- 1Division of Colorectal Surgery, Department of surgery, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
- 2Graduate Institute of Medical Sciences, National Defense Medical Center, Taipei, Taiwan
- 3Medical Informatics Office, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 4Artificial Intelligence Center, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 5School of Public Health, National Defense Medical Center, Taipei, Taiwan
- 6Department of Medical Research, Tri-Service General Hospital, National Defense Medical Center, Taipei, Taiwan
- 7Taiwanese Injury Prevention and Safety Promotion Association, Taipei, Taiwan
- 8Big Data Research Center, College of Medicine, Fu-Jen Catholic University, New Taipei City, Taiwan
- 9Department of Public Health, College of Medicine, Fu-Jen Catholic University, New Taipei City, Taiwan
- 10Department of Psychiatry, Tri-Service General Hospital, School of Medicine, National Defense Medical Center, Taipei, Taiwan
- 11Student Counseling Center, National Defense Medical Center, Taipei, Taiwan
- 12Graduate Institute of Life Sciences, National Defense Medical Center, Taipei, Taiwan
Background: The association between attention-deficit hypersensitivity disorder (ADHD) and the risk of developing colorectal cancer (CRC) is, as yet, to be investigated, and thus, we have conducted this nationwide, cohort study to examine the association in patients from Taiwan.
Methods: In this study, 798 individuals with newly diagnosed ADHD and 2,394 (1:3) age-, gender-, and index year- matched controls without ADHD were enrolled, between 2000 and 2013, from the Longitudinal Health Insurance Database, a subset of the National Health Insurance Research Database in Taiwan. The cumulative incidence of CRC was assessed in each cohort by the Kaplan–Meier method. The multivariate Cox proportional hazards model was used to estimate the crude, and the adjusted hazards ratios (HRs) with 95% confidence intervals (CIs), was conducted to estimate the association between ADHD and CRC.
Results: The Kaplan–Meier analysis revealed that the cumulative incidence of CRC was significantly higher in patients with ADHD than in those without it (log rank test, p < 0.001). After adjustments for age, gender, comorbidities, and other covariates, the ADHD group was associated with an increased risk of CRC in comparison to the non-ADHD group (adjusted HR = 3.458, 95% CI = 1.640–7.293, p < 0.001). In addition, the usage of methylphenidate was not associated with the risk of developing CRC in patients with ADHD.
Conclusion: This retrospective cohort study depicts the evidence that ADHD was associated with the increased risk of CRC. Further studies are needed to confirm the association and the underlying mechanisms.
Introduction
Colorectal cancer (CRC) is a major health challenge, representing the most common cancer and the third most frequent cause of cancer-related deaths in Taiwan (5,698 estimated deaths in 2012) (1). Several risk factors for CRC have been identified, such as smoking, dietary fat intake, obesity, and physical inactivity, and some studies have confirmed an excess of alcohol consumption may be a risk factor for a variety of cancers at the colorectal system (2). In addition, the average lifetime alcohol intakes were linearly associated with the risk of cancer and cancer-related death (3). Some recent evidence has suggested that specific gut microbiota contributes to the dysbiosis, mucosal integrity, immune deregulation, and immune-inflammatory alterations, and may contribute to the carcinogenesis (4). However, the mechanisms in the carcinogenesis of CRC are not, as yet, completely understood.
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common pediatric neurodevelopmental and neurobehavioral disorders with a worldwide average prevalence estimated at 5% in children and 3.4–4.4% in adults, which results in attention deficit, hyperactivity, and increased impulsivity (5, 6), and up to 78% of ADHD patients have persisting symptoms into their adulthood (7). ADHD affects the health-related quality of life and leads to considerable school absences, family stress, and health care expenses (8–10). In addition, several studies have found that patients with ADHD are at risk of health problems, such as alcohol abuse (11, 12), cigarette smoking (13–15), and injury (16–18). Therefore, the identification, treatment, and management of ADHD are important and challenging in both children and adults. Psychostimulants are the first-line pharmacological treatment choice and have shown beneficial short-term efficacy (19). In addition, non-pharmacological treatments, such as parent training (20), behavioral interventions (20, 21), and neuro-feedback (22), could also be effective. However, several researchers have depicted that the varied relations between the ADHD medication methylphenidate (MPH) and cancer in human (23, 24) and animal studies (19, 25). However, the association between ADHD itself, with or without methylphenidate usages, and the risk of the development of cancer, is yet to be studied.
ADHD have bidirectional relations (26), for example, the increased gut microbiome predicts the function of the dopamine precursor synthesis between the ADHD cases and the controls (27), and the microbiota-gut-brain axis might play a role in the complex network of communication between the gut, intestinal microbiota, and the brain, by modulating the immune, gastrointestinal, and central nervous system functions (28). As aforementioned, gradual gut microbiota alterations are also linked to CRC in the initiation and progression during colorectal carcinogenesis (29, 30). Therefore, we hypothesize that there might be a link between ADHD and CRC, and we have thus conducted an explorative study to investigate the association between ADHD and CRC, by using a nationwide, population-based claims database, being Taiwan's National Health Insurance Research Database (NHIRD).
Methods
Data Source
The NHIRD was established in 1995, and as of June 2009, included contracts with 97% of the medical providers with ~23 million people in the program, or more than 99% of the entire population in Taiwan (31). The details of the program have been documented in previous studies (32–43). It contains comprehensive information, including the demographic data, inpatient and ambulatory claims, prescriptions, surgery, and other medical procedures claim records. The NHIRD, with multiple data sources, could represent a powerful research engine with enriched dimensions, and thereby serve as a guiding light for real-world evidence-based medicine in Taiwan (44). Diseases in the database are defined according to the International Classification of Disease, 9th Revision (ICD-9) codes.
Study Subjects
This was a retrospective cohort study. Patients with ADHD between January 2000, and December 2013, were selected from the LHID and categorized according to the ICD as 314. All diagnoses of ADHD were made by board-certified specialists such as psychiatrists, pediatricians, neurologists, or physiatrists with specialty in child and adolescent development. The subject selection process is as presented in Figure 1. We also enrolled a 1:3 age-, gender-, and index year- matched control group of patients from the NHIRD, without a diagnosis of ADHD throughout the study period, as the unexposed group. We excluded patients who were younger than 18 years, data with unknown gender, drug abuse, and those diagnosed with cancer before the beginning of the follow-up from January 1, 2000.
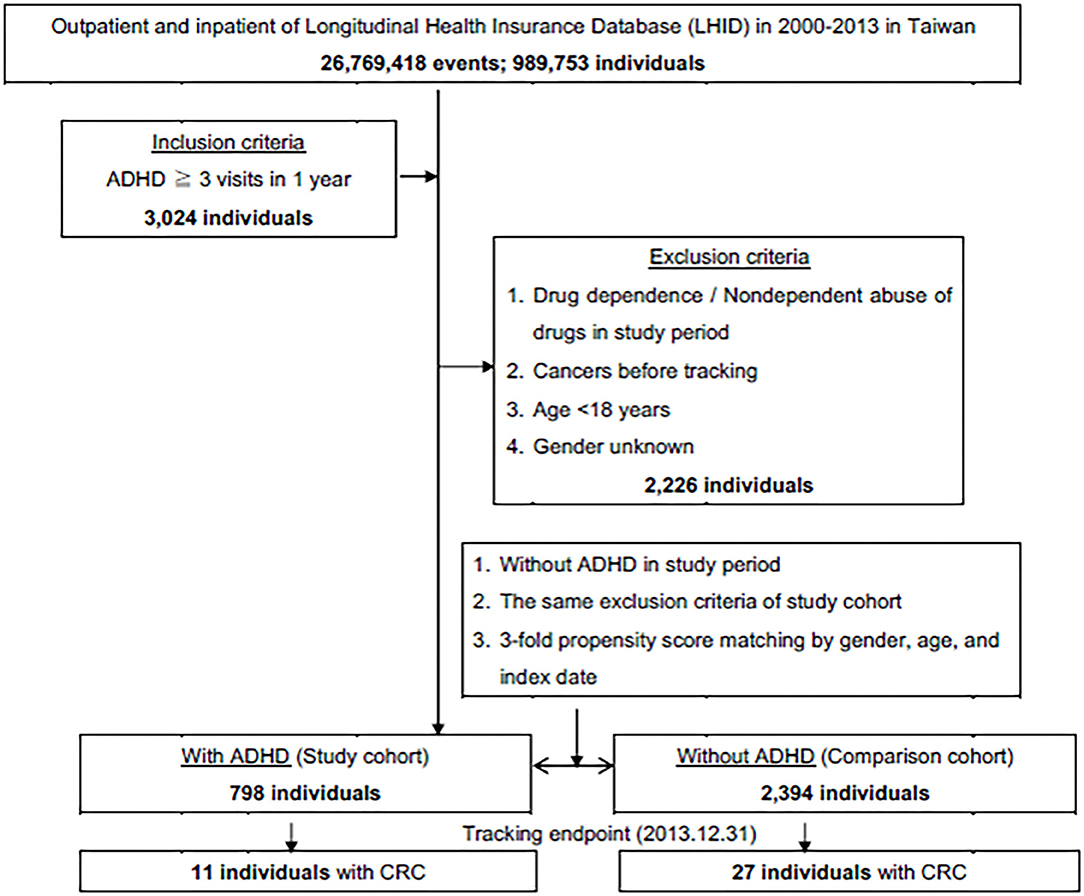
Figure 1. The flowchart of study sample selection from National Health Insurance Research Database in Taiwan.
Outcome
We followed up both cohorts from January 1, 2000, until the date of CRC diagnosis (ICD-9-CM codes: 153, 153.0, 153.1, 153.2, 153.3, 153.6, 153.7, 153.8, 153.9,154, 154.0, 154.1, 154.2, 154.3, 154.8, and 159.0), withdrawal from the NHI, or the end of 2013.
Covariates
The covariates included the age, gender, monthly insured premiums, comorbidities, locations, urbanization levels of residence, and the levels of hospitals for medical help. In the analysis, since the north Taiwan is the center of politics and economics, in the country. In addition, most of the healthcare resources, for example, 12 medical centers among total 23, located in the northern Taiwan. Therefore, the northern Taiwan was listed as the reference in our study for the locations.
Comorbidities
We noticed the covariates that were potential confounders in the association between ADHD and CRC including age, gender, and the underlying chronic diseases related to the risk of developing CRC. Those chronic diseases, which were taken into account, included chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes: 490, 491, 492, 493, 494, 495, and 496), diabetes mellitus (DM) (ICD-9-CM code: 250), coronary artery disease (CAD, ICD-9-CM codes: 410, 411, 412, 413, and 414), hypertension (HTN, ICD-9-CM codes:401, 402, 403, 404, and 405), hypercholesterolemia (ICD-9-CM codes: 272.0, 272.1, 272.2, and 272.4), alcohol-related diseases (ARD, ICD-9-CM codes: alcoholic liver disease, 571.0, 571.1, 571.2, and 571.3, and alcohol dependence, 303), peptic ulcer (ICD-9-CM codes: 531, 532, and 533); liver cirrhosis and chronic hepatitis (ICD-9-CM code: 571), inflammatory bowel disease (ICD-9-CM codes: 555 and 556), and psychiatric comorbidities such as oppositional defiant disorder (ODD, ICD-9-CM code: 313.81), conduct disorder (CD, ICD-9-CM code: 312), autism spectrum disorder (ASD, ICD-9-CM code: 299); tic disorder (ICD-9-CM code: 307.2); intellectual disabilities (ICD-9-CM codes: 317, 318, and 319); anxiety (ICD-9-CM code: 300); depression (ICD-9-CM codes: 296.2, 296.3, 300.4, and 311); and bipolar disorder (ICD-9-CM code: 296.0, 296.4-296.8). All the diagnosis of the psychiatric disorders was conducted by the board-certified psychiatrists, pediatricians, neurologists, and physiatrists.
Statistical Analysis
We examined the descriptive statistics of the demographic characteristics and baseline comorbidities between the exposed and non-exposed cohorts by conducting chi-square tests or Student's-t-tests when appropriate. In addition, we used the Kaplan-Meier method to estimate the cumulative incidence of CRC in the study cohorts and performed the log-rank test to compare the difference between these two curves. We computed the hazard ratios (HRs) presented together with 95% confidence intervals (CIs) using the Cox proportional hazards models after adjusting for the potential confounders mentioned above. All the confounders, as covariates and comorbidities, including the psychiatric diagnoses, were calculated separately. All analyses were performed using the SAS version 9.4 (SAS Institute, Cary, NC) and the statistical significance was set to 0.05 in the 2-tailed tests.
Results
Sample Characteristics
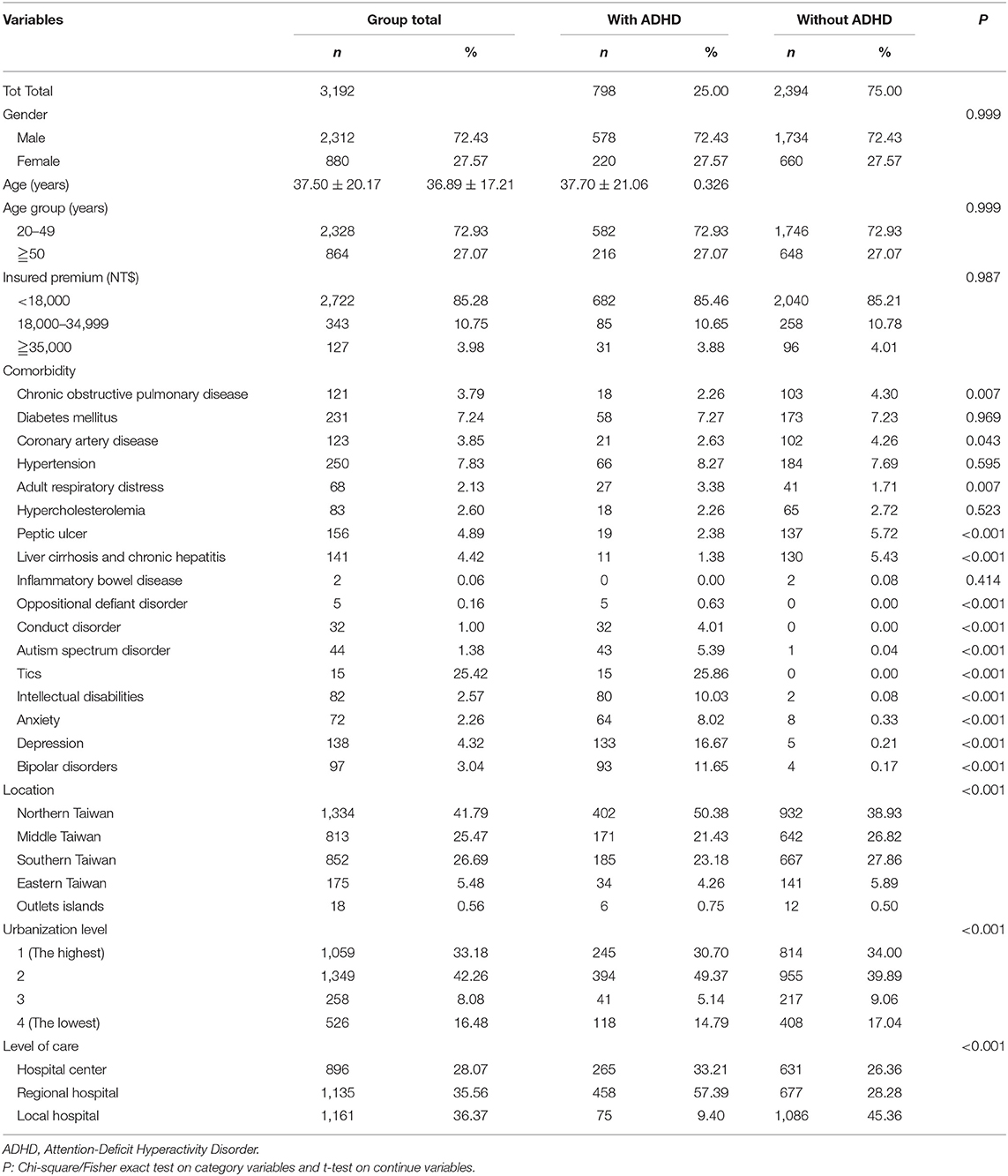
A total of 3,192 patients were enrolled in this study, including 798 adult patients with ADHD and 2,394 patients in the non-ADHD control cohort. The age, gender, monthly insured premiums, comorbidities, locations and urbanization levels of residence, and the levels of hospitals for medical help are as summarized in Table 1. Most of the patients were in the age group of 20–49. The ADHD group tended to have higher rates of DM. HIN, ARD, and psychiatric comorbidities, such as ODD, CD, ASD, tics, intellectual disabilities, anxiety, depression, and bipolar disorders. In addition, the ADHD group tended to live in the North and the outlet islands of Taiwan, urbanization level 2, and seek their medical help from the regional hospitals.
Kaplan-Meier Model for the Cumulative Incidence of CRC
The Kaplan-Meier analysis for the cumulative incidence of CRC in the ADHD and non-ADHD cohorts with the log-rank test revealed a significant difference over the 13-year follow-up period (p < 0.001) (Figure 2). In the 10th year of the study, the difference between the groups became significant (log-rank test p = 0.03).
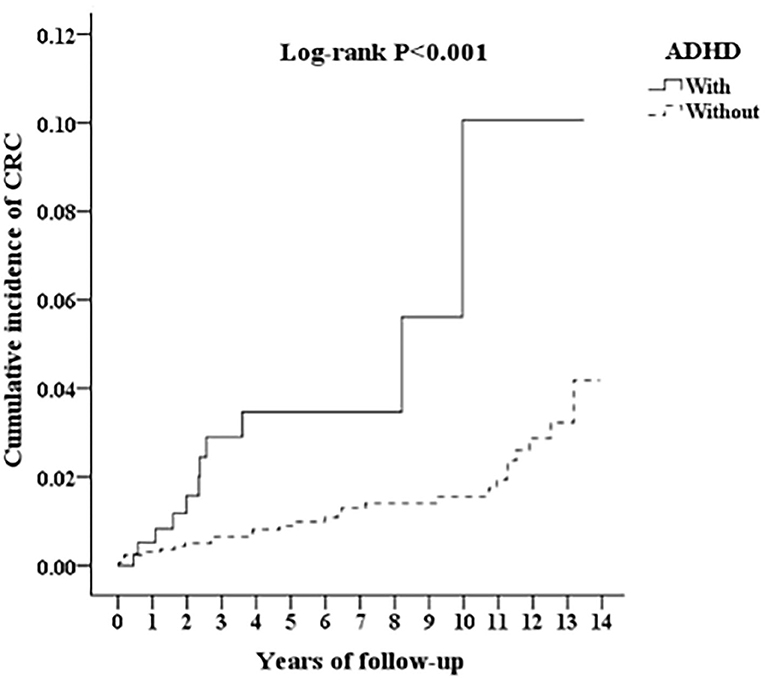
Figure 2. Kaplan-Meier for cumulative incidence of CRC among aged 18 and over stratified by ADHD with log-rank test.
Hazard Ratios Analysis of CRC in the Patients With ADHD
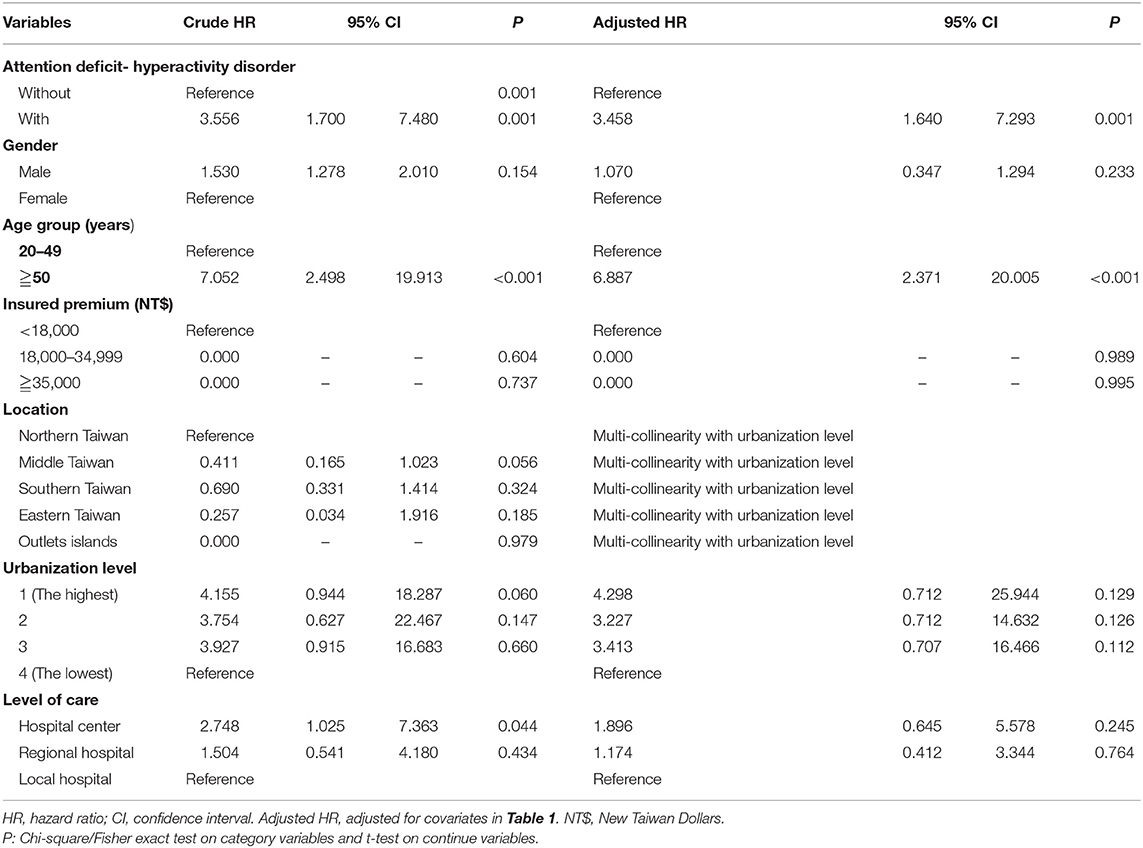
Table 2 depicts that the multivariate Cox regression revealed a significantly higher risk of developing CRC in the ADHD cohort. The crude HR was 3.556 (95% CI = 1.700–7.480, p < 0.001), and after adjusting for age, gender, comorbidities, geographical area of residence, urbanization level of residence, and monthly income, the adjusted HR was 3.458 (95% CI = 1.640–7.293; p < 0.001). For the participants older than 50 years, in both the ADHD and non-ADHD cohort, the risk of CRC was 6.887 (95% CI = 2.371–20.005, p < 0.001), in comparison to the subgroup aged from 20 to 49. In contrast, the ADHD group was uniformly associated with an increased risk of CRC for all the factors.
Subgroup Analysis of CRC in the Patients With ADHD
In Table 3, after the stratification according to age, gender, and covariates, the risk of development of CRC was higher in the ADHD group than the control group. We found that adults with ADHD were associated with an increased risk of developing CRC no matter the age group, gender, and with or without comorbidities, such as COPD, DM, CAD, and HTN, in comparison with the controls. Besides, the patients in the ADHD group without comorbidities similar to ARD, hypercholesterolemia, peptic disease, liver cirrhosis, and chronic hepatitis, ODD/CD, ASD, tics, intellectual disabilities, anxiety, and bipolar disorder, were associated with an increased risk of developing CRC when compared to the controls. However, we did not find any association between the usage of MPH and the risk of developing CRC in the patients with ADHD (Figure 3).
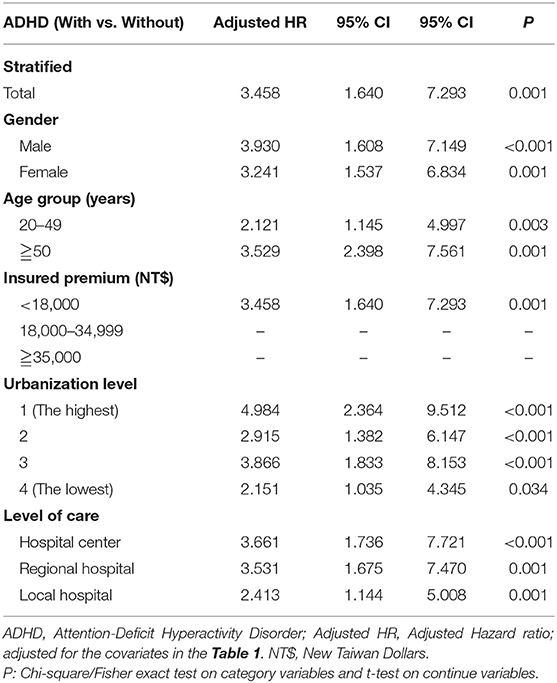
Table 3. Factors of Colorectal cancer stratified by variables listed in the table by using Cox regression.
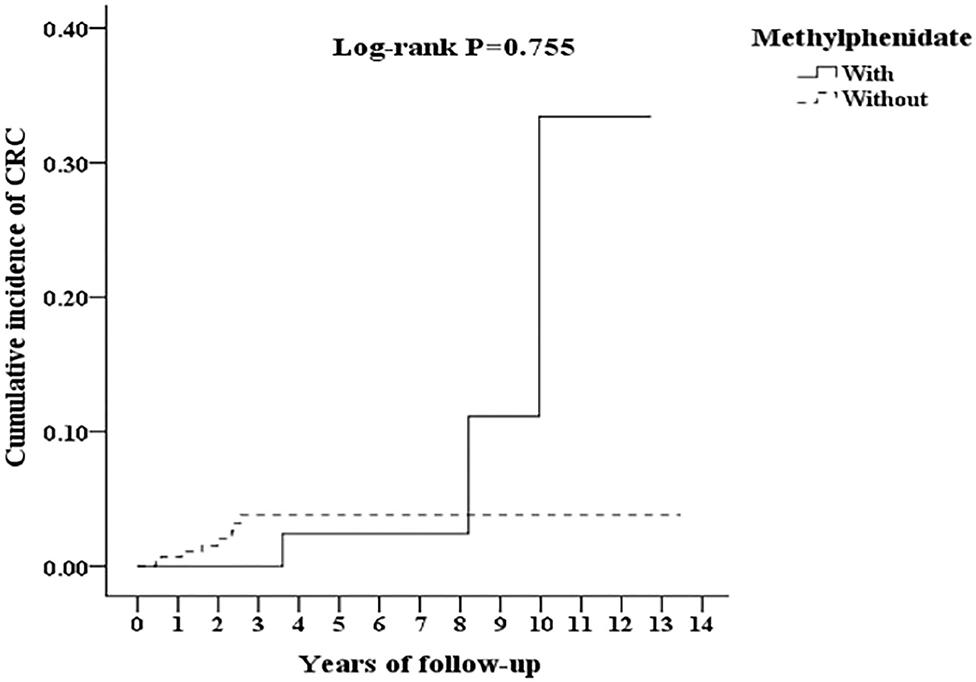
Figure 3. Kaplan-Meier for cumulative incidence of CRC among ADHD aged 18 and over stratified by Methylphenidate with log-rank test.
Discussion
Association Between ADHD and the Risk of CRC
In this study, we examined the association between ADHD and the risk of CRC. After adjusting the covariates, the adjusted HR was 3.458 for the ADHD group (95% CI = 1.640–7.293; p < 0.001), when compared with the non-ADHD control group. The Kaplan-Meier analysis revealed that the study subjects had a significantly higher 14-year cumulative incidence of ADHD than the controls. Furthermore, the ADHD-cohorts older than 50 years had a nearly 6.9-fold increased risk of CRC (adjusted HR: 6.887 [95% CI: 2.371–20.005; p < 0.001]). This study revealed that patients with ADHD had a nearly 3.5-fold risk of CRC, and this report could be a reminder for the clinicians who care the patients of ADHD in the follow-up. To the best of our knowledge, this is the first nationwide, population-based cohort on the topic of the association between ADHD and the risk of developing CRC.
Comparison of This Study to Previous Literatures
Several previous studies have shown that the overall psychiatric disorders were associated with the increased risk of cancer (45, 46). Additionally, some studies have found that depression (47), eating disorders (48), and posttraumatic disorder (49) are associated with the increased risk of different types of cancer, but the risk of cancer in schizophrenia and bipolar disorder varied with gender and age (50). Prior studies have revealed that psychotropic medications, such as antidepressants, were associated with several cancers, such as breast cancer (51, 52), nasopharyngeal cancer (53), and Hodgkin's lymphoma (54). The usage of MPH and other drugs for ADHD have suggested an increase in the risk of developing cancers, especially in vulnerable elderly patients and in high-dosage groups (24, 25), but there is some controversy in this association (24, 55).
Possible Mechanisms for the Increased Risk of CRC in Patients With ADHD
The underlying mechanisms of the association between ADHD and CRC remain unknown. We hypothesize several factors for this association: Owing to the lifestyles, behavioral problems, and comorbidities related to ADHD (16, 18, 57–59), they are at risk of alcohol abuse (11, 12), cigarette smoking (13–15), and obesity (60), and these problems are, in turn, the risk factors of CRC (61–63). In addition, the socio-economic disadvantages might well-contribute to the risk of ADHD (64, 65) and CRC (66, 67). Our results from the subgroup analyses showed that the urbanization level-specific ADHD in comparison to the HR of CRC was significant for patients living in the residence of urbanization level 1, having higher monthly insurance premiums, and visiting hospital centers. Moreover, patients living in higher urbanization levels had a greater risk of CRC. However, further studies are needed to clarify the role of the interactions among the lifestyles, behavioral problems, and socio-economic disadvantages in the risk of developing CRC in the ADHD patients.
Furthermore, ADHD and CRC share several common links such as gut microbiota, hypothalamic-pituitary-adrenal axis, chemokines, cytokines, short-chain fatty acids, autonomic nervous system, and enteric nervous systems (28). Several animal model studies have suggested that gut microbiota may be involved in the development of brain-related disorders (68). The gut microbiota could influence the reward centers of the brain with dopamine (DA) that have been found in people with ADHD (27).
Several studies have found a higher presence of asthma (69), eczema, and rhinitis (70, 71) in patients with ADHD. Studies also identified a higher prevalence of autoimmune diseases, such as thyrotoxicosis, type 1 diabetes, autoimmune hepatitis, psoriasis, and ankylosing spondylitis in ADHD patients (72). Thus, inflammation might play an important role in the mechanism in ADHD. Moreover, the connection between inflammation and CRC tumorigenesis is well-established (73, 74). Therefore, inflammation might be a common link between ADHD and CRC.
Prior studies have found that peripheral DA plays an important role of the tumor's immunity (75), and dopamine significantly enhances the efficacies of the commonly used anticancer drugs (76). In addition, one of the potential underlying mechanisms is the imbalance of the dopaminergic system (77, 78). Therefore, further studies are needed to investigate as to whether there is a common link between ADHD and CRC.
There was a possible pathophysiology role correlation between MPH treatment and cancer, for an elevated incidence of chromosomal anomalies related to MPH (24). However, the present study does not support the association between MPH and the risk of CRC. As aforementioned, inflammation might play a role as the common link between ADHD and CRC. One finding suggests that MPH could down-regulate the inflammatory markers, and thus might be one of the reasons that MPH is noted associated with CRC (56). This discrepancy between these two studies warrants a further study.
Strengths
Our study has several strengths. First, it was conducted by using the NHIRD, a claims database widely used for academic research, was retrieved from the NHI program, a universal, single-payer health insurance system, which comprises comprehensive information, including the demographic data, dates of medical visits, and medical services (79). Second, in this database with a high coverage of people in Taiwan, which is a large, nationwide, and population-based sample, that avoids the selection and participation biases (79). Third, the criteria of ADHD and CRC were defined according to ICD-9-CM, which were monitored and strictly evaluated by the NHI Administration for the reimbursement agency, and we could have also adjusted the covariates from this nationwide database to estimate the association between ADHD and the risk of developing CRC (80).
Limitations
We are aware of the limitations of our study. First, the diagnoses of ADHD and CRC were based on the diagnostic codes recorded manually by the physicians into the National Health Insurance (NHI) claim database system; therefore, some registry bias may have been involved in the calculation of the CRC risk. In addition, the family history of colorectal cancer is present, up to one third of patients (81). The lack of the information of the genetic-family risk factor in this claims database study is an important limitation. Second, the socio-economic status, which is an important contributory factor to ADHD and CRC, as aforementioned, could only be represented by the monthly insurance premiums, urbanization levels and the location of the residences, and the hospitals of the patients seeking medical help. Third, the severity of ADHD and CRC and the functional status of the ADHD patients were not evaluated in the NHIRD.
Conclusion
This retrospective cohort study provided evidence of a nearly 3.5-fold increased risk of CRC in ADHD. The results of this study could serve as a reminder for the clinicians who care for the patients of ADHD in the follow-up. Further prospective studies are necessary for confirming our findings, we therefore recommend meticulous evaluation and aggressive risk reduction for CRC for the patients with ADHD.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to Data are available from the National Health Insurance Research Database (NHIRD) published by the Taiwan National Health Insurance (NHI) Administration. Due to legal restrictions imposed by the government of Taiwan in relation to the “Personal Information Protection Act”, data cannot be made publicly available. Requests for data can be sent as a formal proposal to the NHIRD (https://nhird.nhri.org.tw/en/index.html).
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Tri-Service General hospital approved the protocol for this study (TSGHIRB NO. 2-106-05-029). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
J-MH, C-CL, N-ST, and W-CC conceived, planned, and conducted this study. C-CL, J-MH, N-ST, C-HC, and W-CC contributed to the data analysis and interpretation. J-MH, T-CL, C-HC, C-YC, P-KC, C-AS, and C-WH contributed to this data interpretation. C-CL wrote the first draft. J-MH has played major role, in this revision, in the concept, data interpretation, data analysis, and the re-writing of this manuscript. N-ST and W-CC conducted the critical revisions of this article. All authors approved this manuscript.
Funding
This study was supported by the Tri-Service General Hospital Research Foundation under the grants from the Medical Affairs Bureau, the Ministry of Defense of Taiwan (MAB-107-084 and MND-MAB-110-087), the Tri-Service General Hospital Research Foundation (TSGH-C108-003, TSGH-C108-151, TSGH-B-109-010, TSGH-E-110240, and TSGH-B-110-012), and the Taoyuan Armed Forces General Hospital (TYAFGH-A-110020). The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We also appreciate the provision of the National Health Insurance Research Database by the Taiwan's Health and Welfare Data Science Center and Ministry of Health and Welfare (HWDC, MOW).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Patel S. The benevolent tyranny of biostatistics: public administration and the promotion of biostatistics at the National Institutes of Health, 1946-1970. Bull History Med. (2013) 87:622–47. doi: 10.1353/bhm.2013.0084
2. Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell Host Microbe. (2014) 15:317–28. doi: 10.1016/j.chom.2014.02.007
3. de Menezes RF, Bergmann A, Thuler LC. Alcohol consumption and risk of cancer: a systematic literature review. Asian Pacific J Cancer Prevent. (2013) 14:4965–72. doi: 10.7314/APJCP.2013.14.9.4965
4. Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. (2015) 1:653–61. doi: 10.1001/jamaoncol.2015.1377
5. Fayyad J, De Graaf R, Kessler R, Alonso J, Angermeyer M, Demyttenaere K, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. (2007) 190:402–9. doi: 10.1192/bjp.bp.106.034389
6. Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. (2014) 43:434–42. doi: 10.1093/ije/dyt261
7. Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. (2010) 177:299–304. doi: 10.1016/j.psychres.2009.12.010
8. Kleinman NL, Durkin M, Melkonian A, Markosyan K. Incremental employee health benefit costs, absence days, and turnover among employees with ADHD and among employees with children with ADHD. J Occu Environ Med. (2009) 51:1247–55. doi: 10.1097/JOM.0b013e3181bca68c
9. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolescent Health. (2010) 46:209–17. doi: 10.1016/j.jadohealth.2009.09.009
10. Sonuga-Barke EJ, Koerting J, Smith E, McCann DC, Thompson M. Early detection and intervention for attention-deficit/hyperactivity disorder. Exp Rev Neurotherap. (2011) 11:557–63. doi: 10.1586/ern.11.39
11. Daurio AM, Aston SA, Schwandt ML, Bukhari MO, Bouhlal S, Farokhnia M, et al. Impulsive personality traits mediate the relationship between adult attention-deficit/hyperactivity symptoms and alcohol dependence severity. Alcohol Clin Exp Res. (2018) 42:173–83. doi: 10.1111/acer.13538
12. Kuppa A, Maysun A. Risk of alcohol abuse in humans with attention-deficit/hyperactivity disorder symptoms. Cureus. (2019) 11:e5996. doi: 10.7759/cureus.5996
13. Glass K, Flory K. Why does ADHD confer risk for cigarette smoking? A review of psychosocial mechanisms. Clin Child Family Psychol Rev. (2010) 13:291–313. doi: 10.1007/s10567-010-0070-3
14. Modesto-Lowe V, Danforth JS, Neering C, Easton C. Can we prevent smoking in children with ADHD: a review of the literature. Connect Med. (2010) 74:229–36.
15. Wilens TE, Morrison NR. The intersection of attention-deficit/hyperactivity disorder and substance abuse. Curr Opin Psychiatry. (2011) 24:280–5. doi: 10.1097/YCO.0b013e328345c956
16. Tai YM, Gau SS, Gau CS. Injury-proneness of youth with attention-deficit hyperactivity disorder: a national clinical data analysis in Taiwan. Res Dev Disabil. (2013) 34:1100–8. doi: 10.1016/j.ridd.2012.11.027
17. Amiri S, Sadeghi-Bazargani H, Nazari S, Ranjbar F, Abdi S. Attention deficit/hyperactivity disorder and risk of injuries: a systematic review and meta-analysis. J Inj Violence Res. (2017) 9:95–105. doi: 10.5249/jivr.v9i2.858
18. Chien WC, Chung CH, Lin FH, Yeh CB, Huang SY, Lu RB, et al. The risk of injury in adults with attention-deficit hyperactivity disorder: a nationwide, matched-cohort, population-based study in Taiwan. Res Dev Disabil. (2017) 65:57–73. doi: 10.1016/j.ridd.2017.04.011
19. Bolea-Alamanac B, Nutt DJ, Adamou M, Asherson P, Bazire S, Coghill D, et al. Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the british association for psychopharmacology. J Psychopharmacol. (2014) 28:179–203. doi: 10.1177/0269881113519509
20. Daley D, Van Der Oord S, Ferrin M, Cortese S, Danckaerts M, Doepfner M, et al. Practitioner review: current best practice in the use of parent training and other behavioural interventions in the treatment of children and adolescents with attention deficit hyperactivity disorder. J Child Psychol Psychiatry Allied Discip. (2018) 59:932–47. doi: 10.1111/jcpp.12825
21. Scholz L, Werle J, Philipsen A, Schulze M, Collonges J, Gensichen J. Effects and feasibility of psychological interventions to reduce inattention symptoms in adults with ADHD: a systematic review. J Ment Health. (2020) 1–14. doi: 10.1080/09638237.2020.1818189
22. Vafaei A, Vafaei I, Noorazar G, Akbarzadeh R, Erfanparast L, Shirazi S. Comparison of the effect of pharmacotherapy and neuro-feedback therapy on oral health of children with attention deficit hyperactivity disorder. J Clin Exp Dent. (2018) 10:e306–e11. doi: 10.4317/jced.54586
23. Oestreicher N, Friedman GD, Jiang SF, Chan J, Quesenberry C Jr, Habel LA, et al. Methylphenidate use in children and risk of cancer at 18 sites: results of surveillance analyses. Pharmacoepidemiol Drug Safety. (2007) 16:1268–72. doi: 10.1002/pds.1519
24. Steinhausen HC, Helenius D. The association between medication for attention-deficit/hyperactivity disorder and cancer. J Child Adolescent Psychopharmacol. (2013) 23:208–13. doi: 10.1089/cap.2012.0050
25. El-Zein RA, Abdel-Rahman SZ, Hay MJ, Lopez MS, Bondy ML, Morris DL, et al. Cytogenetic effects in children treated with methylphenidate. Cancer Lett. (2005) 230:284–91. doi: 10.1016/j.canlet.2005.01.003
26. Cenit MC, Nuevo IC, Codoner-Franch P, Dinan TG, Sanz Y. Gut microbiota and attention deficit hyperactivity disorder: new perspectives for a challenging condition. Europ Child Adolescent Psychiatry. (2017) 26:1081–92. doi: 10.1007/s00787-017-0969-z
27. Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE. (2017) 12:e0183509. doi: 10.1371/journal.pone.0183509
28. Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. (2011) 12:453–66. doi: 10.1038/nrn3071
29. Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. (2012) 22:299–306. doi: 10.1101/gr.126516.111
30. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. (2013) 14:207–15. doi: 10.1016/j.chom.2013.07.007
31. Ho Chan WS. Taiwan's healthcare report 2010. EPMA J. (2010) 1:563–85. doi: 10.1007/s13167-010-0056-8
32. Chien WC, Chung CH, Lin FH, Chang HA, Kao YC, Tzeng NS. Is weight control surgery associated with increased risk of newly onset psychiatric disorders? A population-based, matched cohort study in Taiwan. J Med Sci. (2017) 37:137–49. doi: 10.4103/jmedsci.jmedsci_94_16
33. Lee HC, Chiu WC, Wang TN, Liao YT, Chien IC, Lee Y, et al. Antidepressants and colorectal cancer: a population-based nested case-control study. J Affect Disord. (2017) 207:353–8. doi: 10.1016/j.jad.2016.09.057
34. Tzeng NS, Chung CH, Lin FH, Yeh CB, Huang SY, Lu RB, et al. Risk of dementia in adults with ADHD: a nationwide, population-based cohort study in Taiwan. J Attent Disord. (2017) 23:995–1006. doi: 10.1177/1087054717714057
35. Kao LC, Chien WC, Chung CH, Yeh HW, Chou YC, Huang SY, et al. The newly diagnosed amnestic disorders and dementia: a nationwide, cohort study in Taiwan. Taiwan J Psychiatry. (2018) 32:18–28.
36. Yang CC, Chien WC, Chung CH, Liu YP, Yeh CB, Chen KH, et al. No association between human immunodeficiency virus infections and dementia: a nationwide cohort study in Taiwan. Neuropsychiatr Dis Treat. (2019) 15:3155–66. doi: 10.2147/NDT.S225584
37. Chen TY, Huang CH, Chung CH, Mao WC, Yeh CB, Yang CCH, et al. Sex and age differences in the association between anxiety disorders and narcolepsy: a nationwide population-based case control study. J Affect Disord. (2020) 264:130–7. doi: 10.1016/j.jad.2019.12.010
38. Lin YC, Chen TY, Chien WC, Chung CH, Chang HA, Kao YC, et al. Stimulants associated with reduced risk of hospitalization for motor vehicle accident injury in patients with obstructive sleep apnea-a nationwide cohort study. BMC Pulmon Med. (2020) 20:28. doi: 10.1186/s12890-019-1041-1
39. Liu YP, Chien WC, Chung CH, Chang HA, Kao YC, Tzeng NS. Are anticholinergic medications associated with increased risk of dementia and behavioral and psychological symptoms of dementia? A nationwide 15-year follow-up cohort study in Taiwan. Front Pharmacol. (2020) 11:30. doi: 10.3389/fphar.2020.00030
40. Wan FJ, Chien WC, Chung CH, Yang YJ, Tzeng NS. Association between traumatic spinal cord injury and affective and other psychiatric disorders–A nationwide cohort study and effects of rehabilitation therapies. J Affect Disord. (2020) 265:381–8. doi: 10.1016/j.jad.2020.01.063
41. Wang DS, Chung CH, Chang HA, Kao YC, Chu DM, Wang CC, et al. Association between child abuse exposure and the risk of psychiatric disorders: a nationwide cohort study in Taiwan. Child Abuse Neglect. (2020) 101:104362. doi: 10.1016/j.chiabu.2020.104362
42. Lin CH, Chien WC, Chung CH, Chiang CP, Wang WM, Chang HA, et al. Increased risk of dementia in patients with genital warts: a nationwide cohort study in Taiwan. J Dermatol. (2020) 47:503–11. doi: 10.1111/1346-8138.15277
43. Yeh TC, Chien WC, Chung CH, Liang CS, Chang HA, Kao YC, et al. Psychiatric disorders after traumatic brain injury: a nationwide population-based cohort study and the effects of rehabilitation therapies. Arch Phys Med Rehabil. (2020) 101:822–31. doi: 10.1016/j.apmr.2019.12.005
44. Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan's national health insurance research database: past and future. Clin Epidemiol. (2019) 11:349–58. doi: 10.2147/CLEP.S196293
45. Whitley E, Batty GD, Mulheran PA, Gale CR, Osborn DP, Tynelius P, et al. Psychiatric disorder as a risk factor for cancer: different analytic strategies produce different findings. Epidemiology. (2012) 23:543–50. doi: 10.1097/EDE.0b013e3182547094
46. Kisely S, Forsyth S, Lawrence D. Why do psychiatric patients have higher cancer mortality rates when cancer incidence is the same or lower? Austr New Zealand J Psychiatry. (2016) 50:254–63. doi: 10.1177/0004867415577979
47. Karamanou M, Tzavellas E, Laios K, Koutsilieris M, Androutsos G. Melancholy as a risk factor for cancer: a historical overview. J Buon. (2016) 21:756–9.
48. Chen VCH, Liu YC, Lu ML, Chen KJ, Yang YH. Risk of cancer in patients with eating disorders: a population-based study. Taiwan J Psychiatry. (2019) 33:76–82. doi: 10.4103/TPSY.TPSY_16_19
49. Gradus JL, Farkas DK, Svensson E, Ehrenstein V, Lash TL, Milstein A, et al. Posttraumatic stress disorder and cancer risk: a nationwide cohort study. Eur J Epidemiol. (2015) 30:563–8. doi: 10.1007/s10654-015-0032-7
50. Lin GM, Chen YJ, Kuo DJ, Jaiteh LE, Wu YC, Lo TS, et al. Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997-2009. Schizophrenia Bullet. (2013) 39:407–16. doi: 10.1093/schbul/sbr162
51. Cotterchio M, Kreiger N, Darlington G, Steingart A. Antidepressant medication use and breast cancer risk. Am J Epidemiol. (2000) 151:951–7. doi: 10.1093/oxfordjournals.aje.a010138
52. Wernli KJ, Hampton JM, Trentham-Dietz A, Newcomb PA. Antidepressant medication use and breast cancer risk. Pharmacoepidemiol Drug Safety. (2009) 18:284–90. doi: 10.1002/pds.1719
53. Lin CF, Chan HL, Hsieh YH, Liang HY, Chiu WC, Lee Y, et al. Antidepressant medication use and nasopharyngeal cancer risk: a nationwide population-based study. Neuropsychiatr Dis Treat. (2018) 14:1101–6. doi: 10.2147/NDT.S161049
54. Bôaventura CS, Guimarães AN, Soares GR, Fraga AMBF, Neves FBCS, Pondé MP. Risk of cancer associated with the use of antidepressants. Pharmacoepidemiol Drug Saf. (2007) 29:63–9. doi: 10.1590/S0101-81082007000100013
55. Walitza S, Werner B, Romanos M, Warnke A, Gerlach M, Stopper H. Does methylphenidate cause a cytogenetic effect in children with attention deficit hyperactivity disorder? Environ Health Perspect. (2007) 115:936–40. doi: 10.1289/ehp.9866
56. Khalid A, Abbasi UA, Amber S, Sumera, Mirza FJ, Asif M, et al. Methylphenidate and Rosmarinus officinalis improves cognition and regulates inflammation and synaptic gene expression in AlCl(3)-induced neurotoxicity mouse model. Mol Biol Rep. (2020) 47:7861–70. doi: 10.1007/s11033-020-05864-y
57. Tong L, Xiong X, Tan H. Attention-deficit/hyperactivity disorder and lifestyle-related behaviors in children. PLoS ONE. (2016) 11:e0163434. doi: 10.1371/journal.pone.0163434
58. Holton KF, Nigg JT. The association of lifestyle factors and ADHD in children. J Attent Disord. (2016) 24:1511–20. doi: 10.1177/1087054716646452
59. Huang YF, Chiou HY, Chung CH, Chien WC, Chang HJ. Psychiatric disorders after attention-deficit/hyperactivity disorder: a nationwide population-based study in Taiwan. J Nursing Scholar. (2019) 51:138–46. doi: 10.1111/jnu.12457
60. Cortese S, Tessari L. Attention-Deficit/Hyperactivity Disorder (ADHD) and obesity: update 2016. Curr Psychiatry Rep. (2017) 19:4. doi: 10.1007/s11920-017-0754-1
61. Shrubsole MJ, Wu H, Ness RM, Shyr Y, Smalley WE, Zheng W. Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. Am J Epidemiol. (2008) 167:1050–8. doi: 10.1093/aje/kwm400
62. Fagunwa IO, Loughrey MB, Coleman HG. Alcohol, smoking and the risk of premalignant and malignant colorectal neoplasms. Best Pract Res Clin Gastroenterol. (2017) 31:561–8. doi: 10.1016/j.bpg.2017.09.012
63. Wong MC, Ding H, Wang J, Chan PS, Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intestinal Res. (2019) 17:317–29. doi: 10.5217/ir.2019.00021
64. Russell AE, Ford T, Russell G. Socioeconomic associations with ADHD: findings from a mediation analysis. PLoS ONE. (2015) 10:e0128248. doi: 10.1371/journal.pone.0128248
65. Rowland AS, Skipper BJ, Rabiner DL, Qeadan F, Campbell RA, Naftel AJ, et al. Attention-Deficit/Hyperactivity Disorder (ADHD): Interaction between socioeconomic status and parental history of ADHD determines prevalence. J Child Psychol Psychiatry Allied Discip. (2018) 59:213–22. doi: 10.1111/jcpp.12775
66. Doubeni CA, Laiyemo AO, Major JM, Schootman M, Lian M, Park Y, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer. (2012) 118:3636–44. doi: 10.1002/cncr.26677
67. Zhang D, Matthews CE, Powell-Wiley TM, Xiao Q. Ten-year change in neighborhood socioeconomic status and colorectal cancer. Cancer. (2019) 125:610–7. doi: 10.1002/cncr.31832
68. Luczynski P, McVey Neufeld KA, Oriach CS, Clarke G, Dinan TG, Cryan JF. Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int J Neuropsychopharmacol. (2016) 19:pyw020. doi: 10.1093/ijnp/pyw020
69. Cortese S, Sun S, Zhang J, Sharma E, Chang Z, Kuja-Halkola R, et al. Association between attention deficit hyperactivity disorder and asthma: a systematic review and meta-analysis and a Swedish population-based study. Lancet Psychiatry. (2018) 5:717–26. doi: 10.1016/S2215-0366(18)30224-4
70. Miyazaki C, Koyama M, Ota E, Swa T, Mlunde LB, Amiya RM, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. (2017) 17:120. doi: 10.1186/s12888-017-1281-7
71. Schans JV, Çiçek R, de Vries TW, Hak E, Hoekstra PJ. Association of atopic diseases and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. Neurosci Biobehav Rev. (2017) 74:139–48. doi: 10.1016/j.neubiorev.2017.01.011
72. Hegvik TA, Instanes JT, Haavik J, Klungsøyr K, Engeland A. Associations between attention-deficit/hyperactivity disorder and autoimmune diseases are modified by sex: a population-based cross-sectional study. Europ Child Adolescent Psychiatry. (2018) 27:663–75. doi: 10.1007/s00787-017-1056-1
73. Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. (2010) 138:2101–14.e5. doi: 10.1053/j.gastro.2010.01.058
74. Long AG, Lundsmith ET, Hamilton KE. Inflammation and colorectal cancer. Curr Colorectal Cancer Rep. (2017) 13:341–51. doi: 10.1007/s11888-017-0373-6
75. Zhang X, Liu Q, Liao Q, Zhao Y. Potential roles of peripheral dopamine in tumor immunity. J Cancer. (2017) 8:2966–73. doi: 10.7150/jca.20850
76. Sarkar C, Chakroborty D, Chowdhury UR, Dasgupta PS, Basu S. Dopamine increases the efficacy of anticancer drugs in breast and colon cancer preclinical models. Clin Cancer Res. (2008) 14:2502–10. doi: 10.1158/1078-0432.CCR-07-1778
77. Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson's disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res. (2006) 10:167–79. doi: 10.1007/BF03033354
78. Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, et al. Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev. (2007) 17:39–59. doi: 10.1007/s11065-007-9019-9
79. Hsing AW, Ioannidis JP. Nationwide population science: lessons from the Taiwan national health insurance research database. JAMA Internal Med. (2015) 175:1527–9. doi: 10.1001/jamainternmed.2015.3540
80. National Health Insurance Reimbursement Regulations. (2014). Available online at: http://law.moj.gov.tw/LawClass/LawAllIf.aspx?PCode=L0060006 (accessed February 12, 2020).
Keywords: attention-deficit hyperactivity disorder, colorectal cancer, retrospective cohort study, National Health Insurance Research Database, Longitudinal Health Insurance Database
Citation: Hu J-M, Lee C-C, Lin T-C, Chung C-H, Chen C-Y, Chang P-K, Hsiao C-W, Sun C-A, Tzeng N-S and Chien W-C (2021) Risk of Colorectal Cancer in Patients With Attention-Deficit Hyperactivity Disorder: A Nationwide, Population-Based Cohort Study. Front. Psychiatry 12:537137. doi: 10.3389/fpsyt.2021.537137
Received: 22 February 2020; Accepted: 11 January 2021;
Published: 05 February 2021.
Edited by:
Andreas Stengel, Charité – Universitätsmedizin Berlin, GermanyReviewed by:
Sam Cortese, University of Southampton, United KingdomMechthild Westhoff-Bleck, Hannover Medical School, Germany
Copyright © 2021 Hu, Lee, Lin, Chung, Chen, Chang, Hsiao, Sun, Tzeng and Chien. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nian-Sheng Tzeng, cGllcnJlbnNAbWFpbC5uZG1jdHNnaC5lZHUudHc=; Wu-Chien Chien, Y2hpZW53dUBuZG1jdHNnaC5lZHUudHc=
†These authors have contributed equally to this work
 Je-Ming Hu1,2,3
Je-Ming Hu1,2,3 Chi-Hsiang Chung
Chi-Hsiang Chung Cheng-Wen Hsiao
Cheng-Wen Hsiao Nian-Sheng Tzeng
Nian-Sheng Tzeng Wu-Chien Chien
Wu-Chien Chien