- 1Department of Psychiatry, University Health Network and University of Toronto, Toronto, ON, Canada
- 2Youthdale Treatment Centre, Toronto, ON, Canada
- 3Department of Psychiatry, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
Objective: Tourette syndrome (TS) is a neuropsychiatric disorder that is highly associated with several comorbidities. Given the complex and multifaceted nature of TS, the condition is managed by a wide variety of practitioners in different disciplines. The goal of this study was to investigate health service delivery and care practices by clinicians who see TS patients across different geographic settings internationally.
Methods: A comprehensive questionnaire was developed to assess clinical care resources for patients with TS and was sent to clinicians in Canada (CA), the United States (US), Europe (EU), and the United Kingdom (UK). Responses were compared quantitatively between geographic regions.
Results: The majority of respondents, regardless of region, reported that fewer than 40% of their case-load are patients with tics. The accessibility of TS services varied among regions, as indicated by differences in wait times, telemedicine offerings, comorbidity management and the availability of behavioral therapies. First-line pharmacotherapy preferences varied among physicians in different geographical regions with CA respondents preferring alpha-2-adrenergic agonists and respondents from the UK and EU preferring dopamine receptor antagonists.
Discussion: The results suggest that there is a scarcity of specialized TS clinics, potentially making access to services challenging, especially for patients newly diagnosed with TS. Differences in regional pharmacotherapeutic preferences are reflected in various published treatment guidelines in EU and North America. The lack of dedicated specialists and telemedicine availability, coupled with differences in comorbidity management, highlight the need for interprofessional care and holistic management to improve health care delivery to patients with TS.
Introduction
Tourette syndrome (TS) is a developmental neuropsychiatric disorder characterized by motor and vocal tics that is estimated to affect 0.52–0.77% of children and 0.05% of adults (1, 2). Approximately 90% of patients with TS have at least one other psychiatric diagnosis; these comorbidities contribute to the wide spectrum of functional impairment and associated distress seen in patients with TS (3).
The most appropriate treatment for TS varies depending on the clinical presentation. When active treatment of tics is required, comprehensive behavioral intervention for tics (CBIT) has been empirically validated by well-designed studies (4, 5). However, its usefulness is limited by a lack of well-trained practitioners, high costs, and by patients' ability to devote the time and effort it requires (6); pharmacological treatment is therefore required for many patients with TS. The dopamine receptor antagonists (DRAs) are often effective but can be associated with potential serious adverse effects (7). Thus, given their more favorable side effect profile, the alpha-2 adrenergic receptor agonists (A2AAs) clonidine and guanfacine are commonly used in the treatment of TS (8), though their efficacy is lower than that of the DRAs, and their utility is further limited by their own side effects (9). Other pharmacological agents have only limited and weak evidence to support their use.
Adults and children with TS have lower quality of life than the general population (10), as evidenced by lower scores on measures of psychological health and adaptive functioning (11–13), as well as lower socioeconomic statuses (14). Lower quality of life measurements in TS samples (15) suggest the need for more health care services in this population. Indeed, compared to the general population, patients with TS are more likely to report greater health care needs, are more likely to need medication, and use more medical, mental health and educational services (16, 17).
While it has been demonstrated that patients with TS require more health care services, little is known about the different models of care available to TS patients. Previous research has shown that TS clinical populations are similar across different countries and continents (18), but it is unclear what clinical resources and therapeutic practices are available to patients with TS and how these are distributed within (19–22) and across different geographical regions. In order to better understand the resources available to patients with TS, we designed and distributed a survey for healthcare practitioners who work with TS patients. The survey included comprehensive questions that sought out details regarding availability and accessibility of health care services to TS patients from different regions.
Methods
Survey Development
A comprehensive questionnaire was developed to assess the clinical care approaches and resources that are available to TS patients internationally. The electronic survey was compiled through SurveyMonkey and reviewed by experienced neurodevelopmental psychiatrists (EAJ and PS) who provided constructive feedback and survey improvements. The revised survey was then distributed for pilot testing amongst the members of the Tourette Syndrome Neurodevelopment Clinic at the Toronto Western Hospital. After further revisions based on feedback from the piloting, the finalized survey consisted of 30 questions, most of which were multiple-choice, but also included rank-order and open-ended questions. The survey questions had two themes, the first one focused on the clinic and setting in which the respondent practices, while the second related specifically to the personal practice of the respondent.
Study Participants
Lists of health care providers that manage TS, including physicians and non-physicians, were obtained through international TS organizations: Tourette Syndrome Foundation of Canada (now called Tourette Canada), Tourette Syndrome Association (now called Tourette Association of America), Tourettes Action, and Tourette Syndrome Portal. The lists included contact numbers for clinicians in English speaking Canada (CA), the United States of America (US), the United Kingdom (UK), and Europe (EU). Clinicians were then contacted by phone to collect email addresses and request permission to distribute the survey link. The survey link was distributed by e-mail via SurveyMonkey to the clinicians who expressed interest in completing the study. Email reminders were sent 1 week apart, for 3 weeks, if the surveys were not completed. The survey took place from December 2014 to May 2015, with the bulk of responses collected in December and January.
Data Analysis
The survey data was collected via SurveyMonkey and exported to SPSS (Statistical Package for the Social Sciences). Survey responses were compared between and within geographical regions. Qualitative and quantitative approaches were used to provide detailed descriptions of the responses to the survey questions. Descriptive analyses were repeated on the subset of respondents who indicated that >60% of their patients have tics, regardless of geographical location, to examine the responses among “tic specialists.”
Results
The survey was distributed to 484 clinicians; 123 clinicians responded, for an overall response rate of 25%. An overwhelming majority of respondents indicated that they practiced in an urban setting (93%), more specifically, at teaching hospitals (51%), private clinics/practices (24%) and community hospitals/clinics (14%). The catchment area for most of the respondents ranged from several hundred thousand to several million people. Respondents included mostly physicians (41%), psychologists (33%) and nurses (6.5%). Of the physician respondents, 46% identified as psychiatrists, 32% as neurologists, 16% as neuropsychiatrists, and 6% as pediatricians.
Regional Health Service Delivery
The respondents were grouped based on their geographical location, so that the overall sample was divided into 4 regional samples: n(CA) = 21, n(US)=33, n(UK)=28 and n(EU)=41. The response rate was 22%, 37%, 47%, and 17% for each of the regions respectively.
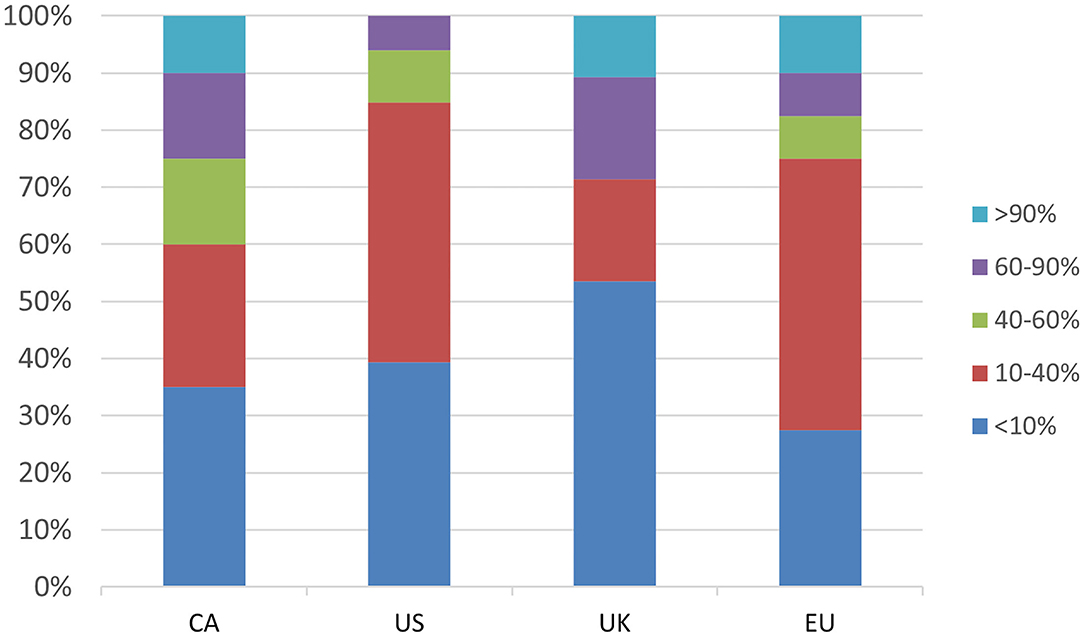
Only a minority of respondents (ranging from 6% in the US to 29% in the UK) reported having a clinic population in which most patients (>60%) have a tic disorder (Figure 1). The majority of respondents in the UK (52%), CA (52%) and US (79%) indicated that they see 0–2 new TS patients/month, whereas the most frequent response in the EU (32% of respondents) was 3–6 new patients per month, and 15% saw more than 15 new TS patients/month (Supplementary Figure 1). The majority of respondents in all regions reported seeing 15 or less follow-up TS patients/month; 5% of CA and EU respondents reported seeing greater than 30 follow-up TS patients/month (Supplementary Figure 2). Respondents from the US reported seeing the fewest number of both new and follow-up TS patients/month.
The most commonly seen age range for TS patients was 6–11, regardless of region, closely followed by ages 12–18. Approximately a third of respondents in the UK and EU reported seeing adult TS patients (>18 years old), whereas just under a quarter of clinicians in CA and the US reported doing so (Supplementary Figure 3).
In terms of health services delivery management, the large majority of respondents, regardless of region, reported that patients are booked in the order referrals are received and are not commonly triaged based on severity or urgency (Supplementary Table 1). The most common average wait time for new TS patients to be seen by a clinician varied greatly by region; 1–3 weeks for the US (61%), 1–3 months for CA (37%) and the EU (42%), with the UK having the longest wait time at 3–6 months (39%) (Figure 2). Survey participants were also asked about the availability of telemedicine services, that is, the use of telecommunication and information technology to provide clinical health care services from a distance. Only in the US were telemedicine services commonly available (84%), whereas access to such services was less common in CA (42%), the UK (21%) and the EU (8%) (Supplementary Table 1).
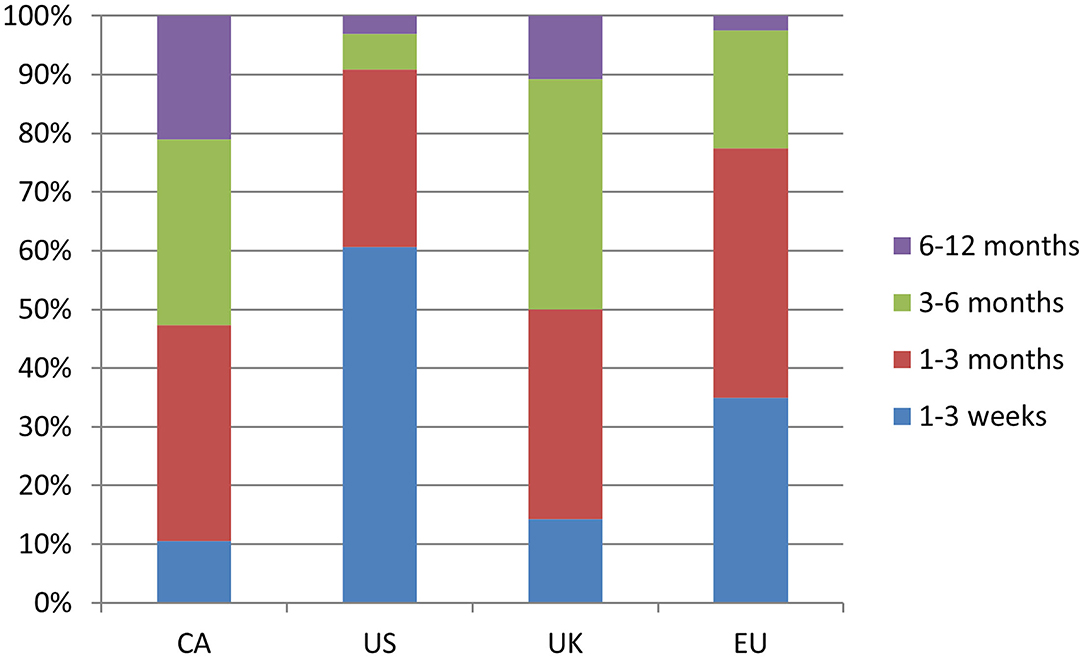
Figure 2. Response break-down for the question: “How long is the wait time on average for new patients with TS?”
Many respondents in the EU, UK, and CA, reported having a diverse interdisciplinary team, often consisting of psychiatrists, psychologists and nurses, with neurologists, social workers and occupational therapists being far less common, especially in the US (Figure 3). There were some common trends in clinical resource management between the geographical regions (see Supplementary Table 1). More than 70% of clinicians in all regions reported using intake questionnaires. Additionally, the average initial consultation length for the vast majority of clinicians was reported as being between 1–2 h, with patients subsequently being followed for as long as needed (Supplementary Figure 4). Most respondents prioritized clinical time and resources to provide comprehensive and continuing treatment to existing patients rather than assessing as many new patients as possible. More than half of all respondents reported tracking patient outcomes, for example, by using quality of life, patient satisfaction or symptom-based measures (Supplementary Table 1).
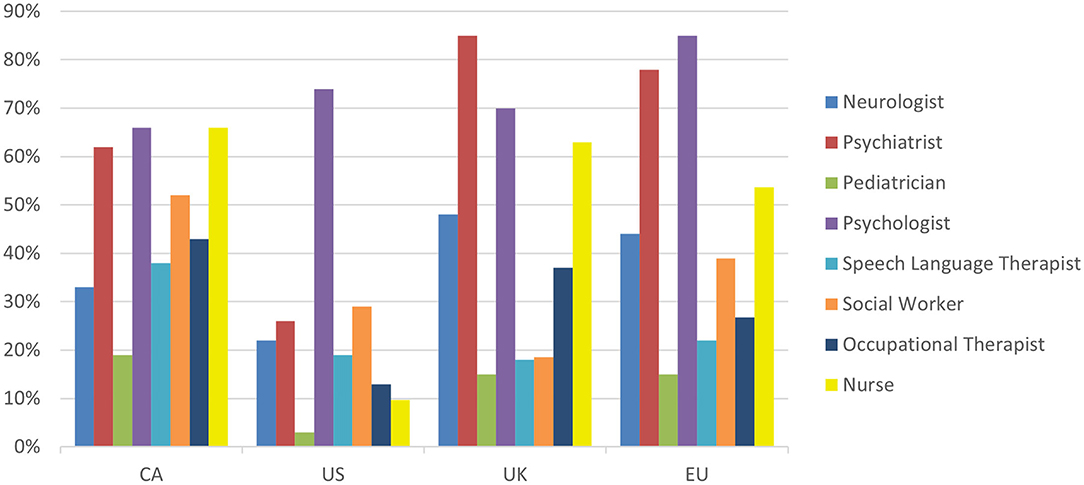
Figure 3. Response break-down for the question: “Who are the healthcare providers of your clinical team?”
Clinical Management
Survey participants were asked to indicate which TS comorbidities they manage (Supplementary Figures 5, 6). The vast majority of respondents reported managing obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD) and anxiety disorders. Most respondents reported managing anger/rage problems, ranging from 52% of US respondents to 87% of EU respondents. As well, nearly 60% of respondents across all regions reported managing skin picking/self-injurious behaviors and sleep disorders with one exception being that only 40% of CA respondents reported managing sleep disorders. Regional differences were also found with autism spectrum disorder (ASD) management; <30% of CA and US respondents reported managing comorbid ASD, compared to the UK and EU in which more than 50% reported doing so.
In terms of TS management, respondents were asked about the therapeutic services offered at their clinic (Figure 4). Individual CBIT therapies were highest among US respondents (91%), when compared with the other regions (43–57%). As well, the majority of respondents in all regions reported offering psychoeducation at their clinic, ranging from 68% of UK respondents to 83% of EU respondents.
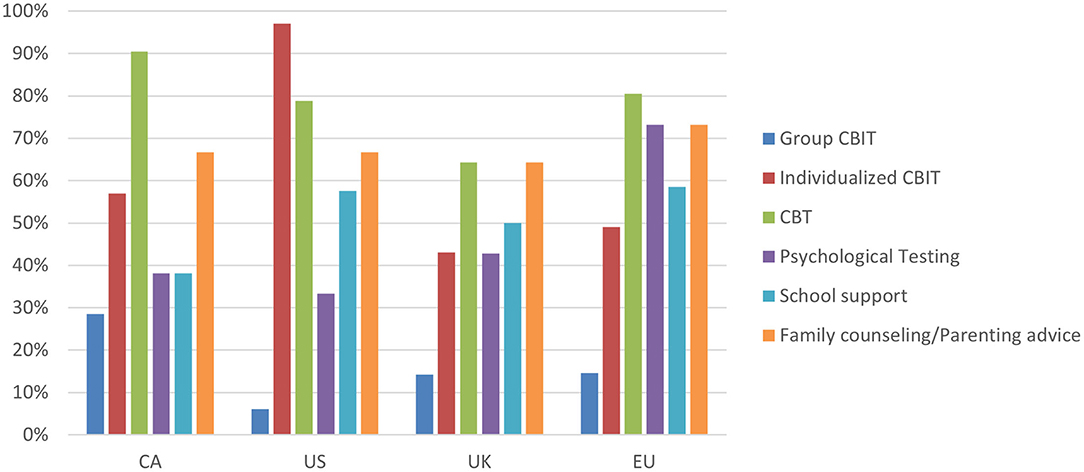
Figure 4. Response break-down for the question: “What services are available for TS patients at your clinic?”
When looking at order of intervention preferences among the respondents, only physician responses were included since in most regions, only physicians are licensed to prescribe pharmacological treatments. Physician responses were then grouped by region: UK (n = 17), EU (n = 26), CA (n = 7) and US (n = 0).
Psychoeducation was the first-line choice of intervention for 57% of CA physicians and three-quarters of physicians in the EU and UK (Supplementary Figure 7). Behavioral interventions were the most popular second-line treatment choice for physicians in all regions—CA (57%), UK (59%), and EU (81%). For third-line treatment choice, pharmacological interventions were the most common choice in each region; CA (57%), UK (71%), EU (84%).
For the first-line medications for tics, 56% of respondents in the UK chose DRAs, and a further 38% chose A2AAs. A similar pattern was observed in the EU with 61% and 27% choosing these options, respectively. Notably the proportion was reversed in Canada, where 86% of CA clinicians chose A2AAs, while none chose antidopaminergic medications as their first-line psychotropic choice (Table 1).
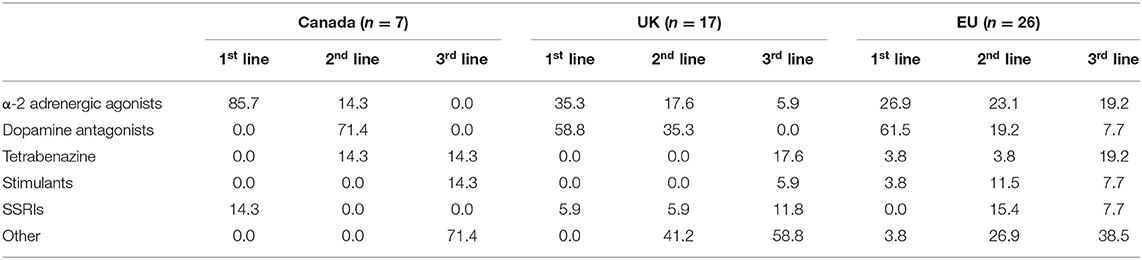
Table 1. Percentages of first, second and third line pharmacotherapeutic preferences of physicians by region.
Dopamine antagonists were the most popular second-line agents for physicians in the UK (31%) and CA (71%), whereas A2AAs were the most popular in the EU (23%). Multiple physicians in the EU chose selective serotonin reuptake inhibitors (SSRIs) (15%), stimulants (12%), and anticonvulsants (8%) as their second-line agent of preference.
The most popular third-line treatment in CA was tetrabenazine (14%). Popular choices for third-line treatment in the UK included tetrabenazine (19%), botulism toxin (13%), anticonvulsants (13%) and SSRIs (13%). Similarly, there was a wide range of preferences in the EU with the most popular third-line choices as follows: A2AAs (19%), tetrabenazine (19%), cannabis (8%), benzodiazepines (8%), stimulants (8%) and SSRIs (8%) (Table 1).
Tic Specialists
The regional breakdown for tic specialists, that is, those whose practice included more than 60% of patients with tics, were as follows: CA n = 5, US n = 2, UK n = 8, EU n = 7; responses were combined (n = 22) for descriptive reporting purposes. The majority of tic specialists (59%) reported having wait times of 3 months or longer for new patients, with wait-list management based on the order referrals are received (73%) and telemedicine services offered by the minority of respondents (41%). As well, the majority of tic specialists reported that they use intake questionnaires and track patient outcomes (73 and 82%, respectively). Greater than 70% of tic specialists reported managing comorbid OCD, anxiety, ADHD and anger/rage issues while approximately 40% or less manage comorbid ASD, learning disabilities and sleep disorders. When asked about the services available for TS patients at their clinic, 32% reported the availability of group CBIT while 73% reported the availability of individual CBIT services. Additionally, the majority (64–68%) of tic specialists reported the availability of cognitive behavioral therapy, family counseling, psychological testing and school support services at their clinic.
Discussion
This study investigated the patterns of health care delivery to patients with TS in different geographic regions. The results of the study suggest that access to services varies greatly and finding timely professional help may be challenging for TS patients in some regions. Nearly all respondents practice in urban settings, potentially limiting access to care for TS patients in rural areas. Furthermore, only a small number of respondents in each region reported having a clinic population in which the majority of their patients have a tic disorder, indicating that there is a lack of dedicated TS clinics. Clinicians, regardless of region, reported seeing more follow-up patients than new patients, with respondents in Europe generally seeing more patients than their counterparts in other regions. Most respondents prioritized clinical resources to providing care to existing patients thus reducing the availability of services for new patients. These findings raise questions about ease of access to care for new TS patients, especially those living outside of large urban centers. With the exception of the US, most new TS patients have to wait 1 month or longer to be seen by a clinician. Difficulties in accessing services for TS patients have also been reported in previous studies from Canada (23), the US (24), Spain (25) and Australia (19), with patient respondents noting a long and difficult process before they receive a TS diagnosis (26) and a lack of knowledge regarding tics among health care specialists (25).
In general, tic specialists reported long wait times for initial consultations. The combination of longer wait times and fewer specialists is such that most patients seek treatment from non-specialized physicians and/or non-physician clinicians. Shortages of experts and knowledge gaps may explain some of the reasons why access to services is limited for TS patients (27–29). Additionally, there is a lack of multidisciplinary teams with expertise to manage TS and its co-morbidities (19, 28) which may be especially relevant to the US, as only a few US respondents reported the availability of such teams in comparison to the other regions. The Tourette Association of America (TAA) recognized this problem a few years ago and encouraged the development of multidisciplinary clinics by offering their Center of Excellence designation to select clinics in the US. The benefits of such interprofessional teams allow for collaborative treatment, pooled expertise and often, shorter wait-times. A feasible solution may be an interprofessional care model (30) where expert health care providers assess a patient and develop a treatment plan that is then implemented by local, non-expert health care providers, with the experts providing ongoing support via communication and re-assessment as required.
The most common age range of TS patients seen by our respondents was 6–11, followed by 12–18, regardless of region. The predominance of child and youth patients is understandable as TS peaks in early puberty and wanes during the late teens, with only a subset of TS patients exhibiting symptoms that require services into adulthood (31, 32). Although only a minority of adults continue to exhibit significant tics, these are often impairing. Our data indicates that adults with TS are even more underserviced than children and adolescents. However, regional differences were apparent regarding services for adult TS patients, with the EU and UK seeing more adult patients than the respondents from North America. These results may also reflect the possibility that many of the health care providers who responded to our survey focus solely on pediatric populations, however this was not directly assessed.
Psychoeducation was reported as the first-line intervention for all the regions and is offered by the majority of all regional respondents. Psychoeducation is crucial in addressing the stigma of TS, understanding the course of the disorder, and teaching acceptance and coping strategies to patients and their families (33–35). Providing TS education and coping mechanisms may be sufficient in reducing tic-related disability, as patients with mild tics often do not require additional treatment.
For patients with more severe tics who require behavioral and/or pharmacological therapies, guidelines have been published in Europe (9, 34, 36), Canada (8, 35) and the US (33, 37) to promote better integration of treatment strategies using evidence-based medicine. Most respondents in the current study indicated that after psychoeducation, behavioral therapy was their preferred treatment choice, followed by pharmacological interventions. Based on the evidence, the guidelines agree that CBIT should be the first-line treatment, even though it is resource intensive and requires a trained therapist, who are not widely available. The majority of CA and US respondents indicated that they offer individual CBIT therapy. The high rates of individual CBIT offerings in the US may speak to the success of the TAA's initiative to train clinicians in CBIT, which was funded by the Center for Disease Control. As well, the recent Treating Tourette Together summit in the US further identified increasing access to CBIT as a priority (38). Since all of our US respondents came from a list provided by the TAA, which includes practitioners who have attended their CBIT training workshops, respondent answers are unlikely to be representative of general access to CBIT for TS patients in the US, which might explain differences with other studies reporting low rates and limited access to psychotherapy in the US (26, 39, 40).
The relatively low availability of CBIT in the UK and EU may result from barriers to implementation, including lack of clinician training and the need for a series of sessions (6). CBIT delivered via telemedicine has been shown to result in similarly significant tic reductions when compared to face-to-face delivery, demonstrating the value of telemedicine services in healthcare delivery (41). With the exception of the US, the majority of all other respondents, especially in the EU and UK, indicated that telemedicine services were not available at their clinics. The implementation of telemedicine services allows for greater dissemination of healthcare services, such as CBIT, to a wider range of patients including those living in rural settings, which is especially relevant as almost all of our respondents practice in urban settings. However, for this technological development to result in increased access to CBIT, a larger number of trained clinicians will be required; additionally, issues of costs and insurance coverage for CBIT will have to be addressed (6).
There were differences among the regions with regards to first-line pharmacotherapy preferences which might reflect the influence of various TS treatment guidelines. Based on a systematic assessment of the evidence, the Canadian guidelines recommend clonidine and guanfacine as first-line pharmacotherapy for tics (8); this is reflected in our data, as the majority of CA clinicians reported prescribing A2AAs first, findings which are further supported by published Canadian TS prescribing trends in which recommendations for A2AAs increased over the study period (42). In contrast, EU and UK respondents preferred DRAs, which is consistent with the published European guidelines. The EU guidelines, driven largely by expert experience and opinion, recommend risperidone, a DRA, as the first-choice agent for the treatment of tics (9). Our results are also supported by investigations of TS pharmacotherapy trends in the UK in which the DRA aripiprazole was the most commonly prescribed medication (43), and in the EU where aripiprazole and risperidone were the most commonly prescribed medications to adult and children patients with TS, respectively (44). While there were no US physicians in the survey sample, the most recent US guidelines recommend A2AAs (33), which is supported by US studies reporting that A2AAs are the most commonly used class of medication in TS (39, 40).
It is clear that DRAs and A2AAs are the first or second choice for most physicians treating patients with TS. However, there was less consensus regarding second- and especially third-line treatment choices, with some physicians choosing SSRIs, stimulants, tetrabenazine, botulism toxin injections and benzodiazepines despite weak or absent evidence of their efficacy against tics. Specifically, there is only low quality evidence for the efficacy of agents such as tetrabenazine in treating tics, while other agents such as SSRIs and stimulants, are rarely used to treat tics on their own and can in fact exacerbate tics in some individuals (45, 46). Alternatively, such responses may have referred to treatment choices for comorbid OCD and ADHD rather than for treatment of tics, even though the survey question specifically asked about pharmacotherapeutic preferences when treating tics. Nevertheless, the current results indicate that there is little consistency in second- and third-line medication preferences and illustrates the need for better treatment options for TS patients.
Our study was not without limitations. We did not include Latin America, Asia or Africa, and while we made efforts to obtain data from Australia, we were unsuccessful. The response rates in all regions were low thus limiting the generalizability of the results, and the overall sample size was small. Many clinicians could not be reached due to outdated and/or incorrect contact information. The questionnaire was only available in English, which made it difficult to get results from non-English speaking clinicians. As well, we reached out only to the contacts that were provided by individual TS associations. This may have biased the sample as the practices reported in our survey may not be representative of the care received by TS patients who see health care professionals not listed on the TS associations' contact lists. For example, family physicians who provide primary care for their patients with TS are likely under-represented in TS association listings. Additionally, the US sample did not include any physicians and thus could not be included in the treatment preference comparisons. However, we believe that the responses collected from the US non-physician clinicians provide important insight about clinical care practices in the US since many of these clinicians take on critical roles in the management of tics. Finally, since our survey was conducted a few years ago, there may have been changes in some practices. For example, there has recently been a markedly accelerated implementation of telemedicine since the COVID-19 pandemic due to self-isolation measures.
TS is a treatable disorder with a good prognosis; thus, it is paramount that appropriate, evidence-based treatments are readily accessible to patients with the condition. Developing an international agreed upon treatment algorithm may be beneficial. Interprofessional care, accessibility of services, and multi-modal management approaches are needed to contribute to an ideal care facility for patients with TS (47–49). These parameters are recommended as the base of any specialized TS clinic with the goal of improving health service delivery to TS patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
TB: design and execution of statistical analyses, writing of first draft of manuscript, as well as reviewing and critiquing subsequent drafts. RE: conception, organization and execution of research project, reviewing and critiquing manuscript drafts. EA-J: conception and organization of research project, reviewing and critiquing statistical analyses and manuscript drafts. PS: conception and organization of research project, reviewing and critiquing statistical analyses and manuscript drafts. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Toronto General & Western Foundation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Peter Szatmari for assisting us with the development of the survey; Tourette Canada, Tourette Association of America, Tourettes Action and Tourette Syndrome Portal for providing us with contact information of clinicians; and all of the respondents who completed the survey.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.621874/full#supplementary-material
References
1. Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. (2012) 47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002
2. Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Benhlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. (2015) 30:221–8. doi: 10.1002/mds.26089
3. Pringsheim T, Lang A, Kurlan R, Pearce M, Sandor P. Understanding disability in Tourette syndrome. Dev Med Child Neurol. (2009) 51:468–472. doi: 10.1111/j.1469-8749.2008.03168.x
4. Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with tourette disorder: a randomized controlled trial. JAMA. (2010) 303 1929–37. doi: 10.1001/jama.2010.607
5. Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch Gen Psychiatry. (2012) 69:795–803. doi: 10.1001/archgenpsychiatry.2011.1528
6. Scahill L, Woods DW, Himle MB, Peterson AL, Wilhelm S, Piacentini JC, et al. Current controversies on the role of behavior therapy in Tourette syndrome. Mov Disord. (2013) 28:1179–1183. doi: 10.1002/mds.25488
7. Sandor P. Pharmacological management of tics in patients with TS. J Psychosom Res. (2003) 55:41–8. doi: 10.1016/S0022-3999(03)00060-6
8. Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry. (2012) 57:133–43. doi: 10.1177/070674371205700302
9. Roessner V, Plessen KJ, Rothenberger A, Ludolph AG, Rizzo R, Skov L, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. (2011) 20:173–196. doi: 10.1007/s00787-011-0163-7
10. Eddy CM, Rizzo R, Gulisano M, Agodi A, Barchitta M, Cal P, et al. Quality of life in young people with Tourette syndrome: a controlled study. J Neurol. (2011) 258:291–301. doi: 10.1007/s00415-010-5754-6
11. Conelea CA, Woods DW, Zinner SH, Budman C, Murphy T, Scahill LD, et al. Exploring the impact of chronic tic disorders on youth: results from the Tourette Syndrome Impact Survey. Child Psychiatry Hum Dev. (2011) 42:219–242. doi: 10.1007/s10578-010-0211-4
12. Elstner K, Selai CE, Trimble MR, Robertson MM. Quality of Life (QOL) of patients with Gilles de la Tourette's syndrome. Acta Psychiatr Scand. (2001) 103:52–9. doi: 10.1111/j.1600-0447.2001.00147.x
13. Storch EA, Merlo LJ, Lack C, Milsom VA, Geffken GR, Goodman WK, et al. Quality of life in youth with Tourette's syndrome and chronic tic disorder. J Clin Child Adolesc Psychol. (2007) 36:217–27. doi: 10.1080/15374410701279545
14. Aldred M, Cavanna AE. Tourette syndrome and socioeconomic status. Neurol Sci. (2015) 36:1643–9. doi: 10.1007/s10072-015-2223-0
15. Cavanna AE, David K, Bandera V, Termine C, Balottin U, Schrag A, et al. Health-related quality of life in Gilles de la Tourette syndrome: a decade of research. Behav Neurol. (2013) 27:83–93. doi: 10.1155/2013/732038
16. Bitsko RH, Holbrook JR, Visser SN, Mink JW, Zinner SH, Ghandour RM, et al. A National Profile of Tourette Syndrome, 2011–2012. J Dev Behav Pediatr. (2014) 35:317–22. doi: 10.1097/DBP.0000000000000065
17. Bitsko R, Danielson M, King M, Visser S, Scahill L, Perou R. Health Care Needs of Children With Tourette Syndrome. J Child Neurol. (2013) 28:1626–36. doi: 10.1177/0883073812465121
18. Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3500 individuals in 22 countries. Dev Med Child Neurol. (2000) 42:436–447. doi: 10.1017/S0012162200000839
19. Efron D, Payne J, Gulenc A, Chan E. Assessment and management of tic disorders and Tourette syndrome by Australian paediatricians. J Paediatr Child Health. (2020) 56:136–141. doi: 10.1111/jpc.14541
20. Bachmann CJ, Roessner V, Glaeske G, Hoffmann F. Trends in psychopharmacologic treatment of tic disorders in children and adolescents in Germany. Eur Child Adolesc Psychiatry. (2015) 24:199–207. doi: 10.1007/s00787-014-0563-6
21. Carulla-Roig M, Isomura K, Perez-Vigil A, Larsson H, Hellner C, Mataix-Cols D, et al. Pharmacoepidemiology of Tourette and Chronic Tic Disorders in Sweden 2005–2013. J Child Adolesc Psychopharmacol. (2018) 28:637–45. doi: 10.1089/cap.2017.0169
22. Hamamoto Y, Fujio M, Nonaka M, Matsuda N, Kono T, Kano Y. Expert consensus on pharmacotherapy for tic disorders in Japan. Brain Dev. (2019) 41:501–6. doi: 10.1016/j.braindev.2019.02.003
23. Munce SEP, Pitzul KB, Guilcher SJT, Bereket T, Kwan M, Conklin J, et al. Health and Community-Based Services for Individuals with Neurological Conditions. Can J Neurol Sci. (2017) 44:670–5. doi: 10.1017/cjn.2017.207
24. Wolicki SB, Bitsko RH, Danielson ML, Holbrook JR, Zablotsky B, Walkup JT, et al. Children with Tourette Syndrome in the United States: Parent-Reported Diagnosis, Co-Occurring Disorders, Severity, and Influence of Activities on Tics. J Dev Behav Pediatr. (2019) 40:407–14. doi: 10.1097/DBP.0000000000000667
25. Rivera-Navarro J, Cubo E, Almazan J. The diagnosis of Tourette's Syndrome: communication and impact. Clin Child Psychol Psychiatry. (2009) 14:13–23. doi: 10.1177/1359104508100127
26. Cuenca J, Glazebrook C, Kendall T, Hedderly T, Heyman I, Jackson G, et al. Perceptions of treatment for tics among young people with Tourette syndrome and their parents: a mixed methods study. BMC Psychiatry. (2015) 15:46. doi: 10.1186/s12888-015-0430-0
27. Marcks B, Woods D, Teng E, Twohig M. What do those who know, know? Investigating providers' knowledge about Tourette's Syndrome and its treatment. Cognit Behav Pract. (2004) 11:298–305. doi: 10.1016/S1077-7229(04)80044-0
28. Woods DW, Conelea CA, Walther MR. Barriers to Dissemination: Exploring the Criticisms of Behavior Therapy for Tics. Clinical Psychology: Science and Practice (2007) 14:279–82. doi: 10.1111/j.1468-2850.2007.00088.x
29. Kim WJ. The American Academy of Child and Adolescent Psychiatry Task Force on, Workforce Needs. Child and adolescent psychiatry workforce: a critical shortage and national challenge. Academic Psychiatry. (2003) 27:277–82. doi: 10.1176/appi.ap.27.4.277
30. S G, P B, J F, D R, AJ S. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med. 2006:2314–21. doi: 10.1001/archinte.166.21.2314
31. Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. (1998) 102:14. doi: 10.1542/peds.102.1.14
32. Rizzo R, Gulisano M, Cali PV, Curatolo P. Long term clinical course of Tourette syndrome. Brain Dev. (2012) 34:667–73. doi: 10.1016/j.braindev.2011.11.006
33. Pringsheim T, Okun MS, Muller-Vahl K, Martino D, Jankovic J, Cavanna AE, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. (2019) 92:896–906. doi: 10.1212/WNL.0000000000007466
34. Verdellen C, Griendt J, Hartmann A, Murphy T. European clinical guidelines for Tourette Syndrome and other tic disorders. Part III: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry. 20:197–207. doi: 10.1007/s00787-011-0167-3
35. Steeves T, McKinlay BD, Gorman D, Billinghurst L, Day L, Carroll A, et al. Canadian guidelines for the evidence-based treatment of tic disorders: behavioural therapy, deep brain stimulation, and transcranial magnetic stimulation. Can J Psychiatry. (2012) 57:144–51. doi: 10.1177/070674371205700303
36. Cath DC, Hedderly T, Ludolph AG, Stern JS, Murphy T, Hartmann A, et al. European clinical guidelines for Tourette Syndrome and other tic disorders. Part I: assessment. Eur Child Adolesc Psychiatry. (2011) 20:155–71. doi: 10.1007/s00787-011-0164-6
37. Murphy TK, Lewin AB, Storch EA, Stock S. Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry. (2013) 52:1341–59. doi: 10.1016/j.jaac.2013.09.015
38. Conelea C, Bennett S, Capriotti M, Hamilton S, Himle M, Hunt C, et al. Treating tourette together: an agenda for patient-centered research focused on comprehensive behavior therapy for tics. PsyArXiv [Preprint]. 2020. doi: 10.31234/osf.io/qhb2z
39. Olfson M, Crystal S, Gerhard T, Huang C, Walkup JT, Scahill L, et al. Patterns and correlates of tic disorder diagnoses in privately and publicly insured youth. J Am Acad Child Adolesc Psychiatry. (2011) 50:119–31. doi: 10.1016/j.jaac.2010.11.009
40. Smith JL, Gregory S, McBride N, Murphy TK, Storch EA. Outpatient Treatment of Tic Disorders Among Children and Adults. Mov Disord Clin Pract. (2017) 4:559–67. doi: 10.1002/mdc3.12472
41. Himle MB, Freitag M, Walther M, Franklin SA, Ely L, Woods DW. A randomized pilot trial comparing videoconference versus face-to-face delivery of behavior therapy for childhood tic disorders. Behav Res Ther. (2012) 50:565–70. doi: 10.1016/j.brat.2012.05.009
42. Cothros N, Martino D, McMorris C, Stewart D, Tehrani A, Pringsheim T. Prescriptions for alpha agonists and antipsychotics in children and youth with tic disorders: a pharmacoepidemiologic study. Tremor Other Hyperkinet Mov. (2019) 9. doi: 10.5334/tohm.459
43. Farag M, Stern JS, Simmons H, Robertson MM. Serial pharmacological prescribing practices for tic management in Tourette syndrome. Hum Psychopharmacol Clin Exp. (2015) 30:435–41. doi: 10.1002/hup.2495
44. Rickards H, Cavanna AE, Worrall R. Treatment practices in Tourette syndrome: the European perspective. Eur J Paediatr Neurol. (2012) 16:361–4. doi: 10.1016/j.ejpn.2011.12.001
45. Osland ST, Steeves TDL, Pringsheim T. Pharmacological treatment for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders. Cochrane Database Syst Rev. (2018) 6:CD007990. doi: 10.1002/14651858.CD007990.pub3
46. Desarkar P, Sinha VK. Sertraline caused tics in a child with obsessive-compulsive disorder. J Pediatr Neurol. (2006) 4:283. doi: 10.1055/s-0035-1557335
47. Abi-Jaoude E, Kideckel D, Stephens R, Lafreniere-Roula M, Deutsch J, Sandor P. Tourette syndrome: a model of integration. Handbook of Integrative Clinical Psychology, Psychiatry and Behavioral Medicine: Perspectives, Practices and Research. New York, NY: Springer Publishing Company (2009). p. 549–88.
48. Reeves G, Anthony B. Multimodal treatments versus pharmacotherapy alone in children with psychiatric disorders: implications of access, effectiveness, and contextual treatment. Paediatr Drugs. (2009) 11:165–9. doi: 10.2165/00148581-200911030-00002
Keywords: Tourette syndrome, pharmacotherapy, health services, health care delivery, comorbidity, clinician survey
Citation: Bhikram T, Elmaghraby R, Abi-Jaoude E and Sandor P (2021) An International Survey of Health Care Services Available to Patients With Tourette Syndrome. Front. Psychiatry 12:621874. doi: 10.3389/fpsyt.2021.621874
Received: 27 October 2020; Accepted: 05 February 2021;
Published: 26 February 2021.
Edited by:
Wolfram Kawohl, University of Zurich, SwitzerlandReviewed by:
James Leckman, Yale University, United StatesJude Uzoma Ohaeri, University of Nigeria, Nsukka, Nigeria
Copyright © 2021 Bhikram, Elmaghraby, Abi-Jaoude and Sandor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elia Abi-Jaoude, ZWxpYS5hYmkuamFvdWRlQHV0b3JvbnRvLmNh
†These authors share first authorship
 Tracy Bhikram
Tracy Bhikram Rana Elmaghraby
Rana Elmaghraby Elia Abi-Jaoude
Elia Abi-Jaoude Paul Sandor
Paul Sandor