
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Psychiatry , 12 February 2021
Sec. Mood Disorders
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.598119
This article is part of the Research Topic Mechanisms Underlying Mood Disorders View all 12 articles
Despite the overwhelming prevalence of anxiety disorders in modern society, medications and psychotherapy often fail to achieve complete symptom resolution. A complementary approach to medicating symptoms is to address the underlying metabolic pathologies associated with mental illnesses and anxiety. This may be achieved through nutritional interventions. In this perspectives piece, we highlight the roles of the microbiome and inflammation as influencers of anxiety. We further discuss the evidence base for six specific nutritional interventions: avoiding artificial sweeteners and gluten, including omega-3 fatty acids and turmeric in the diet, supplementation with vitamin D, and ketogenic diets. We attempt to integrate insights from the nutrition science-literature in order to highlight some practices that practitioners may consider when treating individual patients. Notably, this piece is not meant to serve as a comprehensive review of the literature, but rather argue our perspective that nutritional interventions should be more widely considered among clinical psychiatrists. Nutritional psychiatry is in its infancy and more research is needed in this burgeoning low-risk and potentially high-yield field.
Anxiety disorders are the most common type of psychiatric condition in the United States, with one-third of individuals suffering from some form of anxiety during their lifetime (1). Standard of care medications and psychotherapy are only successful in treating about half of patients, and only one-quarter experience complete symptomatic resolution (2).
While medications and behavioral therapies certainly have their place as part of a multifaceted approach to treat anxiety, the relatively high failure rate of such approaches is consistent with the broader failure of drug treatments for most neurological conditions. For example, antidepressants are efficacious in only about one-third of clinical cases (3) and there are no established disease-modifying medications for major neurodegenerative conditions like Parkinson's disease or Alzheimer's disease. With respect to the latter, the drug discovery failure rate for mere symptomatic management is 99.6% (4). It is therefore feasible, if not probable, that we are approaching neurological conditions with the wrong paradigm. As neurological conditions and mental illnesses (5) are characterized by a subset of fundamental metabolic disturbances [such as oxidative stress (6), insulin resistance (7), inflammation (8), and microbiome dysbiosis (9)] to which lifestyle factors are a contributor, it would makes sense that mental illnesses deserve complementary lifestyle approaches. In effect, lifestyle interventions for mental illness are a form of metabolic medicine complementary to metabolic disease (5). Nutrition is one such metabolic medicine.
Herein, we discuss the pathological correlates of anxiety disorders specifically, emphasizing the possible roles of microbiome dysbiosis and inflammation. We chose to structure this perspective piece as follows: First, we discuss microbiome dysbiosis and inflammation, pathologies that are particularly relevant to anxiety disorders in order to establish anxiety as a metabolic disease. Second, we discuss six nutritional strategies for which there is emerging evidence of their efficacy in anxiety. These are elimination of (i) artificial sweeteners and (ii) gluten, inclusion (iii) omega-3 fatty acids and (iv) turmeric (curcumin), maintaining adequate levels of (v) vitamin D, and (vi) and ketogenic diets. Within each section, we build up the evidence hierarchy from a mechanistic metabolic perspective, to animal models, to human studies. The purpose of this piece is not to delve into all the mechanisms of interventions (for which there is currently limited data), but demonstrate that anxiety is a metabolic disease and that nutritional therapy can be efficacious in its treatment.
The gut contains ~40 trillion microorganisms and is the largest endocrine organ in the body. By communicating to the brain via the Vagus nerves, regulating hormones, and influencing inflammation, the gut can impact mental health (10). More specifically, the compositions of individuals' gut microbial ecosystems can regulate mental status and anxiety (11). It is, therefore, unsurprising that microbiome dysbiosis is associated with anxiety (9).
As a comprehensive description of the mechanisms by which the microbiome and gut-brain axis influence the neuroanatomy and neurochemistry of anxiety is beyond the scope of this piece, we will emphasize the role of the amygdala, short chain fatty acids (SCFAs), and gut peptides as examples [Please see the following references as starting points for further reading on the Vagus nerve (12) or microbiome influence of cytokine production (13)].
The amygdala is a structure in the brain largely responsible for the threat response that is hyperactive in anxiety disorders (14). Interestingly, germ-free mice exhibit larger and more active amygdalae (15, 16). Furthermore, fecal transplantation, or the introduction Bifidobacterium infantis, has been shown to correct excessive stress response in such germ-free mice (17), implicating the microbiome in amygdala dysfunction.
The amygdala has receptors for gut peptides, including neuropeptide Y (NPY), pancreatic polypeptide (PP), and glucagon-like peptide 1 (GLP-1) (11, 18). Addressing each of these, there is evidence that the NPY system affects anxiety (19, 20); the PP Y4 receptor has been shown to modulate anxiety in rodents (21); and multiple GLP-1 receptor agonists have been used to address anxiety in animal models (22, 23). The release of each of these gut peptides is regulated by SCFAs produced by certain gut bacteria, which act through the G-protein coupled receptors, free fatty acid receptors 2 (FFAR2) and FFAR3 (11). Notably, populations of SCFA producing species tend to be reduced in individuals with anxiety (9).
In review, food influences the microbiome (24) and microbe-derived SCFAs bind to receptors on enteroendocrine cells to regulate the secretion of gut peptides, which themselves bind to receptors on the amygdala to influence the stress response and anxiety. This is just one cascade by which diet can influence the brain. SCFAs from gut microbes can also act through immune, inflammatory, and other endocrine mechanisms (25, 26), and lipopolysaccharide (LPS) from gram-negative bacteria can induce anxiety when leaked into circulation through a compromised gut barrier (27, 28). The mechanisms are many, but the point is simple: diet and nutrition influence anxiety by modulating the microbiome.
It is also to be emphasized that the microbiome-brain axis is a bidirectional relationship. Negative emotions can shift the microbial ecosystem by the release of stress hormones sympathetic neurotransmitters (29). Therefore, even if the current state of science does not enable precision medicine aimed at the microbiome, it is still important to consider the role that positive feedback loops between the gut and brain may be playing in anxiety disorders.
Chronic inflammation is a feature of almost all neurological and neurodegenerative disorders, including anxiety (30). Individuals suffering from anxiety and anxiety-related disorders, like panic disorder (31), generalized anxiety disorder (32), and post-traumatic stress disorders (PTSD), exhibit elevated levels of inflammatory markers in their circulation and cerebral spinal fluid (33). These include C-reactive protein (CRP), IL-1β, IL-6, and TNFα (31, 34–38). These cytokines contribute to neurotransmitter imbalances in the brain (including, serotonin, dopamine, glutamate/GABA) and can pathologically increase amygdala responsivity (30).
Suggestions of causality exist in the literature and, because the existing literature is most highly focused on PTSD, we too will focus on PTSD as a case in point of potential causality. As examples, polymorphisms in CRP predict increased likelihood of being diagnosed with PTSD, and predict worse symptoms if diagnosed (36); a study on immune cells taken from patients with anxiety showed increased reactivity and secretion of the cytokines, IL-17 and TNFα (39); and, administration of LPS to 39 healthy subjects doubled amygdala activity, as measured by fMRI, in response to socially threatening images (40). Admittedly, the state of research on the mechanisms of inflammation-induced anxiety is in its infancy. Nevertheless, it is probable that inflammation contributes to anxiety in at least some, and possibly a majority, of patients.
The menu of “inflammatory foods” is extensive, but generally includes foods associated with the Standard American Diet (SAD). Holistically speaking, the two most metabolically challenging components of SAD are refined sugars and processed vegetable oils, both of which can contribute to inflammation through myriad mechanisms (41–43). To mention a few as illustrative points, refined sugars, and in particular high fructose corn syrup, contribute to de novo lipogenesis of pro-inflammatory visceral fat (43), and fructose now composes 10% of caloric intake in the United States (42). Sugar also attaches to molecules throughout the body to generate inflammatory advanced glycation end products (AGEs). It has even been demonstrated that sugar can increase the production of AGEs in the brain and that these AGEs increase neuroinflammation and contribute to metabolic diseases (41).
Processed vegetable oils, such as corn oil and soybean oil, that contain high levels of the omega-6 fatty acids, linoleic acid, are likewise inflammatory. Having been stripped of the antioxidants that protect omega-6 fats in whole foods, the linoleic acid in processed vegetable oils incorporates into cells and tissue throughout the body, gets oxidized, and can initiate a vicious cycle of oxidation, insulin resistance, and inflammation that perpetuates metabolic and inflammatory diseases from the gut to the brain (7, 44–46). Increased consumption of linoleic acid-containing vegetable oils has even been proposed as a driver of cardiovascular disease (47), an inflammatory disease and comorbidity of anxiety disorders (46, 48, 49). Elimination of refined sugars and processed vegetable oils from the diet, and their replacement with whole foods, is foundational for good physical, cognitive, and mental health. However, more specific dietary and nutritional interventions have been explored in the context of anxiety, and it is to these which we turn.
Administration of artificial sweeteners to animals has been shown to precipitate anxiety (50). The anxiolytic effects of sweeteners are likely mediated by their adverse impacts on the microbiome and inflammation. Negative effects of certain sweeteners on systemic metabolism have been shown to be causal in animal models and humans, although the precise pathways are unknown (51, 52). Other mechanisms exist as well. For example, aspartame given to rats increased the levels of stress hormones in the animals' amygdalae (53). Aspartame can also block the transport of dopamine and serotonin precursors into the brain and can increase the levels of excitatory neurotransmitters, shifting brain chemistry toward an anxiety prone state (54).
In humans, artificial sweeteners have been associated with neuropsychiatric problems, including anxiety (55). Further, it has been proposed that individuals suffering from mental disorders may be particularly susceptible to the adverse effects of artificial sweeteners. For example, a randomized, placebo-controlled, crossover study designed to assess the impact of aspartame on mood was prematurely terminated because of the severity of reactions in patients with a history of depression (56), which is highly comorbid with anxiety (57).
Unfortunately, the literature is currently limited to the investigation of only a narrow range of sweeteners (and predominantly the sweetener, aspartame, found under the trade names Equal and NutraSweet, and in popular low-fat snacks and drinks, like Diet Coke). Future human studies will hopefully reveal associations between specific sweeteners and specific neurological disorders so that nutritional psychiatrists can provide more specific recommendations.
For patients unwilling to give up sweeteners, stevia (a natural non-caloric, non-insulinogenic sweetener) and erythritol [a non-insulinogenic sugar alcohol that gets absorbed in the small intestine and is not fermented by gut bacteria (58)] may be reasonable alternatives to recommend to patients in a practical clinical setting because they are presumed to have minimal negative impact on insulin sensitivity and the microbiome and are, therefore, less likely to cause metabolic dysfunction. However, as absence of evidence does not equate to evidence of absence, the most conservative approach is still the complete elimination of sugar and sweeteners.
Gluten can induce inflammation by causing “leaky gut.” Gluten proteins increase zonulin expression, which increases gut permeability (59, 60). Thereafter, immune stimulating compounds, like LPS, leak from the gut into the bloodstream, leading to inflammation.
Zonulin protein is overexpressed in celiac disease, a condition that itself is associated with social phobias, panic disorder, and other forms of anxiety (61–63). Generalizing beyond celiac disease, zonulin has been linked as a biomarker of mental illnesses such as autism, attention deficient hyperactivity disorder, and schizophrenia (64). Even in anxiety patients with no reported history of gastrointestinal disturbances, zonulin and LPS are found at elevated levels in the blood relative to non-anxious control subjects (65). This is consistent with the hypothesis that gluten can cause “leaky gut” to precipitate inflammation and anxiety and suggests patients with anxiety may be particularly sensitive to gluten.
At this time, a gluten-free diet has been shown to decrease anxiety only in celiac patients (66). Nevertheless, we feel it is reasonable to include a gluten-free diet in the arsenal of metabolic treatments for anxiety, given the mechanistic link to “leaky gut” and associations between zonulin and mental illness and zonulin levels and anxiety.
Omega-3 fatty acids, particularly the long-chain omega-3s, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are potent anti-inflammatory signaling molecules that support the microbiome (67, 68) and are important in cognition and mental health (69, 70). Direct evidence that omega-3s themselves are healthful, in addition to their whole food sources, comes from the comparison of genetically engineered mice that can biosynthesize omega-3 and/or omega-6 fats. On identical diets, mice that biosynthesize omega-3s and have lower omega-6/omega-3 ratios and exhibit healthier microbiomes, less inflammation, and less chronic disease (71). In preclinical studies on rats suffering from inflammation-induced anxiety, omega-3-rich diets have been shown to normalize dopamine levels (72) and reduce anxiety-like behaviors (73). And, in mice, omega-3s have been shown to improve serotonergic neurotransmission and increase levels of brain-derived neurotrophic factor (BDNF) (74). Thus, while the mechanisms by which omega-3s assist in addressing the metabolic foundations of anxiety are manifold, they likely include improving microbiome balance, decreasing inflammation, and balancing neurochemistry.
Turning to humans, Green et al. demonstrated that, in patients with social anxiety disorder, erythrocyte EPA and DHA levels are reduced 18–34%. Moreover, an inverse correlation exists between levels of these omega-3s and severity of anxiety (75). Similar observations have been made by others (76), and these associations are backed by interventional trials.
A randomized, double-blinded, placebo-controlled trial on 68 medical students showed that 12 weeks of omega-3 supplementation lowered anxiety by 20%. This study also revealed that lower omega-6/omega-3 ratios predicted lower levels of inflammatory markers and anxiety (77). Lastly, a meta-analysis of nineteen clinical trials, including 2,240 participants across eleven countries, concluded that omega-3 treatment is effective in reducing anxiety (78).
The aforementioned meta-analysis also highlights the fact that dose and omega-3s type are important to consider. Studies that used doses lower than 2 grams per day tended not to be effective in treating anxiety. Furthermore, subgroups analyses found that supplements with lower proportions of DHA were less effective in reducing anxiety, with supplements containing more than 60% EPA having no significant effect (78).
Practically speaking, on the topic of omega-3 types and sources, plant sources of omega-3 (such as flax seeds and chia seeds) contain primarily alpha linolenic acid (ALA), a shorter chain omega-3 that is converted in to the more bioactive EPA and DHA only at very low levels, on the order of 5% conversion (79–81). Fatty fish, such as mackerel, sardines, and Alaskan sockeye salmon are far richer in EPA and DHA. Salmon, in particular, includes the antioxidant, astaxanthin, which not only gives salmon their pink-red color but also protects omega-3s from oxidation and itself has neuroprotective properties (82). It is also worth mentioning that there is diversity among DHA forms. Specifically, lysophosphatidylcholine-conjugated DHA, found at its highest levels in fish roe and krill oil, has privileged transport to the brain via the major facilitatory superfamily domain-containing protein (MSFD2A) transporter, a transmembrane protein that exists within endothelial cells at the blood-brain barrier. Whereas, free DHA bound in the blood crosses into the brain via passive diffusion, the MSFD2A transporter actively shuttles lysophosphatidylcholine-conjugated DHA into the brain using energy derived from the sodium electrochemical gradient (83). This active transport mechanisms may be particularly beneficial in inflamed brains in which the blood-brain barrier is compromised. Therefore, when recommending omega-3 sources to patients, fish roe and krill oil may be the best options, followed by salmon and other fatty fish.
Thus, there is mechanistic rationale, animal and human data supporting the emphasis of dietary omega-3 for the treatment of anxiety.
Turmeric is probably the most heavily studied spices for brain health. Its active component, curcumin, has been explored as a treatment for Alzheimer's disease, Parkinson's disease, depression, comorbidities of anxiety, and anxiety itself (84, 85). Curcumin's mechanisms of action are many and include improving the gut microbial ecosystem (86), decreasing inflammation by inhibiting NFκB and the NLRP3 inflammasome (87–90), altering dopamine, serotonin, and cortisol levels (91), and regulating microRNAs and histone deacetylases (HDACs) (92).
Preclinical trials of curcumin for anxiety in rodent models add to the promise of curcumin as an anti-anxietolytic. In rats treated with a food preservative to induce anxiety, curcumin treatment completed rescued anxiety-like behaviors (93). Similar findings have been reported in other animal models of anxiety (94, 95). In these and other studies, curcumin significantly reduced anxiety-like behaviors concomitant with complementary improvements in neurotransmitter and hormone levels (91, 94, 95).
Multiple randomized, double-blinded, placebo-controlled trials have shown that curcumin supplementation can reduce anxiety in human patients. In patients with diabetes, 8 weeks of curcumin supplementation decreased anxiety (96). A crossover trial on 30 obese individuals likewise found that curcumin supplementation for 30 days reduced anxiety scores (97). And, a meta-analysis of five studies reported an overall significant effect of curcumin on anxiety with a large effect size [Hedge's g = −2.62 (84)].
Admittedly, there are limitations to the curcumin literature. Some have challenged that the health benefits of turmeric and its active components are over sensationalized. Specifically, Nelson et al. performed a careful analysis of the medical chemistry of curcumin and make a compelling case that the positive results in model systems may be confounded by curcumin's chemical instability and potential for interfering with assay readouts. Furthermore, they point out that there is a great degree of variability among studies with respect to supplement purity and formulations, which confound the reproducibility of studies (98). For example, curcuminoids are fat-soluble and exhibit <1% bioavailability when administered alone or an aqueous solution (99). For this reason, curcuminoids should be consumed with fats, and lipid-based delivery systems have been and are being developed for the administration of curcumin, including liposomes and nanoparticles (100). Indeed, the two randomized controlled trials referenced in the previous paragraph that reported positive findings for curcumin on anxiety each employed techniques to increase the bioavailability of curcumin, including nano-curcumin (96) and co-administration of bioperine (97) (also known as piperine), which enhances curcumin absorption 20-fold (101). Thus, future research resources should be devoted to the study of these more bioavailable forms of curcumin, and also to the impact of curcumin on the human microbiome, as this does not require systemic absorption.
Turmeric also relates to the previous section on omega-3s. Recall that humans are inefficient at converting ALA into EPA and DHA. Curcumin can increase ALA to DHA conversion by increasing levels of the DHA synthesis enzymes (102). This not only increases DHA in the brain but has direct functional implications on anxiety. Rodents treated with a combination of ALA and curcumin exhibit decreased anxiety (102).
As most modern humans spend most of their time inside, fully clothed, or simply living at high latitudes, endogenous vitamin D production is often inadequate. It is also difficult to get enough vitamin D from the diet. Even using the most favorable numbers for vitamin D content of milk, one would need to consume five gallons of whole milk daily to meet the recommended 600 IU (103), ignoring that many practitioners believe higher doses may be optimal. On a population level, vitamin D insufficiency (<30 ng/mL) has been estimated at 77% in the United States (104), making low vitamin D levels a hormonal epidemic.
In the brain, vitamin D regulates calcium homeostasis and ion channels (105, 106), neurotransmitter levels, including dopamine and serotonin (107–110), and the secretion of nerve growth factor and BDNF (111, 112). The benefits of vitamin D are also likely mediated by its role in shaping the microbiome and reducing inflammation (113–117).
Lower levels of vitamin D are associated with multiple mental disorders, including schizophrenia (118), depression (119), and anxiety (120–122). One such association study found that vitamin D levels in patients with a wide range of anxiety disorders were <60% those of healthy controls (120).
In interventional studies, vitamin D supplementation to those with vitamin D deficiency has been effective in addressing anxiety. In a study of 30 anxiety patients, once weekly vitamin D supplementation at 50,000 IU for 3 months significantly improved symptoms (123). A similar study in 51 women with type II diabetes also showed that 50,000 IU vitamin D fortnightly decreased inflammation and reduced symptoms of anxiety over 4 months (124).
It may be that vitamin D supplementation for anxiety is only effective in those with vitamin D insufficiency, with one association study finding elevated anxiety only on those with extreme vitamin D deficiency (<10 ng/mL) (125). Nonetheless, vitamin D insufficiency persists amongst Americans, sufficient vitamin D intake is difficult to obtain through diet or sun exposure, when living at higher latitudes, and adequate vitamin D levels are important to overall health. Therefore, vitamin D supplementation should be considered for patients with anxiety as most will be vitamin D insufficient and the collateral effects on patient health are likely to be positive.
Ketogenic diets—high-fat, low-carbohydrate diets that induce the body to produce ketones, a fuel source for the brain—are gaining traction as a metabolic treatment for a wide range of chronic metabolic diseases (126–131). Ketogenic diets have been used for a century to treat drug-resistant pediatric epilepsy, are still widely used for epilepsy (132, 133), and are gaining in popularity for the treatment of neurodegenerative conditions, such as Parkinson's disease (131, 134, 135) and Alzheimer's disease (128, 130, 136). For reviews specifically on the topic of ketogenic diets for neurological diseases and mental illnesses, we recommend the following recent reviews, both published this year, which cover the literature supporting the therapeutic implementation of ketogenic diets for a wide range of conditions including attention deficit hyperactivity disorder (ADHD), bipolar disorder, schizophrenia, autism spectrum disorder, major depressive disorder, and binge eating disorder (5, 137).
Ketogenic diets help to address many of the biopathological foundations of chronic neurological diseases and mental illnesses, including glucose hypometabolism, neurotransmitter imbalances, oxidative stress, and inflammation (5). Ketones produced by the liver during carbohydrate restriction are not only a more efficient fuel substrate for the brain, but are also signaling molecules that bind their own G-protein coupled receptors, inhibit HDACs, directly modify histones, shift the gut microbiome and improve gut barrier function, and reduce oxidative stress and inflammation (134, 138–142).
Preclinical models offer promise. Rats orally administered exogenous ketone supplements to achieve ketone levels comparable to those achieved by patients on ketogenic diets (~0.8 mmol/L) exhibited significant decreases in anxiety (143). Another rat study found that ketosis induced anti-anxiolytic changes in brain metabolism in association with a reduction of anxiety-like behaviors (144). There is also evidence that intermittent fasting, an intervention that induces ketosis, induces neurological adaptations overtime (including an upregulation of the mitochondrial sirtuin, SIRT3, and GABAergic activity) that and neuroprotective and reduce anxiety (145). No clinical trials assessing the efficacy of ketogenic diets for anxiety have yet been conducted. We include ketogenic diets as a potentially promising future option and to provide clinicians with a perspective on the emerging research on nutrition and mental health.
Additional nutritional strategies hold potential for the treatment of anxiety disorders, including caffeine reduction (146, 147), prebiotics (148, 149), and probiotics (150) to support the microbiome, and supplementation with or magnesium (151, 152) or tryptophan (153) to potentially increase serotonin synthesis.
Herein, we provide biological rationales and translational evidence that nutritional strategies aimed at addressing disturbances in metabolism and brain function can protect against anxiety disorders (Figure 1). Anxiety and other mental illnesses are metabolic diseases as much as they are psychological. And, in our opinions, metabolic diseases deserve metabolic medicine. Nutrition is one form of metabolic medicine, and one which patients and clinicians interact with every day. It is important to leverage this metabolic tool to better offer persons suffering with anxiety a full spectrum of relief.
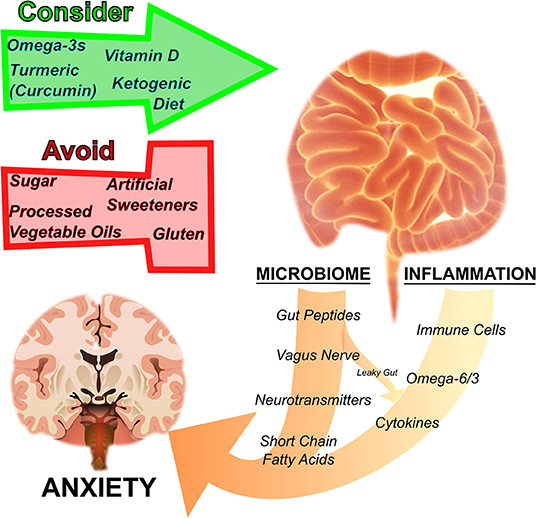
Figure 1. Nutrition regulates anxiety disorders by influencing the microbiome and inflammation. The gut microbiome and inflammation are interrelated and influence anxiety. By acting on the gut microbial ecosystem, regulating inflammation, as well as through other pathways mentioned in the text, particular nutritional strategies have been suggested to either harm or help disorders of anxiety. Sugar, processed vegetable oils rich in inflammatory omega-6 fatty acids, artificial sweeteners, and gluten have a negative effect on anxiety, whereas omega-3 fatty acids, turmeric (curcumin), vitamin D, and ketogenic diets are thought to have a therapeutic effect.
However, the clinical challenge of bioindividuality persists. Different patients are afflicted with different deficiencies and comorbidities. We each carry genetic polymorphisms and have distinct microbiomes. Therefore, future research should be focused on determining the mechanisms by which various interventions operate such that the medical community can turn nutritional psychiatry from a shotgun approach into precision personalized medicine.
In closing, we pose the question, “if patients needs to eat everyday anyway, why not turn a gustatory pleasure into an experimental one as well?”
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Both authors contributed to the work and approved it for publication.
NN's research at the University of Oxford is graciously funded by the Keasbey Memorial Foundation.
NN and UN each declare that they each stand to receive royalties from their respective books, “The New Mediterranean Diet Cookbook” and “This is Your Brain of Food”.
Advancements in Nutritional Psychiatry would not be possible without strong effort from patients. Both authors would like to thank those struggling with any mental illness for their resilience against these biologically based conditions. You make the field move forward.
AGEs, advanced glycation end products; ALA, alpha-linolenic acid; BDNF, brain-derived neurotrophic factor; CRP, C-reactive protein; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FFARs, free fatty acid receptors; GABA, gamma-aminobutyric acid; GLP-1, glucagon-like peptide 1; HDACs, histone deacetylases; IBDs, inflammatory bowel diseases; IBS, irritable bowel syndrome; IL, interleukin; LPS, lipopolysaccharide; MSFD2A, major facilitator superfamily domain-containing protein 2 A; NFκB, nuclear factor κ-light-chain enhancer of activated B cells; NLRP3, NOD-LRR-and pyrin domain-containing protein 3; NPY, neuropeptide Y; PP, pancreatic polypeptide; PTSD, post-traumatic stress disorder; SAD, Standard American Diet; SCFAs, short chain fatty acids; TNFα, tumor necrosis factor α.
1. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. (2015) 17:327–35. doi: 10.31887/DCNS.2015.17.3/bbandelow
2. Roy-Byrne P. Treatment-refractory anxiety; definition, risk factors, and treatment challenges. Dialogues Clin Neurosci. (2015) 17:191–206. doi: 10.31887/DCNS.2015.17.2/proybyrne
3. Blackburn TP. Depressive disorders: treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect. (2019) 7:e00472. doi: 10.1002/prp2.472
4. Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. (2014) 6:37. doi: 10.1186/alzrt269
5. Norwitz NG, Sethi SD, Palmer CM. Ketogenic diet as a metabolic treatment for mental illness. Curr Opin Endocrinol Diabetes Obes. (2020) 27:269–74. doi: 10.1097/MED.0000000000000564
6. Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety: relationship and cellular pathways. Oxid Med Cell Longev. (2009) 2:63–7. doi: 10.4161/oxim.2.2.7944
7. Norwitz NG, Mota AS, Norwitz SG, Clarke K. Multi-loop model of Alzheimer disease: an integrated perspective on the Wnt/GSK3beta, alpha-synuclein, and type 3 diabetes hypotheses. Front Aging Neurosci. (2019) 11:184. doi: 10.3389/fnagi.2019.00184
8. Firth J, Veronese N, Cotter J, Shivappa N, Hebert JR, Ee C, et al. What is the role of dietary inflammation in severe mental illness? A review of observational and experimental findings. Front Psychiatry. (2019) 10:350. doi: 10.3389/fpsyt.2019.00350
9. Jiang HY, Zhang X, Yu ZH, Zhang Z, Deng M, Zhao JH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. (2018) 104:130–6. doi: 10.1016/j.jpsychires.2018.07.007
10. Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The Microbiota-gut-brain axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
11. Lach G, Schellekens H, Dinan TG, Cryan JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. (2018) 15:36–59. doi: 10.1007/s13311-017-0585-0
12. Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. (2018) 9:44. doi: 10.3389/fpsyt.2018.00044
13. Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. (2016) 167:1125–36 e1128. doi: 10.1016/j.cell.2016.10.020
14. Engel K, Bandelow B, Gruber O, Wedekind D. Neuroimaging in anxiety disorders. J Neural Transm. (2009) 116:703–16. doi: 10.1007/s00702-008-0077-9
15. Hoban AE, Stilling RM, Moloney G, Shanahan F, Dinan TG, Clarke G, et al. The microbiome regulates amygdala-dependent fear recall. Mol Psychiatry. (2018) 23:1134–44. doi: 10.1038/mp.2017.100
16. Luczynski P, Whelan SO, O'Sullivan C, Clarke G, Shanahan F, Dinan TG, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. (2016) 44:2654–66. doi: 10.1111/ejn.13291
17. Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558(Pt. 1):263–75. doi: 10.1113/jphysiol.2004.063388
18. Anesten F, Dalmau Gasull A, Richard JE, Farkas I, Mishra D, Taing L, et al. Interleukin-6 in the central amygdala is bioactive and co-localised with glucagon-like peptide-1 receptor. J Neuroendocrinol. (2019) 31:e12722. doi: 10.1111/jne.12722
19. Farzi A, Reichmann F, Holzer P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol. (2015) 213:603–27. doi: 10.1111/apha.12445
20. Reichmann F, Holzer P. Neuropeptide Y: a stressful review. Neuropeptides. (2016) 55:99–109. doi: 10.1016/j.npep.2015.09.008
21. Painsipp E, Wultsch T, Edelsbrunner ME, Tasan RO, Singewald N, Herzog H, et al. Reduced anxiety-like and depression-related behavior in neuropeptide Y Y4 receptor knockout mice. Genes Brain Behav. (2008) 7:532–42. doi: 10.1111/j.1601-183X.2008.00389.x
22. Komsuoglu Celikyurt I, Mutlu O, Ulak G, Uyar E, Bektas E, Yildiz Akar F, et al. Exenatide treatment exerts anxiolytic- and antidepressant-like effects and reverses neuropathy in a mouse model of type-2 diabetes. Med Sci Monit Basic Res. (2014) 20:112–7. doi: 10.12659/MSMBR.891168
23. Sharma AN, Pise A, Sharma JN, Shukla P. Glucagon-like peptide-1 (GLP-1) receptor agonist prevents development of tolerance to anti-anxiety effect of ethanol and withdrawal-induced anxiety in rats. Metab Brain Dis. (2015) 30:719–30. doi: 10.1007/s11011-014-9627-z
24. Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. (2021). doi: 10.1038/s41591-020-01183-8. [Epub ahead of print].
25. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
26. Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. (2020) 11:25. doi: 10.3389/fendo.2020.00025
27. Sulakhiya K, Keshavlal GP, Bezbaruah BB, Dwivedi S, Gurjar SS, Munde N, et al. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett. (2016) 611:106–11. doi: 10.1016/j.neulet.2015.11.031
28. Yang TY, Jang EY, Ryu Y, Lee GW, Lee EB, Chang S, et al. Effect of acupuncture on Lipopolysaccharide-induced anxiety-like behavioral changes: involvement of serotonin system in dorsal Raphe nucleus. BMC Complement Altern Med. (2017) 17:528. doi: 10.1186/s12906-017-2039-y
29. Malan-Muller S, Valles-Colomer M, Raes J, Lowry CA, Seedat S, Hemmings SMJ. The gut microbiome and mental health: implications for anxiety- and trauma-related disorders. OMICS. (2018) 22:90–107. doi: 10.1089/omi.2017.0077
30. Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. (2018) 16:533–58. doi: 10.2174/1570159X15666171123201142
31. Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. (2009) 26:447–55. doi: 10.1002/da.20564
32. Bankier B, Barajas J, Martinez-Rumayor A, Januzzi JL. Association between C-reactive protein and generalized anxiety disorder in stable coronary heart disease patients. Eur Heart J. (2008) 29:2212–7. doi: 10.1093/eurheartj/ehn326
33. Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. (2017) 42:254–70. doi: 10.1038/npp.2016.146
34. Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, et al. Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation. (2001) 9:209–17. doi: 10.1159/000049028
35. Bersani FS, Wolkowitz OM, Lindqvist D, Yehuda R, Flory J, Bierer LM, et al. Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun. (2016) 52:153–60. doi: 10.1016/j.bbi.2015.10.015
36. Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry. (2015) 172:353–62. doi: 10.1176/appi.ajp.2014.14020263
37. Oganesyan LP, Mkrtchyan GM, Sukiasyan SH, Boyajyan AS. Classic and alternative complement cascades in post-traumatic stress disorder. Bull Exp Biol Med. (2009) 148:859–61. doi: 10.1007/s10517-010-0836-0
38. von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. (2007) 41:744–52. doi: 10.1016/j.jpsychires.2006.06.009
39. Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araujo-Lima CF, et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol. (2010) 229:212–8. doi: 10.1016/j.jneuroim.2010.07.018
40. Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. (2012) 59:3222–6. doi: 10.1016/j.neuroimage.2011.10.090
41. Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab. (2017) 6:897–908. doi: 10.1016/j.molmet.2017.06.008
42. Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, et al. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. (2018) 27:351–61 e353. doi: 10.1016/j.cmet.2017.12.016
43. Softic S, Cohen DE, Kahn CR. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig Dis Sci. (2016) 61:1282–93. doi: 10.1007/s10620-016-4054-0
44. Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. (1993) 328:238–44. doi: 10.1056/NEJM199301283280404
45. Guyenet SJ, Carlson SE. Increase in adipose tissue linoleic acid of US adults in the last half century. Adv Nutr. (2015) 6:660–4. doi: 10.3945/an.115.009944
46. Lopez-Candales A, Hernandez Burgos PM, Hernandez-Suarez DF, Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci. (2017) 3:e341.
47. DiNicolantonio JJ, O'Keefe JH. Omega-6 vegetable oils as a driver of coronary heart disease: the oxidized linoleic acid hypothesis. Open Heart. (2018) 5:e000898. doi: 10.1136/openhrt-2018-000898
48. Easton K, Coventry P, Lovell K, Carter LA, Deaton C. Prevalence and measurement of anxiety in samples of patients with heart failure: meta-analysis. J Cardiovasc Nurs. (2016) 31:367–79. doi: 10.1097/JCN.0000000000000265
49. Grace SL, Abbey SE, Irvine J, Shnek ZM, Stewart DE. Prospective examination of anxiety persistence and its relationship to cardiac symptoms and recurrent cardiac events. Psychother Psychosom. (2004) 73:344–52. doi: 10.1159/000080387
50. Ashok I, Sheeladevi R, Wankhar D. Effect of long-term aspartame (artificial sweetener) on anxiety, locomotor activity and emotionality behavior in Wistar Albino rats. Biomed Prev Nutr. (2014) 4:39–43. doi: 10.1016/j.bionut.2013.04.002
51. Bian X, Chi L, Gao B, Tu P, Ru H, Lu K. Gut microbiome response to sucralose and its potential role in inducing liver inflammation in mice. Front Physiol. (2017) 8:487. doi: 10.3389/fphys.2017.00487
52. Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. (2014) 514:181–6. doi: 10.1038/nature13793
53. Yokogoshi H, Wurtman RJ. Acute effects of oral or parenteral aspartame on catecholamine metabolism in various regions of rat brain. J Nutr. (1986) 116:356–64. doi: 10.1093/jn/116.3.356
54. Rycerz K, Jaworska-Adamu JE. Effects of aspartame metabolites on astrocytes and neurons. Folia Neuropathol. (2013) 51:10–7. doi: 10.5114/fn.2013.34191
55. Choudhary AK, Lee YY. Neurophysiological symptoms and aspartame: what is the connection? Nutr Neurosci. (2018) 21:306–16. doi: 10.1080/1028415X.2017.1288340
56. Walton RG, Hudak R, Green-Waite RJ. Adverse reactions to aspartame: double-blind challenge in patients from a vulnerable population. Biol Psychiatry. (1993) 34:13–7. doi: 10.1016/0006-3223(93)90251-8
57. Zhou Y, Cao Z, Yang M, Xi X, Guo Y, Fang M, et al. Comorbid generalized anxiety disorder and its association with quality of life in patients with major depressive disorder. Sci Rep. (2017) 7:40511. doi: 10.1038/srep40511
58. Arrigoni E, Brouns F, Amado R. Human gut microbiota does not ferment erythritol. Br J Nutr. (2005) 94:643–6. doi: 10.1079/BJN20051546
59. Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, et al. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. (2003) 52:218–23. doi: 10.1136/gut.52.2.218
60. Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann N Y Acad Sci. (2012) 1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x
61. Addolorato G, Mirijello A, D'Angelo C, Leggio L, Ferrulli A, Vonghia L, et al. Social phobia in coeliac disease. Scand J Gastroenterol. (2008) 43:410–5. doi: 10.1080/00365520701768802
62. Carta MG, Hardoy MC, Boi MF, Mariotti S, Carpiniello B, Usai P. Association between panic disorder, major depressive disorder and celiac disease: a possible role of thyroid autoimmunity. J Psychosom Res. (2002) 53:789–93. doi: 10.1016/S0022-3999(02)00328-8
63. Jackson JR, Eaton WW, Cascella NG, Fasano A, Kelly DL. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatr Q. (2012) 83:91–102. doi: 10.1007/s11126-011-9186-y
64. Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. (2020) 9:F1000 Faculty Rev-69. doi: 10.12688/f1000research.20510.1
65. Stevens BR, Goel R, Seungbum K, Richards EM, Holbert RC, Pepine CJ, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut. (2018) 67:1555–7. doi: 10.1136/gutjnl-2017-314759
66. Addolorato G, Capristo E, Ghittoni G, Valeri C, Masciana R, Ancona C, et al. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: a longitudinal study. Scand J Gastroenterol. (2001) 36:502–6. doi: 10.1080/00365520119754
67. Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, et al. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep. (2017) 7:11079. doi: 10.1038/s41598-017-10382-2
68. Parolini C. Effects of fish n-3 PUFAs on intestinal microbiota and immune system. Mar Drugs. (2019) 17:374. doi: 10.3390/md17060374
69. Lauritzen L, Brambilla P, Mazzocchi A, Harslof LB, Ciappolino V, Agostoni C. DHA Effects in brain development and function. Nutrients. (2016) 8:6. doi: 10.3390/nu8010006
70. Weiser MJ, Butt CM, Mohajeri MH. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients. (2016) 8:99. doi: 10.3390/nu8020099
71. Kaliannan K, Li XY, Wang B, Pan Q, Chen CY, Hao L, et al. Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun Biol. (2019) 2:276. doi: 10.1038/s42003-019-0521-4
72. Song C, Li X, Kang Z, Kadotomi Y. Omega-3 fatty acid ethyl-eicosapentaenoate attenuates IL-1beta-induced changes in dopamine and metabolites in the shell of the nucleus accumbens: involved with PLA2 activity and corticosterone secretion. Neuropsychopharmacology. (2007) 32:736–44. doi: 10.1038/sj.npp.1301117
73. Song C, Li X, Leonard BE, Horrobin DF. Effects of dietary n-3 or n-6 fatty acids on interleukin-1beta-induced anxiety, stress, and inflammatory responses in rats. J Lipid Res. (2003) 44:1984–91. doi: 10.1194/jlr.M300217-JLR200
74. Zemdegs J, Rainer Q, Grossmann CP, Rousseau-Ralliard D, Grynberg A, Ribeiro E, et al. Anxiolytic- and antidepressant-like effects of fish oil-enriched diet in brain-derived neurotrophic factor deficient mice. Front Neurosci. (2018) 12:974. doi: 10.3389/fnins.2018.00974
75. Green P, Hermesh H, Monselise A, Marom S, Presburger G, Weizman A. Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder. Eur Neuropsychopharmacol. (2006) 16:107–13. doi: 10.1016/j.euroneuro.2005.07.005
76. Liu JJ, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, Mann JJ, et al. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. J Clin Psychiatry. (2013) 74:732–8. doi: 10.4088/JCP.12m07970
77. Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. (2011) 25:1725–34. doi: 10.1016/j.bbi.2011.07.229
78. Su KP, Tseng PT, Lin PY, Okubo R, Chen TY, Chen YW, et al. Association of use of omega-3 polyunsaturated fatty acids with changes in severity of anxiety symptoms: a systematic review and meta-analysis. JAMA Netw Open. (2018) 1:e182327. doi: 10.1001/jamanetworkopen.2018.2327
79. Anderson BM, Ma DW. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. (2009) 8:33. doi: 10.1186/1476-511X-8-33
80. Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care. (2002) 5:127–32. doi: 10.1097/00075197-200203000-00002
81. Brenna JT, Salem N Jr, Sinclair AJ, Cunnane SC, International Society for the Study of Fatty, Acide and Lipids. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids. (2009) 80:85–91. doi: 10.1016/j.plefa.2009.01.004
82. Barros MP, Poppe SC, Bondan EF. Neuroprotective properties of the marine carotenoid astaxanthin and omega-3 fatty acids, and perspectives for the natural combination of both in krill oil. Nutrients. (2014) 6:1293–317. doi: 10.3390/nu6031293
83. Patrick RP. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer's disease. FASEB J. (2019) 33:1554–64. doi: 10.1096/fj.201801412R
84. Fusar-Poli L, Vozza L, Gabbiadini A, Vanella A, Concas I, Tinacci S, et al. Curcumin for depression: a meta-analysis. Crit Rev Food Sci Nutr. (2019) 60:2643–53. doi: 10.1080/10408398.2019.1653260
85. Zhou H, Beevers CS, Huang S. The targets of curcumin. Curr Drug Targets. (2011) 12:332–47. doi: 10.2174/138945011794815356
86. Di Meo F, Margarucci S, Galderisi U, Crispi S, Peluso G. Curcumin, gut microbiota, and neuroprotection. Nutrients. (2019) 11:2426. doi: 10.3390/nu11102426
87. Ambegaokar SS, Wu L, Alamshahi K, Lau J, Jazayeri L, Chan S, et al. Curcumin inhibits dose-dependently and time-dependently neuroglial cell proliferation and growth. Neuro Endocrinol Lett. (2003) 24:469–73.
88. Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, Pinna F, Lee YH, Kitade M, et al. Curcumin effectively inhibits oncogenic NF-kappaB signaling and restrains stemness features in liver cancer. J Hepatol. (2015) 63:661–9. doi: 10.1016/j.jhep.2015.04.018
89. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: implications for the treatment of osteoarthritis. Biochem Pharmacol. (2007) 73:1434–45. doi: 10.1016/j.bcp.2007.01.005
90. Yin H, Guo Q, Li X, Tang T, Li C, Wang H, et al. Curcumin Suppresses IL-1beta secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J Immunol. (2018) 200:2835–46. doi: 10.4049/jimmunol.1701495
91. Xia X, Cheng G, Pan Y, Xia ZH, Kong LD. Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J Ethnopharmacol. (2007) 110:356–63. doi: 10.1016/j.jep.2006.09.042
92. Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. (2011) 6:93–108. doi: 10.1007/s12263-011-0222-1
93. Noorafshan A, Vafabin M, Karbalay-Doust S, Asadi-Golshan R. Efficacy of curcumin in the modulation of anxiety provoked by sulfite, a food preservative, in rats. Prev Nutr Food Sci. (2017) 22:144–8. doi: 10.3746/pnf.2017.22.2.144
94. Benammi H, El Hiba O, Romane A, Gamrani H. A blunted anxiolytic like effect of curcumin against acute lead induced anxiety in rat: involvement of serotonin. Acta Histochem. (2014) 116:920–5. doi: 10.1016/j.acthis.2014.03.002
95. Lee B, Lee H. Systemic administration of curcumin affect anxiety-related behaviors in a rat model of posttraumatic stress disorder via activation of serotonergic systems. Evid Based Complement Alternat Med. (2018) 2018:9041309. doi: 10.1155/2018/9041309
96. Asadi S, Gholami MS, Siassi F, Qorbani M, Sotoudeh G. Beneficial effects of nano-curcumin supplement on depression and anxiety in diabetic patients with peripheral neuropathy: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res. (2020) 34:896–903. doi: 10.1002/ptr.6571
97. Esmaily H, Sahebkar A, Iranshahi M, Ganjali S, Mohammadi A, Ferns G, et al. An investigation of the effects of curcumin on anxiety and depression in obese individuals: a randomized controlled trial. Chin J Integr Med. (2015) 21:332–8. doi: 10.1007/s11655-015-2160-z
98. Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem. (2017) 60:1620–37. doi: 10.1021/acs.jmedchem.6b00975
99. Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. (2007) 853:183–9. doi: 10.1016/j.jchromb.2007.03.010
100. Sun M, Su X, Ding B, He X, Liu X, Yu A, et al. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine. (2012) 7:1085–100. doi: 10.2217/nnm.12.80
101. Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. (1998) 64:353–6. doi: 10.1055/s-2006-957450
102. Wu A, Noble EE, Tyagi E, Ying Z, Zhuang Y, Gomez-Pinilla F. Curcumin boosts DHA in the brain: implications for the prevention of anxiety disorders. Biochim Biophys Acta. (2015) 1852:951–61. doi: 10.1016/j.bbadis.2014.12.005
103. Schmid A, Walther B. Natural vitamin D content in animal products. Adv Nutr. (2013) 4:453–62. doi: 10.3945/an.113.003780
104. Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population. 1988-2004. Arch Intern Med. (2009) 169:626–32. doi: 10.1001/archinternmed.2008.604
105. Annweiler C. Vitamin D in dementia prevention. Ann N Y Acad Sci. (2016) 1367:57–63. doi: 10.1111/nyas.13058
106. Brewer LD, Thibault V, Chen KC, Langub MC, Landfield PW, Porter NM. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. (2001) 21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001
107. Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care. (2007) 10:12–9. doi: 10.1097/MCO.0b013e328010ca18
108. Kesby JP, Turner KM, Alexander S, Eyles DW, McGrath JJ, Burne THJ. Developmental vitamin D deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Int J Dev Neurosci. (2017) 62:1–7. doi: 10.1016/j.ijdevneu.2017.07.002
109. Lima LAR, Lopes MJP, Costa RO, Lima FAV, Neves KRT, Calou IBF, et al. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J Neuroinflammation. (2018) 15:249. doi: 10.1186/s12974-018-1266-6
110. Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. (2015) 29:2207–22. doi: 10.1096/fj.14-268342
111. Gezen-Ak D, Dursun E, Yilmazer S. The effect of vitamin D treatment on Nerve Growth Factor (NGF) release from hippocampal neurons. Noro Psikiyatr Ars. (2014) 51:157–62. doi: 10.4274/npa.y7076
112. Khairy EY, Attia MM. Protective effects of vitamin D on neurophysiologic alterations in brain aging: role of brain-derived neurotrophic factor (BDNF). Nutr Neurosci. (2019) 16:1–10. doi: 10.1080/1028415X.2019.1665854
113. Aranow C. Vitamin D and the immune system. J Investig Med. (2011) 59:881–6. doi: 10.2310/JIM.0b013e31821b8755
114. Gominak SC. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med Hypotheses. (2016) 94:103–7. doi: 10.1016/j.mehy.2016.07.007
115. Tabatabaeizadeh SA, Tafazoli N, Ferns GA, Avan A, Ghayour-Mobarhan M. Vitamin D, the gut microbiome and inflammatory bowel disease. J Res Med Sci. (2018) 23:75. doi: 10.4103/jrms.JRMS_606_17
116. Yamamoto EA, Jorgensen TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front Immunol. (2019) 10:3141. doi: 10.3389/fimmu.2019.03141
117. Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. (2014) 7:69–87. doi: 10.2147/JIR.S63898
118. Cieslak K, Feingold J, Antonius D, Walsh-Messinger J, Dracxler R, Rosedale M, et al. Low vitamin D levels predict clinical features of schizophrenia. Schizophr Res. (2014) 159:543–5. doi: 10.1016/j.schres.2014.08.031
119. Maddock J, Berry DJ, Geoffroy MC, Power C, Hypponen E. Vitamin D and common mental disorders in mid-life: cross-sectional and prospective findings. Clin Nutr. (2013) 32:758–64. doi: 10.1016/j.clnu.2013.01.006
120. Bicikova M, Duskova M, Vitku J, Kalvachova B, Ripova D, Mohr P, et al. Vitamin D in anxiety and affective disorders. Physiol Res. (2015) 64:S101–103. doi: 10.33549/physiolres.933082
121. Han B, Zhu FX, Yu HF, Liu S, Zhou JL. Low serum levels of vitamin D are associated with anxiety in children and adolescents with dialysis. Sci Rep. (2018) 8:5956. doi: 10.1038/s41598-018-24451-7
122. Wu C, Ren W, Cheng J, Zhu B, Jin Q, Wang L, et al. Association between serum levels of vitamin D and the risk of post-stroke anxiety. Medicine. (2016) 95:e3566. doi: 10.1097/MD.0000000000003566
123. Eid A, Khoja S, AlGhamdi S, Alsufiani H, Alzeben F, Alhejaili N, et al. Vitamin D supplementation ameliorates severity of generalized anxiety disorder (GAD). Metab Brain Dis. (2019) 34:1781–6. doi: 10.1007/s11011-019-00486-1
124. Fazelian S, Amani R, Paknahad Z, Kheiri S, Khajehali L. Effect of vitamin D supplement on mood status and inflammation in vitamin D deficient type 2 diabetic women with anxiety: a randomized clinical trial. Int J Prev Med. (2019) 10:17. doi: 10.4103/ijpvm.IJPVM_174_18
125. Armstrong DJ, Meenagh GK, Bickle I, Lee AS, Curran ES, Finch MB. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. (2007) 26:551–4. doi: 10.1007/s10067-006-0348-5
126. Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year non-randomized clinical trial. Front Endocrinol. (2019) 10:348. doi: 10.3389/fendo.2019.00348
127. Cunnane SC, Courchesne-Loyer A, Vandenberghe C, St-Pierre V, Fortier M, Hennebelle M, et al. Can ketones help rescue brain fuel supply in later life? Implications for cognitive health during aging and the treatment of Alzheimer's disease. Front Mol Neurosci. (2016) 9:53. doi: 10.3389/fnmol.2016.00053
128. Fortier M, Castellano CA, Croteau E, Langlois F, Bocti C, St-Pierre V, et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers Dement. (2019) 15:625–34. doi: 10.1016/j.jalz.2018.12.017
129. Hyde PN, Sapper TN, Crabtree CD, LaFountain RA, Bowling ML, Buga A, et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight. (2019) 4:e128308. doi: 10.1172/jci.insight.128308
130. Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer's disease. Alzheimers Dement. (2015) 11:99–103. doi: 10.1016/j.jalz.2014.01.006
131. Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord. (2018) 33:1306–14. doi: 10.1002/mds.27390
132. Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. (2012). 2:CD001903. doi: 10.1002/14651858.CD001903.pub2
133. Liu H, Yang Y, Wang Y, Tang H, Zhang F, Zhang Y, et al. Ketogenic diet for treatment of intractable epilepsy in adults: a meta-analysis of observational studies. Epilepsia Open. (2018) 3:9–17. doi: 10.1002/epi4.12098
134. Norwitz NG, Hu MT, Clarke K. The mechanisms by which the ketone body D-beta-hydroxybutyrate may improve the multiple cellular pathologies of Parkinson's disease. Front Nutr. (2019) 6:63. doi: 10.3389/fnut.2019.00063
135. Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology. (2005) 64:728–30. doi: 10.1212/01.WNL.0000152046.11390.45
136. Rusek M, Pluta R, Ulamek-Koziol M, Czuczwar SJ. Ketogenic diet in Alzheimer's disease. Int J Mol Sci. (2019) 20:3892. doi: 10.3390/ijms20163892
137. Kraeuter A-K, Phillips R, Sarnyai Z. Ketogenic therapy in neurodegenerative and psychiatric disorders: from mice to men. Prog Neuro Psychopharmacol Biol Psychiatry. (2020) 101:109913. doi: 10.1016/j.pnpbp.2020.109913
138. Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. (2019) 178:1115–31 e1115. doi: 10.1016/j.cell.2019.07.048
139. Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. (2008) 43:65–77. doi: 10.1007/s11745-007-3132-7
140. Lindefeldt M, Eng A, Darban H, Bjerkner A, Zetterstrom CK, Allander T, et al. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. NPJ Biofilms Microbiomes. (2019) 5:5. doi: 10.1038/s41522-018-0073-2
141. Xie Z, Zhang D, Chung D, Tang Z, Huang H, Dai L, et al. Metabolic regulation of gene expression by histone lysine beta-hydroxybutyrylation. Mol Cell. (2016) 62:194–206. doi: 10.1016/j.molcel.2016.03.036
142. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. (2015) 21:263–9. doi: 10.1038/nm.3804
143. Ari C, Kovacs Z, Juhasz G, Murdun C, Goldhagen CR, Koutnik AP, et al. Exogenous ketone supplements reduce anxiety-related behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk rats. Front Mol Neurosci. (2016) 9:137. doi: 10.3389/fnmol.2016.00137
144. Hollis F, Mitchell ES, Canto C, Wang D, Sandi C. Medium chain triglyceride diet reduces anxiety-like behaviors and enhances social competitiveness in rats. Neuropharmacology. (2018) 138:245–56. doi: 10.1016/j.neuropharm.2018.06.017
145. Liu Y, Cheng A, Li YJ, Yang Y, Kishimoto Y, Zhang S, et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat Commun. (2019) 10:1886. doi: 10.1038/s41467-019-09897-1
146. Smith JE, Lawrence AD, Diukova A, Wise RG, Rogers PJ. Storm in a coffee cup: caffeine modifies brain activation to social signals of threat. Soc Cogn Affect Neurosci. (2012) 7:831–40. doi: 10.1093/scan/nsr058
147. Wikoff D, Welsh BT, Henderson R, Brorby GP, Britt J, Myers E, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxicol. (2017) 109(Pt. 1):585–648. doi: 10.1016/j.fct.2017.04.002
148. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. (2013) 36:305–12. doi: 10.1016/j.tins.2013.01.005
149. Taylor AM, Holscher HD. A review of dietary and microbial connections to depression, anxiety, and stress. Nutr Neurosci. (2020) 23:237–50. doi: 10.1080/1028415X.2018.1493808
150. Selhub EM, Logan AC, Bested AC. Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol. (2014) 33:2. doi: 10.1186/1880-6805-33-2
151. Boyle NB, Lawton C, Dye L. The effects of magnesium supplementation on subjective anxiety and stress-a systematic review. Nutrients. (2017) 9:429. doi: 10.3390/nu9050429
152. Grases G, Perez-Castello JA, Sanchis P, Casero A, Perello J, Isern B, et al. Anxiety and stress among science students. Study of calcium and magnesium alterations. Magnes Res. (2006) 19:102–6.
Keywords: anxiety, inflammation, microbiome, nutrition, mental illness
Citation: Norwitz NG and Naidoo U (2021) Nutrition as Metabolic Treatment for Anxiety. Front. Psychiatry 12:598119. doi: 10.3389/fpsyt.2021.598119
Received: 23 August 2020; Accepted: 20 January 2021;
Published: 12 February 2021.
Edited by:
Mario F. Juruena, King's College London, United KingdomReviewed by:
Chien-Han Lai, National Yang-Ming University, TaiwanCopyright © 2021 Norwitz and Naidoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas G. Norwitz, bndpdHo5OUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.