, Teppei H, Mit Y, Keele L, State P, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. (2014) 59:1–38. doi: 10.18637/jss.v059.i05
55. Gunzler D, Chen T, Wu P, Zhang H. Introduction to mediation analysis with structural equation modeling. Shanghai Arch Psychiatry. (2013) 25:390–4. doi: 10.3969/j.issn.1002-0829.2013.06.009
56. VanderWeele TJ. Mediation analysis: a practitioner's guide. Annu Rev Public Health. (2016) 37:17–32. doi: 10.1146/annurev-publhealth-032315-021402
57. Lee H, Herbert R, McAuley J. Mediation analysis. JAMA. (2019) 321:697–8. doi: 10.1001/jama.2018.21973
58. Zhang Z, Zheng C, Kim C, Van Poucke S, Lin S, Lan P. Causal mediation analysis in the context of clinical research. Ann Transl Med. (2016) 4:425. doi: 10.21037/atm.2016.11.11
59. Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. (2002) 7:422–45. doi: 10.1037/1082-989X.7.4.422
60. Lee H, Wiggers J, Kamper SJ, Williams A, O'Brien KM, Hodder RK, et al. Mechanism evaluation of a lifestyle intervention for patients with musculoskeletal pain who are overweight or obese: protocol for a causal mediation analysis. BMJ Open. (2017) 7:e014652. doi: 10.1136/bmjopen-2016-014652
61. Krebs EE, Bair MJ, Damush TM, Tu W, Wu J, Kroenke K. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med Care. (2010) 48:1007–14. doi: 10.1097/MLR.0b013e3181eaf835
62. Ostelo RWJG, de Vet HCW. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. (2005) 19:593–607. doi: 10.1016/j.berh.2005.03.003
63. Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. (2006) 61:102–9. doi: 10.1016/j.jclinepi.2007.03.012
64. Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice AS, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. (2016) 157:1382–6. doi: 10.1097/j.pain.0000000000000507
65. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–7. doi: 10.1097/j.pain.0000000000000160
66. Hainline B, Turner JA, Caneiro JP, Stewart M, Moseley GL. Pain in elite athletes—neurophysiological, biomechanical and psychosocial considerations: a narrative review. Br J Sports Med. (2017) 51:1259–64. doi: 10.1136/bjsports-2017-097890
67. Hellhammer D, Hellhammer J. Stress: the Brain-Body Connection. Basel: Karger Medical and Scientific Publishers (2008). doi: 10.1159/isbn.978-3-8055-7969-8
68. Slade GD, Sanders AE, By K. Role of allostatic load in sociodemographic patterns of pain prevalence in the US population. J Pain. (2012) 13:666–75. doi: 10.1016/j.jpain.2012.04.003
69. Rohleder N. Stress and inflammation – The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. (2019) 105:164–71. doi: 10.1016/j.psyneuen.2019.02.021
70. Santini G, Patrignani P, Sciulli MG, Seta F, Tacconelli S, Panara MR, Ricotti E, Capone ML, Patrono C: The human pharmacology of monocyte cyclooxygenase 2 inhibition by cortisol and synthetic glucocorticoids. Clinical Pharmacology & Therapeutics (2001) 70:475-483. doi: 10.1067/mcp.2001.119213
71. Lee H, Mansell G, McAuley JH, Kamper SJ, Hübscher M, Moseley GL, et al. Causal mechanisms in the clinical course and treatment of back pain. Best Pract Res Clin Rheumatol. (2016) 30:1074–83. doi: 10.1016/j.berh.2017.04.001
72. Hayden JA, Wilson MN, Stewart S, Cartwright JL, Smith AO, Riley RD, et al. Exercise treatment effect modifiers in persistent low back pain: an individual participant data meta-analysis of 3514 participants from 27 randomised controlled trials. Br J Sports Med. (2019) 2019:1–16. doi: 10.1136/bjsports-2019-101205
73. Baadjou VA, Lee H, Smeets RJ, Kamper SJ. How much of the effect of exercise and advice for subacute low back pain is mediated by depressive symptoms? Musculoskelet Sci Pract. (2019) 44:102055. doi: 10.1016/j.msksp.2019.102055
74. Hall AM, Kamper SJ, Maher CG, Latimer J, Ferreira ML, Nicholas MK. Symptoms of depression and stress mediate the effect of pain on disability. Pain. (2011) 152:1044–51. doi: 10.1016/j.pain.2011.01.014
75. Mansell G, Hill JC, Main CJ, Von Korff M, van der Windt D. Mediators of treatment effect in the back in action trial: using latent growth modeling to take change over time into account. Clin J Pain. (2017) 33:811–9. doi: 10.1097/AJP.0000000000000463
76. Wippert P-M, Puschmann A-K, Drießlein D, Arampatzis A, Banzer W, Beck H, et al. Development of a Risk Stratification and Prevention Index for stratified care in chronic low back pain. Focus: yellow flags (MiSpEx Network). Pain Rep. (2017) e623:1–11. doi: 10.1097/PR9.0000000000000623
77. Wippert P-M, Puschmann A-K, Arampatzis A, Schiltenwolf M, Mayer F. Diagnosis of psychosocial risk factors in prevention of back pain in athletes (MiSpEx Network). BMJ Open Sports Exerc Med. (2017) 3:1–7. doi: 10.1136/bmjsem-2017-000295
78. Niederer D, Vogt L, Wippert P-M, Puschmann A-K, Pfeifer A-C, Schiltenwolf M, et al. Medicine in spine exercise (MiSpEx) for nonspecific low back pain patients: study protocol of a multicentre, single-blind randomised controlled trial. Trials. (2016) 17:1–12. doi: 10.1186/s13063-016-1645-1
79. Niederer D, Engel T, Vogt L, Arampatzis A, Banzer W, Beck H, et al. Motor control stabilisation exercise for patients with non-specific low back pain: a prospective meta-analysis with multilevel meta regressions on intervention effects. J Clin Med. (2020) 9:3058. doi: 10.3390/jcm9093058
Appendix A. Abbreviations
Appendix B. Intervention
The training was supervised for a 3-week center-based period by therapists, followed by a 9-week self-administered home-based training by DVD. Participants were scheduled to train three times a week (30 min duration, four different sensorimotor exercises: Basic: (1) quadrupedal/all-fours stability, (2) deadlift/rowing, (3) double leg – single leg heel-pad-stance and (4) side planks, three series of 10 repetitions, 2 min break). For individualization purposes, all exercises comprised 12 different levels of difficulty (at higher level with additional weights). Beyond this standard program, the SMT group received a standardized stretching program and SMT-BT group three additional behavioral modules (“cognitive distraction during exercising,” “Body Scan-Unit,” and “Psychoeducation” after exercising). Home-based training adherence was monitored by a self-administered training log, kept by the participant. For details, please see (35, 78) and further (79).
Keywords: low-back-pain, motor-control-exercise, multidisciplinary-therapy, MiSpEx-network, yellow flags
Citation: Wippert P-M, Niederer D, Drießlein D, Beck H, Banzer W, Schneider C, Schiltenwolf M and Mayer F (2021) Psychosocial Moderators and Mediators of Sensorimotor Exercise in Low Back Pain: A Randomized Multicenter Controlled Trial. Front. Psychiatry 12:629474. doi: 10.3389/fpsyt.2021.629474
Received: 04 December 2020; Accepted: 31 May 2021;
Published: 29 July 2021.
Edited by:
Andreas Stengel, Charité – Universitätsmedizin Berlin, GermanyReviewed by:
Lianne Wood, Keele University, United KingdomBárbara Oliván Blázquez, University of Zaragoza, Spain
Copyright © 2021 Wippert, Niederer, Drießlein, Beck, Banzer, Schneider, Schiltenwolf and Mayer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pia-Maria Wippert, d2lwcGVydEB1bmktcG90c2RhbS5kZQ==
 Pia-Maria Wippert
Pia-Maria Wippert Daniel Niederer
Daniel Niederer David Drießlein
David Drießlein Heidrun Beck5
Heidrun Beck5 Frank Mayer
Frank Mayer