- 1Deakin University, Institute for Mental and Physical Health and Clinical Translation, School of Medicine, Geelong, VIC, Australia
- 2University Hospital Geelong, Barwon Health, Geelong, VIC, Australia
- 3Institute of Clinical Medicine/Psychiatry, University of Eastern Finland, Kuopio, Finland
- 4Kuopio Musculoskeletal Research Unit, Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland
- 5Departments of Psychiatry, South-Savonia Hospital District, Mikkeli, Finland
- 6Departments of Psychiatry, North Karelia Central Hospital, Joensuu, Finland
- 7Departments of Psychiatry, SOTE, Iisalmi, Finland
- 8Department of Psychiatry, Oulu University Hospital, Oulu, Finland
- 9Department of Medicine-Western Health, The University of Melbourne, St. Albans, VIC, Australia
- 10Department of Psychiatry, The University of Melbourne, Parkville, VIC, Australia
- 11Orygen the National Center of Excellence in Youth Mental Health, Center for Youth Mental Health, Florey Institute of Neuroscience and Mental Health, University of Melbourne, Parkville, VIC, Australia
We examined whether personality disorders (PDs) (any, cluster A/B/C) were associated with bone mineral density (BMD) in a population-based sample of Australian women (n = 696). Personality and mood disorders were assessed using semi-structured diagnostic interviews. BMD was measured at the spine, hip, and total body using dual-energy x-ray absorptiometry (GE-Lunar Prodigy). Anthropometrics, medication use, physical conditions, and lifestyle factors were documented. The association between PDs (any, cluster A/B/C) and BMD (spine/hip/total body) was examined with multiple linear regression models. The best models were identified by backward elimination including age, weight, physical activity, smoking status, alcohol consumption, dietary calcium intake, mood disorders, physical multimorbidity, socioeconomic status, and medications affecting bone. The variables were retained in the model if p < 0.05. All potential interactions in final models were tested. Those with cluster A PD, compared to those without, had 6.7% lower hip BMD [age, weight adjusted mean 0.853 (95% CI 0.803–0.903) vs. 0.910 (95% CI 0.901–0.919) g/cm2, p = 0.027] and 3.4% lower total body BMD [age, weight, smoking, alcohol, calcium adjusted mean 1.102 (95% CI 1.064–1.140) vs. 1.139 (95% CI 1.128–1.150) g/cm2, p = 0.056]. No associations were observed between cluster B/C PDs and hip/total body BMD or between any of the PD clusters and spine BMD. To our knowledge, this study is the first to investigate the bone health of women with PD in a population-based sample. Given the paucity of literature, replication and longitudinal research including the examination of underlying mechanisms and sex differences are warranted.
Introduction
Emerging early in the life span, a personality disorder (PD) presents as enduring patterns of maladaptive thinking, emotional and inner experiences, and behaviors—causing significant distress and impairment (1). PDs have traditionally been conceptualized as 10 distinct disorders that are organized within three clusters: A, “odd-eccentric” features; B, “dramatic/emotional/erratic” features; and C, “anxious/fearful” features (1).
Growing evidence points to adverse physical health comorbidities among people with PD, including chronic physical, pain, and sleep conditions (2, 3)—however, little is known regarding the factors underlying these associations (2). PD also appears to be associated with musculoskeletal problems (2, 3); however, there is a paucity of research examining the underlying bone health of people with PD.
Psychological/behavioral factors including impulsivity and self-sabotaging type behaviors, which are typical of “cluster B” symptomatology, appear to lead to difficulties with general health treatment compliance (4) and thus plausibly influence the physical health of people with these PDs. Biological/other health-related factors are suggested to include (but may not be limited to) multiple and long-term poor health-related lifestyle choices and medication use (5, 6) and metabolic syndrome (3). However, little is known whether different PD clusters (i.e., different presentations of PD symptomatology) are independently associated with poorer bone health compared to people without PDs.
Kahl et al. (7, 8) conducted the first clinical-based studies showing that comorbid borderline PD (a cluster B PD) and depressive disorders were associated with reduced bone mineral density (BMD) in younger women. Yet, there are no studies from the population-based setting or across the wider adult age range. Therefore, we explored direct associations between PD and BMD in a large population-based sample of Australian women—exploring the role of age, anthropometrics, lifestyle factors, multimorbidity, medication use, and mood disorders.
Materials and Methods
This study examined data from women participating in the population-based Geelong Osteoporosis Study (GOS) in Australia (9). Originally, 1,494 women (response rate 77.1%) were randomly selected from the electoral rolls for the Barwon Statistical Division during 1994–1996. Complete details have been published elsewhere (9–11). We utilized data collected from participants who returned for assessment during 2011–2014. Participants who did not complete a psychiatric assessment (n = 80), had missing BMD data (n = 50), or with multiple PD clusters (n = 22) were excluded. This study consisted of n = 696 women aged ≥28 years. Barwon Health Human Research Ethics Committee approved the study, and written informed consent was obtained from all participants.
Areal BMD (g/cm2) was measured at the posterior–anterior (PA) spine (L2–4), femoral neck (hip), and total body including head using dual-energy X-ray absorptiometry (Prodigy; GE Lunar, Madison, WI, USA). Technicians carried out all examinations and performed daily calibrations of the densitometer with equipment-specific phantoms. Osteoporosis was determined by a BMD T-score of <-2.5 at the spine and/or hip (12).
The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (DSM) Axis I Disorders, non-patient edition (SCID-I/NP) identified lifetime mood disorders [i.e., major depressive disorder (MDD), minor depression, bipolar disorder, dysthymia, mood disorders due to a general medical condition, and substance-induced mood disorder] (13). The Structured Clinical Interview for DSM-IV Axis II personality disorders (SCID-II) identified PDs (14). Consistent with the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), the 10 PDs were categorized into three clusters: cluster A PDs (i.e., schizoid, paranoid, and schizotypal PDs); cluster B PDs (i.e., antisocial, borderline, narcissistic, and histrionic PDs); and cluster C PDs (i.e., avoidant, dependent, and obsessive–compulsive PDs) (1). As previously published, all interviews were conducted by trained personnel with post-graduate qualifications in psychology (10).
Weight and height were measured to the nearest 0.1 kg or 0.1 cm, respectively. Current smoking and mobility were self-reported. Participants were deemed active if vigorous or light exercise was performed regularly; otherwise, participants were classified as sedentary. Alcohol consumption and calcium intake were estimated from a validated food frequency questionnaire (15). Current medication use affecting bone was recorded, including antiresorptive agents (bisphosphonates/selective estrogen receptor modulators), oral hormone replacement therapy, oral glucocorticoids, and antidepressants. Participants brought lists of medications or containers to the assessment to ensure accurate recordings. Participants self-reported the presence of physical conditions. These were confirmed by medical record, medication use, or clinical data, where possible, and included arthritis, cardiovascular disease, thyroid disorders, metabolic disorders, gastrointestinal disease, gastroesophageal reflux disease, syncope and seizures, recurrent headaches, pulmonary diseases, psoriasis, liver diseases, and cancers (16). Utilizing these data, we defined physical multimorbidity as the presence of ≥5 lifetime physical health disorders (17, 18). Area-based socioeconomic status (SES) was determined using the Index of Relative Socio-economic Advantage and Disadvantage (IRSAD). A lower score (Quintile 1) indicated greater disadvantage; a higher score, greater advantage (Quintile 5) (9).
Statistical analyses were performed using Minitab (version 18). Group differences were determined using t-tests for parametric variables, Kruskal–Wallis for nonparametric continuous variables—for discrete variables, chi-square tests or Fisher's exact test when expected cell counts were less than five. The association between PD (any, cluster A/B/C) and BMD (spine/hip/total body) was examined with multiple linear regression in unadjusted models (Model I) and “best models” (Model II). The best models were identified by backward elimination including age, weight, physical activity, smoking status, alcohol consumption, dietary calcium intake, mood disorders, physical multimorbidity, SES, and medications affecting bone (c.f. above). The variables were retained in the model if p < 0.05. All potential interactions in final models were tested.
Results
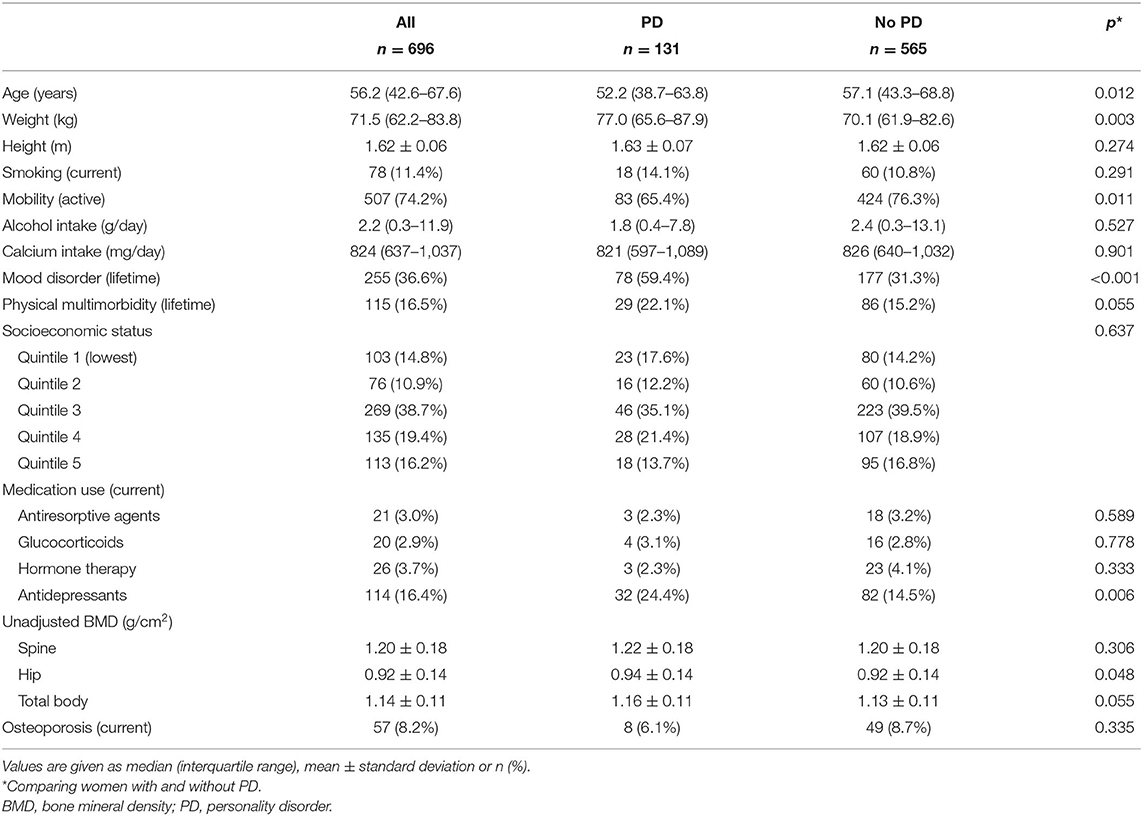
Of 696 women, 131 (18.8%) met the criteria for a PD. Women with PD were more likely to be younger, heavier, sedentary, use antidepressants, had a lifetime mood disorder, and had higher hip and total body BMD than those without PD (all p < 0.05); otherwise, the groups were similar (Table 1).
After age, weight adjustments, no differences were detected in spine, hip, or total body BMD between those with and without any PD (all p > 0.05).
The frequency of PD clusters was: 2.7%, cluster A (n = 19); <1%, cluster B (n = 5); and 15.4%, cluster C (n = 107).
Unadjusted and best models showing associations between PD clusters and BMD are presented in Table 2. Those with cluster A PD, compared to those without, had 6.7% lower hip BMD [age, weight adjusted mean 0.853 (95% CI 0.803–0.903) vs. 0.910 (95% CI 0.901–0.919) g/cm2, p = 0.027] and 3.4% lower total body BMD [age, weight, smoking, alcohol, calcium adjusted mean 1.102 (95% CI 1.064–1.140) vs. 1.139 (95% CI 1.128–1.150) g/cm2, p = 0.056]. No differences were detected in spine BMD between those with and without cluster A PD. Finally, no differences were detected in spine, hip, and total body BMD for those with and without cluster B or C PDs.
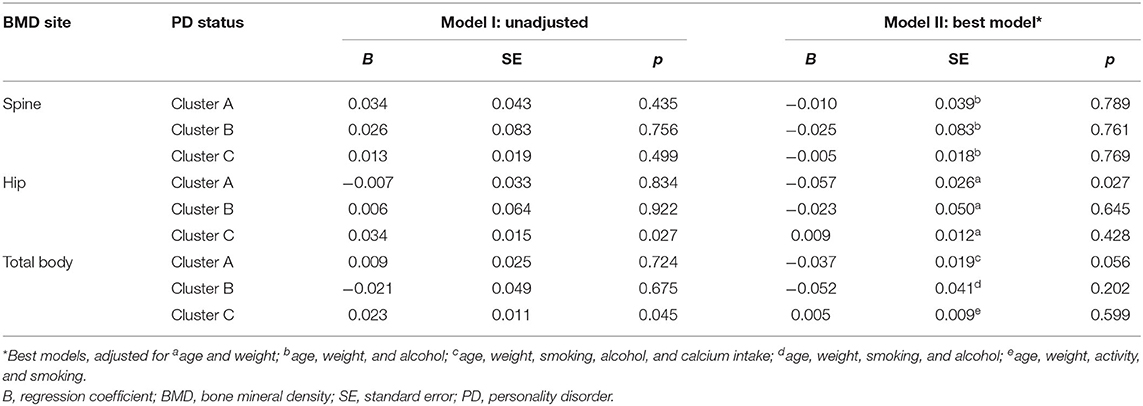
Table 2. Unadjusted and best models presenting associations between PD clusters and BMD using multiple regression.
Discussion
To our knowledge, this study is the first to investigate bone health of women with PD in a population-based sample. It showed that those with cluster A PD have lower hip/total body BMD than those without. There were no associations between pooled PDs, cluster B or cluster C PD and BMD at any site.
Kahl et al. (7, 8) suggested that young women with borderline PD and current major depression may be a group at risk of developing osteoporosis. In their clinical study, the BMD of 24 women with MDD, 16 with borderline PD, 23 with MDD and comorbid borderline PD, and 20 healthy control participants were compared (7). The MDD only group were further divided into younger/older age groups [mean age 30 years (MDD30); 43 years (MDD43), respectively]. Women with MDD and comorbid borderline PD had significantly lower BMD at the lumbar spine compared with women with borderline PD alone and the younger women with MDD (MDD30) (7). No significant associations were reported between the groups at the other sites (i.e., right and left femur and forearm of non-dominant hand) (7). Markers of bone turnover were also measured, and compared with healthy control participants, elevated levels of osteocalcin were reported among women with MDD and borderline PD group (7). However, C-terminal degradation products of type I collagen (CTx) were highest among the MDD43 group, and tumor necrosis factor-α, interleukin-6, and osteoprotegerin were highest among the MDD30 group (7). To summarize, they postulated that rather than the presence of borderline PD alone, the acuteness of a co-occurring MDD may contribute to bone loss through associated immune/endocrine disturbances (7, 8). Though not directly comparable, we detected no direct association between cluster B and BMD. However, the lack of associations between cluster B PD and BMD detected in the present study could also be due to power constraints.
The novelty of the current research precludes meaningful comparisons with other studies on cluster A/C in relation to bone health. It is possible that psychological/social factors/processes that are characteristic of cluster A PDs, including enduring social isolation and avoidance coping, may be a barrier to engaging with healthcare providers for preventative, ongoing monitoring of physical health and/or engagement with treatments (2, 19)—including bone health. It is also plausible that vitamin D intake could be a biological mechanism underlying the associations.
In terms of study limitations, power limitations prevented analyses investigating specific PDs, other psychiatric disorders, and underlying biological mechanisms. Specifically, we did not collect and, therefore, did not examine the role of vitamin D, which is a known regulator of bone and mineral metabolism, as a confounder/moderator of associations between PD and BMD. This may be an area that warrants further research, given the suggestion that vitamin D may play a role in the manifestation of reduced quality of life via poorer health status among postmenopausal women referred for evaluation for osteopenia/osteoporosis (20) and, separately, with poorer psychological and somatic symptomatology (21). We also acknowledge that the classification of PD is undergoing significant reform, and dimensional measures of personality were not examined. Strengths of this study include the wide adult age range, gold standard tool for assessing psychiatric disorders, and adjustment for many known confounders.
Our preliminary study supports an association between PD and bone health. A more substantive evidence base is needed to further uncover the clinical implications of a relationship between PD and bone health. It may be important to determine whether monitoring the bone health of people with PD and comorbidities is warranted, given polypharmacotherapy is often prescribed for the treatment of PD (22), and specific agents, such as selective serotonin reuptake inhibitors, anticonvulsants, and antipsychotics, are associated with decreased bone mass and increased risk for osteoporosis and related fractures (23). This may require a coordinated effort between primary and endocrinological care, given indications that psychological/behavioral symptoms of PD may be a barrier to general health treatment/compliance for some individuals (4).
Given the paucity of literature, replication and longitudinal research including the examination of underlying mechanisms and sex differences are warranted.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Barwon Health Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LW conceived and designed the study. LW and AS performed the analyses. All authors contributed to the interpretation of the data and drafting of the article, provided critical revisions to the article, and approved the final version to be published.
Funding
This work was supported by the National Health and Medical Research Council (NHMRC), Australia (454356 and 628582). LW was supported by an NHMRC Career Development Fellowship (1064272) and an NHMRC Investigator grant (1174060). HKH and SQ are supported by the Päivikki and Sakari Sohlberg Foundation and the Gyllenberg Foundation, Finland. BK was supported by the Australian Postgraduate Research Training Program and an Ian Scott Mental Health PhD Scholarship, Australian Rotary Health. MB was supported by an NHMRC Senior Principal Research Fellowship (1059660 and 1156072).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Professor Graham Giles of the Cancer Epidemiology Center of The Cancer Council Victoria for permission to use the Dietary Questionnaire for Epidemiological Studies (Version 2), Melbourne: The Cancer Council Victoria 1996.
Abbreviations
BMD, bone mineral density; CI, confidence interval; DSM, Diagnostic and Statistical Manual of Mental Disorders; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage; MDD, major depressive disorder; PD, personality disorder; SCID-I/NP, The Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders Axis I Disorders, non-patient edition; SCID-II, The Structured Clinical Interview for DSM-IV Axis II personality disorders; SES, socioeconomic status.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association (2013).
2. Dixon-Gordon KL, Conkey LC, Whalen DJ. Recent advances in understanding physical health problems in personality disorders. Curr Opin Psychol. (2018) 21:1–5. doi: 10.1016/j.copsyc.2017.08.036
3. Dixon-Gordon K, DWhalen D, Layden B, Chapman A. A systematic review of personality disorders and health outcomes. Can Psychol. (2015) 56:188–90. doi: 10.1037/cap0000024
4. Sansone RA, Bohinc RJ, Wiederman MW. Borderline personality symptomatology and compliance with general health care among internal medicine outpatients. Int J Psychiatry Clin Pract. (2015) 19:132–6. doi: 10.3109/13651501.2014.988269
5. Frankenburg FR, Zanarini MC. The association between borderline personality disorder and chronic medical illnesses, poor health-related lifestyle choices, and costly forms of health care utilization. J Clin Psychiatry. (2004) 65:1660–5. doi: 10.4088/JCP.v65n1211
6. Keuroghlian AS, Frankenburg FR, Zanarini MC. The relationship of chronic medical illnesses, poor health-related lifestyle choices, and health care utilization to recovery status in borderline patients over a decade of prospective follow-up. J Psychiatr Res. (2013) 47:1499–506. doi: 10.1016/j.jpsychires.2013.06.012
7. Kahl KG, Greggersen W, Rudolf S, Stoeckelhuber BM, Bergmann-Koester CU, Dibbelt L, et al. Bone mineral density, bone turnover, and osteoprotegerin in depressed women with and without borderline personality disorder. Psychosom Med. (2006) 68:669–74. doi: 10.1097/01.psy.0000237858.76880.3d
8. Kahl KG, Rudolf S, Stoeckelhuber BM, Dibbelt L, Gehl H-BB, Hohagen F, et al. Bone mineral density, markers of bone turnover, and cytokines in young women with borderline personality disorder with and without comorbid major depressive disorder. Am J Psychiatry. (2005) 162:168–74. doi: 10.1176/appi.ajp.162.1.168
9. Pasco JA, Nicholson GC, Kotowicz MA. Cohort profile: Geelong osteoporosis study. Int J Epidemiol. (2012) 41:1565–75. doi: 10.1093/ije/dyr148
10. Quirk SE, Berk M, Pasco JA, Brennan-Olsen SL, Chanen AM, Koivumaa-Honkanen H, et al. The prevalence, age distribution and comorbidity of personality disorders in Australian women. Aust N Z J Psychiatry. (2017) 51:141–50. doi: 10.1177/0004867416649032
11. Williams L, Jacka F, Pasco J, Henry M, Dodd S, Nicholson G, et al. The prevalence of mood and anxiety disorders in Australian women. Aust Psychiatry. (2010) 18:250–5. doi: 10.3109/10398561003731155
12. Henry MJ, Pasco JA, Pocock NA, Nicholson GC, Kotowicz MA. Reference ranges for bone densitometers adopted Australia-wide: Geelong osteoporosis study. Aust Radiol. (2004) 48:473–5. doi: 10.1111/j.1440-1673.2004.01351.x
13. First M, Spitzer RL, Gibbon ML, Williams J. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. New York, NY: New York State Psychiatric Institute, Biometrics Research (2002).
14. Spitzer R, Williams J, Gibbon M, First M. User's guide for the structured clinical interview for DSM-IV Axis II personality disorders (SCID-II). Washington, DC: American Psychiatry Press (1990).
15. Giles GG, Ireland PD. Dietary Questionnaire for Epidemiological Studies (Version 2). Melbourne, VIC: The Cancer Council Victoria (1996).
16. Quirk SE, Stuart AL, Brennan-Olsen SL, Pasco JA, Berk M, Chanen AM, et al. Physical health comorbidities in women with personality disorder: data from the Geelong Osteoporosis Study. Eur Psychiatry. (2016) 34:29–35. doi: 10.1016/j.eurpsy.2015.12.007
17. Quirk SE, Stuart AL, Berk M, Pasco JA, Brennan Olsen SL, Koivumaa-Honkanen H, et al. Personality disorder is an excess risk factor for physical multimorbidity among women with mental state disorders. Psychiatry Res. (2017) 257:546–9. doi: 10.1016/j.psychres.2017.08.010
18. Lukkala PS, Honkanen RJ, Rauma PH, Williams LJ, Quirk SE, Kröger H, et al. Life satisfaction and morbidity among postmenopausal women. PLoS ONE. (2016) 11:1–10. doi: 10.1371/journal.pone.0147521
19. Sanatinia R, Middleton SM, Lin T, Dale O, Crawford MJ. Quality of physical health care among patients with personality disorder. Personal Ment Health. (2015) 9:319–29. doi: 10.1002/pmh.1303
20. Martino G, Catalano A, Bellone F, Langher V, Lasco C, Penna A, et al. Quality of life in postmenopausal women: which role for vitamin D? Mediterr J Clin Psychol. (2018) 6:1–14. doi: 10.6092/2282-1619/2018.6.1875
21. Martino G, Catalano A, Bellone F, Sardella A, Lasco C, Caprì T, et al. Vitamin D status is associated with anxiety levels in postmenopausal women evaluated for osteoporosis. Mediterr J Clin Psychol. (2018) 6:1–16. doi: 10.6092/2282-1619/2018.6.1740
22. Mazza M, Marano G, Janiri L. An update on pharmacotherapy for personality disorders. Expert Opin Pharmacother. (2016) 17:1977–9. doi: 10.1080/14656566.2016.1220542
Keywords: comorbidity, personality disorder, psychiatry, physical health, medical condition, bone
Citation: Williams LJ, Quirk SE, Koivumaa-Honkanen H, Honkanen R, Pasco JA, Stuart AL, Kavanagh BE, Heikkinen J and Berk M (2020) Personality Disorder and Physical Health Comorbidities: A Link With Bone Health? Front. Psychiatry 11:602342. doi: 10.3389/fpsyt.2020.602342
Received: 03 September 2020; Accepted: 05 November 2020;
Published: 08 December 2020.
Edited by:
Gabriella Martino, University of Messina, ItalyReviewed by:
Antonino Catalano, University of Messina, ItalyFederica Bellone, University of Messina, Italy
Marianna Mazza, Catholic University of the Sacred Heart, Italy
Copyright © 2020 Williams, Quirk, Koivumaa-Honkanen, Honkanen, Pasco, Stuart, Kavanagh, Heikkinen and Berk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lana J. Williams, bC53aWxsaWFtc0BkZWFraW4uZWR1LmF1
 Lana J. Williams1,2*
Lana J. Williams1,2* Shae E. Quirk
Shae E. Quirk Heli Koivumaa-Honkanen
Heli Koivumaa-Honkanen Bianca E. Kavanagh
Bianca E. Kavanagh Jeremi Heikkinen
Jeremi Heikkinen