- 1Department of Addictive Disorders, Psychiatric University Clinic Basel, Basel, Switzerland
- 2Department of Addictive Disorders, Psychiatric Services Thurgau, Münsterlingen, Switzerland
- 3Center for Affective, Stress and Sleep Disorders (ZASS), Psychiatric University Hospital Basel, Basel, Switzerland
- 4Department of Clinical Research, University of Basel, Basel, Switzerland
- 5Division of Sport and Psychosocial Health, Department of Sport, Exercise, and Health, University of Basel, Basel, Switzerland
- 6Substance Abuse Prevention Research Center and Sleep Disorders Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 7School of Medicine, Tehran University of Medical Sciences (TUMS), Tehrren, Iran
- 8Department for Psychiatry, Psychotherapy and Psychosomatic, Psychiatric Hospital, University of Zurich, Zurich, Switzerland
Attention-deficit and hyperactivity disorder (ADHD) is a widespread neurodevelopmental disorder in children and adolescents, persisting into adulthood in a majority of them. ADHD and substance use disorders (SUDs) commonly co-occur in the clinical adult population. The higher-than-normal prevalence rates of SUDs in people with ADHD indicate increased risk for developing SUD. This narrative review deals with the question of whether or not adults with both disorders should be treated with methylphenidate (MPH), addressing specific issues surrounding this form of treatment. MPH is considered as first-line pharmacotherapy for ADHD. However, because of its stimulant-like reinforcing properties, MPH has a significant addictive potential to which persons with SUDs are especially susceptible. Appropriate treatment is therefore complex. Because of concerns about misuse and diversion of MPH medication, clinicians may be reluctant to use MPH to manage ADHD symptoms in these patients. However, it is essential to diagnose and treat ADHD adequately as appropriate therapy reduces the impairments, as well as the risk of developing comorbid disorders and poor treatment response. MPH should not be deprived of these patients because of the risk for misuse, especially as several strategies can be applied to minimize this risk. To conclude, carefully applied guideline-based diagnostics to clarify the potential presence of ADHD as well as a responsible prescription practice in a well-defined therapeutic setting with reliable monitoring of medication intake and regular consultations are essential conditions for a safe and proficient MPH treatment of ADHD in patients with SUD.
Introduction
Attention-Deficit and Hyperactivity Disorder
The prevalence of attention-deficit and hyperactivity disorder (ADHD) in children and adolescents is estimated to be between 2 and 7% (1), whereby some studies found prevalence rates up to 16% in certain age groups (2). The prevalence rates vary depending on the underlying classification system, and boys are diagnosed about two to three times more often with ADHD than girls (3–5). Already during early infancy, people with ADHD often exhibit signs, such as frequent crying, feeding and sleeping problems, restless sleep, and excessive unrest (6–8). Subsequently, the key features of ADHD manifest inattention, impulsivity, and hyperactivity.
ADHD is a multifactorial, clinically heterogeneous neurodevelopmental disorder (9) caused by interplay between genetic and environmental factors (10). It is assumed to result from suboptimal dopamine levels in the synaptic cleft due to overexpression of the presynaptic dopamine transporter (DAT) (11). Numerous studies have proven a familial aggregation of ADHD (5, 12). Environmental risk factors are, e.g., prenatal exposure to alcohol and tobacco, premature birth, critical birth circumstances, and incongruities in parent–child interactions, such as difficulties in feeding the infant, etc. (2, 12). Based on genetic predisposition and psychosocial risks, ADHD leads, among others, to neurocognitive/behavioral problems due to neurobiological dysregulation. These problems manifest mainly in the area of attention and executive functions (2, 13). People with ADHD show deficits in attention intensity and selectivity, in executive inhibitory control (of, e.g., motor action or prepotent responses) and therefore in self-regulation, as well as in memory functions, especially in short-term/working memory (2). They therefore struggle with continuous vigilance and attention, are easily distracted by internal and/or external stimuli, experience themselves as “forgetful,” and express difficulty in self-organizing and performing their daily routines. Other neurocognitive performances are usually not affected.
The long-prevailing view that ADHD is a disorder of childhood and adolescence, phasing out in adulthood, has been refuted (14–16). Even in adulthood ADHD is a disorder that leads to several mental problems and serious social issues (17). According to current knowledge, in 60% of the affected individuals, some or even all symptoms of the disorder will persist into adulthood. Depending on the study, the prevalence of ADHD in adults ranges from 1 to 4% (18, 19), with less pronounced gender differences than in children and adolescents (20).
Although the neurobiological functional deficits remain the central problem for all age groups, the severity and course of the disorder in adults are extremely heterogeneous (19). Impairments in everyday life are often more multifaceted than in children, whereas hyperactivity fades often into the background. Issues resulting from attention deficits are much more characteristic in adults so that difficulties in performing daily routine tasks often lead to severe problems at home, at work, and in social relationships (13). Moreover, the suboptimal regulation of affect and impulse control often impair social interactions, increasing the psychological strain of the affected people and their living environment and favoring the occurrence of other mental disorders.
This article briefly reviews existing literature on methylphenidate (MPH) treatment of adult ADHD in persons with substance use disorders (SUDs), especially with opioid use disorder (OUD). It takes up on specific problems, such as misuse and diversion of MPH, which are associated with MPH therapy in these patients. It also aims to give recommendations on diagnostics, therapy, and treatment settings, when dealing with these issues.
Methods
This narrative review is predominantly based on a MEDLINE database search. Additionally, importance was given to include guidelines for clinical practice, such as NICE guidelines (21) or consensus statements of experts (22, 23), in order to address specific issues, which arise during the treatment of patients with ADHD as well as SUD.
Results
ADHD and SUDs
Co-occurring ADHD and SUDs are routinely encountered in clinical settings (24). Studies have shown that the prevalence rates of SUDs are two to four times higher in people with ADHD than in the normal population (25, 26). In clinical samples, one-fifth of all alcohol-dependent and up to one-third of all cocaine-dependent patients meet criteria for adult ADHD (27, 28). In patients receiving opioid agonist treatment (OAT), the prevalence rates range between 20 and 25% (17, 29, 30). Overall, the prevalence rates of adult ADHD in SUD treatment settings range up to 24% (30). Both the severity and persistence of ADHD symptoms seem to influence the risk of developing an SUD and to reduce the effectiveness of treatment (31).
The relationship between ADHD and SUD is complex. As psychoactive substances reduce, imitate, or aggravate the symptoms of ADHD, differentiating between both disorders is challenging, which impedes the research in this field (32, 33). There is an ongoing debate whether common genetic factors or the attempt of self-medication causes the increased vulnerability for SUDs in persons with ADHD (15, 34). However, there seems to be no preference for any specific SUD (28, 35, 36).
Studies exploring common neuronal pathways in ADHD and SUDs indicate that anomalies in circuits related to reward processing, especially delayed reward processing (37, 38), might be present in both disorders (39, 40). Circuits related to reward processing are the mesolimbic and mesocortical pathways. The mesolimbic pathway involves mainly dopaminergic projections from the ventral tegmental area (VTA) in the midbrain through the median forebrain bundle to the nucleus accumbens and the limbic system with amygdala, hypothalamus, and hippocampus (41). Particularly, the mesoaccumbens dopamine pathway projecting from the VTA to the nucleus accumbens is associated with reward and motivation (42). The nucleus accumbens, a major component of the ventral striatum, is associated with the outcome evaluation of the reward (43). The mesocortical pathway involves mainly dopaminergic projections from the VTA to the prefrontal cortex (PFC) (44) and the anterior cingulate cortex (ACC). These structures are involved in executive functioning, such as decision-making or impulse control (45). In ADHD and SUDs, similar deficits in dopamine activity in these regions were found (39, 46). Positron emission tomography studies showed that ADHD and SUD seem to be associated with a reduced D2/D3 receptor availability in the midbrain, caudate nucleus (part of dorsal striatum), and hypothalamus (39, 42, 46). DAT binding was diffusely decreased across nucleus accumbens, midbrain, left caudate nucleus, and hypothalamus (39). PFC is also interconnected to the caudate nucleus, which plays an important role in procedural learning and inhibitory control of action (47). Functional magnetic resonance imaging studies suggest a reduced activation in frontostriatal brain regions (46). Furthermore, underactivity in the ACC and orbitofrontal cortex was associated with both ADHD and SUD symptoms (39). A meta-analysis reported reduced ACC gray matter volumes in adults with ADHD (48). Also, smaller right putamen and right cerebellum gray matter volumes were reported in adult ADHD (49). In people with ADHD and cocaine use disorder, Wingen et al. reported smaller occipital cortical gray matter volumes and reduced volumes in the putamen compared to people with ADHD (49).
Diagnostics and Therapy
Appropriate diagnostic workup and therapy of adult ADHD is important and necessary. First, the disorder is common but often remains unrecognized (22). Second, it leads to severe mental health and social impairments. Third, ADHD increases the risk of developing many other mental disorders, especially SUDs, affective disorders, anxiety disorders, and personality disorders (19). Fourth, the syndrome is well-treatable, and therapy reduces the risk for comorbid disorders, psychosocial problems, and treatment failure due to poor treatment response (2, 50).
Compared to children and adolescents, there is a great backlog in the diagnostics and therapy of ADHD in adults (32). To diagnose ADHD in adults, it is essential that the core symptoms started before the age of 7 [or 12 according to Diagnostic and Statistical Manual of Mental Disorders (DSM-5)] and are, at least in part, still present in adulthood. The diagnostic process should be performed, if possible, under abstinent conditions. Also, the individuals in OAT should be well-adjusted to the medication (51), so that the present symptoms can be clarified in-depth and assessed reliably according to valid diagnostic criteria [DSM-IV/DSM-5/International Classification of Diseases, 10th Revision (ICD-10)].
To ensure a careful and comprehensive evaluation for possible ADHD, a guideline-based diagnostic procedure is recommended in current clinical practice (21). Although ICD-10 offers the possibility to code the presence of ADHD, the criteria for adults are not explicitly mentioned. Diagnosis based on guidelines includes the developmental psychopathology of ADHD by stating the collection of retrospective symptoms as an integral part of the diagnosis.
According to NICE guidelines (21), the diagnostic evaluation consists of multiple phases with different aims. It requires patience, time, and the inclusion of several methods and sources of information. For diagnosis and treatment, it is always important to reconstruct the individual course of the disorder as precisely as possible. The purpose of a thorough and detailed psychiatric history is to identify individual problems considering possible comorbid mental disorders, the history of development of the individuals, and their family of origin. Already the history may indicate the presence of ADHD. The clinical impression is very important, but it is not sufficient to diagnose ADHD. For differential diagnosis and for assessing other mental disorders apart from the clinical impression, the use of structured instruments (e.g., Structured Clinical Interview for DSM-IV/DSM-5) is recommended. In patients with SUD, a thorough assessment of the current SUDs is essential to evaluate the influence of substance use on ADHD symptoms (52).
The next step is to collect a complete medical history and to perform a physical examination to exclude organic causes, such as thyroid disease, seizure disorder, or sleep disorder. To assess former and current symptoms, parents/siblings, and other important persons of trust should be interviewed. Validated structured interviews, such as Conners' Adult ADHD Diagnostic Interview for DSM-IV (53) or the Diagnostic Interview for ADHD in adults (54) can be used for this purpose.
In a following step, disorder-relevant symptoms and their manifestation in individuals are assessed by using standardized methods for detailed assessment. For this purpose, there are several validated instruments available in different languages, such as Adult ADHD Self-Report Scale and the short version for screening, Wender-Utah-Rating-Scale for childhood symptoms, or Conners' or Brown's scale for current symptoms. The Conners' or Brown's scale is recommended to assess the magnitude of the impairments caused by ADHD. As they are normed (55), a severity classification (mild, moderate, and severe) can be conducted by comparing the results with the norm population. Alternatively, there are also batteries (e.g., Homburger ADHD scales for adults) available, which cover all relevant instruments (56).
Finally, a neuropsychological assessment provides relevant information about the general level of cognitive performance and existing neurocognitive deficits. Mainly neuropsychological tests aiming at attentional and executive functions as well as tests to measure various aspects of intelligence are used.
The decision for a therapy, respectively, or the choice of a specific treatment depends on the severity of the current situation, mental and social impairments, present comorbid disorders, the relevance of the symptoms in performing daily routine tasks, and existing resources (23). Ideally, the treatment should include several components, such as pharmacotherapy, cognitive behavioral therapy in individual or group format, psychoeducation, and peer support (57). Pharmacotherapy and behavioral therapy seem to have similar therapeutic effects on ADHD symptoms in adolescents (23). In adults with ADHD and SUD, a combination of pharmacotherapy and psychotherapy is recommended (22, 58, 59). When it comes to pharmacotherapy, however, treatment with stimulants is the therapy of first choice (2, 50).
Methylphenidate
MPH is a centrally acting psychostimulant (60) that is subject to the narcotics law. It is approved for the treatment of ADHD in children from 6 years of age, adolescents, and adults, as well as for the treatment of sleep disorders (e.g., excessive daytime sleepiness, narcolepsy) (61). In addition, off-label use of MPH to treat depression is also practiced (61). There are various immediate- and sustained-release preparations of multiple brands available for oral or transdermal administration.
MPH unfolds its stimulant, indirectly sympathomimetic effects by inhibiting presynaptic reuptake of dopamine and noradrenaline (61). Unlike classical reuptake inhibitors, it also induces rapid and significant rises in striatal (62) and accumbal (63) dopamine efflux, which seems to play a key role for the therapeutic effect of MPH (11). MPH leads to an upregulation of the frontoparietal executive function network and the temporoparietal attentional network, which is associated with improved attention in children with ADHD and better inhibitory control in the PFC (64). The calming effect of MPH in patients with ADHD is most likely connected with the improvement of dopamine deficiency (65). MPH has a high affinity toward the DAT, which is comparable to that of cocaine (65). According to Heal (62), “MPH and cocaine act as ‘inverse agonists,' reversing the usual direction of dopamine transport by DAT” (66).
Neuropharmacobehavioral studies of injected MPH and cocaine revealed not only similarities, but also significant differences between the two substances (67–69). For both drugs, the fast uptake in the striatum paralleled the “high” experience but only for cocaine the decline in the “high” corresponded to the brain clearance rate. In contrast, for MPH, the “high” decreased as rapidly as for cocaine despite significant striatal binding of the drug, suggesting that acute tolerance to the reinforcing effects of MPH had occurred (67, 69). The slow brain clearance of MPH may therefore limit its misuse potential (70).
Misuse Potential of MPH
The misuse liability of MPH is well-known. In the literature, mostly intravenous and nasal administrations, which led to substance- or administration-specific complications, are described as cases of misuse (70). Complications of such administrations, particularly intravenous, with crushed tablets include local or vascular infections, foreign body reactions, granulomas in the lungs, and pulmonary arterial hypertension due to the blockage of the lung circulation (71–73). Studies that investigated the narrow path between the therapeutic impact and the misuse-supporting reinforcing effect of MPH found strong hints for the euphoric potential of MPH in people with as well as without histories of SUD (74, 75). While a slow increase in serum concentrations shows therapeutic effects, a rapid and steep increase results in subjective reinforcing effects (76, 77). Therefore, a rapid onset of action and high concentrations in the body are essential to get the “high,” which can be achieved by intravenous or nasal administration or by the intake of very high doses (76). Recent data collected in a sample of patients in OAT suggest that the need for a rapid onset of action is an important reason for MPH misuse in this population (68). However, an analysis of two studies, which investigated the subjective effects of osmotic-release oral systems MPH (OROS-MPH) showed substance use severity did not significantly affect the euphoric effects of OROS-MPH (78).
Misuse and Diversion of MPH in ADHD Patients
Misuse and diversion are inherent risks of prescribing controlled substances, and a substantial minority of patients with prescribed MPH will misuse their own prescription or divert their medications to others (75). In recent years, an increase of cases of misuse and diversion has been reported, which goes along with an increase of ADHD diagnoses and thus prescription of MPH (71). However, the frequency of misuse in people with ADHD is not clear. In a prospective cohort study with patients who received prescribed MPH, 22% of the patients with ADHD reported to have misused MPH at least once (79). In another survey of a specialized institution, 14% of the patients with ADHD reported to have misused MPH (predominantly nasal) or another prescribed stimulant (80). In a national US survey, 8.9% of the participants with a prescription for ADHD medication reported to have sold, traded, or given away their medication (81). Mostly older adolescents and adults who consume also other psychotropic substances or have other behavioral disorders are at risk of doing so (71, 79, 82). A recent study suggests that MPH may also be misused by a substantial proportion of methadone-maintained patients (83). A survey in France showed that ~46% of MPH acquisitions by persons with substance dependence or on OAT were illegal (84). However, the diversion/misuse of OROS-MPH seems not to be affected by the substance use severity of the patients (78).
Discussion and Conclusion
MPH Therapy for Patients With ADHD and SUD
It has not yet been clarified to what extent ADHD treatment with MPH contributes to the development of SUDs. Current evidence is based on secondary analyses as prospective studies, to our knowledge, are still missing. The existing results are inconsistent; however, they do not indicate that MPH contributes to the development of SUDs. On the contrary, treating ADHD patients with MPH may reduce their risk for SUD (77, 85, 86). According to a meta-analysis, ADHD patients treated with MPH in their childhood show a reduced risk of developing SUD by a factor of 1.9 compared to untreated patients (79). Studies using data from large databases and registries showed that medication treatment of ADHD, mostly with stimulants, was associated with a significantly reduced risk of SUD outcomes (87–89) and that within individuals this reduction in risk of SUD outcomes is related to periods of adherence to the medication prescribed (87, 89). However, stimulant medication was neither associated with an increased nor decreased risk for SUD diagnoses in another study (90). A meta-analysis with data from two of these studies (88, 89) showed that the risk of substance use outcomes was reduced; however, it did not attain statistical significance (91). Research among patients in OAT with ADHD suggests that a cotherapy with MPH not only reduces ADHD symptoms and improves psychosocial functioning but also does not worsen the course of substance use (92).
The question is whether adults with both disorders should be treated with MPH despite its misuse potential and the risks connected to it. In due consideration of the scientific literature, the benefits of ADHD treatment with MPH outweigh its risks; therefore, in our opinion, treatment with MPH should be considered as first-line treatment also in adults with ADHD and SUD. However, these risks should be seriously taken into account when planning the therapy. Furthermore, a reasonable therapeutic setting should be arranged with patients for the prescription of MPH. Additionally, patients should be closely monitored for possible side effects and misuse/diversion of MPH.
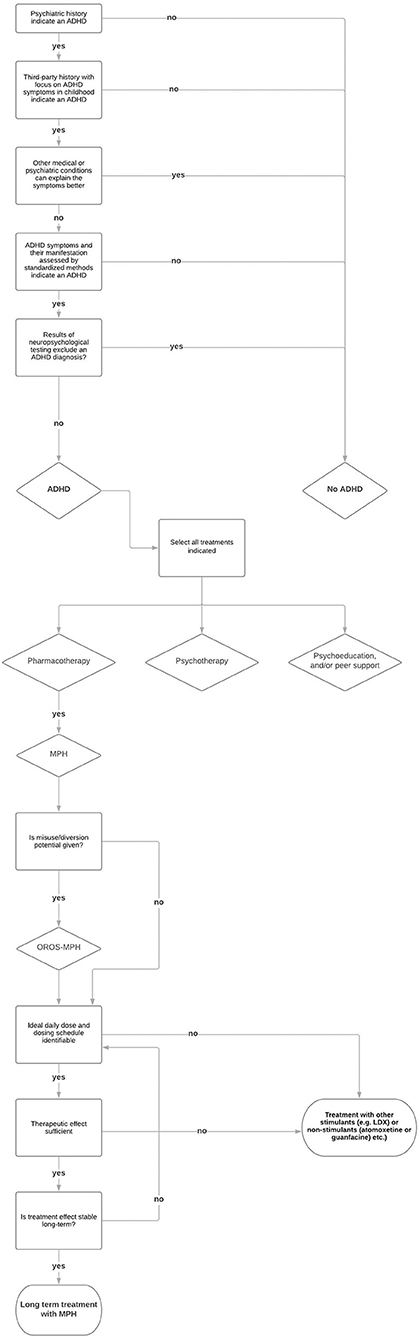
In any case, a verified guideline-based diagnosis is the basic requirement for the treatment of ADHD. Insights from this diagnostic procedure can be used to plan the treatment and to determine the indication of therapy offers (93). The treatment of ADHD should be adjusted individually for patients with SUDs as for any other patient. The treatment and its duration can vary from patient to patient (94). The decision for a treatment depends on the current severity of ADHD, the psychosocial impairments, the existing comorbid disorders, and the relevance of symptoms in the context of existing resources (23, 95). The treatment with MPH is important, but often not enough. Regular psychotherapy sessions and specific interventions improve the success of the treatment significantly (96). Some patients need treatment with MPH only temporarily or periodically, e.g., when other compensation strategies are not sufficient anymore or when they need to face important private or job-related challenges. It is also relevant to find the adequate formulation, daily dose, and dosing schedule of MPH to achieve optimal therapeutic results, which should be verified regularly. However, the approved drugs and recommended dosages are often not enough to cover the therapeutic need (95). Because of the lower DAT availability and occupancy (97), MPH may have reduced efficacy in persons with SUD (98), especially in cocaine-dependent ADHD patients (98, 99). Lisdexamfetamine dimesylate (LDX), as the first long-acting prodrug stimulant for the treatment of ADHD (100), is possibly another safe and more potent option for adults with ADHD (101–103). LDX has low misuse potential due to its biological mechanism of enzymatic hydrolysis to obtain the active compound D-amphetamine (101). Also, non-stimulant α2-adrenoceptor agonists, such as guanfacine are an effective treatment option to be considered, but mainly in the combined hyperactive/impulsive-inattentive ADHD subgroup (66). Atomoxetine, a non-stimulant medication, might be effective for patients with ADHD and alcohol use disorder (104). However, studies determining the efficacy of these drugs in adults with ADHD and SUD are scarce. Figure 1 depicts a step-by-step procedure of diagnosing and treating SUD patients for ADHD.
In general, extended-release formulations of MPH are recommended for the treatment of adult ADHD (105), which may also prevent possible misuse of MPH, due to the lower misuse potential (77, 78, 106). Luo and Levin state that also from clinical experience, the misuse liability of long-acting formulations of prescribed psychostimulants is low (39). Other methods to prevent misuse are prescribing non-stimulant drugs (e.g., atomoxetine or guanfacine) or prescribing LDX. Regular clinical evaluation of MPH adherence and supervising the intake of medication are additional methods to prevent misuse (75). In cases of suspected diversion, regular rapid urine tests can be considered as an instrument to confirm the intake of medication by the patients. However, the rationale of these interventions must be clear and should be discussed with patients in detail as they will experience them as a violation of their autonomy, which could undermine the therapeutic relationship.
Author Contributions
SC, JS, MV, and KD did the literature review and wrote the draft. MW and KD commented on the first draft. SB, MW, and KD commented on the second draft. All authors commented on the final manuscript, which was completed by SC, JS, MV, SB, MW, and KD.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADHD, attention-deficit and hyperactivity disorder; SUD, substance use disorder; DAT, dopamine transporter; MPH, methylphenidate; OUD, opioid use disorder; OAT, opioid agonist treatment; VTA, ventral tegmental area; PFC, prefrontal cortex; ACC, anterior cingulate cortex; OROS-MPH, osmotic-release oral systems methylphenidate; LDX, Lisdexamfetamine dimesylate; ASRS v1.1, adult self-report scale.
References
1. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. (2018) 5:175–86. doi: 10.1016/S2215-0366(17)30167-0
2. Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. (2005) 366:237–48. doi: 10.1016/S0140-6736(05)66915-2
3. Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry. (2010) 49:217–28.e1–3. doi: 10.1097/00004583-201003000-00005
4. Arnett AB, Pennington BF, Willcutt EG, Defries JC, Olson RK. Sex differences in ADHD symptom severity. J Child Psychol Psychiatry Allied Discipl. (2015) 56:632–9. doi: 10.1111/jcpp.12337
5. Chen Q, Brikell I, Lichtenstein P, Serlachius E, Kuja-Halkola R, Sandin S, et al. Familial aggregation of attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry. (2017) 58:231–9. doi: 10.1111/jcpp.12616
6. Lemcke S, Parner ET, Bjerrum M, Thomsen PH, Lauritsen MB. Early development in children that are later diagnosed with disorders of attention and activity: a longitudinal study in the Danish National Birth Cohort. Eur Child Adolesc Psychiatry. (2016) 25:1055–66. doi: 10.1007/s00787-016-0825-6
7. Schmid G, Wolke D. Preschool regulatory problems and attention-deficit/hyperactivity and cognitive deficits at school age in children born at risk: different phenotypes of dysregulation? Early Hum Dev. (2014) 90:399–405. doi: 10.1016/j.earlhumdev.2014.05.001
8. Hemmi MH, Wolke D, Schneider S. Associations between problems with crying, sleeping and/or feeding in infancy and long-term behavioural outcomes in childhood: a meta-analysis. Arch Dis Child. (2011) 96:622–9. doi: 10.1136/adc.2010.191312
9. Friedman LA, Rapoport JL. Brain development in ADHD. Curr Opin Neurobiol. (2015) 30:106–11. doi: 10.1016/j.conb.2014.11.007
10. Pineda-Cirera L, Shivalikanjli A, Cabana-Domínguez J, Demontis D, Rajagopal VM, Børglum AD, et al. Exploring genetic variation that influences brain methylation in attention-deficit/hyperactivity disorder. Transl Psychiatry. (2019) 9:242. doi: 10.1038/s41398-019-0574-7
11. Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. (2000) 285:107–10. doi: 10.1016/S0304-3940(00)01040-5
12. Thapar A, Cooper M, Eyre O, Langley K. What have we learnt about the causes of ADHD? J Child Psychol Psychiatry. (2013) 54:3–16. doi: 10.1111/j.1469-7610.2012.02611.x
13. Renner TJ, Gerlach M, Romanos M, Herrmann M, Reif A, Fallgatter AJ, et al. Neurobiology of attention-deficit hyperactivity disorder. Nervenarzt. (2008) 79:771–81. doi: 10.1007/s00115-008-2513-3
14. Wigal SB, Wigal TL. Special considerations in diagnosing and treating attention-deficit/hyperactivity disorder. CNS Spectr. (2007) 12:1–14; quiz 5–6. doi: 10.1017/S1092852900026092
15. Wilson JJ, Levin FR. Attention deficit hyperactivity disorder (ADHD) and substance use disorders. Curr Psychiatry Rep. (2001) 3:497–506. doi: 10.1007/s11920-001-0044-8
16. Adler LA, Shaw D. Diagnosing ADHD in adults. In: Buitelaar JK, Kan CC, Asherson PJ, editors. ADHD in Adults: Characterization, Diagnosis, and Treatment, 1st ed. New York, NY: Cambridge University Press (2011). p. 91–105. doi: 10.1017/CBO9780511780752.009
17. Carpentier PJ, van Gogh MT, Knapen LJ, Buitelaar JK, De Jong CA. Influence of attention deficit hyperactivity disorder and conduct disorder on opioid dependence severity and psychiatric comorbidity in chronic methadone-maintained patients. Eur Addict Res. (2011) 17:10–20. doi: 10.1159/000321259
18. Philipsen A, Limberger MF, Lieb K, Feige B, Kleindienst N, Ebner-Priemer U, et al. Attention-deficit hyperactivity disorder as a potentially aggravating factor in borderline personality disorder. Br J Psychiatry. (2008) 192:118–23. doi: 10.1192/bjp.bp.107.035782
19. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. (2007) 32:631–42. doi: 10.1093/jpepsy/jsm005
20. Biederman J, Faraone SV, Monuteaux MC, Bober M, Cadogen E. Gender effects on attention-deficit/hyperactivity disorder in adults, revisited. Biol Psychiatry. (2004) 55:692–700. doi: 10.1016/j.biopsych.2003.12.003
21. National Institute for Health Care Excellence N. Attention Deficit Hyperactivity Disorder: Diagnosis and Management. (2018) Available online at: https://www.nice.org.uk/guidance/ng87
22. Crunelle CL, van den Brink W, Moggi F, Konstenius M, Franck J, Levin FR, et al. International consensus statement on screening, diagnosis and treatment of substance use disorder patients with comorbid attention deficit/hyperactivity disorder. Eur Addict Res. (2018) 24:43–51. doi: 10.1159/000487767
23. Ebert D, Krause J, Roth-Sackenheim C. ADHD in adulthood–guidelines based on expert consensus with DGPPN support. Nervenarzt. (2003) 74:939–46.
24. Schubiner H, Tzelepis A, Milberger S, Lockhart N, Kruger M, Kelley BJ, et al. Prevalence of attention-deficit/hyperactivity disorder and conduct disorder among substance abusers. J Clin Psychiatry. (2000) 61:244–51. doi: 10.4088/JCP.v61n0402
25. Biederman J, Wilens TE, Mick E, Faraone SV, Spencer T. Does attention-deficit hyperactivity disorder impact the developmental course of drug and alcohol abuse and dependence? Biol Psychiatry. (1998) 44:269–73. doi: 10.1016/S0006-3223(97)00406-X
26. Cumyn L, French L, Hechtman L. Comorbidity in adults with attention-deficit hyperactivity disorder. Can J Psychiatry. (2009) 54:673–83. doi: 10.1177/070674370905401004
27. Levin FR, Evans SM, Kleber HD. Prevalence of adult attention-deficit hyperactivity disorder among cocaine abusers seeking treatment. Drug Alcohol Depend. (1998) 52:15–25. doi: 10.1016/S0376-8716(98)00049-0
28. Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. (2007) 87:20–9. doi: 10.1016/j.drugalcdep.2006.07.004
29. Lugoboni F, Levin FR, Pieri MC, Manfredini M, Zamboni L, Somaini L, et al. Co-occurring attention deficit hyperactivity disorder symptoms in adults affected by heroin dependence: patients characteristics and treatment needs. Psychiatry Res. (2017) 250:210–6. doi: 10.1016/j.psychres.2017.01.052
30. van Emmerik-van Oortmerssen K, Crunelle CL, Carpentier PJ. Substance use disorders and ADHD: an overview of recent Dutch research. Tijdschr Psychiatr. (2013) 55:861–6.
31. Molina BS, Pelham WE Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. (2003) 112:497–507. doi: 10.1037/0021-843X.112.3.497
32. Levin FR, Upadhyaya HP. Diagnosing ADHD in adults with substance use disorder: DSM-IV criteria and differential diagnosis. J Clin Psychiatry. (2007) 68:e18. doi: 10.4088/JCP.0707e18
33. Lynskey MT, Hall W. Attention deficit hyperactivity disorder and substance use disorders: is there a causal link? Addiction. (2001) 96:815–22. doi: 10.1046/j.1360-0443.2001.9668153.x
34. Mariani JJ, Khantzian EJ, Levin FR. The self-medication hypothesis and psychostimulant treatment of cocaine dependence: an update. Am J Addict. (2014) 23:189–93. doi: 10.1111/j.1521-0391.2013.12086.x
35. Carpentier PJ, Arias Vasquez A, Hoogman M, Onnink M, Kan CC, Kooij JJ, et al. Shared and unique genetic contributions to attention deficit/hyperactivity disorder and substance use disorders: a pilot study of six candidate genes. Eur Neuropsychopharmacol. (2013) 23:448–57. doi: 10.1016/j.euroneuro.2012.07.003
36. Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry. (1995) 152:1652–8. doi: 10.1176/ajp.152.11.1652
37. Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. (2006) 31:1290–4. doi: 10.1016/j.addbeh.2005.09.005
38. Kollins SH, Newland MC, Critchfield TS. Human sensitivity to reinforcement in operant choice: how much do consequences matter? Psychon Bull Rev. (1997) 4:208–20. doi: 10.3758/BF03209395
39. Luo SX, Levin FR. Towards precision addiction treatment: new findings in co-morbid substance use and attention-deficit hyperactivity disorders. Curr Psychiatry Rep. (2017) 19:14. doi: 10.1007/s11920-017-0769-7
40. Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. (2011) 31:328–41. doi: 10.1016/j.cpr.2011.01.006
41. Pariyadath V, Gowin JL, Stein EA. Resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks. Prog Brain Res. (2016) 224:155–73. doi: 10.1016/bs.pbr.2015.07.015
42. Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. (2009) 302:1084–91. doi: 10.1001/jama.2009.1308
43. Mannella F, Gurney K, Baldassarre G. The nucleus accumbens as a nexus between values and goals in goal-directed behavior: a review and a new hypothesis. Front Behav Neurosci. (2013) 7:135. doi: 10.3389/fnbeh.2013.00135
44. Buchta WC, Riegel AC. Chronic cocaine disrupts mesocortical learning mechanisms. Brain Res. (2015) 1628:88–103. doi: 10.1016/j.brainres.2015.02.003
45. Siddiqui SV, Chatterjee U, Kumar D, Siddiqui A, Goyal N. Neuropsychology of prefrontal cortex. Indian J Psychiatry. (2008) 50:202–8. doi: 10.4103/0019-5545.43634
46. Frodl T. Comorbidity of ADHD and substance use disorder (SUD): a neuroimaging perspective. J Atten Disord. (2010) 14:109–20. doi: 10.1177/1087054710365054
47. Venkataraman SS, Claussen CM, Kharas N, Dafny N. The prefrontal cortex and the caudate nucleus respond conjointly to methylphenidate (Ritalin). Concomitant behavioral and neuronal recording study. Brain Res Bull. (2020) 157:77–89. doi: 10.1016/j.brainresbull.2019.10.009
48. Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. (2012) 125:114–26. doi: 10.1111/j.1600-0447.2011.01786.x
49. van Wingen GA, van den Brink W, Veltman DJ, Schmaal L, Dom G, Booij J, et al. Reduced striatal brain volumes in non-medicated adult ADHD patients with comorbid cocaine dependence. Drug Alcohol Depend. (2013) 131:198–203. doi: 10.1016/j.drugalcdep.2013.05.007
50. Fredriksen M, Halmøy A, Faraone SV, Haavik J. Long-term efficacy and safety of treatment with stimulants and atomoxetine in adult ADHD: a review of controlled and naturalistic studies. Eur Neuropsychopharmacol. (2013) 23:508–27. doi: 10.1016/j.euroneuro.2012.07.016
51. Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk, and treatment issues. Psychiatr Clin North Am. (2004) 27:283–301. doi: 10.1016/S0193-953X(03)00113-8
52. Crunelle C, Matthys F. Good Clinical Practice in the Recognition and Treatment of ADHD in Adults With Substance Use Dependence. Brussel: Vrije Universiteit Brussel (2016).
53. Epstein JN, Johnson DE, Conners CK. The Conner's Adult ADHD Diagnostic Interview for DSM-IV (CAADID). Washington DC: American Psychological Association (2001). doi: 10.1037/t04960-000
54. Kooij JJS, Francken MH. Diagnostic Interview for ADHD in Adults 2.0 (DIVA 2.0). DIVA Foundation :1–19.
55. Hechtman L. Adult ADHD. In: Brown TE, editor. ADHD Comorbidities: Handbook for ADHD Complications in Children and Adults, 1st ed. Washington, DC: American Psychiatric Publishing Inc. (2009). p. 81–95.
56. Rösler M, Retz-Junginger P, Retz W, Stieglitz RD. Homburger ADHS-Skalen für Erwachsene. Göttingen: Hogrefe Verlag (2008).
57. Goossensen MA, van de Glind G, Carpentier PJ, Wijsen RM, van Duin D, Kooij JJ. An intervention program for ADHD in patients with substance use disorders: preliminary results of a field trial. J Subst Abuse Treat. (2006) 30:253–9. doi: 10.1016/j.jsat.2005.12.004
58. Zulauf CA, Sprich SE, Safren SA, Wilens TE. The complicated relationship between attention deficit/hyperactivity disorder and substance use disorders. Curr Psychiatry Rep. (2014) 16:436. doi: 10.1007/s11920-013-0436-6
59. Torrens M, Rossi PC, Martinez-Riera R, Martinez-Sanvisens D, Bulbena A. Psychiatric co-morbidity and substance use disorders: treatment in parallel systems or in one integrated system? Subst Use Misuse. (2012) 47:1005–14. doi: 10.3109/10826084.2012.663296
60. Patrick KS, Straughn AB, Perkins JS, González MA. Evolution of stimulants to treat ADHD: transdermal methylphenidate. Hum Psychopharmacol. (2009) 24:1–17. doi: 10.1002/hup.992
61. Rush CR, Stoops WW. Agonist replacement therapy for cocaine dependence: a translational review. Future Med Chem. (2012) 4:245–65. doi: 10.4155/fmc.11.184
62. Heal DJ, Smith SL, Kulkarni RS, Rowley HL. New perspectives from microdialysis studies in freely-moving, spontaneously hypertensive rats on the pharmacology of drugs for the treatment of ADHD. Pharmacol Biochem Behav. (2008) 90:184–97. doi: 10.1016/j.pbb.2008.03.016
63. Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. (2001) 21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001
64. Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry. (2013) 70:857–68. doi: 10.1001/jamapsychiatry.2013.1129
65. Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. (1987) 237:1219–23. doi: 10.1126/science.2820058
66. Heal DJ, Smith SL, Findling RL. ADHD: current and future therapeutics. Curr Top Behav Neurosci. (2012) 9:361–90. doi: 10.1007/7854_2011_125
67. Volkow ND, Ding YS, Fowler JS, Wang GJ, Logan J, Gatley JS, et al. Is methylphenidate like cocaine? Studies on their pharmacokinetics and distribution in the human brain. Arch Gen Psychiatry. (1995) 52:456–63. doi: 10.1001/archpsyc.1995.03950180042006
68. Vogel M, Bucher P, Strasser J, Liechti ME, Krähenbühl S, Dürsteler KM. Similar and different? Subjective effects of methylphenidate and cocaine in opioid-maintained patients. J Psychoact Drugs. (2016) 48:93–100. doi: 10.1080/02791072.2015.1130883
69. Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, et al. Blockade of striatal dopamine transporters by intravenous methylphenidate is not sufficient to induce self-reports of “high”. J Pharmacol Exp Ther. (1999) 288:14–20.
70. Parran TV Jr., Jasinski DR. Intravenous methylphenidate abuse. Prototype for prescription drug abuse. Arch Intern Med. (1991) 151:781–3. doi: 10.1001/archinte.1991.00400040119027
71. Bruggisser M, Bodmer M, Liechti ME. Methylphenidate misuse. Praxis. (2012) 101:299–305. doi: 10.1024/1661-8157/a000856
72. Haglund RM, Howerton LL. Ritalin: consequences of abuse in a clinical population. Int J Addict. (1982) 17:349–56. doi: 10.3109/10826088209071018
73. Arnett EN, Battle WE, Russo JV, Roberts WC. Intravenous injection of talc-containing drugs intended for oral use. A cause of pulmonary granulomatosis and pulmonary hypertension. Am J Med. (1976) 60:711–8. doi: 10.1016/0002-9343(76)90508-8
74. Mariani JJ, Levin FR. Psychostimulant treatment of cocaine dependence. Psychiatr Clin North Am. (2012) 35:425–39. doi: 10.1016/j.psc.2012.03.012
75. Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. (2014) 126:64–81. doi: 10.3810/pgm.2014.09.2801
76. Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. (2003) 160:1909–18. doi: 10.1176/appi.ajp.160.11.1909
77. Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. (2008) 24:1345–57. doi: 10.1185/030079908X280707
78. Winhusen TM, Lewis DF, Riggs PD, Davies RD, Adler LA, Sonne S, et al. Subjective effects, misuse, and adverse effects of osmotic-release methylphenidate treatment in adolescent substance abusers with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. (2011) 21:455–63. doi: 10.1089/cap.2011.0014
79. Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. (2003) 111:179–85. doi: 10.1542/peds.111.1.179
80. Bright GM. Abuse of medications employed for the treatment of ADHD: results from a large-scale community survey. Medscape J Med. (2008) 10:111.
81. Cassidy TA, Varughese S, Russo L, Budman SH, Eaton TA, Butler SF. Nonmedical use and diversion of ADHD stimulants among U.S. adults ages 18–49: a National Internet Survey. J Atten Disord. (2015) 19:630–40. doi: 10.1177/1087054712468486
82. Wilkins C, Sweetsur P, Griffiths R. Recent trends in pharmaceutical drug use among frequent injecting drug users, frequent methamphetamine users and frequent ecstasy users in New Zealand, 2006–2009. Drug Alcohol Rev. (2011) 30:255–63. doi: 10.1111/j.1465-3362.2011.00324.x
83. Peles E, Schreiber S, Linzy S, Domani Y, Adelson M. Differences in methylphenidate abuse rates among methadone maintenance treatment patients in two clinics. J Subst Abuse Treat. (2015) 54:44–9. doi: 10.1016/j.jsat.2014.12.010
84. Frauger E, Nordmann S, Orleans V, Pradel V, Pauly V, Thirion X, et al. Which psychoactive prescription drugs are illegally obtained and through which ways of acquisition? About OPPIDUM survey. Fundam Clin Pharmacol. (2012) 26:549–56. doi: 10.1111/j.1472-8206.2011.00950.x
85. Biederman J, Wilens T, Mick E, Spencer T, Faraone SV. Pharmacotherapy of attention-deficit/hyperactivity disorder reduces risk for substance use disorder. Pediatrics. (1999) 104:e20. doi: 10.1542/peds.104.2.e20
86. Konstenius M, Jayaram-Lindström N, Guterstam J, Beck O, Philips B, Franck J. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: a 24-week randomized placebo-controlled trial. Addiction. (2014) 109:440–9. doi: 10.1111/add.12369
87. Quinn PD, Chang Z, Hur K, Gibbons RD, Lahey BB, Rickert ME, et al. ADHD medication and substance-related problems. Am J Psychiatry. (2017) 174:877–85. doi: 10.1176/appi.ajp.2017.16060686
88. Chang Z, Lichtenstein P, Halldner L, D'Onofrio B, Serlachius E, Fazel S, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. (2014) 55:878–85. doi: 10.1111/jcpp.12164
89. Steinhausen HC, Bisgaard C. Substance use disorders in association with attention-deficit/hyperactivity disorder, co-morbid mental disorders, and medication in a nationwide sample. Eur Neuropsychopharmacol. (2014) 24:232–41. doi: 10.1016/j.euroneuro.2013.11.003
90. Sundquist J, Ohlsson H, Sundquist K, Kendler KS. Attention-deficit/hyperactivity disorder and risk for drug use disorder: a population-based follow-up and co-relative study. Psychol Med. (2015) 45:977–83. doi: 10.1017/S0033291714001986
91. Boland H, DiSalvo M, Fried R, Woodworth KY, Wilens T, Faraone SV, et al. A literature review and meta-analysis on the effects of ADHD medications on functional outcomes. J Psychiatr Res. (2020) 123:21–30. doi: 10.1016/j.jpsychires.2020.01.006
92. Abel KF, Bramness JG, Martinsen EW. Stimulant medication for ADHD in opioid maintenance treatment. J Dual Diagn. (2014) 10:32–8. doi: 10.1080/15504263.2013.867657
93. Luderer M, Kiefer F, Reif A, Moggi F. ADHD in adult patients with substance use disorders. Nervenarzt. (2019) 90:926–31. doi: 10.1007/s00115-019-0779-2
94. Perugi G, Pallucchini A, Rizzato S, De Rossi P, Sani G, Maremmani AG, et al. Pharmacotherapeutic strategies for the treatment of attention-deficit hyperactivity (ADHD) disorder with comorbid substance-use disorder (SUD). Expert Opin Pharmacother. (2019) 20:343–55. doi: 10.1080/14656566.2018.1551878
95. Carpentier PJ, Levin FR. Pharmacological treatment of ADHD in addicted patients: what does the literature tell us? Harv Rev Psychiatry. (2017) 25:50–64. doi: 10.1097/HRP.0000000000000122
96. Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH. Pharmacological and psychosocial treatments for adolescents with ADHD: an updated systematic review of the literature. Clin Psychol Rev. (2014) 34:218–32. doi: 10.1016/j.cpr.2014.02.001
97. Crunelle CL, van den Brink W, Veltman DJ, van Emmerik-van Oortmerssen K, Dom G, Schoevers RA, et al. Low dopamine transporter occupancy by methylphenidate as a possible reason for reduced treatment effectiveness in ADHD patients with cocaine dependence. Eur Neuropsychopharmacol. (2013) 23:1714–23. doi: 10.1016/j.euroneuro.2013.05.002
98. Skoglund C, Brandt L, Almqvist C, D'Onofrio BM, Konstenius M, Franck J, et al. Factors associated with adherence to methylphenidate treatment in adult patients with attention-deficit/hyperactivity disorder and substance use disorders. J Clin Psychopharmacol. (2016) 36:222–8. doi: 10.1097/JCP.0000000000000501
99. Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. (2007) 164:622–9. doi: 10.1176/ajp.2007.164.4.622
100. Goodman DW. Lisdexamfetamine dimesylate (vyvanse), a prodrug stimulant for attention-deficit/hyperactivity disorder. P T. (2010) 35:273–87.
101. Adler LA, Lynch LR, Shaw DM, Wallace SP, O'Donnell KE, Ciranni MA, et al. Effectiveness and duration of effect of open-label lisdexamfetamine dimesylate in adults with ADHD. J Atten Disord. (2017) 21:149–57. doi: 10.1177/1087054713485421
102. Soutullo C, Banaschewski T, Lecendreux M, Johnson M, Zuddas A, Anderson C, et al. A post-hoc comparison of the effects of lisdexamfetamine dimesylate and osmotic-release oral system methylphenidate on symptoms of attention-deficit hyperactivity disorder in children and adolescents. CNS Drugs. (2013) 27:743–51. doi: 10.1007/s40263-013-0086-6
103. Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2018) 5:727–38. doi: 10.1016/S2215-0366(18)30269-4
104. Wilens TE, Adler LA, Weiss MD, Michelson D, Ramsey JL, Moore RJ, et al. Atomoxetine treatment of adults with ADHD and comorbid alcohol use disorders. Drug Alcohol Depend. (2008) 96:145–54. doi: 10.1016/j.drugalcdep.2008.02.009
105. Katzman MA, Sternat T. A review of OROS methylphenidate (Concerta(®)) in the treatment of attention-deficit/hyperactivity disorder. CNS Drugs. (2014) 28:1005–33. doi: 10.1007/s40263-014-0175-1
Keywords: ADHD, methylphenidate, substance use disorder, diagnosis, therapy, misuse
Citation: Chamakalayil S, Strasser J, Vogel M, Brand S, Walter M and Dürsteler KM (2021) Methylphenidate for Attention-Deficit and Hyperactivity Disorder in Adult Patients With Substance Use Disorders: Good Clinical Practice. Front. Psychiatry 11:540837. doi: 10.3389/fpsyt.2020.540837
Received: 06 March 2020; Accepted: 16 December 2020;
Published: 26 January 2021.
Edited by:
Jasmin Vassileva, Virginia Commonwealth University, United StatesReviewed by:
Paul Geoffrey Overton, The University of Sheffield, United KingdomAlessandra Maria Passarotti, University of Illinois at Chicago, United States
Copyright © 2021 Chamakalayil, Strasser, Vogel, Brand, Walter and Dürsteler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sunsha Chamakalayil, c3Vuc2hhLmNoYW1ha2FsYXlpbEB1cGsuY2g=
 Sunsha Chamakalayil
Sunsha Chamakalayil Johannes Strasser
Johannes Strasser Marc Vogel
Marc Vogel Serge Brand
Serge Brand Marc Walter
Marc Walter Kenneth M. Dürsteler
Kenneth M. Dürsteler