
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 14 January 2021
Sec. Addictive Disorders
Volume 11 - 2020 | https://doi.org/10.3389/fpsyt.2020.624012
This article is part of the Research Topic Purple Haze: Issues on Cannabis Legalization View all 17 articles
Background: With the passing of the 2018 Agriculture Improvement Act that legalized hemp-derived products, i.e., cannabidiol (CBD), the use of CBD has increased exponentially. To date, the few studies that have characterized individuals who use CBD suggest that co-use of CBD and tetrahydrocannabinol (THC)-dominant cannabis, i.e., marijuana, is highly prevalent. It is, therefore, important to investigate the relationship between CBD use and marijuana use to understand the antecedents and consequences of co-use of these two cannabis products.
Methods: We conducted an online survey using structured questionnaires to determine differences in CBD users with (CBD+MJ) and without co-morbid marijuana use. Group comparisons were carried out using chi-square tests and ANOVA. Multiple correspondence analysis (MCA) with bootstrap ratio testing was performed to examine the relationship between the categorical data.
Results: We received 182 survey responses from current CBD users. CBD+MJ had more types of CBD administration (F = 17.07, p < 0.001) and longer lifetime duration of CBD use (χ2 = 12.85, p < 0.05). Results from the MCA yielded two statistically significant dimensions that accounted for 77% of the total variance. Dimension 1 (representing 57% of the variance) associated CBD+MJ with indication of CBD use for medical ailments, use of CBD for more than once a day for longer than 2 years, applying CBD topically or consuming it via vaping or edibles, being female, and, having lower educational attainment. Dimension 2 (representing 20% of the variance) separated the groups primarily on smoking-related behaviors where CBD+MJ was associated with smoking CBD and nicotine.
Conclusions: Identifying the factors that influence use of CBD and marijuana can inform future studies on the risks and benefits associated with each substance as well as the impacts of policies related to cannabis-based products.
The cannabis sativa plant species contains a multitude of varieties, such as hemp and marijuana (MJ), with various active elements known as phyto-cannabinoids. Hemp and MJ are commonly differentiated according to their concentration levels of delta-9 tetrahydrocannabinol (THC), the main psychoactive phyto-cannabinoid found in cannabis sativa. Hemp is classified as cannabis sativa with a THC concentration lower than 0.03%, while those with a concentration >0.03% are classified as MJ (1, 2). Prior to 2018, both hemp and MJ were classified as schedule I substances. In December of 2018, the United States Senate passed The Agriculture Improvement Act. Under this new law, hemp was rescheduled from a DEA schedule I substance to a schedule V substance. This reclassification identifies hemp and hemp-derived products, such as cannabidiol (CBD), as a substance of medicinal value with no addictive properties and legalizes it nationally. CBD's appeal as a medicinal agent is based upon its favorable tolerance in both human and animal models (3–5). These models found a lack of habit-forming potential (6, 7) and rare incidents of adverse side-effects (8) from CBD use compared to THC (9–11).
To date, cannabis research has focused largely on THC and CBD given that they are the main phyto-cannabinoids found in cannabis sativa (7, 10, 12)]. In isolation, THC has been shown to induce psychoactive and appetitive effects (13) and impact cognitive abilities, including but not limited to attention, and episodic memory (14, 15). CBD, on the other hand, has been shown to have anxiolytic (16), antipsychotic (17), and neuroprotective effects (18–21).
Studies have found high co-use of THC and CBD, i.e., >50% in CBD users (22, 23) that highlight the need to understand how the two substances interact. To date, however, our knowledge of this interaction remains largely inconclusive. While it has been suggested that CBD does not impact THC's subjective and reinforcing properties (24), its modulatory role on THC's effects on cognition is mixed. For example, some studies have found that CBD has a protective effect on THC-related episodic memory deficits (25), such that cannabis users who smoked cannabis high in cannabidiol content showed no memory impairment. On the other hand, CBD was not found to modulate THC's effect on attention (26, 27). Timing of administration and THC/CBD ratio further complicates this interaction (28). For example, when CBD is administered prior to THC it has been shown to potentiate its effects, but this potentiation does not occur when they are administered concurrently (28).
Thus, there is a critical gap in the knowledge surrounding co-use of CBD and THC. This paucity in the literature combined with the increasing prevalence of both CBD and MJ use, highlight the importance of examining simultaneous use of CBD and MJ. The purpose of the present study was to investigate multivariate patterns that are associated with isolated use vs. co-use of MJ and CBD.
The present cross-sectional survey study was conducted with Internal Review Board approval from the University of Texas at Dallas.
We recruited adults who self-reported CBD use via online advertisements posted on Dallas-Fort Worth and CBD forums (Reddit, Craigslist, Discord, and NextDoor). Inclusion criteria for all respondents was as follows: the endorsement of current CBD use, aged 18 years or older, and, written informed consent.
The study was conducted online in its entirety via Qualtrics Research Software (29). Respondents from the advertisements were directed to the web-based survey in order to participate in the study. The first page of the survey described the informed consent procedures. In order to ensure understanding of the purpose and procedure of the study, the respondents were asked to answer three multiple choice questions about the study. Answering all of the questions correctly was a pre-requisite for informed consent. Those who answered all of the questions correctly were then asked to provide a digital signature to document informed consent to participate in the study. After the digital signature page was completed, the survey assessments began. Those who did not provide a digital signature could not progress with the survey assessments. No identifying information was collected in this survey.
Compensation for study participation was optional. Those who opted for compensation were directed to a different survey. This kept the “data collection” survey and “optional compensation” survey separate such that information could not be linked to respondents' identifying information, thus ensuring anonymity. Following compensation, information from the “optional compensation” survey was destroyed.
The survey used in the present study was adapted from Corroon and Phillips (22) and was created using Qualtrics survey software (29). This survey included questions designed to measure respondent history of use, rate of use, method of self-administration, and the medical indication of CBD use. We also collected sociodemographic data including biological sex, age, and highest level of education. In order to measure respondents' cannabis, nicotine, and alcohol use behavior the following assessments were included in the survey: the Cannabis Use Disorders Identification Test—Revised [CUDIT-R (30)], The Fagerstrom Test for Nicotine Dependence—Revised, [FTND-R; (31)], and the Alcohol Use Disorder Identification Test [AUDIT; (32)]. Quality control of participant responses was carried out using recommendations from Teitcher et al. (33) that examined response times as a metric to detect outliers and examining response patterns to detect dubious responses.
All analyses were conducted in RStudio (34) using R 3.6.3 (35). Descriptive statistics were calculated to examine CBD use characteristics, sociodemographic variables, methods of CBD administration, medicinal CBD use, cannabis, nicotine, and alcohol use characteristics. Chi-square and ANOVA tests were used for comparisons of MJ endorsement groups across variables.
To elucidate possible relationships between multiple variables, multiple correspondence analysis (MCA) in the ExPosition package (36) was used. MCA is an extension of correspondence analysis (CA) and a generalization of principal component analysis (PCA). It is a multivariate analysis technique that allows for the investigation of potential relationships between multiple categorical variables (37–40). Similar to PCA, MCA dimensions are orthogonal to each other and independently explain as much of the variance as possible (41, 42). The Kaiser line test was performed to determine the number of dimensions to retain for further analysis. This test is based on the Kaiser criterion, which recommends retention of dimensions with eigenvalues ≥1. The purple points in Figure 1, show dimensions with eigenvalues that meet Kaiser criterion. The line is generated based on the relative location of the “elbow” of the scree plot where the variance represented by one dimension is not statistically different than that of the next (43). MCA reduces the number of dimensions seen in a given dataset and converts both variables and respondents into factor scores. This factor score calculation and data dimension reduction allow for the visual representation of both variables and respondents along a two-dimensional plane. When examining the factor plots, points (representing variables or respondents) that are plotted closer together have a greater association with each other (38, 39, 44, 45). Variable stability and statistical inferences pertaining to MJ group differences were evaluated via bootstrap resampling (46), bootstrap ratio and 95% confidence interval calculation (47), all of which were carried out with the InPosition package (36). The significance threshold for all analyses was set at p < 0.05.
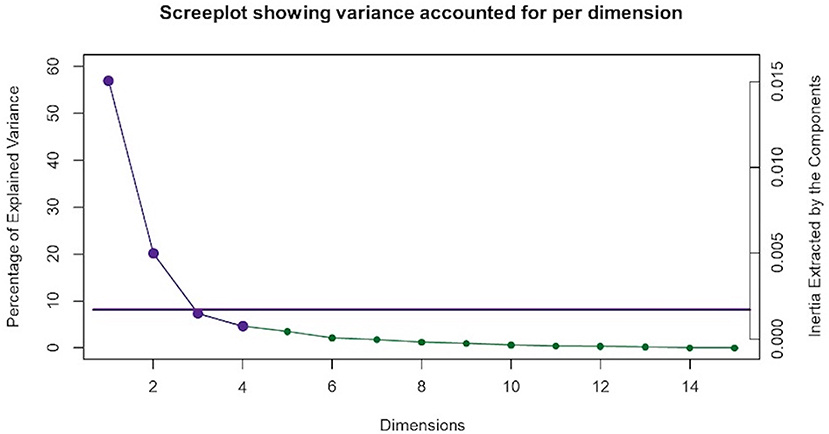
Figure 1. Multiple correspondence analysis (MCA) screeplot. MCA identified four significant dimensions accounting for a combined total of 89% of the variance. The screeplot shows two statistically significant dimensions (dimensions 1 and 2) that survived the Kaiser line test and accounted for 77% of the variance. The purple Kaiser line is a visual representation of the “screetest.”
Two hundred and forty-five individuals responded to the online survey. Of these, 53 had partial data and were excluded. Of the complete surveys, nine had response times classified as outliers using the graphics package from R 3.6.3 (35) and were consequently excluded. Lastly, one respondent's response pattern exhibited signs of malingering and was also excluded. In this study, malingering was defined as having the same response (e.g., all “yes” or “10”) to all of the survey questions that also then conflict with each other. In this particular case, the respondent endorsed the most extreme answer in the Likert scale questions and answered “yes” for every yes or no binary question. This pattern revealed inconsistent responses across similar questions. After these quality control steps, a total of 182 respondents were included in further analyses (112 males, 70 females). See Table 1 for respondents' demographic and drug use information. Respondents were classified into concurrent MJ and CBD use (CBD + MJ) (N = 105), and, CBD only use (N = 77). The two groups were significantly different in age [χ2(5) = 15.67, p = 0.008], education [χ2(7) = 15.30, p = 0.032], and nicotine use [χ2(1) = 15.67, p = 0.007]. CBD+MJ users were younger, had less years of education and greater nicotine use than CBD only users. CBD+MJ users reported greater number of CBD self-administration methods [F(1,180) = 16.73, p < 0.001, = 0.09]. Specifically, there were significant differences between CBD+MJ users and CBD only users in the following CBD self-administration methods: sublingual [χ2(1) = 4.45, p = 0.035, vaping χ2(1) = 6.07, p = 0.014], smoking [χ2(1) = 21.49, p = 0.001] and edible [χ2(1) = 5.39, p = 0.020] administration (Table 2).
MCA identified four significant dimensions accounting for a combined total of 89% of the variance (see Figure 1). Dimensions 1 and 2 survived the Kaiser line test and were retained for further analyses. Together these two dimensions accounted for 77% of the variance. Dimension 1 accounted for 57%, while dimension 2 accounted for 20% of the variance. 95% mean confidence intervals via bootstrap resampling showed that dimension 2 best separated CBD+MJ respondents from CBD only respondents (see Figure 2). Based on the variable factor score map (see Figure 3), dimension 1 separated respondents primarily based on ailments indicated for the use of CBD. CBD+MJ use was associated with endorsement of ailments (anxiety, depression, physical pain, arthritis, migraines, and sleep disorders), high school level of education, being female, administration of CBD via topical, edible, and vaping, and using CBD more than once a day for longer than 2 years (see top right quadrant of Figure 3 and Table 3). CBD only use was associated with absence of ailments related to CBD use, possession of advanced graduate degrees (i.e., master's degree), fewer types of CBD administration, and use of CBD less than once a day and <3 months (see lower left quadrant of Figure 2 and Table 3).
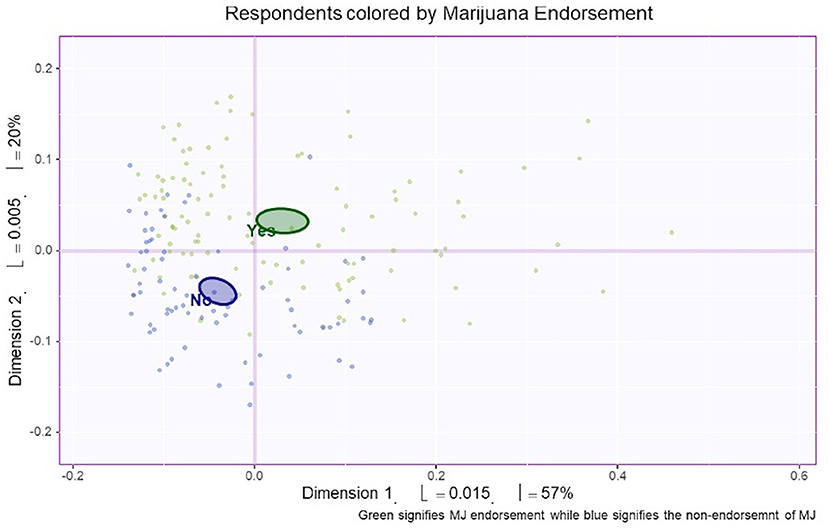
Figure 2. Bootstrap confidence interval comparisons between CBD users with and without marijuana co-use. Mean confidence intervals were created from the bootstrap resampling. Respondents were classified according to endorsement of marijuana use. Based on this figure, dimension 1 (the horizontal line) and dimension 2 (the vertical line) separated CBD users with (green) and without (purple) concurrent marijuana use.
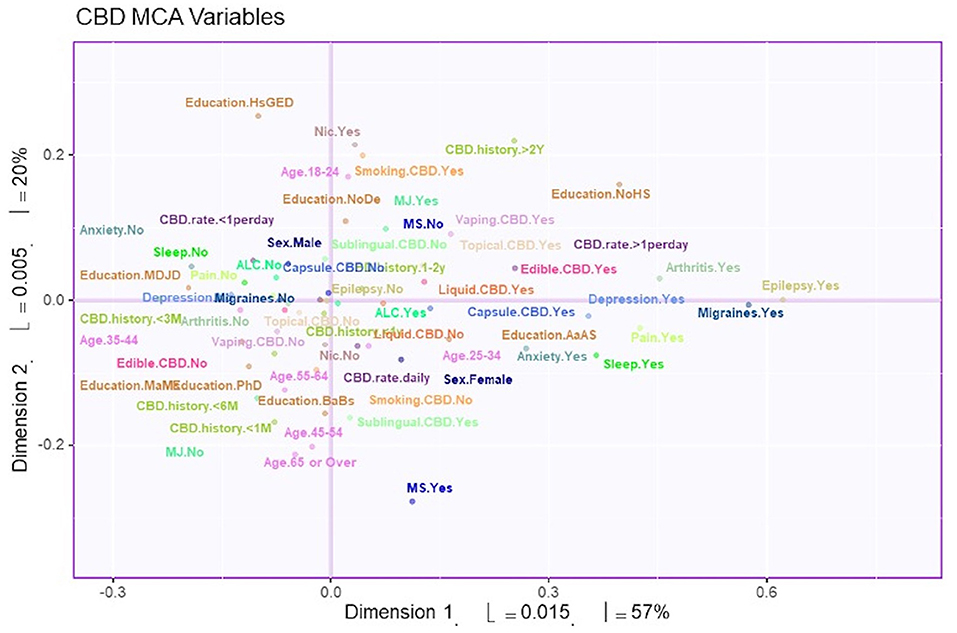
Figure 3. Survey variables plotted on dimensions 1 and 2. The variable factor scores plotted to show dimensions 1 and 2. These two dimensions account for 77% of the total variance. Distance from the axis indicates the association of the variable to the dimension. In addition, two points that are close to each other have greater association with each other.
Dimension 2 primarily separated respondents based on CBD and nicotine smoking behaviors. CBD+MJ use was associated with smoking and vaping CBD, use of CBD for more than 2 years at a rate of less than once day, smoking nicotine, <2 years of college level education, being male and between the ages of 18–24. CBD only use was associated with using CBD sublingually daily for <6 months, possession of a college education, being between the ages of 25–64, and self-reported anxiety, sleep disorders, MS (Figure 4).
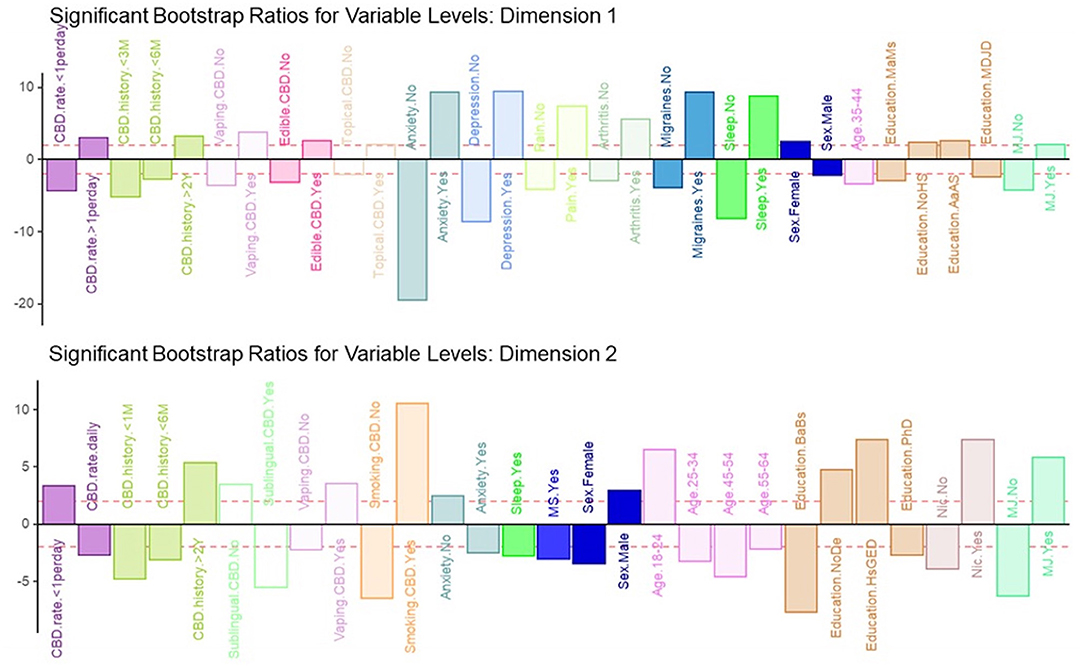
Figure 4. Significant bootstrap ratios for dimensions 1 & 2. Illustration of the significant bootstrap ratios for the variables for dimensions 1 and 2. Bars that are filled-in represent variables with a bootstrap ratio >2. Variables in the same side of the y-axis are positively associated with each other.
The present study sought to elucidate the factors that contribute to co-use of CBD and MJ. MCA was used to explore multivariate relationships within the data, yielding two MCA dimensions, which accounted for the majority of variance. Dimension one separated the CBD only users from CBD+MJ users primarily on ailments for which CBD was used for—anxiety, depression, physical pain, arthritis, migraines, and sleep disturbances. Dimension two separated the groups based on smoking CBD and nicotine.
Our results suggest that co-use of MJ in CBD users is associated with indication of CBD use for medical ailments, use of CBD for more than once a day for longer than 2 years, applying CBD topically or consuming it via vaping or edibles, being female, and having lower educational attainment. Regarding the medical ailments found in MCA dimension 1—anxiety, depression, physical pain, arthritis, migraines, and sleep disorders -, we found that the presence of one ailment was associated with the possible presence of other ailments. Given high co-morbidity between psychiatric disorders, it isn't surprising that anxiety and depression were associated in CBD+MJ users. For example, anxiety has been linked with both depression and substance use (48, 49) and is particularly prevalent in marijuana users (50, 51). Although CBD is more widely considered to provide relief from symptoms related to pain, arthritis and sleep disturbances, we found that MJ use in CBD users was associated with presence of these conditions. It is possible that these individuals either experience or have expectancies that MJ use in addition to CBD provides greater relief for these ailments. There is existing literature that describes the “entourage effect” in cannabis where full spectrum cannabis products that maintain the full profile of the cannabis plant leads to increased endogenous cannabinoid levels that are above and beyond that of the individual phytocannabinoid's isolated components, making them more efficacious for a variety of medical ailments (52). Indeed, medical MJ that contain a variety of cannabinoids including THC, CBD, as well as other cannabinoids and terpenes is often indicated for relief of epilepsy, movement disorders, and pain (53–55). In pain studies, 1:1 THC:CBD (Sativex) combinations have been shown to be more efficacious for cancer-related, arthritis, and other chronic pain compared to both placebo and THC isolate (56–58). In studies involving MS patients, THC (2.7 mg Tetranabinex) and CBD (2.5 mg Nabidiolex) dominant medications were shown to produce pain relief, but a 1:1 THC:CBD combination drug (Sativex) significantly improved sleep symptoms and pain above the other two (59). These initial studies demonstrate that 1:1 THC:CBD combination drugs provide greater symptom relief than isolates in clinical populations. It is also possible that this association could be due to known associations of mood disorders with medical conditions such as chronic pain, arthritis, sleep disturbances (60–62) and may play a mediating role between pain and sleep disturbances in arthritis patients (61). In this instance, pain may contribute to exacerbated depression symptoms in the long-term which, in turn, can result in sleep disturbances. Given the large literature on the associations between marijuana use and mood disorders, we speculate that this may also explain why mood disorders and medical conditions were associated with CBD+MJ users.
Previous results demonstrating that the use of both MJ and CBD is associated with a need for pain relief are consistent with our findings, as the bootstrap ratios indicated that both physical pain and endorsement of MJ co-use were related. The underlying mechanisms for the analgesic effect of CBD are subject to debate. However, previous studies have proposed CBD's interaction with the glycine and serotonergic systems as possible vehicles (63). In animal models of arthritis, locally applied CBD has been found to lessen joint pain and inflammation (64–66). This finding may explain why the endorsement of administering CBD topically was associated with the indication of CBD use for ailments such as chronic pain and arthritis.
We also found that CBD+MJ users are more likely to be female, which is concordant with results showing that female MJ users were more likely to report MJ use for the treatment of pain compared to male MJ users (67). Previous studies have shown using CBD more than once a day is associated with medicinal use (22). The perceived medicinal benefits could be a contributing factor to high rates of CBD use, despite a likelihood of a deep overestimation about the efficacy of CBD has been demonstrated (22, 23). Nevertheless, the literature corroborates our finding that co-use of CBD and MJ is more related to co-existing medical ailments than CBD use alone.
Our results for dimension 2 from the MCA suggest that being young (18–24 years old), male, having an associate degree or less, and the use of nicotine products is associated with the endorsement of MJ co-use. The findings are in accordance with previous research showing that 18–25-year-olds have the highest rate of MJ use (68), and that MJ users tend to have lower levels of education compared to non-users (69, 70). Previous studies have found that earlier initiation of MJ use was associated with lower academic and career attainment (71, 72), suggesting that CBD use may not mitigate the detrimental effects of MJ use.
Nicotine use was found to be a significant a variable associated with MJ co-use. The co-use of nicotine with MJ has been shown in previous research, with data suggesting that greater exposure to one, is associated with greater exposure to the other (73). When examining the CBD history variables, it was found that using CBD less than once a day for longer than 2 years was associated with the endorsement of MJ co-use. The sporadic use history of CBD seen in MJ users could be due to CBD exerting a non-effect on the subjective rewarding effects of THC (24). From the bootstrap ratios, smoking CBD seemed to have the highest association with the endorsement of MJ co-use. This finding makes sense pharmacologically speaking, as smoking has been found to yield the highest plasma concentration in the shortest amount time in both CBD (74) and MJ use (75, 76). In this instance, smoking and vaping methods of administration could be associated with MJ and CBD co-use due to increased familiarity with these methods in MJ users. This is in line with previous studies showing that both vaping and smoking are popular methods of administration in experienced MJ users (77, 78).
Previous findings have suggested that even though the effects of THC and CBD do not physiologically influence each other, the high rate of MJ co-use in the CBD using population may in part be due to MJ users having greater familiarity with CBD (22). The results of the present study support this claim as co-use was associated with using CBD longer but infrequently. Additionally, the methods of CBD administration that were associated with MJ use were methods that are most commonly seen in MJ use (e.g., edibles, vaping, and smoking) (79, 80).
Our findings suggest that co-use of MJ in CBD users may be influenced by several factors, with medical ailments and smoking behavior being primary factors. Although the co-use of MJ in CBD users is associated with factors that have been widely reported to be associated with MJ use, it is surprising to note that the presence of both psychological and medical conditions is more associated with CBD+MJ use than CBD use alone. This suggests that the use of these substances for symptom relief should be an important consideration for future studies.
Due to the cross-sectional nature of the present study, the temporal relationship between CBD use and MJ use cannot be established. The present study also relied on self-reported measures and must take into account issues with reliability. Several studies have explored the reliability and validity of survey measures, including those performed online via similar platforms such as those used in this study. These studies have found that respondents tend to use satisficing or choosing “good enough” answers which increases consistency, reliability, and convergent validity of measures but decreases discriminant validity (81). This, along with our quality control procedures and our use of previously validated questionnaires may mitigate some of the potential limitations of the survey approach. Furthermore, we followed recommendations from previous studies such as: designing the questionnaire in such a way to improve response rates, piloting the survey prior to distribution, and only asking questions that are applicable toward our research goal (82). Based on these recommendations and guidance provided by previous research on using survey approaches to measure substance use (83), we constructed our measurements and analytic approach to avoid common pitfalls. For example, in the survey we emphasized the confidentiality of all information provided by respondents and only used validated measures to minimize measurement error.
Additionally, there is no certainty that the survey respondents truly were diagnosed with the psychiatric conditions they endorsed. In this instance, we assume respondents are taking CBD for symptoms related to endorsed ailments, but these statements cannot be confirmed without professional diagnoses. Moreover, it is likely that due to the nature of the study respondents may have under-estimated their frequency of self-administration, tolerance, and other dependence symptoms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Internal Review Board, University of Texas at Dallas. The patients/participants provided their written informed consent to participate in this study.
JV developed the study concept and design, conducted the acquisition, analyses and interpretation of the data, and drafted the manuscript. MT contributed to the data analyses and interpretation, and drafted the manuscript. FF contributed to the concept and design of the study and critical revisions and approval of the submitted manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Corroon J, Kight R. Regulatory status of cannabidiol in the united states: a perspective. Cannabis Cannabinoid Res. (2018) 3:190–4. doi: 10.1089/can.2018.0030
3. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. (2017) 2:139–54. doi: 10.1089/can.2016.0034
4. Valim Brigante TA, Abe FR, Zuardi AW, Hallak JEC, Crippa JAS, de Oliveira DP. Cannabidiol did not induce teratogenicity or neurotoxicity in exposed zebrafish embryos. Chem Biol Interact. (2018) 291:81–6. doi: 10.1016/j.cbi.2018.06.008
5. Cerne K. Toxicological properties of Δ9-tetrahydrocannabinol and cannabidiol. Arh Hig Rada Toksikol. (2020) 71:1–11. doi: 10.2478/aiht-2020-71-3301
6. Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend. (2017) 172:9–13. doi: 10.1016/j.drugalcdep.2016.11.030
7. Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epil Behav. (2018) 88:162–71. doi: 10.1016/j.yebeh.2018.07.027
8. Dos Santos RG, Guimarães FS, Crippa JAS, Hallak JEC, Rossi GN, Rocha JM, et al. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. (2020) 16:517–26. doi: 10.1080/17425255.2020.1754793
9. Cocchetto DM, Cook LF, Cato AE. A critical review of the safety and antiemetic efficacy of delta-9-tetrahydrocannabinol. Drug Intell Clin Pharm. (1981) 15:867–75. doi: 10.1177/106002808101501104
10. McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. (2015) 172:737–53. doi: 10.1111/bph.12944
11. Withey SL, Bergman J, Huestis MA, George SR, Madras BK. THC and CBD blood and brain concentrations following daily administration to adolescent primates. Drug Alcohol Depend. (2020) 213:108129. doi: 10.1016/j.drugalcdep.2020.108129
12. Campos AC, Fogaça MV, Sonego AB, Guimarães FS. Cannabidiol, neuroprotection neuropsychiatric disorders. Pharmacol Res. (2016) 112:119–127. doi: 10.1016/j.phrs.2016.01.033
13. Curran VH, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Δ 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. (2002) 164:61–70. doi: 10.1007/s00213-002-1169-0
14. Freeman AM, Petrilli K, Lees R, Hindocha C, Mokrysz C, Curran HV, et al. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci Biobeh Rev. (2019) 107:696–712. doi: 10.1016/j.neubiorev.2019.09.036
15. Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. (2016) 73:292–7. doi: 10.1001/jamapsychiatry.2015.3278
16. Zuardi AW, de Souza Crippa JA, Hallak JEC, Campos AC, Guimarães FS. Chapter e13—the anxiolytic effects of Cannabidiol (CBD). In: Preedy VR, editors. Handbook of Cannabis and Related Pathologies. Academic Press (2017) p. e131–9. doi: 10.1016/B978-0-12-800756-3.00097-1
17. Kloft L. Review: the efficacy of Cannabidiol (CBD) as potential antipsychotic medication. Maastr Student J Psychol Neurosci. (2017) 6:1.
18. Crippa JA, Guimarães FS, Campos AC, Zuardi AW. Translational investigation of the therapeutic potential of Cannabidiol (CBD): toward a new age. Front Immunol. (2018) 9:2009. doi: 10.3389/fimmu.2018.02009
19. Elsaid S, Kloiber S, Le Foll B. Chapter Two - Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre-clinical clinical findings. Progr Mol Biol Transl Sci. (2019) 167:25–75. doi: 10.1016/bs.pmbts.2019.06.005
20. Hermann D, Schneider M. Potential protective effects of cannabidiol on neuroanatomical alterations in cannabis users psychosis: a critical review. Curr Pharm Des. (2012) 18:4897–905. doi: 10.2174/138161212802884825
21. Li H, Liu Y, Tian D, Tian L, Ju X, Qi L, et al. Overview of cannabidiol (CBD) its analogues: structures, biological activities, neuroprotective mechanisms in epilepsy Alzheimer's disease. Euro J Med Chem. (2020) 192:112163. doi: 10.1016/j.ejmech.2020.112163
22. Corroon J, Phillips JA. A cross-sectional study of cannabidiol users. Cannabis Cannabinoid Res. (2018) 3:152–61. doi: 10.1089/can.2018.0006
23. Wheeler M, Merten JW, Gordon BT, Hamadi H. CBD (Cannabidiol) product attitudes, knowledge, and use among young adults. Subst Use Misuse. (2020) 55:1–8. doi: 10.1080/10826084.2020.1729201
24. Haney M, Malcolm RJ, Babalonis S, Nuzzo PA, Cooper ZD, Bedi G, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. (2016) 41:1974–82. doi: 10.1038/npp.2015.367
25. Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [corrected]. Br J Psychiatry. (2010) 197:285–90. doi: 10.1192/bjp.bp.110.077503
26. Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJA, et al. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol. (2015) 25:325–34. doi: 10.1016/j.euroneuro.2014.11.014
27. Arkell TR, Lintzeris N, Kevin RC, Ramaekers JG, Vandrey R, Irwin C, et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology. (2019) 236:2713–24. doi: 10.1007/s00213-019-05246-8
28. Zuardi AW, Hallak JEC, Crippa JAS. Interaction between cannabidiol (CBD) Δ9-tetrahydrocannabinol (THC): influence of administration interval dose ratio between the cannabinoids. Psychopharmacology. (2012) 219:247–9. doi: 10.1007/s00213-011-2495-x
30. Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. (2010) 110:137–43. doi: 10.1016/j.drugalcdep.2010.02.017
31. Korte KJ, Capron DW, Zvolensky M, Schmidt NB. The Fagerström test for nicotine dependence: do revisions in the item scoring enhance the psychometric properties? Addict Behav. (2013) 38:1757–63. doi: 10.1016/j.addbeh.2012.10.013
32. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption: II. Addiction. (1993) 88:1–804. doi: 10.1111/j.1360-0443.1993.tb02093.x
33. Teitcher JE, Bockting WO, Bauermeister JA, Hoefer CJ, Miner MH, Klitzman RL. Detecting, preventing, and responding to “fraudsters” in internet research: ethics and tradeoffs. J Law Med Ethics. (2015) 43:116–33. doi: 10.1111/jlme.12200
35. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2020). Retrieved from: https://www.R-project.org/
36. Beaton D, Chin Fatt CR, Abdi H. An ExPosition of multivariate analysis with the singular value decomposition in R. Comput Stati Data Analysis. (2014) 72:176–89. doi: 10.1016/j.csda.2013.11.006
37. Ayele D, Zewotir T, Mwambi H. Multiple correspondence analysis as a tool for analysis of large health surveys in African settings. Afr Health Sci. (2014) 14:1036–45. doi: 10.4314/ahs.v14i4.35
38. Costa PS, Santos NC, Cunha P, Cotter J, Sousa N. The use of multiple correspondence analysis to explore associations between categories of qualitative variables in healthy ageing. J Aging Res. (2013) 2013:302163. doi: 10.1155/2013/302163
39. Greenacre M, Blasius J. Multiple Correspondence Analysis and Related Methods. CRC Press. (2006).
40. Sourial N, Wolfson C, Zhu B, Quail J, Fletcher J, Karunananthan S, et al. Correspondence analysis is a useful tool to uncover the relationships among categorical variables. J Clin Epidemiol. (2010) 63:638–46. doi: 10.1016/j.jclinepi.2009.08.008
41. Abdi H, Valentin D. Multiple correspondence analysis. In: Salkind N, editor. Encyclopedia of Measurement Statistics. Thousand Oaks, CA: Sage (2007).
43. Braeken J. An empirical Kaiser Criterion. Psychol Methods. (2017) 22:450–66. doi: 10.1037/met0000074
45. Pei R, Ji-Ke C, Yang S, Nan L, Wang Q, Zhang S, et al. Risk factors for HIV infection among 15 to 25-year-old rural unmarried Yi adolescents in an ethnic minority region of China. Medicine. (2018) 97:e12279. doi: 10.1097/MD.0000000000012279
47. Abdi H, Dunlop JP, Williams LJ. How to compute reliability estimates and display confidence and tolerance intervals for pattern classifiers using the Bootstrap and 3-way multidimensional scaling (DISTATIS). NeuroImage. (2009) 45:89–95. doi: 10.1016/j.neuroimage.2008.11.008
48. Lai HMX, Cleary M, Sitharthan T, Hunt GE. Prevalence of comorbid substance use, anxiety mood disorders in epidemiological surveys, 1990–2014: a systematic review meta-analysis. Drug Alcohol Depend. (2015) 154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031
49. Mohammadi MR, Salehi M, Khaleghi A, Hooshyari Z, Mostafavi SA, Ahmadi N, et al. Social anxiety disorder among children adolescents: a nationwide survey of prevalence, socio-demographic characteristics, risk factors co-morbidities. J Affect Disord. (2020) 263:450–7. doi: 10.1016/j.jad.2019.12.015
50. Gukasyan N, Strain EC. Relationship between cannabis use frequency and major depressive disorder in adolescents: findings from the National Survey on Drug Use and Health 2012–2017. Drug Alcohol Depend. (2020) 208:107867. doi: 10.1016/j.drugalcdep.2020.107867
51. Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population–a meta-analysis of 31 studies. BMC Psychiatry. (2014) 14:136–6. doi: 10.1186/1471-244X-14-136
52. Russo EB. The case for the entourage effect conventional breeding of clinical Cannabis: no “strain,” no gain. Front Plant Sci. (2019) 9:1969. doi: 10.3389/fpls.2018.01969
53. Mouhamed Y, Vishnyakov A, Qorri B, Sambi M, Frank SS, Nowierski C, et al. Therapeutic potential of medicinal marijuana: an educational primer for health care professionals. Drug Healthcare Patient Safety. (2018) 10:45–66. doi: 10.2147/DHPS.S158592
54. National Academies of Sciences Engineering and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. Therapeutic Effects of Cannabis and Cannabinoids. Washington, DC: National Academies Press. (2017).
55. Sznitman SR, Zolotov Y. Cannabis for therapeutic purposes public health safety: a systematic critical review. Int J Drug Policy. (2015) 26:20–9. doi: 10.1016/j.drugpo.2014.09.005
56. Baron EP, Lucas P, Eades J, Hogue O. Patterns of medicinal cannabis use, strain analysis, substitution effect among patients with migraine, headache, arthritis, chronic pain in a medicinal cannabis cohort. J Headache Pain. (2018). 19:37. doi: 10.1186/s10194-018-0862-2
57. Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manag. (2010) 39:167–79. doi: 10.1016/j.jpainsymman.2009.06.008
58. Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety tolerability of THC/CBD oromucosal spray oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manag. (2013) 46:207–18. doi: 10.1016/j.jpainsymman.2012.07.014
59. Russo EB, Guy GW, Robson PJ. Cannabis, pain, sleep: lessons from therapeutic clinical trials of Sativex©, a cannabis-based medicine. Chem Biodiv. (2007) 4:1729–43. doi: 10.1002/cbdv.200790150
60. Annagür BB, Uguz F, Apiliogullari S, Kara I, Gunduz S. Psychiatric disorders and association with quality of sleep and quality of life in patients with chronic pain: a SCID-based study. Pain Med. (2014) 15:772–81. doi: 10.1111/pme.12390
61. Nicassio PM, Ormseth SR, Kay M, Custodio M, Irwin MR, Olmstead R, et al. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. (2012) 153:107–12. doi: 10.1016/j.pain.2011.09.024
62. Seow LSE, Tan XW, Chong SA, Vaingankar JA, Abdin E, Shafie S, et al. Independent and combined associations of sleep duration and sleep quality with common physical and mental disorders: results from a multi-ethnic population-based study. PLoS ONE. (2020) 15:e0235816–e0235816. doi: 10.1371/journal.pone.0235816
63. Argueta DA, Ventura CM, Kiven S, Sagi V, Gupta K. A balanced approach for cannabidiol use in chronic pain. Front Pharmacol. (2020) 11:561. doi: 10.3389/fphar.2020.00561
64. Fitzcharles MA, Clauw DJ, Hauser W. A cautious hope for cannabidiol (CBD) in rheumatology care. Arthritis Care Res. (2020). doi: 10.1002/acr.24176. [Epub ahead of print].
65. Philpott HT, O'Brien M, McDougall JJ. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain. (2017) 158:2442–51. doi: 10.1097/j.pain.0000000000001052
66. Xu DH, Cullen BD, Tang M, Fang Y. The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol. (2020) 21:390–402. doi: 10.2174/1389201020666191202111534
67. Fales JL, Ladd BO, Magnan RE. Pain relief as a motivation for cannabis use among young adult users with and without chronic pain. J Pain. (2019) 20:908–16. doi: 10.1016/j.jpain.2019.02.001
68. SAMHSA. 2019 National Survey of Drug Use Health (NSDUH). Releases | CBHSQ Data (2019). Available online at: https://www.samhsa.gov/data/release/2019-national-survey-drug-use-and-health-nsduh-releases (accessed December 18, 2020).
69. Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. (2015) 110:19–35. doi: 10.1111/add.12703
70. Hall W. Alcohol cannabis: comparing their adverse health effects regulatory regimes. Int J Drug Policy. (2017) 42:57–62. doi: 10.1016/j.drugpo.2016.10.021
71. Melchior M, Bolze C, Fombonne E, Surkan PJ, Pryor L, Jauffret-Roustide M. Early cannabis initiation and educational attainment: is the association causal? Data from the French TEMPO study. Int J Epidemiol. (2017) 46:1641–50. doi: 10.1093/ije/dyx065
72. Williams J, van Ours JC. Hazardous or not? Cannabis use and early labor market experiences of young men. Health Econ. (2020) 29:1148–60. doi: 10.1002/hec.4125
73. Doran N, Myers MG, Correa J, Strong DR, Tully Y, Pulvers K. Marijuana use among young adult non-daily cigarette smokers over time. Add Behav. (2019) 95:91–7. doi: 10.1016/j.addbeh.2019.03.007
74. Millar SA, Stone NL, Yates AS, O'Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. (2018) 9:1365. doi: 10.3389/fphar.2018.01365
75. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. (2003) 42:327–60. doi: 10.2165/00003088-200342040-00003
76. Heuberger JA, Guan Z, Oyetayo OO, Klumpers L, Morrison PD, Beumer TL, et al. Population pharmacokinetic model of THC integrates oral, intravenous, and pulmonary dosing and characterizes short- and long-term pharmacokinetics. Clin Pharmacokinet. (2015) 54:209–19. doi: 10.1007/s40262-014-0195-5
77. Aston ER, Farris SG, Metrik J, Rosen RK. Vaporization of marijuana among recreational users: a qualitative study. J Stud Alcohol Drugs. (2019) 80:56–62. doi: 10.15288/jsad.2019.80.56
78. Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ. Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int J Drug Policy. (2016) 36:141–7. doi: 10.1016/j.drugpo.2016.02.022
79. Knapp AA, Lee DC, Borodovsky JT, Auty SG, Gabrielli J, Budney AJ. Emerging trends in cannabis administration among adolescent cannabis users. J Adolescent Health. (2019) 64:487–93. doi: 10.1016/j.jadohealth.2018.07.012
80. Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use - basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. (2018) 52:87–96. doi: 10.1016/j.drugpo.2017.11.008
81. Hamby T, Taylor W. Survey satisficing inflates reliability validity measures: an experimental comparison of College amazon mechanical turk samples. Educ Psychol Meas. (2016) 76:912–32. doi: 10.1177/0013164415627349
82. Jones T, Baxter M, Khanduja V. A quick guide to survey research. Annals R College Surg Engl. (2013) 95:5–7. doi: 10.1308/003588413X13511609956372
Keywords: cannabidiol, marijuana, multiple correspondance analysis, cbd, thc, HEMP
Citation: Vilches JR, Taylor MB and Filbey FM (2021) A Multiple Correspondence Analysis of Patterns of CBD Use in Hemp and Marijuana Users. Front. Psychiatry 11:624012. doi: 10.3389/fpsyt.2020.624012
Received: 30 October 2020; Accepted: 14 December 2020;
Published: 14 January 2021.
Edited by:
Marc N. Potenza, Yale University, United StatesReviewed by:
Raffaele Giorgetti, Marche Polytechnic University, ItalyCopyright © 2021 Vilches, Taylor and Filbey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca M. Filbey, ZnJhbmNlc2NhLmZpbGJleUB1dGRhbGxhcy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.