- 1Department of Psychiatry, The Second Xiangya Hospital, Central South University, Changsha, China
- 2Hunan Key Laboratory of Psychiatry and Mental Health, China National Clinical Research Center on Mental Disorders (Xiangya), China National Technology Institute on Mental Disorders, Hunan Technology Institute of Psychiatry, Mental Health Institute of Central South University, Changsha, China
- 3Zhumadian Psychiatric Hospital, Zhumadian, China
Background: Cognitive deficits have shown progressive feature in major depressive disorder (MDD). However, it remains unknown which component of cognitive function is progressively impaired across episodes of MDD. Here we aim to identify the progressively impaired cognitive components in patients with MDD.
Methods: A comprehensive neurocognitive test battery was used to assess the cognitive components (executive function, attention, processing speed, memory, working memory, inhibition, shifting, and verbal fluency) in 35 patients with first-episode MDD (FED), 60 patients with recurrent MDD (RD) and 111 matched healthy controls (HCs). After 6 months of treatment with antidepressant, 20 FED and 36 RD patients achieved clinical remission and completed their second-time neurocognitive tests. Statistical analyses were conducted to identify the impaired cognitive components in the FED and RD groups before and after treatment, and to assess the relationship between the cognitive components and the number of episodes and total illness duration in the MDD patient group.
Results: At baseline, both the FED and RD groups showed impairments in all of the cognitive components; the FED and RD groups showed no significant difference in all of the components except for shifting. After remission, only shifting in the RD group showed no significant improvement and remained in an impaired status. Furthermore, shifting was the only component negatively correlated with the number of episodes as well as the total illness duration.
Conclusions: Shifting may serve as the progressive cognitive deficit across episodes of MDD.
Clinical Trials Registration: Registry name: HPA function and MRI study of trauma-related depression; Registration number: ChiCTR1800014591; URL: http://www.chictr.org.cn/edit.aspx?pid=24669&htm=4.
Introduction
Major depressive disorder (MDD) is a debilitating mental disorder characterized by life-long recurring episodes. Among patients who achieved full remission after a major depressive episode, 16% will develop at least one new episode in the next 6 months, 26% will develop an episode in 1 year (1), and 85% will have that experience again in 15 years (2). According to some studies, the increasing number of episodes is positively correlated with increasing severity of symptoms, longer duration of illness, increasing vulnerability of developing new episodes, and increasing risk of relapse (3–5), suggesting a progressive nature of depression pathology. The progressive nature of MDD may be a major cause of its poor prognosis and chronicity, which have been reported to be associated with impairment in social function and high disease burden (6–9).
It is well-established that cognitive deficits, mainly involving executive function, attention, processing speed, and memory (10, 11), are currently acknowledged as a most pronounced clinical feature of MDD (12–14). Progressive cognitive deficits are the deficits deteriorating with increasing number of episodes and cumulative length of illness duration. Given the enduring and accumulating characteristics of progressive deficits of MDD, it is noteworthy that the progressive cognitive deficits tend to develop into residual cognitive symptoms even when patients have achieved clinical remission, which has been repeatedly reported to be associated with poor prognosis and a higher relapse rate (5, 15–17). Therefore, the identification of the progressive cognitive deficits will not only provide new insight into the chronicity of MDD, but will also help in developing therapeutic strategy to treat residual cognitive symptoms in MDD.
Although many studies have investigated various cognitive features in MDD, only a small number of studies addressed the progressive deteriorating characteristic of cognitive deficits related to this condition. Some previous cross-sectional studies revealed deficits in different cognitive domains in remitted patients, which were associated with prior episodes (18–21). Talarowska et al. (22) reported that patients with recurrent depression (RD) performed worse in tasks on executive function, processing speed, visual-spatial and auditory-verbal memory than patients with first-episode depression (FED), while Roca et al. (23) found no difference in overall cognitive tests between FED and RD patients, even when they were experiencing an acute episode. An earlier longitudinal study by Maeshima et al. (24) found that memory deficits in remitted FED patients disappeared after 3 years of remission, while still persisted in remitted RD patients. Although there is emerging evidence indicating that there might be potential progressive cognitive deficits in MDD, most of those studies had small sample size, were cross-sectional designed, or only assessed one or a small number of cognitive domains, resulting in controversies and insufficient information to identify the cognitive deficits with clinical progressive characteristics. Specifically, it is still unclear which cognitive deficits will deteriorate with increasing episodes and whether these cognitive deficits are the result of enduring and accumulating effects of previous depressive episodes.
Using a comprehensive battery of neurocognitive tests in 35 FED, 60 RD patients and 111 matched healthy controls (HC), the present study aims to investigate the progressive cognitive deficit in MDD. Dual analytic strategies were adopted: firstly, we analyzed the differences in cognitive performance between the FED and RD patients under their episode and remission phases, respectively; and the changes in the patients' cognitive performance across 6 months of treatment were also observed simultaneously. Secondly, we analyzed the correlation between the cognitive performance and the number of episodes as well as the total illness duration. We hypothesized that the cognitive deficit with progressive characteristics might result from the aberrance of the cognitive domain which was associated with poorer performance in RD patients than FED patients both under the acute episode and the remission phase. Moreover, the improvement of progressive cognitive component would fall behind other depression-related symptoms in time and degree and might deteriorate with increasing episodes.
Methods and Materials
Participants
One hundred two medication-free MDD patients and 111 HCs were recruited from the Zhumadian Psychiatric Hospital (Henan, China) and its nearby communities from 2013 to 2018. All the MDD patients met the following inclusion criteria: (1) 18–60 years old, with an education of ≥6 years, and right-handed; (2) meet the criteria for MDD in the Structured Clinical Interview for DSM-IV (SCID-IV); (3) with a current episode of depression for at least 2 weeks, indicated by a 24-item Hamilton Depression Rating Scale (HAM-D24) score ≥20; (4) with no psychotropic drug use for over 2 weeks (6 weeks for fluoxetine) before recruitment. Detailed inclusion criteria and exclusion criteria for the MDD and HC groups were reported in our previous studies (25, 26).
This study was approved by the medical ethics committees of the Second Xiangya Hospital of Central South University and the Zhumadian Psychiatric Hospital. Written informed consent form was obtained from all the participants.
Treatment and Efficacy Assessment
All the subjects received the clinical assessment and neurocognitive tests at baseline, and MDD patients underwent all the neurocognitive tests again after 6 months of treatment with paroxetine. For all the MDD patients, clinical symptoms were assessed using the HAM-D24 at the end of the 0.5, 1st, 2nd, 3rd, 4th, 5th and 6th months. Clinical remission was considered when a patient achieved a HAM-D24 score of ≤ 7, which lasted for at least 2 months. During the treatment period, each patient received 10 mg of paroxetine in the first week and 20 mg or higher in the following weeks, depending on their treatment response and side effects. The maximum dose was 60 mg. Of the 102 patients initially enrolled, 7 had manic onset during the treatment, and thus were excluded. Therefore, 95 patients (35 with FED and 60 with RD) were included for the baseline analysis. Additionally, 7 patients received electroconvulsive therapy or other antidepressant drugs, and 25 patients withdrew from the study. Therefore, 63 patients completed the 6 months of treatment; among them, 56 patients (20 with FED and 36 with RD) who achieved clinical remission were finally included for post-treatment analyses, while 7 unremitted patients were not included because of the unproportionally small sample size.
Neurocognitive Assessment
All the participants underwent a comprehensive battery of neurocognitive tests involving executive function, attention, processing speed, and memory (25, 26). The standardized Z-scores of different tests were summed up to form cognitive domains. The internal consistency of different tests in each cognitive domain was assessed using Cronbach's alpha.
Executive Function
The digit span backward test in the Wechsler Adult Intelligence Scale-Revised (WAIS-R), the color-word interference condition of the Stroop test, the Trail-Making Test part B (TMT-B), and the semantic Verbal Fluency (animals) test were used to assess the four subcomponents of executive function, i.e., working memory, inhibition, shifting, and verbal fluency, respectively (27). It was notable that the patients who performed significantly poorly in TMT-B always needed more time to complete the Trail Making Task part A (TMT-A). The final performance of TMT-B was affected by the performance of TMT-A because the performance of TMT-A reflects the initial processing speed. The shifting components were obtained by subtracting the TMT-A result from the TMT-B result (28) (Cronbach's alpha 0.616).
Attention
The Stroop Word Test and the digit span forward test were used to assess the subjects' sustained attention (27) (Cronbach's alpha 0.576).
Processing Speed
TMT-A and the Stroop Color Test were used to assess the subjects' processing speed (29) (Cronbach's alpha 0.597).
Memory
Three tests from the Wechsler memory scale, i.e., the Visual Memory Test, the Intelligent Memory Test and the Associative Memory Test, were used to assess the subjects' visual memory, intelligent memory, and associative memory, respectively (30) (Cronbach's alpha 0.718).
Statistical Analysis
In this study, the average z-score per subtest for each domain was calculated to level out the difference in the number of subtests across domains (25). Z-scores were calculated using the equation Xindividual-controls/σcontrols. Xindividual represents the raw scores of each individual, Xcontrols represents the mean value of the controls, and σcontrols refers to the standard deviation of the controls. Higher Z-scores indicated better performance for all variables, and this value should be reversed when lower values indicated better performance (in TMT-A and TMT-B). The normality of the variables was tested using Kolmogorov–Smirnov test. Log or square root transformations were used for skewed variables to achieve normality.
One-way analyses of variance (ANOVA) was performed to make sure that the age and education were comparable between the FED, RD, and HC groups, and between the remitted FED (rFED), remitted RD (rRD) and HC groups. Two-sample t-test was used to compare the HAM-D24 score, total illness duration and the number of episodes between the FED and RD groups, and the rFED and rRD groups. Chi-square test was used to ensure that the gender ratio of the groups was matched. Multivariate analysis of covariance (MANCOVA) was conducted to compare the overall cognitive domain between the FED, RD and HC groups, and between the rFED, rRD, and HC groups, with age, gender and education as covariates. Paired t-test was used to compare the test results between pre-treatment and remission phases for both FED and RD patients. The significance level (two-tailed) was set at p < 0.05. All statistical analyses were conducted using SPSS 24.0.
Results
Demographic and Clinical Characteristics
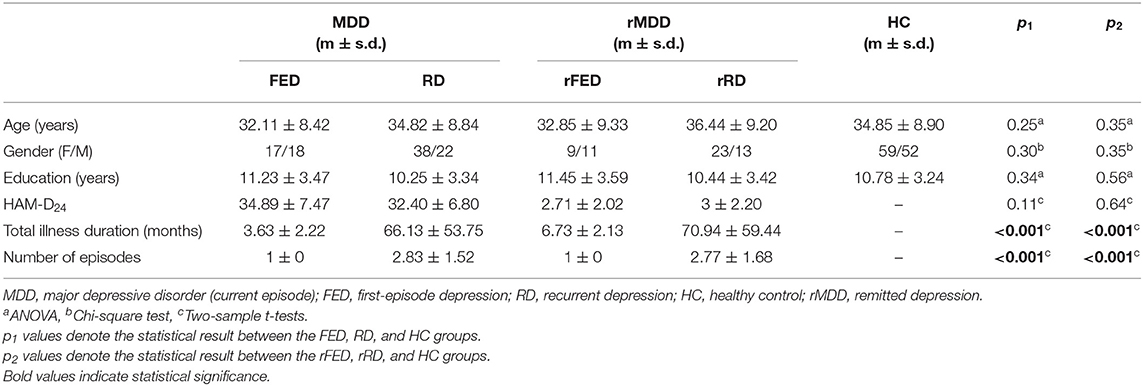
Table 1 showed the demographic and clinical characteristics of the MDD (FED and RD), rMDD (rFED and rRD), and HC groups. There were no inter-group differences between the FED, RD and HC groups, and between the rFED, rRD, and HC groups regarding age, gender, and education. And there were also no significant differences in HAM-D24 total score between the FED and RD groups, and between the rFED and rRD groups.
Cognitive Performance Between FED, RD, and HC Group
MANCOVA analyses revealed significant overall differences between the FED, RD, and HC groups in all the four cognitive domains (F = 10.60, p < 0.001; see Table 2), with age, gender and education as covariates. Post-hoc comparisons confirmed that both the FED and RD groups performed significantly more poorly in all the four cognitive domains, compared with the HC group (all p < 0.01). However, no significant differences were found between the FED and RD groups in all the four cognitive domains (all p > 0.05).
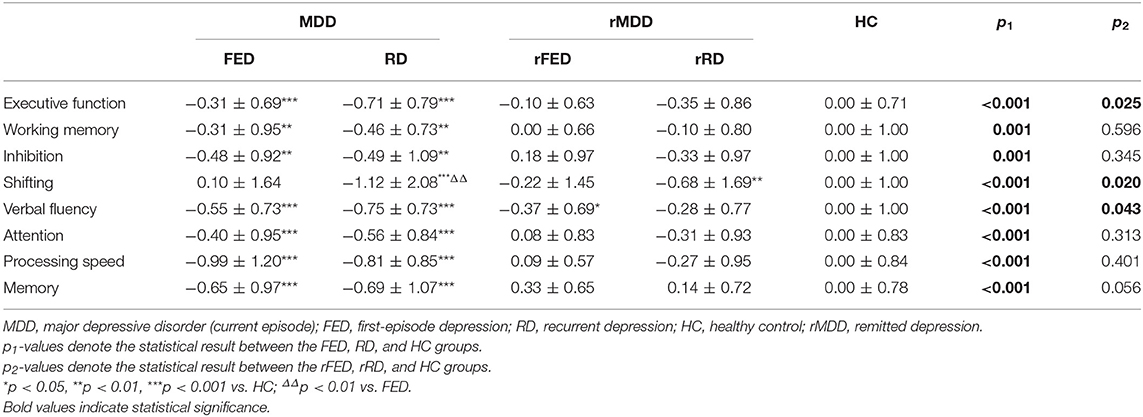
Table 2. Cognitive Performance (Z-score) of the FED and RD patients in episode and remission phases (MANCOVA test).
Although there was no significant statistical difference between the FED and RD groups in term of executive function, the gap in the mean value between these two groups was relatively large (−0.31 ± 0.69 vs. −0.71 ± 0.79, p = 0.088). Therefore, we further investigated four core sub-components of executive function. The MANCOVA analyses revealed significant overall differences between the FED, RD, and HC groups in all the four sub-components of executive function (F = 7.82, p < 0.001; see Table 2), with age, gender and education as covariates. Post-hoc comparisons confirmed that both the FED and RD groups performed significantly more poorly in all the four sub-components of executive function, compared with the HC group (all p < 0.01). However, a significant difference was only found between the FED and RD groups regarding the shifting component (p < 0.01), while no significant differences were found regarding working memory, inhibition and verbal fluency (all p > 0.05).
Cognitive Performance Across Episodes and Remission States
Fifty six patients achieved clinical remission at the end of the 6th month. Paired t-test was used to compare the test results between the pre-treatment and remission phases for both the FED and RD groups, respectively (Table 3). For the FED group, significant improvements were found in attention, processing speed and memory (p < 0.05), while no significant improvement was noticed in executive function (p = 0.055) after the 6-month treatment period. Among the four sub-components of executive function, significant improvements were found in working memory (p = 0.048) and inhibition (p < 0.001), while no significant improvement was found in the shifting component (p = 0.886) and verbal fluency (p = 0.404).
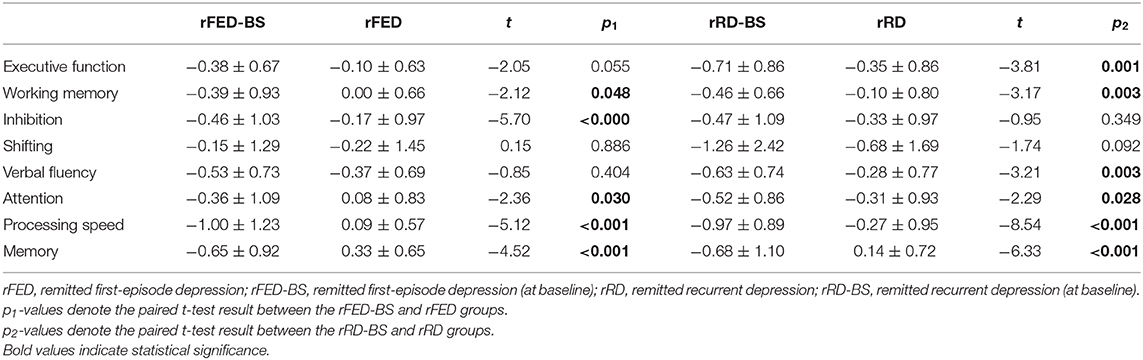
Table 3. Cognitive Performance (Z-score) of the FED and RD patients in episode and remission phases.
In the RD group, significant improvements were found in all the four cognitive domains after 6 months of treatment. Among the four sub-components of executive function, significant improvements were found in working memory (p = 0.003) and verbal fluency (p = 0.003), while no significant improvement was found in the shifting component (p = 0.092) and inhibition (p = 0.349).
Cognitive Performance of the rFED, rRD, and HC Groups
MANCOVA analyses revealed significant overall differences between the three groups in all the four cognitive domains (F = 2.88, p = 0.04, see Table 2) and all the four sub-components of executive function (F = 1.99, p = 0.047, see Table 2), with age, gender and education as covariates. Post-hoc comparisons showed no significant difference in the performance regarding all the four cognitive domains between the rFED, rRD, and HC groups (all p > 0.05). Among the four sub-components of executive function, only the performance of verbal fluency in the rFED group (p = 0.043) and the shifting component in the rRD group (p = 0.02) were significantly worse than the HCs. No significant difference was found between the rFED and rRD groups in all the four cognitive domains and four sub-components of executive function (all p > 0.05).
Correlation Between Cognitive Performance and the Number of Episodes as Well as Total Illness Duration in MDD Patients
Pearson correlation analyses were conducted to reveal the relationships between the cognitive performance and the number of episodes as well as total illness duration in MDD patients. Among all the cognitive domains, only a negative correlation between executive function and the number of episodes (r = −0.257, p = 0.012) and total illness duration (r = −0.238, p = 0.020), and a negative correlation between the shifting component and the number of episodes (r = −0.302, p = 0.003) and total illness duration (r = −0.276, p = 0.007) were found (see Figure 1).
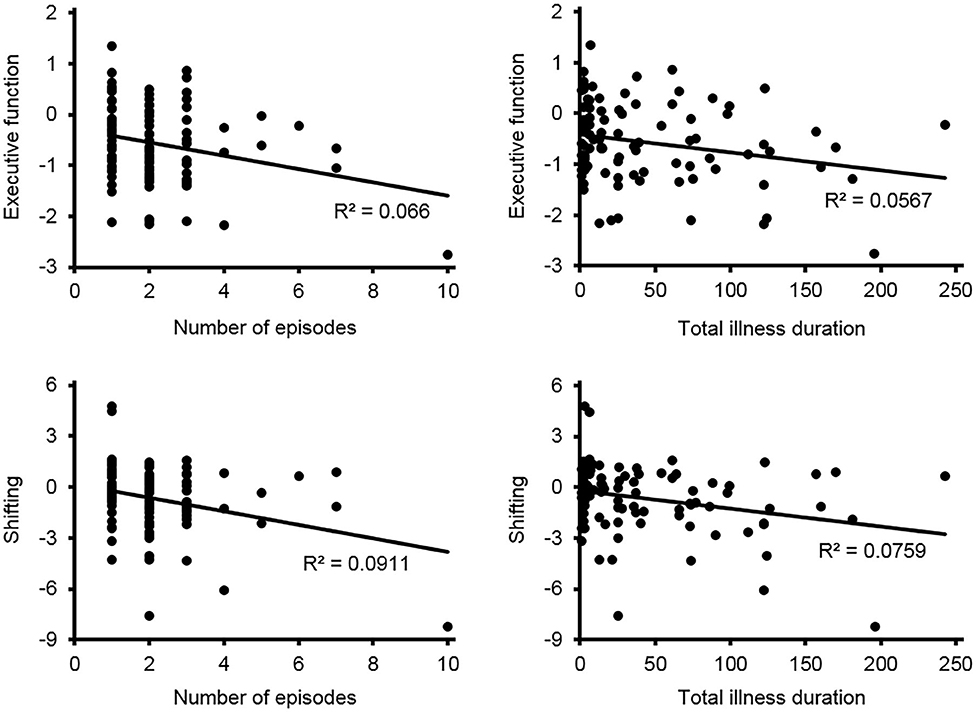
Figure 1. The correlation between cognitive performance and the number of episodes as well as total illness duration in patients with MDD.
Discussion
Using a comprehensive cognitive assessment battery, we have investigated the cognitive deficit with progressive characteristics in MDD in the present study. To our knowledge, this is the first study providing two critical pieces of evidence to identify the progressive cognitive deficit across the course of depression. The main outcome was that both the FED and RD groups showed impairments in all of the cognitive components, compared with the HC group, but there was no significant difference in all of the components except for one sub-component of executive function, shifting, between the two patient groups. After remission, only the shifting component in the RD group showed no significant improvement and remained in an impaired status. Notably, the shifting component was the only component negatively correlated with increasing episodes and cumulated illness duration. These results are consistent and revealed that the chronicity of depression exerts an extended and cumulative effect on shifting.
Executive function is a significant component found through the analyses. Although no significant difference was found between the FED and RD groups in all the four cognitive domains, the domain of executive function showed a notable gap in the mean value between these two groups. In addition, correlation analyses showed that executive function was negatively correlated with the number of episodes as well as total illness duration. These findings indicated that among these four cognitive domains, executive dysfunction might be the impairment most likely to be progressive in MDD. Being the most common and prominent cognitive deficit in MDD, executive dysfunction mainly comprises of four sub-components: working memory, inhibition, shifting and verbal fluency (27). Previous studies have proposed that these four sub-components were well-separated and the impairment of each of them had unique patterns (14, 27). Therefore, we further investigated these four sub-components of executive function and found that the impairment of shifting was most significant in the aspect of progressive feature.
Shifting is defined as the ability to respond to the switching between different tasks or situations and to adapt to changing demands (31). Involved in a variety of cognitive processes, such as working memory, inhibition, and attention, shifting is considered a superior and more comprehensive subcomponent of executive function (32). The wide involvement of shifting might be one of the reasons why the improvement of shifting impairment falls behind other depression-related symptoms, even when the MDD patient has achieved clinical remission. Our findings are in line with previous studies, which reported the impairment in shifting both in acute and in remitted MDD patients (14, 27). As an extension of previous findings, our results also suggest that the impairment in shifting might deteriorate with increasing number of episodes; presumably, the impairment in shifting might not be a state-like deficit, and thus might improve slower and to a lesser degree, compared with those state-related symptoms. Taken together, the evidence provided further insight into the dynamic shifting impairment process following a major depressive episode. Accumulating with future depressive episodes, such shifting impairment might become persistent and progressive.
The neurobiological basis of MDD is essential for us to understand the progressive nature of cognitive deficits. Previous neuroimaging findings reported that widespread structural and functional alterations were associated with the number of episodes and influenced by cumulated illness durations (33–35). Besides, Yan et al. found that patients with RD showed more severe functional connectivity disruption and more extensive functional connectivity network abnormality than those with FED (36). These findings provided evidence for the progressive feature of depression across the course of the illness. In addition, structural and functional alterations in the thalamus, anterior cingulate cortex, putamen, and prefrontal cortex were associated with shifting (37, 38), and the alterations in these brain regions have been reported to be associated with the number of episodes and the cumulated illness durations (33, 39–42). Therefore, it is conceivable that shifting is progressively impaired along with the disruption of the related brain regions.
Widespread cognitive deficits were detected in all the four cognitive domains and all the four subcomponents of executive function at the acute episode phase. After 6 months of treatment, significant improvement of attention, speed of processing, memory, and working memory was found in the rFED and rRD patients, while no significant difference was found between the remitted MDD patients and HCs in all the cognitive domains except for shifting in the rRD patients and except for verbal fluency in the rFED patients. There is a number of previous studies indicating that the improvement of these cognitive performances is state-related and the relation between clinical remission and these cognitive domains is remarkable (23, 43). Additionally, no statistical difference was found in all cognitive domains except for shifting between the FED and RD patients both in the acute and remitted phases. These results were in line with the findings of Roca et al. (23), but inconsistent with the study by Talarowska et al. (22), which reported that patients with RD performed more poorly than those with FED in various cognitive domains during depression episode. We presume that the reason for the inconsistency mentioned above might be the significantly older age of the RD patients than the FED patients in that study, and age is generally considered as a crucial risk factor for cognitive performance (29, 44–47). We have also found that the performance related to these cognitive domains (except for executive function and shifting) was not associated with the number of episodes and total illness duration, which provided another convincing piece of evidence that the impairment in these cognitive domains might be state-related.
It is notable that verbal fluency in patients with rFED was worse than HCs, while no statistical difference was found between the rRD patients and HCs. The association between clinical remission and verbal fluency was obvious in patients with RD while unapparent in those with FED. Similar results were also found by Roca et al. (23). They speculated that patients with rFED performed worse in shifting than those with rRD may be due to the greater relevance of cognitive bias in onset of FED patients rather than RD patients. Besides, it is possible that patients with RD have developed more effective compensatory mechanisms for major depressive episode. Previous studies suggested that clinical remission was associated with the compensation (48). The compensatory mechanisms might be more thorough as the illness progress. Nonetheless, we could not rule out the possibility that the small sample size of the rFED group affected the power of statistical analyses. To further clarify the association between clinical remission and verbal fluency in FED and RD patients, prospective design is essential in future works.
Strengthened by the longitudinal design in a cohort of medication-free MDD patients, the present study still has several limitations. Firstly, a relevant number of patients dropped out during the 6-month treatment course due to unavoidable reasons (such as poor response to paroxetine, severe gastrointestinal reactions, inconvenience of visiting the site, etc.). However, there are no statistically significant differences in demographic and clinical features in baseline between remitted patients and other participants (non-remitted patients and dropouts). The relatively high dropout rate resulted in a quite small number of non-remitted patients included in this study, which limited the exploration of the change trajectory of cognitive performance under the episode, non-remission, and remission states. Secondly, the neurocognitive performance of the HCs was assessed only once at baseline, which limited the time × group statistical comparison. Therefore, we could not rule out the possible effects of time and test-retest practice in this study. Thirdly, the small sample size, especially for the rFED group, might have affected the statistical power and increased the chance of type II errors. Fourthly, the correlation analyses of the association between cognitive performance and the number of episodes as well as total illness duration have made it difficult to make causal inferences. According to our inference, the possibility that poor shifting ability increases relapse rate cannot be ruled out, which is also supported by the evidence for progressive feature of the impairment in cognitive performance. Finally, recurrent patients had used different antidepressants before entering this study. To eliminate the influence of previous antidepressant use, we only enrolled patients who were not taking psychotropic drugs for at least 2 weeks (6 weeks for fluoxetine) before inclusion.
In conclusion, to our knowledge, the present study has, for the first time, investigated the progressive cognitive deficit of MDD by combining the evidence of cognitive performance in different phases of the disease and the association with the number of episodes in a relatively large cohort. The results provided new evidence supporting the progressive characteristics of shifting impairment across the course of depression. Besides, the results also provided moderate evidence for the state-related feature of attention, processing speed, memory, and working memory impairments in MDD. These findings not only provide evidence to understand the progressive feature of cognitive impairments, but also help in the development of therapeutic strategy for residual cognitive symptoms in MDD. Future studies with a longer period, especially longitudinal studies including different phases of the disease are needed to further identify the state-related, trait-related and progressive features of cognitive impairments.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the medical ethics committees of the Second Xiangya Hospital of Central South University and the Zhumadian Psychiatric Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JL: conceptualization, data curation, formal analysis, investigation, writing–original draft, and writing–review & editing. BL: data curation, investigation, and writing–review & editing. MW, YJ, QD, XL, JS, LiaZ, HG, and FZ: data curation and investigation. WL, LiZ, and ZL: investigation. YZ: investigation and writing–review & editing. ML: conceptualization, funding acquisition, methodology, project administration, supervision, and writing–review & editing. LL: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, and writing–review & editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (2019YFA0706200), the National Natural Science Foundation of China (81171286, 91232714, and 81601180), the Natural Science Foundation of Hunan Province (2020JJ5844).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the participants for participating in this study.
References
1. Judd LL, Schettler PJ, Rush AJ, Coryell WH, Fiedorowicz JG, Solomon DA. A new empirical definition of major depressive episode recovery and its positive impact on future course of illness. J Clin Psychiatry. (2016) 77:1065–73. doi: 10.4088/JCP.15m09918
2. Mueller TI, Leon AC, Keller MB, Solomon DA, Endicott J, Coryell W, et al. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am J Psychiatry. (1999) 156:1000–6. doi: 10.1176/ajp.156.7.1000
3. Kessing LV, Andersen PK. Evidence for clinical progression of unipolar and bipolar disorders. Acta Psychiatr Scand. (2017) 135:51–64. doi: 10.1111/acps.12667
4. Hollon SD, Shelton RC, Wisniewski S, Warden D, Biggs MM, Friedman ES, et al. Presenting characteristics of depressed outpatients as a function of recurrence: preliminary findings from the STAR*D clinical trial. J Psychiatr Res. (2006) 40:59–69. doi: 10.1016/j.jpsychires.2005.07.008
5. Buckman JEJ, Underwood A, Clarke K, Saunders R, Hollon SD, Fearon P, et al. Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clin Psychol Rev. (2018) 64:13–38. doi: 10.1016/j.cpr.2018.07.005
6. Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. (2011) 475:27–30. doi: 10.1038/475027a
7. Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. (2013) 10:e1001547. doi: 10.1371/journal.pmed.1001547
8. Ten Have M, de Graaf R, van Dorsselaer S, Tuithof M, Kleinjan M, Penninx B. Recurrence and chronicity of major depressive disorder and their risk indicators in a population cohort. Acta Psychiatr Scand. (2018) 137:503–15. doi: 10.1111/acps.12874
9. Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. (2013) 18:595–606. doi: 10.1038/mp.2012.33
10. Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. (2012) 140:113–24. doi: 10.1016/j.jad.2011.10.023
11. Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. (2014) 75:8–14. doi: 10.4088/JCP.13r08710
12. Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. (2011) 41:1165–74. doi: 10.1017/s0033291710001911
13. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. (2014) 44:2029–40. doi: 10.1017/s0033291713002535
14. Semkovska M, Quinlivan L, O'Grady T, Johnson R, Collins A, O'Connor J, et al. Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry. (2019) 6:851–61. doi: 10.1016/s2215-0366(19)30291-3
15. Bruder GE, Alvarenga JE, Alschuler D, Abraham K, Keilp JG, Hellerstein DJ, et al. Neurocognitive predictors of antidepressant clinical response. J Affect Disord. (2014) 166:108–14. doi: 10.1016/j.jad.2014.04.057
16. Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. (2000) 60:13–23. doi: 10.1016/s0165-0327(99)00157-3
17. Gallagher P, Robinson LJ, Gray JM, Porter RJ, Young AH. Neurocognitive function following remission in major depressive disorder: potential objective marker of response? Aust N Z J Psychiatry. (2007) 41:54–61. doi: 10.1080/00048670601057734
18. Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. (1996) 26:591–603. doi: 10.1017/s0033291700035662
19. Bhardwaj A, Wilkinson P, Srivastava C, Sharma M. Cognitive deficits in euthymic patients with recurrent depression. J Nerv Ment Dis. (2010) 198:513–5. doi: 10.1097/NMD.0b013e3181e4c5ba
20. Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV. The cumulative load of depressive illness is associated with cognitive function in the remitted state of unipolar depressive disorder. Eur Psychiatry. (2013) 28:349–55. doi: 10.1016/j.eurpsy.2012.03.004
21. Paelecke-Habermann Y, Pohl J, Leplow B. Attention and executive functions in remitted major depression patients. J Affect Disord. (2005) 89:125–35. doi: 10.1016/j.jad.2005.09.006
22. Talarowska M, Zajaczkowska M, Galecki P. Cognitive functions in first-episode depression and recurrent depressive disorder. Psychiatr Danub. (2015) 27:38–43.
23. Roca M, Lopez-Navarro E, Monzon S, Vives M, Garcia-Toro M, Garcia-Campayo J, et al. Cognitive impairment in remitted and non-remitted depressive patients: a follow-up comparison between first and recurrent episodes. Eur Neuropsychopharmacol. (2015) 25:1991–8. doi: 10.1016/j.euroneuro.2015.07.020
24. Maeshima H, Baba H, Nakano Y, Satomura E, Namekawa Y, Takebayashi N, et al. Residual memory dysfunction in recurrent major depressive disorder–a longitudinal study from Juntendo University mood disorder project. J Affect Disord. (2012) 143:84–8. doi: 10.1016/j.jad.2012.05.033
25. Liu J, Dong Q, Lu X, Sun J, Zhang L, Wang M, et al. Influence of comorbid anxiety symptoms on cognitive deficits in patients with major depressive disorder. J Affect Disord. (2020) 260:91–6. doi: 10.1016/j.jad.2019.08.091
26. Liu J, Dong Q, Lu X, Sun J, Zhang L, Wang M, et al. Exploration of major cognitive deficits in medication-free patients with major depressive disorder. Front Psychiatry. (2019) 10:836. doi: 10.3389/fpsyt.2019.00836
27. Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. (2013) 139:81–132. doi: 10.1037/a0028727
28. Nilsson J, Thomas AJ, Stevens LH, McAllister-Williams RH, Ferrier IN, Gallagher P. The interrelationship between attentional and executive deficits in major depressive disorder. Acta Psychiatr Scand. (2016) 134:73–82. doi: 10.1111/acps.12570
29. Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. (2006) 60:58–65. doi: 10.1016/j.biopsych.2005.09.019
30. Chelune GJ, Bornstein RA, Prifitera A. The wechsler memory scale—revised. In: McReynolds P, Rosen JC, Chelune GJ, editors. Advances in Psychological Assessment. Volume 7. Boston, MA: Springer US (1990). p. 65–99.
31. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. (2000) 41:49–100. doi: 10.1006/cogp.1999.0734
32. Diamond A. Executive functions. Annu Rev Psychol. (2013) 64:135–68. doi: 10.1146/annurev-psych-113011-143750
33. Meng C, Brandl F, Tahmasian M, Shao J, Manoliu A, Scherr M, et al. Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain. (2014) 137 (Pt. 2):598–609. doi: 10.1093/brain/awt290
34. Liu CH, Ma X, Yuan Z, Song LP, Jing B, Lu HY, et al. Decreased resting-state activity in the precuneus is associated with depressive episodes in recurrent depression. J Clin Psychiatry. (2017) 78:e372–82. doi: 10.4088/JCP.15m10022
35. Jacobs RH, Barba A, Gowins JR, Klumpp H, Jenkins LM, Mickey BJ, et al. Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol Med. (2016) 46:1055–67. doi: 10.1017/s0033291715002615
36. Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci USA. (2019) 116:9078–83. doi: 10.1073/pnas.1900390116
37. Respino M, Jaywant A, Kuceyeski A, Victoria LW, Hoptman MJ, Scult MA, et al. The impact of white matter hyperintensities on the structural connectome in late-life depression: Relationship to executive functions. Neuroimage Clin. (2019) 23:101852. doi: 10.1016/j.nicl.2019.101852
38. Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. (2004) 22:1679–93. doi: 10.1016/j.neuroimage.2004.03.052
39. Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. (1998) 9:2023–8. doi: 10.1097/00001756-199806220-00021
40. Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jäger M, Groll C, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol Psychiatry. (2003) 53:338–44. doi: 10.1016/s0006-3223(02)01474-9
41. Monkul ES, Hatch JP, Nicoletti MA, Spence S, Brambilla P, Lacerda AL, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. (2007) 12:360–6. doi: 10.1038/sj.mp.4001919
42. Belleau EL, Treadway MT, Pizzagalli DA. The impact of stress and major depressive disorder on hippocampal and medial prefrontal cortex morphology. Biol Psychiatry. (2019) 85:443–53. doi: 10.1016/j.biopsych.2018.09.031
43. Ahern E, Semkovska M. Cognitive functioning in the first-episode of major depressive disorder: a systematic review and meta-analysis. Neuropsychology. (2017) 31:52–72. doi: 10.1037/neu0000319
44. Bora E, Harrison BJ, Yucel M, Pantelis C. Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med. (2013) 43:2017–26. doi: 10.1017/s0033291712002085
45. Eraydin IE, Mueller C, Corbett A, Ballard C, Brooker H, Wesnes K, et al. Investigating the relationship between age of onset of depressive disorder and cognitive function. Int J Geriatr Psychiatry. (2019) 34:38–46. doi: 10.1002/gps.4979
46. Grady C. The cognitive neuroscience of ageing. Nat Rev Neurosci. (2012) 13:491–505. doi: 10.1038/nrn3256
47. Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. (1996) 103:403–28.
Keywords: major depressive disorder, cognitive deficit, progression, recurrent depression, the number of episodes, executive function, shifting
Citation: Liu J, Liu B, Wang M, Ju Y, Dong Q, Lu X, Sun J, Zhang L, Guo H, Zhao F, Li W, Zhang L, Li Z, Zhang Y, Liao M and Li L (2021) Evidence for Progressive Cognitive Deficits in Patients With Major Depressive Disorder. Front. Psychiatry 12:627695. doi: 10.3389/fpsyt.2021.627695
Received: 10 November 2020; Accepted: 25 January 2021;
Published: 16 February 2021.
Edited by:
Agorastos Agorastos, Aristotle University of Thessaloniki, GreeceReviewed by:
Anna Rita Atti, University of Bologna, ItalyJosé Manuel Rodríguez-Sánchez, Biocruces Bizkaia Health Research Institute, Spain
Copyright © 2021 Liu, Liu, Wang, Ju, Dong, Lu, Sun, Zhang, Guo, Zhao, Li, Zhang, Li, Zhang, Liao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingjiang Li, TExKMjkyMEBjc3UuZWR1LmNu; Mei Liao, bGlhb21laTEyM0Bjc3UuZWR1LmNu
 Jin Liu
Jin Liu Bangshan Liu
Bangshan Liu Mi Wang1,2
Mi Wang1,2 Yumeng Ju
Yumeng Ju Qiangli Dong
Qiangli Dong Liang Zhang
Liang Zhang Yan Zhang
Yan Zhang Mei Liao
Mei Liao Lingjiang Li
Lingjiang Li