- 1Wuxi Mental Health Center Affiliated to Nanjing Medical University, Wuxi, China
- 2Nanjing Brain Hospital Affiliated to Nanjing Medical University, Nanjing, China
Background: Effortful cognition processing is an intentionally initiated sequence of cognitive activities, which may supply top-down and goal-oriented reassessment of specific stimuli to regulate specific state-driven responses contextually, whereas automatic cognitive processing is a sequence of cognitive activities that is automatically initiated in response to an input configuration. The effortful–automatic perspective has implications for understanding the nature of the clinical features of major depressions. The aim of this study was to investigate the influence of problem solving therapy (PST) on effortful cognition in major depression (MD).
Methods: The participants included an antidepressant treatment (AT) group (n = 31) or the combined antidepressant treatment and PST (CATP) group (n = 32) and healthy controls (HCs) (n = 30). Hamilton Depression Rating Scale (HAMD, 17-item version) and the face–vignette task (FVT) were measured for AT group and CATP group at baseline (before the first intervention) and after 12 weeks of interventions. The HC group was assessed with the FVT only once. At baseline, both patients and HCs were required to complete the basic facial emotion identification test (BFEIT).
Results: The emotion identification accuracy of the HC group was higher than that of the patient group when they performed BFEIT; patients with MD present poor FVT performances; compared to the antidepressant treatment, PST plus antidepressant treatment decreased HAMD scores and improved FVT performances in patients with MD.
Conclusions: Patients with MD present effortful cognition dysfunction, and PST can improve effortful cognitive dysfunction. These findings suggest that the measurement of effortful cognition might be one of the indexes for the therapeutic effect of PST in MD.
Introduction
Major depression (MD) is a common mental disorder with a higher disability rate, affecting 10–15% of the worldwide population every year. To date, some antidepressants, including several typical antidepressants and several atypical antidepressants, have been used to treat major depression; however, only 60–70% of patients respond to antidepressant treatment. Furthermore, 10–30% of these patients exhibit treatment-resistant symptoms such as suicidal thought, a low mood, a decline in interest, and a loss of happiness (1).
To improve the symptoms of MD, several treatment options have been developed, such as switching therapies, augmentation, combination, optimization, psychotherapies, modified electro-convulsive therapy (MECT), repetitive transcranial magnetic stimulation therapies, deep brain stimulation therapies, vagal nerve stimulation therapies, light-based therapies, acupuncture treatment, and yoga; these approaches have been considered and tailored for individual patients (2–4). Most important for the improvement of depressed patients' symptoms, many studies had reported that physical activity interventions are helpful to improve major depressive disorders because physical activity is associated with many mental health benefits (5–11). Assessments to determine symptom improvement for patients with MD often depend on decreased total Hamilton Depression Rating Scale (HAMD, 17 or 24 items) scores.
Problem solving therapy (PST) belongs to a type of cognitive behavioral therapy that mainly concentrates on training in appropriate problem-solving notions as well as skills. PST has been used for major depression (12–15). It has been confirmed that, in the depressed patient group, PST was equally effective as antidepressant treatments and more effective than no treatment and support or attention control patients (16). In clinical practice, the effective treatment program of PST in MD includes three aspects: [1] training in a positive problem orientation, [2] training in problem definition and formulation, the generation of alternatives, decision making, and solution implementation and verification, and [3] training in problem orientation plus problem definition and formulation, the generation of alternatives, decision making, and solution implementation and verification (16).
Cognitive function refers to mental processes involved in working memory, problem-solving, decision-making, the acquisition of knowledge, regulation of information, and reasoning. As a major symptom, cognitive function impairment is acknowledged as a clinical characteristic of major depression. Additionally, many studies of major depression have suggested a role for cognitive measures in predicting those at risk for poor outcomes (17). A previous study indicated that patients with major depression present negatively valanced emotional symptoms that are accompanied by cognitive deficits, and the emotional processing dysfunctions of the prefrontal cortex might lead to cognitive deficits in patients with MD (18). Adaptive emotional responding relies on both effortful cognition processing and automatic cognition processing. Effortful cognition processing is a controlled process and refers to an intentionally initiated sequence of cognitive activities, which may supply top-down as well as goal-oriented reassessment of emotional stimuli to regulate emotion-driven responses contextually (19). Effortful cognition was measured by the face–vignette task (FVT) (19). Relative to effortful cognitive processing, automatic cognitive processing is a sequence of cognitive activities that is automatically initiated in response to an input configuration (20). Automatic cognition processing requires near-zero attention for the task at hand and, in many instances, is executed in response to a specific stimulus.
Previous studies have shown that patients with MD present effortful cognitive dysfunction. For example, a previous study reported that, when patients with MD performed two contrasting cognitive tasks (i.e., one requiring sustained effort and information processing and the other requiring only superficial information processing that could be accomplished automatically), only the effort-demanding cognitive task was performed poorly (21). Additionally, two previous studies investigated the functions of automatic and effortful information processing in a visual search paradigm, and the results showed that the patients with MD exhibited longer reaction times on the tasks requiring more effortful information processing than the controls. However, there were no differences on tasks requiring automatic information processing (22, 23).
Since cognitive function impairment plays a critical role in MD, the assessment of cognitive function is a better way to determine the treatment effect for MD. The effortful–automatic perspective has implications for understanding the nature of the clinical features of MD. Furthermore, the investigation of the influence of PST on effortful cognition in MD is helpful for improving the present understanding of the therapeutic mechanism and assess the therapeutic effect of PST. To date, no studies of PST on effortful cognition in MD have been reported. In this study, the participants included patients with MD and healthy controls (HCs). The MD group was treated with antidepressants or the combination of antidepressants with PST, and effortful cognition was rated by the FVT. The hypothesis of this study is that depressed patients display poor effortful cognition performance, and PST can improve effortful cognitive dysfunctions. The aim of this study was to investigate the effect of PST on effortful cognition in MD.
Materials and Methods
Time and Setting
This study was conducted in Wuxi Mental Health Center Affiliated to Nanjing Medical University, No. 156 Qianrong Road, Rongxiang Street, Binhu District, Wuxi City, P.R. China, from February 1, 2016 to February 27, 2020.
Diagnostic Approaches and Subjects
A total of 80 patients meeting the American Psychiatric Association's fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for major depression were recruited as the research group. The MD patients were randomly assigned to the antidepressant treatment (AT) group or the combined antidepressant treatment and PST (CATP) group. The allocation schedule was generated by using a list of random numbers. Thirty healthy persons were admitted to the HC group. All HCs had no personal history of mental disorders. Patients with MD were selected from Wuxi Mental Health Center Affiliated to Nanjing Medical University, No. 156 Qianrong Road, Rongxiang Street, Binhu District, Wuxi City, P.R. China; the normal controls were citizens of Wuxi City, Jiangsu Province, P.R. China, recruited by online and local community advertisements. Patients with MD and HC subjects were excluded from the study if they had been diagnosed with nicotine addiction or other psychoactive substance dependence, had suffered any systemic disease that may affect the central nervous system, or had received electroconvulsive therapy (including MECT) in the past 24 weeks. All patients and HC subjects were Chinese. All patients and HC subjects were paid 42.12 Euros plus travel costs.
Seven subjects in AT group and five subjects in CATP group were all diagnosed with bipolar disorder in the follow-up survey, and they were ultimately excluded from this study. Two subjects in AT group and three subjects in CATP group were also excluded from this study because they could not finish the follow-up assessment. Finally, the data from 31 subjects in AT group and 32 subjects in CATP group were used in the statistical analyses.
Measurements of Automatic and Effortful Cognition
Basic Facial Emotion Identification Test
The basic facial emotion identification test (BFEIT) consists of eight examples of each of the seven basic facial emotions, e.g., happy, angry, sad, fear, surprise, disgust, and calm, which were taken from the Chinese affective picture system (24). Male and female face pictures were balanced across each emotion category.
Face–Vignette Task
FVT was designed based on an effortful cognitive task that was used in the study on effortful vs. automatic emotional processing in patients with schizophrenia by Patrick et al. (19). E-Prime 2.0 software (Psychology software tools, INC, USA) was used to implement the experimental procedure. The face pictures were white and black photographs and included six emotional expressions, i.e., happy, angry, sad, fear, surprise, and disgust, which were taken from the Chinese affective picture system (24). In each emotion, the male and female faces were equal. Within a given emotion category, the same identity was used only once. The situational vignettes communicated the six special emotions, i.e., guilty, smug, hopeful, insulted, pain, and determined. Before the experiment, the intended emotion for each story (vignette) was verified by seven undergraduates, and the mean accuracy was 0.91 [standard deviation (SD) = 0.08], and the observed inter-rater reliability κ value was 0.75. The face–story pairs were matched such that each story was inconsistent with the facial expression according to the specially appointed emotional category (e.g., a happy facial expression paired with a smug story). Each specific emotion category depended on the situational context (see the listed example in Figure 1). The specially appointed face–story pairs included sad vs. guilty, happy vs. smug, fearful vs. painful, angry vs. determined, disgusted vs. insulted, and surprised vs. hopeful. During the FVT, the participants viewed a series of 24 face–story (vignette) pairs and were informed that each facial expression represented the subject of the vignette. The faces and vignettes were presented simultaneously. All participants were required to read the vignettes aloud. In each trial, all participants answered the question accompanied by face–vignette pairs through a specially appointed keypad in a multiple choice pattern. The 13 obtainable choices for each trial were as follows: angry, happy, sad, fearful, disgusted, surprised, smug, guilty, hopeful, determined, pain, insulted as well as no emotion.

Figure 1. Example of a trial on the face–vignette task. The situational vignettes in English are as follows: This is a story about a girl's birthday. The girl stayed in her room. She received a call from her beloved boyfriend: “You're waiting for me at home. I'll bring your favorite flowers to your birthday!” Several minutes later, she heard the knock of her boyfriend's arrival. The question was “What emotion is the person feeling?” Responding with “surprise” will be recorded as a face response and responding with “hopeful” will be recorded as a vignette response. Additionally, any other response will be recorded as a random response.
On the FVT, the responses of the participants were labeled as face responses, vignette responses, and random responses. The response data were converted to proportions, which were used for statistical analysis.
Problem Solving Therapy Procedure
The PST was performed as described in a previous study (25). All the patients with MD were scheduled for PST, which consists of six sessions administered every other week. The treatment sessions were conducted at the psychological therapy room of the Psychiatry Department. The PST was conducted by six psychotherapists, and visits were conducted by two psychiatric resident physicians. All the psychotherapists owned a therapy handbook and underwent training, including a short theoretical course, role playing in a clinical background as well as watching a training videotape. The PST includes three steps: [1] the patient's symptoms are linked with their problems in daily living, [2] the problems are defined and clarified, and [3] an attempt is made to solve the problems in a structured way. The sessions lasted 1 h for the first visit and half an hour for the subsequent visits.
Clinical Interventions and Clinical Assessment
Two psychiatric residents examined all the participants to confirm or exclude a major depression diagnosis based on DSM-5 criteria and to collect medication and sociodemographic data. A HAMD (17-item version) was applied to assess the depressive severity for patients. A decrease of more than 50% in HAMD (17-item version) scores from baseline to follow-up was defined as a treatment response, and HAMD (17-item version) scores <7 at follow-up were defined as clinical remission.
HAMD (17-item version) and the FVT data were measured for the AT group and CATP group at baseline (before the first intervention, time 1) and after 12 weeks of interventions (time 2). The HC group was assessed using the face–vignette task only once. At baseline, both patients and HCs were required to complete the BFEIT.
Statistical Analysis
Data are presented as mean (SD), and all data were analyzed with Statistical Product and Service Solution 18.0 statistical software (SPSS 18.0, International Business Machines Corporation). Comparisons of the demographic data, basic facial emotion identification test scores, face response proportions, vignette response proportions, and random response proportions at baseline among patients and healthy controls were conducted using the method of one-way analysis of variance (ANOVA) or the chi-square test. Comparisons of HAMD (17-item version) scores, face response proportions, vignette response proportions, and random response proportions between baseline (time 1) and after 12 weeks of interventions (time 2) in the patient group were performed using 2 × 2 repeated-measures ANOVA. In this study, all alpha values of 0.05 were considered as statistically significant throughout. Cohen's d effect sizes were used for t-tests. The cutoff values for Cohen's d's were defined as trivial effect size when d < 0.19, small effect size when 0.2 < d < 0.49, medium effect size when 0.5 < d < 0.79, and large effect size when d > 0.8. Partial eta-square (ηp2) effect sizes were used for F-tests. Similarly, the cutoff values for ηp2 were set as trivial effect size when ηp2 < 0.019, small effect size when 0.02 < ηp2 < 0.059, medium effect size when 0.06 < ηp2 < 0.139, and large effect size when ηp2 > 0.14. Phi (ϕ) effect sizes were used for chi-square test. The cutoff values for ϕ were set as trivial effect size when ϕ < 0.09, small effect size when 0.10 < ϕ < 0.29, medium effect size when 0.30 < ϕ < 0.49, and large effect size when ϕ > 0.50.
Results
The Demographic Data of All Participants
The demographic data of the participants are described in Table 1. No significant differences were observed in sex ratio, mean age, age range, or mean education years among the AT group, CATP group, and HC group.
Antidepressant Treatments
In the AT group, 20 patients with MD were antidepressant-naïve, and 11 patients with MD were antidepressant-free (six for at least 24 weeks and five for at least 4 weeks); patients with MD received fluoxetine (n = 8), paroxetine (n = 7), fluvoxamine (n = 7), sertraline (n = 6), or escitalopram (n = 3). The mean fluoxetine-equivalent dose was 30.5 (8.8) mg/day. In the CATP group, 19 patients with MD were antidepressant-naïve, and 13 patients with MD were antidepressant-free (eight for at least 24 weeks and five for at least 4 weeks); patients with MD received fluoxetine (n = 9), paroxetine (n = 8), fluvoxamine (n = 8), sertraline (n = 3), or escitalopram (n = 4). According to a previous report (26), the mean fluoxetine-equivalent dose was 30.1 (7.9) mg/day. Neither of the patient groups used concomitant medications.
Comparisons of BFEIT Performance Among the AT Group, CATP Group, and HC Group
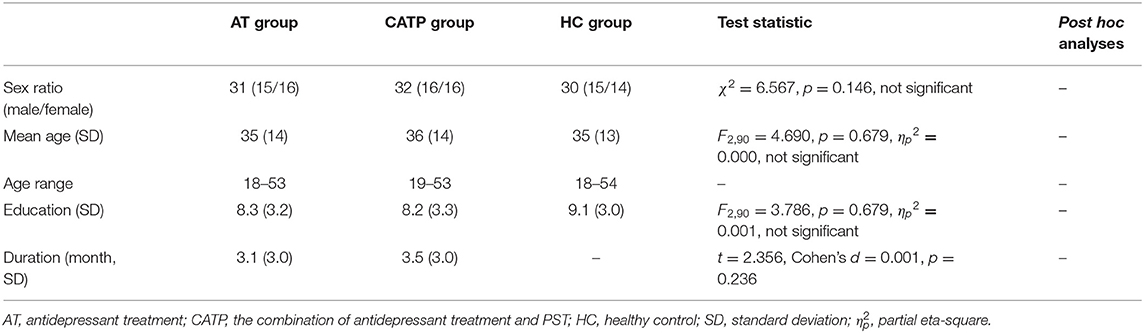
As shown in Figure 2, one-way ANOVA revealed that there were significant differences in BFEIT performance (emotion identification accuracy) among the AT group, CATP group, and HC group (F2,90 = 27.729, df = 2, ηp2 = 0.33, p = 0.000). Least square difference tests were performed as post hoc analyses and showed significant differences between the HC group, AT group, and CATP group (all p = 0.000). The emotion identification accuracy of the HC group was higher than that of the AT group or CATP group. However, no significant difference was observed between the AT group and the CATP group (p = 0.951).
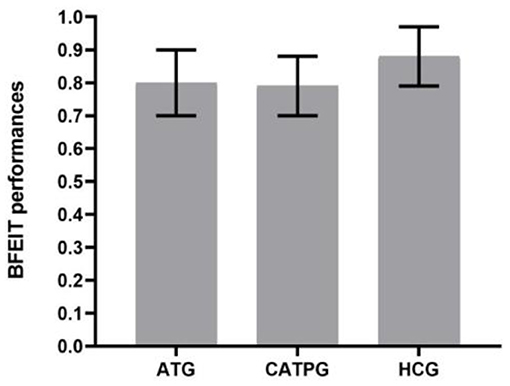
Figure 2. Comparisons of BFEIT performance among the AT group, CATP group, and HC group. BFEIT, basic facial emotion identification test; ATG, antidepressant treatment group; CATPG, the combination of antidepressant treatment and PST group; HC, healthy control; SD, standard deviation.
Comparisons of HAMD (17-Item Version) Scores Before and After Clinical Interventions
As shown in Figure 3, using HAMD (17-item version) scores as dependent variables, a 2 × 2 repeated-measures ANOVA with group (AT group vs. CATP group) as a between-subjects factor and time point (time 1 vs. time 2) as a within-subjects factor revealed that the interaction effect for group × time point was not significant (F1,61 = 1.697, ηp2 = 0.003, p = 0.198); however, the main effect for time point was significant (F1,61 = 206.419, ηp2 = 0.35, p = 0.000), and the main effect for group was significant (F1,61 = 170.914, ηp2 = 0.18, p = 0.038). The 12-week interventions decreased HAMD (17-item version) scores in the two patient groups.
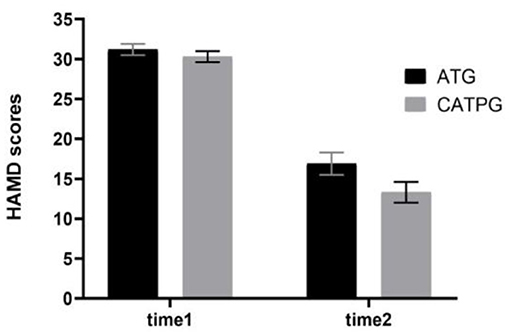
Figure 3. Comparisons of HAMD scores before and after clinical interventions between the AT group and CATP group. HAMD, Hamilton Depression Rating Scale (17-item version); ATG, antidepressant treatment group; CATPG, the combination of antidepressant treatment and PST group; time 1, baseline; time 2, after 12 weeks of intervention; SD, standard deviation.
There were significant differences in the remission rate between the CATP group (19/32) and the AT group (14/31); the remission rate in the CATP group was higher than that of the AT group (χ2 = 6.123, ϕ = 0.29, p = 0.028). There were significant differences in the treatment response rate between the CATP group (25/32) and AT group (18/31); the treatment response rate in the CATP group was higher than that of the AT group (χ2 = 4.370, ϕ = 0.26, p = 0.035).
Comparisons of FVT Performance Among the AT Group, CATP Group, and HC Group
Baseline Level
As shown in Table 2, one-way ANOVA revealed that there were significant differences in face response proportions and vignette response proportions among the AT group, CATP group, and HC group (F2,90 = 27.861, 18.234, all df = 2; ηp2 = 0.32, 0.36, all p = 0.000). Least square difference tests were performed as post hoc analyses and showed significant differences between the HC group and AT group or between the HC group and the CATP group (all p = 0.000). The face response proportions of the HC group were lower than those of the AT group and CATP group, and the vignette response proportions of the HC group were higher than those of the AT group and CATP group. For the above-mentioned two variables, no differences between the AT group and CATP group were observed (p = 0.951, 0.913).
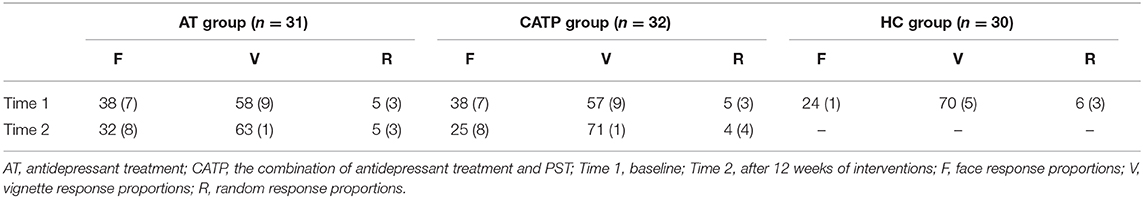
Table 2. Face–vignette task performances (%, SD) among the AT group, CATP group, and healthy control group.
However, there were no significant differences in random response proportions among the AT group, CATP group, and HC group (F2,90 = 0.979, df = 2, ηp2 = 0.006, p = 0.380).
Before and After Interventions
As shown in Table 2, using face response proportions, vignette response proportions, and random response proportions as dependent variables, a 2 × 2 repeated-measures ANOVA with group (AT group vs. CATP group) as the between-subjects factor and time point (time 1 vs. time 2) as the within-subjects factor was performed.
Face Response Proportions
The interaction effect for group × time point was significant (F1,61 =25.174, df =1, ηp2 = 0.30, p = 0.000), the main effect for time point was significant (F1,61 = 138.086, df = 1, ηp2 = 0.32, p = 0.000), and the main effect for group was significant (F1,61 = 4.853, df = 1, ηp2 = 0.24, p = 0.031).
Vignette Response Proportions
The interaction effect for group × time point was significant (F1,61 = 29.450, df = 1, ηp2 = 0.31, p = 0.000), the main effect for time point was significant (F1,61 = 144.130, df = 1, ηp2 = 0.32, p = 0.000), and the main effect for group was significant (F1,61 = 3.083, df = 1, ηp2 = 0.18, p = 0.041).
Random Response Proportions
The interaction effect for group × time point was not significant (F1,61 = 1.003, df = 1, ηp2 = 0.001, p = 0.320), the main effect for time point was not significant (F1,61 = 1.519, df = 1, ηp2 = 0.001, p = 0.223), and the main effect for group was not significant (F1,61 = 0.017, df = 1, ηp2 = 0.000, p = 0.897).
Discussion
This study is the first to survey the effect of problem-solving therapy on effortful cognition in MD using FVT; measurements of the basic facial emotion identification were also conducted. Our data showed that the emotion identification accuracy of HCs was higher than that of patients with MD; patients with MD exhibited poor FVT performance. Compared to antidepressant treatment, PST plus antidepressant treatment resulted in lower HAMD (17-item version) scores and better FVT performance.
This study also investigated the ability of patients with MD to employ contextual information when determining the intended or expressed or signified message of facial emotional expressions. In the FVT, target facial emotional expressions are preceded by stories describing situational messages which are discrepant in affective valence. What both patients with MD and HCs had judged reflects either the dominance of the emotional context or the facial emotional expression. Many studies on cognitive processing by patients with MD reported that depressive symptoms interfere with effortful processing, and the degree of interference is determined by the degree of effort required for the task, the severity of depression, and the valence of the stimulus material to be processed. However, depressive symptoms only interfere minimally with automatic processes (27).
Consistent with the findings of previous studies (21–23), our results showed that patients with MD could not utilize contextual information for specific face–vignette pairs. However, HCs more extensively made good judgments on emotion in line with contextual information, which indicates that patients with MD display poor effortful cognition performance. Cognition dysfunctions in MD include impairments of social cognition and neurocognition (28, 29). Social cognition refers to a process or a function for an individual's mental operations underlying social behavior, while neurocognition refers to those basic information processing functions such as attention and executive processes. Effortful cognitive processing was involved in either social cognition or neurocognition. We verified our hypothesis, i.e., patients with MD present effortful cognitive dysfunction.
In this study, we confirmed that PST plus antidepressant treatments leads to a greater reduction of depressive symptoms, a greater response rate, and a greater remission rate over a period of 12 weeks than antidepressant treatments only in patients with MD. We also indirectly verified our previous hypothesis, i.e., PST can improve effortful cognitive dysfunction, namely, PST improved the severity of MD by improving effortful cognition. Our data provide supporting evidence for the conclusion that the facial affect processing ability could be a valuable predictor of successful social context integration in FVT in MD.
Conclusions
In conclusion, patients with MD present effortful cognitive dysfunction, and PST can improve effortful cognitive dysfunction. The measurement of effortful cognition might be one of the indexes for the therapeutic effect of PST in MD.
There are some limitations in the study. First, the findings must be considered preliminary due to the small sample size. Second, healthy controls were assessed with the FVT only once; therefore, the results of the FVT would be influenced by the practice effect in patients with MD. Future studies should augment the sample size and eliminate the practice effect to further confirm the relationship between effortful cognition and PST in MD. Finally, this study investigated the effect of PST plus antidepressant treatment on effortful cognition in MD. Therefore, no outcome of the pure PST effect on effortful cognition was obtained. The examination of the pure PST effect on effortful cognition in MD is necessary in a future study.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Affiliated Wuxi Mental Health Center of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CJ, HZ, and ZZ designed the study and wrote the paper. CJ, HZ, LC, and ZZ acquired and analyzed the data. All authors reviewed the content and approved the final version for publication.
Funding
This research was supported by the Wuxi Taihu Talent Project (No. WXTTP2020008) and the Key Medical Talent Training Project of Jiangsu Province (No. ZDRCC2016019).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the Key Medical Talent Training Project of Jiangsu Province for providing support (project Grant No. ZDRCC2016019) for this research.
References
1. McAllister-Williams RH, Arango C, Blier P, Demyttenaere K, Falkai P, Gorwood P, et al. The identification, assessment and management of difficult-to-treat depression: an international consensus statement. J Affect Disord. (2020) 267:264–82. doi: 10.1016/j.jad.2020.02.023
2. Strawbridge R, Carter B, Marwood L, Bandelow B, Tsapekos D, Nikolova VL, et al. Augmentation therapies for treatment-resistant depression: systematic review and meta-analysis. Br J Psychiatry. (2019) 214:42–51. doi: 10.1192/bjp.2018.233
3. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adher. (2012) 6:369–88. doi: 10.2147/PPA.S29716
4. Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dial Clin Neurosci. (2015) 17:111–26. doi: 10.31887/DCNS.2015.17.2/dionescu
5. Bailey AP, Hetrick SE, Rosenbaum S, Purcell R, Parker AG. Treating depression with physical activity in adolescents and young adults: a systematic review and meta-analysis of randomised controlled trials. Psychol Med. (2017) 48:1068–83. doi: 10.1017/S0033291717002653
6. Gerber M, Holsboer-Trachsler E, Puhse U, Brand S. Exercise is medicine for patients with major depressive disorders: but only if the “pill” is taken! Neuropsychiatr Dis Treat. (2016) 12:1977–81. doi: 10.2147/NDT.S110656
7. Mota-Pereira J, Carvalho S, Silverio J, Fonte D, Pizarro A, Teixeira J, et al. Moderate physical exercise and quality of life in patients with treatment-resistant major depressive disorder. J Psychiatr Res. (2011) 45:1657–59. doi: 10.1016/j.jpsychires.2011.08.008
8. Rosenbaum S, Tiedemann A, Sherrington C, Curtis J, Ward PB. Physical activity interventions for people with mental illness: a systematic review and meta-analysis. J Clin Psychiatry. (2014) 75:964–74. doi: 10.4088/JCP.13r08765
9. Rosenbaum S, Tiedemann A, Stanton R, Parker A, Waterreus A, Curtis J, et al. Implementing evidence-based physical activity interventions for people with mental illness: an Australian perspective. Austr Psychiatry. (2016) 24:49–54. doi: 10.1177/1039856215590252
10. Saligheh M, Hackett D, Boyce P, Cobley S. Can exercise or physical activity help improve postnatal depression and weight loss? A systematic review. Arch Womens Ment Health. (2017) 20:595–611. doi: 10.1007/s00737-017-0750-9
11. Stubbs B, Rosenbaum S, Vancampfort D, Ward P, Schuch FB. Exercise improves cardiorespiratory fitness in people with depression: a meta-analysis of randomized control trials. J Affect Disord. (2016) 190:249–53. doi: 10.1016/j.jad.2015.10.010
12. Alexopoulos GS, Raue PJ, Kiosses DN, Mackin RS, Kanellopoulos D, McCulloch C, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. (2011) 68:33–41. doi: 10.1001/archgenpsychiatry.2010.177
13. Garland A, Harrington J, House R, Scott J. A pilot study of the relationship between problem-solving skills and outcome in major depressive disorder. Br J Med Psychol. (2000) 73:303–9. doi: 10.1348/000711200160525
14. Zhang DX, Lewis G, Araya R, Tang WK, Mak WW, Cheung FM, et al. Prevention of anxiety and depression in Chinese: a randomized clinical trial testing the effectiveness of a stepped care program in primary care. J Affect Disord. (2014) 169:212–20. doi: 10.1016/j.jad.2014.08.015
15. Waller JM, Silk JS, Stone LB, Dahl RE. Co-rumination and co-problem solving in the daily lives of adolescents with major depressive disorder. J Am Acad Child Adolesc Psychiatry. (2014) 53:869–78. doi: 10.1016/j.jaac.2014.05.004
16. Bell AC, D'Zurilla TJ. Problem-solving therapy for depression: a meta-analysis. Clin Psychol Rev. (2009) 29:348–53. doi: 10.1016/j.cpr.2009.02.003
17. Bortolato B, Miskowiak KW, Köhler CA, Maes M, Fernandes BS, Berk M, et al. Cognitive remission: a novel objective for the treatment of major depression? BMC Med. (2016) 14:9. doi: 10.1186/s12916-016-0560-3
18. Heinzel A, Northoff G, Boeker H, Boesiger P, Grimm S. Emotional processing and executive functions in major depressive disorder: dorsal prefrontal activity correlates with performance in the intra-extra dimensional set shift. Acta Neuropsychiatr. (2010) 22:269–79. doi: 10.1111/j.1601-5215.2010.00494.x
19. Patrick RE, Rastogi A, Christensen BK. Effortful versus automatic emotional processing in schizophrenia: insights from a face-vignette task. Cogn Emot. (2015) 29:767–83. doi: 10.1080/02699931.2014.935297
20. Ruff RM, Evans RW, Light RH. Automatic detection vs. controlled search: a paper-and-pencil approach. Percept Mot Skills. (1986) 62:407–16. doi: 10.2466/pms.1986.62.2.407
21. Roy-Byrne PP, Weingartner H, Bierer LM, Thompson K, Post RM. Effortful and automatic cognitive processes in depression. Arch Gen Psychiatry. (1986) 43:265–7. doi: 10.1001/archpsyc.1986.01800030083008
22. Hammar A, Lund A, Hugdahl K. Selective impairment in effortful information processing in major depression. J Int Neuropsychol Soc. (2003) 9:954–9. doi: 10.1017/S1355617703960152
23. Hammar A. Automatic and effortful information processing in unipolar major depression. Scand J Psychol. (2003) 44:409–13. doi: 10.1046/j.1467-9450.2003.00361.x
24. Yan W. Standardization and assessment of college students' facial expression of emotion. Chin J Clin Psychol. (2005) 13:396–8. (In Chinese).
25. Williams JW, Barrett J, Oxman T, Frank E, Katon W, Sullivan M, et al. Treatment of dysthymia and minor depression in primary care: a randomized controlled trial in older adults. JAMA. (2000) 284:1519–26. doi: 10.1001/jama.284.12.1519
26. Yu H, Marianna P, Laura RM, Yusuke O, Nozomi T, Andrea C. Dose equivalents of antidepressants: Evidence-based recommendations from randomized controlled trials-Science Direct. J Affect Disord. (2015) 180:179–84. doi: 10.1016/j.jad.2015.03.021
27. Hartlage S, Alloy LB, Vázquez C, Dykman B. Automatic and effortful processing in depression. Psychol Bull. (1993) 113:247–78. doi: 10.1037/0033-2909.113.2.247
28. Michael JW, Tracy Michele A, Bernhard TB. A review of the role of social cognition in major depressive disorder. Front Psychiatry. (2014) 5:179. doi: 10.3389/fpsyt.2014.00179
Keywords: major depression, problem solving therapy, effortful cognition, the face-vignette task, cognitive function
Citation: Jiang C, Zhou H, Chen L and Zhou Z (2021) Problem Solving Therapy Improves Effortful Cognition in Major Depression. Front. Psychiatry 12:607718. doi: 10.3389/fpsyt.2021.607718
Received: 18 September 2020; Accepted: 26 February 2021;
Published: 07 April 2021.
Edited by:
Brisa Fernandes, University of Texas Health Science Center at Houston, United StatesReviewed by:
Serge Brand, University Psychiatric Clinic Basel, SwitzerlandXiaoyun Guo, Shanghai Jiao Tong University, China
Copyright © 2021 Jiang, Zhou, Chen and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhe Zhou, emhvdXpoJiN4MDAwNDA7bmptdS5lZHUuY24=
†These authors share first authorship
 Chenguang Jiang
Chenguang Jiang Hongliang Zhou
Hongliang Zhou Limin Chen1
Limin Chen1 Zhenhe Zhou
Zhenhe Zhou