
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 18 February 2021
Sec. Child and Adolescent Psychiatry
Volume 12 - 2021 | https://doi.org/10.3389/fpsyt.2021.519840
Background: Deficient decision-making (DM) in attention deficit/hyperactivity disorder (ADHD) is marked by altered reward sensitivity, higher risk taking, and aberrant reinforcement learning. Previous meta-analysis aggregate findings for the ADHD combined presentation (ADHD-C) mostly, while the ADHD predominantly inattentive presentation (ADHD-I) and the predominantly hyperactive/impulsive presentation (ADHD-H) were not disentangled. The objectives of the current meta-analysis were to aggregate findings from DM for each presentation separately.
Methods: A comprehensive literature search of the PubMed (Medline) and Web of Science Database took place using the keywords “ADHD,” “attention-deficit/hyperactivity disorder,” “decision-making,” “risk-taking,” “reinforcement learning,” and “risky.” Random-effects models based on correlational effect-sizes were conducted. Heterogeneity analysis and sensitivity/outlier analysis were performed, and publication biases were assessed with funnel-plots and the egger intercept.
Results: Of 1,240 candidate articles, seven fulfilled criteria for analysis of ADHD-C (N = 193), seven for ADHD-I (N = 256), and eight for ADHD-H (N = 231). Moderate effect-size were found for ADHD-C (r = 0.34; p = 0.0001; 95% CI = [0.19, 0.49]). Small effect-sizes were found for ADHD-I (r = 0.09; p = 0.0001; 95% CI = [0.008, 0.25]) and for ADHD-H (r = 0.1; p = 0.0001; 95% CI = [−0.012, 0.32]). Heterogeneity was moderate for ADHD-H. Sensitivity analyses show robustness of the analysis, and no outliers were detected. No publication bias was evident.
Conclusion: This is the first study that uses a meta-analytic approach to investigate the relationship between the different presentations of ADHD separately. These findings provide first evidence of lesser pronounced impairment in DM for ADHD-I and ADHD-I compared to ADHD-C. While the exact factors remain elusive, the current study can be considered as a starting point to reveal the relationship of ADHD presentations and DM more detailed.
ADHD is a neurodevelopmental disorder associated with inappropriate levels of inattention and/or hyperactivity/impulsivity (1). Subtypes are categorized depending on the degree of inattention and/or hyperactivity in predominantly inattentive presentation (ADHD-I), predominantly hyperactive/impulsive presentation (ADHD-H), and the combined presentation (ADHD-C) (2). ADHD as a disorder has been considered by some to be primarily a disorder of executive dysfunction (3). Among these, dysfunction in inhibition, working memory, and task switching are most consistently reported (4). However, the relationship between executive dysfunction and ADHD is not that simple because not all of those with ADHD exhibit executive dysfunction (5). There are now several other conceptualizations that address this heterogeneity and propose multiple pathway models (5, 6). According to these models, ADHD can arise from dysfunction across several different pathways that include executive and non-executive dysfunctions as well pathways that emphasize motivational aspects accompanied with suboptimal reward processes, delay aversion, that is, the drive toward immediate reinforcement, and to escape the negative affect induced by delay (7). These latter two pathways are mediated by deficits on mesocortical control circuits for the cognitive pathway and by alterations in meso-limbic reward circuits for the motivational pathway.
Decision-making (DM) can be viewed as choosing one specific action among others after evaluating the potential outcomes, preferences, and context. This encompasses scenarios ranging from simple perceptual decisions to complex learned situations (e.g., reinforcement learning) as well as risky DM, all of which have been studied empirically (8, 9). To optimally decide for the best outcome, an interplay of cognitive functions have to take place. These comprise of self-referential processes, for example, reflections on autobiographical past and prospection about possible future events. Further, working memory, inhibition, and planning, as well as value estimation, outcome appraisal, and learning need to work together, further underline the complexity of the DM process. However, a decision does not necessarily always depend on all aforementioned cognitive functions. Dependent on the task, different types of DM can be distinguished: those who predominantly require “cool aspects” of cognitive control mediated by dorsolateral prefrontal cortex (DLPFC) can be differentiated by those scenarios predominantly requiring affect regulation, that is, motivational aspects mediated by ventral medial prefrontal cortex (VMPFC) (4, 10, 11). Tasks involving cool aspects of DM are the Cambridge gambling task, game of dice task, make-a-match game, and probabilistic discounting task. All these tasks share the feature that a fast-intuitive strategy can be applied, since the learned outcome is based on associations (4, 12, 13). Tasks involving hot aspects are, for example, the Iowa gambling task, balloon analog risk task, door-opening task, and card-playing task. These tasks are solved best by applying a slow analytical strategy, that is, based on rule learning. Cool aspects of DM are deficient in ADHD in terms of less rational choices and lower risk adjustment compared to controls (14). Also, when the task is more progressed and the participants become more used to it, those with ADHD perform worse than controls, that is, riskier (15). In tasks that involve more analytical rule learning, for example, the Iowa gambling task, children/adolescents with ADHD show more risky behavior, choose less often the advantageous decks compared to controls, and are sensitive to the frequency and not to the magnitude of a punishment (16). However, there are studies showing no differences in terms of risky behavior and the amount of choices for the advantageous decisions. Further, ADHD-subtype comparisons revealed no differences. Other tasks, for example, the door-opening task and the balloon analog risk task, show higher risk-taking behavior in ADHD. While Groen et al. reported weaker evidence for adults compared to children/adolescents, no age moderation effect was reported in a meta-analytic study by Groen et al. (14) and Jackson and MacKillop (17). In their case-control delay discounting the meta-analytic model, an effect-size (ES) of d = 0.43 was reported (17). Another meta-analysis reported an odd ratio of 1.9 for single choice paradigms (i.e., choose one option among two rewards with different size and delay to delivery) and standardized mean difference for temporal discounting paradigms of 0.43 for ADHD vs. controls (18). In conclusion, patients with ADHD show deficient DM on both the cool and hot aspects of executive functioning. Despite methodological differences in task design and heterogeneity in study samples, the effects found appear to be relatively robust throughout development in ADHD. We are currently lacking a systematic investigation of the single presentations since reviews and meta-analyses and most of the single studies have considered all groups in one analysis and do not differentiate between the different ADHD presentations (14, 17). Some studies have reported that ADHD subtypes differ in their correlation to DM (19, 20). Since there is no analysis available, the aim of the current review is to provide a quantitative overview of DM in the ADHD-I, ADHD-H and, ADHD-C separately. We hypothesize stronger ESs for the ADHD-C presentation.
This study followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 1) to achieve a high standard of reporting (21). A comprehensive literature search of the PubMed (Medline) and Web of Science Database took place using the keywords “ADHD,” “attention-deficit/hyperactivity disorder,” “decision-making,” “risk-taking,” “reinforcement learning,” and “risky” in all possible combinations. The search was conducted in July 2020 with no time interval specified. Reference lists of obtained articles were also considered.
Studies were included if they met the following criteria: (1) a publication of the research paper in a peer-reviewed journal, (2) standardized ADHD-diagnostic/assessment procedures (i.e., structured or semi-structured interviews or ADHD-specific questionnaires) according to DSM IV/V [see (22, 23) for an overview], (3) tasks including risk-taking behavior, rewards and DM, and (4) comparison to a healthy, typically developing group. Studies were excluded if (1) the predominant presentations of ADHD subtypes were not considered in the analysis or (2) ESs were not reported. See Figure 2 for a schematic overview of study inclusion/exclusion.
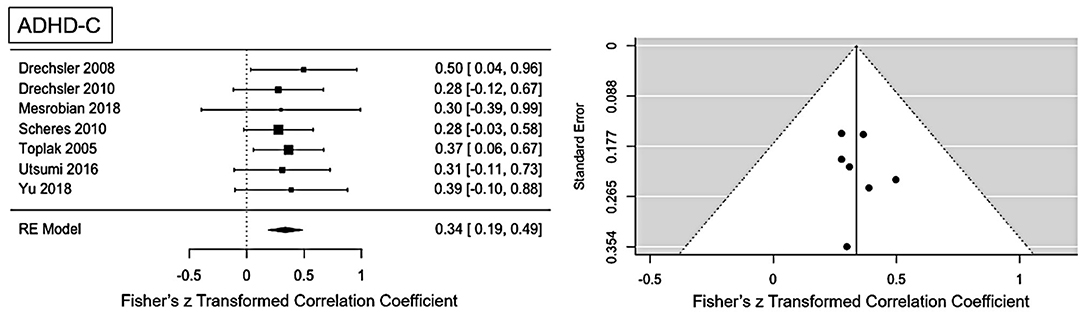
Figure 2. Forest plot of the effect-sizes and 95% confidence intervals for ADHD-combined presentation.
The recorded variables from each study were: sample size, mean age of the participants, type of control group, kind of DM-task, and metric for calculation of ES. Data were extracted from each study by one of the authors (MS) and checked by another author (SL) to minimize data selection errors.
To calculate a random-effects model, correlational ESs were extracted. If only F-or t-values were provided, correlation coefficients were obtained according to the following formula (24):
If partial eta squared were provided, it was transformed to Cohens' f/Cohens' d and finally to the correlation coefficients using the following formula (25, 26):
where, a is a correction factor for cases of unbalanced numbers of participants between the groups (27). The meta-analytic procedure was realized using R-software library package metafor [version 2.0-0 (28)]. Sampling variances weighted by sample size were determined by Hunter and Schmidt method because this method estimates the average correlation with the least error with comparative accuracy as, for example, Hedges and Vevea (29). Heterogeneities were assessed with Q and I2 statistics (30). Conventions were followed by the interpretation of I2: values of 0.25, 0.50, and 0.75 correspond to low, moderate, and high between-trial heterogeneities (30). To evaluate the influence of an individual study on the overall effect, leave-one-out analysis (repeat the ES-calculation while omitting a study at a time) using dmetar was performed (31). Outlier detection was performed using “find.outlier” -function in dmetar. In the case of the presence of outlier, the study was excluded, and the ES-calculation was repeated. The publication bias was assessed with funnel-plots and an egger intercept. Since study inclusion is not determined by age, age was included as a moderator variable in a mixed-effects model if heterogeneity is present.
The literature search resulted in a final set of seven studies for ADHD-C (mean age: 12.9, SD: 4.7), eight studies for ADHD-H presentation (man age: 12.2, SD: 4.7), and seven studies for ADHD-I presentation (mean age: 12.5, SD: 5.0) (see search flow diagram in Figure 2). Total sample size comprised of 193 patients for ADHD-C presentation, 265 patients for ADHD-I, and 231 patients for ADHD-H presentation. Except for one study, where performance of DM was compared to oppositional defiant disorder (32), all studies used a healthy age-matched control group. The following paradigms were applied: temporal discounting task (19, 33, 34), Iowa gambling task (32), game of dice task (35), make a match game (15), probabilistic game task (36), and card task (37) (see Table 1 for an overview).
Moderate ES for ADHD-C presentation was found: r = 0.34 (95% CI = [0.19, 0.49], p = <0.001). No significant heterogeneity is present (Q = 0.81, p = 0.99, I2 = 0%). Visual inspection of the funnel-plots and the egger intercept (z = 0.2, p = 0.84) suggesting the absence of a publication bias (see Figure 2). Influence analysis, that is, leave one out sensitivity analysis, showed that the result is not driven by a single study (see Figure 5). No outlier was identified.
The main ES for the ADHD-H presentation was r = 0.1 (95% CI = [−0.012, 0.32], p = 0.0001). The forest plot with the within-study ES and aggregated ES is presented in Figure 3. Moderate heterogeneity is present in the study sample (Q = 21.1, p = 0.003, I2 = 61.02%, df = 7). Since we included pediatric and adult studies in the analysis, we performed a moderator analysis with age, suggesting that age has no influence on the heterogeneity, Q(df = 1)=1.24, p = 0.26. Visual inspection of the funnel-plots and the egger intercept (z = −0.47, p = 0.64) suggesting the absence of a publication bias (see Figure 5 for the funnel-plots). Influence analysis, that is, leave-one out analysis, suggests general robustness of the result. Outlier analysis could not detect any outlier in the current analysis.
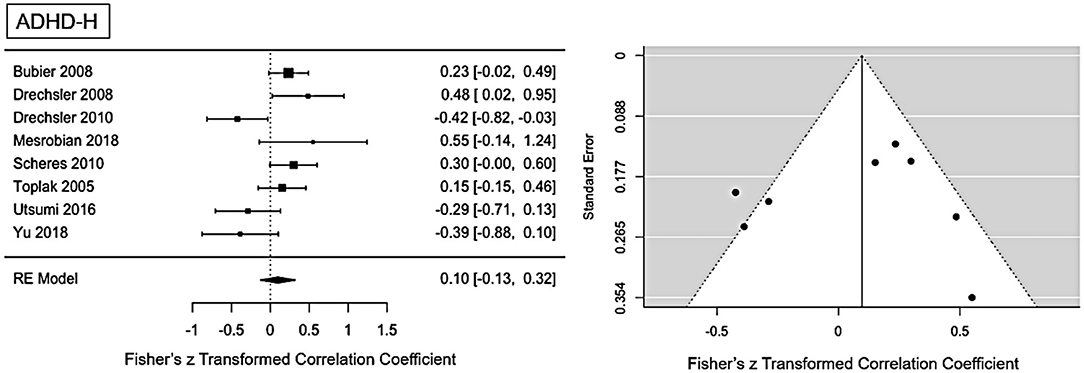
Figure 3. Forest plot of the effect-sizes and 95% confidence intervals for ADHD-hyperactivity/impulsive-presentation.
For ADHD-I presentation an ES of r = 0.09 (95% CI = [0.008, 0.25], p = 0.0001) was found (see Figure 4 for forest plot). Low heterogeneity is present in this sample (Q = 10.1, p = 0.12, I2 = 29.59%, df = 6). No publication bias is evident as visual inspection of the funnel-plots (see Figure 5) and Eggers intercept (z = 0.11, p = 0.9) suggest. Moderator with age as regressor show no moderate influence, Q(df = 1) = 0.03, p = 0.85. Influence analysis showed general robustness of the results, and no outlier was detected.
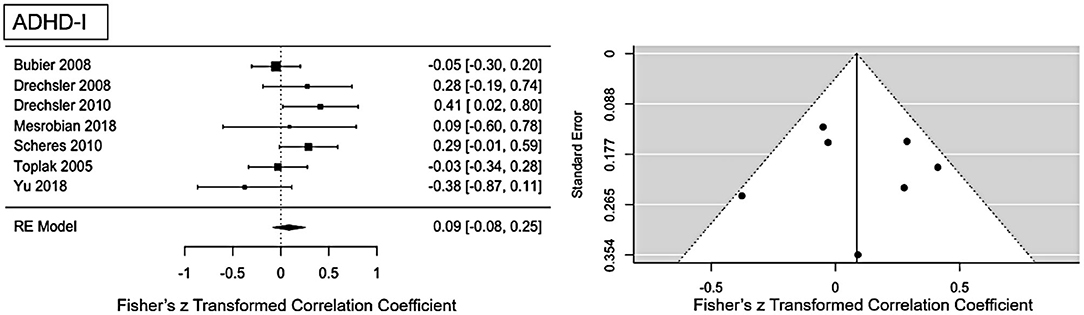
Figure 4. Forest plot of the effect-sizes and 95% confidence intervals for ADHD-inattention-presentation.
Since the majority of published studies report associations of DM and ADHD-C presentation, the aim of the current study was to investigate DM separately for the ADHD-C, ADHD-I, and ADHD-H presentation. Moderate ES was found for ADHD-C (r = 0.34), small ES for ADHD-H (r = 0.1), and ADHD-I (r = 0.09). Heterogeneity was moderate in ADHD-H and low for ADHD-I and ADHD-C. In all analyses, no outlier studies were evident. The ES for ADHD-C were comparable to other meta-analyses, which also report medium ES, for example, d = 0.43 (17), SMD = 0.43, and OR = 1.9 (18). For the first time, we report ES for the predominant presentations separately. The smaller ES for ADHD-I and ADHD-H might be a hint that these presentations are less impaired dependent on the context DM is needed. According to Sonuga-Barke's dual pathway model, ADHD is associated with deficits on the motivational and cognitive/executive pathways. More specifically, the model proposes that delay aversion and poor inhibitory control are independent coexisting characteristics of ADHD. Deficient delay aversion is mediated by the mesolimbic structures that are associated with dopaminergic reward circuits (e.g., nucleus accumbens). Poor inhibitory control is mediated by aberrant connectivity from mesocortical structures to the prefrontal cortex (6). Delay aversion and poor inhibitory control are differentially expressed dependent on the DM type. When confronted with a delay, ADHD patients with delay aversion react with a negative emotional response and tend to choose the impulsive element of a decision, that is, the smaller sooner reward over a larger longer reward (38). In scenarios, where the delay cannot be overcome with an impulsive choice, the perception might switch away from the task and to the environment. In such delay situations, an increase in general activity and inattention arise (38, 39). As suggested by Sonuga-Barke (38), the context of delay-rich scenarios decides whether ADHD patients may be a delay-averse subtype (hence, reacting with impulsivity), whereas more pervasive expressions might reflect other executive deficits associated with inhibitory dysfunction.
In our sample, three studies used delay-rich paradigms, that is, temporal discounting (19, 33, 34), whereas the other studies modulated the height of an immediate reward dependent on the decision made. In delay-rich scenarios, the ADHD-C presentations seem to show more deficient DM-performance compared to ADHD-I and ADHD-C. In other words, ADHD-C presentations may be more sensitive to delay-rich scenarios; hence, it may be more associated with a delay-averse subtype as suggested by Sonuga-Barke. When the delay is rather small as in Scheres et al. (19), where a small delay of 60 s is used, the difference in DM-performance between the ADHD presentations decreases. Future studies are needed to explore the specific effect of the delay-length on ADHD-presentations. Delay-discounting paradigms are associated with hot executive functions that encompass emotions, motivation, and the interplay of desire for an immediate gratification and long-term rewards (40). Carefully interpreted, the delay in DM scenarios may have higher impact on performance in ADHD-C presentations compared to ADHD-I/H presentations. The cool aspects of executive functions are associated with slow, analytical strategies and are dependent of inhibition, planning, and working memory (40). In our analysis, different paradigms were used that rely on cool executive functions: game of dice task (35), make-a-match game (15), and probabilistic-game tasks (36). While the ADHD-I presentation was associated with the smallest ES for the game of dice task (r = 0.28) and probabilistic-game task (r = 0.09), it was also associated with highest ES for the make-a-match task (r = 0.41). These differences could be due to diagnostical differences, that is, used instruments and experience in rating ADHD-symptoms across studies (22). Alternatively, the paradigms used in the studies may differ in the requirements of executive functions, for example, the game of dice task and the probabilistic-game task rely more on guessing the outcome and may be lower working memory demanding. In contrast, the make-a-match game, where two matching cards have to be found, needs higher working memory capacity.
According to the neuroeconomic model proposed by Sonuga-Barke and Fairchild (41), deficient DM in ADHD can be mediated by disruptions in three neuronal subsystems, which interact with each other: disrupted connectivity within the default mode network, dorsal-frontostriatal activations, and dopamine dysregulation in ventral frontostriatal networks (7, 41). In brief, an aberrant connectivity pattern in the default-mode network is associated with impaired self-referential thoughts, poor goal setting/implementation, and unstable value hierarchies. Also, impaired cognitive functioning is associated with deficient DLPFC-activation (part of the dorsal frontostriatal system), as for instance difficulties in updating working memory about different choice options. Impairments in the ventral frontostriatal network are associated with prospective behavior, that is, predicting future rewards. Taken together, DM in ADHD can be associated with impairment in the different functions necessary for DM. Distinctive neuronal activation patterns between ADHD-C and ADHD-I presentations compared to healthy controls could be differentiated. While both ADHD-I and ADHD-H presentations have been characterized by atypical connectivity throughout the brain, the combined presentation has been associated with more deviant connectivity in the default-mode network (42). In terms of DM, this could mean that the degree of impairment of the default-mode network in the ADHD-I and ADHD-H presentation might not result in the same behavioral consequences compared to ADHD-C presentation. This may lead to better goal setting/implementation and more stable value hierarchies in ADHD-I/H, compared to ADHD-C presentation. Further, ADHD-I has been shown to be associated with aberrant activation in dorsolateral prefrontal regions, which is proposed as a region that is important for cognitive functioning, for example, inhibition, working memory, and planning in the neuroeconomic model. To address the relationship of ADHD presentations, DM, and core separable neuropsychological functions, such as working memory, inhibition, and sustained attention, it seems prudent to calculate ES between DM and neuropsychological functions and between ADHD symptoms and neuropsychological functions, respectively. However, most of the included studies did not provide appropriate measurements, and therefore it was not possible to extract metrics in a sufficient manner for a proper ES calculation.
The relationship between neuropsychological functioning and ADHD symptoms seem not to be as directly dependent on each other as usually often assumed. Based on heterogeneity of impairment in cognitive functions, studies have identified distinct ADHD groups (independent of ADHD presentations). While some patients do show intact neuropsychological functioning in some aspects of cognition, others do not appear to have any cognitive deficits in commonly assessed domains (5, 43). Coghill and colleagues have proposed a model that considers that ADHD symptoms do not arise as a direct consequence of cognitive deficits rather that symptoms and cognition are relatively independent constructs with their causal roots in distinct aspects of brain structure and function (44). Therefore, it will be important to investigate the different neuropsychological functions needed for DM separately in the three different ADHD presentations. It will also be important to investigate the ways that the different ADHD presentations and their associated patterns of DLPFC activation, as well as frontostriatal systems (as mentioned above) contribute to problems with DM.
Since the majority of published studies include mostly the combined type or fail to differentiate between the different ADHD presentations, the number of included studies in the current analysis and the total sample size is rather small. In future studies, researchers should include data relating to the different ADHD presentations and provide metrics on the association of ADHD presentations and DM. Another limitation is the absence to control for diagnostical instruments used to assess ADHD symptoms. There may be certain variability in the diagnostical validity of the symptoms due to the use of different diagnostical tools and also the experience of the staff to rate symptoms for interview-based assessments. Unfortunately, due to the limited number of included studies, we could not assess the impact of the used diagnostical instruments. Further, ADHD subtypes are not discrete entities that are constant over time. When followed over time, patients may switch from one subtype to another, for example, from ADHD-C to ADHD-I (45, 46). This suggests when looking at DM in ADHD, subtype classification might not necessarily be the primary factor for a differentiation of DM performance across patients. We argue to also consider, for example, neuropsychological scores and comorbidities for a more comprehensive analysis. Further, the number of subjects per ADHD presentation might be imbalanced in the original studies, considering the prevalence of each ADHD presentation (47). Another limitation is that most of the studies (32, 37) include DM tasks that require cool aspects of executive functioning. It cannot be ruled out whether the current results are biased for this type of DM tasks. The small sample size hindered subanalysis on task designs that could potentially separate tasks requiring cool and hot aspects.
This is the first study to use a meta-analytic approach to investigate the relationship of ADHD-C, ADHD-I, and ADHD-H presentations in DM separately. While meta-analytic evidence in the literature shows deficient DM in ADHD-C presentations, it appears less clear in comparison to ADHD-I and ADHD-H presentations. The current meta-analysis provides rather limited evidence, but cautiously interpreted, it might be that patients with ADHD-I and ADHD-H presentations show less impairment in DM skills. However, the interplay between the triad of ADHD-presentation-specific symptoms, DM skills and neuropsychological functions are complex and not yet fully understood. The current study is considered as a starting point to clarify the relationship of ADHD presentations and DM. Since the current evidence is rather limited, future work is needed to support our findings and clarify the interplay.
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed tobWFyY2VsLnNjaHVsemVAdWtib25uLmRl.
MS and SL: literature search, figures, data analysis, data interpretation, and writing. DC and AP: data interpretation, writing, and supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition. Arlington, VA: American Psychiatric Association (2013).
2. LeRoy A, Jacova C, Young C. Neuropsychological performance patterns of adult ADHD subtypes. J Attent Disord. (2019) 23:1136–47. doi: 10.1177/1087054718773927
3. Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry. (2002) 41:59–66. doi: 10.1097/00004583-200201000-00012
4. Geurts HM, van der Oord S, Crone EA. Hot and cool aspects of cognitive control in children with ADHD: decision-making and inhibition. J Abnorm Child Psychol. (2006) 34:813–24. doi: 10.1007/s10802-006-9059-2
5. Coghill DR, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol Med. (2014) 44:1989–2001. doi: 10.1017/S0033291713002547
6. Sonuga-Barke EJS. Psychological heterogeneity in AD/HD—a dual pathway model of behaviour and cognition. Behav Brain Res. (2002) 130:29–36. doi: 10.1016/S0166-4328(01)00432-6
7. Sonuga-Barke EJS, Cortese S, Fairchild G, Stringaris A. Annual research review: transdiagnostic neuroscience of child and adolescent mental disorders - differentiating decision making in attention-deficit/hyperactivity disorder, conduct disorder, depression, and anxiety. J Child Psychol Psychiatry. (2016) 57:321–49. doi: 10.1111/jcpp.12496
8. Mowinckel AM, Pedersen ML, Eilertsen E, Biele G. A meta-analysis of decision-making and attention in adults with ADHD. J Attent Disord. (2015) 19:355–67. doi: 10.1177/1087054714558872
9. Matthies S, Philipsen A, Svaldi J. Risky decision making in adults with ADHD. J Behav Ther Exp Psychiatry. (2012) 43:938–46. doi: 10.1016/j.jbtep.2012.02.002
10. Hongwanishkul D, Happaney KR, Lee WSC, Zelazo PD. Assessment of hot and cool executive function in young children: age-related changes and individual differences. Dev Neuropsychol. (2005) 28:617–44. doi: 10.1207/s15326942dn2802_4
11. Zelazo PD, Muller U, Frye D, Marcovitch S. I. The development of executive function. Monogr Soc Res Child Dev. (2003) 68:1–27. doi: 10.1111/j.0037-976X.2003.00261.x
12. Evans JSBT. Dual-processing accounts of reasoning, judgment, and social cognition. Ann Rev Psychol. (2008) 59:255–78. doi: 10.1146/annurev.psych.59.103006.093629
13. Del Missier F, Mäntylä T, De Bruin WB. Decision-making competence, executive functioning, and general cognitive abilities. J Behav Decis Mak. (2012) 25:331–51. doi: 10.1002/bdm.731
14. Groen Y, Gaastra GF, Lewis-Evans B, Tucha O. Risky behavior in gambling tasks in individuals with ADHD – a systematic literature review. PLoS ONE. (2013) 8:e74909. doi: 10.1371/journal.pone.0074909
15. Drechsler R, Rizzo P, Steinhausen HC. Decision making with uncertain reinforcement in children with attention deficit/hyperactivity disorder (ADHD). Child Neuropsychol. (2010) 16:145–61. doi: 10.1080/09297040903190774
16. Luman M, Oosterlaan J, Knol DL, Sergeant JA. Decision-making in ADHD: sensitive to frequency but blind to the magnitude of penalty? J Child Psychol Psychiatry. (2008) 49:712–22. doi: 10.1111/j.1469-7610.2008.01910.x
17. Jackson JN, MacKillop J. Attention-deficit/hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging. (2016) 1:316–25. doi: 10.1016/j.bpsc.2016.01.007
18. Marx I, Hacker T, Yu X, Cortese S, Sonuga-Barke E. ADHD and the choice of small immediate over larger delayed rewards: a comparative meta-analysis of performance on simple choice-delay and temporal discounting paradigms. J Attent Disord. (2021) 25:171–87. doi: 10.1177/1087054718772138
19. Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry. (2010) 67:641–8. doi: 10.1016/j.biopsych.2009.10.033
20. Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke EJS, et al. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. (2006) 44:2092–103. doi: 10.1016/j.neuropsychologia.2005.10.012
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2010) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. Posserud M-B, Ullebø AK, Plessen KJ, Stormark KM, Gillberg C, Lundervold AJ. Influence of assessment instrument on ADHD diagnosis. Eur Child Adolesc Psychiatry. (2014) 23:197–205. doi: 10.1007/s00787-013-0442-6
23. Murphy KR, Adler LA. Assessing attention-deficit/hyperactivity disorder in adults: focus on rating scales. J Clin Psychiatry. (2004) 65(Suppl. 3):12–7.
24. Kock A, Gemünden HG. A guideline to meta-analysis. TIM Working Paper Series. Berlin: Technische Universitat Berlin (2009).
25. Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge. (2013).
27. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Converting among effect sizes. In: Borenstein M, editor. Introduction to Meta-Analysis. Chichester: John Wiley & Sons, Ltd. (2009). p. 45–9.
28. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
29. Field AP. Meta-analysis of correlation coefficients: a Monte Carlo comparison of fixed-and random-effects methods. Psychol Methods. (2001) 6:161. doi: 10.1037/1082-989X.6.2.161
30. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I 2 Index? Psychol Methods. (2006) 11:193–206. doi: 10.1037/1082-989X.11.2.193
31. Harrer M, Cuijpers P, Furukawa T, Ebert DD. Doing meta-analysis in R: a hands-on guide. Protect Lab. (2019) 0.0:9000. doi: 10.5281/zenodo.2551803
32. Bubier JL, Drabick DA. Affective decision-making and externalizing behaviors: the role of autonomic activity. J Abnorm Child Psychol. (2008) 36:941–53. doi: 10.1007/s10802-008-9225-9
33. Yu X, Sonuga-Barke E, Liu X. Preference for smaller sooner over larger later rewards in ADHD: contribution of delay duration and paradigm type. J Attent Disord. (2018) 22:984–93. doi: 10.1177/1087054715570390
34. Utsumi DA, Miranda MC, Muszkat M. Temporal discounting and emotional self-regulation in children with attention-deficit/hyperactivity disorder. Psychiatry Res. (2016) 246:730–7. doi: 10.1016/j.psychres.2016.10.056
35. Drechsler R, Rizzo P, Steinhausen HC. Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. J Neural Transm. (2008) 115:201–9. doi: 10.1007/s00702-007-0814-5
36. Mesrobian SK, Villa AEP, Bader M, Gotte L, Lintas A. Event-related potentials during a gambling task in young adults with attention-deficit/hyperactivity disorder. Front Hum Neurosci. (2018) 12:79. doi: 10.3389/fnhum.2018.00079
37. Toplak ME, Jain U, Tannock R. Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD). Behav Brain Funct. (2005) 1:8. doi: 10.1186/1744-9081-1-8
38. Sonuga-Barke EJS. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry. (2005) 57:1231–8. doi: 10.1016/j.biopsych.2004.09.008
39. Antrop I, Buysse A, Roeyers H, van Oost P. Stimulation seeking and hyperactive behavior in children with ADHD: a re-analysis. Percept Motor Skills. (2002) 95:71–90. doi: 10.2466/pms.2002.95.1.71
40. Peterson E, Welsh MC. The development of hot and cool executive functions in childhood and adolescence: are we getting warmer? In: Goldstein S, Naglieri JA, editors. Handbook of Executive Functioning. New York, NY: Springer New York. p. 45–65.
41. Sonuga-Barke EJS, Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol Psychiatry. (2012) 72:126–33. doi: 10.1016/j.biopsych.2012.04.004
42. Fair D, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NUF, et al. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front Syst Neurosci. (2013) 6:80. doi: 10.3389/fnsys.2012.00080
43. Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal heterogeneity in attention-deficit/hyperactivity disorder: do we need neuropsychologically impaired subtypes? Biol Psychiatry. (2005) 57:1224–30. doi: 10.1016/j.biopsych.2004.08.025
44. Coghill DR, Hayward D, Rhodes SM, Grimmer C, Matthews K. A longitudinal examination of neuropsychological and clinical functioning in boys with attention deficit hyperactivity disorder (ADHD): improvements in executive functioning do not explain clinical improvement. Psychol Med. (2014) 44:1087–99. doi: 10.1017/S0033291713001761
45. Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, et al. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol. (2012) 121:991–1010. doi: 10.1037/a0027347
46. Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV subtypes of ADHD from preschool through elementary school. Arch Gen Psychiatry. (2005) 62:896. doi: 10.1001/archpsyc.62.8.896
Keywords: attention deficit and hyperactivity disorder, decision making, meta-analysis, inattention and hyperactivity, risk behavior
Citation: Schulze M, Coghill D, Lux S and Philipsen A (2021) Disentangling ADHD's Presentation-Related Decision-Making—A Meta-Analytic Approach on Predominant Presentations. Front. Psychiatry 12:519840. doi: 10.3389/fpsyt.2021.519840
Received: 13 December 2019; Accepted: 06 January 2021;
Published: 18 February 2021.
Edited by:
Kerstin Jessica von Plessen, Center Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Lin Sørensen, University of Bergen, NorwayCopyright © 2021 Schulze, Coghill, Lux and Philipsen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcel Schulze, bWFyY2VsLnNjaHVsemVAdWtib25uLmRl
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.