- 1Department of Psychiatry, The First Affiliated Hospital of China Medical University, Shenyang, China
- 2Central Laboratory, The First Affiliated Hospital of China Medical University, Shenyang, China
Major depressive disorder (MDD) is a complex psychiatric disease requiring multidisciplinary approaches to identify specific risk factors and establish more efficacious treatment strategies. Although the etiology and pathophysiology of MDD are not clear until these days, it is acknowledged that they are almost certainly multifactorial and comprehensive. Monoamine neurotransmitter system dysfunction and specific personality traits are independent risk factors for depression and suicide. These factors also demonstrate complex interactions that influence MDD pathogenesis and symptom expression. In this review, we assess these relationships with the aim of providing a reference for the development of precision medicine.
Introduction
Major depressive disorder (MDD) is the most prevalent mood disorder and the most common disabling psychiatric disease across the globe. In the United States, the lifetime prevalence of MDD is 20.6% (1), and the associated healthcare and economic burdens are surpassed only by cardiomyopathy (2). The most clinically significant symptom of MDD is suicidality (3, 4). Over the years, MDD has been explained in genetic, biological, psychosocial, personality and other terms. No definite explanation accounts for the mechanism of MDD, however. Reducing the morbidity and mortality associated with MDD requires a more complete understanding of disease pathophysiology. Evidence accrued over many decades strongly implicates dysregulation of monoamine neurotransmitter systems in MDD development. Further, there is compelling evidence that MDD risk is strongly associated with certain personality traits. In this review, we expound the underlying relationships among monoamine neurotransmitter systems, personality traits, and MDD.
A biological basis for MDD risk is strongly supported by genetic studies demonstrating moderate heritability (ranging from about 37% and 45%) (5–9). Thus, gene–environment interactions are likely crucial to disease etiology, such as stressful life events (10, 11), childhood maltreatment (including emotional abuse, sexual abuse, emotional neglect, and physical neglect) (12, 13), and in fact these interactions result in an underestimation of the overall genetic influence (14). Kendler et al. reported a genetic correlation for liability to major depression of 0.63 in both males and females (9), and a similar estimate was reported in a population-based twin study (0.55) (15), consistent with several earlier studies suggesting that genetic risk factors are not sex-specific (16–19). However, the largest-sample twin study reported greater heritability in females (0.49, 95%CI = 0.31─0.56 vs. 0.41, 95%CI = 0.21─0.49), as well as 0.36 (95%CI = 0.31─0.38) in full siblings and 0.51 (95%CI = 0.51─0.53) in half-siblings (20). Several other studies have found a similarly elevated genetic propensity in females (9, 21, 22). These observed differences in MDD heritability between males and females are particularly interesting because recent neuroimaging and molecular genetic studies have also shown potential biological differences in MDD etiology between men and women. Edvardsen et al. reported a higher monozygotic/dizygotic ratio among male twins compared to female twins (8). Alternatively, a sex-limitation model suggested that the same genes influence MDD in males and females (19), although others have found that different genes impacted depressive illness (23). Thus, there is still no consensus on sex differences in the genetics of MDD.
Monoamine Neurotransmitters and MDD
Multiple studies have implicated the monoamine neurotransmitters 5-hydroxytryptamine (5-HT or serotonin), dopamine (DA), and norepinephrine (NE) as the primary contributors to MDD etiology. In the mammalian central nervous system (CNS), the major sources of the three monoamines are the raphe nuclei (24), substantia nigra and ventral tegmentum area (VTA) (25), and locus coeruleus, respectively.
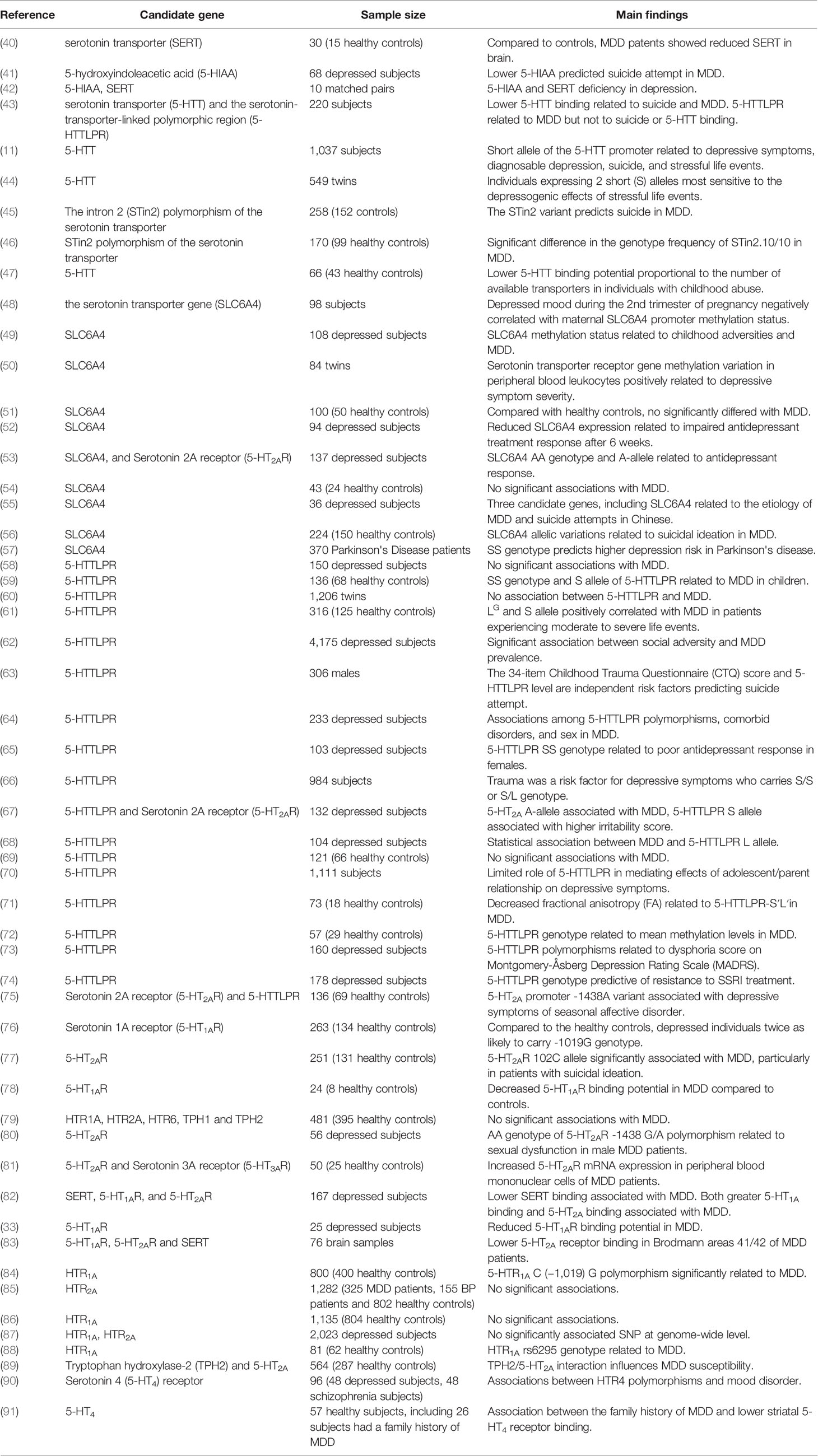
Raphe serotonergic neurons project to the caudate, putamen, pallidus, amygdala, limbic forebrain, and neocortex, where 5-HT signaling contributes to motivation, emotion stress processing (26), and regulation of other limbic functions (27). Acute depletion of the 5-HT precursor tryptophan (acute tryptophan depletion, ATD) markedly influences affective experience and emotional regulation in subjects with a family history of MDD (28). Challis et al. reported sensitization of inhibitory GABAergic neurons within the dorsal raphe nuclei and concomitant inhibition of serotonergic activity following social defeat in mice (29). Collectively, human and animal studies of tryptophan depletion (30) and associated serotonergic signaling deficiency strongly implicate 5-HT in mood regulation and MDD pathogenesis. Such insufficient 5-HT signaling may result from both reduced release and lower postsynaptic sensitivity as MDD patients demonstrate both decreased plasma and platelet levels of 5-HT, as well as blunted prefrontal cortical responses to 5-HT (31). Barton et al. reported elevated brain serotonin turnover before antidepressant therapy and markedly reduced turnover after antidepressant therapy and condition improvement, suggesting brain serotonin turnover as a potential biomarker for MDD (32). Further, a recent positron emission tomography (PET) study found reduced binding potential of the 5-HT1A receptor subtype in MDD patients relative to controls, and the authors suggested that lower 5-HT1A activity may result in “decreased engagement of the cognitive control network and impaired resolution of interfering cognitive stimuli” (33). Also consistent with a major contribution of 5-HT signaling dysfunction to MDD, elevated brain turnover of 5-HT is strongly influenced by 5-HT transporter (5-HTT) genotype (32), which in turn is associated with MDD risk. The urine serotonin/dopamine ratio may also be a useful diagnostic indicator for patients with MDD (34). Alternatively, selective serotonergic reuptake inhibitors (SSRIs) like fluoxetine, fluvoxamine, paroxetine, sertraline, and citalopram can enhance brain serotonin levels and are considered the first-line therapies for MDD patients based on demonstrated efficacy in the majority of placebo-controlled clinical studies (35). Growing evidence supports the hypothesis that epigenetic mechanisms, such as DNA methylation, play an important role in psychiatric diseases (36) such as MDD and personality disorders (37, 38), where epigenetic factors bridge the environmental and genetic mechanisms. A multitude of reports have considered the DNA methylation of the serotonin transporter gene (SLC6A4), located on chromosome 17 (39), as the major research target in investigation and evaluation in depression (Table 1). In summary, 5-HT is the biogenic amine most strongly associated with depression, as evidenced by the negative influence of 5-HT depletion on mood, the antidepressant efficacy of SSRIs, the perturbed 5-HT turnover and neuronal sensitivity in MDD patients and animal models, and the numerous associations between 5-HT pathway gene polymorphisms and MDD (Table 1).
Changes in 5-HT signaling may also predict suicidality. Patients with suicidal impulses exhibited lower cerebrospinal fluid (CSF) concentrations of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIIA) and fewer 5-HT uptake sites on platelets (92, 93). Weissmann et al. reported increased editing of the 5-HT2C receptor (5-HT2CR) mRNA in cortical areas of depressed suicides compared to non-psychiatric controls, suggesting that region-specific changes in 5-HT2CR function may contribute to MDD etiology (94). Further, altered activities of the major 5-HT biosynthetic enzymes tryptophan hydroxylase 1 and 2 (TPH 1 and TPH 2) (95), of 5-HTT (96), and of serotonin receptors, especially HTR1A (97), HTR2A (98), and HTR2C (99), are associated with suicidal impulses and violent suicidal behavior. However, contradictory findings have been reported (98, 100, 101), possibly due to low statistical power or heterogeneity of study populations. Larger-scale studies of different clinical and ethnic populations may resolve these controversies.
In animal models, genetic and pharmacological manipulation of serotonergic signaling can induce acute depression- and anxiety-like behaviors (102). Further, manipulating serotonergic and dopaminergic signaling during development can affect later-life somatosensory, anxiety/depression-like, and aggressive behavior (103). A recent study found generally lower levels of all three monoamines in a Wistar–Kyoto (WKY) animal model of maternal depression compared to matched control Sprague–Dawley (SD) rats (104).
Norepinephrine (NE) secreted from the locus coeruleus (LC) is a critical modulator of neural circuits involved in learning and memory (105–107), mood, sleep, appetite, and neuroendocrine function (108). Moreover, the antidepressant actions of monoamine oxidase (MAO) inhibitors and non-selective monoamine reuptake blockers suggest that NE plays a major role in the neurobiology of MDD (109). One potential pathogenic mechanism is elevated NE sensitivity of α2-adrenoceptors, which can inhibit NE release from the LC via negative feedback (110, 111). Indeed, elevated density and enhanced activity of α2-adrenoceptors have been reported in the brain tissues and platelets of MDD patients (112, 113). Elevated α2-adrenoceptor density has also been found in the frontal cortex and hippocampus of depressed suicides (114, 115). Moreover, Rivero and co-workers found that the elevated α2-adrenoceptors density in the prefrontal cortex of suicidal depressed subjects was resistant to antidepressant therapy, whereas elevated β1-adrenoceptor density was reduced by such therapy (116).
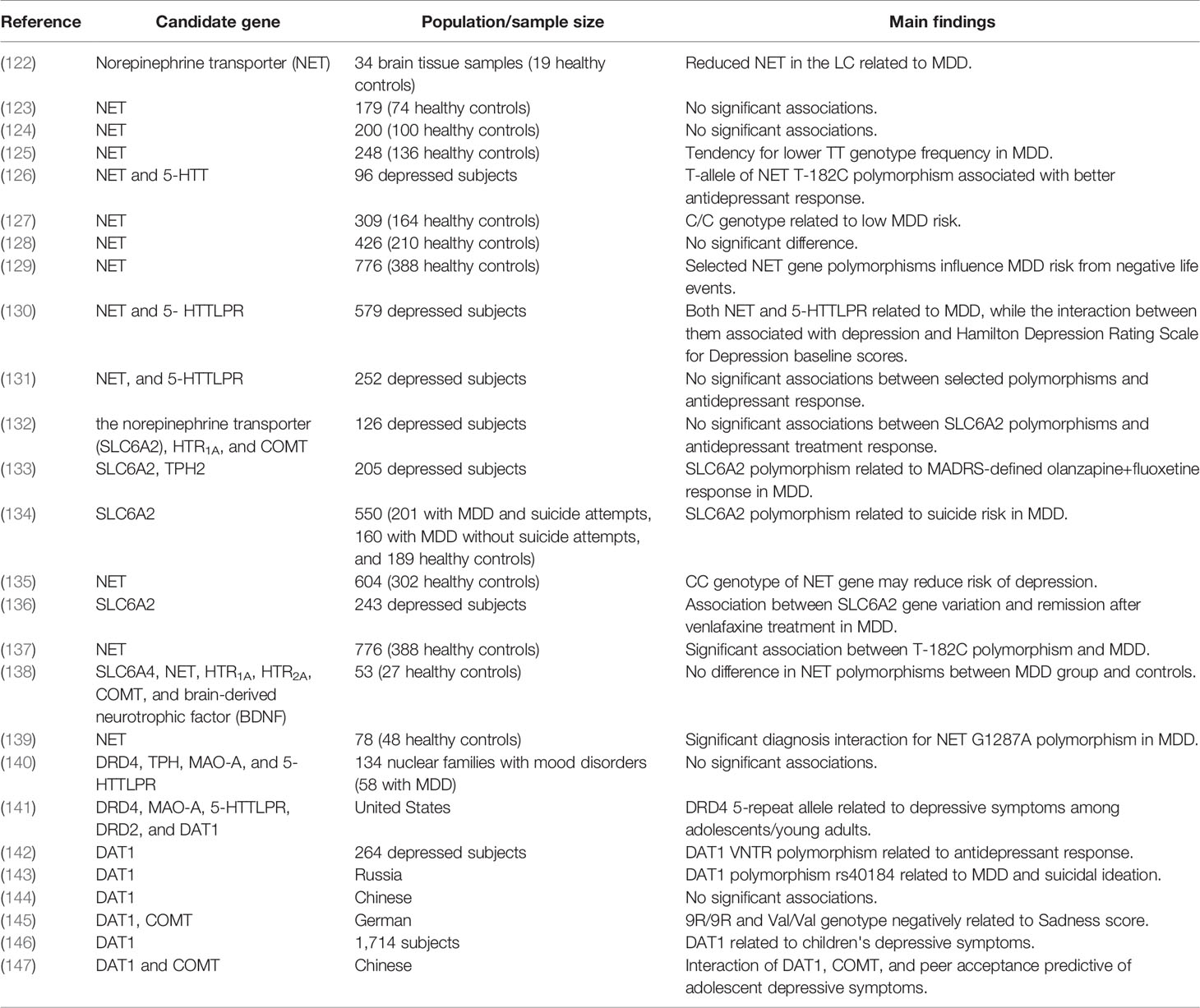
The efficacy of selective norepinephrine reuptake inhibitors (SNRIs) provides the strongest evidence for a direct contribution of deficient NE transmission to depression. A recent systematic review concluded that the SNRI duloxetine hydrochloride was effective against MDD as well as panic disorder, obsessive–compulsive disorder, and other psychiatric disorders (117), indicating broad involvement of NE in psychopathology. Another review suggested that duloxetine may be safe for older adults with MDD (118), although this agent has not been suggested for use as first-line acute therapy for MDD (119). Nonetheless, the norepinephrine transporter (NET) is well documented therapeutic target for MDD and like SSRIs (120), nonselective 5-HT/NE reuptake inhibitors such as venlafaxine (121) are widely used for MDD treatment. Many studies have also implicated NET gene polymorphism in MDD pathogenesis (Table 2). Abnormalities of noradrenergic function may also be involved in the pathogenesis of suicide (148). Several earlier studies reported upregulation of β-adrenoceptors in the brains of suicides (114, 149, 150), although several others reported the opposite (150, 151). Aside for receptor abnormalities, excessive stress could trigger depletion of NE and the onset of MDD (152).
While 5-HT and NE are the biogenic amines most consistently associated with MDD, abnormalities in DA signaling have also been implicated. For instance, depletion of DA has also been reported in MDD patients (153). The medial part of the VTA projects mainly to the nucleus accumbens and ventral striatum, which are central hubs of the brain reward system (154, 155). Allelic variation of DA-related genes modulate brain circuitry involved in the regulation of negative emotional stimuli (156), and DA system dysfunction has been associated with many symptoms of MDD such as anhedonia and low motivation (157, 158), as well as with cognitive symptoms such as impaired concentration (159, 160).
A dopamine deficiency has also been reported in MDD. One study measuring monoamine neurotransmitters and related metabolites in the cortex of rats detected DA only in the control group (161). A multi-data source-based prioritization (MDSP) study by Liu et al. identified 143 depression-related genes, including the DA receptor 4 (DRD4), as well 16 significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, including the ‘dopaminergic synapse' as well as the ‘serotonergic synapse' and ‘glutamatergic synapse'. The neuroactive ligand–receptor interaction list from KEGG pathway analysis also included the dopaminergic synapse (162). Further, a number of dopaminergic gene polymorphisms are associated with MDD (Table 2).
Reduced NE, 5-HT, and DA have been identified as significant biomarkers for depression in animal studies (163, 164). Advances in imaging techniques, including PET and single-photon emission computed tomography (SPECT), have also provided valuable insights into the contributions of DA to MDD. For instance, a recent study reported significantly reduced DA transporter (DAT) availability in the bilateral putamen and VTA of patients compared to healthy controls (Cohen d range, −0.62 to −0.71) (158). Moreover, this same study found lowest DAT availability in the VTA of patients reporting the greatest stress-related fatigue (165). While this relationship was replicated (166), the findings of a meta-analysis were contradictory (167).
In summary, the evidence is very strong that dysregulation of NE, DA, and 5-HT signaling contributes to MDD development and symptom expression. However, prospective studies are required to establish causal relationships between these deficiencies and MDD.
Personality Traits and MDD
Personality can be described as a composite of multiple, relatively stable traits and specific trait profiles, as measured using instruments such as the Neuroticism, Extraversion, Openness Five-Factor Inventory (NEO-FFI) questionnaire, Temperament and Character Inventory (TCI), and Eysenck Personality Questionnaire (EPQ) for associations with MDD risk.
A large-scale longitudinal cohort study using baseline and 2-year follow-up data found that increased neuroticism scores on the NEO-FFI were associated with both anxiety and depressive disorders. Higher agreeableness has also been associated with the occurrence of MDD, while openness demonstrated no association with the occurrence of, or recovery from, any depressive or anxiety disorder (168). In contrast, extraversion trait scores were associated with lower depressive disorder incidence and increased rate of recovery (169). Pair-wise genome-wide association studies (GWASs) have also found that numerous genetic variants overlap between depression and trait neuroticism (170). Further, high trait neuroticism has been confirmed as a dominant risk factor for depression (104). Also, low extraversion scores were a predictor of depression during the remission period of bipolar disorder (BP), the other main subtype of mood disorder (171). A recent resting-state dynamic functional network connectivity analysis found that state 4 was positively correlated with trait extraversion and negatively correlated with neuroticism, as measured by the EPQ, and that MDD patients showed significantly reduced dwell time and fractional time in state 4 compared to healthy controls, with lowest centrality degree in hippocampus and ventral striatum (172).
Neuroticism can improve the ability to cope with negative emotional stimuli (173) and has been linked to panic disorder (174), schizophrenia (175), and obsessive–compulsive disorder (OCD) (176) as well as to MDD. According to twin studies, the heritability of trait neuroticism is approximately 40%, with 15% to 37% caused by single-nucleotide polymorphism (SNP) variations (177). High trait neuroticism is associated with sensitivity to stress and negative emotional experiences, as well as with excessive worry, emotional vulnerability, and increased emotional exhaustion (178), all of which can impact an individual's physical activity (179), perception (180, 181), and emotion (182). An early meta-analysis of GWASs analyzing over 106,000 individuals identified nine neuroticism-associated loci (including the ionotropic kainate 3 glutamate receptor, Kelch-like protein 2, and corticotropin-releasing hormone receptor 1). This same study also found a strong association between neuroticism and MDD (genetic correlation = 0.64), but no sex difference in the heredity of neuroticism (177). Another meta-analysis of GWASs identified the Membrane-associated guanylate kinase inverted repeat member 1 (MAGI1) gene as a novel locus for neuroticism, both among the entire cohort of 63,661 individuals as well as in the combined Netherlands Twin Registry (NTR)/Netherlands Study of Depression and Anxiety (NESDA) cohort, with significant polygenic risk scores associated with MDD for SNP sets at P-value thresholds of 0.01 and 0.05, again providing compelling evidence that higher neuroticism is strongly correlated with MDD (183).
Harm avoidance (HA), a core personality trait defined by Cloninger, reflects a tendency to avoid potential danger, and like neuroticism, is related to traits such as pessimism, anxiousness, insecurity, bashfulness, and unusual susceptibility to fatigue (184). Trait HA has a high degree of stability throughout life (185), and is strongly associated with OCD (186), eating disorders (187), and other psychiatric disorders. High HA scores are also considered predictive of MDD (188). Bipolar disease patients demonstrating high HA scores on the TCI also showed a strong tendency for poor antidepressant treatment response during depressive episodes (189). A meta-analysis focusing on the associations between personality traits and MDD recovery found that patients with high novelty seeking (NS), high self-directedness (SD), and low HA exhibited better antidepressant responses (190). Alternatively, higher HA scores and lower SD scores were significantly correlated with non-remission in MDD patients (191), these findings have been replicated (192–194). Interestingly, a meta-analysis from Zaninotto et al. not only found such correlations, but the team reported the influence of HA in MDD vs healthy subjects was significantly greater than that found in BP vs healthy subjects (195), although there was marked heterogeneity among the included studies. Additional longitudinal studies are needed to confirm the association between HA and MDD.
Personality traits are also the major focus of suicide research. Garcia Herrero et al. concluded that high neuroticism can predict suicidal ideation (196). Similarly, Peters and his colleagues followed a large sample population in the United Kingdom for 10 years and found that neuroticism was related to suicide risk in both males and females and that neuroticism was a major predictor of suicide in females with mood disorders (197). An earlier study also found that neuroticism and openness were risk factors for suicide specifically in females, while extraversion and conscientiousness reduced the risk in males (198).
A recent study using the TCI to assess personality traits found that higher HA increased the risk of suicidal ideation in depression (199). Eric et al. also reported significantly higher HA scores, as well as low SD scores in subjects with suicidal ideation (192). Further, several studies have found that higher HA and NS scores are significant risk factors for suicidal behavior (200–202), while others have linked lower SD and higher self-transcendence (ST) to suicidality (203, 204).
Mood state may also impact personality traits, at least as measured at specific times, which complicates these association results. Nonetheless, the relatively consistent relationships between specific traits and MDD, including suicidal MDD, and the overlap between several trait-related and MDD-related genes suggest that investigations of the genetic and physiological attributes underlying specific traits may provide additional clues to the pathophysiology of MDD.
Monoamine Neurotransmitters and Personality Traits
Twin, family, and genomic studies have shown that personality traits are strongly influenced genetics, with estimated heritability ranging from 40% to 60% (205–208). Cloninger's Tridimensional Personality Questionnaire (TPQ) traits NS, HA, and reward-dependence (RD) have all been associated with monoamine functions (209, 210), as have the so called “the Big Five” personality traits assessed by NEO, NEO-PI-R, and NEO-FFI (neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness) (211) and the three personality traits of the EPQ (psychoticism, extraversion, and neuroticism) (212).
Extraversion, a higher-order personality trait, has been linked to reward system function in several studies (213–215). Furthermore, evidence strongly suggests that DA modulation is involved in both reward system function and extroversion (216). Smillie et al. (208) and co-workers reported that subjects with the DA receptor 2 (DRD2) gene A1-allele had significantly higher extroversion scores. In contrast, however, a functional magnetic resonance imaging (fMRI) study reported that A1-allele carriers exhibited lower extraversion scores, although the difference between carriers and non-carriers was not significant (217). A cross-national study of personality differences by Fischer et al. found a positive correlation between dopaminergic brain function index score and extraversion as well as a negative association between dopaminergic function and neuroticism score in those under high stress (218). A meta-analysis also found a relationship between self-consciousness (one facet of neuroticism) and the domain receptor 1 (DDR1) gene (219). Again, these relationships may be complicated by covariables. For instance, a previous study reported a negative correlation between neuroticism scores and quality of life in schizophrenia (175).
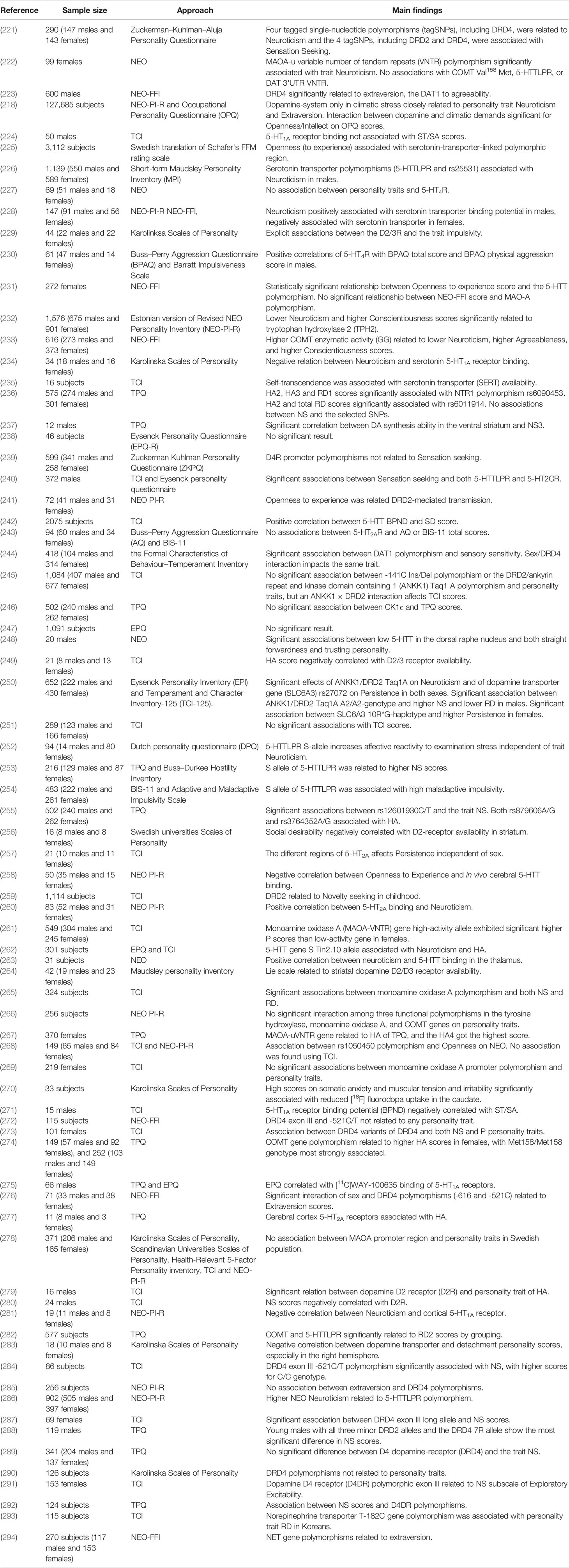
The opponent interactions between serotonin and DA makes the relationship between serotonin and personality traits was interesting and complex (220). Several studies have looked at the relationship, but the results have been inconsistent (Table 3). For example, most evidence to date support a link between the serotonin-transporter-linked polymorphic region (5-HTTLPR) and neuroticism (252, 286), meanwhile the different result were obtained using NEO-FFI (225). Interesting, in Swedish cohort study, they observed openness was significantly associated with 5-HTTLPR, while they also found that the positive association between openness and childhood adversity in the gene-environment model regardless of 5-HTTLPR genotype (225). Paaver et al. demonstrated S allele carriers with adverse family relations were related to higher thoughtlessness, disinhibition and impulsivity using the Barratt Impulsiveness Scale 11 (BIS-11) solely among girls (254), they also indicated that, in agreement with other studies, the influence of 5-HTTLPR genotype on affect is related to environmental adversity (61, 66). Indeed, environmental adversity, such as childhood adversity, can have a negative effect on child's expectations and present strained interpersonal relationships, which can affect personality or temperature (295), as well as associate with a range of psychopathology, including MDD (11). This factor has not been considered in some studies, which might be one of the fundamental reasons for the inconsistent results. Some studies on children have demonstrated significant association between 5-HTTLPR short (S) allele and higher NS scores (253), and S allele closely related to higher prevalence of substance use (296). In addition, the study of the relationship between personality trait and NE is rather little.
A number of monoaminergic transmitter-related genes are linked to personality traits, such as those encoding catechol-O-methyl-transferase (COMT) (297), monoamine oxidase A (MAOA) (222), and glutathione peroxidase 1 (GP × 1) (268). Furthermore, polymorphisms in monoamine receptors, for example 5-HTTLPR(226) and DRD4 (221), are associated with personality traits (Table 3). Recent studies in our laboratory have demonstrated associations between personality traits and Neurotensin receptor 1 (NTR1) (236), Dopamine- and cAMP-regulated phosphoprotein (DARPP-32) (255), and casein kinase 1ϵ (CK1ϵ) (246), all of which can affect monoaminergic signaling.
Undoubtedly, it is important that any assessment of the role of monoamines in personality traits should involve precise neural circuits associated with the relevant behavioral processes from the examples provided above (298). However, in many studies, there are some limitations, such as the small sample size with low statistical power, still need more participants to provide high quality evidence in further analysis.
Conclusions
MDD, therapeutic strategy still remain unclear, is one of the most prevalent medical disorder which causes life-threatening conditions, like suicides tend and suicidal behaviors. Although the precise etiology is not known, several studies support the fact that MDD is the severe mental disease that involves disturbance of chemical neurotransmitters, psychosocial factors, genetic factors, personality traits and other formulations. In our study, numerous strong associations have been identified among monoamine signaling deficits, detrimental personality traits, and major depressive disorder, providing potential clues to disease pathogenesis. And through incredible advancements in medical technology, these independent and interactive dimensions may be promising targets for precision medicine. Suicide is a massive public problem in depressed patients, thus research regarding the prevention and intervenient countermeasures of suicide should be thoroughly investigated in the field of biogenic amines changes and personality traits. Moreover, such studies have identified potential biomarkers for MDD risk that could aid in the early identification of at-risk individuals (299). Clinical programs should focus on early identification and intervention for emotional problems and high-risk behaviors among children and adolescents. Notably, the evidences for the relationship between monoamines, MDD and personality traits are confused and contradictory. Small sample size (significantly drop the accuracy rate and lead bias), unified analyzing methods, differences in tissues, depressive phenotypes, ethnicities, and others may lead to these inconsistent data. These factors should be considered in future studies.
Author Contributions
GZ planned and directed the paper, and XS wrote it.
Funding
This project was supported by a grant from the Major Project of the Department of Science & Technology of Liaoning Province (2019JH8/10300019) and a grant from the Major Project of the Science and Technology Ministry in China (2017YFC0820200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry (2018) 75(4):336–46. doi: 10.1001/jamapsychiatry.2017.4602
2. Ogbo FA, Mathsyaraja S, Koti RK, Perz J, Page A. The burden of depressive disorders in South Asia, 1990-2016: findings from the global burden of disease study. BMC Psychiatry (2018) 18(1):333. doi: 10.1186/s12888-018-1918-1
3. Hoertel N, Franco S, Wall MM, Oquendo MA, Kerridge BT, Limosin F, et al. Mental disorders and risk of suicide attempt: a national prospective study. Mol Psychiatry (2015) 20(6):718–26. doi: 10.1038/mp.2015.19
4. Knipe D, Williams AJ, Hannam-Swain S, Upton S, Brown K, Bandara P, et al. Psychiatric morbidity and suicidal behaviour in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med (2019) 16(10):e1002905–e. doi: 10.1371/journal.pmed.1002905
5. Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry (2000) 157(10):1552–62. doi: 10.1176/appi.ajp.157.10.1552
6. Bierut LJ, Heath AC, Bucholz KK, Dinwiddie SH, Madden PA, Statham DJ, et al. Major depressive disorder in a community-based twin sample: are there different genetic and environmental contributions for men and women? Arch Gen Psychiatry (1999) 56(6):557–63. doi: 10.1001/archpsyc.56.6.557
7. Glowinski AL, Madden PA, Bucholz KK, Lynskey MT, Heath AC. Genetic epidemiology of self-reported lifetime DSM-IV major depressive disorder in a population-based twin sample of female adolescents. J Child Psychol Psychiatry Allied Disciplines (2003) 44(7):988–96. doi: 10.1111/1469-7610.00183
8. Edvardsen J, Torgersen S, Roysamb E, Lygren S, Skre I, Onstad S, et al. Unipolar depressive disorders have a common genotype. J Affect Disord (2009) 117(1-2):30–41. doi: 10.1016/j.jad.2008.12.004
9. Kendler KSMD, Gatz M, Gardner C Ph.D., Pedersen NL Ph.D.. A Swedish National Twin Study of Lifetime Major Depression. Am J Psychiatry (2006) 163(1):109–14. doi: 10.1176/appi.ajp.163.1.109
10. Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. Jama (2009) 301(23):2462–71. doi: 10.1001/jama.2009.878
11. Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Sci (New York NY) (2003) 301(5631):386–9. doi: 10.1126/science.1083968
12. Humphreys KL, LeMoult J, Wear JG, Piersiak HA, Lee A, Gotlib IH. Child maltreatment and depression: A meta-analysis of studies using the Childhood Trauma Questionnaire. Child Abuse Negl (2020) 102:104361. doi: 10.1016/j.chiabu.2020.104361
13. Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, et al. Stressful life events and depression symptoms: the effect of childhood emotional abuse on stress reactivity. J Clin Psychol (2014) 70(3):209–23. doi: 10.1002/jclp.22011
14. Duncan AE, Munn-Chernoff MA, Hudson DL, Eschenbacher MA, Agrawal A, Grant JD, et al. Genetic and environmental risk for major depression in African-American and European-American women. Twin Res Hum Genet : Off J Int Soc Twin Stud (2014) 17(4):244–53. doi: 10.1017/thg.2014.28
15. Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med (2001) 31(4):605–16. doi: 10.1017/s0033291701003907
16. Foley DL, Neale MC, Gardner CO, Pickles A, Prescott CA, Kendler KS. Major depression and associated impairment: same or different genetic and environmental risk factors? Am J Psychiatry (2003) 160(12):2128–33. doi: 10.1176/appi.ajp.160.12.2128
17. Forlani C, Morri M, Ferrari B, Dalmonte E, Menchetti M, De Ronchi D, et al. Prevalence and gender differences in late-life depression: a population-based study. Am J Geriatr Psychiatry : Off J Am Assoc Geriatr Psychiatry (2014) 22(4):370–80. doi: 10.1016/j.jagp.2012.08.015
18. Gillespie NA, Kirk KM, Evans DM, Heath AC, Hickie IB, Martin NG. Do the genetic or environmental determinants of anxiety and depression change with age? A longitudinal study of Australian twins. Twin Res : Off J Int Soc Twin Stud (2004) 7(1):39–53. doi: 10.1375/13690520460741435
19. Petkus AJ, Beam CR, Johnson W, Kaprio J, Korhonen T, McGue M, et al. Gene-environment interplay in depressive symptoms: moderation by age, sex, and physical illness. Psychol Med (2017) 47(10):1836–47. doi: 10.1017/s0033291717000290
20. Kendler KS, Ohlsson H, Lichtenstein P, Sundquist J, Sundquist K. The Genetic Epidemiology of Treated Major Depression in Sweden. Am J Psychiatry (2018) 175(11):1137–44. doi: 10.1176/appi.ajp.2018.17111251
21. Jansson M, Gatz M, Berg S, Johansson B, Malmberg B, McClearn GE, et al. Gender differences in heritability of depressive symptoms in the elderly. Psychol Med (2004) 34(3):471–9. doi: 10.1017/s0033291703001375
22. McGue M, Christensen K. Genetic and environmental contributions to depression symptomatology: evidence from Danish twins 75 years of age and older. J Abnormal Psychol (1997) 106(3):439–48. doi: 10.1037//0021-843x.106.3.439
23. Kendler KS, Gardner CO. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am J Psychiatry (2014) 171(4):426–35. doi: 10.1176/appi.ajp.2013.13101375
24. Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience (1981) 6(4):557–618. doi: 10.1016/0306-4522(81)90146-9
25. Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res (1987) 434(2):117–65. doi: 10.1016/0165-0173(87)90011-7
26. Evers EA, Sambeth A, Ramaekers JG, Riedel WJ, van der Veen FM. The effects of acute tryptophan depletion on brain activation during cognition and emotional processing in healthy volunteers. Curr Pharma Design (2010) 16(18):1998–2011. doi: 10.2174/138161210791293060
27. Waselus M, Valentino RJ, Van Bockstaele EJ. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat (2011) 41(4):266–80. doi: 10.1016/j.jchemneu.2011.05.011
28. van der Veen FM, Evers EA, Deutz NE, Schmitt JA. Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol (2007) 32(1):216–24. doi: 10.1038/sj.npp.1301212
29. Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, et al. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci : Off J Soc Neurosci (2013) 33(35):13978–88, 88a. doi: 10.1523/jneurosci.2383-13.2013
30. Feder A, Skipper J, Blair JR, Buchholz K, Mathew SJ, Schwarz M, et al. Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol Psychiatry (2011) 69(8):804–7. doi: 10.1016/j.biopsych.2010.12.033
31. Anderson AD, Oquendo MA, Parsey RV, Milak MS, Campbell C, Mann JJ. Regional brain responses to serotonin in major depressive disorder. J Affect Disord (2004) 82(3):411–7. doi: 10.1016/j.jad.2004.04.003
32. Barton DA, Esler MD, Dawood T, Lambert EA, Haikerwal D, Brenchley C, et al. Elevated brain serotonin turnover in patients with depression: effect of genotype and therapy. Arch Gen Psychiatry (2008) 65(1):38–46. doi: 10.1001/archgenpsychiatry.2007.11
33. Langenecker SA, Mickey BJ, Eichhammer P, Sen S, Elverman KH, Kennedy SE, et al. Cognitive Control as a 5-HT1A-Based Domain That Is Disrupted in Major Depressive Disorder. Front Psychol (2019) 10:691. doi: 10.3389/fpsyg.2019.00691
34. Wijaya CS, Lee JJZ, Husain SF, Ho CSH, McIntyre RS, Tam WW, et al. Differentiating Medicated Patients Suffering from Major Depressive Disorder from Healthy Controls by Spot Urine Measurement of Monoamines and Steroid Hormones. Int J Environ Res Public Health (2018) 15(5):865. doi: 10.3390/ijerph15050865
35. National Collaborating Centre for Mental H. National Institute for Health and Clinical Excellence: Guidance. In: . Depression in Children and Young People: Identification and Management in Primary, Community and Secondary Care. Leicester (UK): British Psychological Society. The British Psychological Society & The Royal College of Psychiatrists (2005).
36. Rizzardi LF, Hickey PF, Rodriguez DiBlasi V, Tryggvadóttir R, Callahan CM, Idrizi A, et al. Neuronal brain-region-specific DNA methylation and chromatin accessibility are associated with neuropsychiatric trait heritability. Nat Neurosci (2019) 22(2):307–16. doi: 10.1038/s41593-018-0297-8
37. Nestler EJ. Epigenetic mechanisms of depression. JAMA Psychiatry (2014) 71(4):454–6. doi: 10.1001/jamapsychiatry.2013.4291
38. Gescher DM, Kahl KG, Hillemacher T, Frieling H, Kuhn J, Frodl T. Epigenetics in Personality Disorders: Today's Insights. Front Psychiatry (2018) 9:579. doi: 10.3389/fpsyt.2018.00579
39. Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U States America (1993) 90(6):2542–6. doi: 10.1073/pnas.90.6.2542
40. Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L, et al. Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry (1998) 44(11):1090–8. doi: 10.1016/s0006-3223(98)00272-8
41. Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid. A Biochem Suicide predictor? Arch Gen Psychiatry (1976) 33(10):1193–7. doi: 10.1001/archpsyc.1976.01770100055005
42. Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology (2001) 25(6):892–903. doi: 10.1016/s0893-133x(01)00310-4
43. Mann JJ, Huang Y-y, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, et al. A Serotonin Transporter Gene Promoter Polymorphism (5-HTTLPR) and Prefrontal Cortical Binding in Major Depression and Suicide. Arch Gen Psychiatry (2000) 57(8):729–38. doi: 10.1001/archpsyc.57.8.729
44. Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry (2005) 62(5):529–35. doi: 10.1001/archpsyc.62.5.529
45. Lopez de Lara C, Dumais A, Rouleau G, Lesage A, Dumont M, Chawky N, et al. STin2 variant and family history of suicide as significant predictors of suicide completion in major depression. Biol Psychiatry (2006) 59(2):114–20. doi: 10.1016/j.biopsych.2005.06.021
46. Sarosi A, Gonda X, Balogh G, Domotor E, Szekely A, Hejjas K, et al. Association of the STin2 polymorphism of the serotonin transporter gene with a neurocognitive endophenotype in major depressive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry (2008) 32(7):1667–72. doi: 10.1016/j.pnpbp.2008.06.014
47. Miller JM, Kinnally EL, Ogden RT, Oquendo MA, Mann JJ, Parsey RV. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse (New York NY) (2009) 63(7):565–73. doi: 10.1002/syn.20637
48. Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PloS One (2010) 5(8):e12201. doi: 10.1371/journal.pone.0012201
49. Kang HJ, Kim JM, Stewart R, Kim SY, Bae KY, Kim SW, et al. Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog Neuro-Psychopharmacol Biol Psychiatry (2013) 44:23–8. doi: 10.1016/j.pnpbp.2013.01.006
50. Zhao J, Goldberg J, Bremner JD, Vaccarino V. Association between promoter methylation of serotonin transporter gene and depressive symptoms: a monozygotic twin study. Psychosom Med (2013) 75(6):523–9. doi: 10.1097/PSY.0b013e3182924cf4
51. Okada S, Morinobu S, Fuchikami M, Segawa M, Yokomaku K, Kataoka T, et al. The potential of SLC6A4 gene methylation analysis for the diagnosis and treatment of major depression. J Psychiatr Res (2014) 53:47–53. doi: 10.1016/j.jpsychires.2014.02.002
52. Domschke K, Tidow N, Schwarte K, Deckert J, Lesch KP, Arolt V, et al. Serotonin transporter gene hypomethylation predicts impaired antidepressant treatment response. Int J Neuropsychoph (2014) 17(8):1167–76. doi: 10.1017/s146114571400039x
53. Matsumoto Y, Fabbri C, Pellegrini S, Porcelli S, Politi P, Bellino S, et al. Serotonin transporter gene: a new polymorphism may affect response to antidepressant treatments in major depressive disorder. Mol Diagn Ther (2014) 18(5):567–77. doi: 10.1007/s40291-014-0110-7
54. Chagnon YC, Potvin O, Hudon C, Preville M. DNA methylation and single nucleotide variants in the brain-derived neurotrophic factor (BDNF) and oxytocin receptor (OXTR) genes are associated with anxiety/depression in older women. Front Genet (2015) 6:230. doi: 10.3389/fgene.2015.00230
55. Rao S, Leung CS, Lam MH, Wing YK, Waye MM, Tsui SK. Resequencing three candidate genes discovers seven potentially deleterious variants susceptibility to major depressive disorder and suicide attempts in Chinese. Gene (2017) 603:34–41. doi: 10.1016/j.gene.2016.12.006
56. Ran L, Ai M, Wang W, Chen J, Wu T, Liu W, et al. Rare variants in SLC6A4 cause susceptibility to major depressive disorder with suicidal ideation in Han Chinese adolescents and young adults. Gene (2019), 144147. doi: 10.1016/j.gene.2019.144147
57. Wang JY, Fan QY, He JH, Zhu SG, Huang CP, Zhang X, et al. SLC6A4 Repeat and Single-Nucleotide Polymorphisms Are Associated With Depression and Rest Tremor in Parkinson's Disease: An Exploratory Study. Front Neurol (2019) 10:333. doi: 10.3389/fneur.2019.00333
58. Camarena B, Alvarez-Icaza D, Hernandez S, Aguilar A, Munch L, Martinez C, et al. Association Study Between Serotonin Transporter Gene and Fluoxetine Response in Mexican Patients With Major Depressive Disorder. Clin Neuropharmacol (2019) 42(1):9–13. doi: 10.1097/wnf.0000000000000315
59. Nobile M, Cataldo MG, Giorda R, Battaglia M, Baschirotto C, Bellina M, et al. A case-control and family-based association study of the 5-HTTLPR in pediatric-onset depressive disorders. Biol Psychiatry (2004) 56(4):292–5. doi: 10.1016/j.biopsych.2004.05.018
60. Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med (2005) 35(1):101–11. doi: 10.1017/s0033291704002727
61. Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry (2006) 163(9):1588–93. doi: 10.1176/ajp.2006.163.9.1588
62. Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry (2006) 59(3):224–9. doi: 10.1016/j.biopsych.2005.07.014.
63. Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology (2007) 32(9):2046–52. doi: 10.1038/sj.npp.1301331
64. Verhagen M, van der Meij A, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B. Effect of the 5-HTTLPR polymorphism in the serotonin transporter gene on major depressive disorder and related comorbid disorders. Psychiat Genet (2009) 19(1):39–44. doi: 10.1097/YPG.0b013e3283208061
65. Gressier F, Bouaziz E, Verstuyft C, Hardy P, Becquemont L, Corruble E. 5-HTTLPR modulates antidepressant efficacy in depressed women. Psychiat Genet (2009) 19(4):195–200. doi: 10.1097/YPG.0b013e32832cef0d
66. Goldman N, Glei DA, Lin YH, Weinstein M. The serotonin transporter polymorphism (5-HTTLPR): allelic variation and links with depressive symptoms. Depression Anxiety (2010) 27(3):260–9. doi: 10.1002/da.20660
67. Kamata M, Suzuki A, Yoshida K, Takahashi H, Higuchi H, Otani K. Genetic polymorphisms in the serotonergic system and symptom clusters of major depressive disorder. J Affect Disord (2011) 135(1-3):374–6. doi: 10.1016/j.jad.2011.08.027
68. Peralta-Leal V, Leal-Ugarte E, Meza-Espinoza JP, Gutierrez-Angulo M, Hernandez-Benitez CT, Garcia-Rodriguez A, et al. Association of serotonin transporter gene polymorphism 5-HTTLPR and depressive disorder in a Mexican population. Psychiat Genet (2012) 22(5):265–6. doi: 10.1097/YPG.0b013e32834f3577
69. Mohamed Saini S, Muhamad Radzi A, Abdul Rahman AH. Polymorphism of the serotonin transporter gene (5-HTTLPR) in major depressive disorder patients in Malaysia. Asia Pac Psychiatry (2012) 4(2):126–30. doi: 10.1111/j.1758-5872.2012.00190.x
70. Van Assche E, Moons T, Van Leeuwen K, Colpin H, Verschueren K, Van Den Noortgate W, et al. Depressive symptoms in adolescence: The role of perceived parental support, psychological control, and proactive control in interaction with 5-HTTLPR. Eur Psychiatry (2016) 35:55–63. doi: 10.1016/j.eurpsy.2016.01.2428
71. Tatham EL, Ramasubbu R, Gaxiola-Valdez I, Cortese F, Clark D, Goodyear B, et al. White matter integrity in major depressive disorder: Implications of childhood trauma, 5-HTTLPR and BDNF polymorphisms. Psychiatry Res Neuroimaging (2016) 253:15–25. doi: 10.1016/j.pscychresns.2016.04.014
72. Iga J, Watanabe SY, Numata S, Umehara H, Nishi A, Kinoshita M, et al. Association study of polymorphism in the serotonin transporter gene promoter, methylation profiles, and expression in patients with major depressive disorder. Hum Psychopharm (2016) 31(3):193–9. doi: 10.1002/hup.2527i
73. Takahashi H, Higuchi H, Sato K, Kamata M, Yoshida K, Nishimura K. Association between serotonin transporter polymorphisms (5-HTTLPR) and the MADRS Dysphoria, Retardation, and Vegetative Subscale scores in the treatment of depression. Neuropsychiatr Dis Treat (2017) 13:1463–9. doi: 10.2147/ndt.s12370
74. Tolahunase MR, Sagar R, Dada R. 5-HTTLPR and MTHFR 677C>T polymorphisms and response to yoga-based lifestyle intervention in major depressive disorder: A randomized active-controlled trial. Indian J Psychiatry (2018) 60(4):410–26. doi: 10.4103/psychiatry.IndianJPsychiatry_398_17
75. Enoch MA, Goldman D, Barnett R, Sher L, Mazzanti CM, Rosenthal NE. Association between seasonal affective disorder and the 5-HT2A promoter polymorphism, -1438G/A. Mol Psychiatry (1999) 4(1):89–92. doi: 10.1038/sj.mp.4000439
76. Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired Repression at a 5-Hydroxytryptamine 1A Receptor Gene Polymorphism Associated with Major Depression and Suicide. J Neurosci (2003) 23(25):8788–99. doi: 10.1523/jneurosci.23-25-08788.2003
77. Du L, Bakish D, Lapierre YD, Ravindran AV, Hrdina PD. Association of polymorphism of serotonin 2A receptor gene with suicidal ideation in major depressive disorder. Am J Med Genet (2000) 96(1):56–60. doi: 10.1002/(sici)1096-8628(20000207)96:1<56::aid-ajmg12>3.0.co;2-l
78. Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol (2007) 34(7):865–77. doi: 10.1016/j.nucmedbio.2007.06.008
79. Illi A, Setala-Soikkeli E, Viikki M, Poutanen O, Huhtala H, Mononen N, et al. 5-HTR1A, 5-HTR2A, 5-HTR6, TPH1 and TPH2 polymorphisms and major depression. Neuroreport (2009) 20(12):1125–8. doi: 10.1097/WNR.0b013e32832eb708
80. Liang CS, Ho PS, Chiang KT, Su HC. 5-HT2A receptor -1438 G/A polymorphism and serotonergic antidepressant-induced sexual dysfunction in male patients with major depressive disorder: a prospective exploratory study. J Sex Med (2012) 9(8):2009–16. doi: 10.1111/j.1743-6109.2012.02769.x
81. Amidfar M, Kim YK, Colic L, Arbabi M, Mobaraki G, Hassanzadeh G, et al. Increased levels of 5HT2A receptor mRNA expression in peripheral blood mononuclear cells of patients with major depression: correlations with severity and duration of illness. Nord J Psychiatry (2017) 71(4):282–8. doi: 10.1080/08039488.2016.1276624
82. Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Dwork AJ, Mann JJ, et al. Serotonin receptors and suicide, major depression, alcohol use disorder and reported early life adversity. Transl Psychiatry (2018) 8(1):279–. doi: 10.1038/s41398-018-0309-1
83. Steinberg LJ, Underwood MD, Bakalian MJ, Kassir SA, Mann JJ, Arango V. 5-HT1A receptor, 5-HT2A receptor and serotonin transporter binding in the human auditory cortex in depression. J Psychiatry Neurosci (2019) 44(5):294–302. doi: 10.1503/jpn.180190
84. Wu Y, Xu Y, Sun Y, Wang YF, Li X, Lang XE, et al. Association between the serotonin 1A receptor C(-1019)G polymorphism and major depressive disorder in the northern Han ethnic group in China. Chin Med J (2008) 121(10):874–6.
85. Kishi T, Kitajima T, Tsunoka T, Ikeda M, Yamanouchi Y, Kinoshita Y, et al. Genetic association analysis of serotonin 2A receptor gene (HTR2A) with bipolar disorder and major depressive disorder in the Japanese population. Neurosci Res (2009) 64(2):231–4. doi: 10.1016/j.neures.2009.03.003
86. Kishi T, Tsunoka T, Ikeda M, Kawashima K, Okochi T, Kitajima T, et al. Serotonin 1A receptor gene and major depressive disorder: an association study and meta-analysis. J Hum Genet (2009) 54(11):629–33. doi: 10.1038/jhg.2009.84
87. Schosser A, Butler AW, Ising M, Perroud N, Uher R, Ng MY, et al. Genomewide association scan of suicidal thoughts and behaviour in major depression. PloS One (2011) 6(7):e20690. doi: 10.1371/journal.pone.0020690
88. Kautzky A, James GM, Philippe C, Baldinger-Melich P, Kraus C, Kranz GS, et al. The influence of the rs6295 gene polymorphism on serotonin-1A receptor distribution investigated with PET in patients with major depression applying machine learning. Transl Psychiatry (2017) 7(6):e1150. doi: 10.1038/tp.2017.108
89. Yang J, Zhao X, Ma J, Qiao Z, Yang X, Zhao E, et al. The Interaction of TPH2 and 5-HT2A Polymorphisms on Major Depressive Disorder Susceptibility in a Chinese Han Population: A Case-Control Study. Front Psychiatry (2019) 10:172. doi: 10.3389/fpsyt.2019.00172
90. Ohtsuki T, Ishiguro H, Detera-Wadleigh SD, Toyota T, Shimizu H, Yamada K, et al. Association between serotonin 4 receptor gene polymorphisms and bipolar disorder in Japanese case-control samples and the NIMH Genetics Initiative Bipolar Pedigrees. Mol Psychiatry (2002) 7(9):954–61. doi: 10.1038/sj.mp.4001133
91. Madsen K, Torstensen E, Holst KK, Haahr ME, Knorr U, Frokjaer VG, et al. Familial risk for major depression is associated with lower striatal 5-HT(4) receptor binding. Int J Neuropsychoph (2014) 18(1). doi: 10.1093/ijnp/pyu034
92. Roy A, Agren H, Pickar D, Linnoila M, Doran AR, Cutler NR, et al. Reduced CSF concentrations of homovanillic acid and homovanillic acid to 5-hydroxyindoleacetic acid ratios in depressed patients: relationship to suicidal behavior and dexamethasone nonsuppression. Am J Psychiatry (1986) 143(12):1539–45. doi: 10.1176/ajp.143.12.1539
93. Jokinen J, Nordstrom AL, Nordstrom P. The relationship between CSF HVA/5-HIAA ratio and suicide intent in suicide attempters. Arch Suicide Res : Off J Int Acad Suicide Res (2007) 11(2):187–92. doi: 10.1080/13811110701250093
94. Weissmann D, van der Laan S, Underwood MD, Salvetat N, Cavarec L, Vincent L, et al. Region-specific alterations of A-to-I RNA editing of serotonin 2c receptor in the cortex of suicides with major depression. Trans Psychiatry (2016) 6(8):e878. doi: 10.1038/tp.2016.121
95. Antypa N, Serretti A, Rujescu D. Serotonergic genes and suicide: a systematic review. Eur Neuropsychopharmacol : J Eur Coll Neuropsychopharmacol (2013) 23(10):1125–42. doi: 10.1016/j.euroneuro.2013.03.013
96. Saiz PA, Garcia-Portilla P, Paredes B, Corcoran P, Arango C, Morales B, et al. Role of serotonergic-related systems in suicidal behavior: Data from a case-control association study. Prog Neuropsychopharmacol Biol Psychiatry (2011) 35(6):1518–24. doi: 10.1016/j.pnpbp.2011.04.011
97. Pompili M, Gentile G, Scassellati C, Bonvicini C, Innamorati M, Erbuto D, et al. Genetic association analysis of serotonin and signal transduction pathways in suicide attempters from an Italian sample of psychiatric patients. Neurosci Lett (2017) 656:94–102. doi: 10.1016/j.neulet.2017.07.020
98. Gonzalez-Castro TB, Tovilla-Zarate C, Juarez-Rojop I, Pool Garcia S, Velazquez-Sanchez MP, Genis A, et al. Association of the 5HTR2A gene with suicidal behavior: case-control study and updated meta-analysis. BMC Psychiatry (2013) 13:25. doi: 10.1186/1471-244x-13-25
99. Lyddon R, Dwork AJ, Keddache M, Siever LJ, Dracheva S. Serotonin 2c receptor RNA editing in major depression and suicide. World J Biol Psychiatry : Off J World Fed Societies Biol Psychiatry (2013) 14(8):590–601. doi: 10.3109/15622975.2011.630406
100. Angles MR, Ocana DB, Medellin BC, Tovilla-Zarate C. No association between the HTR1A gene and suicidal behavior: a meta-analysis. Rev Bras Psiquiatr (Sao Paulo Brazil : 1999) (2012) 34(1):38–42. doi: 10.1590/s1516-44462012000100008
101. Yoon HK, Kim YK. Association between serotonin-related gene polymorphisms and suicidal behavior in depressive patients. Prog Neuropsychopharmacol Biol Psychiatry (2008) 32(5):1293–7. doi: 10.1016/j.pnpbp.2008.04.004
102. Borg J. Molecular imaging of the 5-HT(1A) receptor in relation to human cognition. Behav Brain Res (2008) 195(1):103–11. doi: 10.1016/j.bbr.2008.06.011
103. Suri D, Teixeira CM, Cagliostro MK, Mahadevia D, Ansorge MS. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol (2015) 40(1):88–112. doi: 10.1038/npp.2014.231
104. Winokur SB, Lopes KL, Moparthi Y, Pereira M. Depression-related disturbances in rat maternal behaviour are associated with altered monoamine levels within mesocorticolimbic structures. J Neuroendocrinol (2019) 31(9):e12766. doi: 10.1111/jne.12766
105. Hagena H, Hansen N, Manahan-Vaughan D. beta-Adrenergic Control of Hippocampal Function: Subserving the Choreography of Synaptic Information Storage and Memory. Cereb Cortex (New York NY : 1991) (2016) 26(4):1349–64. doi: 10.1093/cercor/bhv330
106. Hansen N. The Longevity of Hippocampus-Dependent Memory Is Orchestrated by the Locus Coeruleus-Noradrenergic System. Neural Plastic (2017) 2017:2727602. doi: 10.1155/2017/2727602
107. Gibbs ME, Hutchinson DS, Summers RJ. Noradrenaline release in the locus coeruleus modulates memory formation and consolidation; roles for alpha- and beta-adrenergic receptors. Neuroscience (2010) 170(4):1209–22. doi: 10.1016/j.neuroscience.2010.07.052
108. Kaye WH, Ballenger JC, Lydiard RB, Stuart GW, Laraia MT, O'Neil P, et al. CSF monoamine levels in normal-weight bulimia: evidence for abnormal noradrenergic activity. Am J Psychiatry (1990) 147(2):225–9. doi: 10.1176/ajp.147.2.225
109. Bunney WE Jr., Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry (1965) 13(6):483–94. doi: 10.1001/archpsyc.1965.01730060001001
110. Kafka MS, Paul SM. Platelet alpha 2-adrenergic receptors in depression. Arch Gen Psychiatry (1986) 43(1):91–5. doi: 10.1001/archpsyc.1986.01800010093012
111. Piletz JE, Schubert DS, Halaris A. Evaluation of studies on platelet alpha 2 adrenoreceptors in depressive illness. Life Sci (1986) 39(18):1589–616. doi: 10.1016/0024-3205(86)90156-6
112. Cottingham C, Wang Q. alpha2 adrenergic receptor dysregulation in depressive disorders: implications for the neurobiology of depression and antidepressant therapy. Neurosci Biobehav Rev (2012) 36(10):2214–25. doi: 10.1016/j.neubiorev.2012.07.011
113. Garcia-Sevilla JA, Ventayol P, Perez V, Rubovszky G, Puigdemont D, Ferrer-Alcon M, et al. Regulation of platelet alpha 2A-adrenoceptors, Gi proteins and receptor kinases in major depression: effects of mirtazapine treatment. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol (2004) 29(3):580–8. doi: 10.1038/sj.npp.1300356
114. Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry (1986) 43(10):954–9. doi: 10.1001/archpsyc.1986.01800100048007
115. Gonzalez AM, Pascual J, Meana JJ, Barturen F, del Arco C, Pazos A, et al. Autoradiographic demonstration of increased alpha 2-adrenoceptor agonist binding sites in the hippocampus and frontal cortex of depressed suicide victims. J Neurochemi (1994) 63(1):256–65. doi: 10.1046/j.1471-4159.1994.63010256.x
116. Rivero G, Gabilondo AM, Garcia-Sevilla JA, La Harpe R, Callado LF, Meana JJ. Increased alpha2- and beta1-adrenoceptor densities in postmortem brain of subjects with depression: differential effect of antidepressant treatment. J Affect Disord (2014) 167:343–50. doi: 10.1016/j.jad.2014.06.016
117. Muscatello MRA, Zoccali RA, Pandolfo G, Mangano P, Lorusso S, Cedro C, et al. Duloxetine in Psychiatric Disorders: Expansions Beyond Major Depression and Generalized Anxiety Disorder. Front Psychiatry (2019) 10:772. doi: 10.3389/fpsyt.2019.00772
118. Dhillon S. Duloxetine: a review of its use in the management of major depressive disorder in older adults. Drugs Aging (2013) 30(1):59–79. doi: 10.1007/s40266-012-0040-1
119. Cipriani A, Koesters M, Furukawa TA, Nose M, Purgato M, Omori IM, et al. Duloxetine versus other anti-depressive agents for depression. Cochrane Database Systematic Rev (2012) 10:Cd006533. doi: 10.1002/14651858.CD006533.pub2
120. Andre K, Kampman O, Illi A, Viikki M, Setala-Soikkeli E, Mononen N, et al. SERT and NET polymorphisms, temperament and antidepressant response. Nordic J Psychiatry (2015) 69(7):531–8. doi: 10.3109/08039488.2015.1012554
121. Marshe VS, Maciukiewicz M, Rej S, Tiwari AK, Sibille E, Blumberger DM, et al. Norepinephrine Transporter Gene Variants and Remission From Depression With Venlafaxine Treatment in Older Adults. Am J Psychiatry (2017) 174(5):468–75. doi: 10.1176/appi.ajp.2016.16050617
122. Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, et al. Reduced Levels of Norepinephrine Transporters in the Locus Coeruleus in Major Depression. J Neurosci (1997) 17(21):8451–8. doi: 10.1523/jneurosci.17-21-08451.1997
123. Owen D, Du L, Bakish D, Lapierre YD, Hrdina PD. Norepinephrine transporter gene polymorphism is not associated with susceptibility to major depression. Psychiatry Res (1999) 87(1):1–5. doi: 10.1016/s0165-1781(99)00050-5
124. Zill P, Engel R, Baghai TC, Juckel G, Frodl T, Muller-Siecheneder F, et al. Identification of a naturally occurring polymorphism in the promoter region of the norepinephrine transporter and analysis in major depression. Neuropsychopharmacology (2002) 26(4):489–93. doi: 10.1016/s0893-133x(01)00386-4
125. Ryu SH, Lee SH, Lee HJ, Cha JH, Ham BJ, Han CS, et al. Association between norepinephrine transporter gene polymorphism and major depression. Neuropsychobiology (2004) 49(4):174–7. doi: 10.1159/000077361
126. Yoshida K, Takahashi H, Higuchi H, Kamata M, Ito K, Sato K, et al. Prediction of antidepressant response to milnacipran by norepinephrine transporter gene polymorphisms. Am J Psychiatry (2004) 161(9):1575–80. doi: 10.1176/appi.ajp.161.9.1575
127. Inoue K, Itoh K, Yoshida K, Shimizu T, Suzuki T. Positive association between T-182C polymorphism in the norepinephrine transporter gene and susceptibility to major depressive disorder in a japanese population. Neuropsychobiology (2004) 50(4):301–4. doi: 10.1159/000080957
128. Chang CC, Lu RB, Chen CL, Chu CM, Chang HA, Huang CC, et al. Lack of association between the norepinephrine transporter gene and major depression in a Han Chinese population. J Psychiatry Neurosci (2007) 32(2):121–8.
129. Sun N, Xu Y, Wang Y, Duan H, Wang S, Ren Y, et al. The combined effect of norepinephrine transporter gene and negative life events in major depression of Chinese Han population. J Neural Transm (Vienna) (2008) 115(12):1681–6. doi: 10.1007/s00702-008-0109-5
130. Min WJ, Ma XH, Li T, Zhang B, Sun XL. [Association of serotonin and norepinephrine transporter gene polymorphisms with the susceptibility to depression]. Zhonghua yi xue yi Chuan xue za zhi = Zhonghua yixue yichuanxue zazhi = Chin J Med Genet (2009) 26(4):388–92.
131. Baffa A, Hohoff C, Baune BT, Muller-Tidow C, Tidow N, Freitag C, et al. Norepinephrine and serotonin transporter genes: impact on treatment response in depression. Neuropsychobiology (2010) 62(2):121–31. doi: 10.1159/000317285
132. Houston JP, Zou W, Aris V, Fijal B, Chen P, Heinloth AN, et al. Evaluation of genetic models for response in a randomized clinical trial of duloxetine in major depressive disorder. Psychiatry Res (2012) 200(1):63–5. doi: 10.1016/j.psychres.2012.06.002
133. Houston JP, Lau K, Aris V, Liu W, Fijal BA, Heinloth AN, et al. Association of common variations in the norepinephrine transporter gene with response to olanzapine-fluoxetine combination versus continued-fluoxetine treatment in patients with treatment-resistant depression: a candidate gene analysis. J Clin Psychiatry (2012) 73(6):878–85. doi: 10.4088/JCP.10m06744
134. Kim YK, Hwang JA, Lee HJ, Yoon HK, Ko YH, Lee BH, et al. Association between norepinephrine transporter gene (SLC6A2) polymorphisms and suicide in patients with major depressive disorder. J Affect Disord (2014) 158:127–32. doi: 10.1016/j.jad.2014.01.018
135. Pan Y, Cheng Q, Shan MS, Yan J. Association between polymorphism of the norepinephrine transporter gene rs2242446 and rs5669 loci and depression disorders. Int J Clin Exp Med (2015) 8(10):18837–42.
136. Yeh YW, Chen CJ, Jang FL, Kuo SC, Chen CY, Liang CS, et al. SLC6A2 variants may predict remission from major depression after venlafaxine treatment in Han Chinese population. J Psychiatr Res (2015) 61:33–9. doi: 10.1016/j.jpsychires.2014.11.017
137. Wang Y, Sun N, Li S, Du Q, Xu Y, Liu Z, et al. A Genetic Susceptibility Mechanism for Major Depression: Combinations of polymorphisms Defined the Risk of Major Depression and Subpopulations. Medicine (2015) 94(23):e778. doi: 10.1097/md.0000000000000778
138. Phillips JL, Batten LA, Tremblay P, Aldosary F, Du L, Blier P. Impact of monoamine-related gene polymorphisms on hippocampal volume in treatment-resistant depression. Acta Neuropsychiatr (2015) 27(6):353–61. doi: 10.1017/neu.2015.25
139. Ueda I, Kakeda S, Watanabe K, Yoshimura R, Kishi T, Abe O, et al. Relationship between G1287A of the NET Gene Polymorphisms and Brain Volume in Major Depressive Disorder: A Voxel-Based MRI Study. PloS One (2016) 11(3):e0150712. doi: 10.1371/journal.pone.0150712
140. Serretti A, Cristina S, Lilli R, Cusin C, Lattuada E, Lorenzi C, et al. Family-based association study of 5-HTTLPR, TPH, MAO-A, and DRD4 polymorphisms in mood disorders. Am J Med Genet (2002) 114(4):361–9. doi: 10.1002/ajmg.10356
141. Adkins DE, Daw JK, McClay JL, van den Oord EJ. The influence of five monoamine genes on trajectories of depressive symptoms across adolescence and young adulthood. Dev Psychopathol. (2012) 24(1):267–85. doi: 10.1017/s0954579411000824
142. Kirchheiner J, Nickchen K, Sasse J, Bauer M, Roots I, Brockmoller J. A 40-basepair VNTR polymorphism in the dopamine transporter (DAT1) gene and the rapid response to antidepressant treatment. Pharmacogenomics J (2007) 7(1):48–55. doi: 10.1038/sj.tpj.6500398
143. Haeffel GJ, Getchell M, Koposov RA, Yrigollen CM, Deyoung CG, Klinteberg BA, et al. Association between polymorphisms in the dopamine transporter gene and depression: evidence for a gene-environment interaction in a sample of juvenile detainees. Psychol Sci (2008) 19(1):62–9. doi: 10.1111/j.1467-9280.2008.02047.x
144. Huang CC, Lu RB, Shih MC, Yen CH, Huang SY. Association study of the dopamine transporter gene with personality traits and major depressive disorder in the Han Chinese population. Pharmacogenet Genom (2011) 21(2):94–7. doi: 10.1097/FPC.0b013e3283424d94
145. Felten A, Montag C, Markett S, Walter NT, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain Behav (2011) 1(2):109–18. doi: 10.1002/brb3.20
146. D'Souza S, Thompson JM, Slykerman R, Marlow G, Wall C, Murphy R, et al. Environmental and genetic determinants of childhood depression: The roles of DAT1 and the antenatal environment. J Affect Disord (2016) 197:151–8. doi: 10.1016/j.jad.2016.03.023
147. Cao Y, Lin X, Chen L, Ji L, Zhang W. The Catechol-O-Methyltransferase and Dopamine Transporter Genes Moderated the Impact of Peer Relationships on Adolescent Depressive Symptoms: A Gene-Gene-Environment Study. J Youth Adolesc (2018) 47(11):2468–80. doi: 10.1007/s10964-018-0925-3
148. Pandey GN, Dwivedi Y. Noradrenergic function in suicide. Arch Suicide Res : Off J Int Acad Suicide Res (2007) 11(3):235–46. doi: 10.1080/13811110701402587
149. Biegon A, Israeli M. Regionally selective increases in beta-adrenergic receptor density in the brains of suicide victims. Brain Res (1988) 442(1):199–203. doi: 10.1016/0006-8993(88)91453-9
150. Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry (1990) 47(11):1038–47. doi: 10.1001/archpsyc.1990.01810230054009
151. De Paermentier F, Crompton MR, Katona CL, Horton RW. beta-adrenoceptors in brain and pineal from depressed suicide victims. Pharmacol Toxicol (1992) 71 Suppl 1:86–95. doi: 10.1111/j.1600-0773.1992.tb01632.x
152. Oquendo MA, Sullivan GM, Sudol K, Baca-Garcia E, Stanley BH, Sublette ME, et al. Toward a biosignature for suicide. Am J Psychiatry (2014) 171(12):1259–77. doi: 10.1176/appi.ajp.2014.14020194
153. Hasler G, Fromm S, Carlson PJ, Luckenbaugh DA, Waldeck T, Geraci M, et al. Neural response to catecholamine depletion in unmedicated subjects with major depressive disorder in remission and healthy subjects. Arch Gen Psychiatry (2008) 65(5):521–31. doi: 10.1001/archpsyc.65.5.521
154. Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol (2010) 35(1):27–47. doi: 10.1038/npp.2009.93
155. Schultz W, Stauffer WR, Lak A. The phasic dopamine signal maturing: from reward via behavioural activation to formal economic utility. Curr Opin Neurobiol (2017) 43:139–48. doi: 10.1016/j.conb.2017.03.013
156. Prata DP, Mechelli A, Fu CH, Picchioni M, Toulopoulou T, Bramon E, et al. Epistasis between the DAT 3' UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proc Natl Acad Sci U States America (2009) 106(32):13600–5. doi: 10.1073/pnas.0903007106
157. Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci (2004) 5(6):483–94. doi: 10.1038/nrn1406
158. Pandit R, Omrani A, Luijendijk MC, de Vrind VA, Van Rozen AJ, Ophuis RJ, et al. Melanocortin 3 Receptor Signaling in Midbrain Dopamine Neurons Increases the Motivation for Food Reward. Neuropsychopharmacol : Off Publ Am Coll Neuropsychopharmacol (2016) 41(9):2241–51. doi: 10.1038/npp.2016.19
159. Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Sci (New York NY) (1979) 205(4409):929–32. doi: 10.1126/science.112679
160. Wang M, Datta D, Enwright J, Galvin V, Yang ST, Paspalas C, et al. A novel dopamine D1 receptor agonist excites delay-dependent working memory-related neuronal firing in primate dorsolateral prefrontal cortex. Neuropharmacology (2019) 150:46–58. doi: 10.1016/j.neuropharm.2019.03.001
161. Huang D, Zhang L, Yang JQ, Luo Y, Cui T, Du TT, et al. Evaluation on monoamine neurotransmitters changes in depression rats given with sertraline, meloxicam or/and caffeic acid. Genes Dis (2019) 6(2):167–75. doi: 10.1016/j.gendis.2018.05.005
162. Liu Y, Fan P, Zhang S, Wang Y, Liu D. Prioritization and comprehensive analysis of genes related to major depressive disorder. Mol Genet Genomic Med (2019) 7(6):e659. doi: 10.1002/mgg3.659
163. Ahmed M, Azmat A. Decreased brain serotonin turnover rate following administration of Sharbat-e-Ahmed Shah produces antidepressant and anxiolytic effect in rats. Metab Brain Dis (2017) 32(6):1785–90. doi: 10.1007/s11011-017-0065-6
164. Tsutsumi H, Yonemitsu K, Sasao A. Cerebrospinal fluid neurotransmitter levels and central nervous system depression in a rat drug overdose model. Toxicol Mech Methods (2019), 1–7. doi: 10.1080/15376516.2019.1672122
165. Pizzagalli DA, Berretta S, Wooten D, Goer F, Pilobello KT, Kumar P, et al. Assessment of Striatal Dopamine Transporter Binding in Individuals With Major Depressive Disorder: In Vivo Positron Emission Tomography and Postmortem Evidence. JAMA Psychiatry (2019) 88. doi: 10.1001/jamapsychiatry.2019.0801
166. Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, et al. Lower dopamine transporter binding potential in striatum during depression. Neuroreport (2001) 12(18):4121–5. doi: 10.1097/00001756-200112210-00052
167. Li Z, He Y, Tang J, Zong X, Hu M, Chen X. Molecular imaging of striatal dopamine transporters in major depression–a meta-analysis. J Affect Disord (2015) 174:137–43. doi: 10.1016/j.jad.2014.11.045
168. Karsten J, Penninx BW, Riese H, Ormel J, Nolen WA, Hartman CA. The state effect of depressive and anxiety disorders on big five personality traits. J Psychiatr Res (2012) 46(5):644–50. doi: 10.1016/j.jpsychires.2012.01.024
169. Grav S, Stordal E, Romild UK, Hellzen O. The relationship among neuroticism, extraversion, and depression in the HUNT Study: in relation to age and gender. Issues Ment Health Nurs (2012) 33(11):777–85. doi: 10.3109/01612840.2012.713082
170. Adams MJ, Howard DM, Luciano M, Clarke T-K, Davies G, Hill WD, et al. Genetic stratification of depression by neuroticism: revisiting a diagnostic tradition. Psychol Med (2019), 1–10. doi: 10.1017/S0033291719002629
171. Barnett JH, Huang J, Perlis RH, Young MM, Rosenbaum JF, Nierenberg AA, et al. Personality and bipolar disorder: dissecting state and trait associations between mood and personality. Psychol Med (2011) 41(8):1593–604. doi: 10.1017/s0033291710002333
172. Wu X, He H, Shi L, Xia Y, Zuang K, Feng Q, et al. Personality traits are related with dynamic functional connectivity in major depression disorder: A resting-state analysis. J Affect Disord (2019) 245:1032–42. doi: 10.1016/j.jad.2018.11.002
173. Silverman MH, Wilson S, Ramsay IS, Hunt RH, Thomas KM, Krueger RF, et al. Trait neuroticism and emotion neurocircuitry: Functional magnetic resonance imaging evidence for a failure in emotion regulation. Dev Psychopathol (2019) 31(3):1085–99. doi: 10.1017/s0954579419000610
174. Zugliani MM, Martin-Santos R, Nardi AE, Freire RC. Personality Traits in Panic Disorder Patients With and Without Comorbidities. J Nervous Ment Dis (2017) 205(11):855–8. doi: 10.1097/nmd.0000000000000745
175. Ridgewell C, Blackford JU, McHugo M, Heckers S. Personality traits predicting quality of life and overall functioning in schizophrenia. Schizophr Res (2017) 182:19–23. doi: 10.1016/j.schres.2016.10.007
176. Inozu M, Kahya Y, Yorulmaz O. Neuroticism and Religiosity: The Role of Obsessive Beliefs, Thought-Control Strategies and Guilt in Scrupulosity and Obsessive-Compulsive Symptoms Among Muslim Undergraduates. J Religion Health (2018). doi: 10.1007/s10943-018-0603-5
177. Smith DJ, Escott-Price V, Davies G, Bailey MES, Colodro-Conde L, Ward J, et al. Genome-wide analysis of over 106 000 individuals identifies 9 neuroticism-associated loci. Mol Psychiatry (2016) 21(6):749–57. doi: 10.1038/mp.2016.49
178. Sosnowska J, De Fruyt F, Hofmans J. Relating Neuroticism to Emotional Exhaustion: A Dynamic Approach to Personality. Front Psychol (2019) 10:2264. doi: 10.3389/fpsyg.2019.02264
179. Lahey BB. Public health significance of neuroticism. Am Psychol (2009) 64(4):241–56. doi: 10.1037/a0015309
180. Ashina S, Bendtsen L, Buse DC, Lyngberg AC, Lipton RB, Jensen R. Neuroticism, depression and pain perception in migraine and tension-type headache. Acta Neurolog Scand (2017) 136(5):470–6. doi: 10.1111/ane.12751
181. Hannuschke M, Gollwitzer M, Geukes K, Nestler S, Back M. Neuroticism and interpersonal perception: Evidence for positive, but not negative, biases. J Pers (2020) 88(2):217–36. doi: 10.1111/jopy.12480
182. Andric S, Maric NP, Knezevic G, Mihaljevic M, Mirjanic T, Velthorst E, et al. Neuroticism and facial emotion recognition in healthy adults. Early Intervention Psychiatry (2016) 10(2):160–4. doi: 10.1111/eip.12212
183. de Moor MH, van den Berg SM, Verweij KJ, Krueger RF, Luciano M, Arias Vasquez A, et al. Meta-analysis of Genome-wide Association Studies for Neuroticism, and the Polygenic Association With Major Depressive Disorder. JAMA Psychiatry (2015) 72(7):642–50. doi: 10.1001/jamapsychiatry.2015.0554
184. Bey K, Lennertz L, Riesel A, Klawohn J, Kaufmann C, Heinzel S, et al. Harm avoidance and childhood adversities in patients with obsessive-compulsive disorder and their unaffected first-degree relatives. Acta Psychiatr Scand (2017) 135(4):328–38. doi: 10.1111/acps.12707
185. Josefsson K, Jokela M, Cloninger CR, Hintsanen M, Salo J, Hintsa T, et al. Maturity and change in personality: developmental trends of temperament and character in adulthood. Dev Psychopathol (2013) 25(3):713–27. doi: 10.1017/s0954579413000126
186. Grisham JR, Fullana MA, Mataix-Cols D, Moffitt TE, Caspi A, Poulton R. Risk factors prospectively associated with adult obsessive-compulsive symptom dimensions and obsessive-compulsive disorder. Psychol Med (2011) 41(12):2495–506. doi: 10.1017/s0033291711000894
187. Giovanni AD, Carla G, Enrica M, Federico A, Maria Z, Secondo F. Eating disorders and major depression: role of anger and personality. Depression Res Treat (2011) 2011:194732. doi: 10.1155/2011/194732
188. Cloninger CR, Svrakic DM, Przybeck TR. Can personality assessment predict future depression? A twelve-month follow-up of 631 subjects. J Affect Disord (2006) 92(1):35–44. doi: 10.1016/j.jad.2005.12.034
189. Mandelli L, Mazza M, Di Nicola M, Zaninotto L, Harnic D, Catalano V, et al. Role of substance abuse comorbidity and personality on the outcome of depression in bipolar disorder: harm avoidance influences medium-term treatment outcome. Psychopathology (2012) 45(3):174–8. doi: 10.1159/000330364
190. Kampman O, Poutanen O. Can onset and recovery in depression be predicted by temperament? A systematic review and meta-analysis. J Affect Disord (2011) 135(1-3):20–7. doi: 10.1016/j.jad.2010.12.021
191. Balestri M, Porcelli S, Souery D, Kasper S, Dikeos D, Ferentinos P, et al. Temperament and character influence on depression treatment outcome. J Affect Disord (2019) 252:464–74. doi: 10.1016/j.jad.2019.04.031
192. Eric AP, Eric I, Curkovic M, Dodig-Curkovic K, Kralik K, Kovac V, et al. The temperament and character traits in patients with major depressive disorder and bipolar affective disorder with and without suicide attempt. Psychiatr Danubina (2017) 29(2):171–8. doi: 10.24869/psyd.2017.171
193. Jylha P, Mantere O, Melartin T, Suominen K, Vuorilehto M, Arvilommi P, et al. Differences in temperament and character dimensions in patients with bipolar I or II or major depressive disorder and general population subjects. psychol Med (2011) 41(8):1579–91. doi: 10.1017/s0033291710002606
194. Jylha P, Ketokivi M, Mantere O, Melartin T, Suominen K, Vuorilehto M, et al. Temperament, character and personality disorders. Eur Psychiatry : J Assoc Eur Psychiatrists (2013) 28(8):483–91. doi: 10.1016/j.eurpsy.2013.06.003
195. Zaninotto L, Solmi M, Toffanin T, Veronese N, Cloninger CR. Correll CU. A meta-analysis of temperament and character dimensions in patients with mood disorders: Comparison to healthy controls and unaffected siblings. J Affect Disord (2016) 194:84–97. doi: 10.1016/j.jad.2015.12.077
196. Garcia Herrero AM, Sanchez-Meca J, Alvarez Munoz FJ, Rubio-Aparicio M, Navarro-Mateu F. [Neuroticism and suicidal thoughts: a meta-analytic study]. Rev Espanola Salud Publica (2018) 92.
197. Peters EM, John A, Bowen R, Baetz M, Balbuena L. Neuroticism and suicide in a general population cohort: results from the UK Biobank Project. BJPsych Open (2018) 4(2):62–8. doi: 10.1192/bjo.2017.12
198. Bluml V, Kapusta ND, Doering S, Brahler E, Wagner B, Kersting A. Personality factors and suicide risk in a representative sample of the German general population. PloS One (2013) 8(10):e76646. doi: 10.1371/journal.pone.0076646
199. Hooijer AAT, Sizoo BB. Temperament and Character as Risk Factor for Suicide Ideation and Attempts in Adults with Autism Spectrum Disorders. Autism Res : Off J Int Soc Autism Res (2019) 13(1):104–11. doi: 10.1002/aur.2221
200. Perroud N, Baud P, Ardu S, Krejci I, Mouthon D, Vessaz M, et al. Temperament personality profiles in suicidal behaviour: an investigation of associated demographic, clinical and genetic factors. J Affect Disord (2013) 146(2):246–53. doi: 10.1016/j.jad.2012.09.012
201. Sarisoy G, Kacar OF, Pazvantoglu O, Ozturk A, Korkmaz IZ, Kocamanoglu B, et al. Temperament and character traits in patients with bipolar disorder and associations with attempted suicide. Compr Psychiatry (2012) 53(8):1096–102. doi: 10.1016/j.comppsych.2012.05.002
202. Ardani AR, Naghibzadeh B, Farid Hosseini F, Asadpour Z, Khabazianzadeh F. Temperament and character personality profile and affective temperaments in self-poisoning nonlethal suicide attempters. Psychiatry Res (2015) 229(1-2):394–400. doi: 10.1016/j.psychres.2015.06.035
203. Woo YS, Jun TY, Jeon YH, Song HR, Kim TS, Kim JB, et al. Relationship of temperament and character in remitted depressed patients with suicidal ideation and suicide attempts–results from the CRESCEND study. PloS One (2014) 9(10):e105860. doi: 10.1371/journal.pone.0105860
204. Lee K, Lee HK, Kim SH. Temperament and character profile of college students who have suicidal ideas or have attempted suicide. J Affect Disord (2017) 221:198–204. doi: 10.1016/j.jad.2017.06.025
205. Bouchard TJ Jr., McGue M. Genetic and environmental influences on human psychological differences. J Neurobiol (2003) 54(1):4–45. doi: 10.1002/neu.10160
206. McCrae RR, Jang KL, Livesley WJ, Riemann R, Angleitner A. Sources of structure: genetic, environmental, and artifactual influences on the covariation of personality traits. J Pers (2001) 69(4):511–35. doi: 10.1111/1467-6494.694154
207. Bouchard TJ Jr., Lykken DT, McGue M, Segal NL, Tellegen A. Sources of human psychological differences: the Minnesota Study of Twins Reared Apart. Sci (New York NY) (1990) 250(4978):223–8. doi: 10.1126/science.2218526
208. Vukasovic T, Bratko D. Heritability of personality: A meta-analysis of behavior genetic studies. Psychol Bull (2015) 141(4):769–85. doi: 10.1037/bul0000017
209. Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry (1987) 44(6):573. doi: 10.1001/archpsyc.1987.01800180093014
210. Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states: a reply to commentaries. Psychiatr Dev (1988) 6(2):83–120.
211. Costa PT Jr., McCrae RR. Stability and change in personality assessment: the revised NEO Personality Inventory in the year 2000. J Pers Assess (1997) 68(1):86–94. doi: 10.1207/s15327752jpa6801_7
212. Rocklin T, Revelle W. The measurement of extroversion: A comparison of the Eysenck Personality Inventory and the Eysenck Personality Questionnaire. Br J Soc Psychol (1981) 20(4):279–84. doi: 10.1111/j.2044-8309.1981.tb00498.x
213. Smillie LD, Cooper AJ, Pickering AD. Individual differences in reward-prediction-error: extraversion and feedback-related negativity. Soc Cogn Affect Neurosci (2011) 6(5):646–52. doi: 10.1093/scan/nsq078
214. Kennis M, Rademaker AR, Geuze E. Neural correlates of personality: an integrative review. Neurosci Biobehav Rev (2013) 37(1):73–95. doi: 10.1016/j.neubiorev.2012.10.012
215. Speed BC, Nelson BD, Levinson AR, Perlman G, Klein DN, Kotov R, et al. Extraversion, neuroticism, and the electrocortical response to monetary rewards in adolescent girls. Biol Psychol (2018) 136:111–8. doi: 10.1016/j.biopsycho.2018.05.017
216. Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: relation of agonist-induced dopamine activity to positive emotionality. J Pers Soc Psychol (1994) 67(3):485–98. doi: 10.1037//0022-3514.67.3.485
217. Cohen MX, Young J, Baek JM, Kessler C, Ranganath C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res Cogn Brain Res (2005) 25(3):851–61. doi: 10.1016/j.cogbrainres.2005.09.018
218. Fischer R, Lee A, Verzijden MN. Dopamine genes are linked to Extraversion and Neuroticism personality traits, but only in demanding climates. Sci Rep (2018) 8(1):1733. doi: 10.1038/s41598-017-18784-y
219. Kim SE, Kim HN, Yun YJ, Heo SG, Cho J, Kwon MJ, et al. Meta-analysis of genome-wide SNP- and pathway-based associations for facets of neuroticism. J Hum Genet (2017) 62(10):903–9. doi: 10.1038/jhg.2017.61
220. Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Networks : Off J Int Neural Network Soc (2002) 15(4-6):603–16. doi: 10.1016/s0893-6080(02)00052-7
221. Aluja A, Balada F, Blanco E, Fibla J, Blanch A. Twenty candidate genes predicting neuroticism and sensation seeking personality traits: A multivariate analysis association approach. Pers Individ Dif (2019) 140:90–102. doi: 10.1016/j.paid.2018.03.041
222. Rodríguez-Ramos Á, Moriana JA, García-Torres F, Ruiz-Rubio M. Emotional stability is associated with the MAOA promoter uVNTR polymorphism in women. Brain Behav (2019) 9(9):e01376–e. doi: 10.1002/brb3.1376
223. Chmielowiec J, Chmielowiec K, Suchanecka A, Trybek G, Mroczek B, Malecka I, et al. Associations Between the Dopamine D4 Receptor and DAT1 Dopamine Transporter Genes Polymorphisms and Personality Traits in Addicted Patients. Int J Environ Res Public Health (2018) 15(10). doi: 10.3390/ijerph15102076
224. Griffioen G, Matheson GJ, Cervenka S, Farde L, Borg J. Serotonin 5-HT1A receptor binding and self-transcendence in healthy control subjects-a replication study using Bayesian hypothesis testing. PeerJ (2018) 6:e5790. doi: 10.7717/peerj.5790
225. Rahman MS, Guban P, Wang M, Melas PA, Forsell Y, Lavebratt C. The serotonin transporter promoter variant (5-HTTLPR) and childhood adversity are associated with the personality trait openness to experience. Psychiatry Res (2017) 257:322–6. doi: 10.1016/j.psychres.2017.07.071
226. Chang C-C, Chang H-A, Fang W-H, Chang T-C, Huang S-Y. Gender-specific association between serotonin transporter polymorphisms (5-HTTLPR and rs25531) and neuroticism, anxiety and depression in well-defined healthy Han Chinese. J Affect Disord (2017) 207:422–8. doi: 10.1016/j.jad.2016.08.055
227. Stenbaek DS, Dam VH, Fisher PM, Hansen N, Hjordt LV, Frokjaer VG. No evidence for a role of the serotonin 4 receptor in five-factor personality traits: A positron emission tomography brain study. PloS One (2017) 12(9):e0184403. doi: 10.1371/journal.pone.0184403
228. Tuominen L, Miettunen J, Cannon DM, Drevets WC, Frokjaer VG, Hirvonen J, et al. Neuroticism Associates with Cerebral in Vivo Serotonin Transporter Binding Differently in Males and Females. Int J Neuropsychoph (2017) 20(12):963–70. doi: 10.1093/ijnp/pyx071
229. Caravaggio F, Fervaha G, Chung JK, Gerretsen P, Nakajima S, Plitman E, et al. Exploring personality traits related to dopamine D2/3 receptor availability in striatal subregions of humans. Eur Neuropsychoph (2016) 26(4):644–52. doi: 10.1016/j.euroneuro.2016.02.010
230. da Cunha-Bang S, Mc Mahon B, Fisher PM, Jensen PS, Svarer C, Knudsen GM. High trait aggression in men is associated with low 5-HT levels, as indexed by 5-HT4 receptor binding. Soc Cognit Affect Neurosci (2016) 11(4):548–55. doi: 10.1093/scan/nsv140
231. Jurczak A, Szkup M, Wieder-Huszla S, Grzywacz A, Samochowiec A, Karakiewicz B, et al. The assessment of the relationship between personality, the presence of the 5HTT and MAO-A polymorphisms, and the severity of climacteric and depressive symptoms in postmenopausal women. Arch Womens Ment Health (2015) 18(4):613–21. doi: 10.1007/s00737-015-0497-0
232. Lehto K, Vaht M, Mäestu J, Veidebaum T, Harro J. Effect of tryptophan hydroxylase-2 gene polymorphism G-703 T on personality in a population representative sample. Prog Neuropsychopharmacol Biol Psychiatry (2015) 57:31–5. doi: 10.1016/j.pnpbp.2014.10.005
233. Kotyuk E, Duchek J, Head D, Szekely A, Goate AM, Balota DA. A genetic variant (COMT) coding dopaminergic activity predicts personality traits in healthy elderly. Pers Individ Dif (2015) 82:61–6. doi: 10.1016/j.paid.2015.03.012
234. Hirvonen J, Tuominen L, Nagren K, Hietala J. Neuroticism and serotonin 5-HT1A receptors in healthy subjects. Psychiatry Res (2015) 234(1):1–6. doi: 10.1016/j.pscychresns.2015.04.007
235. Kim JH, Son YD, Kim JH, Choi EJ, Lee SY, Joo YH, et al. Self-transcendence trait and its relationship with in vivo serotonin transporter availability in brainstem raphe nuclei: An ultra-high resolution PET-MRI study. Brain Res (2015) 1629:63–71. doi: 10.1016/j.brainres.2015.10.006
236. Ma H, Huang Y, Zhang B, Jin L, Cong Z, Wang Y, et al. Neurotensin receptor 1 gene polymorphisms are associated with personality traits in healthy Chinese individuals. Neuropsychobiology (2014) 69(1):11–8. doi: 10.1159/000356966
237. Lawrence AD, Brooks DJ. Ventral striatal dopamine synthesis capacity is associated with individual differences in behavioral disinhibition. Front Behav Neurosci (2014) 8:86. doi: 10.3389/fnbeh.2014.00086
238. Stokes PR, Benecke A, Puraite J, Bloomfield MA, Shotbolt P, Reeves SJ, et al. Does human presynaptic striatal dopamine function predict social conformity? J Psychopharmacol (Oxford) (2014) 28(3):237–43. doi: 10.1177/0269881113512037
239. Thomson CJ, Rajala AK, Carlson SR, Rupert JL. Variants in the dopamine-4-receptor gene promoter are not associated with sensation seeking in skiers. PloS One (2014) 9(4):e93521. doi: 10.1371/journal.pone.0093521
240. Egorova MS, Alfimova MV, Parshikova OV. Pyankova SD. A Serotonin Transporter Gene Promoter Polymorphism (5-HTTLPR), Serotonin 2C Receptor (5-HT2CR) and Sensation Seeking. Proc Soc Behav Sci (2013) 86:24–8. doi: 10.1016/j.sbspro.2013.08.519
241. Peciña M, Mickey BJ, Love T, Wang H, Langenecker SA, Hodgkinson C, et al. DRD2 polymorphisms modulate reward and emotion processing, dopamine neurotransmission and openness to experience. Cortex (2013) 49(3):877–90. doi: 10.1016/j.cortex.2012.01.010
242. Tuominen L, Salo J, Hirvonen J, Nagren K, Laine P, Melartin T, et al. Temperament, character and serotonin activity in the human brain: a positron emission tomography study based on a general population cohort. Psychol Med (2013) 43(4):881–94. doi: 10.1017/s003329171200164x
243. da Cunha-Bang S, Stenbaek DS, Holst K, Licht CL, Jensen PS, Frokjaer VG, et al. Trait aggression and trait impulsivity are not related to frontal cortex 5-HT2A receptor binding in healthy individuals. Psychiatry Res (2013) 212(2):125–31. doi: 10.1016/j.pscychresns.2012.09.007
244. Oniszczenko W, Dragan WL. Association between temperament in terms of the Regulative Theory of Temperament and DRD4 and DAT1 gene polymorphisms. Compr Psychiatry (2012) 53(6):789–96. doi: 10.1016/j.comppsych.2012.01.001
245. Tsuchimine S, Yasui-Furukori N, Sasaki K, Kaneda A, Sugawara N, Yoshida S, et al. Association between the dopamine D2 receptor (DRD2) polymorphism and the personality traits of healthy Japanese participants. Prog Neuro-Psychopharmacol Biol Psychiatry (2012) 38(2):190–3. doi: 10.1016/j.pnpbp.2012.03.008
246. Li J, Ma H, Huang Y, Wu L, Li J, Zhao X, et al. No association of a casein kinase 1epsilon (CK1epsilon) gene polymorphism with personality traits in healthy Chinese-Han subjects. J Mol Neurosci : MN (2012) 47(3):437–41. doi: 10.1007/s12031-011-9680-6
247. Luciano M, MacLeod AK, Payton A, Davies G, Ke X, Tenesa A, et al. Effects of gene copy number variants on personality and mood in ageing cohorts. Pers Individ Dif (2012) 53(4):393–7. doi: 10.1016/j.paid.2011.12.019
248. Takahashi H, Takano H, Camerer CF, Ideno T, Okubo S, Matsui H, et al. Honesty mediates the relationship between serotonin and reaction to unfairness. Proc Natl Acad Sci U S A (2012) 109(11):4281–4. doi: 10.1073/pnas.1118687109
249. Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, et al. Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: a high resolution PET study. Biol Psychol (2011) 87(1):164–7. doi: 10.1016/j.biopsycho.2011.02.01160
250. Kazantseva A, Gaysina D, Malykh S, Khusnutdinova E. The role of dopamine transporter (SLC6A3) and dopamine D2 receptor/ankyrin repeat and kinase domain containing 1 (DRD2/ANKK1) gene polymorphisms in personality traits. Prog Neuropsychopharmacol Biol Psychiatry (2011) 35(4):1033–40. doi: 10.1016/j.pnpbp.2011.02.013
251. Calati R, Porcelli S, Giegling I, Hartmann AM, Möller H-J, De Ronchi D, et al. Catechol-o-methyltransferase gene modulation on suicidal behavior and personality traits: review, meta-analysis and association study. J Psychiatr Res (2011) 45(3):309–21. doi: 10.1016/j.jpsychires.2010.07.004
252. Verschoor E, Markus CR. Affective and neuroendocrine stress reactivity to an academic examination: Influence of the 5-HTTLPR genotype and trait neuroticism. Biol Psychol (2011) 87(3):439–49. doi: 10.1016/j.biopsycho.2011.06.001
253. Gerra G, Garofano L, Castaldini L, Rovetto F, Zaimovic A, Moi G, et al. Serotonin transporter promoter polymorphism genotype is associated with temperament, personality traits and illegal drugs use among adolescents. J Neural Transm (2005) 112(10):1397–410. doi: 10.1007/s00702-004-0268-y
254. Paaver M, Kurrikoff T, Nordquist N, Oreland L, Harro J. The effect of 5-HTT gene promoter polymorphism on impulsivity depends on family relations in girls. Prog Neuropsychopharmacol Biol Psychiatry (2008) 32(5):1263–8. doi: 10.1016/j.pnpbp.2008.03.021
255. Li J, Ma H, Zhou H, Huang Y, Wu L, Li J, et al. Association between DARPP-32 gene polymorphism and personality traits in healthy Chinese-Han subjects. J Mol Neurosci : MN (2011) 44(1):48–52. doi: 10.1007/s12031-011-9505-7
256. Cervenka S, Gustavsson JP, Halldin C, Farde L. Association between striatal and extrastriatal dopamine D2-receptor binding and social desirability. NeuroImage (2010) 50(1):323–8. doi: 10.1016/j.neuroimage.2009.12.006
257. Soloff PH, Price JC, Mason NS, Becker C, Meltzer CC. Gender, personality, and serotonin-2A receptor binding in healthy subjects. Psychiatry Res (2010) 181(1):77–84. doi: 10.1016/j.pscychresns.2009.08.007
258. Kalbitzer J, Frokjaer VG, Erritzoe D, Svarer C, Cumming P, Nielsen FA, et al. The personality trait openness is related to cerebral 5-HTT levels. NeuroImage (2009) 45(2):280–5. doi: 10.1016/j.neuroimage.2008.12.001
259. Keltikangas-Jarvinen L, Pulkki-Raback L, Elovainio M, Raitakari OT, Viikari J, Lehtimaki T. DRD2 C32806T modifies the effect of child-rearing environment on adulthood novelty seeking. Am J Med Genet B Neuropsychiatr Genet (2009) 150b(3):389–94. doi: 10.1002/ajmg.b.30830
260. Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, et al. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry (2008) 63(6):569–76. doi: 10.1016/j.biopsych.2007.07.009
261. Tsuchimine S, Yasui-Furukori N, Kaneda A, Saito M, Nakagami T, Sato K, et al. Association between monoamine oxidase A (MAOA) and personality traits in Japanese individuals. Prog Neuropsychopharmacol Biol Psychiatry (2008) 32(8):1932–5. doi: 10.1016/j.pnpbp.2008.09.012
262. Kazantseva AV, Gaysina DA, Faskhutdinova GG, Noskova T, Malykh SB, Khusnutdinova EK. Polymorphisms of the serotonin transporter gene (5-HTTLPR, A/G SNP in 5-HTTLPR, and STin2 VNTR) and their relation to personality traits in healthy individuals from Russia. Psychiat Genet (2008) 18(4):167–76. doi: 10.1097/YPG.0b013e328304deb8
263. Takano A, Arakawa R, Hayashi M, Takahashi H, Ito H, Suhara T. Relationship between neuroticism personality trait and serotonin transporter binding. Biolog Psychiatry (2007) 62(6):588–92. doi: 10.1016/j.biopsych.2006.11.007
264. Huang CL, Yang YK, Chu CL, Lee IH, Yeh TL, Chen PS, et al. The association between the Lie scale of the Maudsley personality inventory and striatal dopamine D2/D3 receptor availability of healthy Chinese community subjects. Eur Psychiatry (2006) 21(1):62–5. doi: 10.1016/j.eurpsy.2005.05.004
265. Shiraishi H, Suzuki A, Fukasawa T, Aoshima T, Ujiie Y, Ishii G, et al. Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants. Psychiat Genet (2006) 16(2):55–8. doi: 10.1097/01.ypg.0000199447.62044.ef
266. Tochigi M, Otowa T, Hibino H, Kato C, Otani T, Umekage T, et al. Combined analysis of association between personality traits and three functional polymorphisms in the tyrosine hydroxylase, monoamine oxidase A, and catechol-O-methyltransferase genes. Neurosci Res (2006) 54(3):180–5. doi: 10.1016/j.neures.2005.11.003
267. Yu YW, Yang CW, Wu HC, Tsai SJ, Hong CJ, Chen MC, et al. Association study of a functional MAOA-uVNTR gene polymorphism and personality traits in Chinese young females. Neuropsychobiology (2005) 52(3):118–21. doi: 10.1159/000087556
268. Matsuzawa D, Hashimoto K, Shimizu E, Fujisaki M, Iyo M. Functional polymorphism of the glutathione peroxidase 1 gene is associated with personality traits in healthy subjects. Neuropsychobiology (2005) 52(2):68–70. doi: 10.1159/000086607
269. Hakamata Y, Takahashi N, Ishihara R, Saito S, Ozaki N, Honjo S, et al. No association between monoamine oxidase A promoter polymorphism and personality traits in Japanese females. Neurosci Lett (2005) 389(3):121–3. doi: 10.1016/j.neulet.2005.03.075
270. Laakso A, Wallius E, Kajander J, Bergman J, Eskola O, Solin O, et al. Personality traits and striatal dopamine synthesis capacity in healthy subjects. Am J Psychiatry (2003) 160(5):904–10. doi: 10.1176/appi.ajp.160.5.904
271. Borg J, Andree B, Soderstrom H, Farde L. The serotonin system and spiritual experiences. Am J Psychiatry (2003) 160(11):1965–9. doi: 10.1176/appi.ajp.160.11.1965
272. Strobel A, Spinath FM, Angleitner A, Riemann R, Lesch KP. Lack of association between polymorphisms of the dopamine D4 receptor gene and personality. Neuropsychobiology (2003) 47(1):52–6. doi: 10.1159/000068876
273. Lee HJ, Lee HS, Kim YK, Kim SH, Kim L, Lee MS, et al. Allelic variants interaction of dopamine receptor D4 polymorphism correlate with personality traits in young Korean female population. Am J Med Genet B Neuropsychiatr Genet (2003) 118b(1):76–80. doi: 10.1002/ajmg.b.10047
274. Enoch MA, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet (2003) 13(1):33–41. doi: 10.1097/00041444-200303000-00006
275. Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, et al. A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. NeuroImage (2002) 15(3):620–32. doi: 10.1006/nimg.2001.0984
276. Bookman EB, Taylor RE, Adams-Campbell L, Kittles RA. DRD4 promoter SNPs and gender effects on Extraversion in African Americans. Mol Psychiatry (2002) 7(7):786–9. doi: 10.1038/sj.mp.4001075
277. Moresco FM, Dieci M, Vita A, Messa C, Gobbo C, Galli L, et al. In vivo serotonin 5HT(2A) receptor binding and personality traits in healthy subjects: a positron emission tomography study. NeuroImage (2002) 17(3):1470–8. doi: 10.1006/nimg.2002.1239
278. Garpenstrand H, Norton N, Damberg M, Rylander G, Forslund K, Mattila-Evenden M, et al. A regulatory monoamine oxidase a promoter polymorphism and personality traits. Neuropsychobiology (2002) 46(4):190–3. doi: 10.1159/000067804
279. Yasuno F, Suhara T, Sudo Y, Yamamoto M, Inoue M, Okubo Y, et al. Relation among dopamine D(2) receptor binding, obesity and personality in normal human subjects. Neurosci Lett (2001) 300(1):59–61. doi: 10.1016/s0304-3940(01)01552-x
280. Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, et al. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. NeuroImage (2001) 13(5):891–5. doi: 10.1006/nimg.2001.0761
281. Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry (2001) 158(8):1326–8. doi: 10.1176/appi.ajp.158.8.1326
282. Benjamin J, Osher Y, Lichtenberg P, Bachner-Melman R, Gritsenko I, Kotler M, et al. An interaction between the catechol O-methyltransferase and serotonin transporter promoter region polymorphisms contributes to tridimensional personality questionnaire persistence scores in normal subjects. Neuropsychobiology (2000) 41(1):48–53. doi: 10.1159/000026632
283. Laakso A, Vilkman H, Kajander J, Bergman J, Paranta M, Solin O, et al. Prediction of detached personality in healthy subjects by low dopamine transporter binding. Am J Psychiatry (2000) 157(2):290–2. doi: 10.1176/appi.ajp.157.2.290
284. Okuyama Y, Ishiguro H, Nankai M, Shibuya H, Watanabe A, Arinami T. Identification of a polymorphism in the promoter region of DRD4 associated with the human novelty seeking personality trait. Mol Psychiatry (2000) 5(1):64–9. doi: 10.1038/sj.mp.4000563
285. Persson ML, Wasserman D, Geijer T, Frisch A, Rockah R, Michaelovsky E, et al. Dopamine D4 receptor gene polymorphism and personality traits in healthy volunteers. Eur Arch Psychiatry Clin Neurosci (2000) 250(4):203–6. doi: 10.1007/s004060070025
286. Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, et al. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet (2000) 96(2):202–16. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j
287. Tomitaka M, Tomitaka S, Otuka Y, Kim K, Matuki H, Sakamoto K, et al. Association between novelty seeking and dopamine receptor D4 (DRD4) exon III polymorphism in Japanese subjects. AmJ Med Genet (1999) 88(5):469–71. doi: 10.1002/(sici)1096-8628(19991015)88:5<469::aid-ajmg6>3.0.co;2-f
288. Noble EP, Ozkaragoz TZ, Ritchie TL, Zhang X, Belin TR, Sparkes RS. D2 and D4 dopamine receptor polymorphisms and personality. Am J Med Genet (1998) 81(3):257–67.
289. Gelernter J, Kranzler H, Coccaro E, Siever L, New A, Mulgrew CL. D4 dopamine-receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am J Hum Genet (1997) 61(5):1144–52. doi: 10.1086/301595
290. Jonsson EG, Nothen MM, Gustavsson JP, Neidt H, Brene S, Tylec A, et al. Lack of evidence for allelic association between personality traits and the dopamine D4 receptor gene polymorphisms. Am J Psychiatry (1997) 154(5):697–9. doi: 10.1176/ajp.154.5.697
291. Ono Y, Manki H, Yoshimura K, Muramatsu T, Mizushima H, Higuchi S, et al. Association between dopamine D4 receptor (D4DR) Exon III polymorphism and novelty seeking in Japanese subjects. Am J Med Genet (1997) 74(5):501–3. doi: 10.1002/(sici)1096-8628(19970919)74:5<501::aid-ajmg9>3.0.co;2-q
292. Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, et al. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of Novelty Seeking. Nat Genet (1996) 12(1):78–80. doi: 10.1038/ng0196-78
293. Ham BJ, Choi MJ, Lee HJ, Kang RH, Lee MS. Reward dependence is related to norepinephrine transporter T-182C gene polymorphism in a Korean population. Psychiat Genet (2005) 15(2):145–7. doi: 10.1097/00041444-200506000-00012
294. Narita S, Iwahashi K, Nagahori K, Numajiri M, Yoshihara E, Ohtani N, et al. Analysis of Association between Norepinephrine Transporter Gene Polymorphisms and Personality Traits of NEO-FFI in a Japanese Population. Psychiatry Invest (2015) 12(3):381–7. doi: 10.4306/pi.2015.12.3.381
295. Belsky J, Steinberg L, Draper P. Childhood experience, interpersonal development, and reproductive strategy: and evolutionary theory of socialization. Child Dev (1991) 62(4):647–70. doi: 10.1111/j.1467-8624.1991.tb01558.x
296. Windle M, Kogan SM, Lee S, Chen YF, Lei KM, Brody GH, et al. Neighborhood x Serotonin Transporter Linked Polymorphic Region (5-HTTLPR) interactions for substance use from ages 10 to 24 years using a harmonized data set of African American children. Dev Psychopathol (2016) 28(2):415–31. doi: 10.1017/s095457941500053x
297. Lehto K, Akkermann K, Parik J, Veidebaum T, Harro J. Effect of COMT Val158Met polymorphism on personality traits and educational attainment in a longitudinal population representative study. Eur Psychiatry (2013) 28(8):492–8. doi: 10.1016/j.eurpsy.2013.06.008
298. Robbins TW. Opinion on monoaminergic contributions to traits and temperament. Philos Trans R Soc London Ser B Biol Sci (2018) 373(1744). doi: 10.1098/rstb.2017.0153
Keywords: personality traits, mood disorder, major depressive disorder, monoamine neurotransmitters, mechanism
Citation: Shao X and Zhu G (2020) Associations Among Monoamine Neurotransmitter Pathways, Personality Traits, and Major Depressive Disorder. Front. Psychiatry 11:381. doi: 10.3389/fpsyt.2020.00381
Received: 19 January 2020; Accepted: 16 April 2020;
Published: 13 May 2020.
Edited by:
Shaohua Hu, Zhejiang University, ChinaReviewed by:
Chun Wang, Nanjing Hospital affiliated to Nanjing Medical University, ChinaJun Chen, Shanghai Jiao Tong University, China
Dubravka Svob Strac, Rudjer Boskovic Institute, Croatia
Copyright © 2020 Shao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Zhu, Z3podUBjbXUuZWR1LmNu
 Xiaojun Shao
Xiaojun Shao Gang Zhu
Gang Zhu